Submitted:
14 May 2025
Posted:
16 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
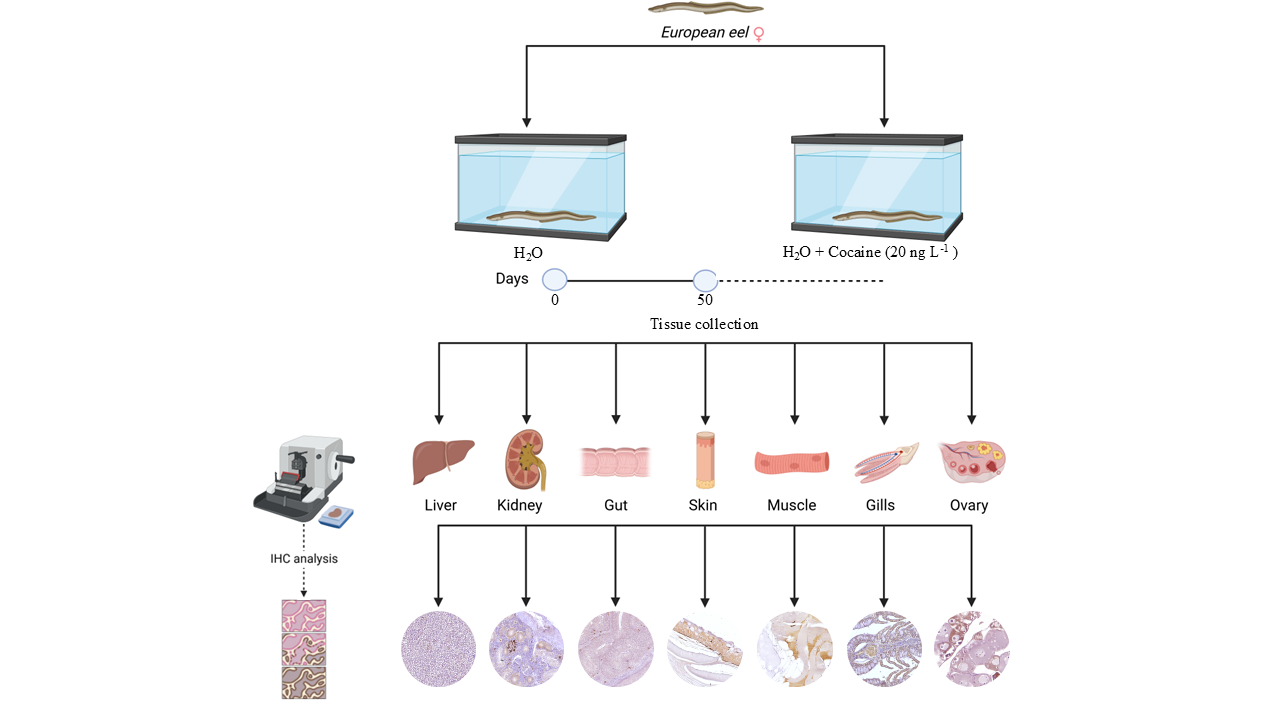
2.1. Animals
2.2. Experimental Design
2.3. Western Blot
2.4. Immunohistochemical Analysis
2.5. Semiquantitative Analysis of the Immunoreactivity
2.6. Statistical Analysis
3. Results
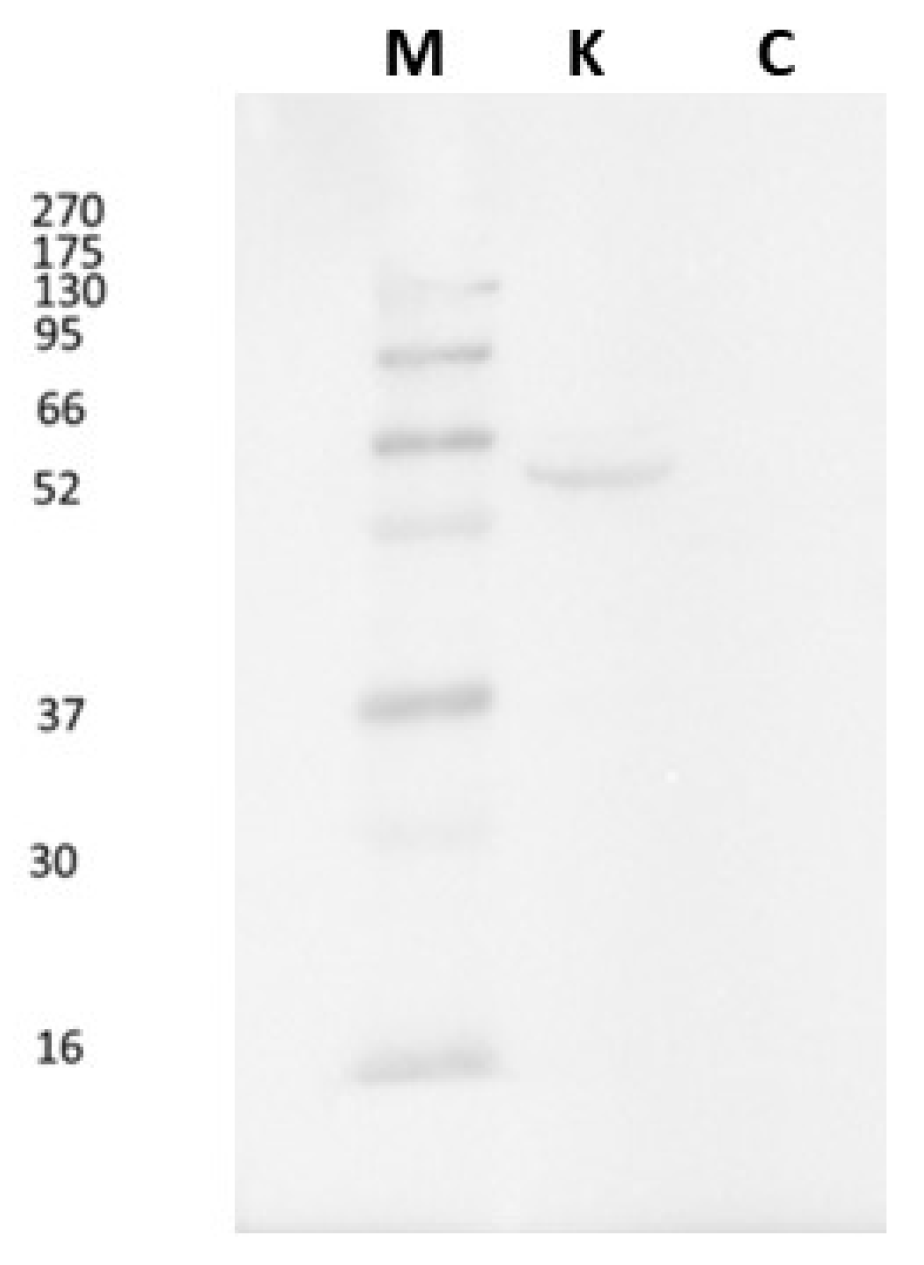
3.1. Western Blot
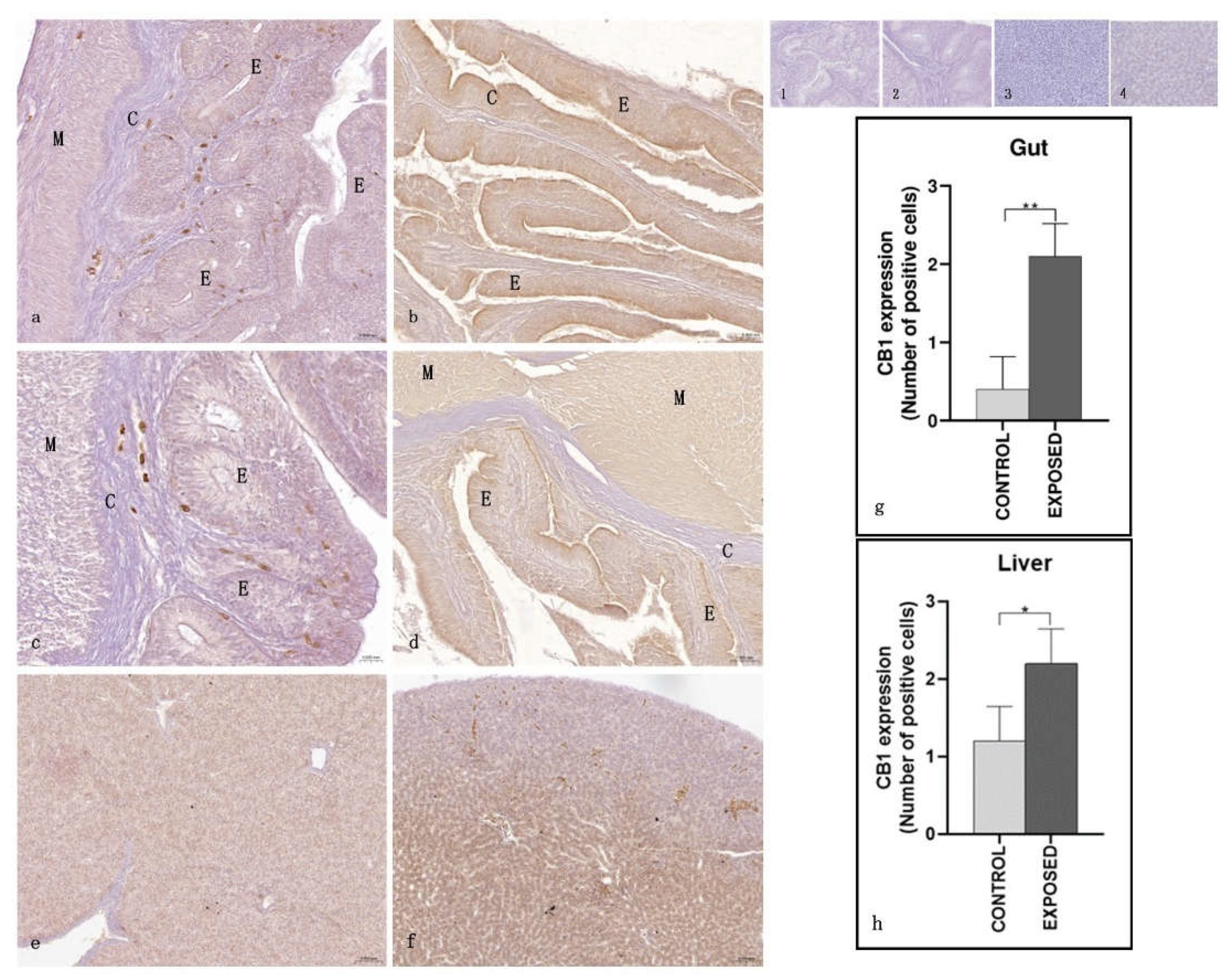
3.2. Gut and Liver Immunoistochemistry
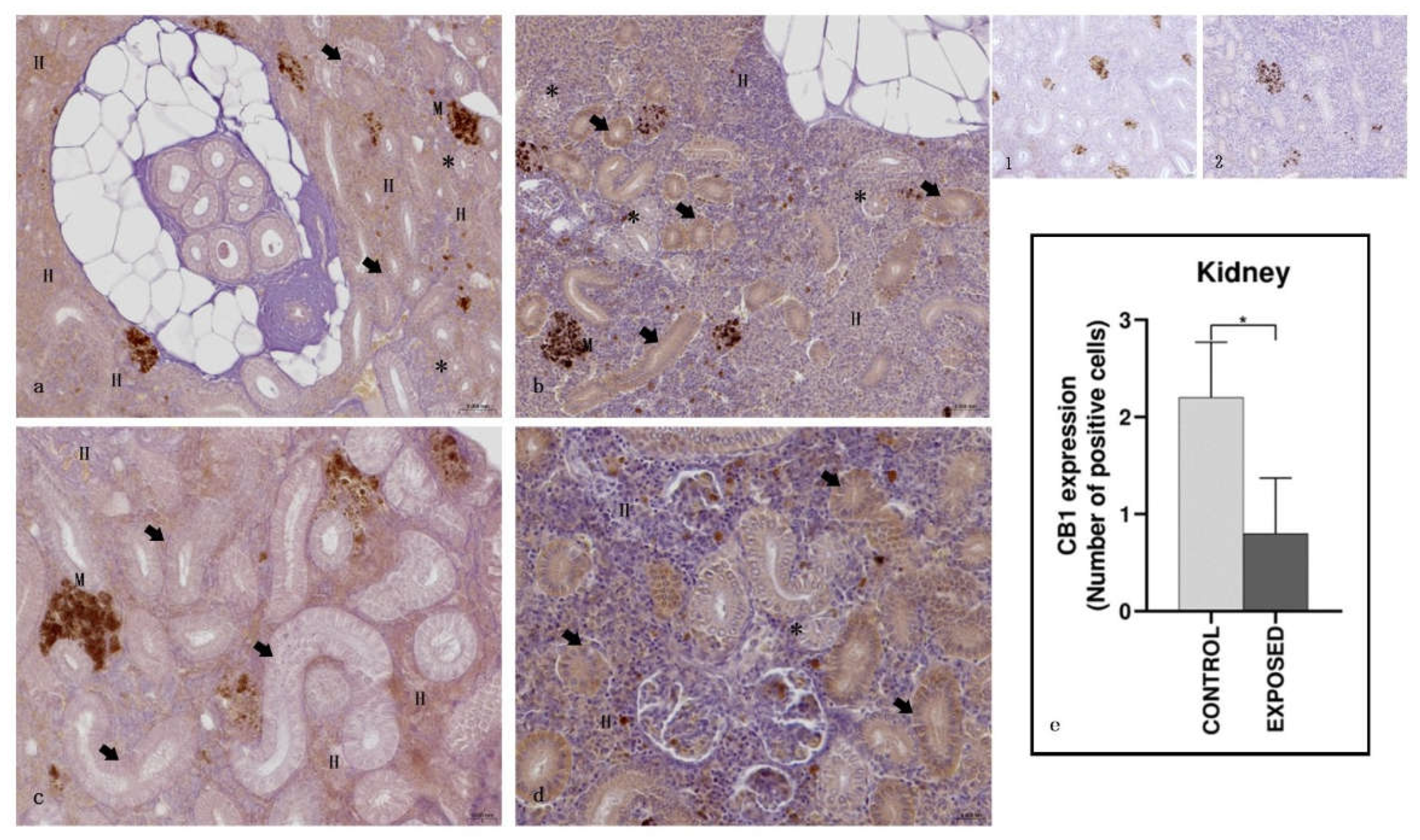
3.3. Kidney Immunoistochemistry
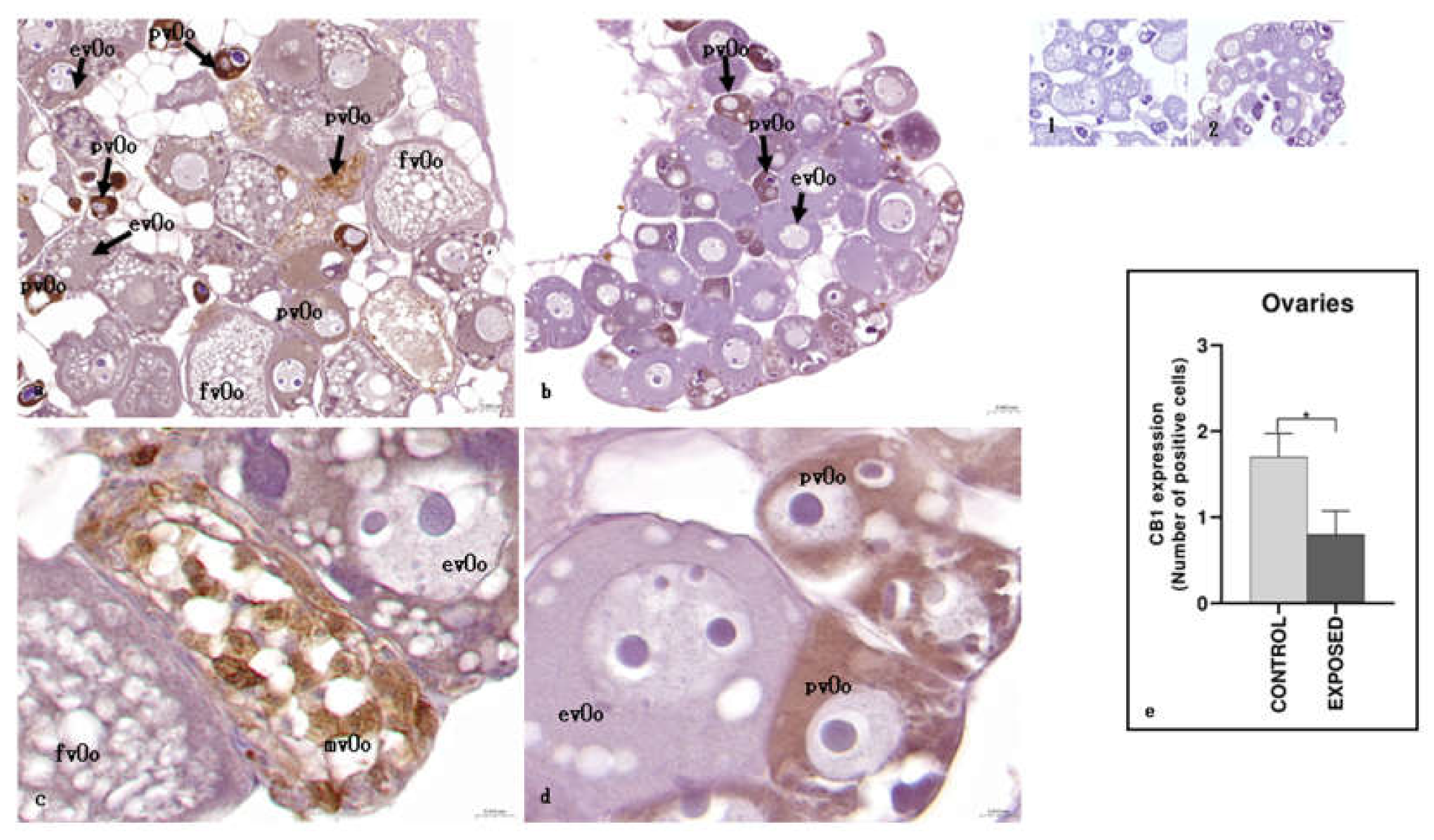
3.4. Ovary Immunoistochemistry
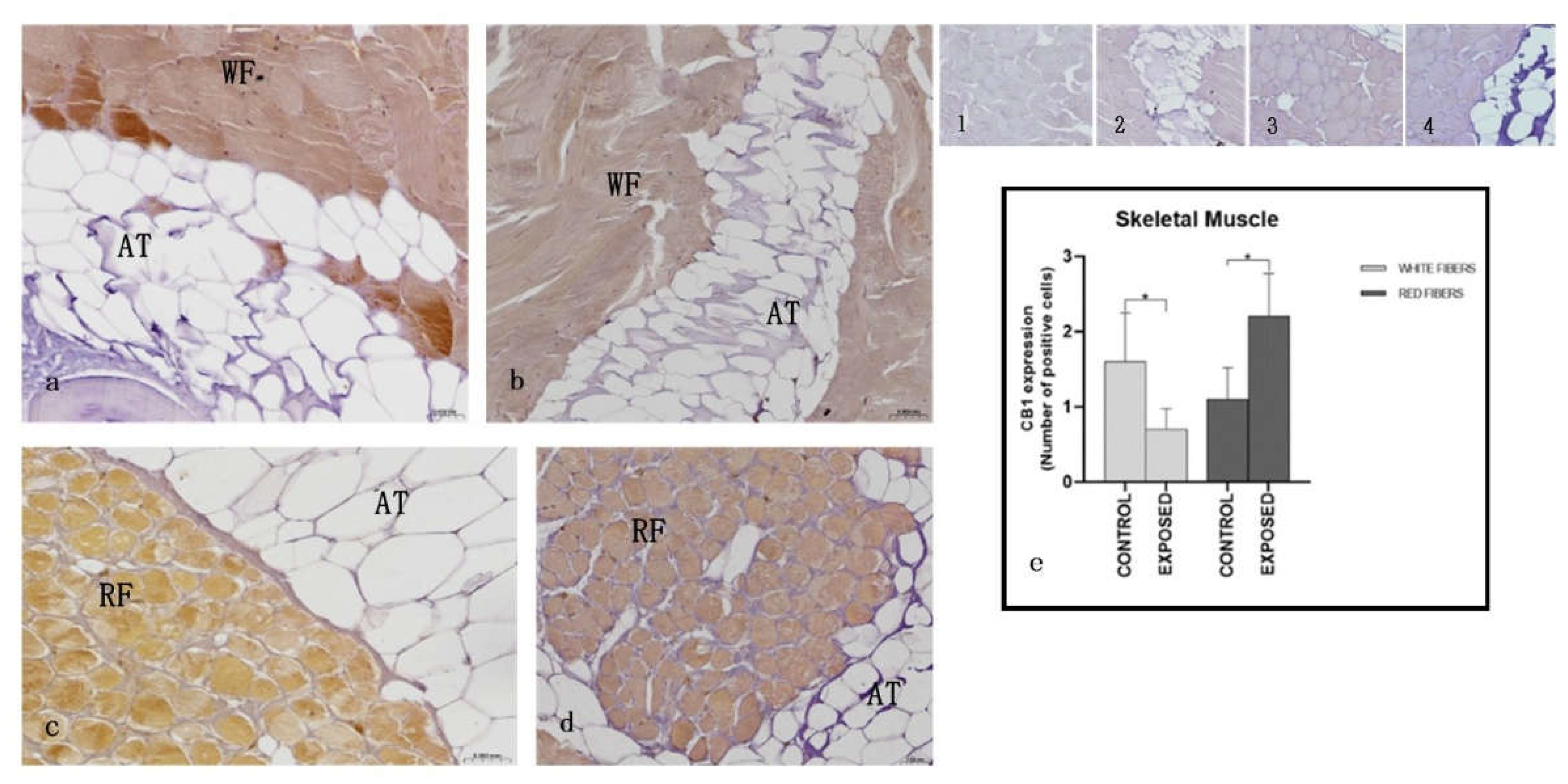
3.5. Skeletal Muscle Immunoistochemistry
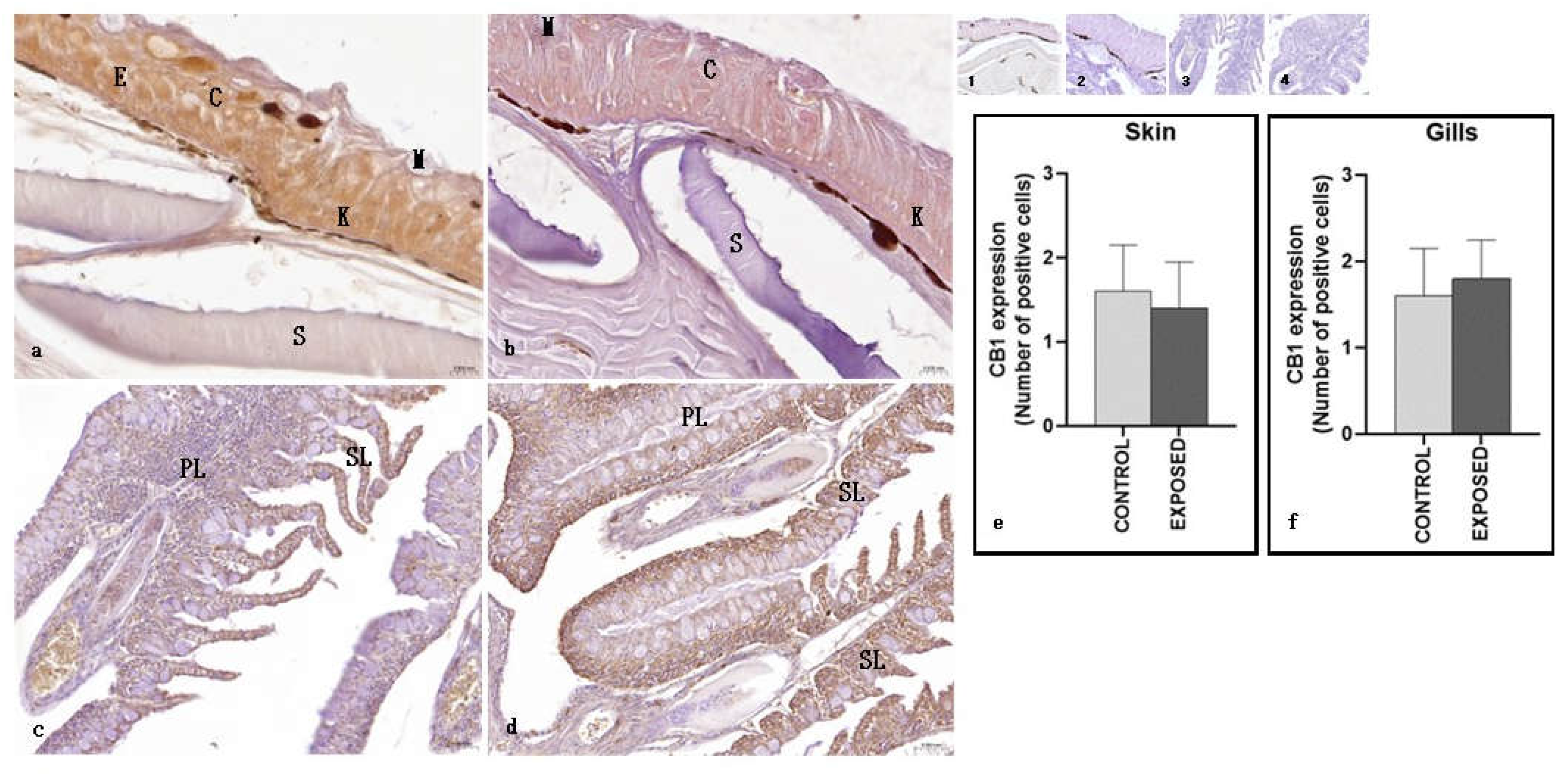
3.6. Skin and Gills Immunoistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, R., Megharaj, M., Kirkbride, K.P., Naidu, R. Illicit drugs and the environment-A review. Sci. Total Environ. 2013, 463-464, 1079-1092. [CrossRef]
- Rosi-Marshall, E.J., Snow, D., Bartelt-Hunt, S.L., Paspalof, A., Tank, J.L. A review of ecological effects and environmental fate of illicit drugs in aquatic ecosystems. J. Hazard. Mater. 2015, 282, 18-25. [CrossRef]
- Aligizakis, N.A., Gago-Ferrero, P., Borova, V.L., Pavlidou, A., Hatzianestis, I., Thomaidis N.S.. Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci. Total Environ. 2016, 541, 1097-1105. [CrossRef]
- Seabra-Pereira, C.D., Maranho, L.A., Cortez, F.S., Pusceddu, F.H., Santos, A.R., Ribeiro, D.A., Cesar, A., Guimarães L.L. Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci. Total Environ. 2016, 548-549, 148-154. [CrossRef]
- Fontes, M.K., Maranho, L., Pereira, C.D.S. Review on the occurrence and biological effects of illicit drugs in aquatic ecosystems. Environ. Sci. Pollut. Res. 2020, 27, 30998–31034. [CrossRef]
- Muñiz-Bustamante, L., Caballero-Casero, N., Rubio, S. Drugs of abuse in tap water from eight European countries: Determination by use of supramolecular solvents and tentative evaluation of risks to human health. Environment International. 2022, 164, 107281. [CrossRef]
- UNODC, World Drug Report 2024 (United Nations Publication 2024). https://www.unodc.org/documents/data-and-analysis/WDR_2024/WDR24_Key_findings_and_conclusions.pdf.
- Kasprzyk-Hordern, B., Dinsdale, R.M., Guwy, A. J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [CrossRef]
- Deng, Y., Guo, C., Zhang, H., Yin, X., Chen, L., Wu, D., Xu, J. Occurrence and removal of illicit drugs in different wastewater treatment plants with different treatment techniques. Environ. Sci. Eur. 2020, 32, 28. [CrossRef]
- Davey, C.J.E., Kraak, M.H.S., Pratorius, A., ter Laak, T.L., van Wezel, A. P. Occurrence, hazard, and risk of psychopharmaceuticals and illicit drugs in European surface waters. Water Res. 2022, 222, 118878. [CrossRef]
- Binelli, A., Pedriali, A., Riva, C., Parolini, M. Illicit drugs as new environmental pollutants: Cyto-genotoxic effects of cocaine on the biological model Dreissena polymorpha. Chemosphere. 2012, 86, 906-911. [CrossRef]
- Chen, L., Guo, C., Sun, Z., Xu, J. Occurrence, bioaccumulation and toxicological effect of drugs of abuse in aquatic ecosystem: A review. Environ. Res. 2021, 200, 111362. [CrossRef]
- Fontes, M.K., Dourado, P.L.R. de Campos, B.G. Maranho, L.A. de Almeida, E.A. de Souza Abessa, D.M. Pereira, C.D.S. Environmentally realistic concentrations of cocaine in seawater disturbed neuroendocrine parameters and energy status in the marine mussel Perna perna. Comp. Biochem. Physiol. 2022a, 251, 109198. [CrossRef]
- Rosati, L., Caputo, I., Lionetti, L., Fontes, M.K., Seabra Pereira, C.D., Capaldo, A. Side effects of human drug use: an overview of the consequences of eels’ exposure to cocaine. Fishes. 2023, 8, 166. [CrossRef]
- Sokołowski, A., Mordec, M., Caban, M., Øverjordet, I.B., Wielogorska, E., Włodarska-Kowalczuk, M., Balazy, P., Chełchowski, M., Lepoint. G. Bioaccumulation of pharmaceuticals and stimulants in macrobenthic food web in the European Arctic as determined using stable isotope approach. Sci. Total Environ. 2024, 909, 168557. [CrossRef]
- De Farias Araujo, G., de Oliveira, L.V.A., Hoff, R.B., Wosnick, N., Vianna, M., Verruck, S., Hauser-Davis, R.A., Saggioro, E.M. “Cocaine Shark”: First report on cocaine and benzoylecgonine detection in sharks. Sci. Total Environ. 2024, 948, 174798. [CrossRef]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Phil. Trans. R. Soc. B. 2012, 367, 3201–3215. [CrossRef]
- Oltrabella F., Melgoza A., Nguyen B., Guo S. Role of the endocannabinoid system in vertebrates: Emphasis on the zebrafish model. Dev Growth Differ. 2017 May;59(4):194-210. [CrossRef]
- Silver, R.J. The endocannabinoid system of animals. Animals. 2019, 9, 686-701. [CrossRef]
- Bailone R.L., Fukushima H.C.S., Kluwe de Agular, Borra R.C. The endocannabinoid system in zebrafish and its potential to study the effects of Cannabis in humans. Laboratory Animal Research. 2022, 38, 5. [CrossRef]
- Pacher, P., Batkai, S., Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [CrossRef]
- Zou, S., Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci., 2018, 19, 833. [CrossRef]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990; 346:561–4. [CrossRef]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993; 365:61–5. [CrossRef]
- Shahbazi, F., Grandi, V., Banerjee, A., Trant, J. Cannabinoids and Cannabinoid Receptors: The Story so Far. IScience. 2020, 23, 101301. [CrossRef]
- Izzo, A.A., Sharkey, K.A. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol. Ther. 2010, 126, 21–38. [CrossRef]
- Di Marzo, V., de Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B 2012, 367, 3216–3228. [CrossRef]
- Mukhopadhyay S., Shim J. Y., Assi A. A., Norford D., Howlett A. C. CB1 cannabinoid receptor-g protein association: a possible mechanism for differential signaling. Chemistry and Physics of Lipids. 2002, 121: 61-65. [CrossRef]
- Cacciola G, Chianese R, Chioccarelli T, Ciaramella V. Fasano S, Pierantoni R, Meccariello R, Cobellis G.Cannabinoids and Reproduction: A Lasting and Intriguing History. Pharmaceuticals. 2010 3: 3275-332. [CrossRef]
- Migliarini, B., Carnevali, O. Anandamide modulates growth and lipid metabolism in the zebrafish Danio rerio. Molecular and Cellular Endocrinology. 2008, 286S, S12–S16. [CrossRef]
- Fattore, L., Martellotta, C., Cossu, G., Mascia, M.S., Fratta, W. CB1 cannabinoid receptor agonist WIN 55, 212-2 decreases intravenous cocaine self-administration in rats. Behavioural Brain Research. 1999, 104, 141–146. [CrossRef]
- Gonzàlez, S., Fernàndez-Ruiz, J., Sparpaglione, V., Parolaro, D., Ramos, J. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB1 receptor binding and mRNA levels. Drug and Alcohol Dependence. 2002, 66, 77–84. [CrossRef]
- Centonze, D., Battista, N., Rossi, S., Mercuri, N.B., Finazzi-Agro, A., Bernardi, G., Calabresi, P., Maccarrone, M. A critical interaction between Dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004, 29, 1488–1497. [CrossRef]
- Soria G., Mendizabal, V., Tourin, C., Robledo, P., Ledent, C., Parmentier, M., Maldonado, R., Valverde, O. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005, 30, 1670–1680. [CrossRef]
- Tanda, G. Modulation of the endo-cannabinoid system: therapeutic potential against cocaine dependence. Pharmacol Res. 2007, 56(5): 406–417. [CrossRef]
- Harvey-Girard, E., Giassi, A.C.C, Ellis, W., Maler, L. Expression of the cannabinoid CB1 receptor in the Gymnotiform fish brain and its implications for the organization of the teleost pallium. The Journal of Comparative Neurology-Research in Systems Neuroscience. 2013, 521:949–975. [CrossRef]
- Henkel C., Jong-Raadsen S.A., Dufour S., Weltzien F.-A., Palstra A.P., Pelster B., Spaink H.P., Van Den Thillart G.E., Jansen H., Zahm M., Klopp C., Cedric C., Louis A., Berthelot C., Parey E., Roest Crollius H., Montfort J., Robinson-Rechavi M., Bucao C., Bouchez O., Gislard M., Lluch J., Milhes M., Lampietro C., Lopez Roques C., Donnadieu C., Braasch I., Desvignes T., Postlethwait J., Bobe J., Guiguen Y., Dirks R. A chromosome-scale assembly of European eel, Anguilla anguilla. UniProt: A0A9D3MIM1_ANGAN; submitted to MBL/GenBank/DDBJ databases JAN-2021. https://www.uniprot.org/citations/CI-4V42E84B85DHN.
- Gheorghe, A., van Nuijs, A., Pecceu, B., Bervoets, L., Jorens, P.G., Blust, R., Neels, R., Covaci, A. Analysis of cocaine and its principal metabolites in waste and surface water using solid-phase extraction and liquid chromatography–ion trap tandem mass spectrometry. Anal Bioanal Chem.2008, 391:1309–1319. [CrossRef]
- Chianese, T., Trinchese, G., Leandri, R., De Falco, M., Mollica, M.P., Scudiero, R., Rosati, L. Glyphosate Exposure Induces Cytotoxicity, Mitochondrial Dysfunction and Activation of ERα and ERβ Estrogen Receptors in Human Prostate PNT1A Cells. Int. J. Mol. Sci. 2024, 25, 7039. [CrossRef]
- d’Aquino, I., Piegari, G., Miletti, G., Sannino, E., Costanza, D., Meomartino, L., Fico, R., Riccio, L., Vaccaro, E., De Biase, D., Paciello, O. Morphometrical and Immunohistochemical Evaluation of Kidney as an Indirect Parameter to Estimate Age in Puppies in Veterinary Forensic Pathology. Animals. 2023, 13(16), 2665. [CrossRef]
- Spampinato, M., Siciliano, A., Travaglione, A., Chianese, T., Mileo, A., Libralato, G., Guida, M., Trifuoggi, M., De Gregorio, V., Rosati L. Unravelling the ecotoxicological impacts of gadolinium (Gd) on Mytilus galloprovincialis embryos and sperm in seawater: A preliminary study. Heliyon. 2024, 17;10(10):e31087. [CrossRef]
- Johnson, S.J., Walker, F. R. Strategies to improve quantitative assessment of immunohistochemical and immunofluorescent labelling. Scientific reports. 2015, 5(1), 10607. [CrossRef]
- Law, A.M., Yin, J.X., Castillo, L., Young, A.I., Piggin, C., Rogers, S., Caldon, C.E., Burgess, A., Millar, E.K.A., O'Toole, S.A., Gallego-Ortega, D., Ormandy, C.J., Oakes, S.R. Andy’s Algorithms: new automated digital image analysis pipelines for FIJI. Scientific reports. 2017, 7(1), 15717. [CrossRef]
- Lutz B. Molecular biology of cannabinoid receptors. Prostaglandins Leukot Essent Fat Acids. 2002, 66(2–3):123–42. [CrossRef]
- Yamaguchi F., Macrae A.D., Brenner S. Molecular cloning of two cannabinoid type 1-like receptor genes from the puffer fish Fugu rubripes. Genomics. 1996, 35:603-605. [CrossRef]
- Elphick M. R. Evolution of cannabinoid receptors in vertebrates: identification of a CB2 gene in the puffer fish Fugu rubripes. Biol Bull. 2002, 202:104-107. [CrossRef]
- Lachowicz, J., Szopa, A., Ignatiuk, K., Swiader, K., Serefko, A. Zebrafish as an animal model in cannabinoid research. Int. J. Mol.Sci. 2023, 24, 10455. [CrossRef]
- Casu, M.A., Porcella, A., Ruiu, S., Saba, P., Marchese, G., Carai, M.A.M., Reali, R., Gessa, G.L., Pani, L. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur. J. Pharmacol. 2003. 459, 97-105. [CrossRef]
- Tesch, S.W. The Eel. Blackwell Science, Ltd, Oxford. 2003. [CrossRef]
- Di Marzo, V., Matias, I. Endocannabinoid control of food intake and energy balance. Nature Neurosci. 2005, 8(5), 585-589. [CrossRef]
- Di Patrizio, N. Endocannabinoids in the Gut. Cannabis and Cann. Res, 2016, 1.1, 67-77. [CrossRef]
- Gay, F., Ferrandino, I., Monaco, A., Cerulo, M., Capasso, G., Capaldo, A. Histological and hormonal changes in the European eel (Anguilla anguilla) after exposure to environmental cocaine concentration. J. Fish Dis. 2016, 39, 295-308. [CrossRef]
- Becker, W., Alrafas, H.R., Busbee, P.B., Walla, M.D., Wilson, K., Miranda, K., Cai, G., Putluri, V., Putluri, N., Nagarkatti, M., Nagarkatti, P. Cannabinoid Receptor Activation on Haematopoietic Cells and Enterocytes Protects against Colitis. J. Chrohn’s Colitis. 2021, 15(6), 1032-1048. [CrossRef]
- Arinç, E., Bozcaarmutlu, A. Catalyzation of Cocaine N-Demethylation by Cytochromes P4502B, P4503A, and P4502D in Fish Liver. J. Biochem. Mol. Toxicol. 2003, 17(3), 169-176. [CrossRef]
- Tam, J., Liu, J., Mukhopadhyay S., Cinar, R., Godlewski, G., Kunos, G. Endocannabinoids in Liver Disease. Hepatology. 2011, 53(1), 346-355. [CrossRef]
- Bazwinsky-Wutschke, I., Zipprich, A., Dehghani, F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int.J.Mol.Sci. 2019, 20(10), 2516. [CrossRef]
- Howlett, A.C., Abood, M.E. CB1 & CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169-206. [CrossRef]
- Pagotto, U., Pasquali, R. Endocannabinoids and energy metabolism. J. Endocrinol. Invest. 2006, 29 (3), 58-68. PMID: 16751710.
- Capaldo A., Gay F., Caputo I., Lionetti L., Paolella G., Di Gregorio I., Martucciello S., Di Lorenzo M., Rosati L., Laforgia V. Effects of environmental cocaine concentrations on COX and caspase-3 activity, GRP-78, ALT, CRP and blood glucose levels in the liver and kidney of the European eel (Anguilla anguilla). Ecotoxicology and Environmental Safety. 2021, 208, 111475. [CrossRef]
- Mumford, S., Heidel, J., Smith, C., Morrison, J., Mac Connell, B., Blazer, V. Fish Histology and Histopathology. U.S. Fish & Wildlife Service-National Conservation Training Center. 2007. https://nctc.fws.gov/resources/course-resources/fish-histology/.
- Watanabe, T., Takei, Y. Molecular physiology and functional morphology of SO4 2– excretion by the kidney of seawater-adapted eels. J. Exper.Biol. 2011, 214, 1783-1790. [CrossRef]
- Jiang, S., Fu, Y., Avraham, H.K. Regulation of hematopoietic stem cell trafficking and mobilization by the endocannabinoid system. Transfusion 2011, 51 supplement, 65S-71S. [CrossRef]
- Cottone, E., Pomatto, V., Cerri, F., Campantico, E., Mackie, K., Delpero, M., Guastalla, A., Dati, C., Bovolin, P., Franzoni, M.F. Cannabinoid receptors are widely expressed in goldfish: molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiol Biochem. 2013, 39:1287–1296. [CrossRef]
- Tam, J. The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J. Basic Clin. Physiol. Pharmacol. 2015, 27(3), 267-276. [CrossRef]
- Dao, M., Francois, H. Cannabinoid Receptor 1 Inhibition in Chronic Kidney Disease: A New Therapeutic Toolbox. Front. Endocrinol. 2021, 12:720734. [CrossRef]
- Arceri, L., Nguyen, T.K., Gibson, S., Baker, S., Wingert, R.A. Cannabinoid Signaling in Kidney Disease. Cells. 2023, 12, 1419. [CrossRef]
- Valente, M.J., Henrique, R., Vilas-Boas, V., Silva, R., de lourdes Bastos, M., Carvalho, F., Guedes de Pinho, P., Carvalho, M. Cocaine-induced kidney toxicity: an in vitro study using primary cultured human proximal tubular epithelial cells. Arch. Toxicol. 2012, 86 (2), 249–261. [CrossRef]
- Cianchi, F., Papucci, L., Schiavone, N., Lulli, M., Magnelli, L., Vinci, M.C., Messerini, L., Manera, C., Ronconi, E., Romagnani, P., Donnini, M., Perigli, G., Trallori, G., Tanganelli, E., Capaccioli, S., Masini, E. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor α-mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 2008, 14(23), 7691-7700. [CrossRef]
- Fontes, M. K., Rosati, L., Di Lorenzo, R., Seabra Pereira, C.D., Maranho, L. A., Laforgia, V., Capaldo, A.Aquatic Pollution and Risks to Biodiversity: The Example of Cocaine Effects on the Ovaries of Anguilla anguilla. Animals 2022b, 12(14), 1766. [CrossRef]
- Walker, O.S., Holloway, A.C., Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovar. Res., 2019, 12, 3. [CrossRef]
- Rosati, L., Chianese, T., Mileo, A., De Falco, M., Capaldo, A. Cocaine Effects on Reproductive Behavior and Fertility: An Overview. Vet. Sci. 2023, 10, 484. [CrossRef]
- Di Blasio, A.M., Vignali, M., Gentilini, D. The endocannabinoid pathway and the female reproductive organs. J Mol Endocrinol. 2013, 11;50(1), R1-9. [CrossRef]
- Mendizabal-Zublaga, J.M., Melser, Su., Bènard, G., Ramos, A., Reguero, L., Arrabal, S., Elezgaral, I., Gerrikagoitia, I., Suarez, J., De Fonseca, F.R., Puente, N., Marsicano, G., Grandes, O. Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front. Physiol. 2016, 7, 476. [CrossRef]
- Cavuoto, P., McAinch, A.J., Hatzinikolas, G., Cameron-Smith, G.A., Wittert, G.A. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell Endocrinol. 2007a, 267, 63-69. [CrossRef]
- Cavuoto, P., McAinch, A.J., Hatzinikolas, G., Janovskà, A., Game, P., Wittert, G.A. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res Comm. 2007b, 364, 105-110. [CrossRef]
- Dalle, S., Schouten, M., Meeus, G., Slagmolen, L., Koppo, K. Molecular networks underlying cannabinoid signaling in skeletal muscle plasticity. J. Cell. Physiol.2022, 237, 3517–3540. [CrossRef]
- de Abreu, N.K., Fabro Feltrin, I., Russiano Pereira, D.B., Penasso bezerra, P., Aguiar Jr, A.S. Impact of CB1 receptor antagonism on skeletal muscle hypertrophy and metabolic health: a systematic review of preclinical studies. Hormones. 2025, 1-12. [CrossRef]
- Tomiyama, K.I, Funada M, Synthetic cannabinoid CP-55,940 induces apoptosis in a human skeletal muscle model via regulation of CB1 receptors and l-type Ca2+channels, Arch. Toxicol. 2021, 95, 617-630. [CrossRef]
- Capaldo, A., Gay, F., Lepretti, M., Paolella, G., Martucciello, S., Lionetti, L., Caputo, I., Laforgia, V. Effects of environmental cocaine concentrations on the skeletal muscle of the European eel (Anguilla anguilla). Sci. Total Environ. 2018, 640-641, 862-873. [CrossRef]
- Birò, T., Tòth, B., Haskò, G., Paus, R., Pacher, P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009, 30(8), 411-420. [CrossRef]
- Kupczyk, P., Reich, A., Szepietowski, J.C. Cannabinoid system in the skin – a possible target for future therapies in dermatology. Exp. Dermatol. 2009, 18, 669-679. [CrossRef]
- Rodriguez-Martin, I., Herrero-Turrion, M.J., Marron Fdez de Velasco, E., Gonzalez-Sarmiento, R., Rodriguez, R.E. Characterization of two duplicate zebrafish Cb2-like cannabinoid receptors. Gene. 2007, 389, 36-44. [CrossRef]
- Capaldo, A., Gay, F., Laforgia, V. Changes in the gills of the European eel (Anguilla anguilla) after chronic exposure to environmental cocaine concentration. Ecotoxicol. Environ. Saf. 2019, 169, 112-119. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




