Highlights
What are the main findings?
The IMU-based system demonstrated high concurrent validity with a gold-standard optoelectronic motion capture system across a wide variety of full body tasks.
The IMU-based system achieved strong correlations (r ≥ 0.77), low RMSE values (generally < 7°), and negligible systematic biases (≤ 3.9°) compared to the optical reference.
What is the implication of the main finding?
The tested IMU-based system provides clinically acceptable range-of-motion estimates and can serve as a reliable alternative to laboratory-based motion analysis tools.
Its portability and ease of use make it particularly suited for in-clinic assessments and home-based rehabilitation programs, supporting remote patient monitoring.
1. Introduction
Motor rehabilitation plays a crucial role in restoring functional abilities in individuals affected by injuries, acute or chronic diseases, and other musculoskeletal or neurological conditions [
1]. It encompasses targeted physical therapies designed to enhance motor coordination, promote neuroplasticity, improve strength, and prevent muscle atrophy, ultimately contributing to improved quality of life [
2,
3,
4]. Given that motor impairments can lead to varying degrees of functional limitations, the precise and objective quantification of joints range of motion (ROM) is fundamental for assessing functional deficits and evaluating the effectiveness of rehabilitation interventions. ROM is defined as the angular displacement of a joint from its initial position to its maximum movement in a specific direction and is traditionally measured using a manual goniometer.
Manual goniometers are widely used in clinical settings due to their simplicity, cost-effectiveness, and ability to provide direct joint ROM measurements [
5]. However, their accuracy is influenced by multiple factors, including the examiner’s expertise and the specific joint assessed [
6,
7]. Inter-rater reliability also varies, generally showing higher consistency in the upper limbs compared to lower limbs [
8]. Despite their practicality, goniometers are limited to measuring static joint angles in a single plane, making them unsuitable for dynamic motion assessments [
9]. Consequently, advanced motion capture technologies have emerged to offer comprehensive assessment of joint kinematics. Among these, optoelectronic marker-based motion capture (MCap) systems have become the gold standard for evaluating body kinematics, providing highly accurate and objective movement analysis [
10,
11,
12]. Nonetheless, their high costs, lack of portability, and dependency on specialized laboratory setups limit their widespread adoption [
13].
In this context, the development of Inertial Measurement Units (IMUs) has introduced new possibilities for human motor assessment, significantly increasing their popularity in motion capture studies [
14,
15,
16,
17]. IMUs are compact, stand-alone devices integrating data from three-axial accelerometers, gyroscopes, and magnetometers through sensor fusion algorithms and biomechanical models, estimating body segments kinematics in three-dimensional space [
18,
19,
20]. Compared to MCap systems, IMUs significantly expand clinical and research applications due to their portability, cost-effectiveness, and ease of use. Furthermore, their independence from external cameras or laboratory setups makes them ideal for in-home or remote rehabilitation, where continuous real-time monitoring is crucial [
21,
22]. Nonetheless, robust calibration and validation protocols are critical: improper sensor placement, ferromagnetic disturbances [
23], and drift errors due to signal integration [
24,
25] may cause cumulative measurement inaccuracies. Therefore, standardized calibration procedures, involving known reference poses or synchronized movements, are essential to minimize drift and enhance accuracy before clinical application [
23,
24,
25,
26].
Over the past decade, IMU-based systems have increasingly been adopted to estimate kinematics across various tasks, including gait analysis [
27,
28], dynamic movements such as jumps [
29,
30,
31], squats [
32,
33,
34], and lifting tasks [
35,
36,
37]. Despite their good concurrent validity compared with traditional MCap systems, IMU-based joint kinematics require further validation. Specifically, tasks involving large ROMs, abrupt directional changes, or multi-joint coordination can exacerbate sensor fusion errors, underscoring the importance of carefully designed validation studies for different movement patterns [
38,
39,
40].
Several studies have examined the accuracy of IMU-based systems with respect to traditional MCap. Cutti et al. reported root mean square error (RMSE) values below 3.6 degrees and a correlation coefficient of 0.99 in scapulothoracic and humerothoracic kinematics [
41]. Similar findings were reported by Parel et al. and Friesen et al. in scapular motions during dynamic tests [
42,
43]. Regarding elbow kinematic, both Cutti et al. and Fang et al. demonstrated reliable IMUs performance in controlled settings and in everyday tasks [
41,
44]. However, the IMUs accuracy in assessing the upper limbs kinematic may vary depending on joint complexity and context of movement [
44,
45,
46]. Regarding lower limbs and trunk tasks, Cerfoglio et al. validated a remote IMU system, finding average ROM errors below 5.0 degrees in hip movements and squats [
47]. Similarly, Leardini et al. reported average discrepancies below 5 degrees for knee flexion and 3 degrees for chest inclination [
48]. IMU-based systems have also demonstrated their effectiveness for inpatient assessment [
15,
49] and remote home-based rehabilitation [
48,
50], enabling automated, objective and real-time evaluations [
51,
52].
However, while IMU-based systems are generally reported to provide reliable descriptions, their accuracy in estimating joint angles can vary depending on system complexity and sensors’ placement [
53,
54]. For successful adoption in clinical and research practice, IMU-based systems require rigorous validation before being integrated into motion analysis and rehabilitation settings. Ensuring consistent calibration, appropriate sensor placement, and accounting for inter-subject variability is critical.
Thus, the aim of this study was to validate a commercially available multi-sensor IMU-based system used in clinical practice against a gold standard MCap system through lower limbs, trunk, and upper limbs motor tasks.
2. Materials and Methods
This prospective validation study aimed to assess and compare motion data recorded simultaneously by an IMU-based system and an optoelectronic motion capture system (MCap). The study was conducted at the “Luigi Divieti” Posture and Movement Analysis Laboratory (Department of Electronics, Information and Bioengineering (DEIB), Politecnico di Milano, Milano, Italy) in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Politecnico di Milano (Protocol No. 22/2021, 14 June 2021).
2.1. Participants
A total of 15 healthy participants (M/F: 6/9; age: 25.9 ± 3.8 years; body mass: 65.1 ± 14.3 kg; height = 170.0 ± 9.2 cm) were included in the study based on the following inclusion criteria: (i) age ≥ 18 years, (ii) absence of pain or musculoskeletal injuries within the 15 days prior to testing, and (iii) no underlying musculoskeletal conditions that could affect test outcomes. Prior to participation, all volunteers received an explanation of the study’s objectives and provided informed written consent.
2.2. Equipment
Data were simultaneously collected from IMU-based and MCap systems.
The IMU-based system consisted of five inertial measurement units (Movella DOT, Enschede, The Netherlands) connected via Bluetooth Low Energy (BLE) to proprietary software (Euleria Lab, Euleria srl, Rovereto, Italy). Each IMU included a tri-axial accelerometer (±16 g full scale), a tri-axial gyroscope (±2000 deg/s full scale), and a tri-axial magnetometer (±8 Gauss full scale). Accelerometer and gyroscope signals were sampled at 800 Hz, whereas magnetometer signals were sampled at 60 Hz. A proprietary strapdown integration sensor fusion algorithm [
55] combined these data to output orientation quaternions at 60 Hz. The IMUs were attached to participants' bodies using non-invasive elastic bands to minimize skin motion artifacts.
The gold-standard MCap system consisted of eight infrared cameras (SMART DX 100, BTS-Bioengineering, Milan, Italy), providing accuracy of <0.2 mm over a 2 × 2 × 2 m volume, operating at 100 Hz.
To synchronize signals from the two systems, MCap data were resampled at 60 Hz to match the IMU output frequency.
2.3. Procedure
Participants were informed of the study’s purpose and anthropometric measurements were recorded, including height, weight, leg length, knee diameter (distance between femoral condyles), ankle diameter (distance between malleoli), distance between the anterior iliac spines, and pelvis thickness.
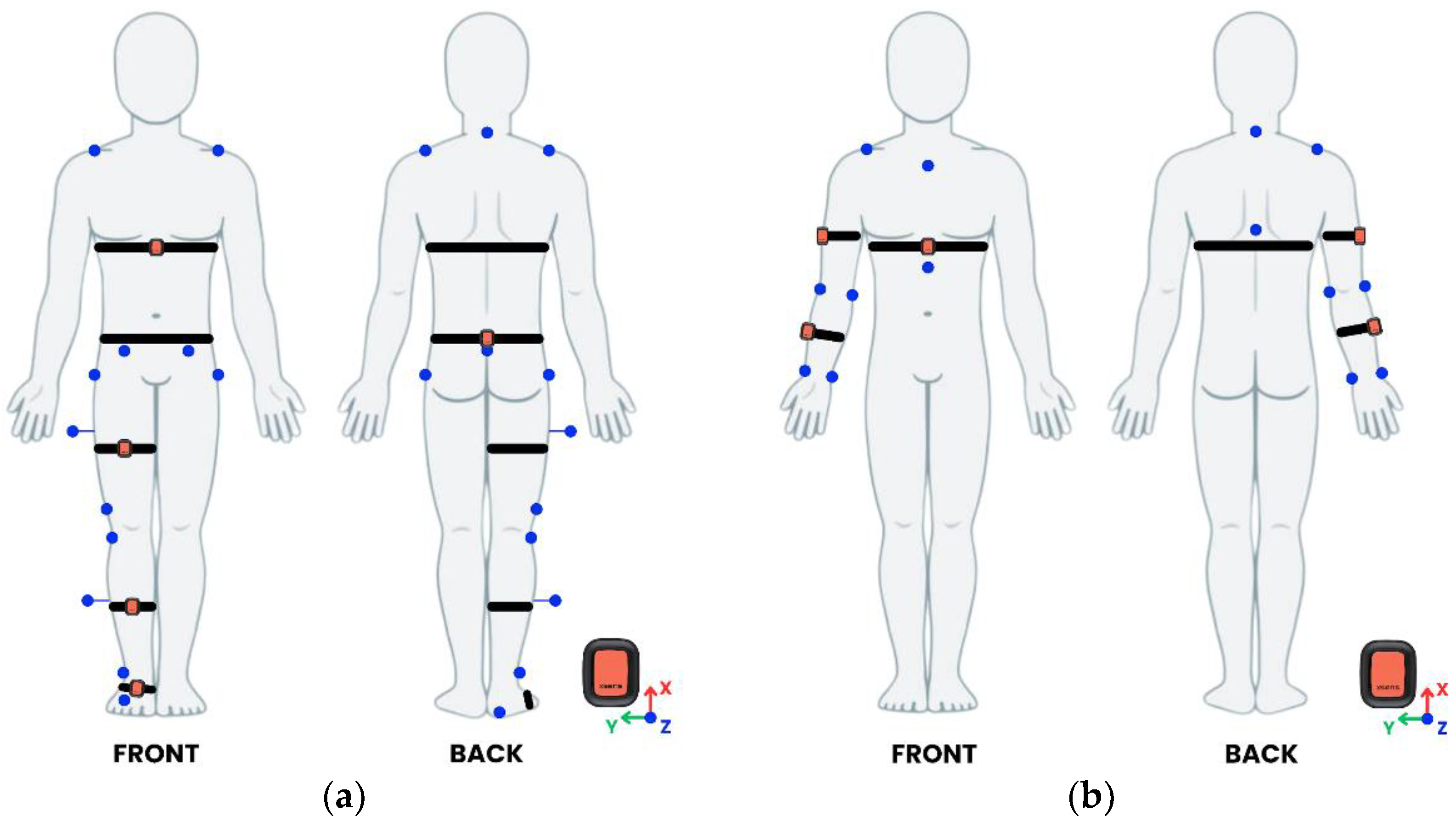
IMUs and reflective markers were positioned following two different configurations based on the assessed tasks.
For lower body and trunk motor tasks, five IMUs were mounted on the chest (below xiphoid process), pelvis (L5 vertebra level, midpoint between posterior superior iliac spines), anterior right thigh, anterior right shank and dorsal right foot. Fifteen reflective markers were placed on anatomical landmarks, according to a modified Davis protocol for the right side only (
Figure 1a) [
56].
For upper body tasks, three IMUs were mounted on the chest (below xiphoid process), lateral right arm, and lateral right forearm. Nine reflective markers were placed to the anatomical landmarks displayed in
Figure 1b [
57].
After positioning IMUs and markers, both the IMU-based and the MCap systems were simultaneously calibrated. Participants assumed an anatomical N-pose calibration position: upright stance, feet parallel (about 20 cm apart), arms slightly abducted from the body, palms facing forward. For the MCap system, calibration was performed to define participant anatomy and joint centers of rotation, as detailed in [
56].
Following calibration, participants performed three repetitions of single-joint and multi-joint tasks described sequentially in
Table 1.
2.4. Data Processing
Raw IMU data were processed internally by Euleria Lab software, automatically providing 3D joint angles from quaternions. During the N-pose calibration, initial calibration quaternions
established the global-to-anatomical frame relationship for each sensor. Each calibration quaternion was converted into a rotation matrix
, expressing the orientation of the IMUs reference frame (
) with respect to the global frame
(Equation (1)):
Adjustments ensured the IMU axes (X,Y,Z) to match standard anatomical directions (anteroposterior, craniocaudal and mediolateral respectively). This refined rotation matrix was reconverted into the quaternion (
) representing the transformation from
to
during the N-pose. During movements, each IMU provided instantaneous orientation quaternion (
) relative to the global frame. By combining
and
according to Equation (2), the quaternions describing the segment orientations relative to the anatomical position (
) are obtained:
where
denotes the Hamilton product, and
the quaternion conjugate.
Joint quaternions (
) were then computed as the difference between proximal and distal segments quaternions (Equation (3)):
Resulting joint quaternions were decomposed into anatomical angles (flexion-extension, abduction-adduction, internal-external rotation) using ZYX Euler sequence.
MCap data were processed with SmartTracker and SmartAnalyzer (BTS Bioengineering, Milan, Italy). Data were tracked, interpolated and filtered (5 Hz Butterworth low-pass filter) to obtain a 3D reconstruction of the coordinates of each marker. Custom routines extracted angular measures.
For lower body tasks, limb rotation algorithm was based on Euler angles, and it defined joint movements in terms of flexion/extension, abduction/adduction, and internal/external rotation [
56]. Trunk and pelvic angles were computed as absolute angles, while hip, knee, and ankle joint angles were expressed as relative angles. For the upper body, shoulder angles were defined as the angle between the humeral axis (acromion-lateral epicondyle) and thorax planes. Elbow angles were computed between humeral axis and forearm axis (lateral elbow-lateral wrist epicondyle).
Finally, ROM values for each task were calculated as the difference between the maximum and minimum joint angles during each repetition. A repetition was defined as a single execution of the task-specific movement, from the calibrated N-pose position to the point of maximal joint excursion and concluding upon the subject’s return to the initial position. Then, the corresponding ROM value for both the IMU-based and MCap systems was obtained as the average result of the three repeated movements.
2.5. Sample Size
The sample size for this study was determined by considering the specific population’s characteristics, feasibility constraints, and statistical requirements. A priori power analysis, conducted with G*Power, indicated that 15 participants would achieve 80% power at an alpha level of 0.05, assuming a large effect size (Cohen’s d = 0.8). This number of participants balanced practical considerations (e.g., participant availability) with the need for sufficient statistical sensitivity.
2.6. Statistical Analysis
Data normality was verified using the Shapiro–Wilk test for each task, and all the ROM values followed a normal distribution (p > 0.05). Consequently, mean and standard deviation (SD) were used for descriptive statistics, and parametric methods were employed [
58,
59]. To test systematic differences between the IMU-based and MCap system for each test, a paired samples t-test was performed, using α = 0.05 as the significance threshold. Effect sizes (Cohen's
d) were computed and classified as small (|
d| < 0.2), moderate (|
d| = 0.5), or large (|
d| = 0.8) [
60].
Accuracy was quantified by calculating the percentage of similarity (PoS, %) and the Root Mean Square Error (RMSE, °) as shown in Equations (4) and (5):
Bland-Altman analyses were then conducted to assess agreement between the two measurement systems, yielding systematic bias and 95% limits of agreement (LoA = bias ±1.96 SD) for each task [
61]. Pearson's correlation coefficients (
) were also computed to evaluate the linear relationship between the two systems, with correlation strength defined as poor (
), fair (
), moderate (
) or strong (
) [
62]. All statistical analyses were carried out using MATLAB (v.2023a MathWorks, Natick, MA, USA) and JASP (JASP Team 2023, Version 0.17.3).
3. Results
For clarity and ease of reference, the results are organized into four sub-sections based on body region and task complexity. The results are reported into tables in which each row reports mean (± SD) ROM values for both the IMU- and MCap-based systems, p-values and Cohen’s d from paired t-tests, accuracy metrics (PoS and RMSE), and Pearson’s correlation coefficients ().
3.1. Lower Body and Trunk
The results for lower body and trunk single-joint tasks are summarized in
Table 2.
The IMU system demonstrated excellent concurrent validity with the MCap system for all single-joint lower-body and trunk tasks. No statistically significant differences were observed (p > 0.05), and the associated Cohen’s d values were uniformly small, indicating that any mean differences were trivial relative to the within-participant variability. Absolute agreement was likewise high (PoS > 98 % and RMSE < 4.5 °), confirming that the IMU estimates lay very close to the reference values. Correlation coefficients are notably high (r ≥ 0.89), indicating that the IMU measurements closely follow the same patterns as those captured by the MCap system. For the multi joint tasks, the pattern was broadly similar. Both the hip and knee ROM during the squat showed no statistically significant differences (p ≥ 0.820, d ≤ 0.07) with the MCap system. High correlations (r ≥ 0.93) between IMU and MCap-based measurements during squat were displayed, reinforcing the close alignment of motion patterns between the two systems. Hip ROM during the lunge exhibited a statistically significant difference (p = 0.034), accompanied by a moderate effect size (Cohen’s d = 0.661), slightly lower accuracy (PoS = 94.6%) and the largest RMSE of the set (6.9°). Despite this, the correlation for lunge hip ROM remains strong (r = 0.93), implying that the IMU-based system generally follows the same trend as the MCap reference. Overall, the IMU system reproduced lower-body ROM with both high accuracy and strong linear association with respect to the reference system.
The agreement between the two systems for lower body and trunk movements was assessed through the Bland-Altman analysis, whose systematic bias and LoA are reported in
Table 3.
Bland–Altman analysis confirmed the close agreement observed in the paired comparisons. Systematic bias was negligible for every single-joint task, ranging from –1.0 ° (trunk flexion) to +1.2 ° (knee flexion). Corresponding LoA ranges were narrow (approximately ±15 °), well within commonly accepted clinical thresholds for lower-limb ROM evaluation. For multi-joint tasks, bias remained small (–3.9 ° to 2.0 °) in the squat and lunge knee, although the LoA ranges widened to about 30 °, reflecting the increased kinematic variability inherent in compound movements. The wider differences were reported in the hip ROM during lunges, where the IMU underestimated the optical measurement by –3.9 ° on average and displayed an asymmetric LoA (–15.0 ° to +7.6 °). This pattern echoes the significant paired-test result and suggests a modest but systematic under-reading for that specific motion.
3.2. Upper Body
The results for upper body tasks are summarized in
Table 4.
For single-joint tasks, the IMU and MCap systems show not statistically differences (p ≥ 0.30, d ≤ 0.32) and high accuracy (PoS > 97 %, RMSE ≈ 3.7–6.6 °). Pearson’s r reveals strong correlation for shoulder abduction task (r = 0.92), whereas moderate correlation for shoulder and elbow flexion (r = 0.77 and r = 0.80, respectively). Both the shoulder and elbow angles during the overhead press task displayed excellent agreement with non-significant differences (p ≥ 0.59) and negligible effect sizes (d ≤ 0.16). Accuracy and correlation results are comparable to the ones for the single-joint tasks (PE ≥ 98.5 %, RMSE < 7°, r > 0.93). These findings indicate that, despite the increased kinematic complexity of a coordinated press, the IMU system reproduces the reference measurements with both high absolute precision and close tracking of inter-individual variability.
The agreement between the two systems for upper body movements was assessed through the Bland-Altman analysis, whose systematic bias and LoA are reported in
Table 5.
The results from the Bland-Altman analysis reported a systematic bias within ±2° with an overall overestimation of the ROM from IMU-based system with respect to the MCap. The task complexity (single-joint or multi-joint task) does not impact systematic bias and LoA. The task showing the narrowest LoA range is the shoulder flexion (14.6°), while multi-joint tasks show wider LoA (~27°).
4. Discussion
The present study aimed to evaluate the concurrent validity of a commercially available multi-sensor IMU-based motion-capture system against a laboratory-grade optoelectronic reference system during a comprehensive battery of single- and multi-joint movements involving the lower limbs, trunk, and upper limbs. Overall, the findings align with the previous literature, demonstrating that the IMU-based system reproduces joint-specific ROM measurements with accuracy and agreement comparable to the reference system.
For all single-joint lower-body and trunk movements, mean biases were ≤ 1.2 °, PoS values exceeded 98 %, and RMSE remained below 4.5 °. These results indicate excellent absolute agreement and are consistent with previous validations reporting RMSE values below 5 ° for lower-limb joints when IMUs were benchmarked against marker-based systems [
41,
47,
48]. Correlation coefficients were uniformly strong (
r ≥ 0.89), underscoring that inter-individual variability captured by the IMU system closely mirrors that of the gold standard.
During compound movements, the IMU showed high accuracy for squat-related hip and knee ROM (RMSE ≤ 7.1 °) and retained strong correlations (
r ≥ 0.93). The only statistically significant discrepancy emerged for hip flexion during the lunge, where the IMU underestimated ROM by 3.9 ° on average and showed a moderate effect size (
d = 0.661). This under-reading likely reflects the increased pelvic tilt and multi-planar motion inherent in the lunge, which may exacerbate soft-tissue artefacts and violate the single-rigid-segment assumption embedded in the sensor-fusion algorithm [
38,
39,
40]. Nevertheless, even for this task the error magnitude (RMSE = 6.9 °) remained within clinically acceptable limits.
In single-joint upper-limb tasks, the IMU reproduced RMSE values between 3.7 ° and 6.6 ° and no significant mean differences with respect to the MCap system. While correlations were variable (
r = 0.77-0.92), the strength of association was still sufficient for clinical trend monitoring and is comparable to earlier reports on humerothoracic kinematics (
r ≥ 0.80, RMSE < 6 °) [
41,
42,
43,
44]. During the multi-joint movements, the IMU reliably tracked coordinated upper-body motions despite their larger excursion and speed, achieving strong correlations (
r = 0.93–0.96) and RMSE values < 6.7 °. The results indicated that the IMU system can reliably track coordinated multi-joint upper-body motions, despite their larger excursion and speed. These upper-limb outcomes again align with published Person’s
r and RMSE benchmarks for IMU-based systems [
46,
47].
Several factors likely contributed to the high level of agreement observed. From a methodological point of view, the adoption of a standardized N-pose calibration protocol, combined with careful alignment of sensor axes to their corresponding anatomical axes, helped reduce the likelihood of initial orientation errors [
23,
24,
25,
26]. Secure sensor positioning on prominent body landmarks minimized axis misalignment and cross-talk—common sources of error in IMU-based measurements. By minimizing systematic offsets at the outset, the accuracy of subsequent joint angle estimations was enhanced.
With respect to task selection, this research included both single-joint and compound movements to fully assess the performance of the IMU-based system. Single-joint tasks (e.g., hip, knee, and ankle flexion/extension) are ideal for assessing the sensors’ ability to capture specific joint movements with minimal interference from other body parts [
13]. In contrast, compound movements (e.g., squats and lunges) involve multiple joints and body segments, mimicking more complex, functional motions seen in daily activities and rehabilitation exercises. Moreover, by imposing a controlled-speed motion, the study reduced potential issues like sensor drift that can distort data during fast movements [
39]. This diversified yet speed-controlled task set therefore enhances the clinical relevance of the results for rehabilitation settings.
Taken together, these results suggest that the tested IMU configuration delivers ROM estimates that are interchangeable with laboratory-grade MCap system for most tasks. Given their portability, affordability, and independence from dedicated spaces or cameras [
21,
22], IMUs offer clear logistical advantages for both in-clinic and at-home rehabilitation programs, where frequent assessments and real-time feedback are desirable.
Despite the highlighted strengths, some limitations warrant caution.
Although the sample size was sufficient based on the power analysis, the restricted number and age range of participants may limit the generalizability of the findings. Indeed, subjects affected by movement disorders (e.g., orthopedic and post-stroke patients) who may exhibit different biomechanical patterns should be included in future studies to understand the sensors’ accuracy under altered motor movements. Moreover, the study was conducted in a controlled laboratory environment, which does not reflect the variability of real-world or clinical settings, while ensuring experimental consistency. External factors such as ferromagnetic disturbances—common outside the lab—or sensor misplacement may affect the accuracy of IMUs [
23,
24,
47].
The restricted sensor configuration may have introduced limitations in capturing complex body movements. The study employed five sensors for lower-body tasks and three for upper-body tasks, in accordance with the manufacturer’s standard clinical workflow. While this sensor placement strategy is practical and aligned with existing protocols, it may not fully account for all sources of segment-interaction errors, particularly during multi-planar motions.
Another methodological limitation is related to the ROM assessment performed from peak-to-peak. While ROM is a commonly used and clinically relevant metric, it does not provide a complete picture of continuous kinematic waveforms—capturing the temporal evolution of movement. A waveform-level analysis (e.g., statistical parametric mapping, SPM) would offer a more nuanced understanding of the IMU-based system’s behavior compared to the MCap system during dynamic or rehabilitative tasks [
63].
Finally, the evaluation of sensor drift was limited to short-duration trials. Each recording captured discrete trials of movement, which may not fully capture the cumulative integration errors that can accrue over prolonged monitoring periods. Drift, a known limitation of IMU-based systems, is often negligible in short bouts but may become clinically significant during continuous or long-term use [
24,
25]. Longer-duration studies are thus essential to characterize drift behaviors under sustained operation and to inform strategies for mitigating its impact in real-world applications.
Future research should focus on assessing the application of the IMU-based system in pathological populations to understand how sensor placement, altered movement patterns, and assistive devices impact measurement accuracy. Additionally, developing adaptive magnetometer-rejection filters could reduce interference from real-environment settings magnetic fields to improve consistency and scalability across clinical settings. Research should also explore the performance of these systems in dynamic tasks, such as gait, running, and jumping, which present challenges like signal noise and rapid joint movements. Comprehensive validation across diverse movements will be essential for confirming the robustness of IMU-based systems for real-world use.
5. Conclusions
The present validation study demonstrated that a commercially available multi-sensor IMU system can replicate the joint-specific range-of-motion measurements of a laboratory-grade optoelectronic reference across a wide variety of movements involving the lower limbs, trunk, and upper limbs. Bland–Altman analysis revealed trivial systematic biases and limits of agreement no wider than ±15 °, which further supports the interchangeability of the two systems for most functional tasks.
Methodological choices—such as a standardized N-pose calibration, precise sensor alignment to anatomical axes, and secure fixation on bony landmarks—likely minimized orientation error, crosstalk, and soft-tissue artefact, thereby underpinning the high level of agreement observed. The evidence indicates that the ROM estimates from IMU sensors are both statistically and clinically equivalent to those obtained with optical motion capture, while conferring practical advantages in cost, portability, and set-up flexibility.
Future work should extend validation to pathological populations, incorporate waveform-level analyses to capture temporal kinematic characteristics, and examine long-duration trials to quantify drift.
Author Contributions
Conceptualization, G.V. and A.B.; methodology, G.V and S.C.; software, G.V, S.C. and A.B.; validation, P.C., M.G. and V.C.; formal analysis, G.V. and S.C.; investigation, G.V. and A.B.; resources, G.V. and A.B.; data curation, G.V. and S.C.; writing—original draft preparation, G.V. and S.C.; writing—review and editing, G.V., S.C., P.C. and V.C.; visualization, G.V. and S.C.; supervision, P.C., M.G. and V.C.; project administration, V.C.; funding acquisition, P.C. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Politecnico di Milano (Protocol No. 22/2021, 14 June 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors would like to acknowledge Euleria srl Società Benefit for providing the material used in the study and Cecilia Petruccelli for her valuable contribution to the project.
Conflicts of Interest
Giacomo Villa and Alessandro Bonfiglio were employed by the company Euleria srl Società Benefit. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IMU |
Inertial Measurement Unit |
| MCap |
Motion Capture |
| ROM |
Range Of Motion |
| Abd |
Abduction |
| Flex |
Flexion |
| Ext |
Extension |
| RMSE |
Root Mean Square Error |
| PoS |
Percentage of Similarity |
| LoA |
Limits of Agreement |
References
- Hochstenbach, J. Rehabilitation Is More than Functional Recovery. Disability and Rehabilitation 2000, 22, 201–204. [Google Scholar] [CrossRef]
- Andersen, L.L.; Saervoll, C.A.; Mortensen, O.S.; Poulsen, O.M.; Hannerz, H.; Zebis, M.K. Effectiveness of Small Daily Amounts of Progressive Resistance Training for Frequent Neck/Shoulder Pain: Randomised Controlled Trial. Pain 2011, 152, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L.; Anson, J.G. Synergies in Health and Disease: Relations to Adaptive Changes in Motor Coordination. Physical Therapy 2006, 86, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Veldema, J.; Jansen, P. Resistance Training in Stroke Rehabilitation: Systematic Review and Meta-Analysis. Clin Rehabil 2020, 34, 1173–1197. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, S.; Leunig, M.; Glatthorn, J.F.; Stauffacher, S.; Gerber, H.; Maffiuletti, N.A. Validity and Test-Retest Reliability of Manual Goniometers for Measuring Passive Hip Range of Motion in Femoroacetabular Impingement Patients. BMC Musculoskelet Disord 2010, 11, 194. [Google Scholar] [CrossRef]
- Muir, S.W.; Corea, C.L.; Beaupre, L. Evaluating Change in Clinical Status: Reliability and Measures of Agreement for the Assessment of Glenohumeral Range of Motion. N Am J Sports Phys Ther 2010, 5, 98–110. [Google Scholar]
- Walmsley, C.P.; Williams, S.A.; Grisbrook, T.; Elliott, C.; Imms, C.; Campbell, A. Measurement of Upper Limb Range of Motion Using Wearable Sensors: A Systematic Review. Sports Med - Open 2018, 4, 53. [Google Scholar] [CrossRef]
- Boone, D.C.; Azen, S.P.; Lin, C.M.; Spence, C.; Baron, C.; Lee, L. Reliability of Goniometric Measurements. Phys Ther 1978, 58, 1355–1360. [Google Scholar] [CrossRef]
- Lim, C.C.; Affandi, M.; Basah, S.N.; Din, M.Y. Evaluating Lower Limb Joint Flexion by Computerized Visual Tracking System and Compared with Electrogoniometer and Universal Goniometer.
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The Reliability of Three-Dimensional Kinematic Gait Measurements: A Systematic Review. Gait & Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Colyer, S.L.; Evans, M.; Cosker, D.P.; Salo, A.I.T. A Review of the Evolution of Vision-Based Motion Analysis and the Integration of Advanced Computer Vision Methods Towards Developing a Markerless System. Sports Medicine - Open 2018, 4, 24. [Google Scholar] [CrossRef]
- Roggio, F.; Ravalli, S.; Maugeri, G.; Bianco, A.; Palma, A.; Di Rosa, M.; Musumeci, G. Technological Advancements in the Analysis of Human Motion and Posture Management through Digital Devices. World J Orthop 2021, 12, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.-S. Validity and Reliability of Wearable Sensors for Joint Angle Estimation: A Systematic Review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef] [PubMed]
- Adesida, Y.; Papi, E.; McGregor, A.H. Exploring the Role of Wearable Technology in Sport Kinematics and Kinetics: A Systematic Review. Sensors 2019, 19, 1597. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lin, W.; He, X.; Zhang, L.; Zhang, M. IMU-Based Motion Capture System for Rehabilitation Applications: A Systematic Review. Biomimetic Intelligence and Robotics 2023, 3, 100097. [Google Scholar] [CrossRef]
- Arlotti, J.S.; Carroll, W.O.; Afifi, Y.; Talegaonkar, P.; Albuquerque, L.; Burch V, R.F.; Ball, J.E.; Chander, H.; Petway, A. Benefits of IMU-Based Wearables in Sports Medicine: Narrative Review. IJKSS 2022, 10, 36–43. [Google Scholar] [CrossRef]
- Horak, F.; King, L.; Mancini, M. Role of Body-Worn Movement Monitor Technology for Balance and Gait Rehabilitation. Physical Therapy 2015, 95, 461–470. [Google Scholar] [CrossRef]
- Seel, T.; Raisch, J.; Schauer, T. IMU-Based Joint Angle Measurement for Gait Analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef]
- Teufl, W.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Validity, Test-Retest Reliability and Long-Term Stability of Magnetometer Free Inertial Sensor Based 3D Joint Kinematics. Sensors 2018, 18, 1980. [Google Scholar] [CrossRef]
- Mundt, M.; Koeppe, A.; David, S.; Witter, T.; Bamer, F.; Potthast, W.; Markert, B. Estimation of Gait Mechanics Based on Simulated and Measured IMU Data Using an Artificial Neural Network. Front. Bioeng. Biotechnol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Komaris, D.-S.; Tarfali, G.; O’Flynn, B.; Tedesco, S. Unsupervised IMU-Based Evaluation of at-Home Exercise Programmes: A Feasibility Study. BMC Sports Sci Med Rehabil 2022, 14, 28. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Jang, S.-H.; Cho, J.-S.; Kim, M.-J.; Lee, H.D.; Lee, S.Y.; Moon, S.-B. Evaluation of Validity and Reliability of Inertial Measurement Unit-Based Gait Analysis Systems. Ann Rehabil Med 2018, 42, 872–883. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.H.K.; Veeger, H.E.J.; Baten, C.T.M.; Van Der Helm, F.C.T. Magnetic Distortion in Motion Labs, Implications for Validating Inertial Magnetic Sensors. Gait & Posture 2009, 29, 535–541. [Google Scholar] [CrossRef]
- Camomilla, V.; Bergamini, E.; Fantozzi, S.; Vannozzi, G. Trends Supporting the In-Field Use of Wearable Inertial Sensors for Sport Performance Evaluation: A Systematic Review. Sensors 2018, 18, 873. [Google Scholar] [CrossRef] [PubMed]
- Filippeschi, A.; Schmitz, N.; Miezal, M.; Bleser, G.; Ruffaldi, E.; Stricker, D. Survey of Motion Tracking Methods Based on Inertial Sensors: A Focus on Upper Limb Human Motion. Sensors 2017, 17, 1257. [Google Scholar] [CrossRef]
- Eichelberger, P.; Ferraro, M.; Minder, U.; Denton, T.; Blasimann, A.; Krause, F.; Baur, H. Analysis of Accuracy in Optical Motion Capture – A Protocol for Laboratory Setup Evaluation. Journal of Biomechanics 2016, 49, 2085–2088. [Google Scholar] [CrossRef]
- Piche, E.; Guilbot, M.; Chorin, F.; Guerin, O.; Zory, R.; Gerus, P. Validity and Repeatability of a New Inertial Measurement Unit System for Gait Analysis on Kinematic Parameters: Comparison with an Optoelectronic System. Measurement 2022, 198, 111442. [Google Scholar] [CrossRef]
- Dorschky, E.; Nitschke, M.; Seifer, A.-K.; van den Bogert, A.J.; Eskofier, B.M. Estimation of Gait Kinematics and Kinetics from Inertial Sensor Data Using Optimal Control of Musculoskeletal Models. J Biomech 2019, 95, 109278. [Google Scholar] [CrossRef]
- Camuncoli, F.; Barni, L.; Nutarelli, S.; Rocchi, J.E.; Barcillesi, M.; Di Dio, I.; Sambruni, A.; Galli, M. Validity of the Baiobit Inertial Measurements Unit for the Assessment of Vertical Double- and Single-Leg Countermovement Jumps in Athletes. IJERPH 2022, 19, 14720. [Google Scholar] [CrossRef]
- Toft Nielsen, E.; Jørgensen, P.B.; Mechlenburg, I.; Sørensen, H. Validation of an Inertial Measurement Unit to Determine Countermovement Jump Height. Asia-Pacific Journal of Sports Medicine, Arthroscopy, Rehabilitation and Technology 2019, 16, 8–13. [Google Scholar] [CrossRef]
- Marković, S.; Dopsaj, M.; Tomažič, S.; Kos, A.; Nedeljković, A.; Umek, A. Can IMU Provide an Accurate Vertical Jump Height Estimate? Applied Sciences 2021, 11, 12025. [Google Scholar] [CrossRef]
- Blandeau, M.; Guichard, R.; Hubaut, R.; Leteneur, S. IMU Positioning Affects Range of Motion Measurement during Squat Motion Analysis. J Biomech 2023, 153, 111598. [Google Scholar] [CrossRef] [PubMed]
- Whelan, D.; O’Reilly, M.; Huang, B.; Giggins, O.; Kechadi, T.; Caulfield, B. Leveraging IMU Data for Accurate Exercise Performance Classification and Musculoskeletal Injury Risk Screening; 2016; Vol. 2016, p. 662;
- Kianifar, R.; Joukov, V.; Lee, A.; Raina, S.; Kulić, D. Inertial Measurement Unit-Based Pose Estimation: Analyzing and Reducing Sensitivity to Sensor Placement and Body Measures. Journal of Rehabilitation and Assistive Technologies Engineering 2019, 6, 2055668318813455. [Google Scholar] [CrossRef]
- Clemente, F.M.; Akyildiz, Z.; Pino-Ortega, J.; Rico-González, M. Validity and Reliability of the Inertial Measurement Unit for Barbell Velocity Assessments: A Systematic Review. Sensors 2021, 21, 2511. [Google Scholar] [CrossRef]
- Khuyagbaatar, B.; Tumurbaatar, M.; Tsenkherjav, K.; Purevsuren, T.; Shambaljamts, T.; Kim, K.; Danjkhuu, T.; Danaa, G.; Hyuk Kim, Y. Kinematic Comparison of Snatch and Clean Lifts in Weightlifters Using Wearable Inertial Measurement Unit Sensors. Physical Activity and Health 2024, 8, 1–9. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Whelan, D.F.; Ward, T.E.; Delahunt, E.; Caulfield, B.M. Classification of Deadlift Biomechanics with Wearable Inertial Measurement Units. Journal of Biomechanics 2017, 58, 155–161. [Google Scholar] [CrossRef]
- Teufl, W.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Validity of Inertial Sensor Based 3D Joint Kinematics of Static and Dynamic Sport and Physiotherapy Specific Movements. PLOS ONE 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Robert-Lachaine, X.; Mecheri, H.; Larue, C.; Plamondon, A. Validation of Inertial Measurement Units with an Optoelectronic System for Whole-Body Motion Analysis. Med Biol Eng Comput 2017, 55, 609–619. [Google Scholar] [CrossRef]
- Unger, T.; De Sousa Ribeiro, R.; Mokni, M.; Weikert, T.; Pohl, J.; Schwarz, A.; Held, J.P.O.; Sauerzopf, L.; Kühnis, B.; Gavagnin, E.; et al. Upper Limb Movement Quality Measures: Comparing IMUs and Optical Motion Capture in Stroke Patients Performing a Drinking Task. Front. Digit. Health 2024, 6, 1359776. [Google Scholar] [CrossRef]
- Cutti, A.G.; Giovanardi, A.; Rocchi, L.; Davalli, A.; Sacchetti, R. Ambulatory Measurement of Shoulder and Elbow Kinematics through Inertial and Magnetic Sensors. Med Bio Eng Comput 2008, 46, 169–178. [Google Scholar] [CrossRef]
- Parel, I.; Cutti, A.G.; Kraszewski, A.; Verni, G.; Hillstrom, H.; Kontaxis, A. Intra-Protocol Repeatability and Inter-Protocol Agreement for the Analysis of Scapulo-Humeral Coordination. Med Biol Eng Comput 2014, 52, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Friesen, K.B.; Wu, L.Z.; Waslen, A.; Lang, A.E. Defining Repeatability for Scapulothoracic and Thoracohumeral Motion during the Novel Work-Related Activities and Functional Task (WRAFT) Protocol. Journal of Biomechanics 2023, 153, 111596. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Woodford, S.; Senanayake, D.; Ackland, D. Conversion of Upper-Limb Inertial Measurement Unit Data to Joint Angles: A Systematic Review. Sensors 2023, 23, 6535. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.M.B.; Lowndes, B.; Fortune, E.; Kaufman, K.; Hallbeck, M. Validation of Inertial Measurement Units for Upper Body Kinematics. Journal of applied biomechanics 2017, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, F.; Gan, L.; Chou, L.-S. Concurrent Validity of Inertial Measurement Units in Range of Motion Measurements of Upper Extremity: A Systematic Review and Meta-Analysis. Wearable Technol 2024, 5, e11. [Google Scholar] [CrossRef]
- Cerfoglio, S.; Capodaglio, P.; Rossi, P.; Conforti, I.; D’Angeli, V.; Milani, E.; Galli, M.; Cimolin, V. Evaluation of Upper Body and Lower Limbs Kinematics through an IMU-Based Medical System: A Comparative Study with the Optoelectronic System. Sensors 2023, 23, 6156. [Google Scholar] [CrossRef]
- Leardini, A.; Lullini, G.; Giannini, S.; Berti, L.; Ortolani, M.; Caravaggi, P. Validation of the Angular Measurements of a New Inertial-Measurement-Unit Based Rehabilitation System: Comparison with State-of-the-Art Gait Analysis. J NeuroEngineering Rehabil 2014, 11, 136. [Google Scholar] [CrossRef]
- Felius, R.A.W.; Geerars, M.; Bruijn, S.M.; Wouda, N.C.; Van Dieën, J.H.; Punt, M. Reliability of IMU-Based Balance Assessment in Clinical Stroke Rehabilitation. Gait & Posture 2022, 98, 62–68. [Google Scholar] [CrossRef]
- Pan, H.; Wang, H.; Li, D.; Zhu, K.; Gao, Y.; Yin, R.; Shull, P.B. Automated, IMU-Based Spine Angle Estimation and IMU Location Identification for Telerehabilitation. J NeuroEngineering Rehabil 2024, 21, 96. [Google Scholar] [CrossRef]
- Lobo, P.; Morais, P.; Murray, P.; Vilaça, J.L. Trends and Innovations in Wearable Technology for Motor Rehabilitation, Prediction, and Monitoring: A Comprehensive Review. Sensors 2024, 24, 7973. [Google Scholar] [CrossRef]
- Ettefagh, A.; Roshan Fekr, A. Technological Advances in Lower-Limb Tele-Rehabilitation: A Review of Literature. Journal of Rehabilitation and Assistive Technologies Engineering 2024, 11, 20556683241259256. [Google Scholar] [CrossRef]
- Al-Amri, M.; Nicholas, K.; Button, K.; Sparkes, V.; Sheeran, L.; Davies, J.L. Inertial Measurement Units for Clinical Movement Analysis: Reliability and Concurrent Validity. Sensors (Basel) 2018, 18, 719. [Google Scholar] [CrossRef] [PubMed]
- Niswander, W.; Wang, W.; Kontson, K. Optimization of IMU Sensor Placement for the Measurement of Lower Limb Joint Kinematics. Sensors (Basel) 2020, 20, 5993. [Google Scholar] [CrossRef] [PubMed]
- Paulich, M.; Schepers, M.; Rudigkeit, N.; Bellusci, G. Xsens MTw Awinda: Miniature Wireless Inertial-Magnetic Motion Tracker for Highly Accurate 3D Kinematic Applications. 2018. [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A Gait Analysis Data Collection and Reduction Technique. Human Movement Science 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Goreham, J.A.; MacLean, K.F.E.; Ladouceur, M. The Validation of a Low-Cost Inertial Measurement Unit System to Quantify Simple and Complex Upper-Limb Joint Angles. Journal of Biomechanics 2022, 134, 111000. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive Statistics and Normality Tests for Statistical Data. Ann Card Anaesth 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int J Endocrinol Metab 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; 2nd ed.; L. Erlbaum Associates: Hillsdale, N.J, 1988; ISBN 978-0-8058-0283-2.
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk J Emerg Med 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Bonfiglio, A.; Petruccelli, C.; Villa, G.; Bongers, M.; Farella, E. Preliminary Validation of an IMU-Based Physiotherapy Assessment System for the Lower Extremities.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).