Submitted:
06 May 2025
Posted:
08 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Proximate Composition and Reducing Sugars
2.2. Dietary Fiber Content
2.3. Fatty Acids Profile and α-Tocopherol Content
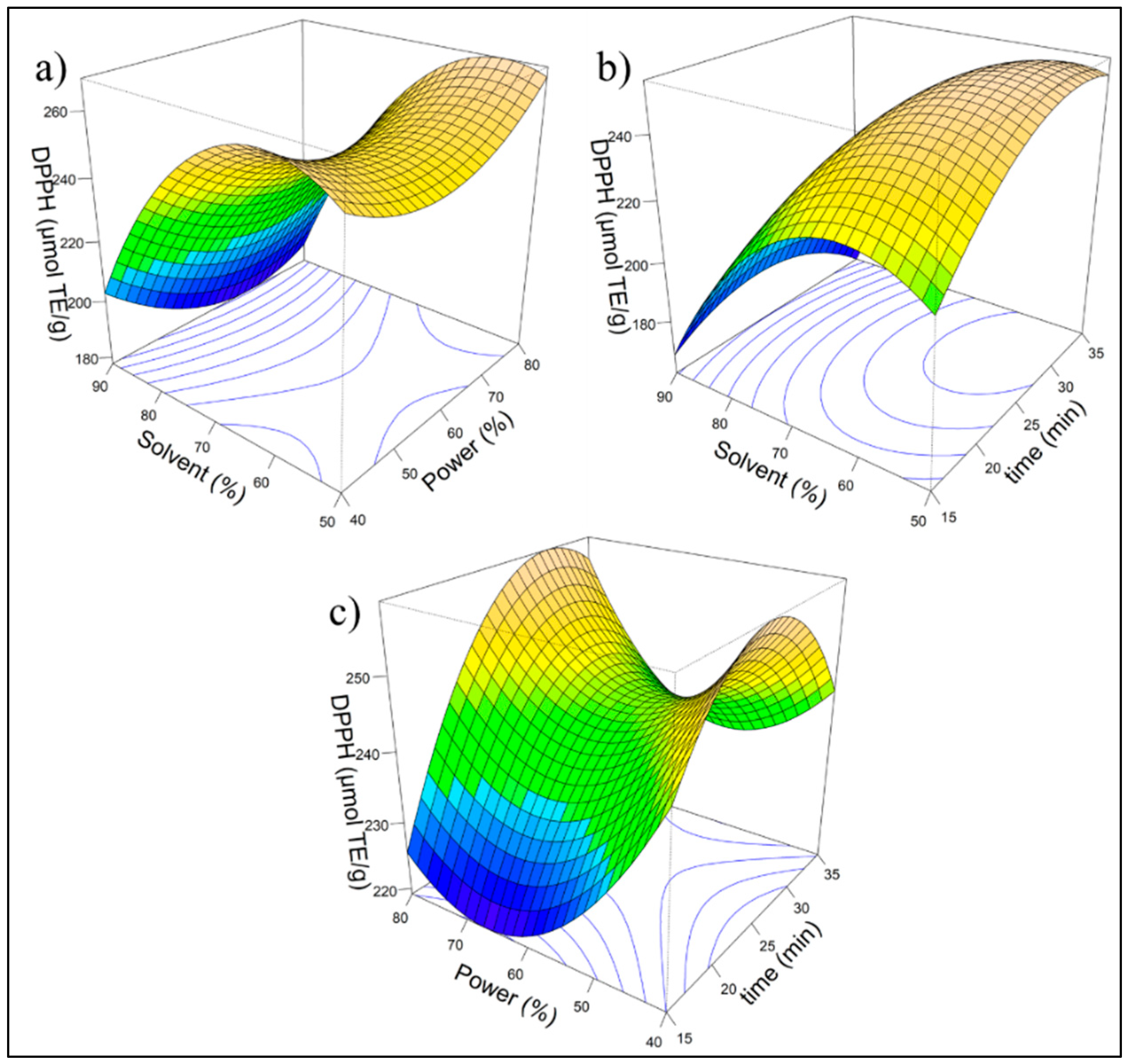
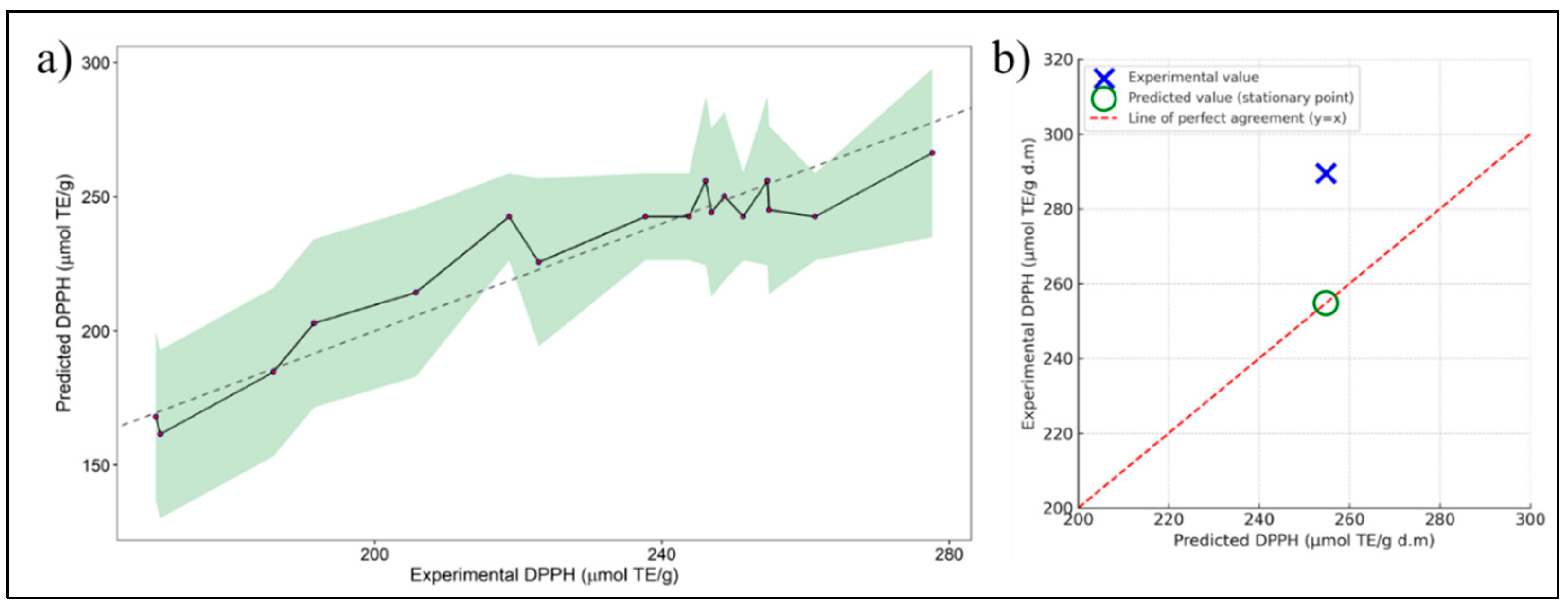
2.4. RSM Method
2.5. Total Bio-Compounds Content
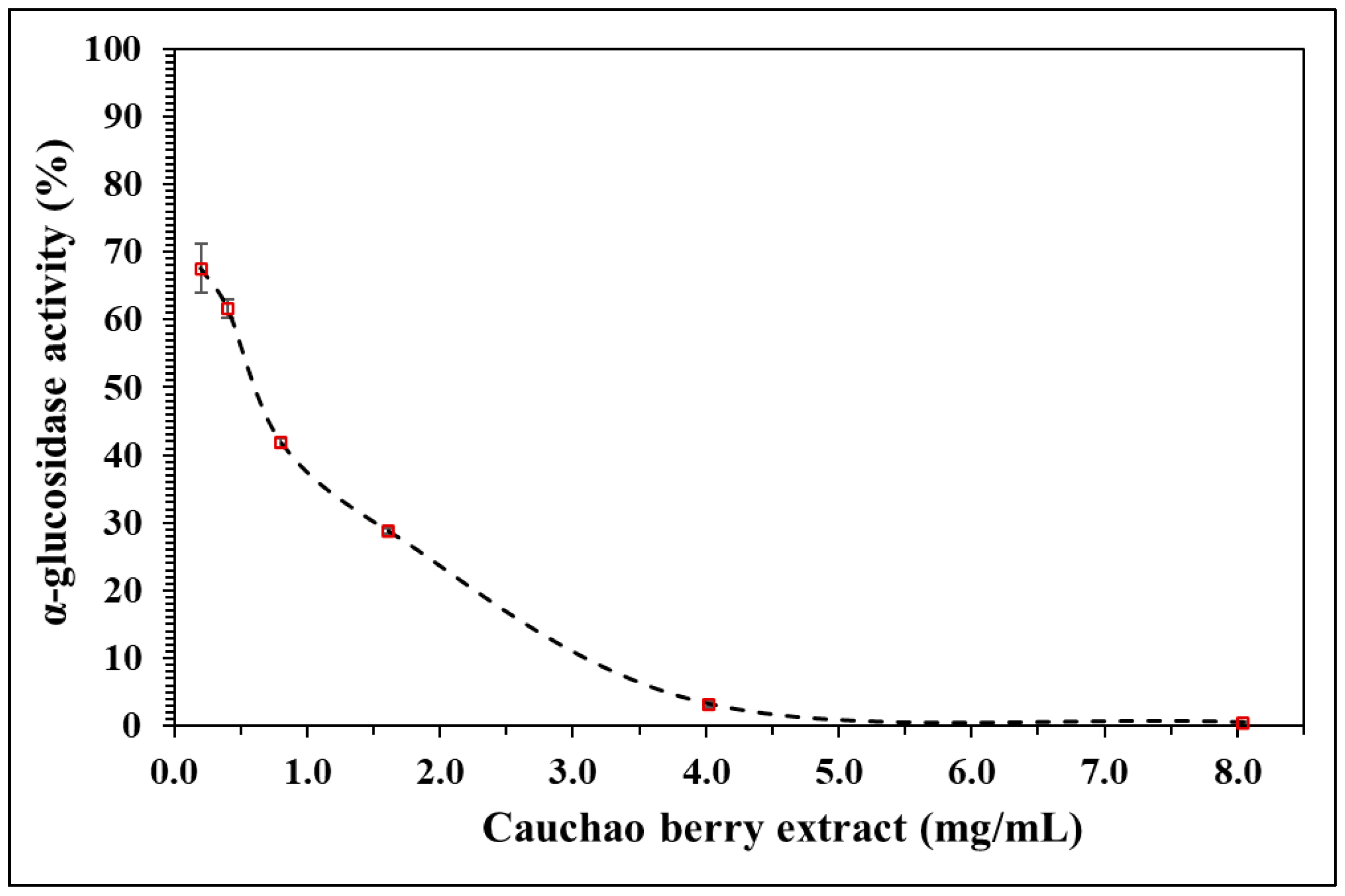
2.6. Antioxidant Potential
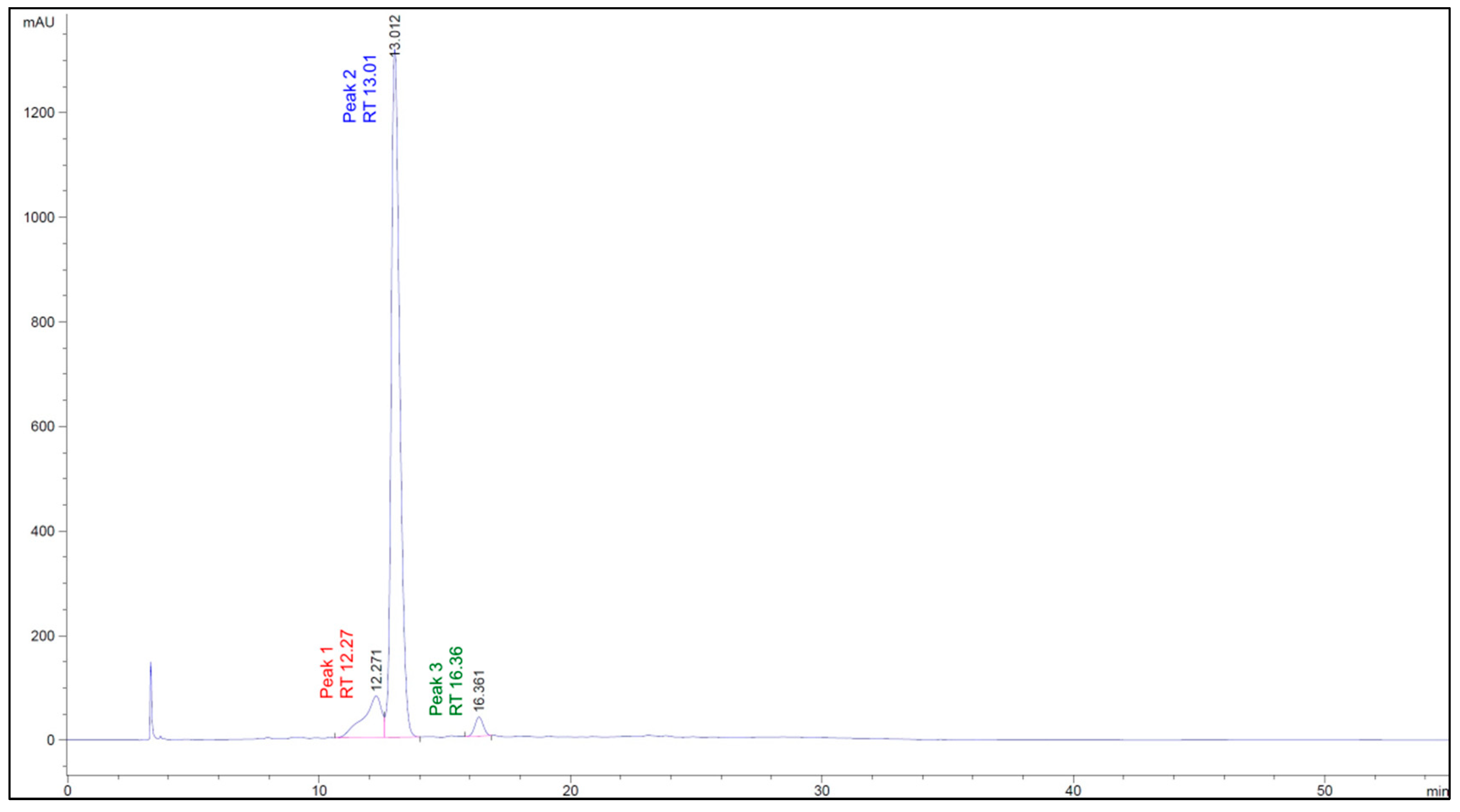
2.8. Phenolics Compound Profile
3. Discussion
4. Materials and Methods
4.1. Raw Material
4.2. Proximate Composition and Reducing Sugars
4.3. Dietary Fiber Content
4.4. Fatty Acids Profile and α-Tocopherols Content
4.5. Ultrasound/Solvent Extraction
4.6. Optimization of Extraction Method
4.7. Total Bio-Compounds Content
4.8. Antioxidant Potential
4.9. α-Glucosidase Activity
4.10. Phenolics Compound Profile
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, Functional Properties and Health-promoting Potential of Native South American Berries: A Review. J Sci Food Agric 2021, 101, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Pap, N.; Fidelis, M.; Azevedo, L.; Do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Current Opinion in Food Science 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Cordero, S.; Abello, L.; & Gálvez, F. Plantas Silvestres Comestibles y Medicinales de Chile y Otras Partes Del Mundo. 2017, Madera ed. Concepción.
- Falkenberg, S.; Tarnow, I.; Guzman, A.; Mølgaard, P.; Simonsen, T. Mapuche Herbal Medicine Inhibits Blood Platelet Aggregation. Hindawi Publishing Corporation, Evidence-Based Complementary and Alternative Medicine 2012, . [CrossRef]
- Weyerstahl, P.; Marschall, H.; Landrum, L.R. Constituents of the Leaf Extract of Amomyrtus Meli (R. A. Philippi) Legrand et Kausel, Amomyrtus Luma (Molina) Legrand et Kausel and of Amomyrtella Guili (Speg.) Kausel. Flavour & Fragrance J 1992, 7, 247–251. [Google Scholar] [CrossRef]
- Archaina, D.; Leiva, G.; Salvatori, D.; Schebor, C. Physical and Functional Properties of Spray-Dried Powders from Blackcurrant Juice and Extracts Obtained from the Waste of Juice Processing. Food sci. technol. int. 2018, 24, 78–86. [Google Scholar] [CrossRef]
- Gagneten, M.; Corfield, R.; Mattson, M.G.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-Dried Powders from Berries Extracts Obtained upon Several Processing Steps to Improve the Bioactive Components Content. Powder Technology 2019, 342, 1008–1015. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrasonics Sonochemistry 2011, 18, 813–835. [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Bioscience 2020, 35, 100547. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; V. González De Peredo, A.; Ferreiro-González, M.; Carrera, C.; Palma, M.; F. Barbero, G.; Espada-Bellido, E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia Chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [CrossRef]
- Watrelot, A.A.; Bouska, L. Optimization of the Ultrasound-Assisted Extraction of Polyphenols from Aronia and Grapes. Food Chemistry 2022, 386, 132703. [Google Scholar] [CrossRef]
- López, J.; Vera, C.; Bustos, R.; Florez-Mendez, J. Native Berries of Chile: A Comprehensive Review on Nutritional Aspects, Functional Properties, and Potential Health Benefits. Food Measure 2021, 15, 1139–1160. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Ah-Hen, K.; Vega-Gálvez, A.; Zura-Bravo, L. Effect of High Hydrostatic Pressure on Rheological and Thermophysical Properties of Murtilla (Ugni Molinae Turcz) Berries. J Food Sci Technol 2016, 53, 2725–2732. [Google Scholar] [CrossRef]
- Al Hasani, S.; Al-Attabi, Z.; Waly, M.; Al-Habsi, N.; Al-Subhi, L.; Shafiur Rahman, M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon Mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods 2023, 12, 871. [Google Scholar] [CrossRef]
- Issis, Q.-F.; Antonio, V.-G.; Elsa, U.; Valeria, V.; Nicole, C.; Jacqueline, P. Vacuum Drying Application to Maqui (Aristotelia Chilensis [Mol] Stuntz) Berry: Weibull Distribution for Process Modelling and Quality Parameters. J Food Sci Technol 2019, 56, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of Dietary Fiber on Human Health. Food Science and Human Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Cabrera-Barjas, G.; Quezada, A.; Bernardo, Y.; Moncada, M.; Zúñiga, E.; Wilkens, M.; Giordano, A.; Nesic, A.; Delgado, N. Chemical Composition and Antibacterial Activity of Red Murta (Ugni Molinae Turcz.) Seeds: An Undervalued Chilean Resource. Food Measure 2020, 14, 1810–1821. [Google Scholar] [CrossRef]
- Gómez-Pérez, L.S.; Moraga, N.; Ah-Hen, K.S.; Rodríguez, A.; Vega-Gálvez, A. Dietary Fibre in Processed Murta (Ugni Molinae Turcz) Berries: Bioactive Components and Antioxidant Capacity. J Food Sci Technol 2022, 59, 3093–3101. [Google Scholar] [CrossRef]
- Bederska-Łojewska, D.; Pieszka, M.; Marzec, A.; Rudzińska, M.; Grygier, A.; Siger, A.; Cieślik-Boczula, K.; Orczewska-Dudek, S.; Migdał, W. Physicochemical Properties, Fatty Acid Composition, Volatile Compounds of Blueberries, Cranberries, Raspberries, and Cuckooflower Seeds Obtained Using Sonication Method. Molecules 2021, 26, 7446. [Google Scholar] [CrossRef]
- Waehler, R. Fatty Acids: Facts vs. Fiction. International Journal for Vitamin and Nutrition Research 2023, 93, 268–288. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary Linoleic Acid and Human Health: Focus on Cardiovascular and Cardiometabolic Effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef]
- Murru, E.; Manca, C.; Carta, G.; Banni, S. Impact of Dietary Palmitic Acid on Lipid Metabolism. Front. Nutr. 2022, 9, 861664. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Shun Ah-Hen, K.; Vega-Gálvez, A.; Morales, A.; García-Segovia, P.; Uribe, E. Effects of Drying Methods on Quality Attributes of Murta ( Ugni Molinae Turcz) Berries: Bioactivity, Nutritional Aspects, Texture Profile, Microstructure and Functional Properties. J Food Process Engineering 2017, 40, e12511. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M.; Poblete, J.; Pasten, A.; Bilbao-Sainz, C.; Wood, D.; McHugh, T.; Delporte, C. Effects of Drying Processes on Composition, Microstructure and Health Aspects from Maqui Berries. J Food Sci Technol 2020, 57, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of Polyphenols and Their Properties. In Polyphenols: Properties, Recovery, and Applications; Elsevier, 2018; pp. 3–44 ISBN 978-0-12-813572-3.
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in Bioactive Components and Antioxidant Capacity of Maqui, Aristotelia Chilensis [Mol] Stuntz, Berries during Drying. LWT 2016, 65, 537–542. [Google Scholar] [CrossRef]
- López, J.; Vega-Gálvez, A.; Ah-Hen, K.S.; Rodríguez, A.; Quispe-Fuentes, I.; Delporte, C.; Valenzuela-Barra, G.; Arancibia, Y.; Zambrano, A. Evaluation of the Antioxidant, Anti-Inflammatory, and Anti-Tumoral Properties of Bioactive Compounds Extracted from Murta Berries (Ugni Molinae T.) Dried by Different Methods. Front. Plant Sci. 2023, 14, 1095179. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Galvez, A.; Pasten, A.; Ah-Hen, K.S.; Mejias, N.; Sepúlveda, L.; Poblete, J.; Gomez-Perez, L.S. Drying: A Practical Technology for Blueberries (Vaccinium Corymbosum L.)—Processes and Their Effects on Selected Health-Promoting Properties. Antioxidants 2024, 13, 1554. [Google Scholar] [CrossRef]
- Lutz, M.; Hernández, J.; Henríquez, C. Phenolic Content and Antioxidant Capacity in Fresh and Dry Fruits and Vegetables Grown in Chile. CyTA Journal of Food 2015, 13, 541–547. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A.; et al. In Vitro Alpha-Amylase and Alpha-Glucosidase Inhibitory Activity and In Vivo Antidiabetic Activity of Withania Frutescens L. Foliar Extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Kawvised, S.; Thukham-mee, W. Encapsulated Mulberry Fruit Extract Alleviates Changes in an Animal Model of Menopause with Metabolic Syndrome. Oxidative Medicine and Cellular Longevity 2019, 2019, 1–23. [Google Scholar] [CrossRef]
- Escobar-Beiza, N.; Pérez-Correa, J.R.; Franco, W. Fermentation of Murta (Ugni Molinae) Juice: Effect on Antioxidant Activity and Control of Enzymes Associated with Glucose Assimilation. IJMS 2023, 24, 15197. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Khatri, D.; Chhetri, S.B.B. Reducing Sugar, Total Phenolic Content, and Antioxidant Potential of Nepalese Plants. BioMed Research International 2020, 2020, 7296859. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. Journal of the Academy of Nutrition and Dietetics 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; He, Y.; Quan, B.; Xia, T.; Zhang, X.; Wang, Y.; Zheng, Y.; Wang, M. Physicochemical Properties, Structure, and Ameliorative Effects of Insoluble Dietary Fiber from Tea on Slow Transit Constipation. Food Chemistry: X 2022, 14, 100340. [Google Scholar] [CrossRef]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host & Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Alarcon-Gil, J.; Sierra-Magro, A.; Morales-Garcia, J.A.; Sanz-SanCristobal, M.; Alonso-Gil, S.; Cortes-Canteli, M.; Niso-Santano, M.; Martínez-Chacón, G.; Fuentes, J.M.; Santos, A.; et al. Neuroprotective and Anti-Inflammatory Effects of Linoleic Acid in Models of Parkinson’s Disease: The Implication of Lipid Droplets and Lipophagy. Cells 2022, 11, 2297. [Google Scholar] [CrossRef]
- Froyen, E.; Burns-Whitmore, B. The Effects of Linoleic Acid Consumption on Lipid Risk Markers for Cardiovascular Disease in Healthy Individuals: A Review of Human Intervention Trials. Nutrients 2020, 12, 2329. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de La Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic Acid Protects against Cadmium Induced Cardiac and Hepatic Tissue Injury in Male Wistar Rats: A Mechanistic Study. Life Sciences 2020, 244, 117324. [Google Scholar] [CrossRef]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Bao, N. Molecular Mechanism of Palmitic Acid and Its Derivatives in Tumor Progression. Front. Oncol. 2023, 13, 1224125. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Nweke, F.N.; Nkeh-Chungag, B.N. Free Radicals, Oxidative Stress-Related Diseases and Antioxidant Supplementation. Alternative Therapies in Health & Medicine 2022, 28, 114–128. [Google Scholar]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory Role in the Cardiovascular System. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef]
- Pelczarski, M.; Wolaniuk, S.; Zaborska, M.; Sadowski, J.; Sztangreciak-Lehun, A.; Bułdak, R.J. The Role of α-Tocopherol in the Prevention and Treatment of Alzheimer’s Disease. Mol Cell Biochem 2025. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. Journal of Food Biochemistry 2022, 46. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; De La Luz Cádiz-Gurrea, M.; Segura Carretero, A.; Mirjalili, M.H. Natural Phenolic Compounds with Neuroprotective Effects. Neurochem Res 2024, 49, 306–326. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Futur J Pharm Sci 2021, 7, 25. [Google Scholar] [CrossRef]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, Characterization, and Health Benefits. Beni-Suef Univ J Basic Appl Sci 2020, 9, 45. [Google Scholar] [CrossRef]
- Hasan, S.; Khatri, N.; Rahman, Z.N.; Menezes, A.A.; Martini, J.; Shehjar, F.; Mujeeb, N.; Shah, Z.A. Neuroprotective Potential of Flavonoids in Brain Disorders. Brain Sciences 2023, 13, 1258. [Google Scholar] [CrossRef]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of Reactive Oxygen Species for the Prevention of Parkinson’s Disease: The Possible Application of Flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Alam, Md.A.; Islam, P.; Subhan, N.; Rahman, Md.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential Health Benefits of Anthocyanins in Oxidative Stress Related Disorders. Phytochem Rev 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Zaa, C.A.; Marcelo, Á.J.; An, Z.; Medina-Franco, J.L.; Velasco-Velázquez, M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules 2023, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch Toxicol 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. IJMS 2021, 22, 4945. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. IJMS 2021, 22, 3380. [Google Scholar] [CrossRef]
- Athauda, D.; Evans, J.; Wernick, A.; Virdi, G.; Choi, M.L.; Lawton, M.; Vijiaratnam, N.; Girges, C.; Ben-Shlomo, Y.; Ismail, K.; et al. The Impact of Type 2 Diabetes in Parkinson’s Disease. Movement Disorders 2022, 37, 1612–1623. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, B.; Zheng, Y.; Liu, X.; Rostyslav, P.; Finiuk, N.; Sik, A.; Stoika, R.; Liu, K.; Jin, M. Neuroprotective Effect of Chlorogenic Acid on Parkinson’s Disease like Symptoms through Boosting the Autophagy in Zebrafish. European Journal of Pharmacology 2023, 956, 175950. [Google Scholar] [CrossRef]
- Majid, A.; Garg, S. Modeling Inhibitory Effects of Chlorogenic Acid on Amyloid Beta Aggregation. Ind. Eng. Chem. Res. 2024, 63, 7636–7645. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and Therapeutic Applications of Sinapic Acid—an Updated Review. Mol Biol Rep 2021, 48, 3733–3745. [Google Scholar] [CrossRef] [PubMed]
- Yasser, M.B.; Hagag, R.S.; El-Sayed, N.M.; Hazem, R.M. Sinapic Acid: A Brief Review of Its Therapeutic Potential and Molecular Targets in Parkinson’s Disease. RECORDS OF PHARMACEUTICAL AND BIOMEDICAL SCIENCES 2025, 9, 1–7. [Google Scholar] [CrossRef]
- Prabhakar, P.; Ahmed, B.A.; Chidambaram, S.B.; Kumar, A.; Pandian, A. In Vitro Ameliorative Effects of Sinapic Acid on Parkinson Related Neurotoxicity in SHSY5Y Cell Lines. International Journal of Nutrition, Pharmacology, Neurological Diseases 2023, 13, 16–24. [Google Scholar] [CrossRef]
- AOAC Official Method of Analysis; 15th Ed.; MA: Association of Official Analytical Chemists: Arlington, 1990.
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A RAPID METHOD OF TOTAL LIPID EXTRACTION AND PURIFICATION. Canadian Journal of Biochemistry and Physiology 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Chandra, M.; Probst, Y.; Price, W.; Kelso, C. Journal of Food Composition and Analysis. p. 104232.
- Rodríguez, K.; Ah-Hen, K.; Vega-Gálvez, A.; López, J.; Quispe-Fuentes, I.; Lemus-Mondaca, R.; Gálvez-Ranilla, L. Changes in Bioactive Compounds and Antioxidant Activity during Convective Drying of Murta (Ugni Molinae T.) Berries. Int J of Food Sci Tech 2014, 49, 990–1000. [Google Scholar] [CrossRef]
- Souza, V.B.D.; Fujita, A.; Thomazini, M.; Da Silva, E.R.; Lucon, J.F.; Genovese, M.I.; Favaro-Trindade, C.S. Functional Properties and Stability of Spray-Dried Pigments from Bordo Grape (Vitis Labrusca) Winemaking Pomace. Food Chemistry 2014, 164, 380–386. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant Activity of Protocatechuates Evaluated by DPPH, ORAC, and CAT Methods. Food Chemistry 2016, 194, 749–757. [Google Scholar] [CrossRef]
- Uribe, E.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Zamorano, M.; Quispe-Fuentes, I.; Pasten, A.; Di Scala, K. Influence of Process Temperature on Drying Kinetics, Physicochemical Properties and Antioxidant Capacity of the Olive-Waste Cake. Food Chemistry 2014, 147, 170–176. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Uribe U., E.; Vega-Gálvez, A.; Poblete G., J.; Olmos C., A.; Pasten C., A. Solar Drying of Flame Seedless (Vitis Vinifera l.) Grape after Different Pretreatments: Characterization of Raisin’s Physicochemical and Functional Properties. Food Measure 2023, 17, 2755–2766. [Google Scholar] [CrossRef]
- Martins, M.S.; Azevedo, R.; Alves, G.; Almeida, A.; De Pinho, P.G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R.; Gonçalves, A.C. Assessment of Chemical Composition of Blackberries and Mulberries from Covilhã Region, Portugal. Journal of Food Composition and Analysis 2025, 137, 106832. [Google Scholar] [CrossRef]
| Parameters (g/100g) | Fresh | Freeze-dried | ||||
| Mositure | 75.31 | ± | 0.41 | 2.78 | ± | 0.10 |
| Fat | 2.15 | ± | 0.15 | 9.15 | ± | 0.16 |
| Ash | 0.57 | ± | 0.03 | 2.50 | ± | 0.15 |
| Crude protein | 1.73 | ± | 0.17 | 7.15 | ± | 0.23 |
| Crude fiber | 3.94 | ± | 0.67 | 15.25 | ± | 0.50 |
| Carbohydrates | 20.24 | ± | 0.32 | 78.42 | ± | 0.29 |
| Reducing sugars | 7.71 | ± | 0.42 | 39.82 | ± | 1.58 |
| Insoluble dietary fiber* | 34.78 | ± | 1.73 | 34.26 | ± | 2.55 |
| Soluble dietary fiber* | 4.60 | ± | 0.50 | 3.66 | ± | 0.81 |
| Total dietary fiber* | 39.37 | ± | 2.23 | 37.92 | ± | 3.36 |
| Fatty acids (g/100 g fatty acid) | Fresh | Freeze-dried | ||||
| C16:0 | 6.99 | ± | 0.02 | 7.18 | ± | 0.13 |
| C18:0 | 2.62 | ± | 0.04 | 2.71 | ± | 0.02 |
| C18:1n9c | 8.83 | ± | 0.05 | 9.24 | ± | 0.17 |
| C18:2n6c | 80.29 | ± | 0.06 | 79.44 | ± | 0.03 |
| C20:0 | 0.35 | ± | 0.07 | 0.41 | ± | 0.01 |
| C20:1 | 0.92 | ± | 0.06 | 1.02 | ± | 0.04 |
| α - tocoferol (µg/g) | 95.51 | ± | 5.42 | 105.41 | ± | 1.39 |
| Factors | Coded factor | Result | ||||
| Solvent (%) | Power (%) | time (min) | X1 | X2 | X3 | DPPH (μmol TE/g d.m) |
| 90 | 80 | 25 | 0 | 1 | 1 | 185.89 |
| 50 | 60 | 35 | 1 | 0 | -1 | 248.70 |
| 70 | 40 | 35 | 1 | -1 | 0 | 246.87 |
| 50 | 40 | 25 | 0 | -1 | -1 | 254.67 |
| 90 | 60 | 35 | 1 | 0 | 1 | 170.16 |
| 90 | 60 | 15 | -1 | 0 | 1 | 169.52 |
| 70 | 60 | 25 | 0 | 0 | 0 | 261.32 |
| 90 | 40 | 25 | 0 | -1 | 1 | 191.53 |
| 70 | 60 | 25 | 0 | 0 | 0 | 237.68 |
| 50 | 60 | 15 | -1 | 0 | -1 | 205.62 |
| 50 | 80 | 25 | 0 | 1 | -1 | 277.65 |
| 70 | 60 | 25 | 0 | 0 | 0 | 243.76 |
| 70 | 60 | 25 | 0 | 0 | 0 | 218.70 |
| 70 | 80 | 15 | -1 | 1 | 0 | 222.84 |
| 70 | 40 | 15 | 0 | -1 | 0 | 254.89 |
| 70 | 80 | 35 | 1 | 1 | 0 | 246.09 |
| 70 | 60 | 25 | 0 | 0 | 0 | 251.31 |
| TPC (mg GAE/g d.m) | 25.43 | ± | 0.85 |
| TFC (mg QE/g d.m) | 46.51 | ± | 1.38 |
| TAC (mg cyanidin-3-glucoside/g d.m) | 5.91 | ± | 0.40 |
| DPPH (μmol TE/g d.m) | 289.54 | ± | 9.05 |
| ORAC (μmol TE/g d.m) | 451.09 | ± | 6.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





