Submitted:
14 May 2025
Posted:
16 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of Microorganisms
2.2. Morphological Identification
2.3. Molecular Identification Through DNA Sequencing
2.4. Ex Vivo Fungal Activity
2.5. In Vitro Antifungal Activity with Essential Oils
3. Results
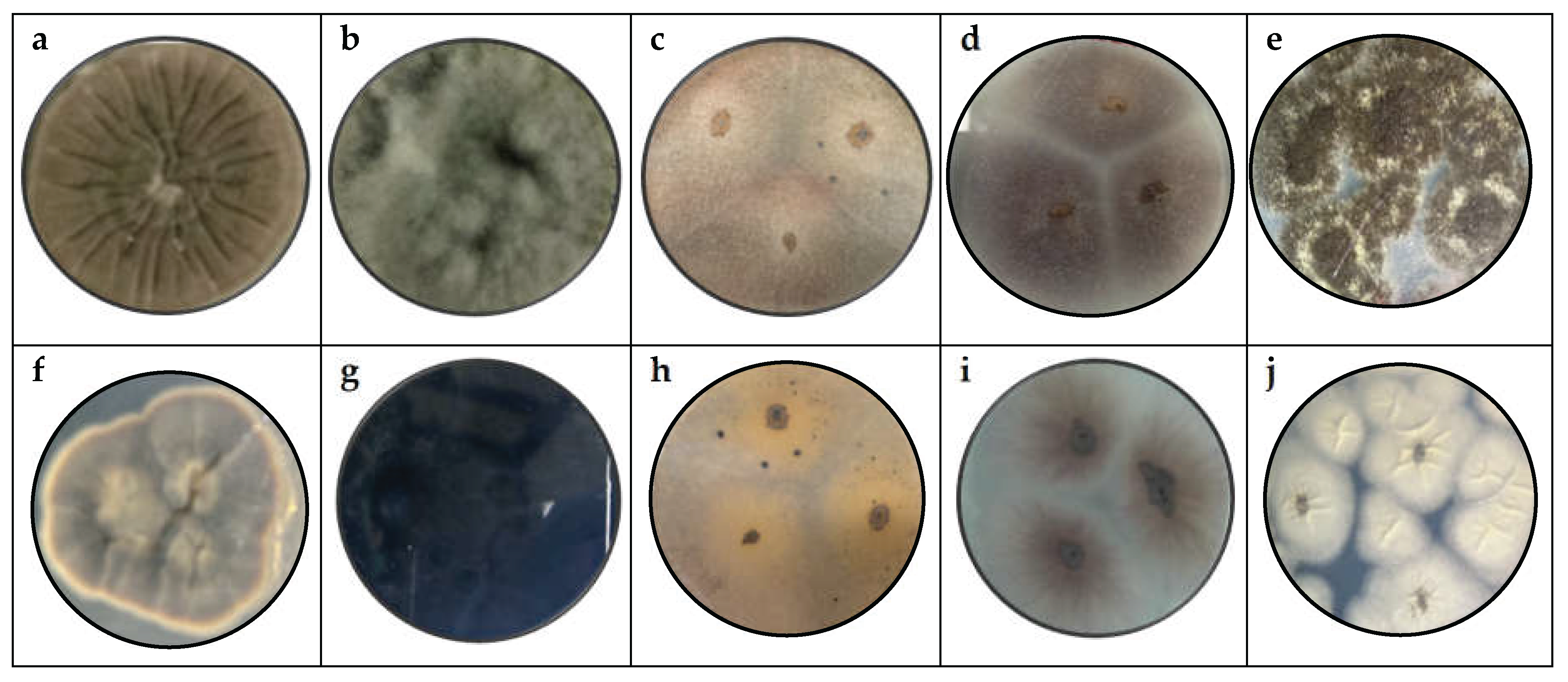
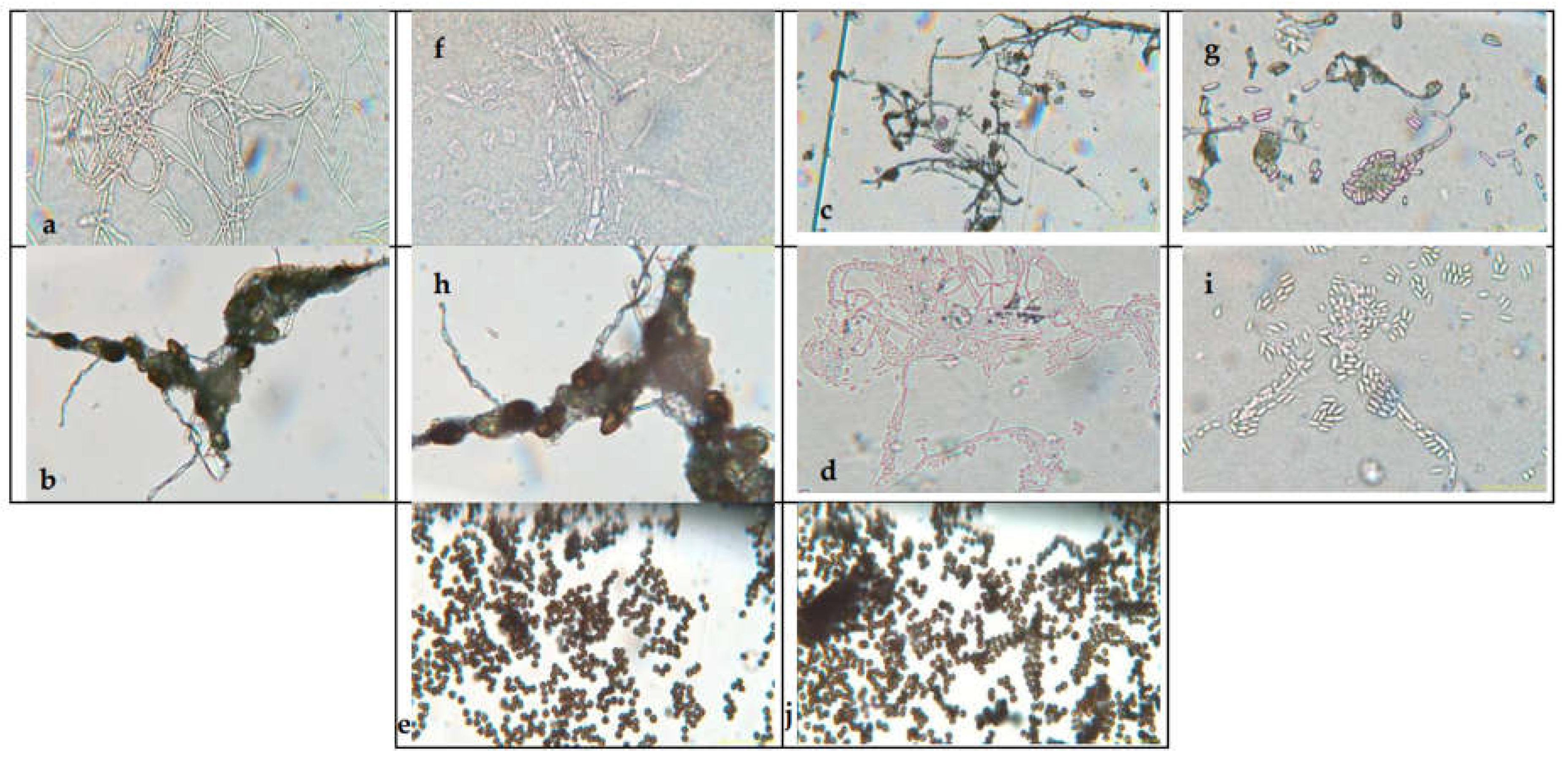
3.1. Morphological Identification
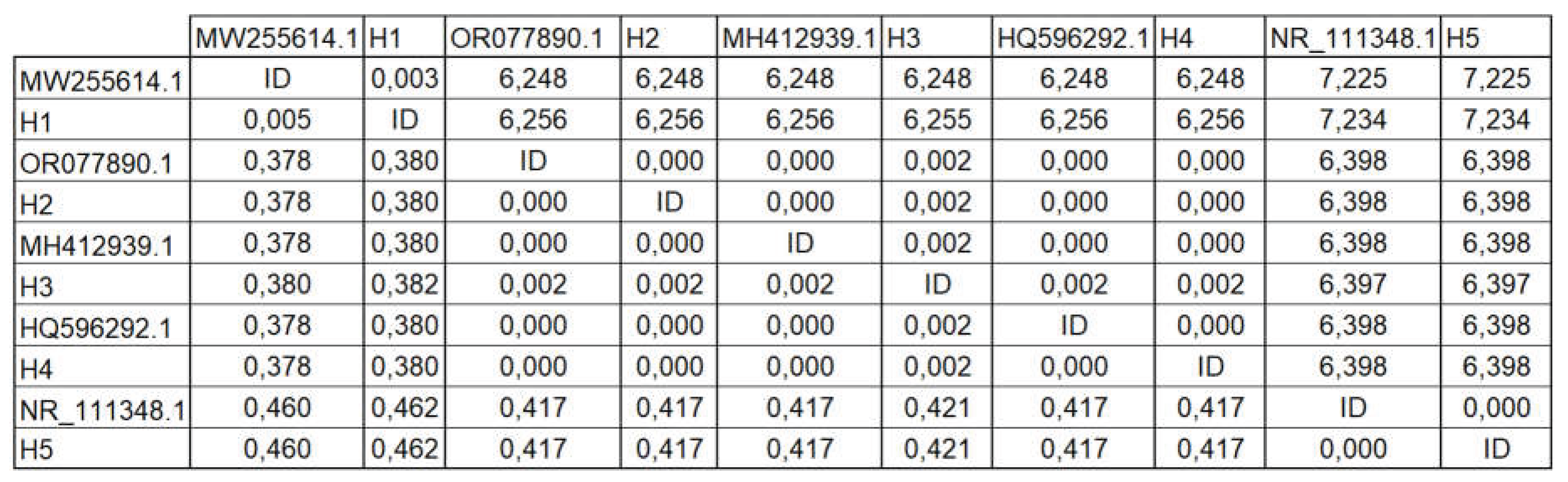
3.2. Molecular Identification Through DNA SEQUENCING
3.3. Fungal Activity Ex Vivo
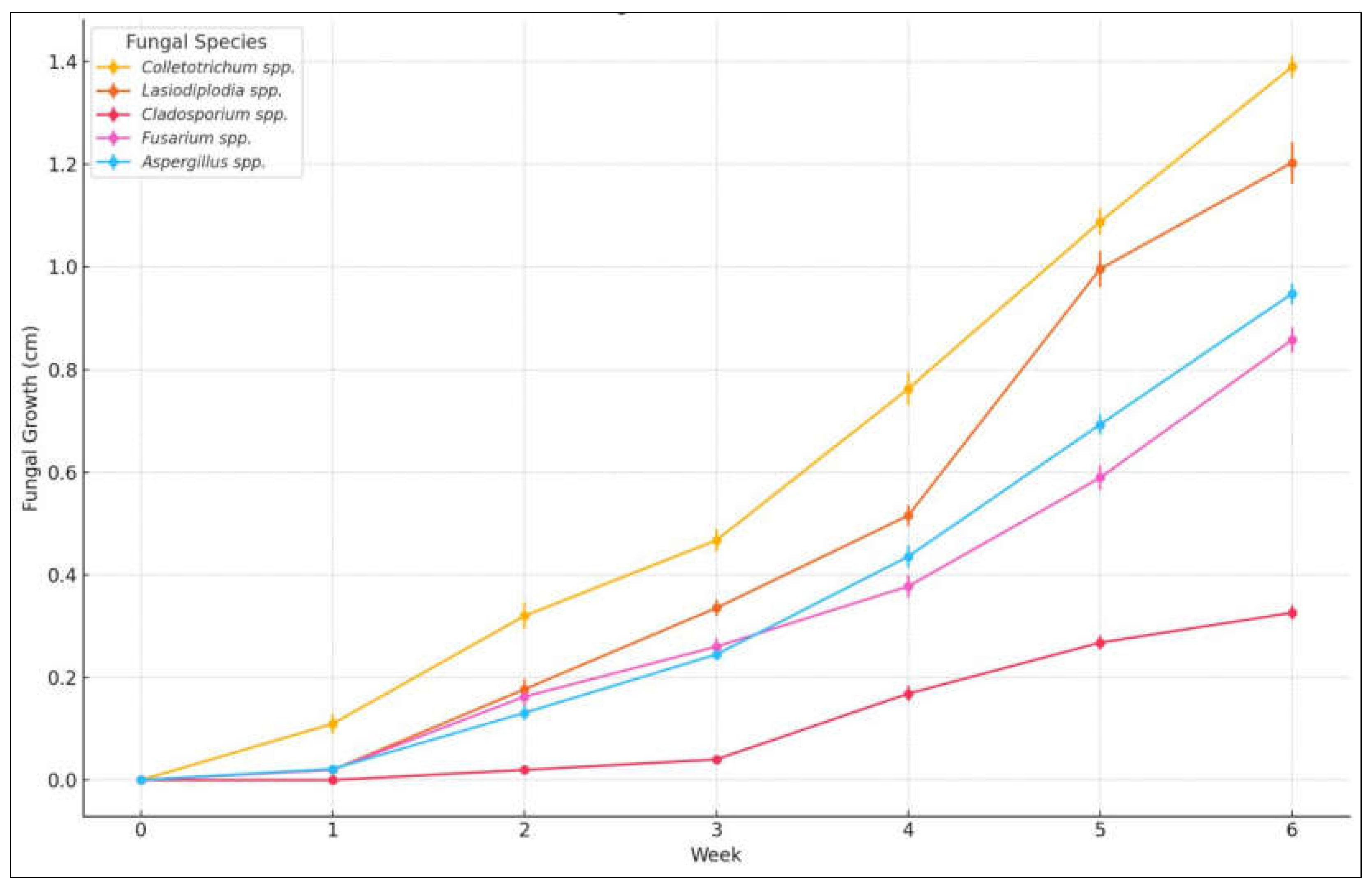
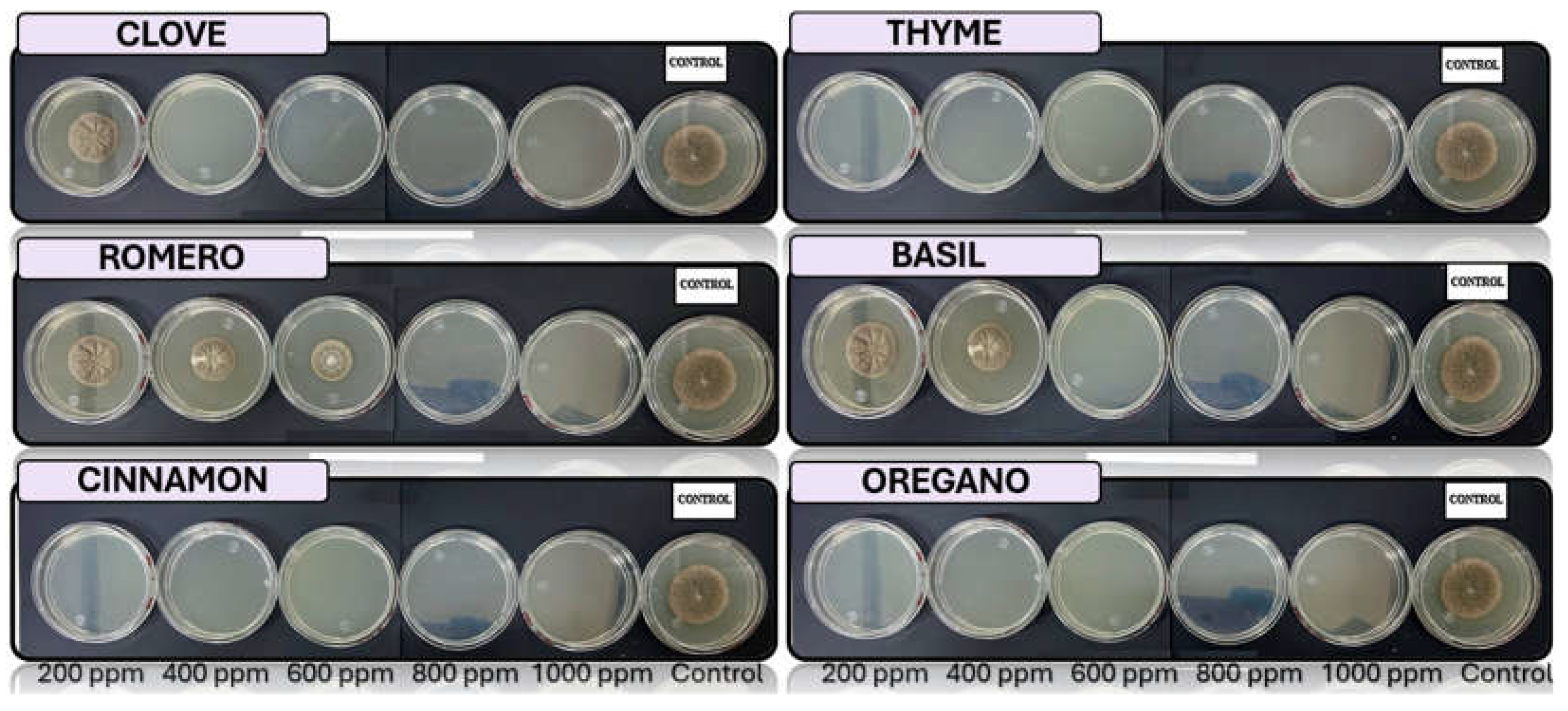
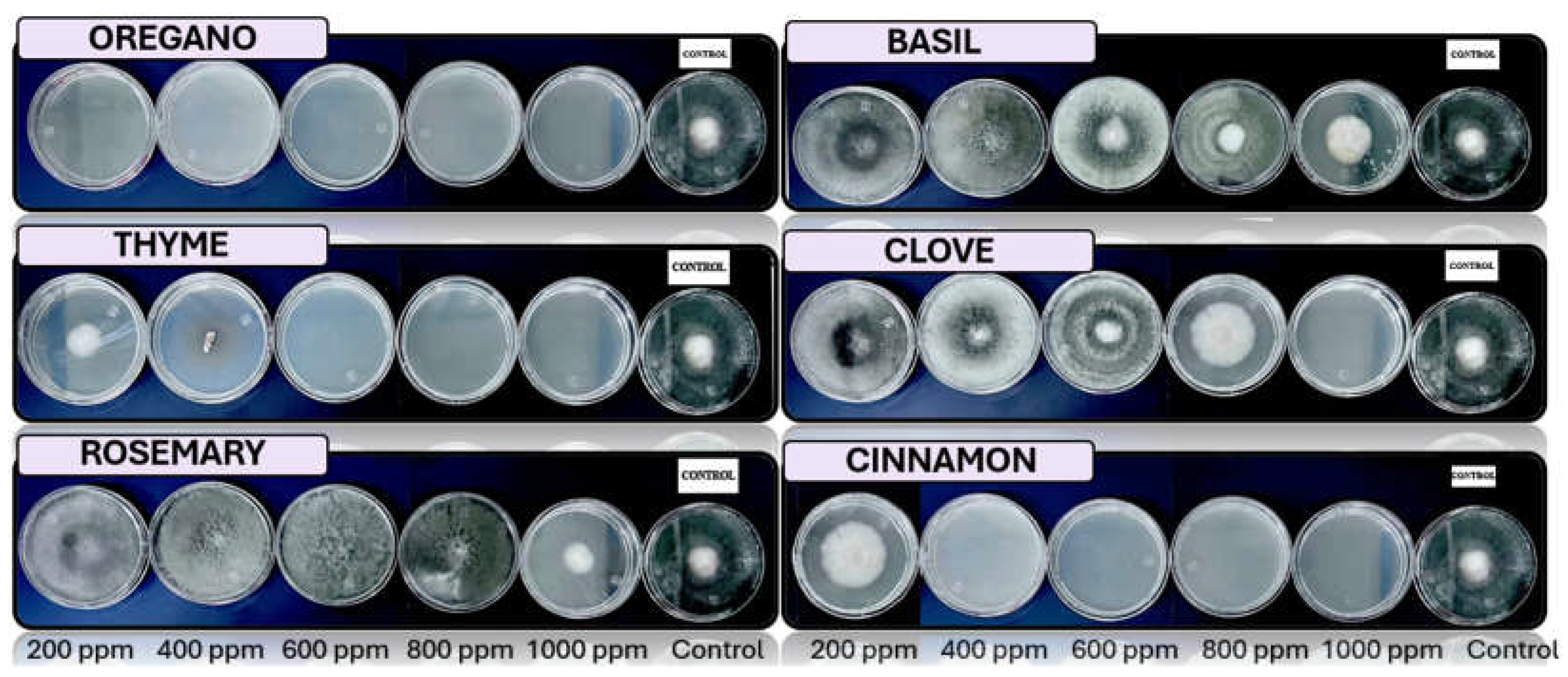
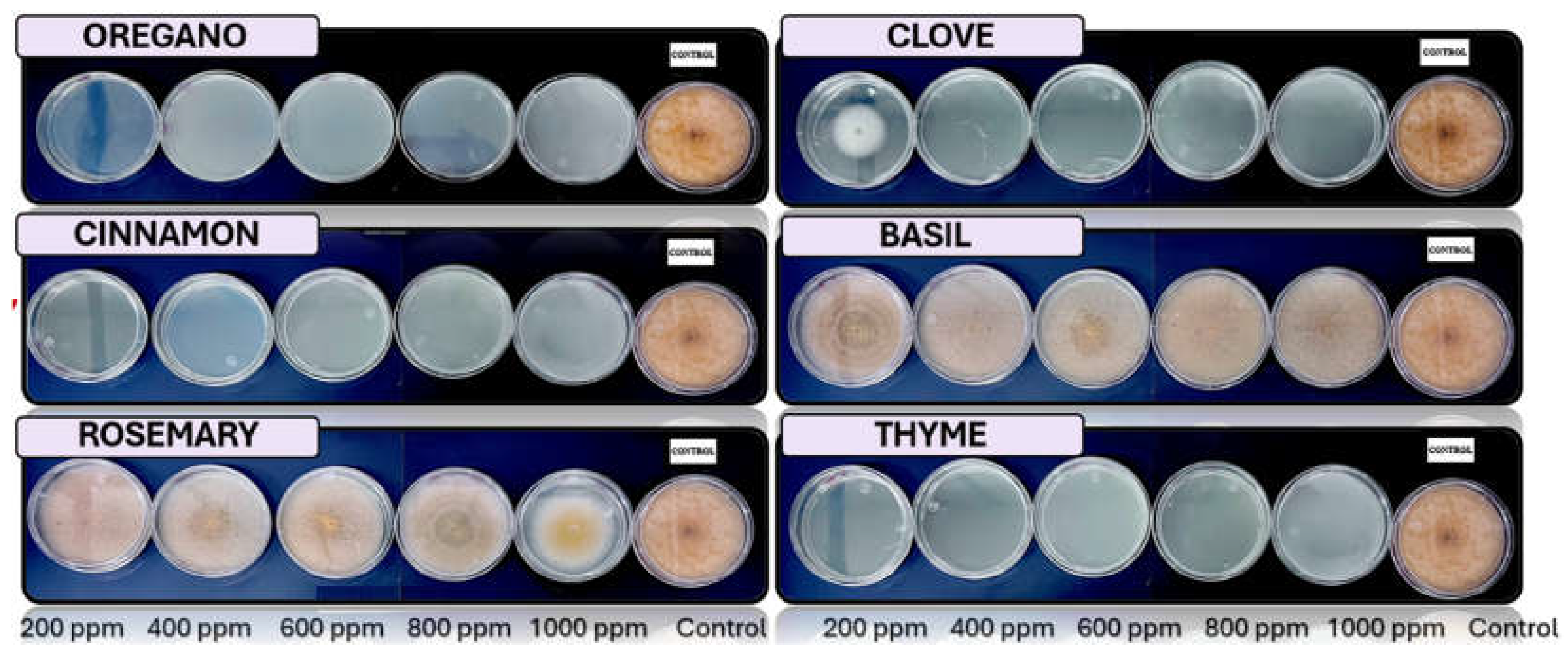
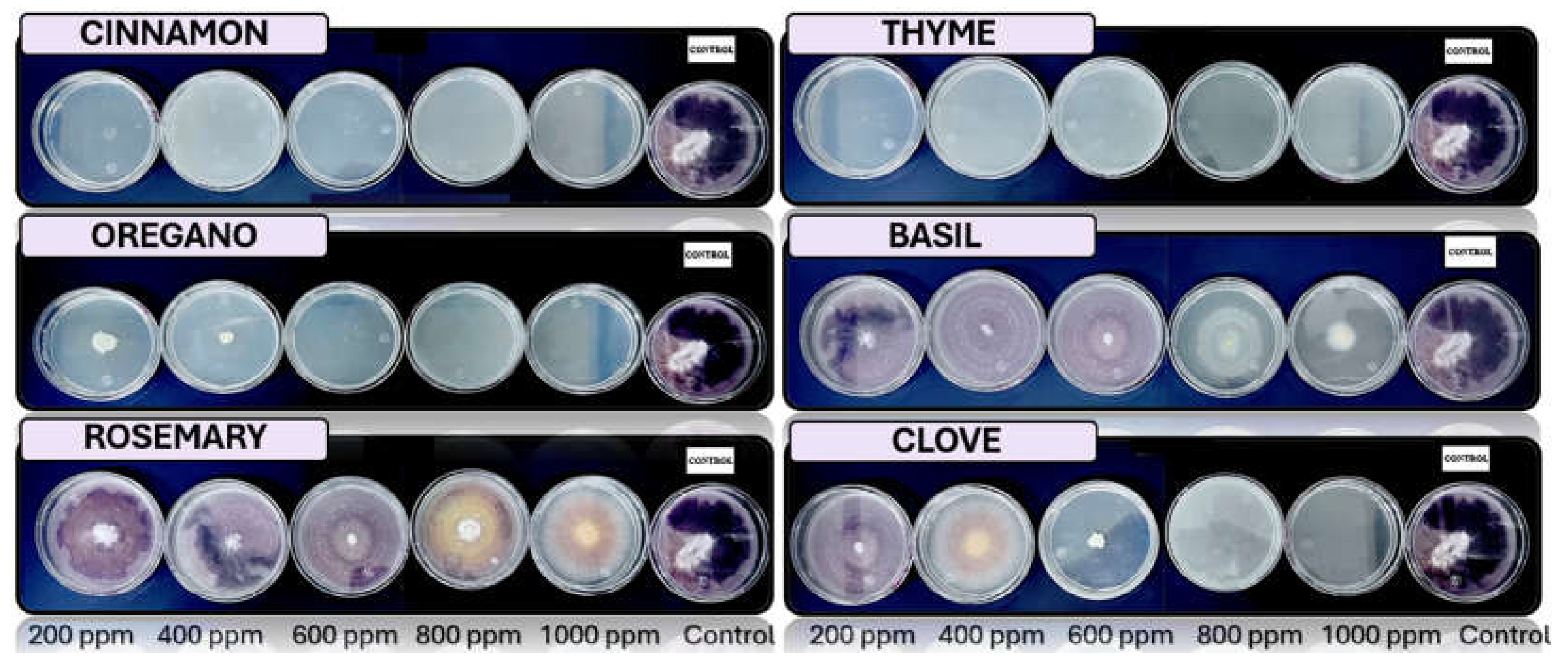
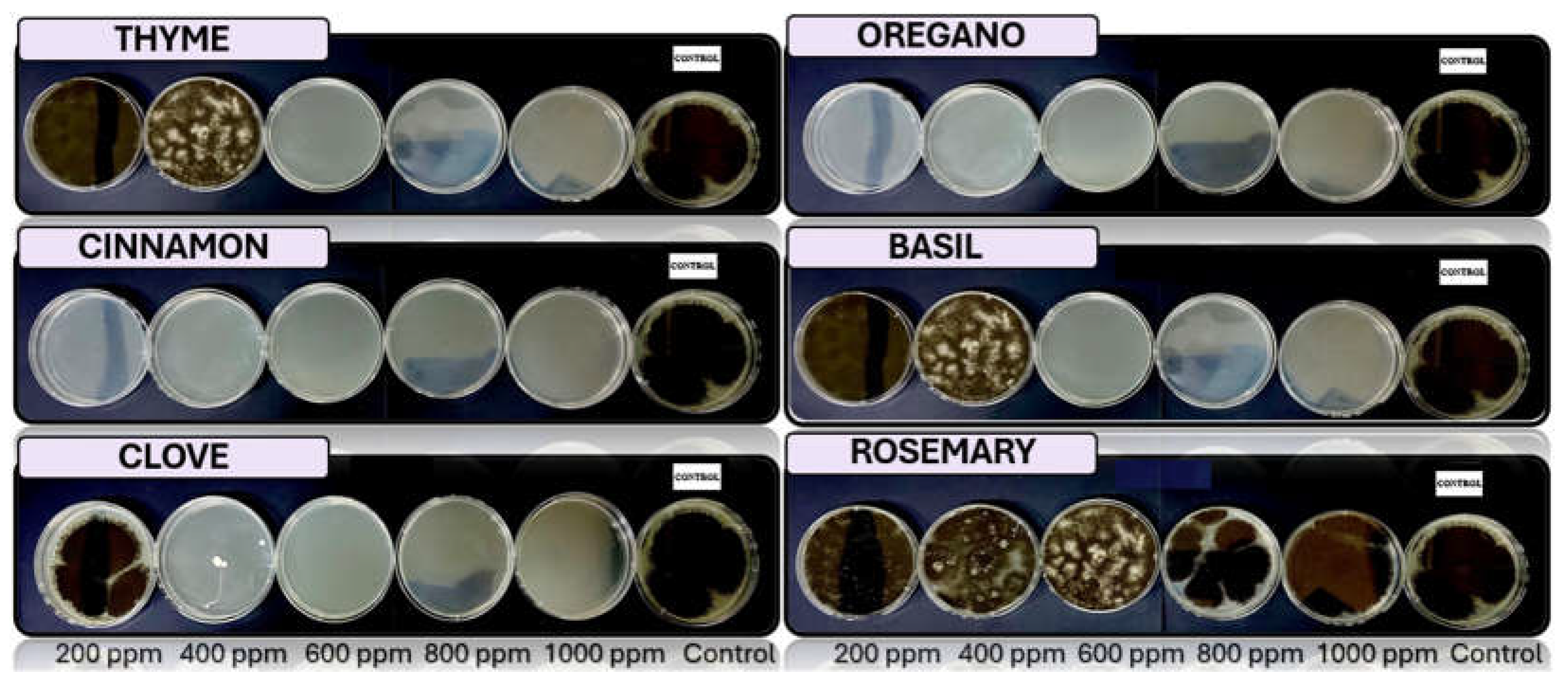
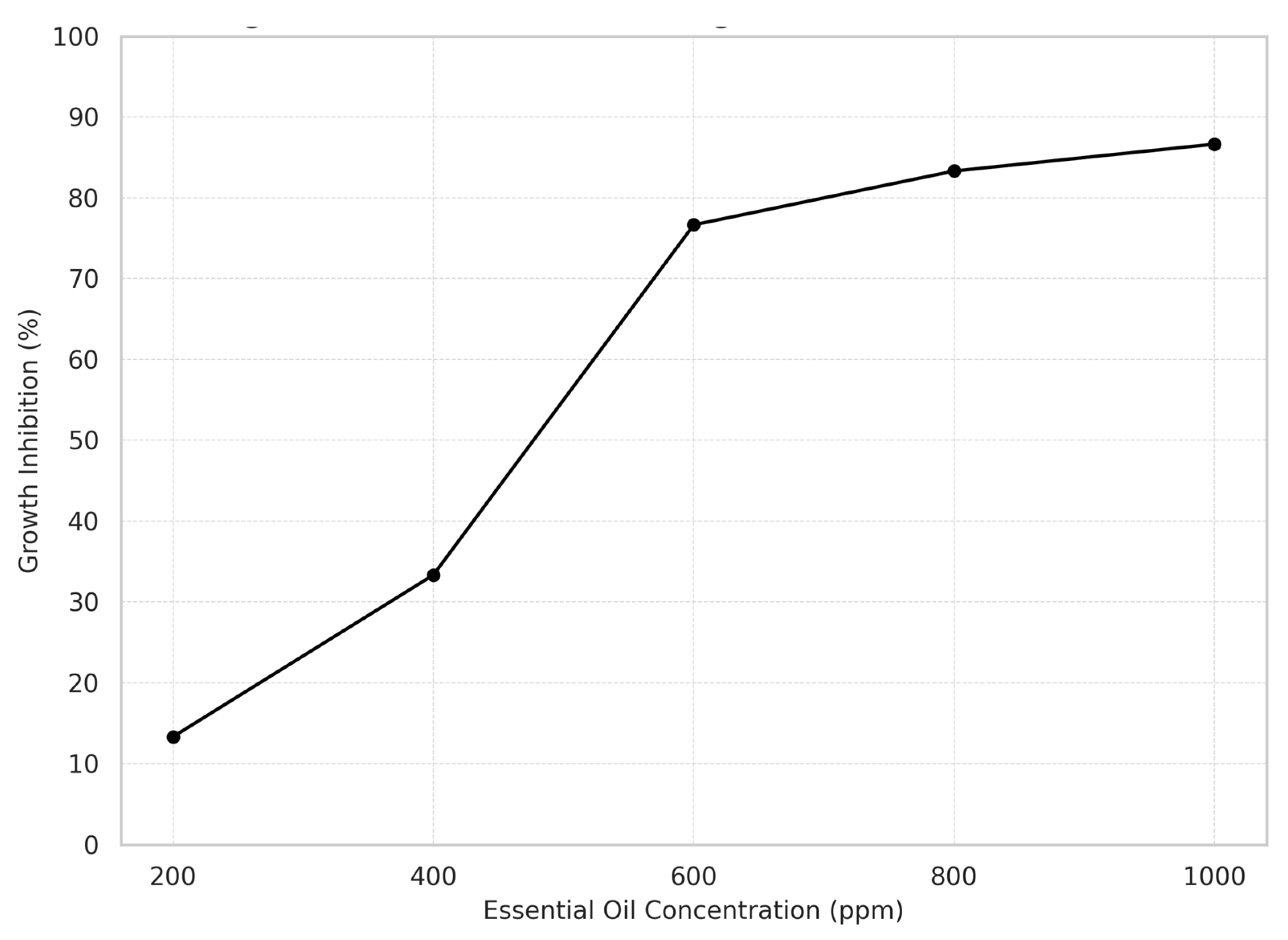
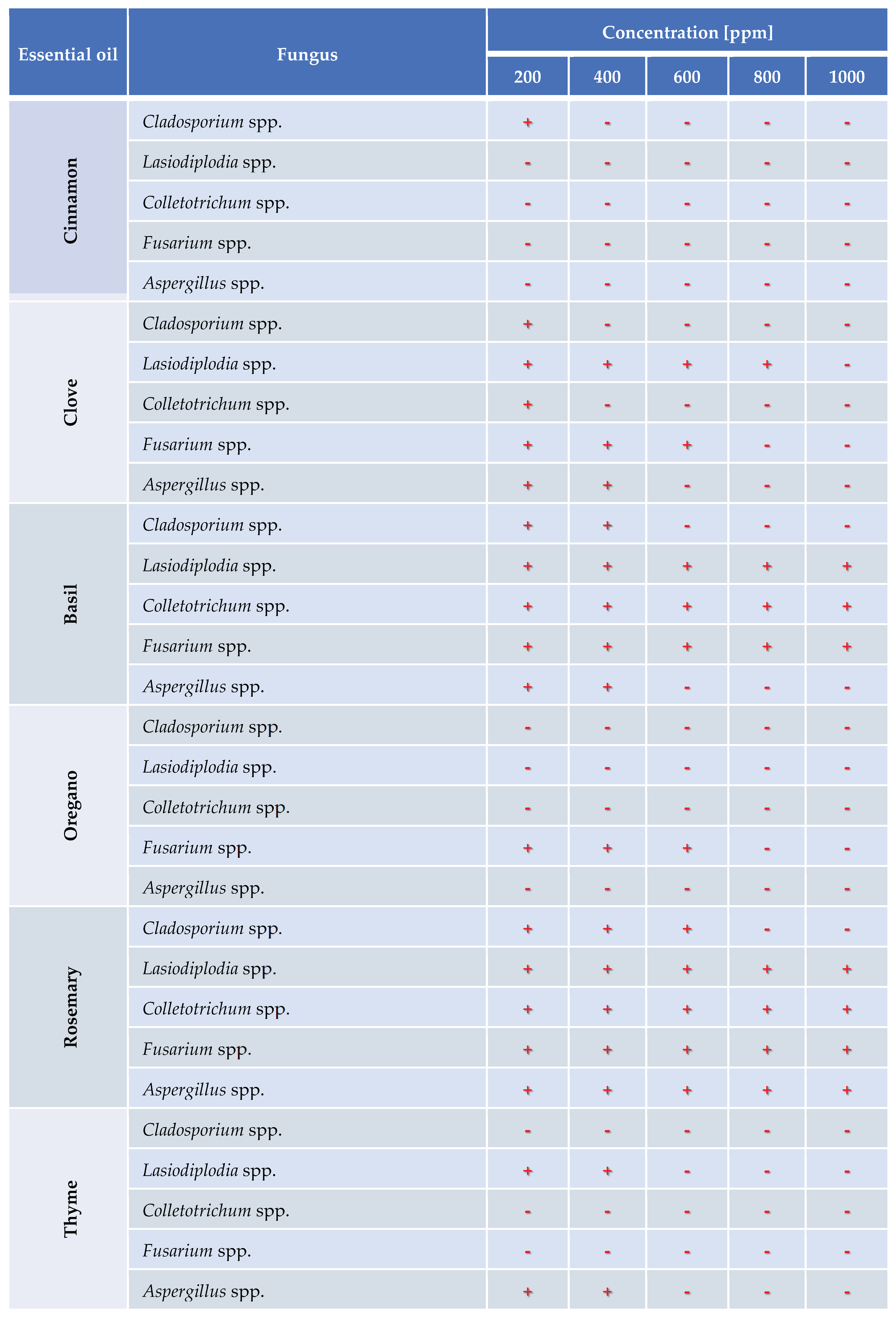
3.4. In Vitro Antifungal Activity with Essential Oils
4. Discussion
4.1. Morphological Identification
4.2. Molecular Identification Through DNA Sequencing
4.3. Ex Vivo Fungal Activity
4.4. In Vitro Antifungal Activity with Essential Oils
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmonds NW, Shepherd K. The taxonomy and origins of the cultivated bananas. Journal of the Linnean Society of London, Botany 1955;55:302–12. [CrossRef]
- Robinson JC, Sauco VG. Bananas and Plantains. 2nd ed. Wallingford, UK: CAB International; 2010.
- Paredes M, Sherwood S. The social life of seeds: The role of networks of relationships in the adoption of new technologies by small farmers in the Ecuadorian Andes. Agriculture and Human Values 2014;31:615–29. [CrossRef]
- Mata Anchundia D, Suatunce Cunuhay P, Poveda Morán R. Análisis económico del banano orgánico y convencional en la provincia Los Ríos, Ecuador. Avances 2021;23:419–30.
- Perrier X, Bakry F, Carreel F, Jenny C, Horry JP, Lebot V, et al. Combining biological approaches to shed light on the evolution of edible bananas. Euphytica 2011;180:51–69. [CrossRef]
- Ruiz Medina MD, Ruales J. Postharvest Alternatives in Banana Cultivation. 2024. [CrossRef]
- Li X, Yu S, Cheng Z, Chang X, Yun Y, Mustaffa S, et al. Origin and evolution of the triploid cultivated banana genome. Nature Genetics 2023;55:118–25. [CrossRef]
- Vilaplana R, Pazmiño L, Valencia-Chamorro S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biology and Technology 2018;138:56–63. [CrossRef]
- Spadaro D, Droby S. Development of biocontrol products for postharvest diseases of fruits: The importance of elucidating the mechanisms of action of yeast antagonists. Trends in Food Science & Technology 2016;47:39–49. [CrossRef]
- Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. International Journal of Food Microbiology 2004;94:223–53. [CrossRef]
- Singh G, Maurya S, de Lampasona MP, Catalan CAN. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food and Chemical Toxicology 2007;45:1650–61. [CrossRef]
- Arcila-Lozano CC, Loarca-Piña G, Lecona-Uribe S, González de Mejía E. El orégano: propiedades, composición y actividad biológica de sus componentes. Archivos Latinoamericanos de Nutrición 2004;54:100–11.
- López Luengo MT. El romero. Planta aromática con efectos antioxidantes. Offarm 2008;27:60–3.
- Villanueva C, Vilca R, Ítalo A, Cotrina G. Aplicación de Aceite Esencial de Canela (cinnamomum verum) y Clavo de Olor (syzygium aromaticum) en la cobertura comestible y tiempo de vida útil de la Fresa (fragaria ananassa). Ciencia Latina Revista Científica Multidisciplinar, vol. 5, Perú: 2021, p. 1504–26. [CrossRef]
- Alzate D, Mier G, Afanador L, Durango D, García C. Evaluación de la fitotoxicidad y la actividad antifúngica contra Colletotrichum acutatum de los aceites esenciales de tomillo (Thymus vulgaris), limoncillo (Cymbopogon citratus), y sus componentes mayoritarios 2009;16.
- Farías C, Cisternas C, Morales G, Muñoz L, Valenzuela Rodrigo. Albahaca: Composición química y sus beneficios en salud. Revista Chilena de nutrición 2022;49. [CrossRef]
- Ultee A, Bennik MHJ, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Applied and Environmental Microbiology 2002;68:1561–8. [CrossRef]
- Prashar A, Locke IC, Evans CS. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Proliferation 2006;39:241–8. [CrossRef]
- Farias APP, Monteiro O dos S, da Silva JKR, Figueiredo PLB, Rodrigues AAC, Monteiro IN, et al. Chemical composition and biological activities of two chemotype-oils from Cinnamomum verum J. Presl growing in North Brazil. J Food Sci Technol 2020;57:3176–83. [CrossRef]
- Bueno J, Sáez-Plaza P, Ramos-Escudero F, Jiménez AM, Fett R, Asuero AG. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Critical Reviews in Analytical Chemistry 2011;42:126–51. [CrossRef]
- Pinto E, Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of essential oils: a review of mechanisms and applications. Journal of Applied Microbiology 2018;124:1089–99. [CrossRef]
- Suppakul P, Miltz J, Sonneveld K, Bigger SW. Antimicrobial properties of basil and its possible application in food packaging. Journal of Agricultural and Food Chemistry 2003;51:3197–207. [CrossRef]
- Bensch K, Braun U, Groenewald JZ, Crous PW. The genus Cladosporium. Studies in Mycology 2012;72:1–401. [CrossRef]
- Crous PW, Braun U, Schubert K, Groenewald JZ. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 2007;58:33–56. [CrossRef]
- Picos-Muñoz PA, García-Estrada RS, León-Félix J, Sañudo-Barajas A, Allende-Molar R, Picos-Muñoz PA, et al. Lasiodiplodia theobromae en Cultivos Agrícolas de México: Taxonomía, Hospedantes, Diversidad y Control. Revista mexicana de fitopatología 2015;33:54–74.
- Sangeetha G, Anandan A, Rani SU. Morphological and molecular characterisation of Lasiodiplodia theobromae from various banana cultivars causing crown rot disease in fruits. Archives Of Phytopathology And Plant Protection 2012.
- Alvindia DG, Natsuaki KT. Biocontrol of crown rot-causing Colletotrichum musae by Burkholderia sp. Crop Protection 2008;27:953–7. [CrossRef]
- Villacís-Aldaz LA, León-Gordon O, Santana-Mayorga R, Mangui-Tobar J, Carranza G, Pazmiño-Miranda P. Actividad anti fúngica (in vitro) de extractos vegetales para el control de antracnosis (Colletotrichum acutatum). Journal of the Selva Andina Biosphere 2017;5:59–64.
- Li W, Li G, Zhang H. Postharvest disease of banana caused by Fusarium musae: A public health concern. Journal of Applied Microbiology 2021;131:828–37. [CrossRef]
- Leslie JF, Summerell BA. The Fusarium Laboratory Manual. Ames, Iowa: Blackwell Publishing; 2006.
- Randy P. Marchites por fusarium del plátano 2015. [CrossRef]
- Ortiz E, Riascos D, Angarita M, Castro O, Rivera C, Romero D, et al. Tópicos taxonómicos para el estudio del género Fusarium 2020;33:61–6.
- Chang PK, Horn BW, Abe K, Gomi K. Aspergillus: Introduction. Encyclopedia of Food Microbiology: Second Edition, Elsevier Inc.; 2014, p. 77–82. [CrossRef]
- Rokas A. Aspergillus. Current Biology 2013;23:R187–8. [CrossRef]
- Hyde KD, Soytong K. The fungal taxonomy boom and its impact on agriculture and human health. Fungal Diversity 2008;33:1–17.
- Sharma RR, Singh D, Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control 2009;50:205–21. [CrossRef]
- Suárez L, Rangel A. Aislamiento de microorganismos para control biológico de Moniliophthora roreri 2013;62.
- Morales R, Henríquez G. Aislamiento e identificación del moho causante de antracnosis en musa paradisiaca l. (plátano) en cooperativa san carlos, el salvador y aislamiento de mohos y levaduras con capacidad antagonista. Crea Ciencia Revista Científica 2021;13:84–94. [CrossRef]
- Bellemain E, Carlsen T, Brochmann C, Coissac E, Taberlet P, Kauserud H. ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiology 2010;10:189.
- Cervantes C, Tarqui M, Huiza P, Quispe A. Determinación de secuencias de ADN en Bioedit. ResearchGate n.d. https://www.researchgate.net/publication/370895529_Determinacion_de_secuencias_de_ADN_en_Bioedit (accessed February 25, 2025).
- Tamura K, Stecher G, Kumar S. Molecular Evolutionary Genetics Analysis version 11. Molecular Biology and Evolution n.d.;38:3022–7. [CrossRef]
- Samarakoon KW, Thong PH, Jeewanthi RKC. Evaluation of antifungal activity of cassia and holy basil essential oils against postharvest banana pathogens. Chemical Papers 2020;74:3113–21. [CrossRef]
- Sivakumar D, Bautista-Baños S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Protection 2014;64:27–37. [CrossRef]
- Castro J, Guzmán M. Identificación molecular de hongos asociados a frutos de banano en Costa Rica mediante la región ITS y análisis de secuencias. Revista de Biología Tropical 2012;60:45–58.
- Mahat NV, Noor A, Pustaka S. Essential oils: A review on the extraction techniques, chemical compositions, and applications. vol. 1. Elsevier; 2020. [CrossRef]
- National Institute of Standards and Technology. Essential oils: The effects of processing and standards for quality control 2011.
- Bautista-Baños S, Bosquez-Molina E, Barrera-Necha LL. The use of fungal antagonists to reduce postharvest fruit decay. Mycopathologia 2003;155:127–32. [CrossRef]
- Tripathi P, Dubey NK, Shukla AK. Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World Journal of Microbiology and Biotechnology 2008;24:39–46. [CrossRef]
- Druzhinina IS, Jurado M. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Diversity 2005;20:227–41. [CrossRef]
- Ogórek R, Pusz W, Lejman A. Characteristic and taxonomy of Cladosporium fungi. Prace Pogladowe 2012;19:80–5.
- Santos JEA, Silva DEM, Vieira RFBS, Cordeiro MVM, Almeida MMM, Lima M a. S, et al. First Report of Lasiodiplodia brasiliensis Causing Crown Rot on Banana in Brazil. Plant Disease 2023;107:2538. [CrossRef]
- Camargo Piñeres Y, Zambrano Montenegro G, Ortega-Cuadros M, Gutierrez Montero DJ, Yepes JA, Camargo Piñeres Y, et al. Actividad antifúngica in vitro del aceite esencial de Swinglea glutinosa Merr sobre Colletotrichum sp. patógeno de mango (Mangifera indica L.). Revista Colombiana de Biotecnología 2021;23:62–71. [CrossRef]
- Aguilar-Anccota R, Apaza-Apaza S, Maldonado E, Calle-Cheje Y, Rafael-Rutte R, Montalvo K, et al. Control in vitro e in vivo de Thielavipsis paradoxa y Collettrichum musae cn biofungicidas en frutos de banano orgánico. Manglar 2024;21:57–63. [CrossRef]
- Mari M, Torres R, Vanneste JL. Biological control of postharvest diseases: Opportunities and challenges. Frontiers in Microbiology 2014;5:1–17. [CrossRef]
- Jiménez M, Logrieco A, Bottalico A. Occurrence and Pathogenicity of Fusarium Species in Banana Fruits. Journal of Phytopathology 1993;137:214–20. [CrossRef]
- Pascal A, Simon T. Aspergillus fumigatus in Poultry 2011;20.
- Kumar D, Gupta S, Dubey SC. Use of molecular markers in identification and characterization of fungi. Indian Phytopathology 2018;71:197–210. [CrossRef]
- Alves A, Crous PW, Correia A, Phillips AJL. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia species associated with grapevine dieback. European Journal of Plant Pathology 2008;122:1–10. [CrossRef]
- Freire FCO, Viana FMP, Lima J. Incidence and pathogenicity of Lasiodiplodia theobromae associated with dieback of tropical fruit crops in Brazil. Tropical Plant Pathology 2018;43:203–10. [CrossRef]
- Aquino-Martínez JG, Vázquez-García LM, Reyes-Reyes BG. Biocontrol in vitro e in vivo de Fusarium oxysporum Schlecht. f. sp. dianthi (Prill. y Delacr.) Snyder y Hans. con Hongos Antagonistas Nativos de la Zona Florícola de Villa Guerrero, Estado de México. Revista mexicana de fitopatología 2008;26:127–37.
- Moretti A, Logrieco AF, Susca A. Mycotoxins: An underhand food problem. Methods in Molecular Biology 2013;1041:3–15. [CrossRef]
- Perrone G, Susca A, Cozzi G, Frisvad JC. Biodiversity of Aspergillus species in some important agricultural products. Studies in Mycology 2011;69:57–71. [CrossRef]
- Saleh A. Mycelial protein production by Aspergillus niger using banana peels. ResearchGate 2024;3. [CrossRef]
- Angarita Merchán M, Torres Caicedo MI, Díaz Torres AK. Técnicas de Biología Molecular en el desarrollo de la investigación. Revisión de la literatura. Revista Habanera de Ciencias Médicas 2017;16:796–807.
- Pérez-Rodríguez LR, Pérez-Moreno L, Guzmán-Mendoza R, Sanzón-Gómez D, Belmonte-Vargas JR, Pérez-Rodríguez LR, et al. Sensibilidad in vitro de hongos fitopatógenos causantes de enfermedades en fresa a controladores biológicos y fungicidas, en el estado de Guanajuato, México. Acta universitaria 2019;29. [CrossRef]
- Ruiz M, Ávila J, Ruales J. Diseño de un recubrimiento comestible bioactivo para aplicarlo en la frutilla (Fragaria vesca) como proceso de postcosecha 2016;17:276–87.
- Ranasinghe L, Jayawardena B, Abeywickrama K. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L.M. Perry against crown rot and anthracnose pathogens isolated from banana. Letters in Applied Microbiology 2002;35:208–11. [CrossRef]
- Spadaro D, Gullino ML. State of the art and future prospects of the biological control of postharvest fruit diseases. International Journal of Food Microbiology 2004;91:185–94. [CrossRef]
- Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013;6:1451–74. [CrossRef]
- Duarte CG, Tomalá CJ, Manzano PI. Valoración de la actividad antifúngica in vitro de dos aceites esenciales para la inhibición del crecimiento de Lasiodiplodia theobromae patógeno de banano y cacao post cosecha. Facultad de Ciencias Naturales y Matemáticas, ESPOL 2022.
- Abadias M, Teixidó N, Usall J, Viñas I. Evaluation of alternative strategies to control postharvest blue mould of apple caused by Penicillium expansum. International Journal of Food Microbiology 2008;122:25–31. [CrossRef]
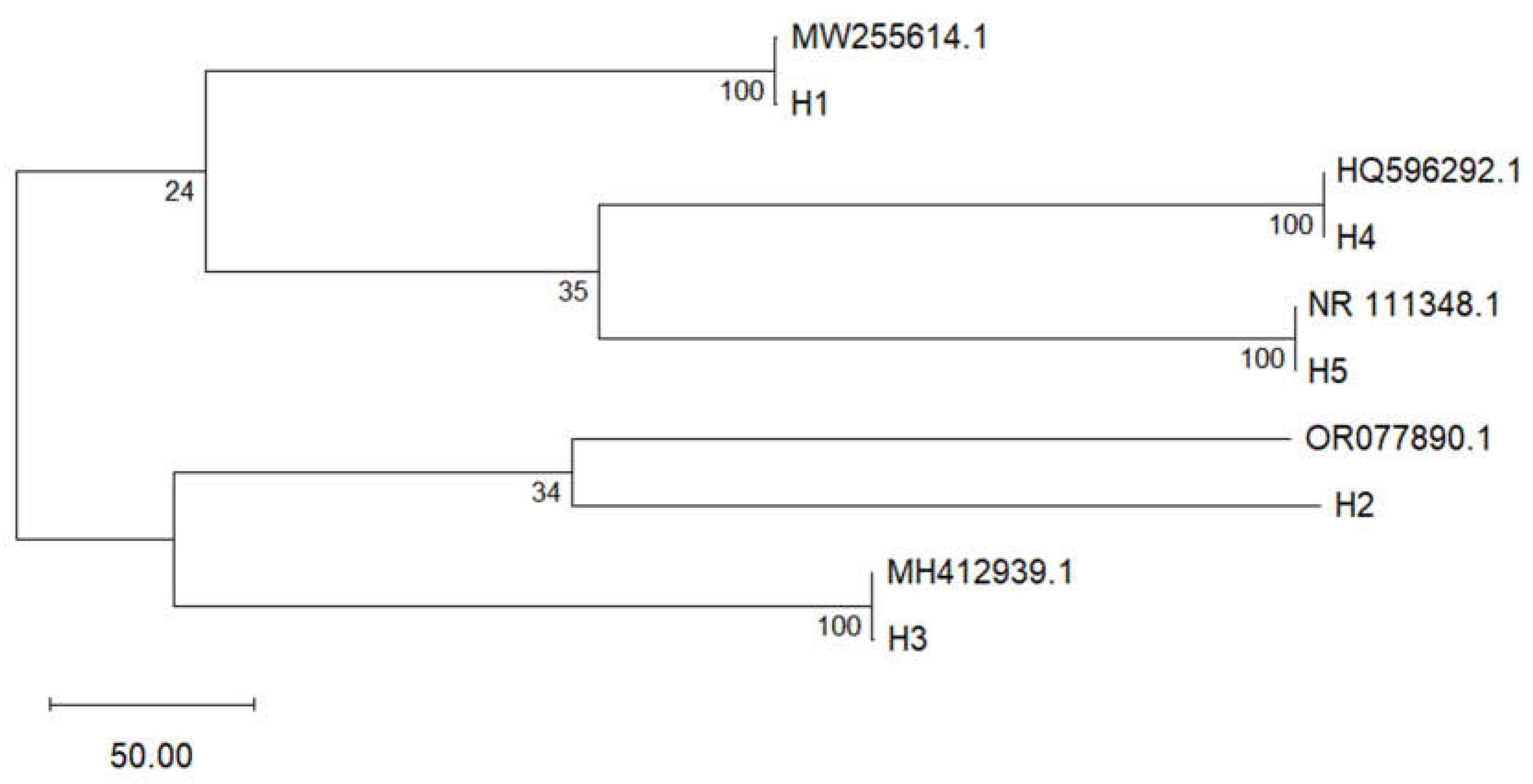
| Organism | Fragment | NCBI | % identity |
| Cladosporium cladosporoides | ITS | MW255614.1 | 99.62% |
| Lasiodiplodia theobromae | ITS | OR077890.1 | 100.00% |
| Colletotrichum musae | ITS | HQ596292.1 | 99.56% |
| Fusarium ophioides | ITS | MH412939.1 | 99.78% |
| Aspergillus niger | ITS | NR_111348.1 | 99.83% |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




