1. Introduction
Despite extensive research in neuroscience, psychology and philosophy, consciousness remains one of the most conceptually and operationally elusive phenomena in the scientific domain. Contemporary models have attempted to localize or characterize conscious states through cognitive correlates, neural substrates or abstract informational structures (Koch et al., 2016; Fazekas and Nemeth, 2018; Bao et al., 2023). Theories such as Global Neuronal Workspace, Integrated Information Theory and Recurrent Processing Theory have significantly advanced our conceptual understanding of consciousness, yet they often lack grounding in measurable physical parameters (Nemirovsky et al., 2022; Mediano et al., 2022; Leung and Tsuchiya. 2023; Zacks and Jablonka, 2023; Naccache and Munoz-Musat, 2024; Cogitate Consortium at al., 2025). Empirical research has provided a range of neural correlates—such as oscillatory synchrony, functional connectivity and specific brain regions involved in awareness—but these remain correlational and variably defined across studies. Moreover, the frequent emphasis on neurons alone overlooks the essential contributions of glial cells, systemic physiological rhythms and metabolic factors supporting the brain’s ability to sustain awareness (Tozzi 2015; Robertson 2018; Damasio and Damasio, 2023). Consequently, the scientific discourse lacks a unifying framework capable of defining consciousness through empirically verifiable, physically grounded requirements.
In response to these limitations, we propose a biophysical model of consciousness defined by measurable thresholds across multiple physiological domains. We argue that consciousness emerges when a biological system maintains sufficient levels of neural integration, temporal precision, metabolic energy and electrochemical homeostasis, all operating within quantifiable ranges. Our approach incorporates not only neuronal signaling but also glial support functions, cardiovascular and respiratory entrainment and gut-brain interactions. This integrated perspective treats consciousness not as a function of abstract information processing, but rather as an emergent, system-level phenomenon governed by physical constraints and specific, experimentally accessible variables.
We proceed by first outlining the methodological approach for identifying physical parameters relevant to consciousness, detailing the mathematical and physiological bases for their selection. We then examine how these parameters vary across different conscious states, such as wakefulness, sleep, anesthesia and coma. Subsequent chapters explore how findings from diverse modalities converge to reinforce core physical constraints, culminating in the formulation of a unified multidimensional threshold model that informs a systems-level definition of consciousness.
1. QUANTITATIVE METHODS FOR EVALUATING BIOPHYSICAL CORRELATES OF CONSCIOUSNESS
We present here the methodological approach for identifying and evaluating the biophysical parameters that define and constrain conscious states. We describe the available quantitative techniques to select, model and interpret key physiological variables—such as electrical activity, metabolic rate, ionic gradients and structural connectivity—within a rigorous analytical framework. Particular emphasis is placed on the mathematical formulations and computational tools enabling the integration of diverse data types into a unified, testable model.
Selection criteria and parameter classification. The first step involves establishing clear criteria for determining when a variable is physiologically meaningful in differentiating conscious from unconscious states. Relevance is determined by the statistical and causal association of a parameter with state transitions such as from wakefulness to anesthesia, sleep or coma (Bonhomme et al., 2019; Mashour et al., 2021; Yang et al., 2022). We divided these parameters into six primary domains:
- 1)
electrophysiological,
- 2)
metabolic,
- 3)
thermodynamic,
- 4)
ionic,
- 5)
structural-connectomic,
- 6)
Systemic and peripheral.
Operationally, parameters may be prioritized using multi-criteria decision analysis (MCDA), where each candidate variable
is assigned a composite score
S(
pi) based on its performance across a set of criteria such as temporal resolution, state specificity, physical measurability and spatial scope (Yuan et al., 2022; Okolie et al., 2023). A general form for the scoring function is:
Here,
is the normalized score of parameter
on the
j-th criterion and
is a weight assigned to that criterion, satisfying
. Once scores are computed, parameters can be ranked and filtered, with those above a defined percentile (e.g., 85th) selected for further examination. This procedure may provide a structured and replicable framework for distinguishing biologically grounded from less informative variables. Overall, by applying formal selection algorithms, researchers may ensure a consistent framework for evaluating the biophysical correlates of consciousness.
Electrophysiological signal analysis and frequency decomposition. Electrophysiological parameters such as neural oscillations and synchronization patterns can be evaluated using high-density electroencephalography (EEG) (Fiedler et al., 2022; Fiedler et al., 2023: Pelc, 2023). Signal preprocessing often involves band-pass filtering in the 0.1–120 Hz range using Butterworth filters and artifact correction via independent component analysis (ICA) (Zhao et al., 2021; Zhang et al., 2025). Oscillatory components of brain activity like amplitude and synchrony can be extracted through time-frequency decomposition methods, including the Morlet wavelet transform, defined as:
where
is a complex Morlet wavelet centered at frequency
f and time t and A is a normalization constant (Spencer and Ghorashi 2014; Wu et al., 2017; Rosenblum et al., 2022). This method provides frequency-specific power and phase information at fine temporal resolutions. Coherence and synchrony can be quantified using phase-locking values (PLVs), calculated across electrode pairs as:
where
is the instantaneous phase at frequency
f for trial n. PLV values close to 1 indicate stable phase relationships across trials, often interpreted as evidence of neural integration. Overall, electrophysiological coherence measures thus offer real-time, physically constrained indicators of global brain states.
Quantification of metabolic and energetic constraints. The brain’s metabolic activity can be assessed through a combination of positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). The cerebral metabolic rate of glucose (CMRglu) is commonly calculated using FDG-PET data interpreted via the Sokoloff model (Lebedev et al., 2002; Dittrich et al., 2012; Dickie et al., 2022):
where
K1,
k2,
k3 are transport and phosphorylation rate constants derived from tracer kinetics and [Glucose]
plasma is the concentration of glucose in plasma. This model allows for the regional mapping of metabolic rates under various states of consciousness. Complementary information can be obtained from fMRI by estimating the cerebral metabolic rate of oxygen consumption (CMRO₂), particularly using calibrated fMRI techniques (Caporale et al., 2021; Chiarelli et al., 2022; Deshpande et al., 2022). These rely on hypercapnic and hyperoxic manipulations and the relationship:
where
M is a calibration factor and
is the arterial partial pressure change in CO₂. These methods yield quantitative values reflecting the energy demand and supply necessary for sustaining neural computation. The reductions in CMRglu and CMRO₂ during unconscious states are consistent and reproducible (Usami et al., 2023), making these measures powerful tools for identifying metabolic thresholds of consciousness.
Analysis of ionic homeostasis and electrodiffusion dynamics. The maintenance of membrane potential and neuronal excitability depends on precise ionic gradients, particularly involving sodium, potassium, calcium and chloride ions. These gradients can be modeled using classical electrochemical equations. The equilibrium potential for a single ion species is determined by the Nernst equation:
where
R is the gas constant,
T is the absolute temperature, z is the ionic valence and
F is Faraday’s constant (Hopper et al., 2022; Tamagawa et al., 2023). In systems with multiple permeant ions, the membrane potential is best approximated by the Goldman-Hodgkin-Katz equation:
Where
Pion denotes membrane permeability for each species (Tamagawa and Ikeda, 2017). Perturbations in these concentrations, such as extracellular potassium rising above ~8 mM, can disrupt action potential generation and lead to depolarization block. Simulations of such dynamics can be carried out using computational platforms like NEURON, which implement Hodgkin-Huxley-type models. Summarizing, the values of ionic concentrations and their fluctuations thus represent another class of physically grounded variables associated with the viability of conscious processing.
Thermodynamic modeling and temperature-linked constraints. Brain temperature influences all aspects of neuronal signaling, enzymatic activity and metabolism (Berger et al., 2022; Horiuchi et al., 2023; Repasky et al., 2024). The relationship between firing rate and temperature often follows an Arrhenius-like dependence:
where
is the firing rate at a reference temperature,
is the effective activation energy, R is the gas constant and T is absolute temperature. Brain thermal dynamics can be further modeled using the Pennes bioheat equation (Lillicrap et al., 2017):
Here,
refer to tissue density, specific heat and thermal conductivity, respectively;
represent blood perfusion and the specific heat of blood;
is arterial temperature; and
is the metabolic heat source term. The equation is solvable through finite difference or finite element methods and allows for dynamic modeling of temperature profiles. Empirical data consistently show cognitive impairment below ~35°C or above ~41°C, establishing a thermodynamic envelope within which consciousness is thermodynamically sustainable (Ashworth et al., 2021; Fischer et al., 2024).
Graph-theoretic modeling of structural and functional connectivity. Structural and functional connectivity are essential to understanding the spatial architecture that supports consciousness. Structural connectivity is typically derived from diffusion MRI data using tractography algorithms, producing adjacency matrices
where each element reflects the strength of anatomical connection between region i and region j (Innocenti et al., 2019; Renauld et al., 2023). Functional connectivity is calculated from time series of neural activity, using the Pearson correlation coefficient:
Once these matrices are obtained, a wide range of graph-theoretic measures can be computed. Global efficiency
, clustering coefficient C and path length
L are commonly used (Abdolalizadeh et al., 2023; Li et al., 2024):
In these expressions, is the shortest path between nodes i and j, is the number of edges among node i’s neighbors and is the degree of node I (van Diessen et al., 2014; Zuo et al., 2023). These metrics reveal the topological efficiency and modularity of brain networks. Consciousness is consistently associated with higher efficiency and clustering (Cacciola et al., 2019; Sun et al., 2023), suggesting that structural integration is a physical precondition for globally available information.
Systemic and peripheral modulators of brain states. In addition to neural parameters, systemic physiological rhythms also play measurable roles in modulating consciousness. Cardiac and respiratory rhythms influence cortical excitability through baroreceptor and vagal afferents (Cavelli et al., 2020; cortices (Zhou et al., 2020b; Choi and Kim, 2022; Väyrynen et al., 2023; Kluger et al., 2025). Heartbeat-evoked potentials (HEPs), measurable via EEG, show phase-coupled fluctuations in amplitude that correlate with interoceptive awareness. These fluctuations are often modeled as time-locked averages of cortical potentials following the R-peak of the ECG signal (Khalsa 2023; Engelen et al., 2023). Similarly, respiratory entrainment of neural oscillations—particularly in the theta and delta bands—has been observed across olfactory and limbic. These are evaluated using cross-spectral coherence techniques:
where is the cross-spectrum between signals x (e.g., respiration) and y (e.g., EEG). Gut-brain interactions, mediated through microbiota-derived metabolites and immune signals, can further modulate neurotransmitter availability and blood-brain barrier permeability (Colombo et al., 2021; Shin and Kim, 2023; Leyrolle et al., 2023). While these peripheral influences operate on slower timescales, they provide a fluctuating physiological substrate that constrains or facilitates neural processes. Such interactions highlight the role of embodied and systemic constraints in shaping the physical state space in which consciousness is instantiated.
Overall, this chapter established the methodological apparatus for identifying and quantifying the biophysical parameters that define and constrain conscious states. By detailing how each parameter can be measured and validated, a technical groundwork was provided for constructing a physically grounded model of consciousness based on empirical observables.
2. PHYSIOLOGICAL DYNAMICS UNDERLYING SHIFTS IN CONSCIOUSNESS
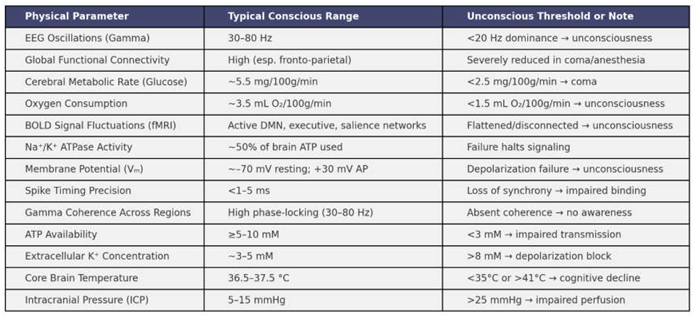
We examine here how key biophysical parameters behave across different states of consciousness, including wakefulness, sleep, anesthesia and coma. We synthesize empirical findings on oscillatory activity, energy metabolism, ionic regulation, thermodynamics, network connectivity and systemic physiological rhythms, highlighting their state-dependent variability. Each parameter is analyzed in terms of its measurable shifts and threshold behavior during transitions into and out of consciousness (
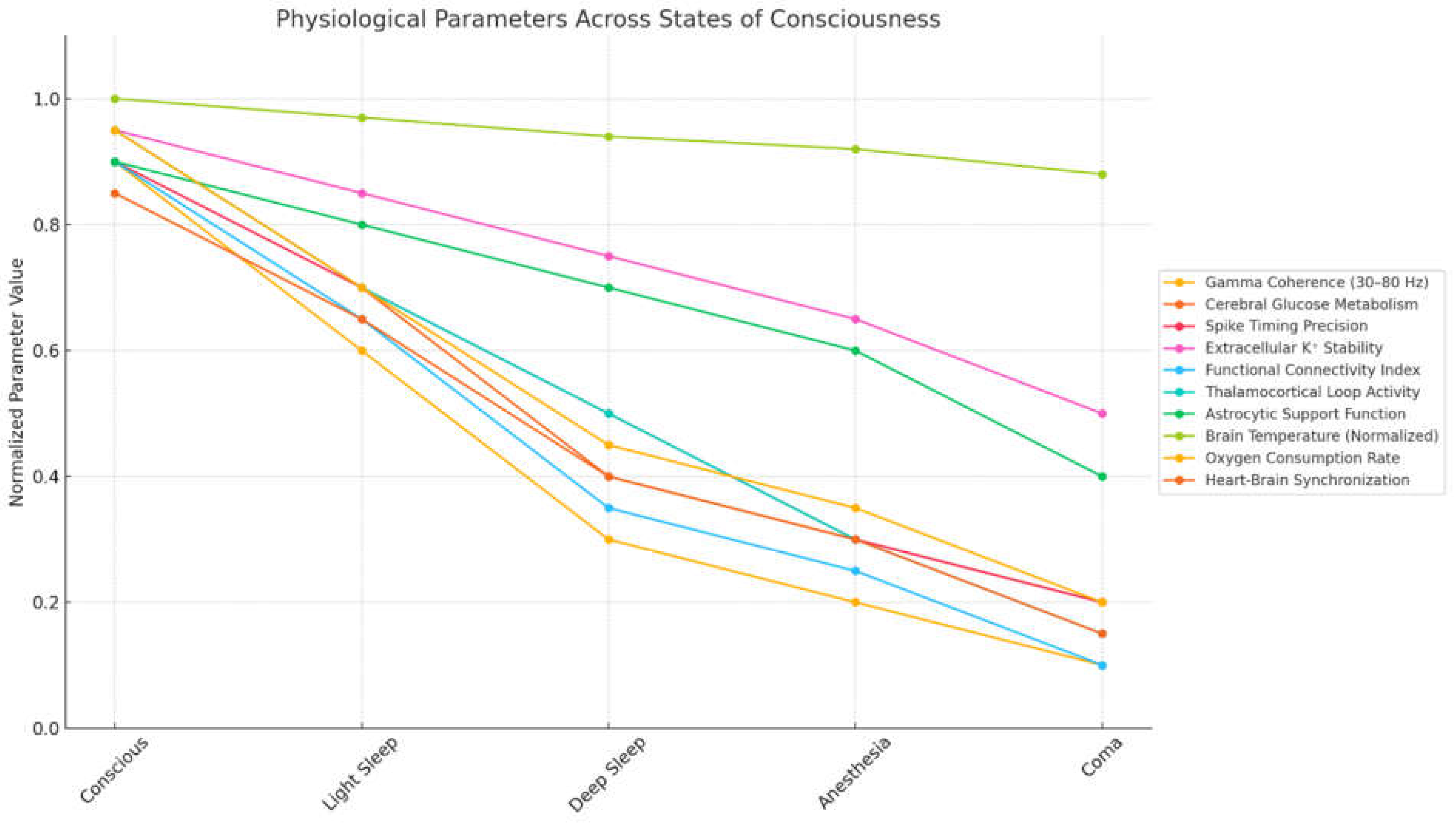
Table 1). A qualitative visualization of how these multiple interdependent parameters tend to degrade progressively as consciousness diminishes is illustrated in
Figure 1.
Oscillatory signatures across conscious states. Neural oscillations are among the most consistent indicators of conscious state transitions. In healthy wakefulness, cortical networks exhibit robust gamma-band activity (30–80 Hz), particularly within frontoparietal circuits (Mikulan et al., 2018). This coherence often decreases progressively during sleep stages and is further suppressed under anesthesia and coma (Mashour 2024). Through multiple studies, gamma power
can be modeled as a decaying function of arousal index α, where:
Here, is the baseline power during conscious wakefulness and k is a state-dependent decay constant. In parallel, phase synchrony between brain regions, quantified by phase-locking values (PLVs), is found to collapse during transitions into non-REM sleep or anesthetic-induced unconsciousness (Zhou et al., 2020a; Leguia et al., 2021; Bardon et al., 2023). The decline in PLV values tends to precede behavioral loss of responsiveness, suggesting that coherent oscillatory activity is not only correlated with consciousness but may serve as a prerequisite. Variability in synchronization is often region-specific; posterior cortical areas retain partial coherence during light sedation, while prefrontal regions disconnect more fully (Liu et al., 2017; Zelmann et al., 2023). These patterns reinforce the notion that high-frequency oscillations and their coherence represent one of the earliest and most sensitive indicators of conscious degradation.
Energy metabolism and the consciousness spectrum. The metabolic demands of consciousness are among the most thoroughly characterized physiological dependencies in neuroscience (Engl and Attwell, 2015; Liu and Prokosch, 2021). Functional neuroimaging shows that conscious wakefulness is associated with elevated cerebral metabolic rates of glucose (CMRglu) and oxygen (CMRO₂), especially in cortical and thalamic regions (Bernier et al., 2017; We et al., 2022; Stankeviciute et al., 2023; Caporale et al., 2023). Quantitatively, the resting-state CMRglu in the awake human brain is approximately 5.5–6.0 mg/100g/min (Maquet et al., 1990). This value drops by 25–35% during non-REM sleep and can decrease by over 50% during deep anesthesia (Liu 2013). Similar patterns are found for CMRO₂, with reductions well below 2.5 mL/100g/min marking the transition into unconsciousness. These data can be represented as sigmoidal functions:
Here, s is the state index (e.g., 0 for coma, 1 for wake), Rmax is the maximal rate in wakefulness and is the midpoint state where the rate is half-maximal. These curves can be used to approximate metabolic thresholds across consciousness conditions. Notably, these reductions are not globally uniform (Koush et al., 2021). Default mode network hubs like the medial prefrontal cortex and posterior cingulate cortex display disproportionate metabolic suppression during unconscious states (Liu et al., 2019). These regional vulnerabilities suggest that sustained consciousness requires not only high total metabolic input, but also its organized regional distribution. The empirical patterns confirm that metabolism operates as a limiting substrate, providing an energetic envelope necessary for neural integration and oscillatory coherence.
Ionic and synaptic stability under fluctuating states of consciousness. During conscious states, extracellular concentrations of potassium, sodium and calcium are tightly regulated by astrocytic uptake, active transport and blood-brain barrier dynamics (Sajib et al., 2018; DiNuzzo 2019; Cheng et al., 2024; Theparambil et al., 2020). Potassium concentration in the extracellular space typically ranges from 3.0 to 5.0 mM (Wellbourne-Wood et al., 2017; Walch et al., 2020). Elevations beyond this—often above 7.5–8.0 mM—are observed in hypoxic, epileptic or anesthetic conditions, correlating with decreased excitability due to depolarization block (Palmer and Clegg, 2017; Lindner et al., 2020). These shifts are modeled using dynamic Nernst potentials, where the membrane voltage is acutely sensitive to ionic drift (Song et al., 2018):
These changes result in altered excitability thresholds, leading to either spurious hyperexcitability or transmission failure. Similarly, the intracellular Ca²⁺ concentration, which supports synaptic vesicle release and plasticity, decreases in anesthetized and deeply sedated states, reducing the likelihood of coordinated neurotransmission (Leitz and Kavalali, 2016; Eisner et al., 2023). These ion fluxes are tightly tied to local metabolic conditions and ATP-dependent pumps such as the Na⁺/K⁺ ATPase consume substantial cellular energy to maintain these gradients. Overall, the breakdown of ionic gradients and the degradation of synaptic precision represent convergent, quantifiable disruptions that underpin many states of unconsciousness.
Temperature regulation and thermodynamic thresholds. Empirical studies of hypothermia, fever and therapeutic cooling demonstrate that even modest deviations from normothermia (~37°C) lead to significant impairments in consciousness and cognition. Temperature effects on neuronal firing can be modeled using the above-mentioned Arrhenius relation, where the rate constantfor enzymatic and synaptic processes depends exponentially on inverse temperature. Lower temperatures reduce the kinetics of ion channel opening, neurotransmitter release and mitochondrial ATP production. The resulting delays in synaptic transmission and integration times disrupt the fast feedback loops needed for high-frequency synchrony and global integration (Burek et al., 2019; Jabbari and Karamati, 2022). In states of hyperthermia (>41°C), excessive kinetic activity can cause denaturation of enzymes and failure of glial buffering systems. Thermoregulation also interacts with cerebral blood flow and oxygen availability; cold temperatures reduce perfusion, while excessive heat accelerates oxidative stress. These dynamics, modeled via the Pennes bioheat equation (Tucci et al., 2021), show how thermodynamic shifts can create physiological conditions incompatible with sustained consciousness. Temperature therefore constitutes a boundary parameter, defining the energetic feasibility space for cognitive activity.
Structural and functional network dynamics. Consciousness is increasingly understood as a network-level phenomenon, requiring both high integration and modular segregation of cortical and subcortical regions (Gonzalez-Escamilla et al., 2023; Wu et al., 2023; Nestor et al., 2024). Structural connectivity derived from diffusion tensor imaging (DTI) defines the anatomical scaffold over which functional interactions occur. In conscious individuals, these networks exhibit small-world properties: short path lengths and high clustering. These features are quantified via graph-theoretical metrics such as the above-mentiones global efficiency, path length L and modularity. Empirical findings show that unconscious states, whether induced pharmacologically or by trauma (Pullon et al., 2022; Choe et al., 2025), are associated with increased path length and reduced efficiency. Resting-state fMRI data confirms that connectivity within the default mode network (DMN) and between DMN and frontoparietal networks becomes attenuated under unconscious conditions (Long et al., 2016; Lemaire et al., 2022). Notably, consciousness seems to depend on the capacity of networks to maintain reciprocal communication— “reentry”—across distant cortical zones. This dynamic coupling is highly dependent on both structural links and phase-coherent oscillations, reinforcing the idea that consciousness is not reducible to local activity alone. Instead, it may reflect a system-wide capability for efficient information transfer across a physically defined architecture.
Overall, by mapping all the above-mentioned changes across physiological states, this chapter established the dynamic nature of consciousness as a function of coordinated, physically constrained variables.
3. CONVERGENCE OF METHODS IN THE PHYSICAL STUDY OF CONSCIOUSNESS
We explore here the convergence of evidence from multiple measurement modalities—such as EEG, fMRI, PET, DTI and physiological monitoring—in identifying the physical correlates of consciousness. We show that structurally and functionally distinct techniques yield overlapping signatures of conscious states, reinforcing the reliability of key parameters like gamma coherence, metabolic rate and network connectivity.
Convergence between electrophysiological and hemodynamic measures. One of the most robust intersections in consciousness research lies in the correlation between electrophysiological recordings (e.g., EEG, MEG) and hemodynamic imaging techniques (e.g., fMRI, PET) (Hall et al., 2014; Gomez-Pilar et al., 2022). Despite their distinct temporal and spatial resolutions, both modalities consistently identify overlapping brain regions and patterns associated with conscious awareness. For instance, studies combining EEG and fMRI during transitions between wakefulness, sleep and anesthesia frequently observe that decreases in gamma-band oscillatory power (30–80 Hz) are paralleled by reductions in BOLD signal amplitude, particularly in frontoparietal and default mode network regions (Yang et al., 2022; Guo et al., 2023; Vijayakrishnan eta l., 2023). These correlations can be quantified using time-lagged canonical correlation analysis (CCA) (Schultze and Grubmüller, 2021), which maps signal covariance structures across modalities:
Here, X and Y are the EEG and fMRI data matrices, respectively. Strong canonical correlations are found particularly when EEG coherence in the gamma band is high and BOLD activation is prominent in higher-order associative cortices (Rodu et al., 2018). These findings indicate that the oscillatory synchrony needed for conscious integration has a vascular metabolic signature, validating both modalities as independent but convergent proxies for the conscious state.
Correlating metabolic demand with electrophysiological dynamics. A critical relationship does exist between cerebral energy metabolism and electrophysiological activity, particularly in the high-frequency gamma band. Metabolic imaging techniques such as FDG-PET and calibrated fMRI offer quantitative estimates of glucose and oxygen consumption, which are then correlated with EEG-derived measures of neural activity. In healthy wakeful subjects, increased gamma coherence in the cortex correlates strongly with higher regional cerebral metabolic rates of glucose (CMRglu). This relationship follows a near-linear trend under normal conditions, as neuronal firing frequency is energetically expensive and gamma-band oscillations require high synchrony and rapid synaptic turnover. The dependence of electrical activity on metabolic support is mathematically formalized in energy-flow coupling models:
Here, is the frequency-dependent time constant for synaptic recovery and ATP replenishment. As the metabolic substrate becomes limited (e.g., under anesthesia or ischemia), high-frequency oscillatory power drops, particularly in associative cortical areas. Conversely, the restoration of metabolic support is necessary for the reappearance of gamma activity during recovery from unconsciousness. The spatial topography of metabolic and electrical changes is consistent, with posterior and medial hubs being particularly energy dependent. This coherence between electrical demand and metabolic supply further validates both types of measures as aligned indices of conscious viability.
Thermodynamic modulation across modalities. The impact of temperature on brain function is observed across various measurement domains, from synaptic kinetics to fMRI signal amplitude. Cooling the brain by only a few degrees reduces both spontaneous EEG activity and BOLD signal fluctuations, particularly in regions involved in conscious processing. Electrophysiological recordings show that synaptic latency and refractory periods increase with decreasing temperature, modeled by the above-mentioned Arrhenius-type exponential relationships. These changes result in longer integration windows and a disruption of the millisecond-scale precision needed for gamma-band synchronization. In parallel, metabolic imaging under hypothermic conditions shows a proportional decrease in glucose and oxygen consumption, particularly in gray matter. The relationship between temperature and functional imaging is also evident in the reduction of fMRI global signal variability and resting-state network modularity. This convergence between electrical, metabolic and thermodynamic measurements demonstrates that temperature imposes a hard physical constraint on the neural dynamics required for consciousness. These effects appear consistently across clinical hypothermia studies and intraoperative human data, suggesting their reliability across modalities.
Linking structural and functional connectivity in conscious states. The relationship between structural and functional connectivity provides another layer of cross-modal convergence, especially in the context of conscious processing. Diffusion tensor imaging (DTI) reveals the underlying anatomical pathways through which functional interactions measured by fMRI or EEG must propagate (Yen et al., 2023). Numerous studies demonstrate that consciousness depends not only on the presence of structural connections but also on their dynamic recruitment into coherent activity patterns (Zhang et al., 2017; Zheng et al., 2017). Functional connectivity matrices (FFF) derived from fMRI time series reveal the highest integration within core hubs of the default mode network (DMN) such as the precuneus, medial prefrontal cortex and posterior cingulate regions that are also structurally well-connected. The degree of alignment between structure and function can be assessed using structural-functional coupling indices:
Where are the structural and functional matrices, respectively (Pan et al., 2023; Huang et al., 2023). In conscious states, tends to be high (~0.5–0.7), whereas in unconscious states (e.g., anesthesia or coma), this coupling decreases markedly (Zarkali et al., 2021; (Rajesh et al., 2024)). Moreover, studies using simultaneous DTI and resting-state fMRI show that reduced structural integrity in long-range white matter tracts correlates with both decreased functional coherence and lowered behavioral responsiveness (Cao et al., 2023). These observations indicate that physical network architecture constrains and partially determines the dynamic patterns of activity associated with consciousness. This means that the anatomy-function relationship operates as a stable cross-modal axis in the evaluation of conscious states.
Integration of peripheral physiology with central measures. A growing body of evidence supports the physiological coupling between peripheral systems—cardiac, respiratory, gastrointestinal—and central neural dynamics relevant to consciousness. Techniques such as electrocardiography (ECG), respiratory plethysmography and electrogastrography (EGG) are now often recorded simultaneously with EEG or fMRI. Heartbeat-evoked potentials (HEPs), measurable by time-locking EEG signals to the R-wave of the ECG, provide a window into cardio-cortical integration. These potentials tend to be larger and more coherent during conscious wakefulness and decrease during deep sleep or under sedation. Cross-modal coherence can be quantified using cross-correlation or mutual information metrics:
Where represent peripheral and cortical signal streams, respectively. Higher mutual information values reflect tighter physiological coupling. Similar findings apply to respiratory-entrained oscillations and gut-brain feedback signals, where increased coherence corresponds to more integrated conscious states. These interactions among diverse neurotechnological approaches confirm that conscious states are shaped not only by intracranial variables but also by broader systemic rhythms, offering additional dimensions for their characterization.
Machine learning models of consciousness across modalities. To synthesize data from heterogeneous modalities, recent studies employ machine learning approaches that integrate EEG, fMRI, PET and physiological data into predictive models of consciousness (Garrido Merchán and Molina, 2020; Wang and Ma, 2023; Mohammadi and Ganjtabesh, 2024; Benitez et al., 2024). Features extracted from each modality—such as gamma-band power, functional connectivity matrices, metabolic rates or peripheral variability—are combined into high-dimensional input vectors
. These are used to train classifiers (e.g., support vector machines, random forests, deep neural networks) with conscious state labels (e.g., wake, sleep, anesthesia) as targets. Model accuracy is typically evaluated using k-fold cross-validation (Gu et al., 2017). A linear Support Vector Machine, for instance, minimizes:
where
are consciousness labels and
are slack variables. Models trained on multimodal features consistently outperform those trained on single modalities, achieving accuracy above 90% in discriminating between conscious and unconscious states. Feature importance rankings also tend to reinforce biological findings, with gamma-band coherence, frontoparietal connectivity and CMRglu among the top contributors. These integrative techniques demonstrate that physical parameters drawn from distinct modalities encode shared and robust information about consciousness.
Overall, through formal comparisons and correlation metrics, this chapter illustrated how the integration of different neuro-techniques strengthens the interpretation of consciousness-related data. This consistency validates the use of diverse techniques within a common analytical framework, setting the stage for a multidimensional model of conscious viability.
4. CONSCIOUSNESS WITHIN A MULTIDIMENSIONAL THRESHOLD SPACE
To formalize the concept of consciousness as a state defined by the convergence of multiple biophysical parameters operating within specific threshold ranges, we introduce here a multidimensional state space model in which consciousness emerges from the simultaneous fulfilment of structural, dynamic and energetic constraints. Mathematical formulations are used to define the geometry, topology and temporal stability of this viability space, including sensitivity analyses and critical boundary conditions.
The multidimensional nature of consciousness constraints. The empirical findings presented in previous sections point to the multidimensional character of consciousness. Unlike binary phenomena or linearly scaled variables, consciousness does not emerge from the presence or absence of a single physiological condition. Rather, it reflects the simultaneous alignment of multiple physical constraints, each operating across distinct dimensions: frequency, energy, ion flux, temperature and structural connectivity. These constraints can be conceptualized within a bounded, multidimensional state space
, where each axis corresponds to a normalized, physically grounded parameter
and where consciousness is defined as any point
(Tozzi 2019; Kosmyna and Lécuyer, 2019). Mathematically, this region can be represented as an intersection of inequality constraints:
Here, are empirically derived lower and upper bounds for the i-th parameter. Points falling outside this region correspond to physiologically unsustainable conditions for conscious processes, such as metabolic insufficiency, desynchronized oscillatory activity or ionic collapse. In this model, loss of consciousness results from the transgression of one or more threshold boundaries, reducing the system’s capacity to maintain integrated neural representation. This framework formalizes the idea that consciousness is a property of a physically constrained subspace within the broader physiological state space.
Mathematical formalization of integration and constraint overlap. To extend the model beyond bounded intervals, we consider interdependence between variables. Some parameters influence others directly—for instance, metabolic rate constrains ATP availability, which in turn regulates ion pump efficacy and synaptic firing. These dependencies can be modeled as coupled inequalities or as conditional mappings in a constraint manifold
(Kingston 2020). Define:
where
represents a constraint function that encodes nonlinear coupling, such as:
This reflects that Na⁺/K⁺ pump activity depends on ATP supply and ionic concentration gradients, modulated by sigmoid kinetics. Another example links functional connectivity f to gamma coherence γ and structural integrity sss:
These formulations express how localized parameter violations may propagate and disrupt global neural integration. Therefore, the effective conscious state space becomes a higher-order constraint surface with system behavior increasingly sensitive near its boundaries. This formalism permits consciousness to be characterized not simply by thresholds, but by their complex interplay and topological relationships.
Parameter sensitivity and critical points. Within the multidimensional constraint surface, some parameters exhibit higher sensitivity, that is, small deviations result in rapid transitions from consciousness to unconsciousness. Sensitivity analysis can be conducted by computing the partial derivatives of a consciousness viability function
, where:
Each is a sigmoidal scaling function mapping parameter values to [0, 1], with 0 representing complete dysfunction. The gradient vector gives the direction of steepest increase in viability and the magnitude of each partial derivative indicates the criticality of the i-th parameter. Parameters such as metabolic rate, gamma coherence and ion gradients show high first-order sensitivity, suggesting that perturbations in these domains exert disproportionate influence on global consciousness viability.
Further, by identifying points such that , one locates critical transition zones where consciousness becomes unstable. These are analogous to bifurcation points in dynamical systems, representing thresholds beyond which compensatory mechanisms fail (Kaszás et al., 2019). Recognizing these critical points allows researchers to distinguish between parameters that are merely correlated with conscious states and those that are causally foundational.
Dimensional reduction and dominant subspace. Although the conscious state space
is inherently high-dimensional, evidence suggests that its effective variation may lie in a low-dimensional manifold embedded within the larger space. Principal component analysis (PCA) or singular value decomposition (SVD) can be applied to multivariate physiological data across states to identify orthogonal axes of maximal variance (Brito et al., 2020; Ben Salem and Ben Abdelaziz, 2021). Let
be a matrix of m samples with n parameters; PCA yields a decomposition:
The first few principal components (columns of V) often account for a majority of variance in consciousness-related transitions. These dominant directions define a consciousness manifold . Empirical studies show that parameters like functional connectivity, cortical metabolism, gamma-band synchrony and K⁺ homeostasis cluster within the first three principal components. The implication is that although consciousness depends on many physiological factors, only a few compound axes explain most of the variability between states.
This dimensional reduction provides a useful simplification for modeling and clinical inference: instead of tracking dozens of biophysical variables, monitoring a small set of key indices may be sufficient to assess the state of consciousness (Perl et al., 2023). The geometry of the subspace also reveals how far a given physiological state lies from the “core” conscious regime. Consciousness, then, becomes not a point, but a stable region within a latent manifold, spanned by physically interpretable vectors.
Temporal stability and dynamical trajectories. Consciousness is not a static condition but a time-evolving process, influenced by endogenous rhythms, environmental stimuli and internal states. To model this temporal behavior, the state vector
can be treated as a trajectory through parameter space governed by a dynamical system:
Here,
encodes the physiological laws and feedback mechanisms determining how parameters evolve over time. Consciousness corresponds to trajectories that remain inside a stability basin
for sufficiently long durations. Transitions into unconsciousness are modeled as escape trajectories crossing the boundary
. These models permit the analysis of resilience, defined by the time-to-boundary under small perturbations
. Linear stability analysis using the Jacobian
around equilibrium points
identifies whether such points are attractors or saddle nodes (Margazoglou and Magri, 2023; Ahmed et al., 2024):
Empirical validation of these dynamics comes from anesthesia studies, where consciousness fades over tens of seconds or from seizure models where it collapses in milliseconds. Dynamical modeling thus provides not only spatial, but also temporal constraints on viable conscious regimes, revealing the importance of maintaining trajectories within slow-changing regions of physiological coherence.
Stochastic fluctuations and boundary uncertainty. Since biological systems are inherently noisy, consciousness thresholds are not perfectly sharp. Small stochastic variations in physical parameters can cause the system to hover near the boundary of the conscious manifold
, creating conditions of unstable awareness. To model this, parameters are treated as stochastic processes:
Here,
is the deterministic drift,
the diffusion coefficient and
a Wiener process. The system evolves as a multidimensional Itô diffusion and the probability of remaining conscious becomes a first-passage problem. The survival function
gives the probability that the system remains within
up to time t:
These models explain the variability in consciousness seen during transitions (e.g., sedation thresholds or partial awareness in sleep) and suggest that proximity to the manifold boundary increases the likelihood of stochastic exits. The geometry and roughness of influence these probabilities, making it essential to account for both deterministic dynamics and stochastic perturbations. These formulations clarify why consciousness may sometimes fluctuate rapidly despite slowly changing physiological inputs.
Overall, by treating consciousness as a constrained trajectory within the space , this chapter established a unified framework for understanding how diverse physiological processes collectively enable or disrupt conscious experience. This represents a step toward constructing a unified physiological model of consciousness.
5. TOWARD A UNIFIED PHYSICAL FRAMEWORK FOR CONSCIOUSNESS
This chapter integrates the empirical and analytical findings of the previous sections into a unified physical framework for understanding consciousness. We propose that consciousness arises from the intersection of structural, dynamic and energetic constraints, each defined by measurable physiological parameters.
Coherence of structural, dynamic and energetic domains. Converging evidence from electrophysiology, metabolic imaging, connectivity analyses, thermodynamics and peripheral integration supports a view of consciousness as a state governed by the concurrent fulfilment of interdependent physical constraints. At a fundamental level, these conditions fall into three interacting domains: structural (connectivity and architecture), dynamic (oscillations, timing and phase synchrony) and energetic (glucose metabolism, oxygen availability, ATP generation). Each domain provides necessary, though not individually sufficient, support for conscious processes. Structural architecture defines the anatomical substrate for long-range communication, but without dynamic synchronization, this substrate cannot support unified representations. Likewise, energy availability sets the operational limit for synaptic and network activity, but does not prescribe the pattern or content of consciousness. These domains can be represented as overlapping constraint sets
, with their intersection defining the viable state space for consciousness:
Each set imposes inequality bounds or dynamic relations over a shared parameter space. The empirical work summarized in previous chapters allows for the parameterization of each domain—such as functional efficiency , gamma coherence PLV > 0.4 for and CMRglu > 3.5 mg/100g/min for . The concept of intersection not only identifies the co-dependence of physical variables, but also defines the boundaries of sustainable conscious states. This triangulated model articulates the unified preconditions for consciousness as an emergent product of structurally supported, dynamically synchronized and energetically viable activity.
Embedding temporal dynamics into a spatial framework. Consciousness is not only defined by the fulfillment of spatial constraints at a given time but by the stability of these constraints over relevant temporal windows. A momentary fulfillment of metabolic or synchronization thresholds may be insufficient if not sustained. Thus, the model must incorporate time as a fourth-dimension governing trajectory stability in the multidimensional state space. Formally, let
represent the system’s evolution in parameter space, governed by dynamics
. A conscious regime is defined not just as
, but as:
for some minimal duration Δt, which may reflect biological constraints on working memory, integration time or neural coding. The requirement of trajectory continuity within prevents transient threshold crossings from qualifying as conscious states. Empirical support for this comes from loss-consciousness studies during anesthesia, where brief returns to metabolic sufficiency or oscillatory coherence do not correspond to behavioral responsiveness or subjective reports. Conversely, gradual transitions out of consciousness show early parameter re-entry without cognitive recovery. Consciousness requires continuous occupancy, not merely isolated visits. This approach reconciles the need for both physical sufficiency and temporal persistence in defining viable conscious activity.
Topology of consciousness in parameter space. The geometry of the conscious state space
reveals further structure when analyzed topologically. Rather than assuming a convex region, empirical fluctuations in physiological parameters suggest that
may consist of nonlinear, multimodal subregions (Tozzi et al., 2017). For example, slow-wave sleep and light sedation may occupy separate lobes of a partially conscious subspace, with distinct metabolic, oscillatory and structural signatures. To describe this, we treat
as a union of locally stable basins
, each with its own centroid
and covariance
, defined empirically from cluster analyses of high-dimensional data:
These ellipsoidal regions describe the local stability of distinct conscious regimes. Transitions between regimes (e.g., light sleep to REM) correspond to inter-basin dynamics, governed by parameter drift and diffusion. Importantly, some unconscious states (deep anesthesia, coma) are associated with attractor basins far removed from any , making spontaneous recovery unlikely. The topological formulation enables a refined classification of consciousness into clusters of viable configurations, rather than a binary awake/asleep dichotomy. It also suggests that transitions between states are path-dependent, with some trajectories requiring sequential re-entry into multiple constraint basins. This view captures the empirical richness of intermediate states, clarifying the structural nature of conscious continuity and disruption.
Multimodal redundancy and degeneracy. A further dimension of unification involves recognizing that multiple physical mechanisms can fulfill the same functional role in sustaining consciousness. This principle of degeneracy—distinct structures producing the same output—explains why different individuals can maintain awareness under varying physiological configurations (Sajid et al., 2023). For example, both gamma synchrony and beta-gamma coupling can support large-scale network coherence, depending on age, pharmacological state, neural architecture or disease (Liu et al., 2022; Hodnik et al., 2024). Likewise, metabolic resilience may be achieved through increased capillary density in one subject or more efficient mitochondrial function in another. This redundancy is formalized using a many-to-one mapping
, where different parameter vectors
yield the same functional state
. Mathematically, the conscious manifold is a fiber bundle over functional equivalence classes:
This structure allows for individual variability and state plasticity while preserving physical consistency. Redundancy also manifests across measurement modalities; gamma power and BOLD signal, though distinct in mechanism and timescale, often reflect the same underlying parameter class—cortical activation and integration. Recognizing this multimodal mapping prevents false dichotomies between data types and encourages a more abstract, system-level modeling of consciousness as a redundant, emergent product of overlapping physical systems.
Constraint violations and boundary classifications. To fully define the limits of the conscious space, it is essential to characterize what occurs at its boundaries. Each boundary violation represents a specific type of failure, e.g., metabolic collapse, network fragmentation, phase desynchronization, ionic instability, thermal dysregulation. These can be formalized as transitions out of
via inequality failure: for example, if
, then
and thus
. Boundary violations can be classified based on their dominant failure mode. Let
measure distance to the upper or lower threshold. The primary constraint violation is then:
This allows the classification of unconscious states not just by behavioral criteria, but by their dominant physical failure. For instance, coma may be categorized by , anesthesia by and hypothermia by . These physical diagnostics unify different pathologies under a shared formal model and suggest that unconsciousness is not a single state but a collection of parameter-specific collapses.
Overall, this chapter modeled consciousness as a stable region within a multidimensional state space, incorporating both spatial and temporal continuity. It explored the topological organization, redundancy and boundary conditions of this space, providing a coherent system-level account of conscious viability.
2. Conclusions
We provide a structured, physically grounded approach to identifying the minimal physiological requirements for consciousness. Through a framework that integrates electrophysiological, metabolic, ionic, thermodynamic, structural and systemic parameters, we have outlined how consciousness can be characterized as an emergent property of a biological system operating within physically definable constraints. Our methodology is based on the rigorous selection of measurable variables, the use of mathematical models to represent their interactions and the synthesis of empirical findings across various states—wakefulness, sleep, anesthesia and coma. Thus, we define consciousness as a dynamic, emergent regime wherein structurally embedded, energy-sufficient and phase-synchronized neural networks enable the selective access and integration of internal representations. This perspective reframes consciousness as a state sustained by physical feasibility rather than symbolic abstraction. Its state space is bounded by critical thresholds in gamma oscillatory coherence, synaptic timing precision, energy metabolism and other biophysical parameters. These variables interact to define a viable region in which the system can generate globally integrated, temporally stable and energetically supported neural activity.
The novelty of this approach lies in its commitment to operational definitions based on physically measurable parameters. Rather than treating consciousness as an undefined or inherently subjective phenomenon, we reframe it as the output of a constrained dynamical system. Our framework aims to unify diverse data types within a consistent systems-theoretic framework, enabling multi-scale integration across cellular, circuit-level and systemic physiological data.
Existing approaches to consciousness research usually fall into two categories: theoretical models that lack empirical traction and empirical studies that lack a unifying framework. Theories such as Global Workspace, Integrated Information and Predictive Coding offer valuable conceptual tools but often suffer from ambiguous operationalization and difficulties in experimental falsifiability (Seth and Bayne, 2022; Cogitate Consortium et al., 2025). On the empirical side, studies of EEG, fMRI and PET imaging produce isolated findings without a common interpretative structure. Our model aims to address these gaps by formalizing the preconditions for consciousness in the language of physical thresholds and dynamic systems. Unlike Global Workspace Theory, which relies on conceptual notions of broadcast and access (Mashour et al., 2020; VanRullen et al., 2021) or Integrated Information Theory, which introduces an abstract measure (Φ) that is difficult to compute and test in vivo (Mediano et al., 2022), our framework employs measurable variables like gamma coherence, functional connectivity and CMRglu. Moreover, unlike neurophenomenological approaches depending on subjective reportability (Timmermann et al., 2023; Lutz et al., 2025), our method situates consciousness within an objective, physically bounded space defined by interdependent constraints. This allows for the integration of diverse techniques—from DTI-based structural analysis to metabolic PET and EEG coherence—into a unified analytic and diagnostic model. Our approach also accommodates factors like the role of astrocytes, peripheral rhythms and thermoregulation, which are often excluded from narrower cognitive models.
Our approach is not without limitations. Chief among these is the absence of a definitive causal mechanism linking individual parameters to conscious experience. While correlations between, e.g., gamma coherence, metabolic rate and conscious state are strong, they do not in themselves confirm that these variables generate consciousness. The multidimensional nature of the model also presents challenges for empirical implementation, requiring simultaneous access to high-fidelity data across modalities, something not always feasible in real-time or clinical settings. Furthermore, while we describe a bounded viability region in parameter space, the precise topological structure and the nature of transitions between states remain only theoretically outlined. Experimental data may not always conform neatly to the geometric or dynamical models proposed and physiological variability between individuals adds complexity to boundary definitions. Another limitation is the reliance on inference from mammalian data; generalizing the model to non-mammalian systems or artificial substrates of cognition remains speculative. Still, some equations (e.g., fiber bundle descriptions or the exact sigmoid formulations in metabolic models) are conceptually plausible but stylized and used here more illustratively than strictly derived from data or primary literature. Finally, although this review outlines a plausible state space for consciousness, it does not yet specify the minimal neural circuitry necessary for entry into such a space, nor the critical duration thresholds for temporal stability.
Our framework opens new directions for applications in clinical assessment, basic research and experimental design. In clinical settings, the model can inform diagnostic criteria for disorders of consciousness by enabling multi-modal evaluations that integrate EEG, metabolic imaging and structural MRI data into a coherent profile. For example, identifying whether a patient lies within or outside the viability region could support differential diagnosis between vegetative and minimally conscious states. In research, our framework provides a structured platform for testing specific hypotheses, for instance, whether transient re-entry into the conscious manifold during anesthesia correlates with recovery quality or behavioral responsiveness. Experiments could test the resilience of specific dimensions—metabolic, ionic or oscillatory—by perturbing each independently and measuring the system’s ability to remain within the viability region. Future studies might use real-time multi-modal monitoring to test whether maintaining specific parameter thresholds over time predicts conscious state continuity. Our approach also suggests comparative studies across species to identify minimal parameter combinations sufficient for conscious processing. More broadly, our model encourages integration across neuroscience subfields—linking glial physiology, neurovascular dynamics and electrophysiology under a unified interpretive lens. These findings suggest potential directions for future research, including the development of multimodal neurodiagnostic tools, greater integration of systemic physiology into consciousness studies and the exploration of time-sensitive models to better account for dynamic transitions and attractor states within physiological space.
In summary, the main research question—what are the physical requisites of consciousness—has been addressed through the development of a multidimensional, empirically anchored model grounded in measurable physiological variables. Consciousness emerges not from a single neural event or structure, but from the synchronized interaction of structural, dynamic and energetic systems operating within a narrow, but identifiable, physical viability region. A key insight is that consciousness may not be defined solely by information content or subjective report, but by the fulfilment of interdependent physical constraints that support coherent neural integration. This physically grounded approach reframes consciousness not as a mysterious emergent phenomenon, but as a constrained systemic state embedded within the laws of biological organization and thermodynamic feasibility.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declarations: Ethics approval and consent to participate
This research does not contain any studies with human participants or animals performed by the Author.
Consent for publication
The Author transfers all copyright ownership, in the event the work is published. The undersigned author warrants that the article is original, does not infringe on any copyright or other proprietary right of any third part, is not under consideration by another journal and has not been previously published.
Availability of data and materials
All data and materials generated or analyzed during this study are included in the manuscript. The Author had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The Author does not have any known or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence or be perceived to influence their work.
Authors' contributions
The Author performed: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtained funding, administrative, technical and material support, study supervision.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the author used ChatGPT 4o to assist with data analysis and manuscript drafting and to improve spelling, grammar and general editing. After using this tool, the author reviewed and edited the content as needed, taking full responsibility for the content of the publication.
References
- Abdolalizadeh, A., M.A.D. Ohadi, A.S.B. Ershadi and M.H. Aarabi. 2023. “Graph Theoretical Approach to Brain Remodeling in Multiple Sclerosis.” Network Neuroscience 7 (1): 148–59. [CrossRef]
- Ahmed, Rizwan, Naveed Tahir and Noor Ali Shah. 2024. "An Analysis of the Stability and Bifurcation of a Discrete-Time Predator-Prey Model with the Slow-Fast Effect on the Predator." Chaos 34 (3): 033127. [CrossRef]
- Ashworth, E. T., J. D. Cotter and A. E. Kilding. 2021. "Impact of Elevated Core Temperature on Cognition in Hot Environments within a Military Context." European Journal of Applied Physiology 121 (4): 1061–71. [CrossRef]
- Bao, W. W., S. Jiang, W. M. Qu, W. X. Li, C. H. Miao and Z. L. Huang. 2023. "Understanding the Neural Mechanisms of General Anesthesia from Interaction with Sleep-Wake State: A Decade of Discovery." Pharmacological Reviews 75 (3): 532–53. [CrossRef]
- Bardon, A. G., J. J. Ballesteros, S. L. Brincat, J. E. Roy, M. K. Mahnke, Y. Ishizawa, E. N. Brown and E. K. Miller. 2024. "Convergent Effects of Different Anesthetics on Changes in Phase Alignment of Cortical Oscillations." bioRxiv, October 9, 2024. 9 October. [CrossRef]
- Ben Salem, Kamel and Abdelaziz Ben Abdelaziz. 2021. "Principal Component Analysis (PCA)." La Tunisie Médicale 99 (4): 383–89.
- Benitez, F., C. Pennartz, and W. Senn. 2024. “The Conductor Model of Consciousness, Our Neuromorphic Twins, and the Human-AI Deal.” AI and Ethics. [CrossRef]
- Bernier, M., E. Croteau, C. A. Castellano, S. C. Cunnane and K. Whittingstall. 2017. "Spatial Distribution of Resting-State BOLD Regional Homogeneity as a Predictor of Brain Glucose Uptake: A Study in Healthy Aging." NeuroImage 150: 14–22. [CrossRef]
- Berger, C., M. Bauer, H. Wittig, E. Scheurer and C. Lenz. 2022. "Post Mortem Brain Temperature and Its Influence on Quantitative MRI of the Brain." MAGMA 35 (3): 375–87. [CrossRef]
- Bonhomme, V., C. Staquet, J. Montupil, A. Defresne, M. Kirsch, C. Martial, A. Vanhaudenhuyse, et al. 2019. "General Anesthesia: A Probe to Explore Consciousness." Frontiers in Systems Neuroscience 13: 36. [CrossRef]
- Brito, J. B. G., G. B. Bucco, D. K. John, M. F. Ferrão, R. S. Ortiz, K. C. Mariotti and M. J. Anzanello. 2020. "Wavenumber Selection Based on Singular Value Decomposition for Sample Classification." Forensic Science International 309 (April): 110191. [CrossRef]
- Burek, M., R. Follmann and E. Rosa. 2019. “Temperature Effects on Neuronal Firing Rates and Tonic-to-Bursting Transitions.” Biosystems 180: 1–6. [CrossRef]
- Cacciola, A., A. Naro, D. Milardi, A. Bramanti, L. Malatacca, M. Spitaleri, A. Leo, M. Muscoloni, C. V. Cannistraci, P. Bramanti, R. S. Calabrò and G. P. Anastasi. 2019. "Functional Brain Network Topology Discriminates between Patients with Minimally Conscious State and Unresponsive Wakefulness Syndrome." Journal of Clinical Medicine 8 (3): 306. [CrossRef]
- Cao, Xiaoying, Zhi Wang, Xiaowei Chen, Yanan Liu, Ibrahima Amadou Abdoulaye, Shanshan Ju, Shuyan Zhang, et al. 2023. "Changes in Resting-State Neural Activity and Nerve Fibres in Ischaemic Stroke Patients with Hemiplegia." Brain Topography 36 (2): 255–68. [CrossRef]
- Caporale, A., H. Lee, H. Lei, H. Rao, M. C. Langham, J. A. Detre, P. H. Wu and F. W. Wehrli. 2021. "Cerebral Metabolic Rate of Oxygen during Transition from Wakefulness to Sleep Measured with High Temporal Resolution OxFlow MRI with Concurrent EEG." Journal of Cerebral Blood Flow & Metabolism 41 (4): 780–92. [CrossRef]
- Caporale, A. S., A. M. Barclay, J. Xu, H. Rao, H. Lee, M. C. Langham, J. A. Detre and F. W. Wehrli. 2023. "Superior Sagittal Sinus Flow as a Proxy for Tracking Global Cerebral Blood Flow Dynamics during Wakefulness and Sleep." Journal of Cerebral Blood Flow & Metabolism 43 (8): 1340–50. [CrossRef]
- Cavelli, M., S. Castro-Zaballa, J. Gonzalez, D. Rojas-Líbano, N. Rubido, N. Velásquez and P. Torterolo. 2020. "Nasal Respiration Entrains Neocortical Long-Range Gamma Coherence during Wakefulness." European Journal of Neuroscience 51 (6): 1463–77. [CrossRef]
- Chiarelli, A. M., M. Germuska, H. Chandler, R. Stickland, E. Patitucci, E. Biondetti, D. Mascali, et al. 2022. "A Flow-Diffusion Model of Oxygen Transport for Quantitative Mapping of Cerebral Metabolic Rate of Oxygen (CMRO₂) with Single Gas Calibrated fMRI." Journal of Cerebral Blood Flow & Metabolism 42 (7): 1192–1209. [CrossRef]
- Choe, M., Y. Choi, J. Kwon, H.P. Park, S.H. Jin, J.S. Kim, S. Lee and C.K. Chung. 2025. “Increased Global and Regional Connectivity in Propofol-Induced Unconsciousness: Human Intracranial Electroencephalography Study.” Anesthesiology, April 3, 2025. 3 April. [CrossRef]
- Choi, S.I. and J.B. Kim. 2022. “Altered Brain Networks in Chronic Obstructive Pulmonary Disease: An Electroencephalography Analysis.” Clinical EEG and Neuroscience 53 (2): 160–64. [CrossRef]
- Cogitate Consortium, O. Ferrante, U. Gorska-Klimowska, et al. 2025. "Adversarial Testing of Global Neuronal Workspace and Integrated Information Theories of Consciousness." Nature. [CrossRef]
- Colombo, A.V., R.K. Sadler, G. Llovera, V. Singh, S. Roth, S. Heindl, L. Sebastian Monasor, et al. 2021. “Microbiota-Derived Short Chain Fatty Acids Modulate Microglia and Promote Abeta Plaque Deposition.” eLife 10: e59826. [CrossRef]
- Damasio, A. and H. Damasio. 2023. "Feelings Are the Source of Consciousness." Neural Computation 35 (3): 277–86. [CrossRef]
- Deshpande, R. S., M. C. Langham, C. C. Cheng and F. W. Wehrli. 2022. "Metabolism of Oxygen via T₂ and Interleaved Velocity Encoding: A Rapid Method to Quantify Whole-Brain Cerebral Metabolic Rate of Oxygen." Magnetic Resonance in Medicine 88 (3): 1229–43. [CrossRef]
- Dickie, B. R., T. Jin, P. Wang, R. Hinz, W. Harris, H. Boutin, G. J. Parker, L. M. Parkes and J. C. Matthews. 2022. "Quantitative Kinetic Modelling and Mapping of Cerebral Glucose Transport and Metabolism Using glucoCESL MRI." Journal of Cerebral Blood Flow & Metabolism 42 (11): 2066–79. [CrossRef]
- Dittrich, A. S., T. Winkler, T. Wellman, N. de Prost, G. Musch, R. S. Harris and M. F. Vidal Melo. 2012. "Modeling 18F-FDG Kinetics during Acute Lung Injury: Experimental Data and Estimation Errors." PLoS ONE 7 (10): e47588. [CrossRef]
- Eisner, D., E. Neher, H. Taschenberger and G. Smith. 2023. “Physiology of Intracellular Calcium Buffering.” Physiological Reviews 103 (4): 2767–2845. [CrossRef]
- Engelen, T., M. Solcà and C. Tallon-Baudry. 2023. “Interoceptive Rhythms in the Brain.” Nature Neuroscience 26 (10): 1670–84. [CrossRef]
- Engl, E. and D. Attwell. 2015. "Non-Signalling Energy Use in the Brain." The Journal of Physiology 593 (16): 3417–29. [CrossRef]
- Fazekas, P. and G. Nemeth. 2018. "Dream Experiences and the Neural Correlates of Perceptual Consciousness and Cognitive Access." Philosophical Transactions of the Royal Society B: Biological Sciences 373 (1755): 20170356. [CrossRef]
- Fiedler, P., C. Fonseca, E. Supriyanto, F. Zanow and J. Haueisen. 2022. "A High-Density 256-Channel Cap for Dry Electroencephalography." Human Brain Mapping 43 (4): 1295–1308. [CrossRef]
- Fiedler, P., U. Graichen, E. Zimmer and J. Haueisen. 2023. "Simultaneous Dry and Gel-Based High-Density Electroencephalography Recordings." Sensors 23 (24): 9745. [CrossRef]
- Fischer, S., K. Naegeli, D. Cardone, C. Filippini, A. Merla, K. U. Hanusch and U. Ehlert. 2024. "Emerging Effects of Temperature on Human Cognition, Affect and Behaviour." Biological Psychology 189: 108791. [CrossRef]
- Garrido Merchán, Eduardo C., and Martín Molina. 2020. A Machine Consciousness Architecture Based on Deep Learning and Gaussian Processes. arXiv preprint. arXiv:2002.00509.
- Gomez-Pilar, Javier, Victor Martínez-Cagigal, David García-Azorín, Carlos Gómez, Álvaro Guerrero and Roberto Hornero. 2022. "Headache-Related Circuits and High Frequencies Evaluated by EEG, MRI, PET as Potential Biomarkers to Differentiate Chronic and Episodic Migraine: Evidence from a Systematic Review." Journal of Headache and Pain 23 (1): 95. [CrossRef]
- Gonzalez-Escamilla, G., V.C. Chirumamilla, N. Koirala, A.R. Anwar, O. Tüscher, J. Vogt, P. Horstmann, et al. 2023. “Modular Segregation Drives Causality of the Dynamic Oscillatory Network Responses during Threat Processing.” Brain Communications 5 (2): fcad035. [CrossRef]
- Gu, B., V.S. Sheng, K.Y. Tay, W. Romano, and S. Li. 2017. “Cross Validation Through Two-Dimensional Solution Surface for Cost-Sensitive SVM.” IEEE Transactions on Pattern Analysis and Machine Intelligence 39 (6): 1103–21. [CrossRef]
- Guo, Yanyan, Yizhou Chen, Yiru Shao, Shuang Hu, Guangya Zou, Jiaqi Chen, Yuting Li, et al. 2023. "Thalamic Network under Wakefulness after Sleep Onset and Its Coupling with Daytime Fatigue in Insomnia Disorder: An EEG-fMRI Study." Journal of Affective Disorders 334 (August 1): 92–99. 1 August. [CrossRef]
- Hall, Emma L., Samuel E. Robson, Peter G. Morris and Matthew J. Brookes. 2014. "The Relationship between MEG and fMRI." NeuroImage 102, Pt 1 (November 15): 80–91. [CrossRef]
- Hodnik, Tanja, Sofia Roytman, Nicholas I. Bohnen and Urska Marusic. 2024. "Beta-Gamma Phase-Amplitude Coupling as a Non-Invasive Biomarker for Parkinson's Disease: Insights from Electroencephalography Studies." Life 14 (3): 391. [CrossRef]
- Hopper, A., H. Beswick-Jones and A. M. Brown. 2022. "The Nernst Equation: Using Physico-Chemical Laws to Steer Novel Experimental Design." Advances in Physiology Education 46 (1): 206–10. [CrossRef]
- Horiuchi, D., T. Shimono, H. Tatekawa, T. Tsukamoto, H. Takita, S. Matsushita and Y. Miki. 2023. "Brain Temperature Remains Stable during the Day: A Study of Diffusion-Weighted Imaging Thermometry in Healthy Individuals." Neuroradiology 65 (8): 1239–46. [CrossRef]
- Huang, Xiaoyang, Yuting Du, Dongming Guo, Feiyu Xie and Cheng Zhou. 2023. "Structural-Functional Coupling Abnormalities in Temporal Lobe Epilepsy." Frontiers in Neuroscience 17 (October 19): 1272514. 19 October. [CrossRef]
- Innocenti, G. M., T. B. Dyrby, G. Girard, E. St-Onge, J. P. Thiran, A. Daducci and M. Descoteaux. 2019. "Topological Principles and Developmental Algorithms Might Refine Diffusion Tractography." Brain Structure and Function 224 (1): 1–8. [CrossRef]
- Jabbari, M.B. and M.R. Karamati. 2022. “The Effects of Temperature on the Dynamics of the Biological Neural Network.” Journal of Biological Physics 48 (1): 111–26. [CrossRef]
- Kaszás, Bálint, Ulrike Feudel and Tamás Tél. 2019. "Tipping Phenomena in Typical Dynamical Systems Subjected to Parameter Drift." Scientific Reports 9: 8654. [CrossRef]
- Khalsa, S.S. 2023. “Rhythms of the Heart, Echoes in the Brain: Exploring Interoception.” JACC: Clinical Electrophysiology 9 (11): 2236–39. [CrossRef]
- Kim, Dongju, Amanda M. Schnakenberg Martin, Yong Wook Shin, Hae-Jeong Jo, Huiling Cheng, Steven D. Newman, Olaf Sporns, William P. Hetrick, Emma Calkins and Brian F. O'Donnell. 2019. "Aberrant Structural-Functional Coupling in Adult Cannabis Users." Human Brain Mapping 40 (1): 252–61. [CrossRef]
- Kingston, Zachary. 2020. "Planning Under Manifold Constraints." In Encyclopedia of Robotics, edited by M.H. Ang, Oussama Khatib and Bruno Siciliano. Berlin, Heidelberg: Springer. [CrossRef]
- Kluger, D.S., T. Erdbrügger, C. Stier, M.B. Höltershinken, O. Abbasi, M. Saltafossi, K. Unnwongse, et al. 2025. “Respiratory Modulations of Cortical Excitability and Interictal Spike Timing in Focal Epilepsy: A Case Report.” Communications Medicine (London) 5 (1): 108. [CrossRef]
- Koch, C., M. Massimini, M. Boly and G. Tononi. 2016. "Neural Correlates of Consciousness: Progress and Problems." Nature Reviews Neuroscience 17 (5): 307–21. [CrossRef]
- Kosmyna, Nathalie and Anatole Lécuyer. 2019. "A Conceptual Space for EEG-Based Brain-Computer Interfaces." PLoS ONE 14 (1): e0210145. [CrossRef]
- Koush, Y., R. A. de Graaf, R. Kupers, L. Dricot, M. Ptito, K. L. Behar, D. L. Rothman and F. Hyder. 2021. "Metabolic Underpinnings of Activated and Deactivated Cortical Areas in Human Brain." Journal of Cerebral Blood Flow & Metabolism 41 (5): 986–1000. [CrossRef]
- Lebedev, S. V., P. W. Van Gelder and W. H. Tsui. 2002. "Use of Linearity of the Sokoloff Model to Improve Performance of Non-Linear Search." Nuclear Medicine Communications 23 (2): 181–85. [CrossRef]
- Leguia, M. G., V. R. Rao, J. K. Kleen and M. O. Baud. 2021. "Measuring Synchrony in Bio-Medical Timeseries." Chaos 31 (1): 013138. [CrossRef]
- Lemaire, J.J., B. Pontier, R. Chaix, Y. El Ouadih, T. Khalil, D. Sinardet, V. Achim, et al. 2022. “Neural Correlates of Consciousness and Related Disorders: From Phenotypic Descriptors of Behavioral and Relative Consciousness to Cortico-Subcortical Circuitry.” Neurochirurgie 68 (2): 212–22. [CrossRef]
- Leung, A. and N. Tsuchiya. 2023. "Separating Weak Integrated Information Theory into Inspired and Aspirational Approaches." Neuroscience of Consciousness 2023 (1): niad012. [CrossRef]
- Leyrolle, Q., L. Prado-Perez and S. Layé. 2023. “The Gut-Derived Metabolites as Mediators of the Effect of Healthy Nutrition on the Brain.” Frontiers in Nutrition 10: 1155533. [CrossRef]
- Li, L., Y. Li, Z. Li, G. Huang, Z. Liang, L. Zhang, F. Wan, et al. 2024. “Multimodal and Hemispheric Graph-Theoretical Brain Network Predictors of Learning Efficacy for Frontal Alpha Asymmetry Neurofeedback.” Cognitive Neurodynamics 18 (3): 847–62. [CrossRef]
- Lillicrap, T., M. Tahtalı, A. Neely, X. Wang, A. Bivard and C. Lueck. 2017. "A Model Based on the Pennes Bioheat Transfer Equation Is Valid in Normal Brain Tissue but Not Brain Tissue Suffering Focal Ischaemia." Australasian Physical & Engineering Sciences in Medicine 40 (4): 841–50. [CrossRef]
- Liu, Thomas T. 2013. "Neurovascular Factors in Resting-State Functional MRI." NeuroImage 80: 339–48. [CrossRef]
- Liu, X., K. K. Lauer, B. Douglas Ward, C. Roberts, S. Liu, S. Gollapudy, R. Rohloff, et al. 2017. "Propofol Attenuates Low-Frequency Fluctuations of Resting-State fMRI BOLD Signal in the Anterior Frontal Cortex upon Loss of Consciousness." NeuroImage 147: 295–301. [CrossRef]
- Liu, H., J. Liu, L. Peng, Z. Feng, L. Cao, H. Liu, H. Shen, D. Hu, L. L. Zeng and W. Wang. 2019. "Changes in Default Mode Network Connectivity in Different Glucose Metabolism Status and Diabetes Duration." NeuroImage: Clinical 21: 101629. [CrossRef]
- Liu, H. and V. Prokosch. 2021. "Energy Metabolism in the Inner Retina in Health and Glaucoma." International Journal of Molecular Sciences 22 (7): 3689. [CrossRef]
- Liu, Xiaojing, Shuo Liu, Meng Li, Fang Su, Shichao Chen, Yu Ke and Delong Ming. 2022. "Altered Gamma Oscillations and Beta-Gamma Coupling in Drug-Naive First-Episode Major Depressive Disorder: Association with Sleep and Cognitive Disturbance." Journal of Affective Disorders 316 (): 99–108. 1 November. [CrossRef]
- Long, J., Q. Xie, Q. Ma, M.A. Urbin, L. Liu, L. Weng, X. Huang, et al. 2016. “Distinct Interactions between Fronto-Parietal and Default Mode Networks in Impaired Consciousness.” Scientific Reports 6: 38866. [CrossRef]
- Lutz, Antoine, Omar Abdoun, Yair Dor-Ziderman, Fynn M. Trautwein and Aviva Berkovich-Ohana. 2025. "An Overview of Neurophenomenological Approaches to Meditation and Their Relevance to Clinical Research." Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 10 (4): 411–24. [CrossRef]
- Maquet, P., D. Dive, E. Salmon, R. von Frenckel and G. Franck. 1990. "Reproducibility of Cerebral Glucose Utilization Measured by PET and the [18F]-2-Fluoro-2-Deoxy-D-Glucose Method in Resting, Healthy Human Subjects." European Journal of Nuclear Medicine 16 (4–6): 267–73. [CrossRef]
- Margazoglou, Georgios and Luca Magri. 2023. "Stability Analysis of Chaotic Systems from Data." Nonlinear Dynamics 111 (9): 8799–819. [CrossRef]
- Mashour, George A., Pieter Roelfsema, Jean-Pierre Changeux and Stanislas Dehaene. 2020. "Conscious Processing and the Global Neuronal Workspace Hypothesis." Neuron 105 (5): 776–98. [CrossRef]
- Mashour, G. A., B. J. Palanca, M. Basner, D. Li, W. Wang, S. Blain-Moraes, N. Lin, et al. 2021. "Recovery of Consciousness and Cognition after General Anesthesia in Humans." eLife 10: e59525. [CrossRef]
- Mashour, G. A. 2024. "Anesthesia and the Neurobiology of Consciousness." Neuron 112 (10): 1553–67. [CrossRef]
- Mediano, Pedro A. M., Fernando E. Rosas, David Bor, Anil K. Seth and Adam B. Barrett. 2022. "The Strength of Weak Integrated Information Theory." Trends in Cognitive Sciences 26 (8): 646–55. [CrossRef]
- Mikulan, E., E. Hesse, L. Sedeño, T. Bekinschtein, M. Sigman, M. D. C. García, W. Silva, C. Ciraolo, A. M. García and A. Ibáñez. 2018. "Intracranial High-Gamma Connectivity Distinguishes Wakefulness from Sleep." NeuroImage 169: 265–77. [CrossRef]
- Mohammadi, Ashena Gorgan, and Mohammad Ganjtabesh. 2024. “On Computational Models of Theory of Mind and the Imitative Reinforcement Learning in Spiking Neural Networks.” Scientific Reports 14: 1945. [CrossRef]
- Naccache, L. and E. Munoz-Musat. 2024. "A Global Neuronal Workspace Model of Functional Neurological Disorders." Dialogues in Clinical Neuroscience 26 (1): 1–23. [CrossRef]
- Nemirovsky, Idan E., Nicholas J. M. Popiel, Jorge Rudas, Matthew Caius, Lorina Naci, Nicholas D. Schiff, Adrian M. Owen and Andrea Soddu. 2023. "An Implementation of Integrated Information Theory in Resting-State fMRI." Communications Biology 6: 692. [CrossRef]
- Nestor, K., J. Rasero, R. Betzel, P.J. Gianaros and T. Verstynen. 2024. “Cortical Network Reconfiguration Aligns with Shifts of Basal Ganglia and Cerebellar Influence.” arXiv preprint, August 15, 2024.
- Okolie, J. A., D. Awotoye, M. E. Tabat, P. U. Okoye, E. I. Epelle, C. C. Ogbaga, F. Güleç and B. Oboirien. 2023. "Multi-Criteria Decision Analysis for the Evaluation and Screening of Sustainable Aviation Fuel Production Pathways." iScience 26 (6): 106944. [CrossRef]
- Pan, Yaping, Xue Li, Yajuan Liu, Xinyi Jia, Shuang Wang, Qing Ji, Wenhao Zhao, Biao Yin, Guanying Bai, Jing Zhang and Lin Bai. 2023. "Hierarchical Brain Structural-Functional Coupling Associated with Cognitive Impairments in Mild Traumatic Brain Injury." Cerebral Cortex 33 (12): 7477–88. [CrossRef]
- Pelc, K. 2023. "High-Density Neonatal Electroencephalography Provides Biomarkers for Developmental Trajectories." Developmental Medicine & Child Neurology 65 (10): 1274–75. [CrossRef]
- Perl, Yair S., Cecilia Pallavicini, Julieta Piccinini, Athena Demertzi, Vincent Bonhomme, Camille Martial, Rakesh Panda, et al. 2023. "Low-Dimensional Organization of Global Brain States of Reduced Consciousness." Cell Reports 42 (5): 112491. [CrossRef]
- Pullon, R.M., C.E. Warnaby and J.W. Sleigh. 2022. “Propofol-Induced Unresponsiveness Is Associated with a Brain Network Phase Transition.” Anesthesiology 136 (3): 420–33. [CrossRef]
- Rajesh, Aishwarya, Nicole A. Seider, Dillan J. Newbold, Babatunde Adeyemo, Scott Marek, Deanna J. Greene, Abraham Z. Snyder, et al. 2024. "Structure-Function Coupling in Highly Sampled Individual Brains." Cerebral Cortex 34 (9): bhae361. [CrossRef]
- Renauld, E., A. Théberge, L. Petit, J. C. Houde and M. Descoteaux. 2023. "Validate Your White Matter Tractography Algorithms with a Reappraised ISMRM 2015 Tractography Challenge Scoring System." Scientific Reports 13 (1): 2347. [CrossRef]
- Repasky, E. A., B. L. Hylander and H. Mohammadpour. 2024. "Temperature Matters: The Potential Impact of Thermoregulatory Mechanisms in Brain-Body Physiology." Genes & Development 38 (17–20): 817–19. [CrossRef]
- Robertson, J. M. 2018. "The Gliocentric Brain." International Journal of Molecular Sciences 19 (10): 3033. [CrossRef]
- Rodu, Jordan, Natalie Klein, Scott L. Brincat, Earl K. Miller and Robert E. Kass. 2018. "Detecting Multivariate Cross-Correlation between Brain Regions." Journal of Neurophysiology 120 (4): 1962–72. [CrossRef]
- Rosenblum, Y., I. Maidan, O. Goldstein, M. Gana-Weisz, A. Orr-Urtreger, N. Bregman, N. Giladi, A. Mirelman and T. Shiner. 2022. "Decreased Delta-Band Event-Related Power in Dementia with Lewy Bodies with a Mutation in the Glucocerebrosidase Gene." Clinical Neurophysiology 143: 14–20. [CrossRef]
- Sajid, Noor andres Gajardo-Vidal, John O. Ekert, Daniel L. Lorca-Puls, Thomas M. H. Hope, David W. Green, Karl J. Friston and Cathy J. Price. 2023. "Degeneracy in the Neurological Model of Auditory Speech Repetition." Communications Biology 6 (1): 1161. [CrossRef]
- Schultze, Steffen and Helmut Grubmüller. 2021. "Time-Lagged Independent Component Analysis of Random Walks and Protein Dynamics." Journal of Chemical Theory and Computation 17: 5766–76. [CrossRef]
- Seth, Anil K. and Tim Bayne. 2022. "Theories of Consciousness." Nature Reviews Neuroscience 23 (7): 439–52. [CrossRef]
- Shin, C. and Y.K. Kim. 2023. “Microbiota-Gut-Brain Axis: Pathophysiological Mechanism in Neuropsychiatric Disorders.” In Advances in Experimental Medicine and Biology, vol. 1411, 17–37. [CrossRef]
- Spencer, K. M. and S. Ghorashi. 2014. "Oscillatory Dynamics of Gestalt Perception in Schizophrenia Revisited." Frontiers in Psychology 5: 68. [CrossRef]
- Stankeviciute, L., C. Falcon, G. Operto, M. Garcia, M. Shekari, Á. Iranzo, A. Niñerola-Baizán, et al. 2023. "Differential Effects of Sleep on Brain Structure and Metabolism at the Preclinical Stages of AD." Alzheimer's & Dementia 19 (12): 5371–86. [CrossRef]
- Sun, F., Y. Wang, Y. Li, Y. Li, S. Wang, F. Xu and X. Wang. 2023. "Variation in Functional Networks between Clinical and Subclinical Discharges in Childhood Absence Epilepsy: A Multi-Frequency MEG Study." Seizure 111: 109–21. [CrossRef]
- Tamagawa, H., T. Nakahata, R. Sugimori, B. Delalande and T. Mulembo. 2023. "The Membrane Potential Has a Primary Key Equation." Acta Biotheoretica 71 (3): 15. [CrossRef]
- Timmermann, Christopher, Peter R. Bauer, Olivia Gosseries, Audrey Vanhaudenhuyse, Franz Vollenweider, Steven Laureys, Tania Singer, et al. 2023. "A Neurophenomenological Approach to Non-Ordinary States of Consciousness: Hypnosis, Meditation and Psychedelics." Trends in Cognitive Sciences 27 (2): 139–59. [CrossRef]
- Tozzi, A. 2015. "Information Processing in the CNS: A Supramolecular Chemistry?" Cognitive Neurodynamics 9 (5): 463–77.
- Tozzi, A. 2019. "The Multidimensional Brain." Physics of Life Reviews 31: 86–103. [CrossRef]
- Tozzi, A., J. F. Peters, A. A. Fingelkurts, A. A. Fingelkurts and P. C. Marijuán. 2017. "Topodynamics of Metastable Brains." Physics of Life Reviews 21: 1–20. [CrossRef]
- Tucci, C., M. Trujillo, E. Berjano, M. Iasiello, A. Andreozzi and G.P. Vanoli. 2021. “Pennes' Bioheat Equation vs. Porous Media Approach in Computer Modeling of Radiofrequency Tumor Ablation.” Scientific Reports 11 (1): 5272. [CrossRef]
- Usami, N., Y. Asano, Y. Ikegame, H. Takei, Y. Yamada, H. Yano and J. Shinoda. 2023. "Cerebral Glucose Metabolism in Patients with Chronic Disorders of Consciousness." Canadian Journal of Neurological Sciences 50 (5): 719–29. [CrossRef]
- van Diessen, E., W. J. Zweiphenning, F. E. Jansen, C. J. Stam, K. P. Braun and W. M. Otte. 2014. "Brain Network Organization in Focal Epilepsy: A Systematic Review and Meta-Analysis." PLoS ONE 9 (12): e114606. [CrossRef]
- VanRullen, Rufin and Ryota Kanai. 2021. "Deep Learning and the Global Workspace Theory." Trends in Neurosciences 44 (9): 692–704. [CrossRef]
- Väyrynen, T., H. Helakari, V. Korhonen, J. Tuunanen, N. Huotari, J. Piispala, M. Kallio, et al. 2023. “Infra-Slow Fluctuations in Cortical Potentials and Respiration Drive Fast Cortical EEG Rhythms in Sleeping and Waking States.” Clinical Neurophysiology 156: 207–19. [CrossRef]
- Vijayakrishnan Nair, V., B. R. Kish, P. L. Chong, H. S. Yang, Y. C. Wu, Y. Tong and A. J. Schwichtenberg. 2023. "Neurofluid Coupling during Sleep and Wake States." Sleep Medicine 110 (October): 44–53. [CrossRef]
- Yang, Q., F. Zhou, A. Li and H. Dong. 2022. "Neural Substrates for the Regulation of Sleep and General Anesthesia." Current Neuropharmacology 20 (1): 72–84. [CrossRef]
- Yang, Yijun, Shuang Wang, Jie Liu, Guangya Zou, Jie Jiang, Bin Jiang, Wei Cao and Qiyong Zou. 2022. "Changes in White Matter Functional Networks during Wakefulness and Sleep." Human Brain Mapping 43 (14): 4383–96. [CrossRef]
- Yen, Chia-Yu, Cheng-Lung Lin and Ming-Chang Chiang. 2023. "Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders." Life 13 (7): 1472. [CrossRef]
- Yuan, Z., B. Wen, C. He, J. Zhou, Z. Zhou and F. Xu. 2022. "Application of Multi-Criteria Decision-Making Analysis to Rural Spatial Sustainability Evaluation: A Systematic Review." International Journal of Environmental Research and Public Health 19 (11): 6572. [CrossRef]
- Wang, Liang, and Ziyi Ma. 2023. “The Construction Approach of Machine Consciousness and Its Limitations.” Computational Science and Mathematics Forum 8 (1): 16. [CrossRef]
- Wu, M., T. Wan, X. Wan, Y. Du and J. She. 2017. "Fast, Accurate Localization of Epileptic Seizure Onset Zones Based on Detection of High-Frequency Oscillations Using Improved Wavelet Transform and Matching Pursuit Methods." Neural Computation 29 (1): 194–219. [CrossRef]
- Wu, P. H., A. E. Rodríguez-Soto, A. Wiemken, E. K. Englund, Z. B. Rodgers, M. C. Langham, R. J. Schwab, J. A. Detre, W. Guo and F. W. Wehrli. 2022. "MRI Evaluation of Cerebral Metabolic Rate of Oxygen (CMRO₂) in Obstructive Sleep Apnea." Journal of Cerebral Blood Flow & Metabolism 42 (6): 1049–60. [CrossRef]
- Wu, X., L. Palaniyappan, G. Yu, K. Zhang, J. Seidlitz, Z. Liu, X. Kong, et al. 2023. “Morphometric Dis-Similarity between Cortical and Subcortical Areas Underlies Cognitive Function and Psychiatric Symptomatology: A Preadolescence Study from ABCD.” Molecular Psychiatry 28 (3): 1146–58. [CrossRef]
- Zacks, O. and E. Jablonka. 2023. "The Evolutionary Origins of the Global Neuronal Workspace in Vertebrates." Neuroscience of Consciousness 2023 (2): niad020. [CrossRef]
- Zarkali, Angeliki, Peter McColgan, Louise-Ann Leyland andrew J. Lees, Geraint Rees and Rimona S. Weil. 2021. "Organisational and Neuromodulatory Underpinnings of Structural-Functional Connectivity Decoupling in Patients with Parkinson's Disease." Communications Biology 4 (1): 86. [CrossRef]
- Zelmann, R., A. C. Paulk, F. Tian, G. A. Balanza Villegas, J. Dezha Peralta, B. Crocker, G. R. Cosgrove, et al. 2023. "Differential Cortical Network Engagement during States of Un/Consciousness in Humans." Neuron 111 (21): 3479–3495.e6. [CrossRef]
- Zhang, Jing, Rui-Li Wei, Guo-Ping Peng, Jia-Jia Zhou, Min Wu, Fu-Ping He, Guang Pan, Jun Gao and Bai-Yun Luo. 2017. "Correlations between Diffusion Tensor Imaging and Levels of Consciousness in Patients with Traumatic Brain Injury: A Systematic Review and Meta-Analysis." Scientific Reports 7 (1): 2793. [CrossRef]
- Zhang, Y., J. Zhao, X. Sun, Y. Zheng, T. Chen and Z. Wang. 2025. "Leveraging Independent Component Analysis to Unravel Transcriptional Regulatory Networks: A Critical Review and Future Directions." Biotechnology Advances 78: 108479. [CrossRef]
- Zheng, Zhi Shan, Nicco Reggente, Eric Lutkenhoff, Adrian M. Owen and Martin M. Monti. 2017. "Disentangling Disorders of Consciousness: Insights from Diffusion Tensor Imaging and Machine Learning." Human Brain Mapping 38 (1): 431–43. [CrossRef]
- Zhao, W., H. Li, G. Hu, Y. Hao, Q. Zhang, J. Wu, B. B. Frederick and F. Cong. 2021. "Consistency of Independent Component Analysis for fMRI." Journal of Neuroscience Methods 351: 109013. [CrossRef]
- Zhou, G., Y. Pan, J. Yang, X. Zhang, X. Guo and Y. Luo. 202a. “Sleep Electroencephalographic Response to Respiratory Events in Patients With Moderate Sleep Apnea-Hypopnea Syndrome.” Frontiers in Neuroscience 14: 310. [CrossRef]
- Zhou, Z., B. Cai, G. Zhang, A. Zhang, V. D. Calhoun and Y. P. Wang. 2020b. "Prediction and Classification of Sleep Quality Based on Phase Synchronization Related Whole-Brain Dynamic Connectivity Using Resting State fMRI." NeuroImage 221: 117190. [CrossRef]
- Zuo, C., X. Suo, H. Lan, N. Pan, S. Wang, G. J. Kemp and Q. Gong. 2023. "Global Alterations of Whole Brain Structural Connectome in Parkinson's Disease: A Meta-Analysis." Neuropsychology Review 33 (4): 783–802. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).