1. Introduction
Groundwater is considered as a very reliable source of drinking water. About 74.9% of rural communities in Malawi depend on water from boreholes, shallow wells and open wells in their day to day lives [
1]. People use groundwater for drinking and cooking with limited or no treatment because it is considered as a safe source of water [
2]. However, groundwater can easily get contaminated by various pollutants thereby degrading the quality of the water and rendering it unsafe [
2]. Various anthropogenic activities, climate change and other natural processes are potential sources of contaminants and they can significantly threaten the quality of groundwater [
3]. Contaminants such as pathogenic microorganisms and antimicrobial resistant bacteria can pollute water through seepage from sewer leaks, poorly constructed septic tanks, pit latrines or improper sewage disposal [
4]. Pathogenic bacteria found in water are responsible for various waterborne diseases. For example, gastrointestinal diseases are caused by S
higella, Salmonella, Campylobacter and Clostridium [
5]. Water borne diseases and pathogenic contamination of water is a great water quality concern. Water borne infections kill about 3.4 million people, mostly children, each year worldwide [
5]. In recent years, there has been an increase in the occurrence of antibiotic resistant bacteria in the environment and groundwater sources due to the high usage of antibiotics. The presence of antibiotics in groundwater overtime eventually results in the development of antibiotic resistant bacteria [
6,
7].
Antimicrobial resistance is one of the major health problems being faced by the global population [
8]. By 2050, it is anticipated that the global cost of antimicrobial-resistant pathogen-related diseases will rise from 700,000 to 10 million fatalities annually and reach USD 100 trillion [
9]. Antimicrobial resistance is one of the major health problems being faced by the global population [
8]. By 2050, it is anticipated that the global cost of antimicrobial-resistant pathogen-related diseases will rise from 700,000 to 10 million fatalities annually and reach USD 100 trillion [
9]. The various complex interactions of antibiotics in the environment have led to the development and spread of antibiotic resistant bacteria [
10]. When an infection resulting from antibiotic resistant bacteria occurs, it can be difficult to treat such infection thereby leading to extended periods of illness or even death. For example,
N. gonorrhoea which used to be treatable by antibiotics such as Penicillins, Tetracyclines, Sulfonamides and Fluoroquinolones has now become resistant to these antibiotics [
11]. Contamination of groundwater by pollutants such as organics, pesticides, pharmaceuticals, micro plastics and other emerging contaminants like antibiotics and antibiotic resistant bacteria (ARB), poses a health threat to consumers and even the entire human population [
3].
According to United Nations general assembly in 2010, access to clean and safe water for human consumption was declared as a human right. Sustainable development goal number 6, target 6.1 states that “By 2030, achieve universal and equitable access to safe and affordable drinking water for all”. “Safe” drinking water means that the water is free of contaminants” [
12]. Contamination of groundwater by emerging contaminants like antibiotic resistant bacteria renders the water unsafe for human consumption and has various health effects. Several studies in Malawi have investigated organic contaminants in groundwater and not on emerging contaminants like antimicrobial resistant bacteria [
13,
14,
15,
16,
17,
18]. A Study evaluating seasonal variation of water quality from shallow wells in Democratic Republic of Congo recommended that “further investigations should be done on emerging contaminants like antibiotics, antibiotic resistant bacteria and antibiotic resistant genes in shallow wells” [
19]. Currently, in Malawi there is a knowledge gap on antibiotic resistant bacteria and genes in groundwater. It is for this reason that the study will investigate the antibiotic susceptibility of pathogenic microorganisms isolated in groundwater from boreholes and wells in T/A Makhwira, Chikwawa.
2. Materials and Methods
This was a descriptive cross-sectional study where quantitative data was collected and analyzed. Water samples were collected in T/A Makhwira, Chikwawa. 13 water samples were collected from boreholes and 7 from protected shallow wells.
2.1. Sample Collection
The researcher collected water samples from boreholes and shallow wells in sterilized 500ml sampling bottles. The sample boreholes were clearly labelled depending on the source of the sample, BH1, BH2 to BH13 for boreholes and SW1, SW2 to SW7 for shallow wells. The collected samples were placed in a cooler box filled with ice after collection and transported to Kamuzu University of Health Sciences laboratory for analysis. The samples were stored in refrigerators at 4oC until all the analysis was completed.
Enumeration of Bacteria Counts
Total plate count was done using spread plate method. The process involved serial dilutions of water samples in distilled water and spread plating 20μl of the distilled samples on nutrient agar in petri dishes. The cultured plates were incubated at 37°C for 24 hours. Total counts were presented as colony-forming units (cfu) per milliliter (cfu/ml).
2.2. Isolation of Pathogenic Microorganisms
Pathogenic microorganisms such as E. coli, K. pneumoniae, salmonella, Shigella, ESBL E. coli and ESBL K. pneumoniae were detected using standard methods. Samples enriched with BPW were cultured on selective media such as CHROMagar™ Orientation, ESBL Chromogenic agar and Xylose Lysine Deoxycholate agar (XLD agar) to isolate the pathogenic bacteria in water. Suspected E. coli and Salmonella spp. colonies were further subjected to biochemical tests using API 20E Kits for confirmation and High resolution melt curve (HRM) PCR was used to confirm suspected K. pneumoniae colonies.
2.3. Antibiotic Susceptibility Testing
Kirby Bauer disk diffusion method was used to determine the resistance and sensitivity of pathogenic bacteria to various selected antibiotics. The antibiotics disks that were used included: Ampicillin 10μg, Amoxicillin-clavulanic acid 30μg, Gentamicin 10μg, Doxycycline 30μg, Ciprofloxacin 5μg and Trimethoprim- sulfamethoxazole 25μg. Pathogenic bacteria isolated from the water samples were inoculated on Mueller-Hilton agar in the presence of antibiotic disks. The Clinical and Laboratory Standards Institute (CLSI) performance standards for antimicrobial susceptibility Testing- 30th edition were used for the interpretation of the zone diameter breakpoints.
2.4. Statistical Analysis
Data was entered and analyzed using IBM SPSS Statistics version 20. Descriptive statistics were used to generate means and frequencies to describe the data. Independent sample T-tests and fishers exact tests were used to assess the significance of associations at 95% confidence interval. Any p-value less than <0.05 was significant.
3. Results
A total of 20 groundwater samples were analyzed in this study. The results of the heterotrophic plate count showed that there was a high bacteria count in all the water samples. The total counts ranged from 1.6x10
3 cfu/ml to 2.35x10
5 cfu/ml for boreholes and 1.2x10
3 cfu/ml to 2.65x10
5 cfu/ml for shallow wells. All the counts were higher than the national [
20] and international [
8] water quality standards. The mean CFU/ml for boreholes was 90708 cfu/ml and 162314 cfu/ml for shallow wells. Further analysis using independent samples T-test found that there was an insignificant difference between the total counts means for the boreholes and shallow wells
(t = -1.426
, p = 0.188
).
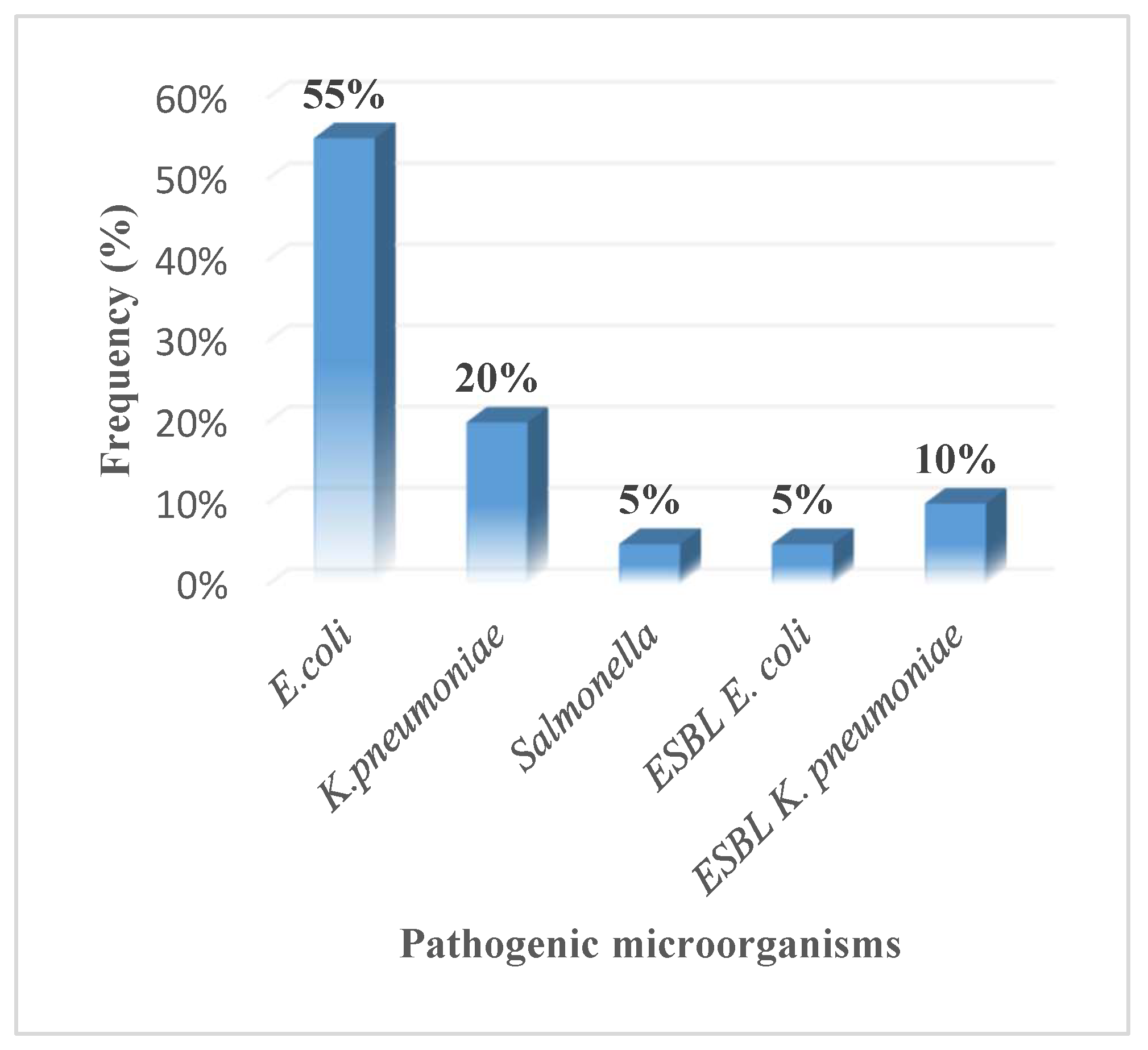
There was a variation in the presence of the selected pathogenic microorganisms among the water samples. E. coli 11(55%) was the most prevalent pathogenic bacteria. K. pneumoniae 4(20%) was the second most prevalent pathogenic bacteria followed by Salmonella 1(5%). There was a low prevalence of ESBL producing enterobacteriaceae in the water sample. ESBL E. coli was present in only 1(5%) of the water samples, i.e., SW3. ESBL K. pneumoniae was also present in just 2 (10%) of the water samples i.e., SW3 and SW7. Shigella was not detected in any of the water samples.
Figure 1.
Frequency of occurrence of pathogenic microorganisms in groundwater.
Figure 1.
Frequency of occurrence of pathogenic microorganisms in groundwater.
Antimicrobial susceptibility testing was done on all the 19 isolates of pathogenic bacteria isolated from the water samples. These isolates included E. coli, Salmonella, K. pneumoniae, ESBL E. coli and ESBL K. pneumoniae. The isolates had varying sensitivities to different antibiotics. Some isolates were resistant to the antibiotics, others had an intermediate resistance whereas others were sensitive to the antibiotics that were used.
Table 1.
Antimicrobial susceptibility of bacteria isolates to selected antibiotics.
Table 1.
Antimicrobial susceptibility of bacteria isolates to selected antibiotics.
| |
|
Antimicrobial susceptibility to selected antibiotics |
| Sample ID |
Bacteria Isolate |
AMP |
SXT |
AMC |
DXT |
CIP |
CN |
| BH1 |
E. coli |
S |
S |
S |
S |
S |
S |
| BH2 |
E. coli |
I |
S |
I |
I |
S |
S |
| BH7 |
E. coli |
I |
S |
S |
S |
S |
S |
| BH8 |
E. coli |
I |
S |
S |
S |
S |
S |
| BH9 |
E. coli |
S |
S |
S |
S |
S |
S |
| SW1 |
E. coli |
I |
S |
S |
S |
S |
S |
| SW2 |
E. coli |
I |
S |
I |
S |
S |
S |
| SW3 |
E. coli |
S |
S |
S |
I |
S |
S |
| SW4 |
E. coli |
S |
S |
S |
S |
R |
S |
| SW6 |
E. coli |
S |
S |
S |
S |
S |
S |
| SW7 |
E. coli |
R |
R |
S |
I |
S |
S |
| SW3 |
ESBL E. coli |
R |
R |
R |
R |
R |
R |
| BH11 |
K. pneumoniae |
R |
S |
S |
I |
S |
S |
| BH2 |
K. pneumoniae |
R |
S |
S |
I |
S |
S |
| SW1 |
K. pneumoniae |
R |
S |
R |
I |
S |
S |
| SW6 |
K. pneumoniae |
R |
R |
S |
R |
I |
S |
| SW3 |
ESBL K. pneumoniae |
R |
R |
R |
I |
R |
S |
| SW7 |
ESBL K. pneumoniae |
R |
R |
I |
R |
R |
R |
| SW7 |
Salmonella |
I |
S |
I |
I |
I |
S |
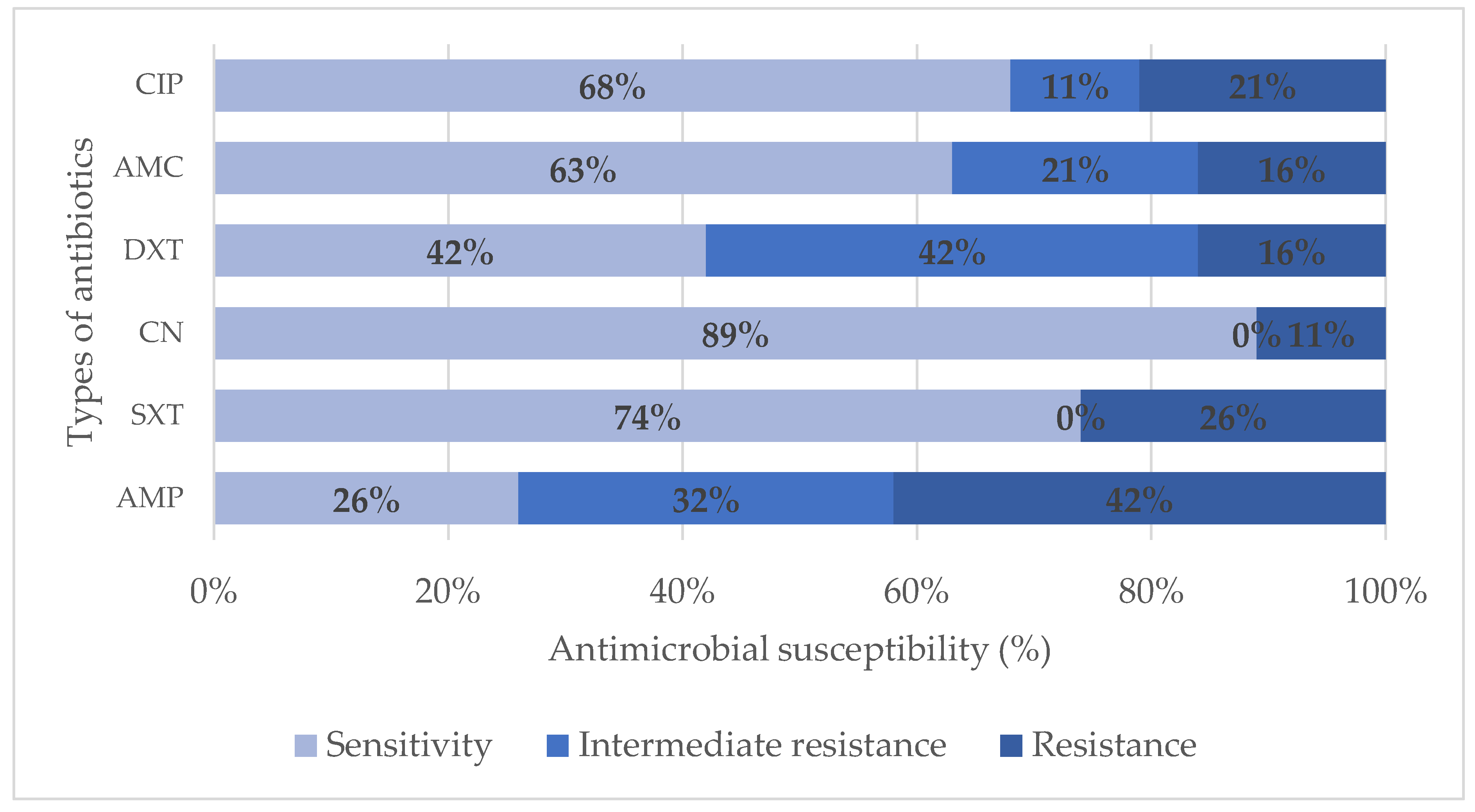
High incidence of resistance was observed in Ampicillin (42%), followed by Trimethoprim- sulfamethoxazole (26%) and Ciprofloxacin (21%). There was a 16% resistance in both Amoxicillin-clavulanic acid and Doxycycline. The lowest resistance was observed in Gentamicin (11%).
The isolates exhibited the highest sensitivity in Gentamicin (89%). There was a 78% sensitivity of the isolates to Trimethoprim- sulfamethoxazole and 68% sensitivity to Ciprofloxacin. Amoxicillin-clavulanic acid had a sensitivity of 63%. Lower sensitivity was observed in Ampicillin (26%) and Doxycycline (42%).
Figure 2.
Antibiotic susceptibility of bacteria isolates from water samples.
Figure 2.
Antibiotic susceptibility of bacteria isolates from water samples.
A few isolates (32%) were resistant to more than one antibiotic. Some were resistant to 1 or 2 antibiotics whereas some were resistant to 3 or more antibiotics. ESBL producing enterobacteriaceae were the ones that showed resistance to more than three antibiotics.
Table 2.
Multi-Drug resistant patterns of isolates from water samples.
Table 2.
Multi-Drug resistant patterns of isolates from water samples.
| Resistance pattern |
Types of antibiotics |
Name of isolate |
| 1 |
AMP, SXT, DXT |
SW6_K. pneumoniae
|
| 2 |
AMP, SXT, AMC, CIP |
SW3_ESBL K. pneumoniae
|
| 3 |
AMP, SXT, DXT, CIP, CN |
SW7_ESBL K. Pneumoniae
|
| 4 |
AMP, SXT, AMC, DXT, CIP, CN |
SW3_ESBL E. coli
|
Fishers exact test showed that there was no significant association between the type of water source and antibiotic susceptibility results as shown in table 3.
Table 3.
Association between antimicrobial susceptibility and type of water source.
Table 3.
Association between antimicrobial susceptibility and type of water source.
| |
Water Source |
|
| Antibiotic |
Borehole1, N = 7 |
Shallow well1, N = 12 |
p-value2
|
| Ampicillin |
|
|
0.8 |
| Intermediate Resistance |
3 (43%) |
3 (25%) |
|
| Resistant |
2 (29%) |
6 (50%) |
|
| Sensitive |
2 (29%) |
3 (25%) |
|
| Sulfamethoxazole-Trimethoprim |
|
|
0.11 |
| Resistant |
0 (0%) |
5 (42%) |
|
| Sensitive |
7 (100%) |
7 (58%) |
|
| Amoxicillin Clavulanic acid |
|
|
0.3 |
| Intermediate Resistance |
1 (14%) |
3 (25%) |
|
| Resistant |
0 (0%) |
3 (25%) |
|
| Sensitive |
6 (86%) |
6 (50%) |
|
| Doxycycline |
|
|
0.4 |
| Intermediate Resistance |
3 (43%) |
5 (42%) |
|
| Resistant |
0 (0%) |
3 (25%) |
|
| Sensitive |
4 (57%) |
4 (33%) |
|
| Ciprofloxacin |
|
|
0.11 |
| Intermediate Resistance |
0 (0%) |
2 (17%) |
|
| Resistant |
0 (0%) |
4 (33%) |
|
| Sensitive |
7 (100%) |
6 (50%) |
|
| Gentamicin |
|
|
0.5 |
| Resistant |
0 (0%) |
2 (17%) |
|
| Sensitive |
7 (100%) |
10 (83%) |
|
|
1n (%) |
|
2Fisher's exact test |
4. Discussion
All samples collected from boreholes and shallow wells had a CFU/ml that was higher than the recommended national [
20] and international [
21] water quality standards. This is consistent with results from [
22,
23] which also found a high colony count above the required standards. This high colony count could be attributed to the proximity of the water points to sanitation facilities and the frequent occurrence of floods in the area. During data collection, it was observed that some of the water points were less than 10 meters away from pit latrines and animal kraals hence the reason for the high colony count. Pit latrines, animal kraals and sporadic presence of wild animals are some of the main contributors to groundwater contamination in the area. T/A Makhwira is an area which has been hit by both cyclone Idai and Freddy in recent years. Flood submergence of both boreholes and shallow wells may also contribute to high microbial contamination. The water samples were collected during rainy season and after part of the area had been affected by cyclone Freddy hence this might have contributed to the high microbial load. Pathogenic bacteria should not be detectable in drinking water [
20,
21]. The presence of these pathogenic bacteria renders the water unsafe for human consumption and increases the risk of waterborne diseases.
E. coli was the most prevalent pathogenic bacteria in the water samples that were collected. This result is consistent with findings reported in Malawi [
23,
24,
25], and Lesotho [
26] that attributed high
E. coli prevalence to proximity of groundwater sources to pit latrines. Studies by have further reported that the presence of
E. coli in water samples is usually an indication of fecal contamination [
27,
28]. A study recent study found the highest level of
E. coli and total coliforms in a water sample from a tube well that was 1m away from a latrine [
29]. This is further evidence that proximity to pit latrines is associated with the presence of
E. coli in groundwater samples.
Salmonella enterica ssp arizonae was found in one sample out of the 20 water samples that were collected. The presence of this species of
salmonella in the water is quite alarming as it is not a common human pathogen and it is usually found in the gut flora of snakes [
30,
31,
32]. This could indicate that there is a potential reservoir of snakes near the protected shallow well where this pathogen was isolated from. This pathogen can be responsible for the occurrence of gastrointestinal diseases such as gastroenteritis in people who consume water from this source. A recent scholarly article discovered that
Salmonella ssp contamination is higher in dry season than in rainy season and this may explain why salmonella was not found in the other water sources [
33]. However, the presence of the
Salmonella enterica ssp arizonae in the sample from this water source could indicate localized contamination possibly linked to snakes accessing the shallow well. The detection of
K. pneumoniae in water samples is of significant concern because
K. pneumoniae is a pathogen that is responsible for causing blood, lung and urinary tract infections [
34]. Consumption of water contaminated with
K. pneumoniae puts individuals at risk of these infections. In this study,
K. pneumoniae was detected in 20% of the water samples. This is consistent with results from [
35,
36] which also detected
K. pneumoniae from groundwater samples. In addition to fecal contamination,
K. pneumoniae can enter groundwater through the use of contaminated irrigation water and the application of manure or wastewater for agricultural reasons. Infiltration and runoff from agricultural areas helps in the spread of these resistant strains [
37]. This study also detected
ESBL E. coli and
ESBL K. pneumoniae in 3 samples collected from shallow wells. This is in line with results from [
38] which detected
ESBL E. coli in shallow wells and attributed the presence of ESBL producing
E. coli to fecal contamination. Drinking water from sources contaminated with ESBL producers can expose individuals to these enterobacteriaceae that are well known for being antimicrobial resistant bacteria. Diseases caused by these antimicrobial resistant bacteria are more severe and harder to treat.
This study showed that there was a high incidence of resistance of isolates to Ampicillin (42%). This is consistent with results from [
39,
40,
41] who also found that isolates had a high resistance to Ampicillin. The high resistance to ampicillin could be because this antibiotic is usually used as a broad-spectrum drug hence the reason why many bacteria species could be resistant to the antibiotic. In the current study,
K. pneumoniae isolates were found to be resistant to a wide range of antibiotics such as Ampicillin, Trimethoprim-sulfamethoxazole, Amoxicillin clavulanic acid and Doxycycline. This is similar with results from [
42] who found that
K. pneumoniae had 100% resistance to both Ampicillin and Amoxicillin among other antibiotics. In clinical settings,
K. pneumoniae is a significant pathogen that causes lower respiratory infections and urinary tract infections hence the concern over antibiotic-resistant
K. pneumoniae is growing [
43]. High resistance of
K. pneumoniae is increasing the burden of antibiotic resistance in humans on a global scale as some of the human infections caused by
K. pneumoniae are becoming resistant to a number of antibiotics [
39]. Some of the
K. pneumoniae isolates in the current study were found to be multi-drug resistant. This is not in line with results by [
44] which found that
K. pneumoniae isolated from groundwater were not multi-drug resistant. This could be because of the different types of antibiotics that were used during the antimicrobial susceptibility testing in the studies. The current study found ESBL producing enterobacteriaceae isolates in 3 water samples, and all these isolates were multi- drug resistant. Several studies have found that ESBL producing enterobacteriaceae are usually multi-drug resistant. For instance, some scholars found that ESBL producing
E.coli from shallow wells were resistant to more than 8 antibiotics that were used in antimicrobial susceptibility testing [
38]. These 8 antibiotics belonged to classes such as beta lactams, aminoglycosides, fluoroquinolones and sulfonamides. Another study found that 77% of ESBL producing
E. coli were multi drug resistant. ESBL producing enterobacteriaceae are usually resistant to the antibiotics that are effective in the treatment of non ESBL producing enterobacteriaceae [
45]. This could be the reason why ESBL producing enterobacteriaceae are widely considered as antimicrobial resistant bacteria due to their multi-drug resistance patterns.
This study also found that ESBL producing
E. coli and
K. pneumoniae isolates were 100% resistant to Ampicillin. Two of the ESBL isolates were also resistant to Amoxicillin- clavulanic acid with one of the isolates having developed intermediate resistance despite clavulanic acid being a Beta-lactames inhibitor. This resistance was expected because Beta-lactam antibiotics cause resistance in Enterobacteriaceae due to production of extended-spectrum beta-lactamases, leading to β-lactam antibiotic resistance worldwide [
46]. ESBL-producing strains frequently display co-resistance to additional antibiotic classes, such as fluoroquinolones, aminoglycosides, and trimethoprim-sulfamethoxazole, in addition to beta-lactam resistance. Treatment choices are further constrained by this multidrug resistant phenotype [
47].
5. Conclusions
The study revealed that the water samples from these boreholes and shallow wells contained pathogenic microorganisms that are of public health importance. This revelation shows that most of the water from these groundwater sources is unsafe for consumption hence needs to be treated before use. Overall, the study has shown that antimicrobial resistant bacteria are becoming more prevalent in groundwater from boreholes and shallow wells and these groundwater sources have become reserves for these antibiotic resistant bacteria (ARB). Drinking water from these sources could transfer the ARB’s to humans as the groundwater acts as a vehicle for the transmission of these Antibiotic resistant bacteria. Therefore, there is a need for regular monitoring of these pathogenic bacteria and antimicrobial resistant bacteria in groundwater to reduce their spread. As a nation, we need to work to protect the integrity of our groundwater and reduce the risks associated with antimicrobial contamination by giving priority to research, putting in place useful policies, and encouraging community involvement in working towards groundwater preservation.
Author Contributions
Conceptualization, B.V.B.; methodology, B.V.B and H.W.T.M.; validation, B.V.B, H.W.T.M. and B.T.; formal analysis, B.V.B.; investigation, B.V.B.; resources, B.V.B, H.W.T.M and B.T.; data curation, B.V.B.; writing—original draft preparation, B.V.B.; writing—review and editing, B.V.B, H.W.T.M and B.T.; visualization, B.V.B.; supervision, H.W.T.M and B.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The National Commission of Science and Technology Ethical Commission (protocol number 23/02/3168) granted ethical clearance for the study. We also sought permission from Chikwawa District Water Development Officer to collect data in the designated area.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is available in a dissertation and can be provided on needs basis.
Acknowledgments
The authors acknowledge material and equipment support from the Malawi University of Business and Applied Sciences, Kamuzu University of Health Sciences microbiology laboratory and Muthi Nhlema, the team leader at BASEflow.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nationl Statistical Office. 2018 Malawi Population and Housing Census Main Report. 2018 [cited 2023 Jul 21]; Available online: https://dataspace.princeton.edu/handle/88435/dsp0105741v60z.
- Mapoma, H.W.T.; Xie, X. Basement and alluvial aquifers of Malawi: An overview of groundwater quality and policies. Afr. J. Environ. Sci. Technol. 2014, 8, 190–202. [Google Scholar] [CrossRef]
- Li, Karunanidhi D, Subramani T, Srinivasamoorthy K. Sources and Consequences of Groundwater Contamination. Archives of Environmental Contamination and Toxicology 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Olalemi, A.O.; Ige, O.M.; James, G.A.; Obasoro, F.I.; Ogunleye, C.O. Detection of enteric bacteria in two groundwater sources and associated microbial health risks. J. Water Heal. 2021, 19, 322–335. [Google Scholar] [CrossRef]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of water resources by pathogenic bacteria. AMB Express 2014, 4, 51. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.-J.; Blanchard, M.; Chevreuil, M. Occurrence of antibiotics in rural catchments. Chemosphere 2017, 168, 483–490. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- World Health Organization Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed June 8).
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Finley, R.L.; Collignon, P.; Larsson, D.G.J.; McEwen, S.A.; Li, X.-Z.; Gaze, W.H.; Reid-Smith, R.; Timinouni, M.; Graham, D.W.; Topp, E. The Scourge of Antibiotic Resistance: The Important Role of the Environment. Clin. Infect. Dis. 2013, 57, 704–710. [Google Scholar] [CrossRef]
- Gelband H, Miller-Petrie M, Pant S, Gandra S, Levinson J, Barter D, et al. The state of the world’s antibiotics 2015. Wound Healing Southern Africa 2015, 8, 83–90. [Google Scholar]
- United Nations. The 2030 Agenda and the Sustainable Development Goals: An opportunity for Latin America and the Caribbean. 2018; Available online: https://www.cepal.org/sites/default/files/events/files/2030_agenda_and_the_sdgs_an_opportunity_for_latin_america_and_the_caribbean.
- Chidya, R.C.; Matamula, S.; Nakoma, O.; Chawinga, C.B. Evaluation of groundwater quality in rural-areas of northern Malawi: Case of Zombwe Extension Planning Area in Mzimba. Phys. Chem. Earth, Parts A/B/C 2016, 93, 55–62. [Google Scholar] [CrossRef]
- Holm RH, Kunkel G, Nyirenda L. A thought leadership piece: Where are the rural groundwater quality data for the assessment of health risks in northern Malawi? Groundwater for Sustainable Development 2018, 7, 157–63. [Google Scholar] [CrossRef]
- Missi, C.; Atekwana, E.A. Physical, chemical and isotopic characteristics of groundwater and surface water in the Lake Chilwa Basin, Malawi. J. Afr. Earth Sci. 2019, 162, 103737. [Google Scholar] [CrossRef]
- Monjerezi, M.; Ngongondo, C. Quality of Groundwater Resources in Chikhwawa, Lower Shire Valley, Malawi. Water Qual. Expo. Heal. 2012, 4, 39–53. [Google Scholar] [CrossRef]
- Rasool, A.; Farooqi, A.; Xiao, T.; Ali, W.; Noor, S.; Abiola, O.; Ali, S.; Nasim, W. A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ. Geochem. Heal. 2017, 40, 1265–1281. [Google Scholar] [CrossRef]
- Wanda, E.M.; Gulula, L.C.; Phiri, A. Hydrochemical assessment of groundwater used for irrigation in Rumphi and Karonga districts, Northern Malawi. Phys. Chem. Earth, Parts A/B/C 2013, 66, 51–59. [Google Scholar] [CrossRef]
- Kapembo, M.L.; Laffite, A.; Bokolo, M.K.; Mbanga, A.L.; Maya-Vangua, M.M.; Otamonga, J.-P.; Mulaji, C.K.; Mpiana, P.T.; Wildi, W.; Poté, J. Evaluation of Water Quality from Suburban Shallow Wells Under Tropical Conditions According to the Seasonal Variation, Bumbu, Kinshasa, Democratic Republic of the Congo. Expo. Heal. 2016, 8, 487–496. [Google Scholar] [CrossRef]
- MBS. Guidelines for Borehole and Shallow Well Water Quality Specifications (MS 733:2005) [Internet]. 2005. Available online: http://www.arso-oran.org/wp-content/uploads/2014/09/Catalogue-of-Malawi-Standards-2011.
- WHO. Guidelines for drinking-water quality [Internet]. 4th Edition. Geneva, Switzerland: WHO Press; 2011 [cited 2024 May 2]. Available online: https://iris.who.int/handle/10665/44584.
- Mkwate, R.C.; Chidya, R.C.G.; Wanda, E.M.M. Assessment of drinking water quality and rural household water treatment in Balaka District, Malawi. Phys. Chem. Earth. Parts A/B/C 2017, 100, 353–362. [Google Scholar] [CrossRef]
- Vunain E, Nkhuzenje C, Mwatseteza J, Sajidu S. Groundwater quality assessment from Phalombe Plain, Malawi. ChemSearch Journal 2019, 10, 1–10. [Google Scholar]
- Jailos, P.; Chimtali, P.J.; Vunain, E. Assessment of Groundwater Quality in Areas Surrounding Thundulu Phosphate Mine, Phalombe District, Malawi. Tanzan. J. Sci. 2021, 47, 1310–1321. [Google Scholar] [CrossRef]
- Kamanula, J.F.; Zambasa, O.J.; Masamba, W.R. Quality of drinking water and cholera prevalence in Ndirande Township, City of Blantyre, Malawi. Phys. Chem. Earth, Parts A/B/C 2014, 72-75, 61–67. [Google Scholar] [CrossRef]
- Gwimbi, P.; George, M.; Ramphalile, M. Bacterial contamination of drinking water sources in rural villages of Mohale Basin, Lesotho: exposures through neighbourhood sanitation and hygiene practices. Environ. Heal. Prev. Med. 2019, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kanyerere, T.; Levy, J.; Xu, Y.; Saka, J. Assessment of microbial contamination of groundwater in upper Limphasa River catchment, located in a rural area of northern Malawi. Water SA 2012, 38, 581–596. [Google Scholar] [CrossRef]
- Mahmud, Z.H.; Islam, S.; Imran, K.M.; Hakim, S.A.I.; Worth, M.; Ahmed, A.; Hossan, S.; Haider, M.; Islam, M.R.; Hossain, F.; et al. Occurrence of Escherichia coli and faecal coliforms in drinking water at source and household point-of-use in Rohingya camps, Bangladesh. Gut Pathog. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Dey, U.; Sarkar, S.; Duttagupta, S.; Bhattacharya, A.; Das, K.; Saha, S.; Mukherjee, A. Influence of Hydrology and Sanitation on Groundwater Coliform Contamination in Some Parts of Western Bengal Basin: Implication to Safe Drinking Water. Front. Water 2022, 4. [Google Scholar] [CrossRef]
- Di Bella, S.; Capone, A.; Bordi, E.; Johnson, E.; Musso, M.; Topino, S.; Noto, P.; Petrosillo, N. Salmonella enterica ssp. arizonae infection in a 43-year-old Italian man with hypoglobulinemia: a case report and review of the literature. J. Med Case Rep. 2011, 5, 323–323. [Google Scholar] [CrossRef]
- Mahajan RK, Khan SA, Chandel DS, Kumar N, Hans C, Chaudhry R. Fatal Case of Salmonella enterica subsp. arizonae Gastroenteritis in an Infant with Microcephaly. J Clin Microbiol 2023, 41, 5830–2. [Google Scholar]
- Shima, N.; Nakamura, J.; Saito, K.; Kamata, Y.; Nagatani, K.; Nagashima, T.; Iwamoto, M.; Akine, D.; Saito, T.; Sato, K.; et al. Salmonella enterica Subspecies arizonae Detected from Bilateral Pleural Fluid in a Patient with Systemic Lupus Erythematosus and Malignant Lymphoma. Intern. Med. 2020, 59, 1223–1226. [Google Scholar] [CrossRef]
- Mahagamage, M.; Pathirage, M.; Manage, P.M. Contamination Status of Salmonella spp., Shigella spp. and Campylobacter spp. in Surface and Groundwater of the Kelani River Basin, Sri Lanka. Water 2020, 12, 2187. [Google Scholar] [CrossRef]
- Muhsin, E.A.; Nimr, H.K. Estimation of the Role of Mrk Genes in Klebsiella pneumoniae Isolated from River Water and Clinical Isolates. Egypt. J. Aquat. Biol. Fish. 2022, 26, 1319–1328. [Google Scholar] [CrossRef]
- Ajobiewe HF, Ajobiewe JO, Mbagwu TT, Ale T, Taimako GA. Assessment of Bacteriological Quality of Borehole Water, Sachet Water and Well Water in Bingham University Community. American Journal of Medicine and Medical Sciences 2019, 9, 96–103. [Google Scholar]
- Fakayode B, Ogunjobi A. Quality assessment and prevalence of antibiotic resistant bacteria in government approved mini-water schemes in Southwest, Nigeria. International Biodeterioration & Biodegradation 2018, 133, 151–8. [Google Scholar]
- Henriot, C.P.; Martak, D.; Cuenot, Q.; Loup, C.; Masclaux, H.; Gillet, F.; Bertrand, X.; Hocquet, D.; Bornette, G. Occurrence and ecological determinants of the contamination of floodplain wetlands with Klebsiella pneumoniae and pathogenic or antibiotic-resistant Escherichia coli. FEMS Microbiol. Ecol. 2019, 95. [Google Scholar] [CrossRef]
- Chishimba K, Libonda L, Bumbangi FN, Maron M, Mulenga E, Hang’ombe B, et al. A Cross-Sectional Study on the Detection of Extended Spectrum Beta-Lactamase (ESBL) E. coli Producers in Groundwater in Lusaka District. Archives of Epidemiology & Public Health Research 2023, 2, 192–7. [Google Scholar]
- Ateba, C.N.; Tabi, N.M.; Fri, J.; Bissong, M.E.A.; Bezuidenhout, C.C. Occurrence of Antibiotic-Resistant Bacteria and Genes in Two Drinking Water Treatment and Distribution Systems in the North-West Province of South Africa. Antibiotics 2020, 9, 745. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, N.; Xiao, C.; Harter, T.; McCowan, B.; Liu, Y.; Atwill, E.R. Antibiotic-resistant E. coli in surface water and groundwater in dairy operations in Northern California. Environ. Monit. Assess. 2013, 186, 1253–1260. [Google Scholar] [CrossRef]
- Wahome, C.N.; Okemo, P.O.; Nyamache, A.K. Microbial quality and antibiotic resistant bacterial pathogens isolated from groundwater used by residents of Ongata Rongai, Kajiado North County, Kenya. Int. J. Biol. Chem. Sci. 2014, 8, 134. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, A.; Thakur, N.; Kumar, V.; Chauhan, A.; Bhardwaj, N.; Sr. , A.T. Changing Trend in the Antibiotic Resistance Pattern of Klebsiella Pneumonia Isolated From Endotracheal Aspirate Samples of ICU Patients of a Tertiary Care Hospital in North India. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Chang, D.; Sharma, L.; Cruz, C.S.D.; Zhang, D. Clinical Epidemiology, Risk Factors, and Control Strategies of Klebsiella pneumoniae Infection. Front. Microbiol. 2021, 12, 750662. [Google Scholar] [CrossRef]
- Ghartimagar, S.; Khatri, P.; Neupane, S.; Joshi, D.R.; Joshi, T.P. Evaluation of Ground Water Quality of Kathmandu Valley and Antibiotic Susceptibility test against Klebsiella pneumoniae. Tribhuvan Univ. J. Microbiol. 2020, 7, 83–90. [Google Scholar] [CrossRef]
- Franz, E.; Veenman, C.; van Hoek, A.H.A.M.; Husman, A.d.R.; Blaak, H. Pathogenic Escherichia coli producing Extended-Spectrum β-Lactamases isolated from surface water and wastewater. Sci. Rep. 2015, 5, srep14372. [Google Scholar] [CrossRef]
- Teklu, D.S.; Negeri, A.A.; Legese, M.H.; Bedada, T.L.; Woldemariam, H.K.; Tullu, K.D. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control. 2019, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tekele, S.G.; Teklu, D.S.; Tullu, K.D.; Birru, S.K.; Legese, M.H. Extended-spectrum Beta-lactamase and AmpC beta-lactamases producing gram negative bacilli isolated from clinical specimens at International Clinical Laboratories, Addis Ababa, Ethiopia. PLOS ONE 2020, 15, e0241984. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).