Submitted:
13 May 2025
Posted:
13 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Fungi Isolate Collection
DNA Extraction and Quality Assessment
Genome Assembly and QC
Genome Annotation
Comparative Genomics and Gene Orthology Analysis
3. Results
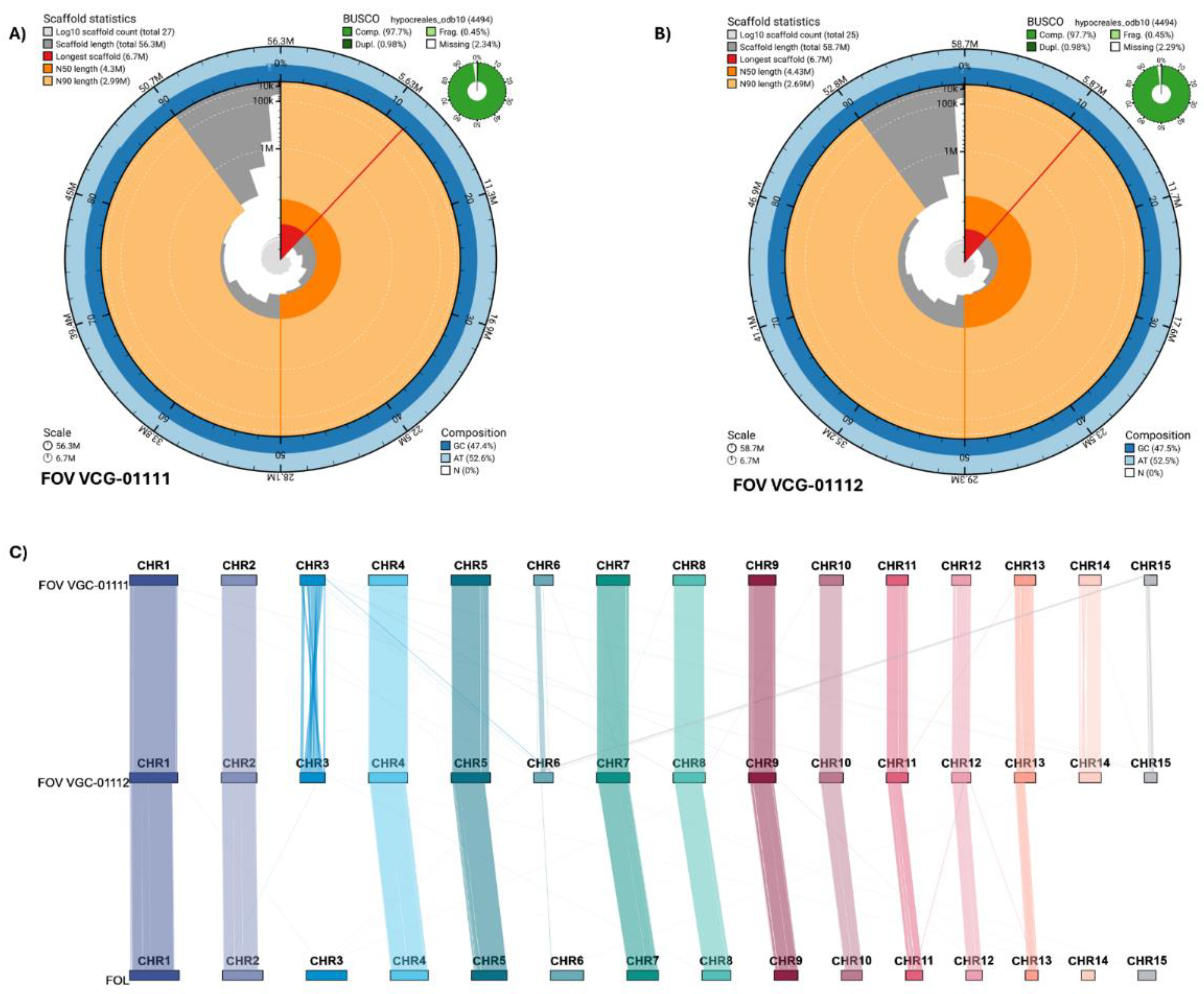
3.1. Genome Assembly
3.2. Genome Annotation and Functional Characteristics
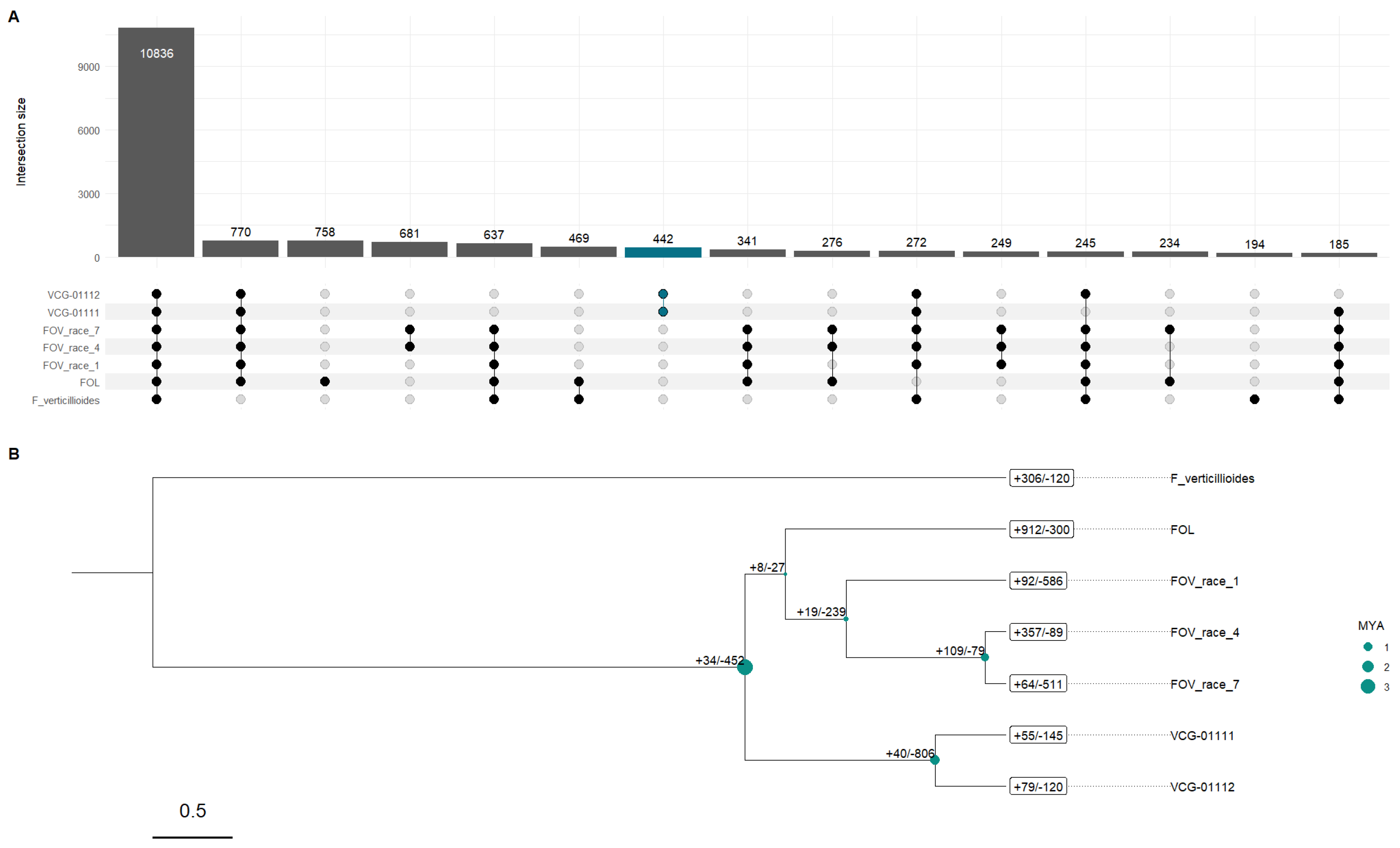
3.3. Evolutionary Differences and Gene Family Evolution
3.4. Identification of Secreted in Xylem Proteins (SIX)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, R.M.; Colyer, P.D.; Rothrock, C.S.; Kochman, J.K. Fusarium Wilt of Cotton: Population Diversity and Implications for Management. Plant Disease 2006, 90, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.D.; Moore, N.Y.; Kochman, J.K. Characterisation of a Population of Fusarium Oxysporum f.Sp. Vasinfectum Causing Wilt of Cotton in Australia. Australian journal of agricultural research 1996, 47, 1143. [Google Scholar] [CrossRef]
- Chakrabarti, A. Fusarium Oxysporum: A “Moving” View of Pathogenicity. In Soil Biology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp. 157–189. ISBN 978-3-642-39338-9. [Google Scholar]
- Bentley, S.; Kochman, J.K.; Moore, N.Y.; Pattemore, J.A.; Gulino, L.; O’Neill, W.T. DNA Diagnostics for Fusarium Wilt of Cotton.; Australian Cotton Growers’ Research Association, January 1 2000; pp. 455–461.
- Molecular Characterization of Races and Vegetative Compatibility Groups in Fusarium Oxysporum f. Sp. Vasinfectum | Applied and Environmental Microbiology. Available online: https://journals.asm.org/doi/abs/10.1128/aem.60.11.4039-4046.1994 (accessed on 13 February 2025).
- Kim, Y.; Hutmacher, R.B.; Davis, R.M. Characterization of California Isolates of Fusarium Oxysporum f. Sp. Vasinfectum. Plant disease 2005, 89, 366–372. [Google Scholar] [CrossRef]
- Fernandez, D.; Assigbese, K.; Dubois, M.P.; Geiger, J.P. Molecular Characterization of Races and Vegetative Compatibility Groups in Fusarium Oxysporum f. Sp. Vasinfectum. Applied and Environmental Microbiology 1994, 60, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Le, D.P.; Tran, T.T.; Gregson, A.; Jackson, R. TEF1 Sequence-Based Diversity of Fusarium Species Recovered from Collar Rot Diseased Cotton Seedlings in New South Wales, Australia. Australasian Plant Pathol. 2020, 49, 277–284. [Google Scholar] [CrossRef]
- Le, D.P.; Nguyen, C.P.T.; Kafle, D.; Scheikowski, L.; Montgomery, J.; Lambeth, E.; Thomas, A.; O’Keeffe, K.; Shakeshaft, B.; Young, A.; et al. Surveillance, Diversity and Vegetative Compatibility Groups of Fusarium Oxysporum f. Sp. Vasinfectum Collected in Cotton Fields in Australia (2017 to 2022). Pathogens 2022, 11, 1537. [Google Scholar] [CrossRef]
- Wang, B.; Brubaker, C.L.; Summerell, B.A.; Thrall, P.H.; Burdon, J.J. Local Origin of Two Vegetative Compatibility Groups of Fusarium Oxysporum f. Sp. Vasinfectum in Australia: Evolutionary Origin of Cotton Wilt in Australia. Evolutionary applications 2010, 3, 505–524. [Google Scholar] [CrossRef]
- Craven, L.; Stewart, J.; Brown, A.; Grace, J. The Australian Wild Species of Gossypium.; January 1995.
- Kochman, J. Fusarium Wilt in Cotton — a New Record in Australia. Australasian Plant Pathology 1995, 24, 74–74. [Google Scholar] [CrossRef]
- Stiller, W.N.; Wilson, I.W. Australian Cotton Germplasm Resources. In World Cotton Germplasm Resources; IntechOpen, 2014; ISBN 978-953-51-1622-6. [Google Scholar]
- Conaty, W.C.; Broughton, K.J.; Egan, L.M.; Li, X.; Li, Z.; Liu, S.; Llewellyn, D.J.; MacMillan, C.P.; Moncuquet, P.; Rolland, V.; et al. Cotton Breeding in Australia: Meeting the Challenges of the 21st Century. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- McFadden, H.; Beasley, D.; Brubaker, C.L. Assessment of Gossypium Sturtianum and G. Australe as Potential Sources of Fusarium Wilt Resistance to Cotton. Euphytica 2004, 138, 61–72. [Google Scholar] [CrossRef]
- Baroncelli, R.; Amby, D.B.; Zapparata, A.; Sarrocco, S.; Vannacci, G.; Le Floch, G.; Harrison, R.J.; Holub, E.; Sukno, S.A.; Sreenivasaprasad, S.; et al. Gene Family Expansions and Contractions Are Associated with Host Range in Plant Pathogens of the Genus Colletotrichum. BMC Genomics 2016, 17, 555. [Google Scholar] [CrossRef] [PubMed]
- Krijger, J.-J.; Thon, M.R.; Deising, H.B.; Wirsel, S.G. Compositions of Fungal Secretomes Indicate a Greater Impact of Phylogenetic History than Lifestyle Adaptation. BMC Genomics 2014, 15, 722. [Google Scholar] [CrossRef]
- Swett, C.L.; Del Castillo Múnera, J.; Hellman, E.; Helpio, E.; Gastelum, M.; Lopez Raymundo, E.; Johnson, H.; Oguchi, R.; Hopkins, A.; Beaulieu, J.; et al. Monitoring for a New I3 Resistance Gene-Breaking Race of F. Oxysporum f. Sp. Lycopersici (Fusarium Wilt) in California Processing Tomatoes Following Recent Widespread Adoption of Resistant (F3) Cultivars: Challenges with Race 3 and 4 Differentiation Methods. Frontiers in plant science 2023, 14, 1088044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Turra, D.; Zhou, S.; Ayhan, D.H.; DeIulio, G.A.; Guo, L.; Broz, K.; Wiederhold, N.; Coleman, J.J.; et al. The Genome of Opportunistic Fungal Pathogen Fusarium Oxysporum Carries a Unique Set of Lineage-Specific Chromosomes. Commun Biol 2020, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.S.; Outram, M.A.; Smith, A.; McCombe, C.L.; Khambalkar, P.B.; Rima, S.A.; Sun, X.; Ma, L.; Ericsson, D.J.; Jones, D.A.; et al. The Structural Repertoire of Fusarium Oxysporum f. Sp. Lycopersici Effectors Revealed by Experimental and Computational Studies. eLife 2024. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Ming, Y.; Liu, C.; Zhang, X.; Liu, S.; Zhu, L. Characterization of the High-Quality Genome Sequence and Virulence Factors of Fusarium Oxysporum f. Sp. Vasinfectum Race 7. Journal of fungi (Basel, Switzerland) 2024, 10, 242. [Google Scholar] [CrossRef]
- Jobe, T.O.; Ulloa, M.; Ellis, M.L. Two de Novo Genome Assemblies from Pathogenic Fusarium Oxysporum f. Sp. Vasinfectum Race 4 (FOV4) Isolates from California. Microbiology resource announcements 2024, 13, e0076023. [Google Scholar] [CrossRef]
- Jobe, T.O.; Ulloa, M.; Ellis, M.L. A High-Quality Whole-Genome Sequence, Assembly, and Gene Annotation of Fusarium Oxysporum f. Sp. Vasinfectum (Fov) Race 1 from California. Microbiology resource announcements 2024, 13, e0070223. [Google Scholar] [CrossRef]
- Nash, S.M.; Snyder, W.C. Quantitative Estimations by Plate Counts of Propagules of the Bean Root Rot Fusarium in Field Soils. Phytopathology 1962, 52, 567–572. [Google Scholar]
- Murray, M.G.; Thompson, W.F. Rapid Isolation of High Molecular Weight Plant DNA. Nucleic Acids Research 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Vurture, G.W.; Sedlazeck, F.J.; Nattestad, M.; Underwood, C.J.; Fang, H.; Gurtowski, J.; Schatz, M.C. GenomeScope: Fast Reference-Free Genome Profiling from Short Reads. Bioinformatics 2017, 33, 2202–2204. [Google Scholar] [CrossRef] [PubMed]
- Schelkunov, M.I. Mabs, a Suite of Tools for Gene-Informed Genome Assembly. BMC Bioinformatics 2023, 24, 377. [Google Scholar] [CrossRef]
- Cheng, H.; Concepcion, G.T.; Feng, X.; Zhang, H.; Li, H. Haplotype-Resolved de Novo Assembly Using Phased Assembly Graphs with Hifiasm. Nat Methods 2021, 18, 170–175. [Google Scholar] [CrossRef]
- Rapid and Sensitive Detection of Genome Contamination at Scale with FCS-GX - PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10898089/ (accessed on 18 February 2025).
- Zhou, C.; McCarthy, S.A.; Durbin, R. YaHS: Yet Another Hi-C Scaffolding Tool. Bioinformatics 2023, 39, btac808. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.C.; Robinson, J.T.; Shamim, M.S.; Machol, I.; Mesirov, J.P.; Lander, E.S.; Aiden, E.L. Juicebox Provides a Visualization System for Hi-C Contact Maps with Unlimited Zoom. Cell Syst 2016, 3, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.M.; Stajich, J. Funannotate v1.8.1: Eukaryotic Genome Annotation 2020.
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- InterProScan 5: Genome-Scale Protein Function Classification | Bioinformatics | Oxford Academic. Available online: https://academic.oup.com/bioinformatics/article/30/9/1236/237988?login=true (accessed on 18 February 2025).
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Research 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models | Nature Biotechnology. Available online: https://www.nature.com/articles/s41587-021-01156-3?utm_campaign=related_content&utm_source=HEALTH&utm_medium=communities (accessed on 18 February 2025).
- Sun, J.; Lu, F.; Luo, Y.; Bie, L.; Xu, L.; Wang, Y. OrthoVenn3: An Integrated Platform for Exploring and Visualizing Orthologous Data across Genomes. Nucleic Acids Research 2023, 51, W397–W403. [Google Scholar] [CrossRef]
- OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics | Genome Biology | Full Text. Available online: https://genomebiology.biomedcentral.com/articles/10.1186/s13059-019-1832-y (accessed on 18 February 2025).
- Kumar, S.; Suleski, M.; Craig, J.M.; Kasprowicz, A.E.; Sanderford, M.; Li, M.; Stecher, G.; Hedges, S.B. TimeTree 5: An Expanded Resource for Species Divergence Times. Molecular Biology and Evolution 2022, 39, msac174. [Google Scholar] [CrossRef]
- Shen, X.-X.; Steenwyk, J.L.; LaBella, A.L.; Opulente, D.A.; Zhou, X.; Kominek, J.; Li, Y.; Groenewald, M.; Hittinger, C.T.; Rokas, A. Genome-Scale Phylogeny and Contrasting Modes of Genome Evolution in the Fungal Phylum Ascomycota. Sci Adv 2020, 6, eabd0079. [Google Scholar] [CrossRef]
- Da Lage, J.-L.; Binder, M.; Hua-Van, A.; Janeček, S.; Casane, D. Gene Make-up: Rapid and Massive Intron Gains after Horizontal Transfer of a Bacterial α-Amylase Gene to Basidiomycetes. BMC Evol Biol 2013, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Van der Nest, M.A.; Steenkamp, E.T.; McTaggart, A.R.; Trollip, C.; Godlonton, T.; Sauerman, E.; Roodt, D.; Naidoo, K.; Coetzee, M.P.A.; Wilken, P.M.; et al. Saprophytic and Pathogenic Fungi in the Ceratocystidaceae Differ in Their Ability to Metabolize Plant-Derived Sucrose. BMC Evol Biol 2015, 15, 273. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic Analyses of RPB1 and RPB2 Support a Middle Cretaceous Origin for a Clade Comprising All Agriculturally and Medically Important Fusaria. Fungal Genet Biol 2013, 52, 20–31. [Google Scholar] [CrossRef]
- Cabanettes, F.; Klopp, C. D-GENIES: Dot Plot Large Genomes in an Interactive, Efficient and Simple Way. PeerJ 2018, 6, e4958. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Davis, R.D.; Moore, N.Y.; Kochman, J.K. Characterisation of a Population of Fusarium Oxysporum f.Sp. Vasinfectum Causing Wilt of Cotton in Australia. Aust. J. Agric. Res. 1996, 47, 1143–1156. [Google Scholar] [CrossRef]
- Taxonomy and Evolution of the Cotton Genus, Gossypium - Wendel - 2015 - Agronomy Monographs - Wiley Online Library. Available online: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2134/agronmonogr57.2013.0020 (accessed on 21 March 2025).
- Wagner, T.A.; Duke, S.E.; Davie, S.M.; Magill, C.; Liu, J. Interaction of Fusarium Wilt Race 4 with Root-Knot Nematode Increases Disease Severity in Cotton. Plant Disease 2022. [Google Scholar] [CrossRef]
- Moore, N.Y.; Kochman, J.K.; Obst, N.R.; O’Neill, W.T.; Bentley, S. Fusarium Wilt of Cotton in Australia.
- Chakrabarti, A.; Rep, M.; Wang, B.; Ashton, A.; Dodds, P.; Ellis, J. Variation in Potential Effector Genes Distinguishing Australian and Non-Australian Isolates of the Cotton Wilt Pathogen Fusarium Oxysporum f.Sp. Vasinfectum. Plant Pathology 2011, 60, 232–243. [Google Scholar] [CrossRef]
- Genetic Variation and Population Structure of Fusarium Oxysporum f.Sp. Vasinfectum in Australia - Wang - 2006 - Plant Pathology - Wiley Online Library. Available online: https://bsppjournals.onlinelibrary.wiley.com/doi/full/10.1111/j.1365-3059.2006.01445.x (accessed on 18 February 2025).
- Disease Ranks. Cotton Seed Distributors.
- Sun, X.; Fang, X.; Wang, D.; Jones, D.A.; Ma, L. Transcriptome Analysis of Fusarium-Tomato Interaction Based on an Updated Genome Annotation of Fusarium Oxysporum f. Sp. Lycopersici Identifies Novel Effector Candidates That Suppress or Induce Cell Death in Nicotiana Benthamiana. Journal of fungi (Basel, Switzerland) 2022, 8, 672. [Google Scholar] [CrossRef]
- Davis, R.D.; Moore, N.Y.; Kochman, J.K. Characterisation of Population of Fusarium Oxysporum f. Sp. Vasinfectum Causing Wilt of Cotton in Australia. Aust. J. Agric. Res 1996, 47, 1143–1156. [Google Scholar] [CrossRef]
- Lopez-Lavalle, L.A.B.; Gillespie, V.J.; Tate, W.A.; Ellis, M.H.; Stiller, W.N.; Llewellyn, D.L.; Wilson, I.W. Molecular Mapping of a New Source of Fusarium Wilt Resistance in Tetraploid Cotton (Gossypium Hirsutum L.). Mol Breeding 2012, 30, 1181–1191. [Google Scholar] [CrossRef]
- Popa-Baez, A.; Smith, L.J.; Stiller, W.; Soliveres, M.; Pandey, G.; Wilson, I. The Complete Genome of Two Australian Isolates of Fusarium Oxysporum f. Sp. Vasinfectum. 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




