Submitted:
07 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
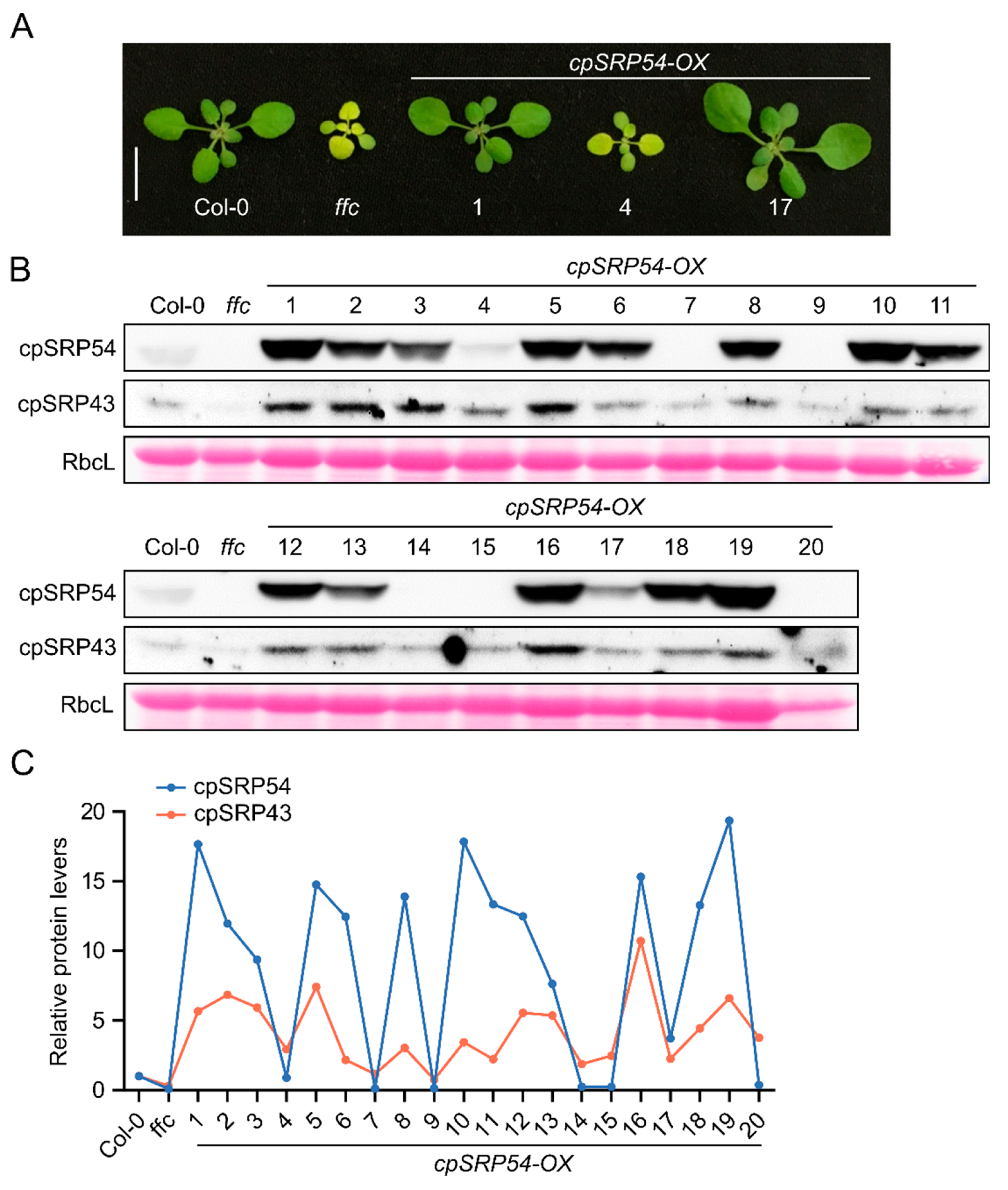
2.1. Correlation of cpSRP43 and cpSRP54 Abundance in cpSRP54 Overexpression Lines
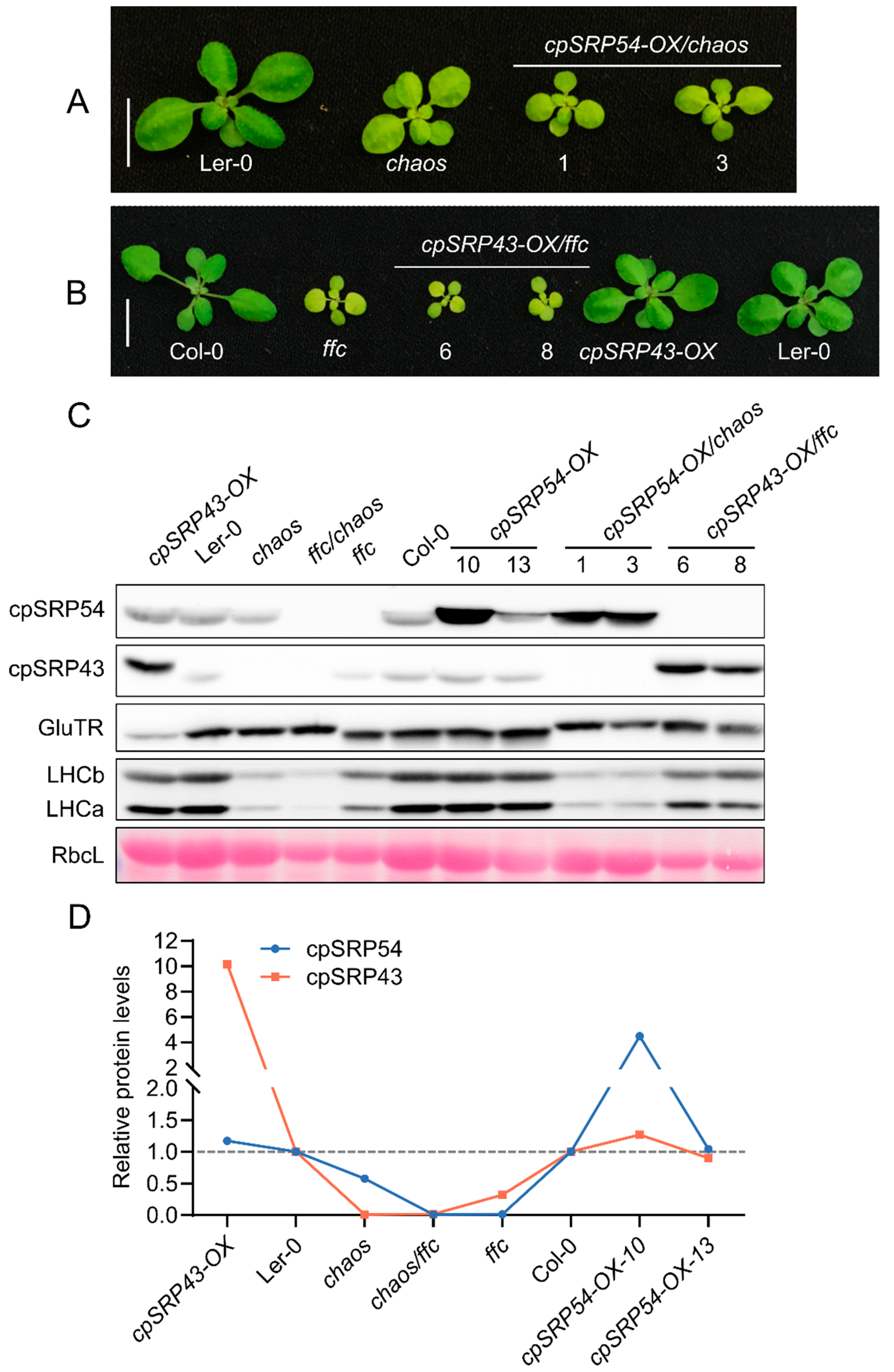
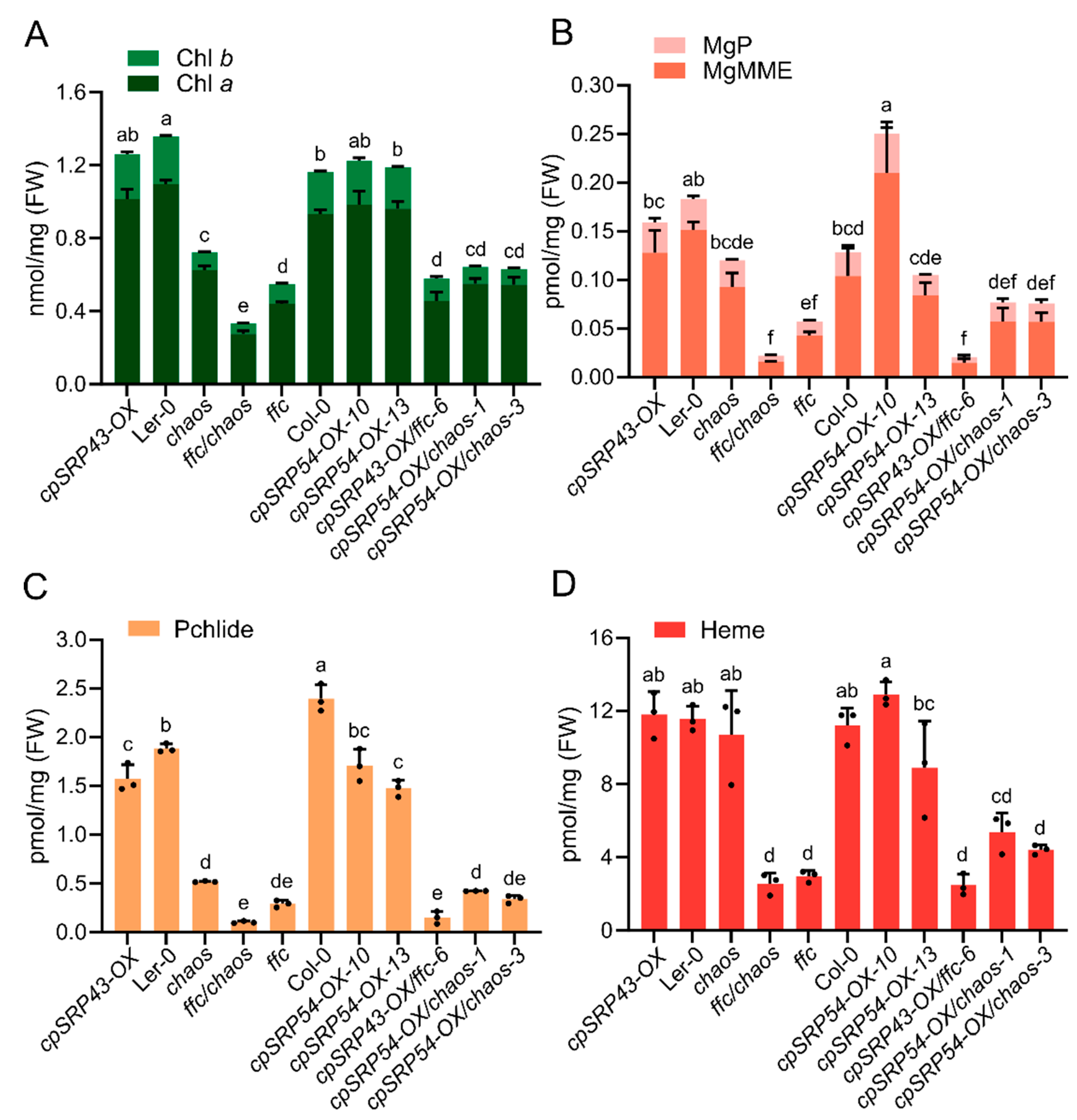
2.2. Disruption of the Correlation Between cpSRP43 and cpSRP54 Abundance Impairs Plant Growth
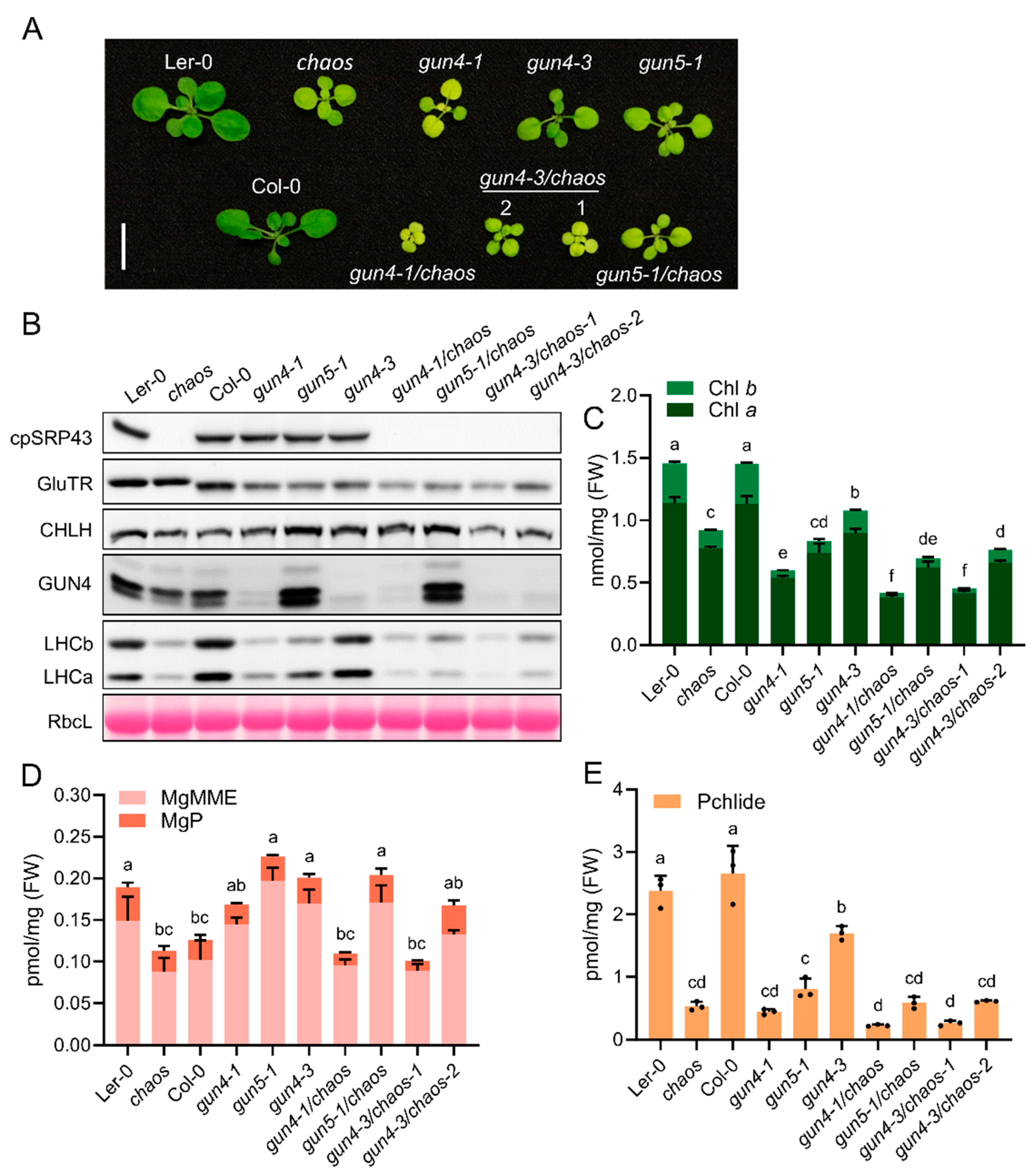
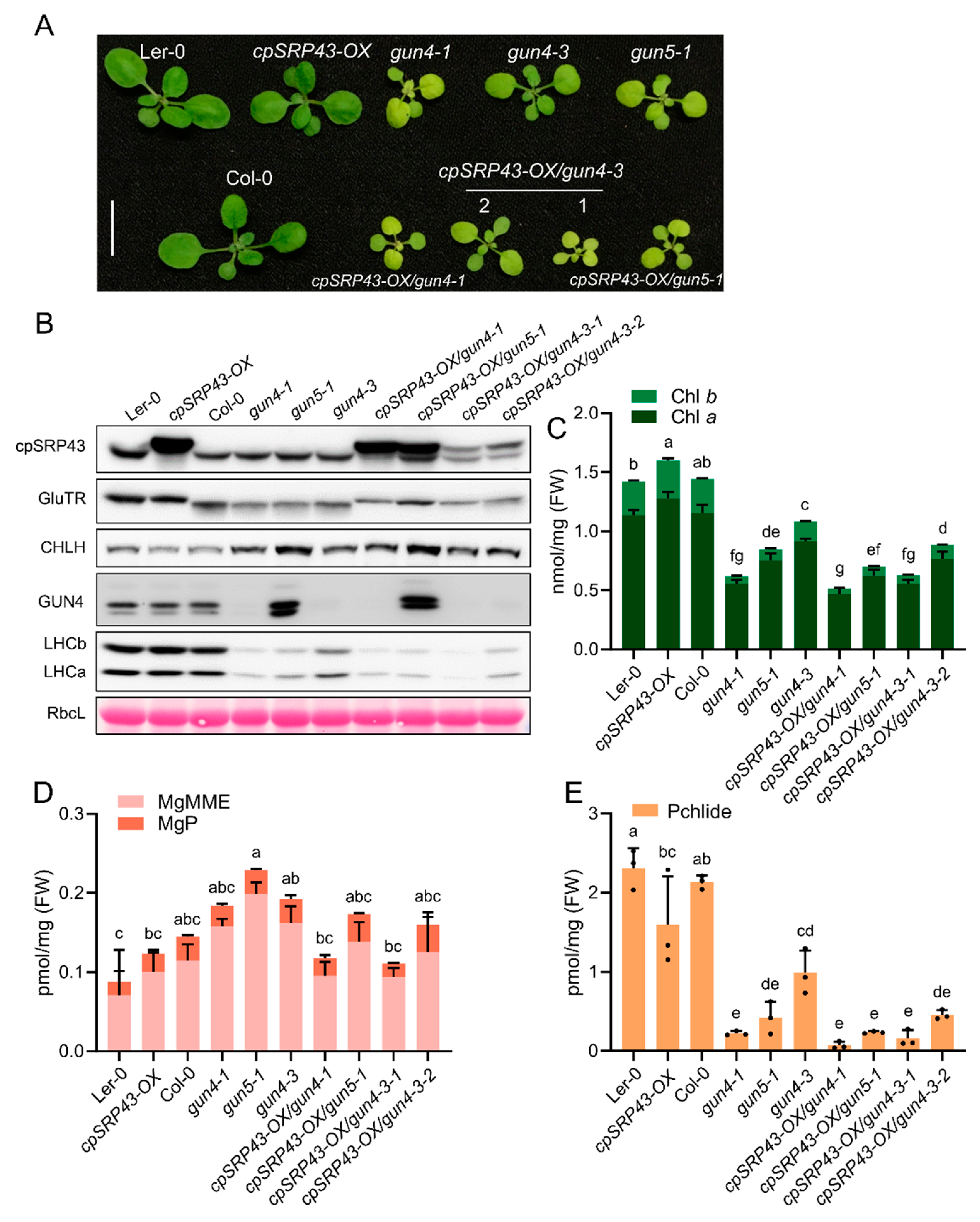
2.3. Double Mutants for cpSRP43 and GUN4/GUN5 are Severely Growth-Retarded and Impaired in Tetrapyrrole Biosynthesis
3. Discussion
3.1. Interdependence of cpSRP43 and cpSRP54 Abundance
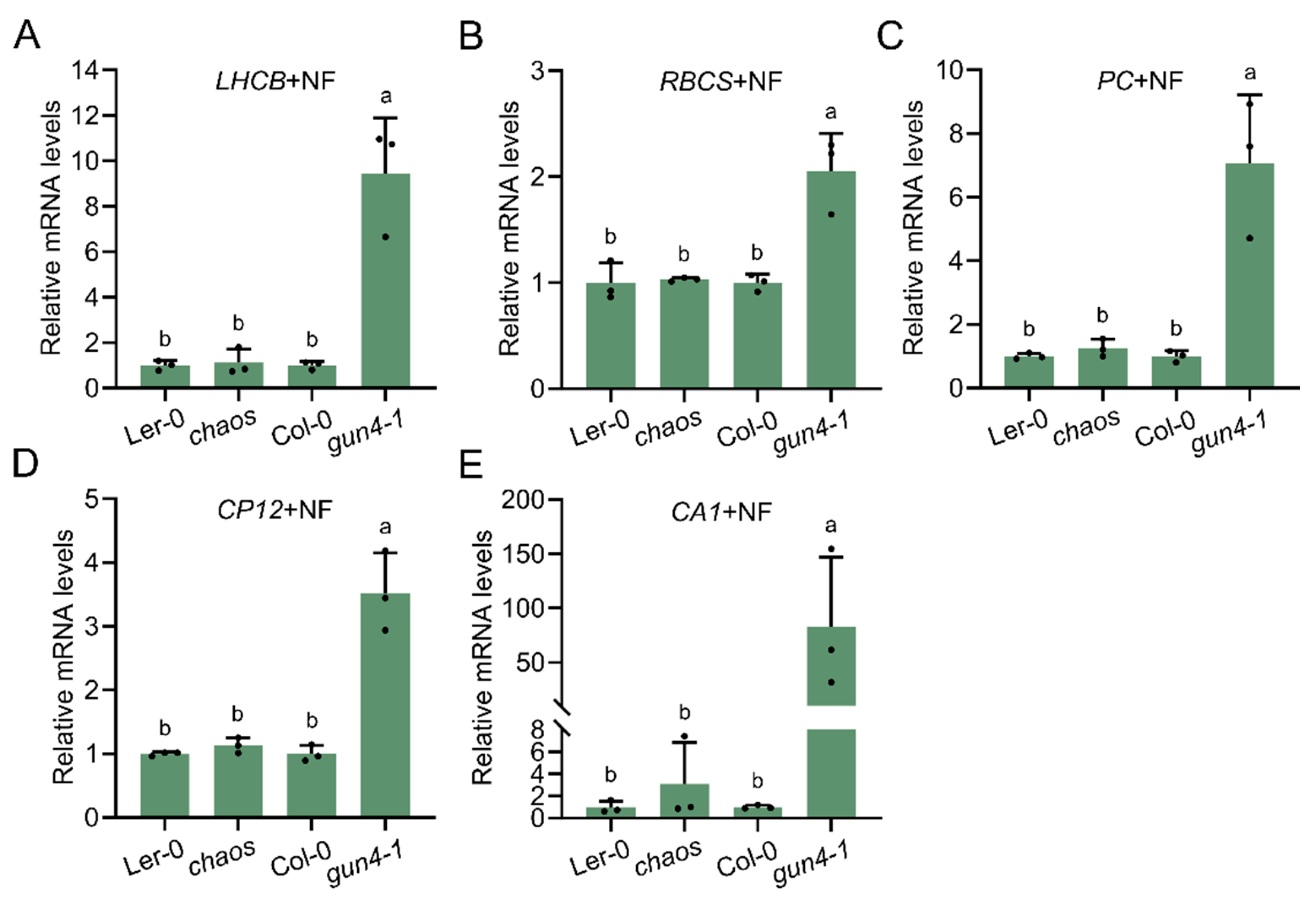
3.2. Synergistic Defects in cpSRP and GUN4/GUN5 Double Mutants
3.5. Coordination of Chloroplast Biogenesis and Future Directions
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Crossing of Arabidopsis thaliana
4.3. Analysis of TBS Intermediates and End-Products
4.4. Norflurazon Treatment
4.5. RNA Extraction and qRT-PCR
4.6. Protein Extraction and Western-Blot Analysis
4.7. Image Processing and Graphic Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franklin, A.; Hoffman, N. Characterization of a chloroplast homologue of the 54-kDa subunit of the signal recognition particle. Journal of Biological Chemistry 1993, 268, 22175–22180. [Google Scholar] [CrossRef] [PubMed]
- Klimyuk, V.I.; Persello-Cartieaux, F.; Havaux, M.; Contard-David, P.; Schuenemann, D.; Meiherhoff, K.; Gouet, P.; Jones, J.D.; Hoffman, N.E.; Nussaume, L. A chromodomain protein encoded by the Arabidopsis CAO gene is a plant-specific component of the chloroplast signal recognition particle pathway that is involved in LHCP targeting. The Plant cell 1999, 11, 87–99. [Google Scholar] [CrossRef]
- Schuenemann, D.; Gupta, S.; Persello-Cartieaux, F.; Klimyuk, V.I.; Jones, J.D.; Nussaume, L.; Hoffman, N.E. A novel signal recognition particle targets light-harvesting proteins to the thylakoid membranes. Proceedings of the National Academy of Sciences 1998, 95, 10312–10316. [Google Scholar] [CrossRef]
- Tu, C.J.; Peterson, E.C.; Henry, R.; Hoffman, N.E. The L18 domain of light-harvesting chlorophyll proteins binds to chloroplast signal recognition particle 43. The Journal of biological chemistry 2000, 275, 13187–13190. [Google Scholar] [CrossRef]
- DeLille, J.; Peterson, E.C.; Johnson, T.; Moore, M.; Kight, A.; Henry, R. A novel precursor recognition element facilitates posttranslational binding to the signal recognition particle in chloroplasts. Proceedings of the National Academy of Sciences 2000, 97, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Goforth, R.L.; Mori, H.; Henry, R. Functional interaction of chloroplast SRP/FtsY with the ALB3 translocase in thylakoids: substrate not required. J Cell Biol 2003, 162. [Google Scholar] [CrossRef] [PubMed]
- Jaru-Ampornpan, P.; Chandrasekar, S.; Shan, S.O. Efficient interaction between two GTPases allows the chloroplast SRP pathway to bypass the requirement for an SRP RNA. Mol. Biol. Cell 2007, 18. [Google Scholar] [CrossRef]
- Falk, S.; Sinning, I. cpSRP43 is a novel chaperone specific for light-harvesting chlorophyll a,b-binding proteins. The Journal of biological chemistry 2010, 285, 21655–21661. [Google Scholar] [CrossRef]
- Falk, S.; Ravaud, S.; Koch, J.; Sinning, I. The C terminus of the Alb3 membrane insertase recruits cpSRP43 to the thylakoid membrane. Journal of Biological Chemistry 2010, 285, 5954–5962. [Google Scholar] [CrossRef]
- Horn, A.; Hennig, J.; Ahmed, Y.L.; Stier, G.; Wild, K.; Sattler, M.; Sinning, I. Structural basis for cpSRP43 chromodomain selectivity and dynamics in Alb3 insertase interaction. Nature communications 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Nilsson, R.; Brunner, J.; Hoffman, N.E.; van Wijk, K.J. Interactions of ribosome nascent chain complexes of the chloroplast-encoded D1 thylakoid membrane protein with cpSRP54. The EMBO journal 1999. [Google Scholar] [CrossRef]
- Hristou, A.; Gerlach, I.; Stolle, D.S.; Neumann, J.; Bischoff, A.; Dunschede, B.; Nowaczyk, M.M.; Zoschke, R.; Schunemann, D. Ribosome-Associated Chloroplast SRP54 Enables Efficient Cotranslational Membrane Insertion of Key Photosynthetic Proteins. The Plant cell 2019, 31, 2734–2750. [Google Scholar] [CrossRef]
- Lei, Y.; Li, B.; Wang, X.; Wei, J.; Wang, P.; Zhao, J.; Yu, F.; Qi, Y. Chloroplast SRP54 and FtsH protease coordinate thylakoid membrane-associated proteostasis in Arabidopsis. Plant physiology 2023, 192, 2318–2335. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liang, F.C.; Wittmann, D.; Siegel, A.; Shan, S.O.; Grimm, B. Chloroplast SRP43 acts as a chaperone for glutamyl-tRNA reductase, the rate-limiting enzyme in tetrapyrrole biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 2018, 115, E3588–E3596. [Google Scholar] [CrossRef]
- Ji, S.; Siegel, A.; Shan, S.-o.; Grimm, B.; Wang, P. Chloroplast SRP43 autonomously protects chlorophyll biosynthesis proteins against heat shock. Nature plants 2021, 7, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Grimm, B.; Wang, P. Chloroplast SRP43 and SRP54 independently promote thermostability and membrane binding of light-dependent protochlorophyllide oxidoreductases. The Plant Journal 2023, 115, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Susek, R.E.; Ausubel, F.M.; Chory, J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 1993, 74, 787–799. [Google Scholar] [CrossRef]
- Mochizuki, N.; Brusslan, J.A.; Larkin, R.; Nagatani, A.; Chory, J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proceedings of the National Academy of sciences 2001, 98, 2053–2058. [Google Scholar] [CrossRef]
- Larkin, R.M.; Alonso, J.M.; Ecker, J.R.; Chory, J. GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 2003, 299, 902–906. [Google Scholar] [CrossRef]
- Woodson, J.D.; Perez-Ruiz, J.M.; Chory, J. Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Current Biology 2011, 21, 897–903. [Google Scholar] [CrossRef]
- Hutin, C.; Havaux, M.; Carde, J.P.; Kloppstech, K.; Meiherhoff, K.; Hoffman, N.; Nussaume, L. Double mutation cpSRP43--/cpSRP54-- is necessary to abolish the cpSRP pathway required for thylakoid targeting of the light-harvesting chlorophyll proteins. The Plant journal : for cell and molecular biology 2002, 29, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Amin, P.; Sy, D.A.; Pilgrim, M.L.; Parry, D.H.; Nussaume, L.; Hoffman, N.E. Arabidopsis mutants lacking the 43-and 54-kilodalton subunits of the chloroplast signal recognition particle have distinct phenotypes. Plant physiology 1999, 121, 61–70. [Google Scholar] [CrossRef]
- Ziehe, D.; Dünschede, B.; Schünemann, D. From bacteria to chloroplasts: evolution of the chloroplast SRP system. Biological chemistry 2017, 398, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Davison, P.A.; Schubert, H.L.; Reid, J.D.; Iorg, C.D.; Heroux, A.; Hill, C.P.; Hunter, C.N. Structural and biochemical characterization of Gun4 suggests a mechanism for its role in chlorophyll biosynthesis. Biochemistry 2005, 44, 7603–7612. [Google Scholar] [CrossRef] [PubMed]
- Tarahi Tabrizi, S.; Sawicki, A.; Zhou, S.; Luo, M.; Willows, R.D. GUN4-Protoporphyrin IX Is a Singlet Oxygen Generator with Consequences for Plastid Retrograde Signaling *. Journal of Biological Chemistry 2016, 291, 8978–8984. [Google Scholar] [CrossRef]
- Espineda, C.E.; Linford, A.S.; Devine, D.; Brusslan, J.A. The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyll b synthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences 1999, 96, 10507–10511. [Google Scholar] [CrossRef]
- Paulsen, H.; Finkenzeller, B.; Kühlein, N. Pigments induce folding of light-harvesting chlorophyll a/b-binding protein. Eur J Biochem 1993, 215, 809–816. [Google Scholar] [CrossRef]
- Yu, B.; Gruber, M.Y.; Khachatourians, G.G.; Zhou, R.; Epp, D.J.; Hegedus, D.D.; Parkin, I.A.; Welsch, R.; Hannoufa, A. Arabidopsis cpSRP54 regulates carotenoid accumulation in Arabidopsis and Brassica napus. Journal of experimental botany 2012, 63, 5189–5202. [Google Scholar] [CrossRef]
- Peter, E.; Grimm, B. GUN4 is required for posttranslational control of plant tetrapyrrole biosynthesis. Molecular plant 2009, 2, 1198–1210. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.W.; Geimer, S.; Grimm, B. Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nature communications 2020, 11, 1254. [Google Scholar] [CrossRef]
- Onate-Sanchez, L.; Vicente-Carbajosa, J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes 2008, 1, 93. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




