Submitted:
06 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
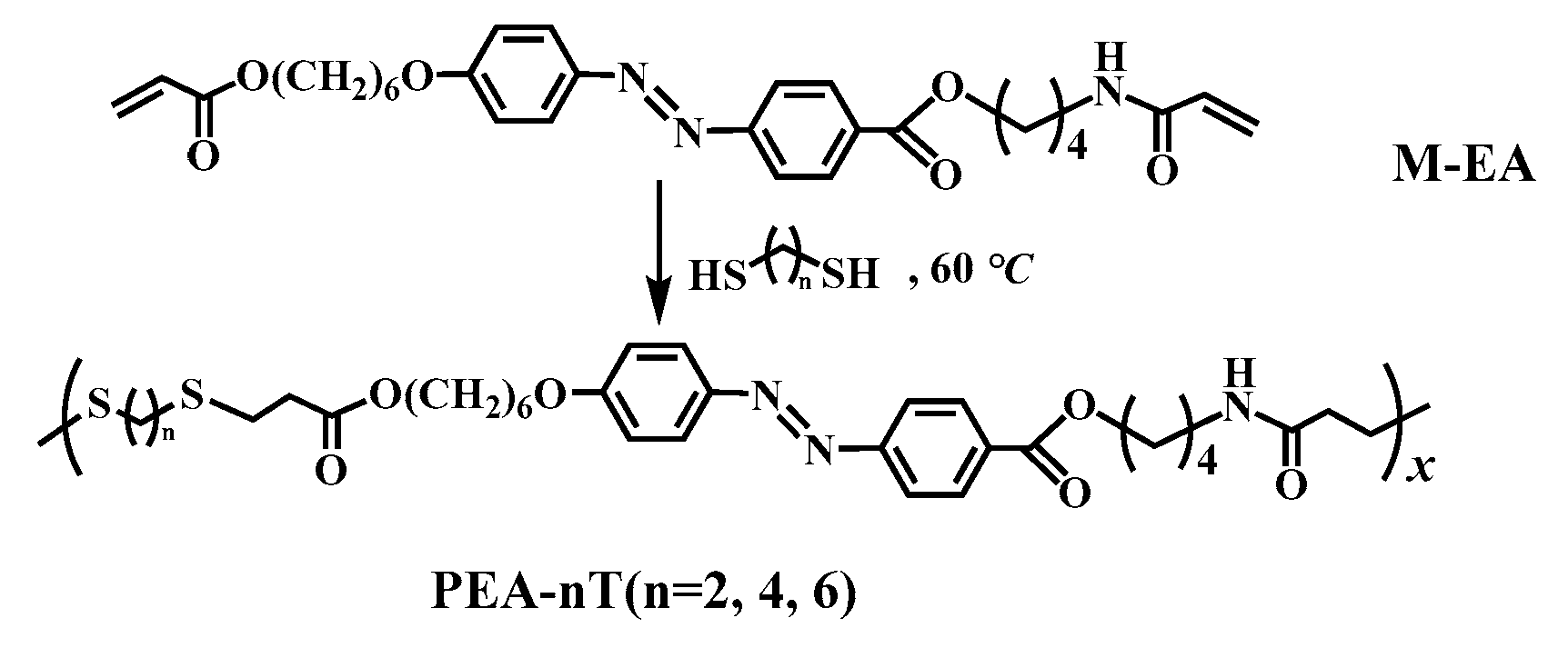
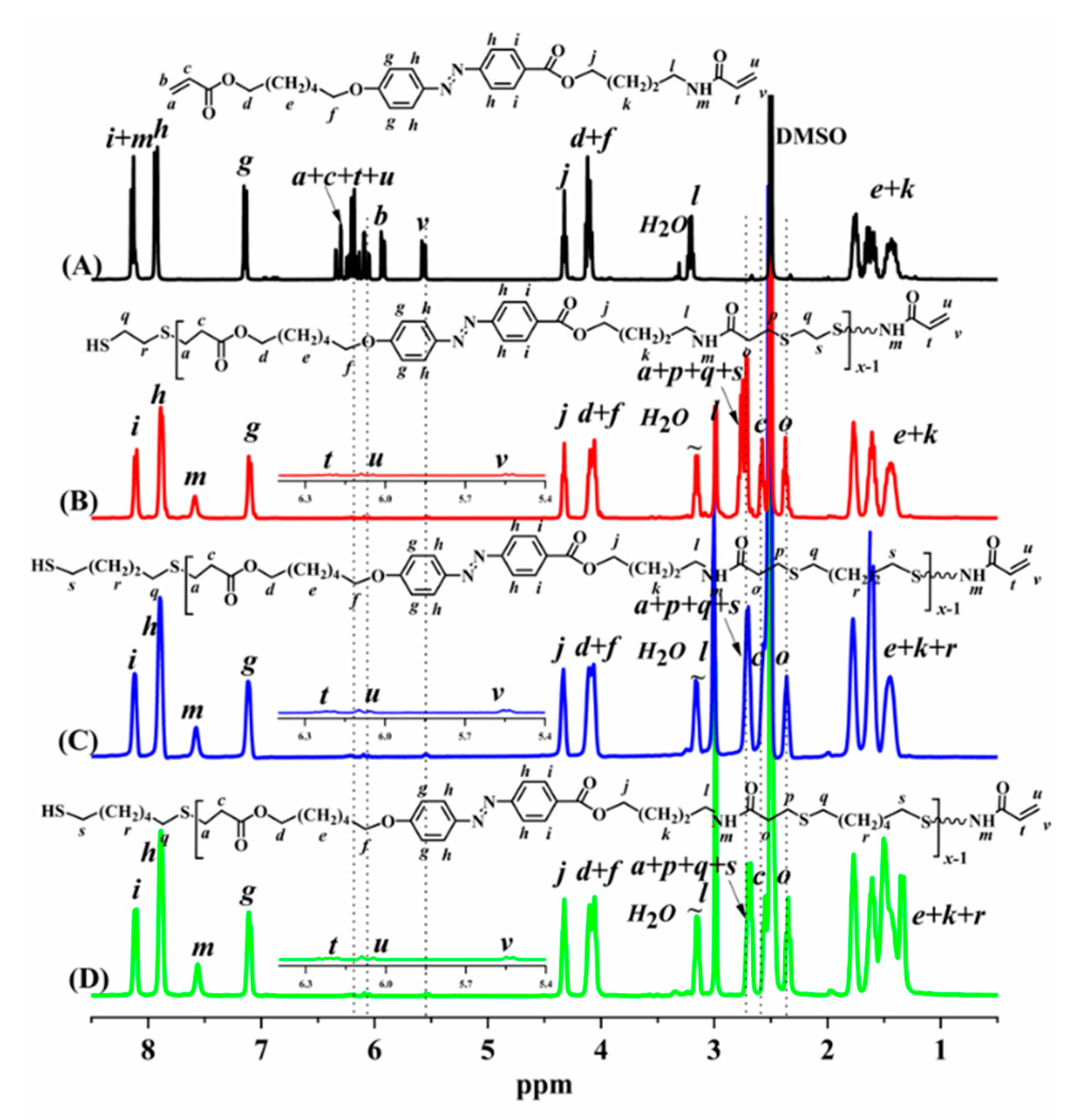
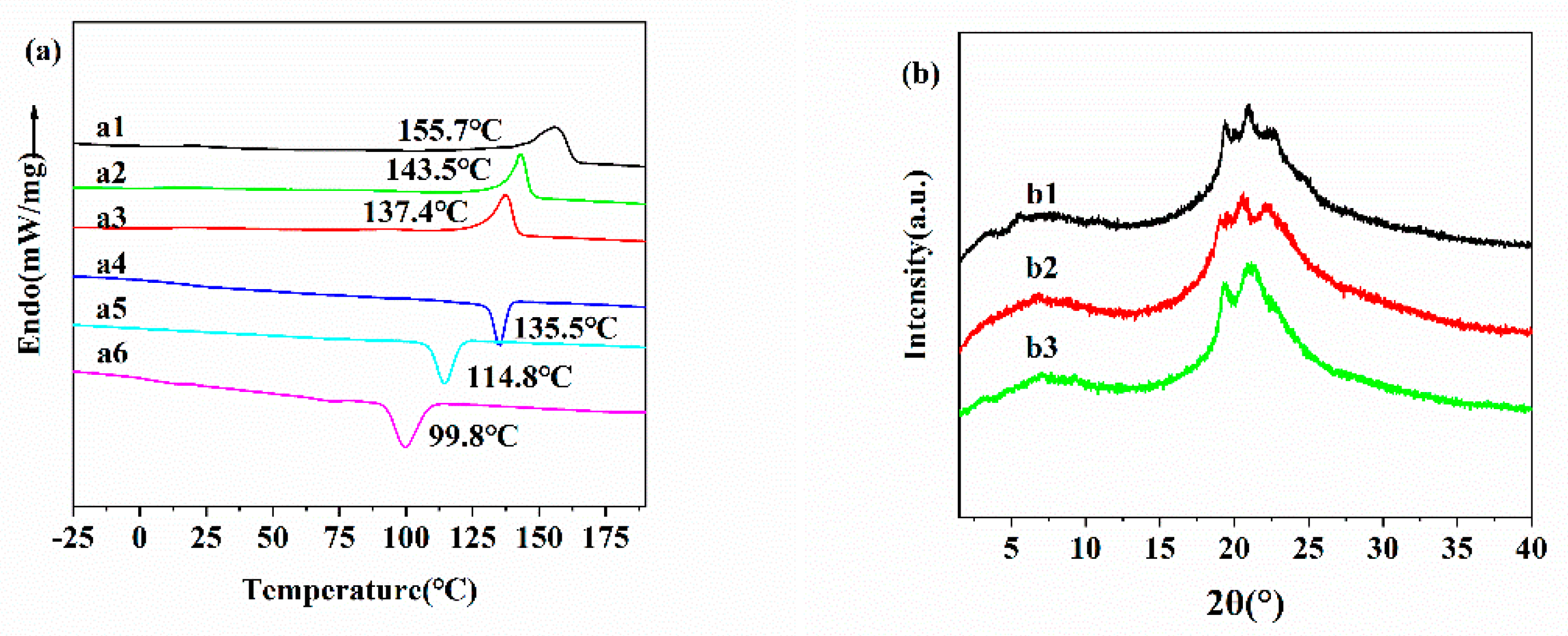
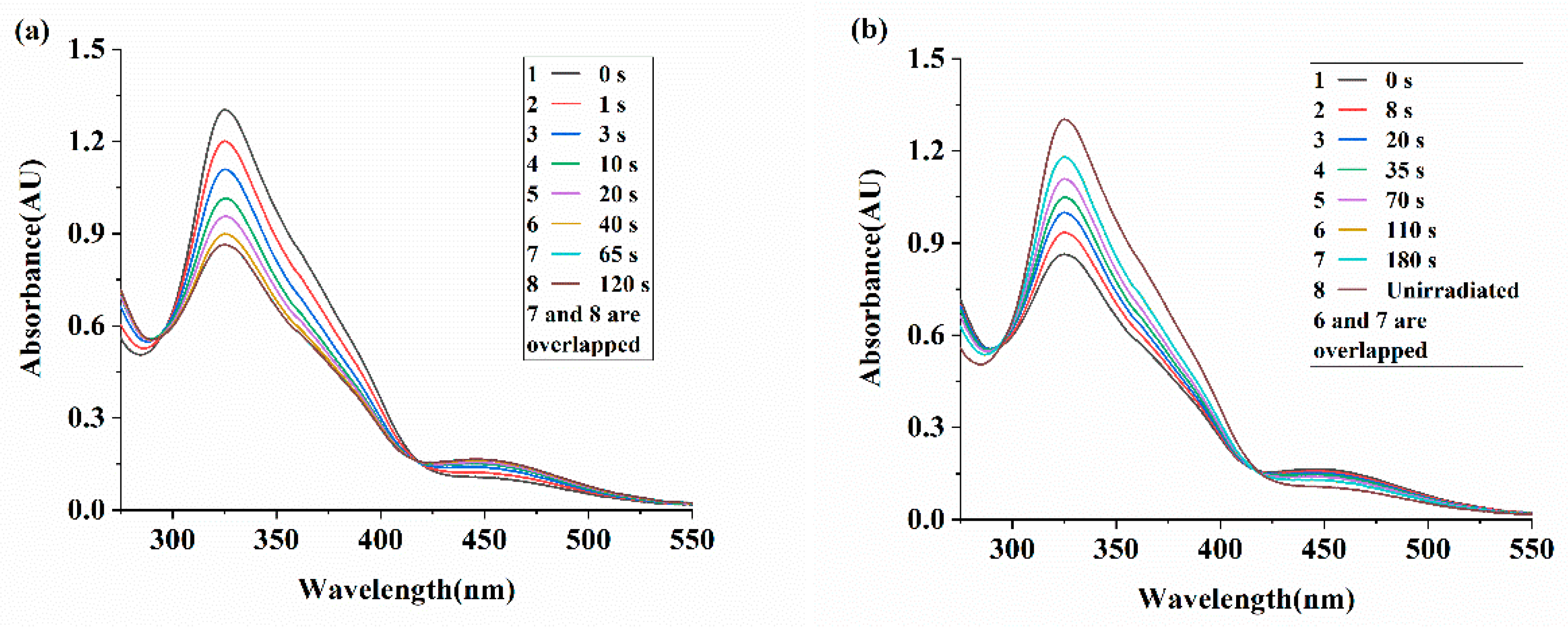
2.1. Synthesis and Characterization of the Main-Chain Azo Semi-Crystalline Poly(ester-amide)S (PEAs)
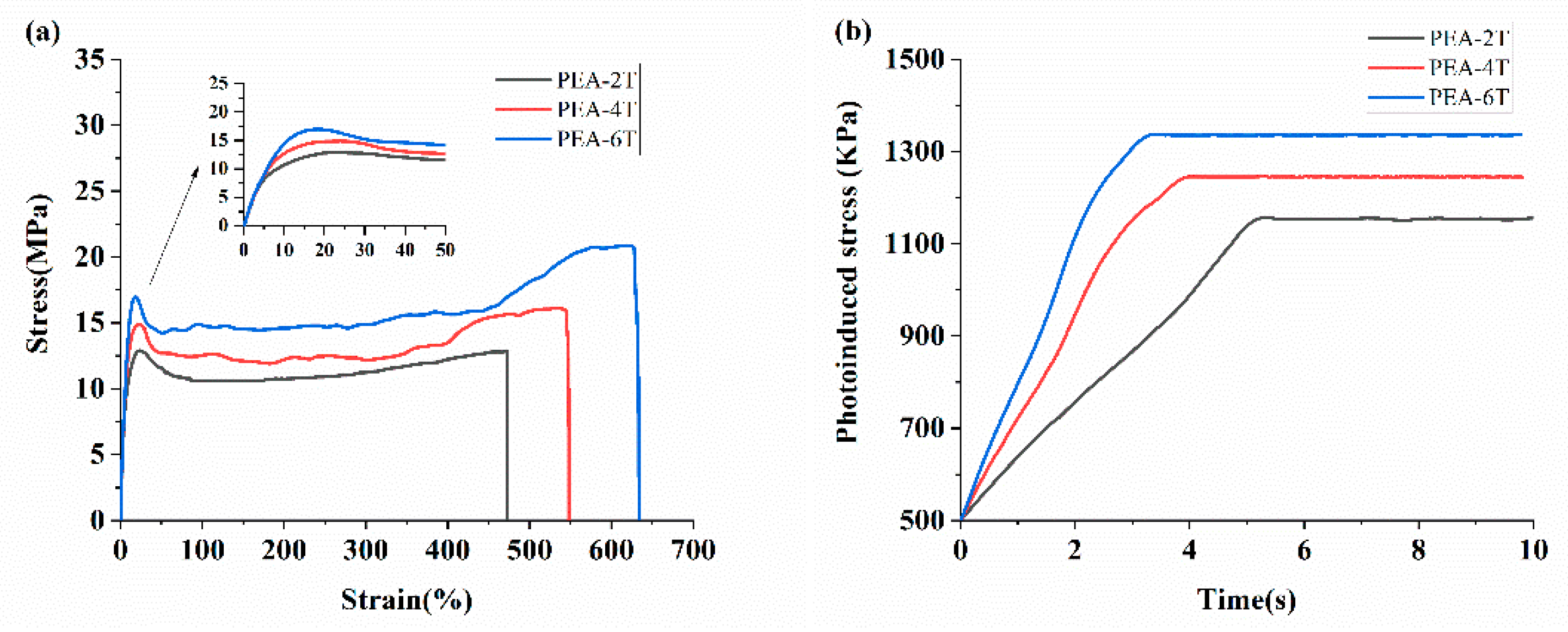
2.2 Fabrication of Uniaxially Oriented Films from Main-Chain Azo PEAs and Study on Mechan-Ical and Photomechanical Properties in Air
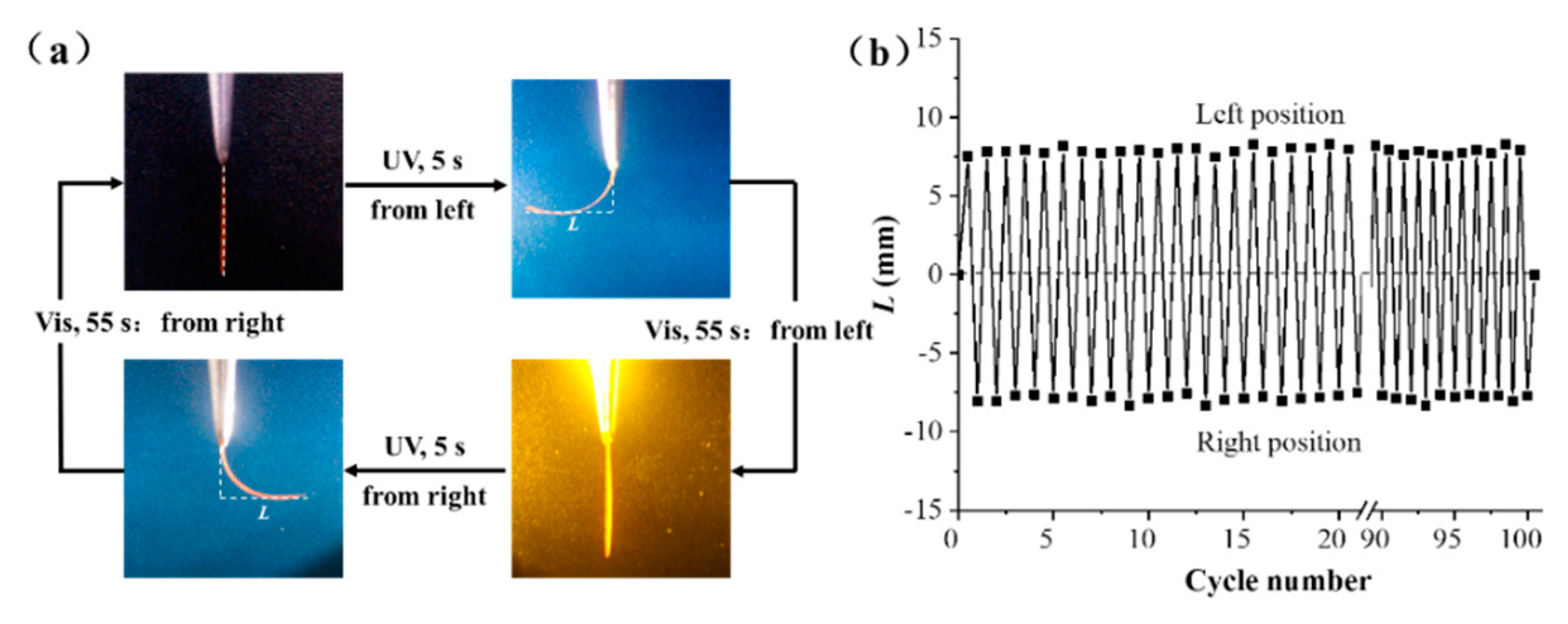
2.3. Room Temperature Three-Dimensional Shape Programmability and Recyclability of Uniaxial Oriented PEA-6T Films
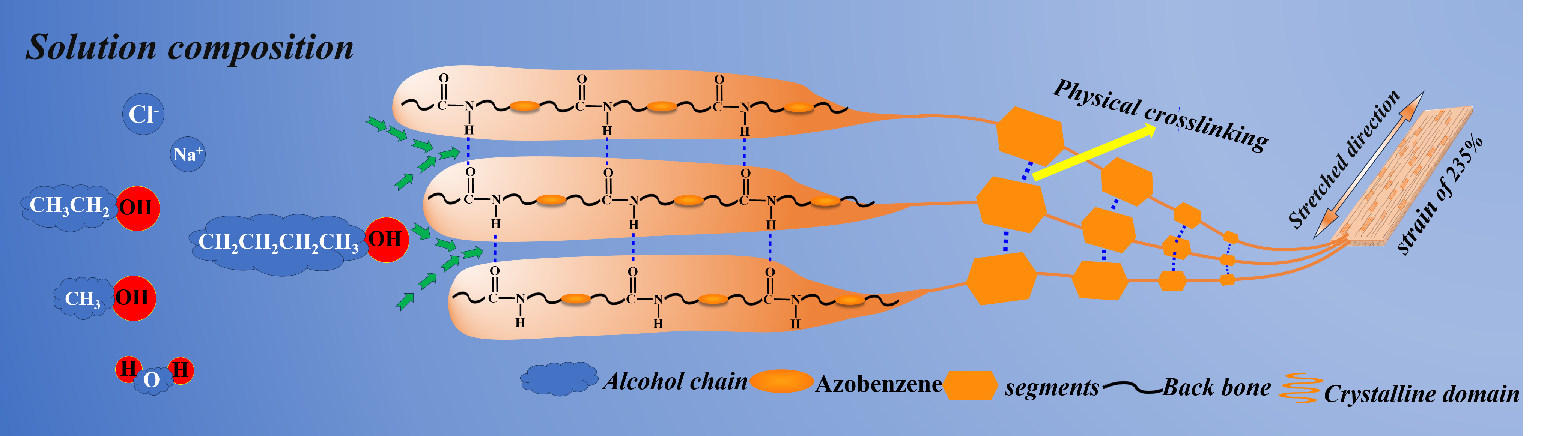
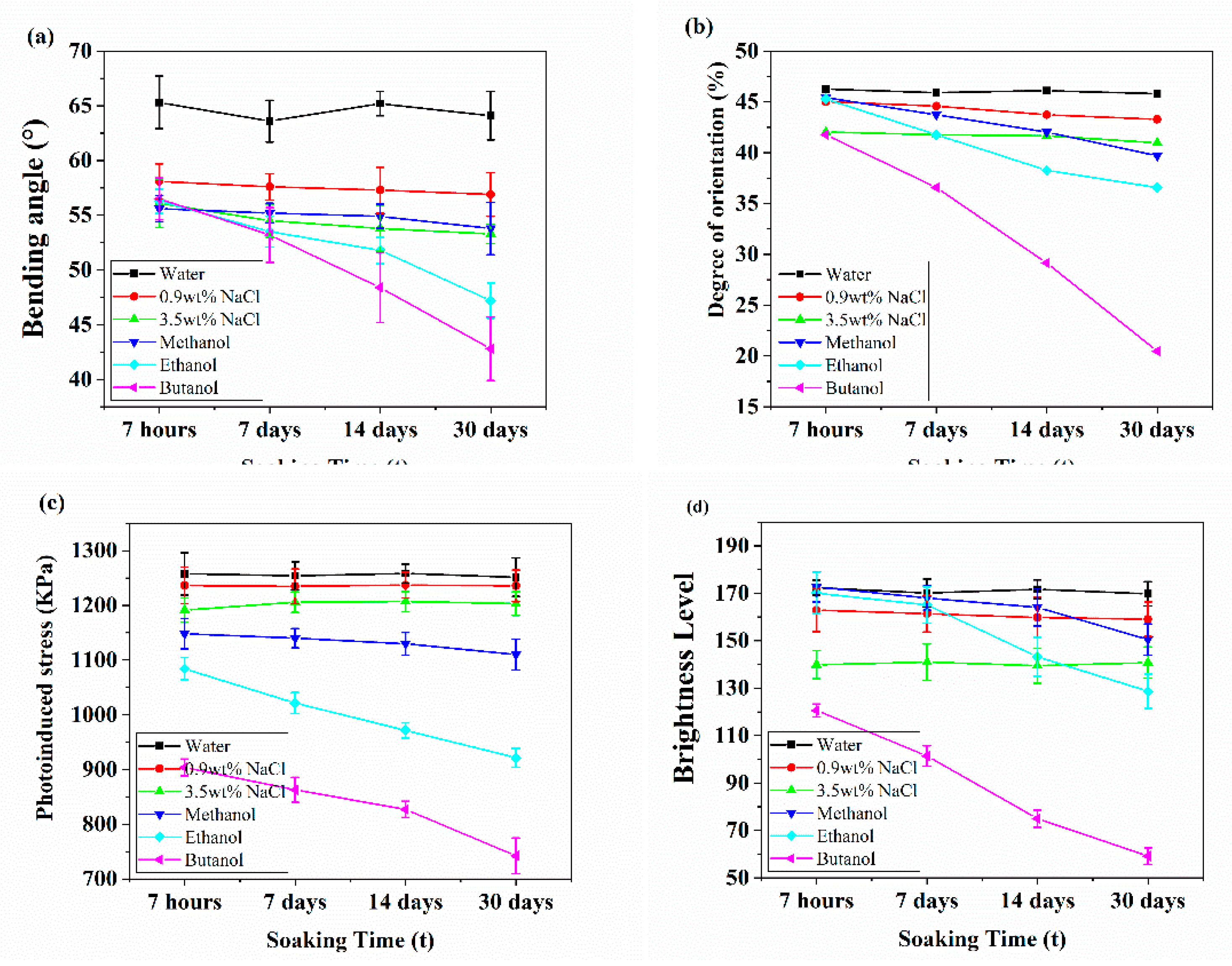
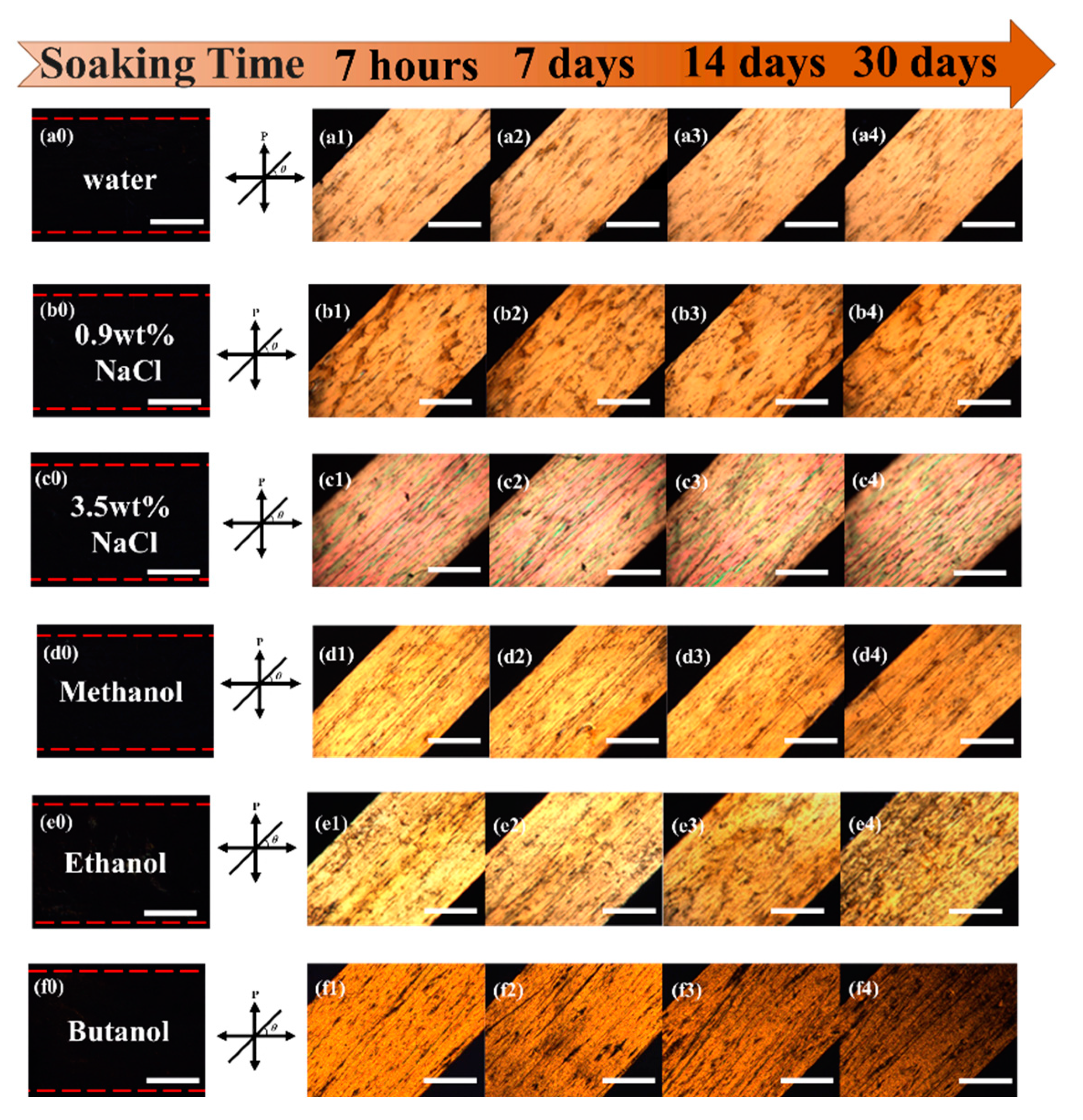
2.4. Photomechanical Properties and Solvent Resistance of Uniaxially Oriented Films of Main-Chain Azo PEAs Under Common Hydrogen Bonding Solvents
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of N- (4-hydroxybutyl) Acrylamide
3.3. Synthesis of M-EA
3.4. Synthesis of PEA-nT (n=2, 4, 6)
3.5. Preparation of the Thin PEA-nT Film for the Photoresponsivity Study
3.6. Preparation of the XRD Characterization for the Azo Polymer Powders
3.7. Preparation of Uniaxially Oriented Main-Chain Azo Polymer Films
3.8. Determination for Degree of Orientation in Uniaxially Oriented Main-Chain Azo Polymer Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yu, H.; Ikeda, T., Photocontrollable liquid-crystalline actuators. Adv. Mater. 2011, 23, (19), 2149-2180. [CrossRef]
- White, T. J.; Broer, D. J., Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, (11), 1087-1098. [CrossRef]
- Natansohn, A.; Rochon, P., Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102(11), 4139-4176. [CrossRef]
- Mysliwiec, J.; Czajkowski, M.; Miniewicz, A.; Bartkiewicz, S.; Kochalska, A.; Polakova, L.; Sedlakova, Z.; Nespurek, S., Dynamics of photoinduced motions in azobenzene grafted polybutadienes. Opt. Mater. 2011, 33, (9), 1398-1404. [CrossRef]
- Iamsaard, S.; Anger, E.; Asshoff, S. J.; Depauw, A.; Fletcher, S. P.; Katsonis, N., Fluorinated azobenzenes for shape-persistent liquid crystal polymer networks. Angew. Chem. Int. Ed. 2016, 55, (34), 9908-12.
- Tkachenko, I. M.; Kurioz, Y. I.; Kravchuk, R. M.; Kobzar, Y. L.; Litoshenko, D. V.; Glushchenko, A. V.; Shevchenko, V. V.; Nazarenko, V. G., Photoinduced birefringence and liquid crystal orientation on polymers with different azobenzene content in the main chain. ACS Appl. Mater. Interfaces 2024, 16, (39), 52945-52957. [CrossRef]
- Wang, D. H.; Lee, K. M.; Yu, Z.; Koerner, H.; Vaia, R. A.; White, T. J.; Tan, L.-S., Photomechanical response of glassy azobenzene polyimide networks. Macromolecules 2011, 44, (10), 3840-3846. [CrossRef]
- Dong, H.; Liu, G.; Zhang, H., Preparation of photodeformable azobenzene polymer fibers by post-crosslinking strategy: Understanding the structure-property relationship. Eur. Polym. J. 2020, 135: 109863. [CrossRef]
- Zhu, Y.; Xu, Z.; Wu, F.; Wang, M.; Chen, L., Liquid-crystal elastomers based on covalent adaptable networks: From molecular design to applications. Science China Materials 2023, 66, (8), 3004-3021. [CrossRef]
- Bin Rusayyis, M. A.; Fenimore, L. M.; Purwanto, N. S.; Torkelson, J. M., Reprocessable, creep-resistant covalent adaptable networks synthesized using conventional free-radical polymerization conditions with piperidine-based and non-piperidine-based dynamic dialkylamino disulfide chemistry. Polym. Chem. 2023, 14, (30), 3519-3534. [CrossRef]
- Zhang, Z. P.; Rong, M. Z.; Zhang, M. Q., Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39-93. [CrossRef]
- Van Zee, N. J.; Nicolaÿ, R., Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104: 101233.
- Chakma, P.; Konkolewicz, D., Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58(29), 9682-9695. [CrossRef]
- Podgórski, M.; Fairbanks, B. D.; Kirkpatrick, B. E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N. J.; Bowman, C. N., Toward stimuli-responsive dynamic thermosets through continuous development and improvements in covalent adaptable networks (CANs). Adv. Mater. 2020, 32(20): 1906876. [CrossRef]
- Jiang, Z. C.; Xiao, Y. Y.; Yin, L.; Han, L.; Zhao, Y., "Self-Lockable" liquid crystalline diels-alder dynamic network actuators with room temperature programmability and solution reprocessability. Angew. Chem. Int. Ed. 2020, 59, (12), 4925-4931.
- Huang, X.; Qin, L.; Wang, J.; Zhang, X.; Peng, B.; Yu, Y., Multiple Shape Manipulation of Liquid Crystal Polymers Containing Diels-Alder Network. Adv. Funct. Mater. 2022, 32(51): 2208312. [CrossRef]
- Han, G.; Nie, J.; Zhang, H., Facile preparation of recyclable photodeformable azobenzene polymer fibers with chemically crosslinked networks. Polym. Chem. 2016, 7, (32), 5088-5092. [CrossRef]
- Qin, C.; Feng, Y.; Luo, W.; Cao, C.; Hu, W.; Feng, W., A supramolecular assembly of cross-linked azobenzene/polymers for a high-performance light-driven actuator. J. Mater. Chem. A 2015, 3, (32), 16453-16460. [CrossRef]
- Qin, C.; Feng, Y.; An, H.; Han, J.; Cao, C.; Feng, W., Tetracarboxylated azobenzene/polymer supramolecular assemblies as high-performance multiresponsive actuators. ACS Appl. Mater. Interfaces 2017, 9, (4), 4066-4073. [CrossRef]
- Vapaavuori, J.; Bazuin, C. G.; Priimagi, A., Supramolecular design principles for efficient photoresponsive polymer–azobenzene complexes. J. Mater. Chem. C 2018, 6, (9), 2168-2188. [CrossRef]
- Yu, H.-T.; Tang, J.-W.; Feng, Y.-Y.; Feng, W., Structural design and application of azo-based supramolecular polymer systems. Chin. J. Polym. Sci. 2019, 37, (12), 1183-1199. [CrossRef]
- Houben, S. J. A.; Lugger, S. J. D.; van Raak, R. J. H.; Schenning, A. P. H. J., A ph-responsive liquid crystal hydrogel actuator with calcium-induced reprogrammable shape fixing. ACS Appl. Polym. Mater. 2022, 4, (2), 1298-1304. [CrossRef]
- Zhong, H.-Y.; Chen, L.; Yang, R.; Meng, Z.-Y.; Ding, X.-M.; Liu, X.-F.; Wang, Y.-Z., Azobenzene-containing liquid crystalline polyester with π–π interactions: diverse thermo- and photo-responsive behaviours. J. Mater. Chem. C 2017, 5, (13), 3306-3314. [CrossRef]
- Zhong, H.-Y.; Chen, L.; Liu, X.-F.; Yang, R.; Wang, Y.-Z., Novel liquid crystalline copolyester containing amphi-mesogenic units toward multiple stimuli-response behaviors. J. Mater. Chem. C 2017, 5, (37), 9702-9711. [CrossRef]
- Zhou, Y.; Wang, L.; Ma, S.; Zhang, H. Fully room-temperature reprogrammable, reprocessable, and photomobile soft actuators from a high-molecular-weight main-chain azobenzene crystalline poly(ester-amide). ACS Appl. Mater. Interfaces 2022, 14, (2), 3264-3273. [CrossRef]
- Lee, W.; Kwak, S.-Y.; Chung, J. W., Arm-length-dependent phase transformation and dual dynamic healing behavior of supramolecular networks consisting of ureidopyrimidinone-end-functionalized semi-crystalline star polymers. Eur. Polym. J. 2020, 138: 109976. [CrossRef]
- Ma, S.; Zhou, Y.; Wang, L.; Zhang, H., Multifunctional UV-NIR dual light-responsive soft actuators from a main-chain azobenzene semi-crystalline poly(ester-amide) doped with polydopamine nanoparticles. Chem. Eur. J. 2024, 30(8): e202303306.
- Li, G.; Xu, M.; Zhang, S.; Yang, G.; Li, W., Reversible controlling the supramolecular chirality of side chain azobenzene polymers: chiral induction and modulation. Macromol. Rapid Commun. 2022, 43(6): 2100904. [CrossRef]
- Zhang, H. Reprocessable photodeformable azobenzene polymers. Molecules 2021, 26(15): 4455. [CrossRef]
- Fang, L.; Han, G.; Zhang, J.; Zhang, H.; Zhang, H., Synthesis of well-defined easily crosslinkable azobenzene side-chain liquid crystalline polymers via reversible addition–fragmentation chain transfer polymerization and photomechanical properties of their post-crosslinked fibers. Eur. Polym. J. 2015, 69, 592-604. [CrossRef]
- Han, G.; Zhang, H.; Chen, J.; Sun, Q.; Zhang, Y.; Zhang, H., Easily crosslinkable side-chain azobenzene polymers for fast and persistent fixation of surface relief gratings. New J. Chem. 2015, 39, (2), 1410-1420. [CrossRef]
- Li, X.; Fang, L.; Hou, L.; Zhu, L.; Zhang, Y.; Zhang, B.; Zhang, H., Photoresponsive side-chain liquid crystalline polymers with amide group-substituted azobenzene mesogens: effects of hydrogen bonding, flexible spacers, and terminal tails. Soft Matter 2012, 8(20), 5532-5542. [CrossRef]
- Li, X.; Wen, R.; Zhang, Y.; Zhu, L.; Zhang, B.; Zhang, H., Photoresponsive side-chain liquid crystalline polymers with an easily cross-linkable azobenzene mesogen. J. Mater. Chem. 2009, 19, (2), 236-245. [CrossRef]
- Li, Z.; Zhang, Y.; Zhu, L.; Shen, T.; Zhang, H., Efficient synthesis of photoresponsive azobenzene-containing side-chain liquid crystalline polymers with high molecular weights by click chemistry. Polym. Chem. 2010, 1(9), 1501-1511. [CrossRef]
- Wani, O. M.; Zeng, H.; Priimagi, A., A light-driven artificial flytrap. Nat. Commun. 2017, 8(1): 15546. [CrossRef]
- Guo, C.; Gao, J.; Ma, S.; Zhang, H., Efficient preparation of chemically crosslinked recyclable photodeformable azobenzene polymer fibers with high processability and reconstruction ability via a facile post-crosslinking method. Eur. Polym. J. 2020, 139: 109998. [CrossRef]
- Nie, J.; Liu, X.; Yan, Y.; Zhang, H. Supramolecular hydrogen-bonded photodriven actuators based on an azobenzene-containing main-chain liquid crystalline poly(ester-amide). J. Mater. Chem. C 2017, 5, (39), 10391-10398. [CrossRef]
- Tan, S.; Sha, Y.; Zhu, T.; Rahman, M. A.; Tang, C., Photoresponsive supramolecular polymers based on quadruple hydrogen-bonding and a photochromic azobenzene motif. Polym. Chem. 2018, 9, (44), 5395-5401. [CrossRef]
- Ube, T.; Nakayama, R.; Ikeda, T., Photoinduced motions of thermoplastic polyurethanes containing azobenzene moieties in main chains. Macromolecules 2022, 55, (2), 413-420. [CrossRef]
- Ma, S.; Wang, L.; Zhou, Y.; Zhang, H., Fully room temperature reprogrammable, recyclable, and photomobile soft actuators from physically cross-linked main-chain azobenzene liquid crystalline polymers. Molecules 2023, 28, (10): 4174. [CrossRef]
- Zhou, Y.; Wang, L.; Zhang, H. Enhancing the performances of physically cross-linked photodeformable main-chain azobenzene poly(ester-amide)s via chemical structure engineering. Polym. Chem. 2022, 13, (24), 3713-3725. [CrossRef]
- Wang, L.; Zhou, Y.; Ma, S.; Zhang, H. Reprocessable and healable room temperature photoactuators based on a main-chain. [CrossRef]
- azobenzene liquid crystalline poly(ester-urea). J. Mater. Chem. C 2021, 9, (38), 13255-13265.
- Pilz da Cunha, M.; van Thoor, E. A. J.; Debije, M. G.; Broer, D. J.; Schenning, A. P. H. J., Unravelling the photothermal and photomechanical contributions to actuation of azobenzene-doped liquid crystal polymers in air and water. J. Mater. Chem. C 2019, 7, (43), 13502-13509. [CrossRef]
- Zhang, P.; Lan, Z.; Wei, J.; Yu, Y., Photodeformable azobenzene-containing polyimide with flexible linkers and molecular alignment. ACS Macro Lett. 2021, 10, (4), 469-475. [CrossRef]
- Kuenstler, A. S.; Clark, K. D.; de Alaniz, J. R.; Hayward, R. C., Reversible actuation via photoisomerization-induced melting of a semicrystal line poly(azobenzene). ACS Macro Lett. 2020, 9, (6), 902-909.
- Niemann, M.; Ritter, H., Comb-like methacrylamide polymers containing condensates of amino acids and azobenzene moieties in the side chains. Makromol. Chem. 1993, 194, (4), 1169-1181.
- Wang, G.-J.; Tong, X.; Zhao, Y. J. M., Preparation of azobenzene-containing amphiphilic diblock copolymers for light-responsive micellar aggregates. Macromolecules 2004, 37, 8911-8917. [CrossRef]
- Akiyama, H.; Tamaoki, N. Synthesis and photoinduced phase transitions of poly(N-isopropylacrylamide) derivative functionalized with terminal azobenzene units. Macromolecules 2007, 40, 5129–5132. [CrossRef]
- Wei, R.; He, Y.; Wang, X., Diblock copolymers composed of a liquid crystalline azo block and a poly(dimethylsiloxane) block: synthesis, morphology and photoresponsive properties. RSC Adv. 2014, 4, (102), 58386-58396. [CrossRef]
- Wie, J. J.; Wang, D. H.; Lee, K. M.; White, T. J.; Tan, L. S., The contribution of hydrogen bonding to the photomechanical response of azobenzene-functionalized polyamides. J. Mater. Chem. C, 2018, 6(22), 5964-5974. [CrossRef]
- Wang, Z.; Huang, H.; Hsu, C.; Wang, X., Azo molecular glass patterning from chiral submicron pillar array to self-organized topographic transition via irradiation with circularly polarized light. Adv. Opt. Mater. 2021, 9, (21): 2100922. [CrossRef]
- Corrado, F.; Bruno, U.; Prato, M.; Carella, A.; Criscuolo, V.; Massaro, A.; Pavone, M.; Muñoz-García, A. B.; Forti, S.; Coletti, C.; Bettucci, O.; Santoro, F., Azobenzene-based optoelectronic transistors for neurohybrid building blocks. Nat. Commun. 2023, 14, (1): 6760.
- Kizhakidathazhath, R.; Geng, Y.; Jampani, V. S. R.; Charni, C.; Sharma, A.; Lagerwall, J. P. F., Facile anisotropic deswelling method for realizing large-area cholesteric liquid crystal elastomers with uniform structural color and broad-range mechanochromic response. Adv. Funct. Mater. 2019, 30(7): 1909537. [CrossRef]
- Kaniyoor, A.; Gspann, T. S.; Mizen, J. E.; Elliott, J. A., Quantifying alignment in carbon nanotube yarns and similar two-dimensional anisotropic systems. J. Appl. Polym. Sci. 2021, 138, (37): 50939. [CrossRef]
- Patil, N.; Balzano, L.; Portale, G.; Rastogi, S., Influence of nanoparticles on the rheological behaviour and initial stages of crystal growth in linear polyethylene. Macromol. Chem. Phys. 2009, 210, (24), 2174-2187. [CrossRef]
- Cinader, D. K.; Burghardt, W. R., X-ray scattering studies of orientation in channel flows of a lyotropic liquid crystalline polymer. Polymer 1999, 40, (15), 4169-4180.
| Entry | Samplea | Yield (%) |
Degree of polymerization (DP)b |
Mn, NMR (g·mol−1)c |
Thermal transition T (℃)d |
Δ Hsi (J·g−1)g |
Td (℃)h |
| 1 | PEA-2T | 90 | 47 | 28400 | G 16.3 Cr 153.7 I e I 134.0 Cr 10.5 G f |
31.3 -26.4 |
288 |
| 2 | PEA-4T | 91 | 48 | 30900 | G 12.6 Cr 143.5 I e I 114.8 Cr 4.5 G f |
29.4 -24.4 |
292 |
| 3 | PEA-6T | 90 | 49 | 32900 | G 8.3 Cr 133.4 I e I 99.8 Cr 1.4 G f |
28.6 -25.5 |
294 |
|
Entry |
Sample |
Elastic modulus (MPa) |
Yield strength (MPa) | Rupture strength (MPa) |
Elongation at break (%) | Toughness (MJ/m3) | Photoinduced stress (kPa) |
| 1 | 1,2-film | 179.2 ± 4.2 | 12.6 ± 1.1 | 12.6 ± 0.7 | 472.9 ± 28.7 | 53.7 ± 8.4 | 582.7 ± 43.1 |
| 2 | 1,4-film | 197.5 ± 3.2 | 14.8 ± 0.9 | 16.1 ± 1.1 | 547.6 ± 22.7 | 72.7± 5.2 | 722.5 ± 48.6 |
| 3 | 1,6-film | 210.3 ± 4.0 | 16.9 ± 1.1 | 22.1 ± 2.1 | 632.9 ± 15.4 | 108.1 ± 2.3 | 835.9 ± 48.2 |
| Entry | Sample | Environment | Temperature (℃) | Induced bending time (s) a | Bending amplitude L(mm) b |
Cycling number (times) c |
|
| UV | Vis | ||||||
| 1 | PEA-2T | Air | 25 | 12 | 110 | 6.8 ± 0.2 | >100 |
| 2 | PEA-4T | Air | 25 | 10 | 90 | 7.0 ± 0.3 | >100 |
| 3 | PEA-6T | Air | 25 | 5 | 55 | 7.9 ± 0.4 | >100 |
| 4 | Water | 25 | 6 | 60 | 7.9 ± 0.3 | >100 | |
| 30 | 6 | 55 | 7.6 ± 0.2 | >100 | |||
| 40 | 5.5 | 51 | 7.6 ± 0.3 | >100 | |||
| 50 | 4 | 46 | 7.5 ± 0.5 | ~40 | |||
| 5 | Methanol | 25 | 6 | 65 | 7.8 ± 0.2 | >100 | |
| 6 | Ethanol | 25 | 6.5 | 73 | 7.8 ± 0.2 | >100 | |
| 7 | N-butanol | 25 | 8 | 90 | 7.6 ± 0.2 | ~40 | |
| 8 | (0.9 wt.%) NaCl | 25 | 5.5 | 55 | 7.6 ± 0.2 | >100 | |
| 50 | 3.5 | 42 | 7.1 ± 0.6 | ~55 | |||
| 9 | (3.5 wt.%) NaCl | 25 | 6 | 65 | 7.7 ± 0.2 | >100 | |
| 50 | 4 | 33 | 6.8 ± 0.7 | ~60 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




