1. Introduction
Obesity represents one of the greatest contemporary challenges in global public health, affecting 2.11 billion adults worldwide in 2021 [
1]. To date, no country has effectively halted the increasing prevalence of obesity [
1]. If current trends persist, it is estimated that by 2050 the global population of adults with obesity will reach approximately 3.80 billion, leading to a continued rise in associated non-communicable diseases [
1].
Current therapeutic options for obesity management encompass lifestyle modifications, as well as pharmacological and surgical interventions [
2,
3,
4,
5]. While lifestyle interventions are considered first-line strategies, they often result in modest weight loss and are difficult to sustain due to low adherence and the tendency to regain weight [
6].
Pharmacological treatments are an established component of comprehensive obesity management and are endorsed by current clinical practice guidelines [
3,
4]. Several drug classes have demonstrated clinical efficacy in promoting weight loss, improving outcomes, and decreasing the risk of obesity-related complications, particularly when combined with lifestyle interventions [
7] However, their effectiveness in real-world settings is often limited by factors such as variable individual response, early discontinuation, adverse effects, and barriers to access [
3,
4,
7]. Cost is also a central barrier. According to a recent analysis by the Congressional Budget Office, if Medicare were to authorize coverage for anti-obesity medications (including GLP-1 receptor agonists, GIP/GLP-1 dual agonists, as well as older agents like orlistat, bupropion/naltrexone, and phentermine/topiramate) starting in 2026, federal spending would increase 35 billion dollars over nine years, while expected savings from improved health outcomes would remain comparatively modest [
8]. The projected annual cost per user would substantially exceed the estimated savings during that period, highlighting the economic challenge of scaling these therapies despite their clinical potential [
8].
In low- and middle-income countries, these economic challenges are even more pronounced. A recent review of the Mexican Guidelines for Obesity Management documented major barriers to equitable access to pharmacological treatments. The authors reported that less than 2% of eligible patients in Mexico receive pharmacotherapy [
3]. This limited uptake is attributed to high out-of-pocket costs, lack of insurance coverage, and limited availability in the healthcare sector.3 These obstacles not only reinforce the existing inequities but also underscore the need to identify complementary or alternative strategies that are effective, safe, financially sustainable, and better aligned with the economic realities of the broader population.
Recently, there has been growing interest in nutraceuticals due to their perceived safety profiles and therapeutic benefits [
9,
10]. These products, which combine nutritional and pharmaceutical properties, have gained recognition for their potential to support health and manage various conditions, in addition to their advantages in terms of affordability and accessibility [
9,
10]. This growing demand is reflected in their expanding market [
9].
In 2014, Mancebo-Molina, et al. patented a polysaccharide-rich hydrolysate from
Saccharomyces cerevisiae (brewing yeast), containing a beta-glucan, chitin and chitosan, which was proposed to promote weight loss through its lipid-binding properties [
11]. Several clinical trials subsequently explored this compound with encouraging results [
12,
13,
14,
15,
16,
17,
18]. However, to date, no systematic review has been conducted to evaluate the evidence in humans.
A recent systematic review by Canaan, et al. (2021) concluded that yeast-derived compounds had no significant effect on body weight [
19]. However, this conclusion is limited because the study was focused on weight gain in animals using these compounds as additives on animal feed, preventing extrapolation to clinical practice in humans [
19]. Thus, a clear gap exists in the clinical evaluation of these compounds when used in humans for weight management purposes.
To address this gap, we conducted a systematic review and meta-analysis, evaluating the effectiveness of a polysaccharide derived from Saccharomyces cerevisiae, commercially known as Stetikal® and LipiGo®, aimed at reducing body weight and adiposity. The findings contribute to positioning this yeast-derived compound as a potentially valuable alternative within the broader spectrum of weight-management strategies.
2. Materials and Methods
Protocol and Registration: This systematic review and meta-analysis were conducted in accordance with standard Cochrane protocols and presented in adherence with Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines for completion of this review [
20]. The study was prospectively registered in PROSPERO on March, 2025: “Systematic Review and Meta-Analysis of the Effect of a Polysaccharide-Rich Hydrolysate Derived from
Saccharomyces cerevisiae on Body Weight Loss” (CRD420251024965).
Search Strategy: We searched for studies registered in Pubmed, SciELO, ClinicalTrials.gov, and CENTRAL databases, and consulted EMBASE through CENTRAL database during April 2025. To identify relevant studies, we used the following search terms: ("Saccharomyces cerevisiae" OR "S. cerevisiae" OR "Saccharomyces" OR "Yeast" OR "Bakers yeast") AND ("beta-glucan-chitin-chitosan" OR "β-Glucan/Chitin-Chitosan" OR "BGCC" OR "beta glucan*" OR "beta-glucan*" OR "water-insoluble glucan*" OR "water insoluble glucan*" OR "polysaccharide*" OR "chitin–chitosan" OR "chitin chitosan" OR "chitosan" OR "βGluCnCs" OR "hydrolysate*") OR "LipiGo". Additionally, a snowballing strategy and expert consultations were employed to identify additional studies.
Selection Criteria: Articles were selected based on the Population, Intervention, Comparison, Outcomes, Study Design (PICOS) principle (
Table 1). We specifically targeted blinded, randomized, placebo-controlled clinical trials that used the specific polysaccharide-rich mixture of beta-glucan, chitin, and chitosan derived from a brewing process described by Mancebo-Molina, et al [
11]. for weight loss in adults. Studies were excluded if they involved non-human experiments, used different yeast extracts, or were conducted in children or breastfeeding women.
Data Extraction and Risk of Bias Assessment: Titles and abstracts were independently screened by 5 reviewers (YCJV, ARC, MHR, CTB, and RRE) and cross-checked. Discrepancies were solved through discussion, and additional reviewer resolution strategy was considered, though no discrepancies remained unsolved. Full-text articles or registry data were downloaded for further review. After removing duplicates, studies were independently assessed by 5 reviewers (YCJV, ARC, MHR, CTB, and RRE) following a similar process. Data was extracted into an XML file via a custom in-house tool. Cochrane’s ROB-2 was used to assess the risk of bias in each publication [
21].
Outcomes: Primary outcomes were changes in body weight, fat composition/mass, BMI, and abdominal perimeter at a 10- or 12- week endpoint. All measurements were standardized to international units, with conversions made where necessary. For all outcomes, changes were expressed as differences of means, with standard mean difference (SMD) as a measure of effect size; when SMD was not originally reported, it was extrapolated from available data. Data presented in graphical form was extracted with the Plot Digitizer [
22] tool and dispersion values were extrapolated as needed.
Data Synthesis and Statistical Analysis (Meta-analysis): Meta-analysis was performed using PyMeta [
23]. Continuous variables were entered as means, with SMD as the effect measure and Hedge’s adjusted g as the algorithm using a random effects model.
3. Results
3.1. Study Selection
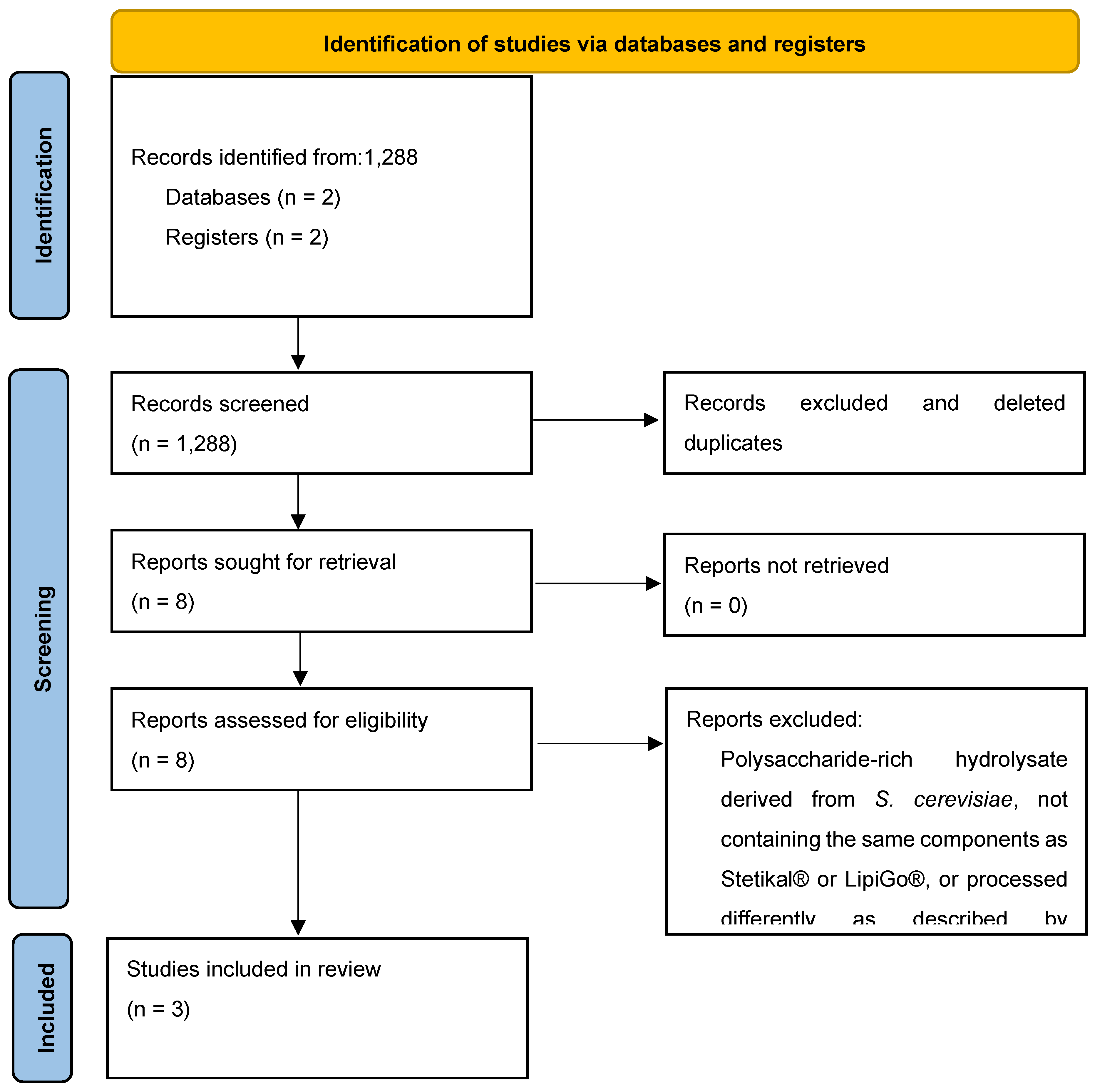
In April 2025 we conducted the previously described strategy, identifying a total of 1,288 abstracts and titles (ClinicalTrials.gov = 40, Pubmed = 70, SciELO = 1055, Central= 123). Following screening and removing duplicates, 1,280 studies did not meet the inclusion criteria. Subsequently, 8 records were kept for full-text review. Only 3 studies were selected for synthesis and meta-analysis, with a pool of 273 patients. Data was extracted in a custom form based on Cochrane’s recommendations and stored in an XML format [
24]. A summary of study selection is displayed in
Figure 1.
3.2. Effect of Polysaccharide-Rich Hydrolysate Derived from Saccharomyces cerevisiae in Body Weight Reduction
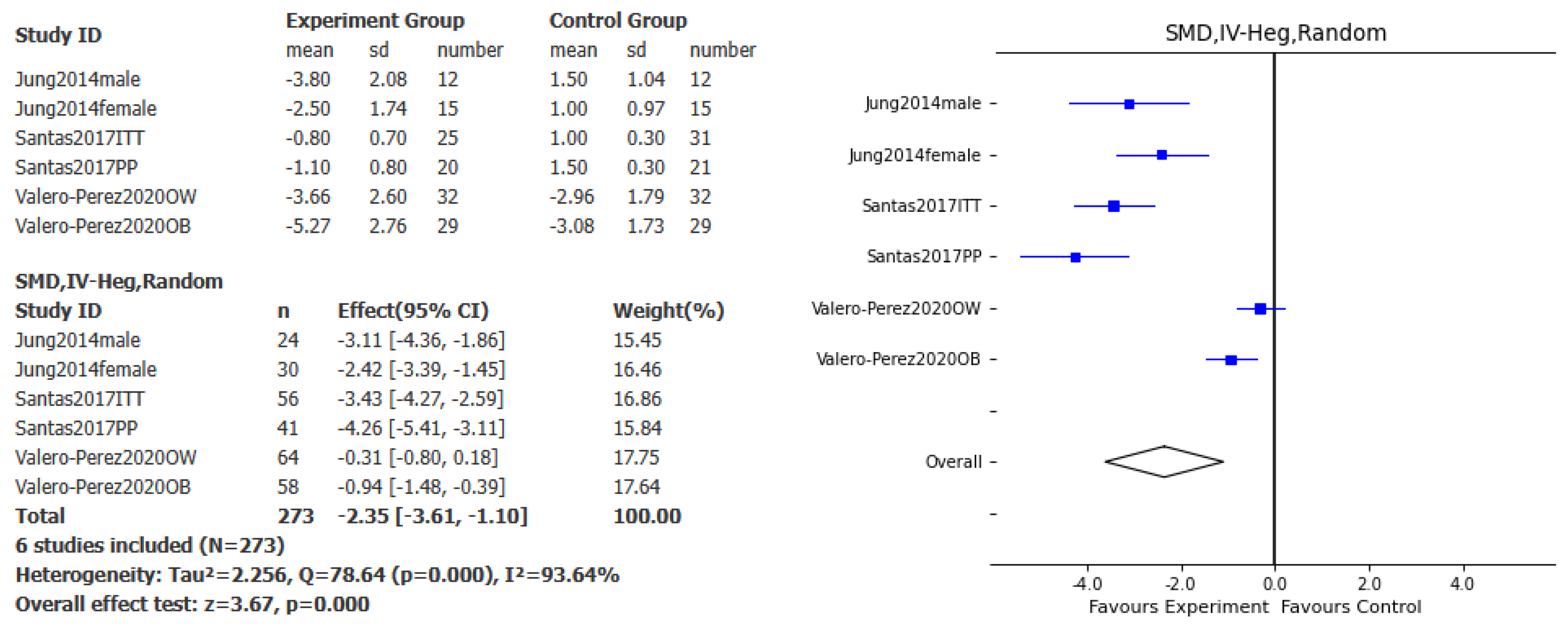
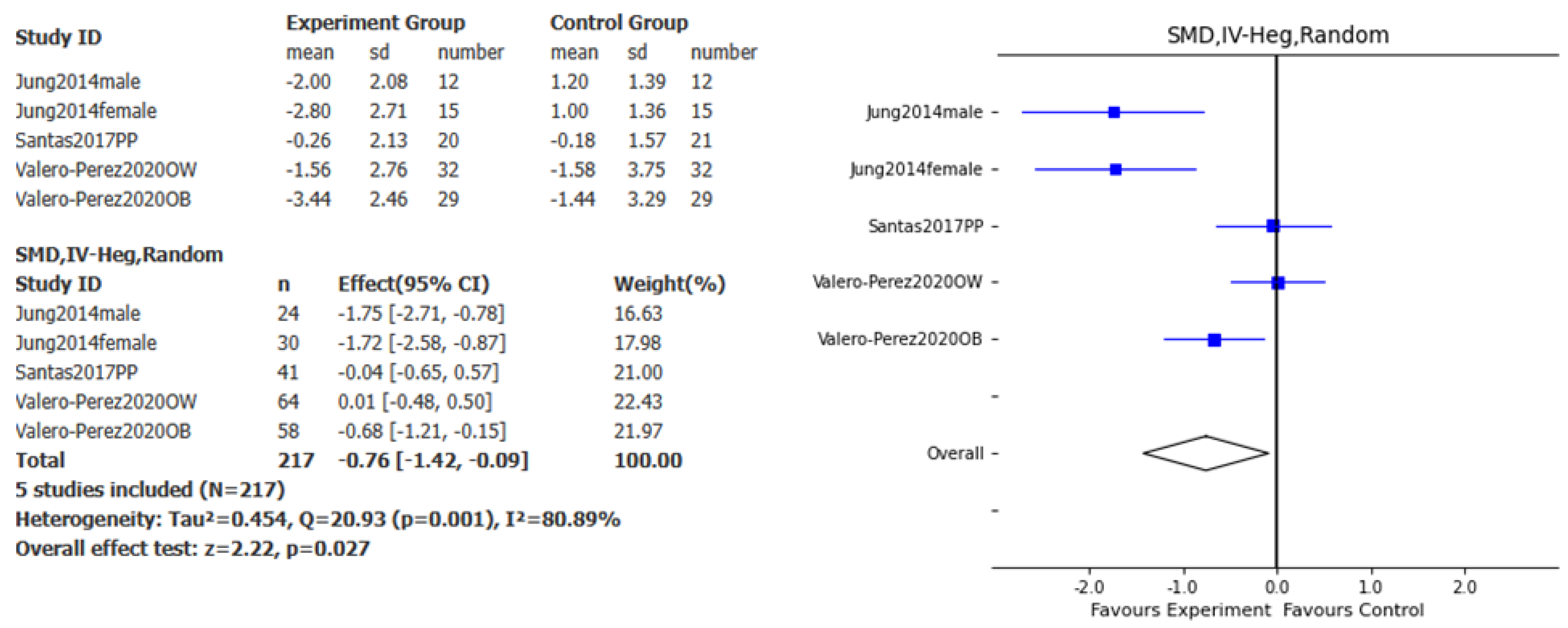
Overall, the effect of this specific Polysaccharide-Rich Hydrolysate on body weight loss was assessed in 3 studies. A significant effect favoring the intervention compared to placebo was found for weight loss (SMD – 2.35kg, 95% CI: -3.61, -1.1, p<0.001, I
2= 93.64%;
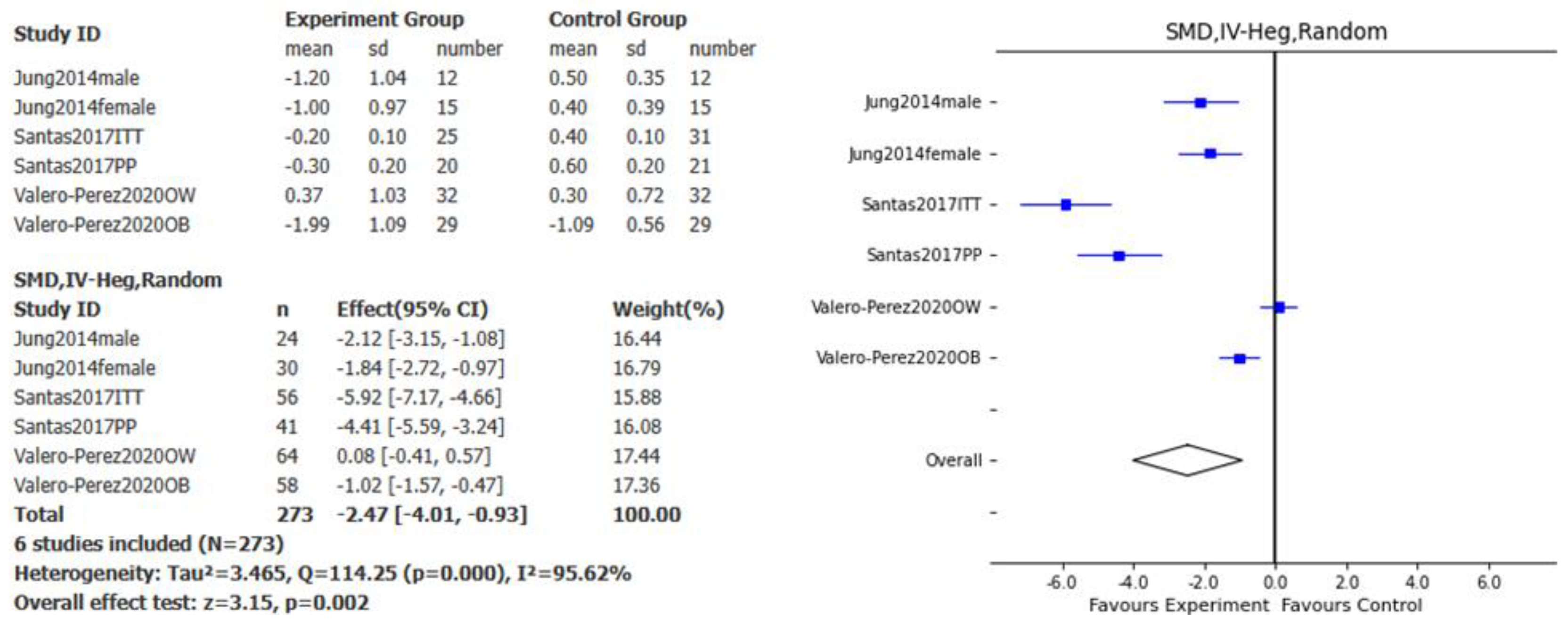
Figure 2), BMI (SMD – 2.47kg/m
2, 95% CI: -4.01, -0.93, p<0.01, I
2= 95.62%;
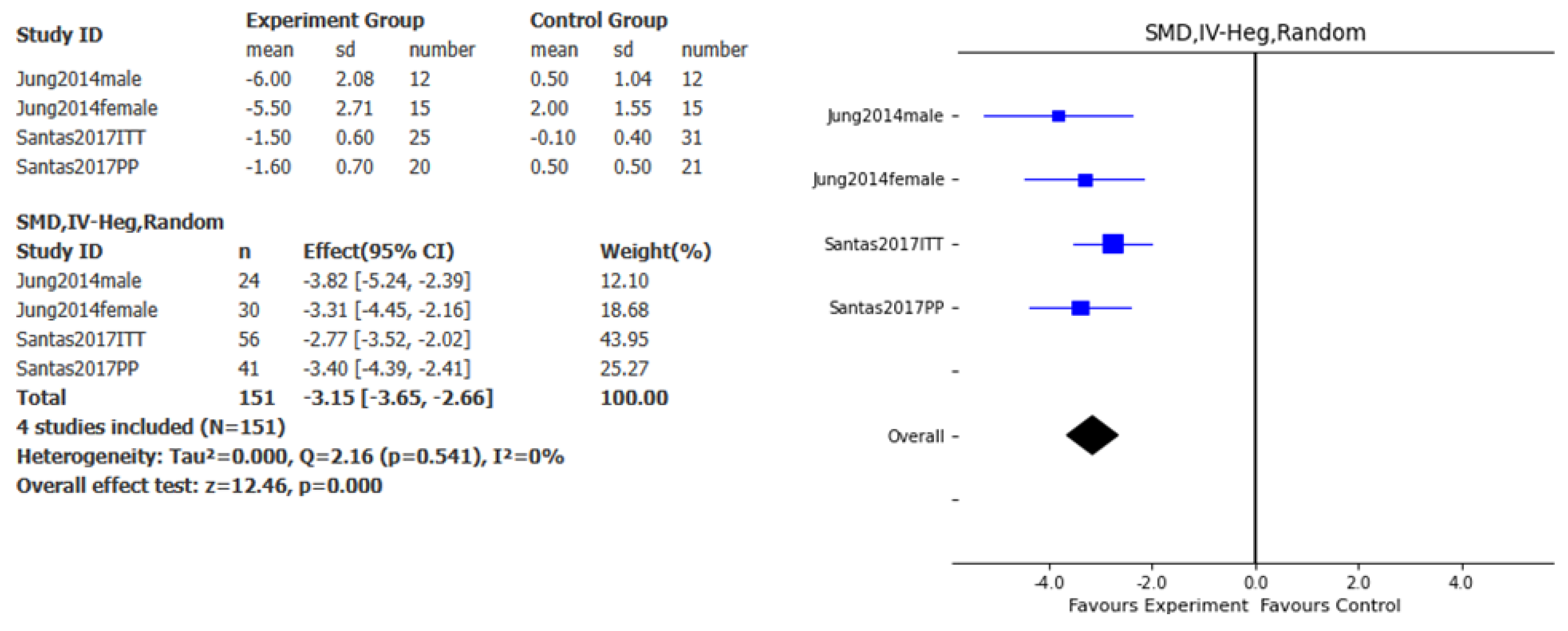
Figure 3), abdominal perimeter (SMD – 3.15cm, 95% CI: - 3.65, -2.66, p<0.001, I
2= 0%;
Figure 4), and fat mass loss (SMD – 0.76kg, 95% CI: - 1.42, -0.09, p<0.05, I
2= 80.89%;
Figure 5).
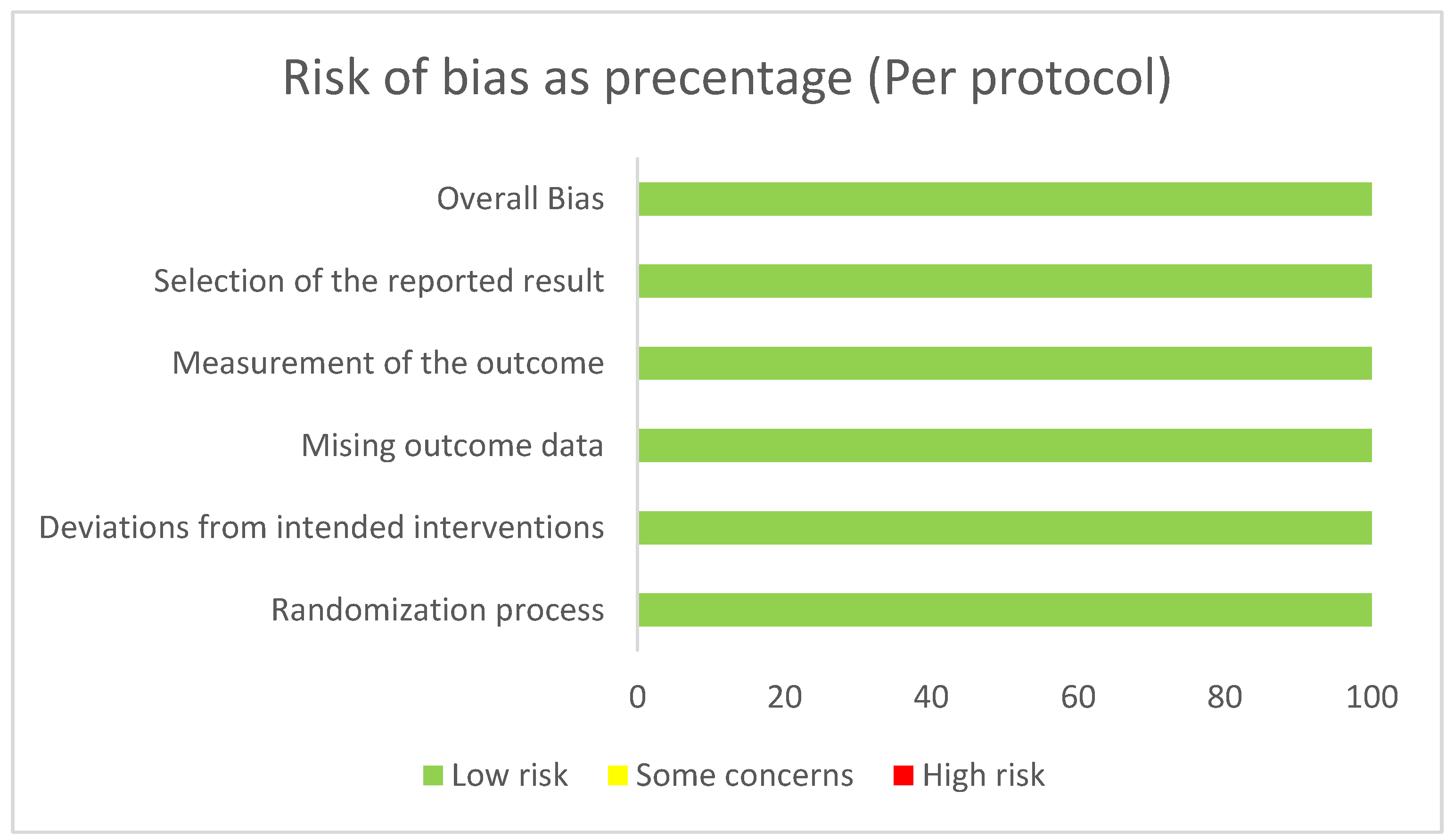
3.3. Risk of Bias
We considered a low risk of bias along all 3 included studies. Results of RoB-2 are displayed in
Figure 6.
4. Discussion
We conducted a systematic review and meta-analysis to evaluate the effects of a commercially available polysaccharide-rich hydrolysate derived from Saccharomyces cerevisiae on body weight reduction. The primary outcomes assessed included changes in body weight, BMI, abdominal circumference, and fat mass over a 10- to 12-week intervention period. Only three studies met the eligibility criteria, as randomized, placebo-controlled clinical trials investigating this specific nutraceutical remain limited. Despite the small number of studies, our meta-analysis revealed a significant favorable effect across all four parameters, with an overall low risk of bias. Notably, high heterogeneity was observed in the meta-analyses of body weight, BMI, and fat mass, likely due to variability in individual study outcomes and the small sample size. To account for this, a random-effects model was applied, which is more appropriate to address results with high heterogeneity.
Several mechanisms by which nutraceuticals contribute to weight management have been documented, including appetite suppression, inhibition of fatty acid synthesis, inhibition of adipocyte differentiation, and activation of lipolysis, among others [
25]. Regarding this
S. cerevisiae-derived hydrolysate, the effect in weight loss may be, at least partially, explained by an appetite-suppressive effect. Previous studies have demonstrated that yeast hydrolysate reduces calorie intake in humans and in animal models [
26,
27]. One rodent study evidenced a lowering in serum ghrelin levels. Although, the effect was not dose-dependent [
27]. In one of the studies included in this systematic review and metanalysis, a clear decrease in calorie intake was demonstrated in patients who received the hydrolysate [
13]. However, this observation was inherently not observable in the other studies due to design of the trials. More studies are required to adequately study the mechanisms underlying the benefits of this intervention.
Notably, a reduction in adiposity was demonstrated through two key measures: fat mass loss and a decrease in abdominal circumference. Excess adiposity is now recognized as a hallmark of clinical and preclinical obesity [
28]. As such, reduction of adiposity can be considered a therapeutic goal for patients with clinical and preclinical obesity. Furthermore, the benefits associated with reducing adiposity may be sufficient to justify the initiation of pharmacological therapy in individuals with preclinical obesity [
28]. Our study found that polysaccharides derived from
S. cerevisiae induced a reduction of 3.15 cm in abdominal circumference and 0.76 kg of fat mass after 10 to 12 weeks of treatment, independent of any additional interventions such as dietary changes or physical activity. Although modest, this reduction is notable, especially considering that semaglutide induces a reduction of 1.5 kg of fat mass or a 3 cm decrease in waist circumference over a similar period of time [
29,
30]. In this context, our findings suggest that polysaccharides from
S. cerevisiae may represent a promising therapeutic option for the reduction of adiposity; either as stand-alone interventions, or as adjuvants with other weight loss interventions such as GLP1 receptor agonists, phentermine, bupropion/topiramate, orlistat, etc. Future studies could be used to test this hypothesis.
Nutraceuticals are bioactive substances present in common food which supply additional benefits to those of providing nutritional components [
10]. They are widely accepted among population in comparison to anti-obesity drugs due to their low toxicity, competitive pricing and lack of requirement of prescription [
25]. Although our study was not designed to assess the safety of this intervention, it has not escaped our attention that minimal adverse events have been reported. For instance, Valero-Pérez, et al. found no differences in adverse events such as bad breath, diarrhea, nausea, and heartburn, when compared to placebo [
16].
While the evidence supporting the benefits of nutraceuticals in obesity management continues to grow, few meta-analyses have evaluated the clinical efficacy of such interventions [
25,
31]. Our study stands out for its rigorous methodology, as it closely followed the recommendations outlined by Cochrane and PRISMA guidelines. Moreover, the studies included in this systematic review and meta-analysis were well-designed, placebo-controlled, appropriately blinded, and demonstrated a low risk of bias. Importantly, our results revealed statistically significant improvements across all assessed parameters. Nevertheless, this study has several limitations. Substantial heterogeneity was observed, likely due to variability in study outcomes and the limited number of included trials. Moreover, there is a lack of head-to-head comparisons among this nutraceutical with drugs usually prescribed for weight loss. Additionally, important outcomes such as patient satisfaction, adverse events, and long-term efficacy were not available for inclusion in the meta-analysis. Future research should aim to address these gaps. Finally, it is noteworthy that no studies involving Latin American populations have been published to date. Post-marketing surveillance and region-specific studies may help clarify the effectiveness and generalizability of these findings in diverse populations.
5. Conclusions
The results of our systematic review and meta-analysis reveal that an intervention with a Polysaccharide-Rich Hydrolysate Derived from Saccharomyces cerevisiae (Stetikal®, LipiGo®) is consistently effective for reducing adiposity, BMI and inducing weight loss in a 10- to 12-week period. Therefore, S. cerevisiae-derived polysaccharides offer a promising therapeutic option for reducing adiposity in patients with preclinical and clinical obesity. Future post marketing studies may provide better evidence of the real-world benefits of this intervention.
Author Contributions
Conceptualization: A.A.P.-G., B.C.-T. and E.R.-R; data curation: J.V.Y.-C., C.A.-R., R.M.H., B.C.-T. and E.R.-R; formal analysis: B.C.-T. and E.R.-R; project administration: A.A.P.-G. and R.M.H.; software, B.C.-T. and E.R.-R; writing—original draft: A.A.P.-G., J.V.Y.-C., C.A.-R., R.M.H., B.C.-T. and E.R.-R; writing—review and editing: A.A.P.-G., J.V.Y.-C., C.A.-R., R.M.H., B.C.-T. and E.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Laboratorios Columbia Comercial, S.A. de C.V., through unrestricted sponsorship. The article processing charge (APC) was also funded by Laboratorios Columbia Comercial, S.A. de C.V. The sponsor had no influence on this study’s methodology, results, or discussion.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors wish to express their sincere gratitude for the unrestricted financial and institutional support provided by Lic. Manuel Martínez Domínguez (President), Lic. Armando del Ángel Murbartian (General Director), and Lic. Mariana Ptacnik Roca (Farma Business Unit Manager) at Laboratorios Columbia. Their invaluable contributions were essential to the completion and publication of this systematic review.
Conflicts of Interest
Palacios-García AA and Moreno-Higareda R, are full time employees of Laboratorios Columbia. Cerón-Trujillo B holds shares at Aequitas Medica. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI |
Body Mass Index |
| CI |
Confidence Interval |
| GIP |
Glucose-dependent Insulinotropic Polypeptide |
| GLP-1 |
Glucagon-Like Peptide-1 |
| ITT |
Intention-To-Treat |
| PICOS |
Population, Intervention, Comparison, Outcomes, Study Design |
| PP |
Per Protocol |
| RoB-2 |
Risk of Bias Tool Version 2 |
| SMD |
Standarized Mean Difference |
| XML |
Extensible Markup Language |
References
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. The Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol 2025. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Manzanera, E.A.; Vera-Zertuche, J.M.; Kaufer-Horwitz, M.; Vázquez-Velázquez, V.; Flores-Lázaro, J.R.; Mireles-Zavala, L.; Calzada-León, R.; Garnica-Cuellar, J.C.; Sánchez-Muñoz, V.; Ramírez-Butanda, E.; et al. Mexican Clinical Practice Guidelines for Adult Overweight and Obesity Management. Curr Obes Rep 2024. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, C.J.; Cooper, K.; Stephens, L.D.; Peeters, A.; Salmon, J.; Porter, J. Clinical Practice Guidelines for the Management of Overweight and Obesity Published Internationally: A Scoping Review. Obesity Reviews 2024, 25. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surgery for Obesity and Related Diseases 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Kheniser, K.; Saxon, D.R.; Kashyap, S.R. Long-Term Weight Loss Strategies for Obesity. Journal of Clinical Endocrinology and Metabolism 2021, 106, 1854–1866. [Google Scholar] [CrossRef]
- Grunvald, E.; Shah, R.; Hernaez, R.; Chandar, A.K.; Pickett-Blakely, O.; Teigen, L.M.; Harindhanavudhi, T.; Sultan, S.; Singh, S.; Davitkov, P. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology 2022, 163, 1198–1225. [Google Scholar] [CrossRef] [PubMed]
- Congressional Budget Office How Would Authorizing Medicare to Cover Anti-Obesity Medications Affect the Federal Budget? Available online: https://www.cbo.gov/publication/60816 (accessed on 9 April 2025).
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Alali, M.; Alqubaisy, M.; Aljaafari, M.N.; Alali, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.H.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Mancebo-Molina, R.; Castañe-Sitjas, F.X.; Cuñé-Castellana, J.; SAntas-Gutiérrez, J.; Rafecas-Martínez, M.; Miralles-Buraglia, M.Á.; Mateos-Aparicio-Cediel, I.; Heras-Caballero, Á.M. LIGANDO DE GRASAS OBTENIDO A PARTIR DE LA BIOMASA DEL PROCEDIMIENTO DE ELABORACIÓN DE LA CERVEZA. 2014; 1–52. [Google Scholar]
- Santamarina, A.B.; Nehmi Filho, V.; de Freitas, J.A.; da Silva, B.F.R.B.; Gusmão, A.F.; Olivieri, E.H.R.; de Souza, E.; da Silva, S.L.; de Miranda, D.A.; Demarque, D.P.; et al. Nutraceutical Composition (Yeast β-Glucan, Prebiotics, Minerals, and Silymarin) Predicts Improvement of Sleep Quality and Metabolic Parameters: A Randomized Pilot Study. Clin Nutr ESPEN 2024, 63, 476–490. [Google Scholar] [CrossRef]
- Jung, E.Y.; Cho, M.K.; Hong, Y.H.; Kim, J.H.; Park, Y.; Chang, U.J.; Suh, H.J. Yeast Hydrolysate Can Reduce Body Weight and Abdominal Fat Accumulation in Obese Adults. Nutrition 2014, 30, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Spigoni, V.; Aldigeri, R.; Antonini, M.; Micheli, M.M.; Fantuzzi, F.; Fratter, A.; Pellizzato, M.; Derlindati, E.; Zavaroni, I.; Bonadonna, R.C.; et al. Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- Nehmi-Filho, V.; Santamarina, A.B.; de Freitas, J.A.; Trarbach, E.B.; de Oliveira, D.R.; Palace-Berl, F.; de Souza, E.; de Miranda, D.A.; Escamilla-Garcia, A.; Otoch, J.P.; et al. Novel Nutraceutical Supplements with Yeast β-Glucan, Prebiotics, Minerals, and Silybum Marianum (Silymarin) Ameliorate Obesity-Related Metabolic and Clinical Parameters: A Double-Blind Randomized Trial. Front Endocrinol (Lausanne) 2023, 13. [Google Scholar] [CrossRef]
- Valero-Pérez, M.; Bermejo, L.M.; López-Plaza, B.; García, M.A.; Palma-Milla, S.; Gómez-Candela, C. Regular Consumption of LIPIGO® Promotes the Reduction of Body Weight and Improves the Rebound Effect of Obese People Undergo a Comprehensive Weight Loss Program. Nutrients 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Santas, J.; Lázaro, E.; Cuñé, J. Effect of a Polysaccharide-Rich Hydrolysate from Saccharomyces Cerevisiae (LipiGo®) in Body Weight Loss: Randomised, Double-Blind, Placebo-Controlled Clinical Trial in Overweight and Obese Adults. J Sci Food Agric 2017, 97, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Mosikanon, K.; Arthan, D.; Kettawan, A.; Tungtrongchitr, R.; Prangthip, P. Yeast β–Glucan Modulates Inflammation and Waist Circumference in Overweight and Obese Subjects. J Diet Suppl 2017, 14, 173–185. [Google Scholar] [CrossRef]
- Canaan, M.M.; Reis-Canaan, J.C.; Zangerônimo, M.G.; Andrade, E.F.; Gonçalves, T.M.S.V.; Pereira, M.C.A.; Lima, R.R.; Pardi, V.; Murata, R.M.; Pereira, L.J. Yeast Beta-Glucans Ingestion Does Not Influence Body Weight: A Systematic Review and Meta-Analysis of Pre-Clinical Studies. Nutrients 2021, 13, 4250. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. The BMJ 2021, 372. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, l4898. [Google Scholar] [CrossRef]
- Portbital PlotDigitizer 3.1.6.
- Deng, H. PyMeta.
- Li, T.; Higgins, J.; Deeks, J. Chapter 5: Collecting Data. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane, 2024.
- Vrânceanu, M.; Hegheş, S.C.; Cozma-Petruţ, A.; Banc, R.; Stroia, C.M.; Raischi, V.; Miere, D.; Popa, D.S.; Filip, L. Plant-Derived Nutraceuticals Involved in Body Weight Control by Modulating Gene Expression. Plants 2023, 12. [Google Scholar] [CrossRef]
- Jung, E.Y.; Lee, J.W.; Hong, Y.H.; Chang, U.J.; Suh, H.J. Low Dose Yeast Hydrolysate in Treatment of Obesity and Weight Loss. Prev Nutr Food Sci 2017, 22, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.B.; Jung, E.Y.; Kim, J.H.; Chang, U.J.; Suh, H.J. Yeast Hydrolysate as a Functional Anti-Obesity Ingredient: Appetite Suppressive Effects of Yeast Hydrolysate in Food Deprived Mice. Progress in Nutrition 2015, 17, 262–264. [Google Scholar]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol 2025. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Sada, Y.; Mihara, S.; Sasaki, Y.; Sone, M.; Tanaka, Y. Oral Semaglutide Induces Loss of Body Fat Mass Without Affecting Muscle Mass in Patients With Type 2 Diabetes. J Clin Med Res 2023, 15, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-W.; Cho, Y.M.; Kim, S.G.; Ko, S.-H.; Lim, S.; Dahaoui, A.; Jeong, J.S.; Lim, H.J.; Yu, J.M. Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin as Metformin Add-on in a Korean Population with Type 2 Diabetes. Diabetes Therapy 2024, 15, 547–563. [Google Scholar] [CrossRef]
- Ammendola, S.; Scotto d’Abusco, A. Nutraceuticals and the Network of Obesity Modulators. Nutrients 2022, 14. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).