1. Introduction
Diabetes is a widespread and complex metabolic disorder, characterized primarily by chronic hyperglycemia, resulting from the impaired insulin secretion, insulin resistance, or both [
1]. Among its various forms, type 2 diabetes is the most prevalent, affecting millions globally and posing a major burden on public health systems due to its progressive nature and associated complications [
2]. High postprandial blood glucose levels are of significant concern in type 2 diabetes management, as they contribute to oxidative stress, inflammation, and tissue damage, ultimately leading to complications such as cardiovascular disease, neuropathy, and retinopathy [
3,
4]. Consequently, managing blood glucose levels, particularly after meals, is crucial to type 2 diabetes treatment strategies [
5].
One of the promising approaches to controlling postprandial blood glucose levels is the inhibition of key carbohydrate-digesting enzymes, α-glucosidase and α-amylase. These enzymes facilitate the breakdown of complex carbohydrates into glucose, which is subsequently absorbed into the bloodstream [
6,
7]. By inhibiting these enzymes, glucose absorption can be delayed, resulting in more controlled blood glucose levels after meals. While synthetic inhibitors such as acarbose have been developed and are widely used for this purpose, they often cause gastrointestinal side effects, including bloating and diarrhea, limiting their long-term use. This has fueled interest in identifying natural alternatives with fewer side effects, particularly those derived from plants known for their health-promoting properties [
7,
8,
9].
In recent years, black elderberry (
Sambucus nigra L.) has gained significant attention for its rich polyphenolic and flavonoid content, which is associated with antioxidant, anti-inflammatory, and potential antidiabetic properties [
10,
11,
12]. Traditionally used in herbal medicine, elderberry flowers and berries contain high amount of bioactive compounds such as phenolic compounds, of which chlorogenic acid and quercetin derivatives occur in the highest concentrations. This translates into various beneficial effects, including reducing oxidative stress and modulating blood glucose levels [
13,
14]. Polyphenols, in particular, have been associated with the inhibition of α-glucosidase and α-amylase, making elderberry a compelling candidate for further research into its antidiabetic potential [
15].
This research aims to establish an optimized extraction protocol to obtain polyphenol-rich extracts from elderberry flowers, to characterize the extracts’ phytochemical composition across different elderberry varieties, and to evaluate their antidiabetic potential through enzyme inhibition assays. By systematically analyzing the relationship between the polyphenolic content and biological activity, this study offers a comprehensive assessment of elderberry flower extracts as potential natural agents for diabetes management, supporting the broader investigation of elderberry and other medicinal plants in chronic disease treatment strategies. Moreover, the differences between the phytochemical content and bioactivity between the different elderberry varieties were revealed during the study, what may facilitate the preselection of the most potent ones for further investigations.
2. Results and Discussion
2.1. Optimization of the Extraction Process
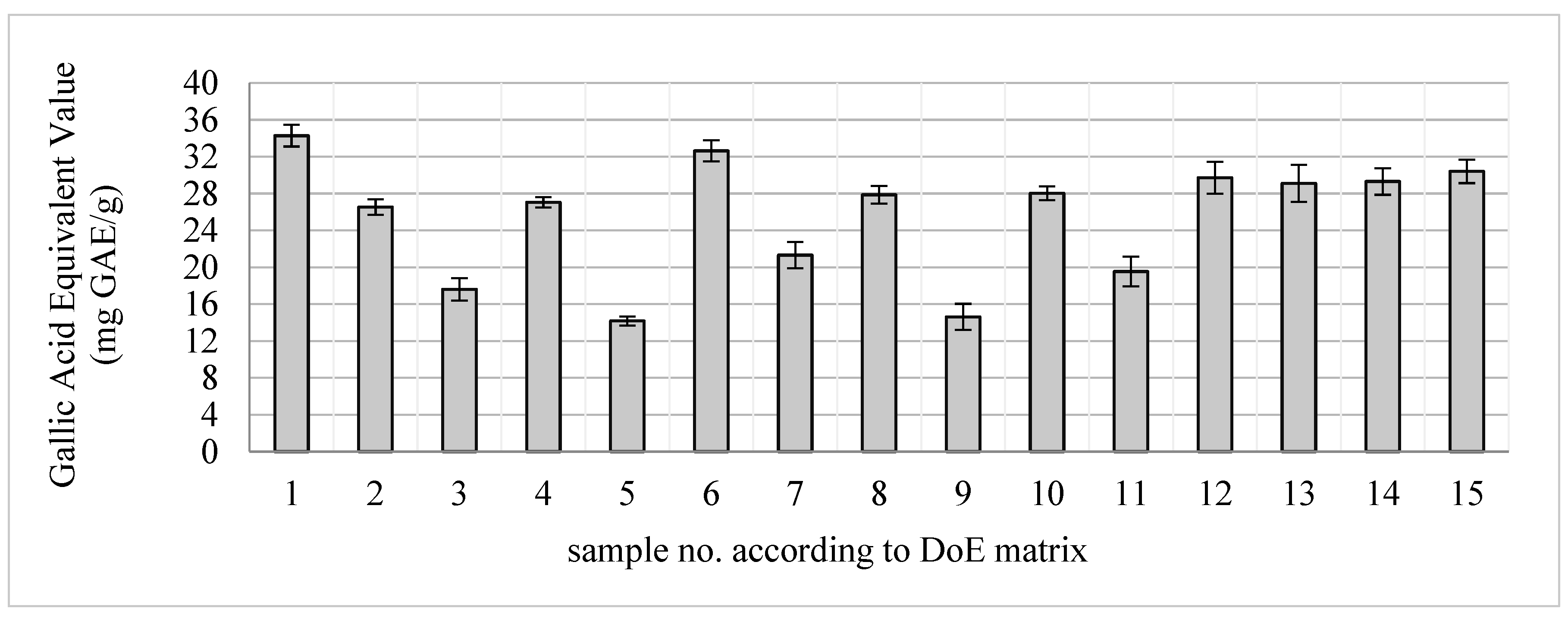
Using the Box-Behnken statistical model, the extraction conditions were optimized to assess the effect of methanol content, process time and solvent volume to plant material ratio. In the extraction optimization process, water, water-methanol and methanol extracts from elderberry flowers were obtained. The extracts were assessed for total polyphenol content using the Folin-Ciocalteau reagent (
Figure 1).
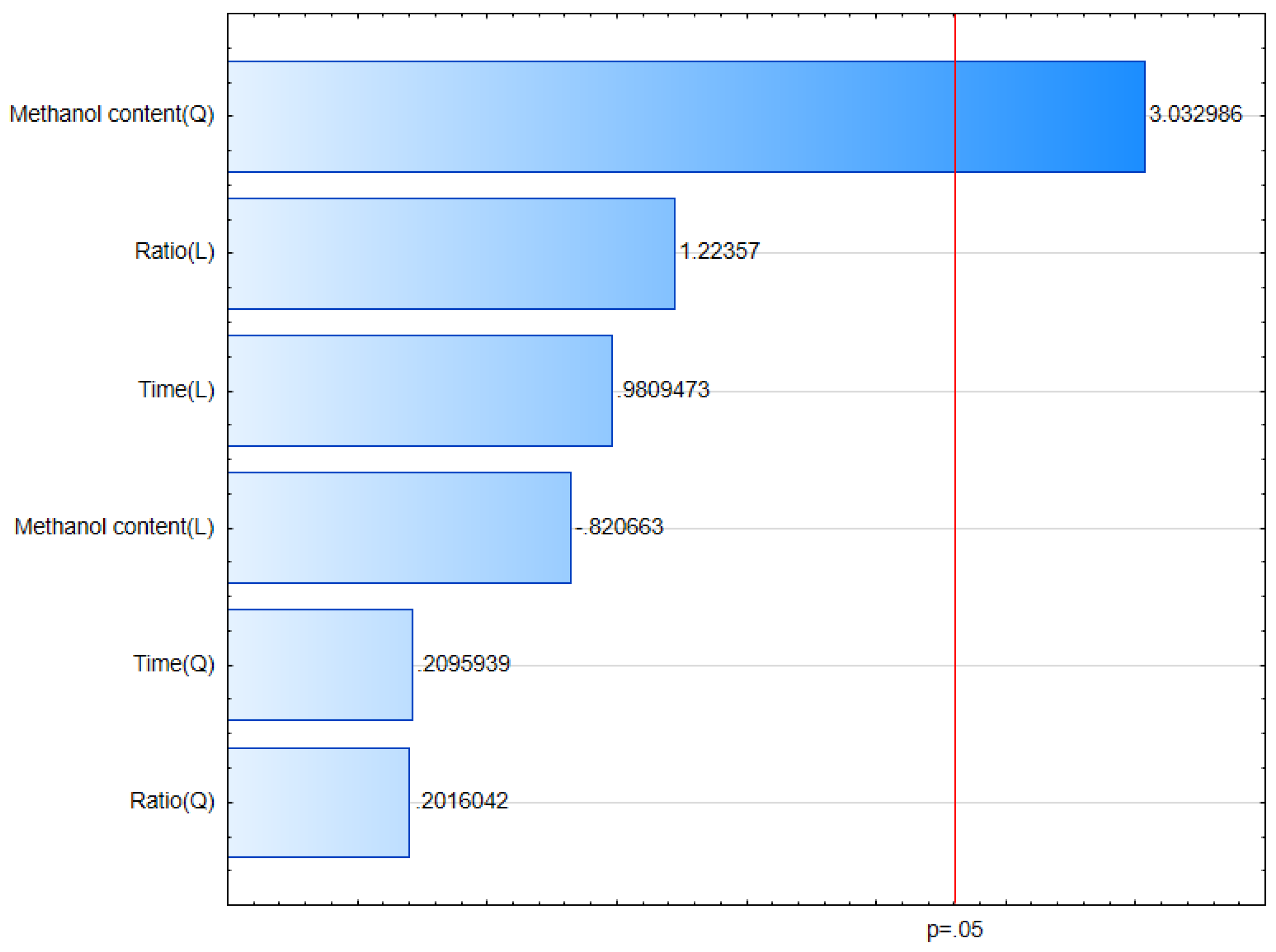
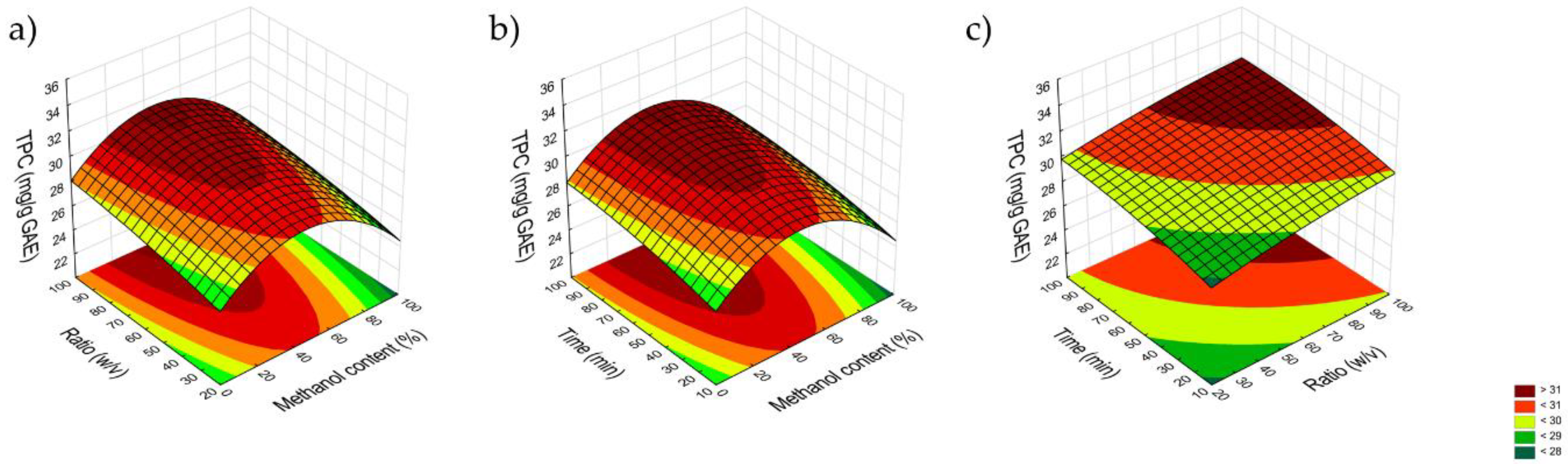
The obtained results were entered into a statistical model to analyze the influence of the following factors: methanol content, process time and solvent volume to sample ratio on the efficiency of the extraction of the active compounds from plant material. The analysis allowed to determine that the optimal solvent for the extraction is methanol 45%. Factors like time and solvent volume did not significantly change the efficiency of the extraction process in relation to the adopted criterion (total polyphenol content), therefore, in order to minimize the use of time and reagents, a decision was made to adopt the lowest values of the tested process parameters (
Figure 2 and
Figure 3).
The optimization of the extraction processes using Box-Behnken design as a response surface methodology has been widely studied, particularly in the context of extracting of bioactive compounds from various plant materials. Using this method, appropriate extraction parameters can be chosen to maximize the amount of the active compounds extracted [
16,
17,
18]. The role of methanol as a significant solvent is further supported by studies such as those by Tai et al., who optimized the extraction conditions for antioxidant compounds from banana peels, where solvent concentration was identified as a key variable [
19]. Attempts have also been made to optimize the extraction of elderflowers. Oziembłowski et al. applied response surface methodology (RSM) to optimize the extraction of chlorogenic acid from elderberry flowers [
20]. Their findings indicated that the extraction process could be efficiently shortened to 20 days with ethanol at a concentration of approximately 68%, without significant losses in chlorogenic acid yield. Domínguez et al. demonstrated that temperature of 60°C, 50% ethanol concentration, and a pH of 2 were the optimal extraction parameters for chlorogenic acid [
21]. The solvent composition identified in their optimization is similar to the results obtained in our study, strongly supporting the use of alcohol around 50% as an efficient solvent for polyphenol extraction from elderberry flowers. Research indicates that
Sambucus nigra flowers possess a significant concentration of polyphenolic compounds. For instance, Viapiana et al. reported a high phenolic content ranging from 15.23 to 35.57 mg GAE/g DW for elderflowers [
22]. The study by Zawiślak et al. specifically utilized the Folin-Ciocalteu method to quantify polyphenols in dried elderflowers [
22]. The results indicated that the drying conditions significantly influenced the polyphenol content, suggesting that optimal processing methods are crucial for maximizing the health benefits of elderflower extracts.
2.2. Phytochemical Studies of the Extracts
High-performance liquid chromatography analysis allowed us to determine the content of chlorogenic acid, rutin and isoquercetin, i.e. the active compounds in water-methanol extracts of black elderberry flowers. The highest content of chlorogenic acid was characterized by extracts from the Black Beauty (23.73±0.01 mg/g of plant material) and Obelisk (21.03±0.00 mg/g of plant material) varieties (
Table 1), while the lowest content in Bez koralowy (9.39 mg/g of plant material). Varieties rich in rutin are Haschberg (38.37±0.01 mg/g of plant material) and Obelisk (30.12±0.18 mg/g of plant material), while the Golden Hybrid variety (4.01 mg/g of plant material) is poor in this compound. Among the compounds tested, the lowest content of isoquercetin was found, regardless of the variety tested. The highest concentration of this flavonoid was noted in the extracts from Sambo flowers (4.69±0.00 mg/g of plant material), the lowest in the Haschberg variety (0.09±0.13 mg/g of plant material). So, it can be stated that there are significant differences in the amount of these secondary metabolites depending on the
S. nigra variety studied.
Analyses of the total content of polyphenols and flavonoids were performed to deepen the phytochemical studies (
Table 1). The results of these studies allowed for a better understanding of the chemical differences between the flowers of the tested
S. nigra varieties. In addition, the obtained data allowed for the conclusion about the potential relationship between the content of polyphenols and flavonoids in the tested raw materials and the biological activity of the tested extracts. The results of the total polyphenol analysis largely matched the results of the HPLC analysis. The Black Beauty variety, characterized by the highest content of chlorogenic acid, had the highest total polyphenol content (44.97 mg GAE/g). Moreover, the Black Tower (40.48 mg GAE/g) and Sambo (38.47 mg GAE/g) varieties were particularly rich in polyphenols. It is worth noting that both the Black Beauty and Black Tower varieties are characterized by purple-red coloration of leaves and flowers, which suggests the presence of other polyphenolic compounds from the anthocyanin group in different parts of this plant [
23]. The results of the conducted evaluation of the total flavonoid content were also consistent with the results of the HPLC analysis. The highest amount of flavonoids was recorded for the flowers of the Haschberg variety richest in rutin (18.19 mg QE/g) and the flowers of the Obelisk variety (16.56 mg QE/g), which was characterized by the second highest rutin content among the extracts tested. The lowest total flavonoid content was recorded for the flowers of the Sampo variety (9.58 mg QE/g). Interestingly, the analysis of the content of active compounds in the pharmacopoeial raw material conducted simultaneously indicated that, in comparison to the other elderberry flower varieties studied, it showed an average content of chlorogenic acid (15.39 ± 0.10), rutin (13.33 ± 0.15) and isoquercetin (1.76 ± 0.09). The extract from
S. nigra flowers also showed a lower total content of polyphenols and flavonoids than the other varieties studied.
The analysis of literature data showed that studies comparing the chemical composition of extracts from flowers of different varieties of black elderberry are scarce and do not concern those examined by us. The assessment of the content of the active compounds in the pharmacopoeial plant material was conducted earlier. It indicated that chlorogenic acid, rutin and isoquercetin are present in Wild elderberry (
Sambuci flos) [
24]. Such analyses were conducted, among others, for alcoholic extracts, where chlorogenic acid was one of the dominant phenolic acids (318.6 ± 4.1 mg/100 g ex), rutin was the dominant flavonoid (573.5 ± 5.0 mg/100 g ex), and the content of isoquercetin was significantly lower (57.5 ± 2.7 mg/100 g ex) [
25]. In turn, Viapiana and Wesołowski [
10] determined the content of chlorogenic acid and rutin for infusions prepared from commercially available plant material – black elderberry flowers (6.26 mg/g of chlorogenic acid and 13.3 mg/g of rutin). Based on the obtained results and chromatograms presented in the paper, it can be stated that both chlorogenic acid and rutin are important components of most black elderberry flowers, regardless of the variety tested. In addition, Viapiana and Wesołowski [
10] determined the total polyphenol content (TPC) for the infusions prepared from Wild elderberry (
Sambuci flos). The determined TPC value ranged from 15.23 to 35.57 mg GAE/g of plant material. The results obtained in the present paper were slightly higher and varied depending on the variety tested (the lowest of the obtained values was 25.05 mg GAE/g of plant material, and the highest was 44.97 mg GAE/g). In the same experiment, the total flavonoid content in the infusions was determined. This value was also lower (from 5.27 to 13.19 mg) than the highest achieved in the present work (18.19 mg QE/g for the Haschberg variety). Considering the results of our research and those cited, it should be noted that the optimization of the extraction process used could have resulted in a higher content of total polyphenols and flavonoids, although the tested elderberry variety was also significant. In addition, regarding the lower quantities of phenolic compounds detected in
S. flos samples, it can be pointed out the interesting potential of other varieties of black elderberry than those used in medicine.
2.3. Studies of Biological Activity of Extracts
Comparative studies of the biological activity of the extracts from twelve varieties of black elderberry flowers were conducted for the first time. For this purpose, antioxidant activity, the ability to reduce copper ions, the ability to inhibit the enzymes α-glucosidase and α-amylase as well as the anti-inflammatory potential were examined.
2.3.1. Extracts from the Flowers of Varieties of Black Elderberry Can Protect Against Free Radicals
Antioxidant activity plays a key role in the prevention of lifestyle diseases such as cardiovascular diseases, cancers, and neurodegenerative diseases, including diabetes control [
26]. In diabetes, excess reactive oxygen species (ROS) resulting from hyperglycemia contributes to oxidative stress, which damages pancreatic cells, reducing insulin secretion and increasing insulin resistance [
27]. Antioxidant activity helps to neutralize ROS, limiting cell and tissue damage and inhibiting inflammatory processes that worsen diabetic complications. Thus, the detection of biological activity studies began with the assessment of antioxidant activity. For this purpose, the ability of antioxidant substances to reduce the DPPH radical was evaluated, and the ability to reduce copper ions was assessed using the CUPRAC method (
Table 2). In the study using the DPPH radical, the highest antioxidant activity was demonstrated by the Black Beauty (53.15 mg TE/g) and Obelisk (49.92 mg TE/g) varieties. Slightly lower activity in this direction was demonstrated by Haschberg flowers (45.01 mg TE/g). These results are consistent with the increased content of the active compounds in the extracts tested. The Black Beauty variety also showed a superior ability to reduce copper ions (77.19 mg TE/g), suggesting that the high chlorogenic acid content increases the antioxidant properties of the raw material, while the low content determined poor antioxidant activity (Bez koralowy: 19.23 mg TE/g and 42.32 mg TE/g, for DPPH and CUPRAC, respectively). The strong antioxidant activity of chlorogenic acid was also confirmed by the standard study (IC
50 0.150 mg/mL). Rutin, even though it works similarly to chlorogenic acid, had a moderate effect on activity of examined extracts.
2.3.2. Extracts from the Flowers of Varieties of Black Elderberry Inhibit α-Glucosidase More Strongly Than α-Amylase.
Inhibiting the enzymes α-glucosidase and α-amylase is an important mechanism in managing blood sugar levels, especially in people with type 2 diabetes [
28]. A-amylase breaks down starch into smaller molecules [
6], which are then digested by α-glucosidase into glucose that is absorbed into the blood [
29]. Blocking these enzymes slows down the process of breaking down carbohydrates, leading to a smaller and slower rise in blood glucose after a meal. Therefore, the aim of the in vitro studies using α-glucosidase and α-amylase enzymes was to determine the hypoglycemic potential of the tested extracts from flowers of different elderberry varieties. In case of the inhibition of α-glucosidase, the flowers of the Black Beauty cultivar showed twice the capacity in this respect than the reference substance (acarbose) at the same concentration (55.89% and 27.85%, respectively) (
Table 3). The next cultivars with high activity were Black Tower (51.39%), Samyl 1 (50.96%) and Obelisk (49.61%). The results of the inhibition of α-glucosidase are consistent with the high content of chlorogenic acid in the active extracts, which has confirmed efficacy in inhibiting this enzyme [
30]. In addition, there are results in the literature suggesting that the extracts from black elderberry flowers inhibited α-glucosidase activity, with this activity being higher for alcoholic than aqueous extracts [
31], while the extracts from black elderberry fruits did not inhibit α-glucosidase [
32]. This suggests that the hydroalcoholic extracts from the flowers of different varieties of black elderberry analyzed in our study may be material for further research in terms of their antidiabetic potential, perhaps more interesting than the anthocyanin-rich fruits of this plant.
In the case of amylase inhibition studies, most of the extracts were found to be ineffective despite the doubled concentration of the extracts. The highest capacity at a concentration of 0.1 g/mL was demonstrated by the flowers of the Obelisk variety (33.95%). This may be due to the high content of chlorogenic acid (21.03 mg/g of plant material) and rutin (30.12 mg/g of plant material), which have a literature-confirmed ability to inhibit amylase [
33,
34].
2.3.3. Extracts from the Flowers of Black Beauty and Balck Tower Varieties Reveal the Higher Anti-Inflammatory Potential
Chronic inflammation is a key factor in the development of many diseases, it can worsen the complications such as neuropathy and retinopathy and impair wound healing [
35,
36]. Hyaluronidase is an enzyme that degrades hyaluronic acid, a crucial component of the extracellular matrix that promotes its breakdown, thereby weakening the protective barrier function of the skin [
37]. Excessive enzyme can also generate low-molecular-weight HA fragments, which may further intensify inflammation [
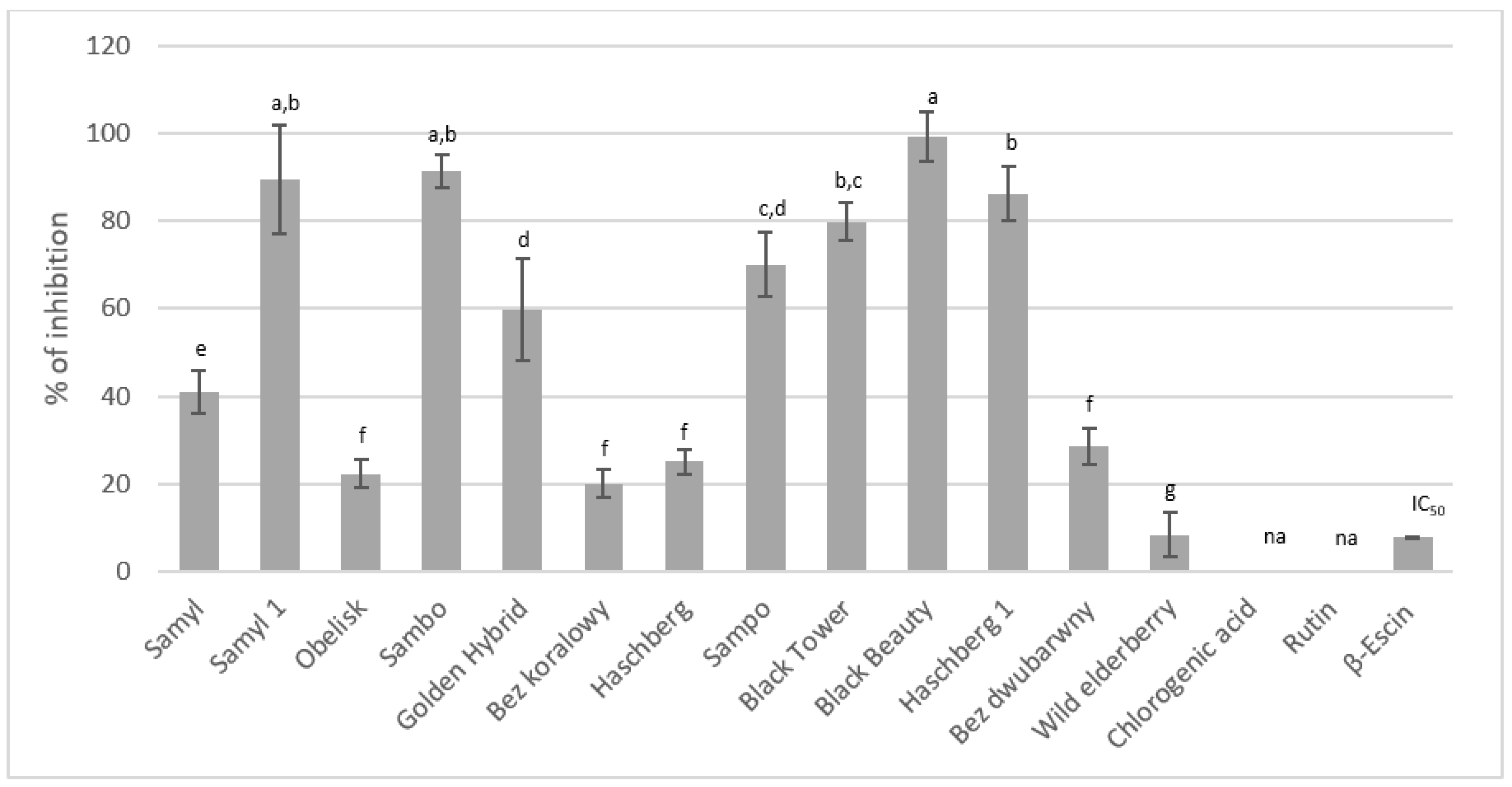
38]. Studies investigating hyaluronidase inhibition by the extracts from the flowers of different elderberry varieties demonstrated varying enzyme inhibition capacities. The highest inhibition rate (99.11 ± 5.64%) was observed for the extract from the "Black Beauty" variety, which also contained the highest polyphenol levels (
Figure 4). Significant inhibitory activity (>80%) was also shown by the "Sambo”, “Samyl”, and “Haeschberg 1” varieties, both rich in isoquercetin, suggesting a substantial role for this compound in inhibiting hyaluronidase. The findings of Kim et al. confirm that isoquercetin exhibits strong inhibitory effects on this enzyme, supporting the conclusions of this study [
39]. Conversely, although rutin and chlorogenic acid were dominant in the samples, they had minimal impact on the inhibition. Studies have demonstrated that neither rutin nor chlorogenic acid at 25 mg/mL concentrations inhibited the enzyme, which aligns with Zhou et al., who observed weak inhibitory activity for chlorogenic acid, and with the findings of Je-Hyuk Lee, who found no inhibitory effect from rutin at low doses [
40].
During the inflammation, large amounts of nitric oxide (NO) are produced, the presence of which is associated with the functioning of factors such as cytokines or bacteria. Therefore, the compounds that inhibit NO production may be inflammatory modulators. Similarly, exposure of macrophages to lipopolysaccharides (LPS) generates an inflammatory-like response [
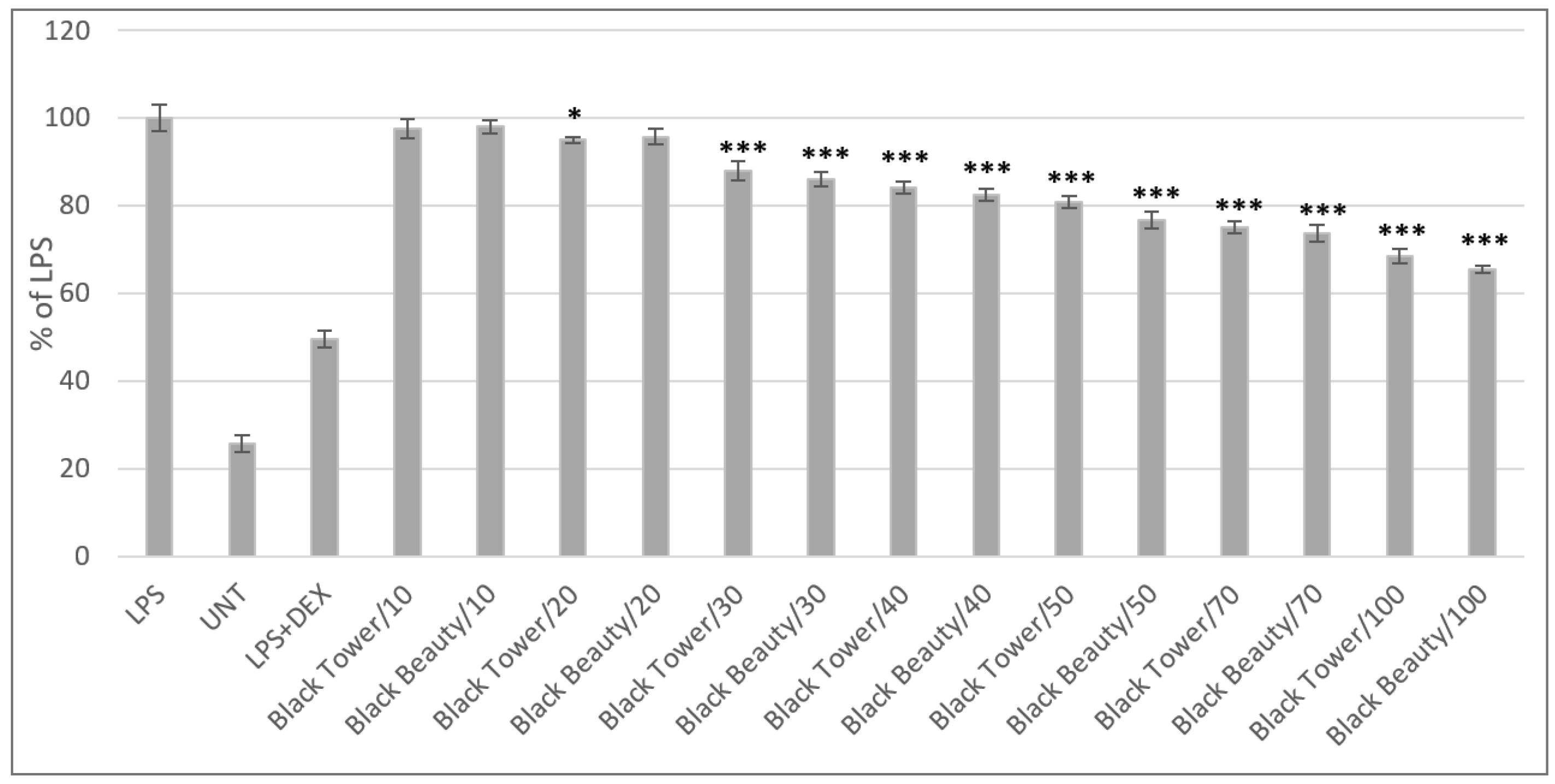
41]. For these reasons, in the next part of the experiment anti-inflammatory potential of the tested extracts was evaluated, using the LPS-stimulated RAW 267.4 murine macrophages model, with nitric oxide as a determined indicator of an inflammatory state. First, the impact of the tested samples on macrophages viability was examined and the results indicated that none of the tested extracts was toxic to the cells, in the tested concentration range. In the highest tested concentration (100 μg/mL) cell viability did not differ significantly from the controlled, untreated cells. Thus, for the anti-inflammatory assay, whole tested concentration range was chosen. Although most of the studied extracts did not reveal anti-inflammatory activity, samples from Black Tower and Black Beauty varieties were the most potent, in terms of the inhibition of nitric oxide release (
Figure 5) and the observed effect was dose-dependent. No significant differences were observed between both active extracts, however the inhibition of NO release by the two extracts was significantly higher when compared to LPS-stimulated cells, except for the lower tested concentrations.
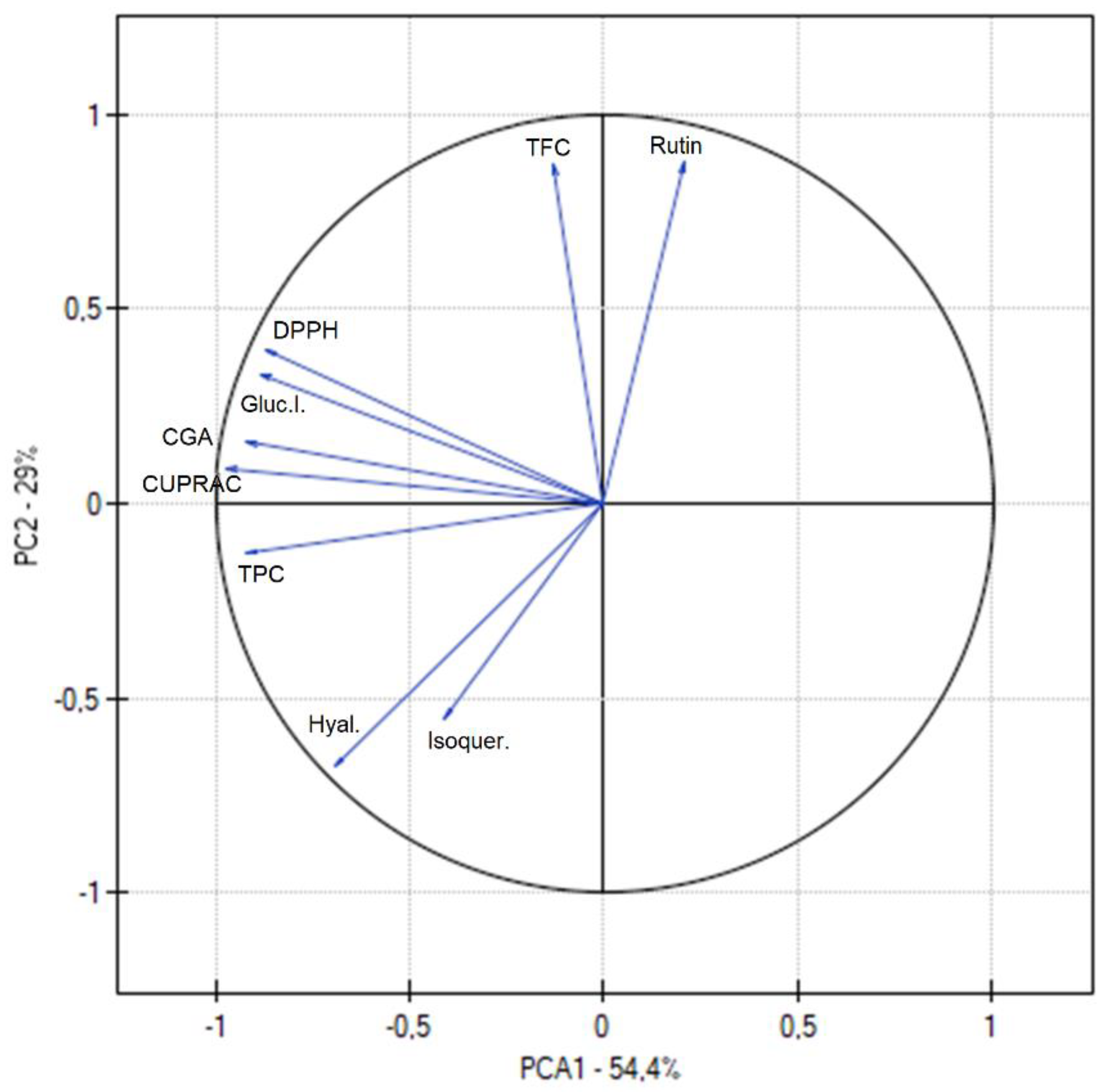
To analyze the relationship between the effects, Principal Component Analysis (PCA) was performed, which is a popular data analysis method that allows observing regularities between the studied variables.
Figure 6 shows the results of PCA analysis of extracts, considering all varieties. A strong positive correlation was observed between chlorogenic acid content, TPC and DPPH, chlorogenic acid content, TPC and CUPRAC as well as between rutin and TFC, which was described previously. Moreover, a strong correlation was noticed between chlorogenic acid content and α-glucosidase inhibition. The above indicates an important role of chlorogenic acid content in the activity of the plant material. It was also noted that between TPC and hyaluronidase inhibition was a strong positive correlation which indicated an important role of polyphenols components in biological activity.
3. Materials and Methods
3.1. Chemical Reagents
Sodium carbonate, sodium hydroxide, DMSO, formic acid, methanol, ammonium acetate, and copper (II) chloride were purchased from Avantor Performance Materials Poland S.A. (Gliwice, Poland). HPLC grade acetonitrile and water , as well as Folin-Ciocalteu phenol reagent, were from Merck (Darmstadt, Germany). Acarbose, β-escin, chlorogenic acid, gallic acid, isoquercetin, quercetin, rutin, and trolox were purchased from Sigma Aldrich (St. Louis, MO USA). All other chemicals were from the Sigma–Aldrich Chemical Co. (St. Louis, MO USA). High-quality pure water and ultra-high-quality pure water were prepared using a Direct-Q 3 UV Merck Millipore purification system.
3.2. Plant Material
The plant material tested was dried flowers of black elderberry varieties: (1) Samyl, (2) Samyl 1, (3) Obelisk, (4) Sambo, (5) Golden Hydrid, (6) Bez koralowy, (7) Haschberg, (8) Sampo, (9) Black Tower, (10) Black Beauty, (11) Haschberg 1, (12) Bez dwubarwny. The Wild elderberry flowers (pharmacopeias material Sambuci flos) were purchased from Polish herb packaging company: Kawon.
The plant material for the tests came from the experimental station area of the Central Center for Research on Cultivar Plant Varieties (COBORU) in Słupia Wielka. The material was provided for the tests thanks to the kindness of Prof. Piotr Szulc from the Department of Agronomy of the University of Life Sciences in Poznań [
42].
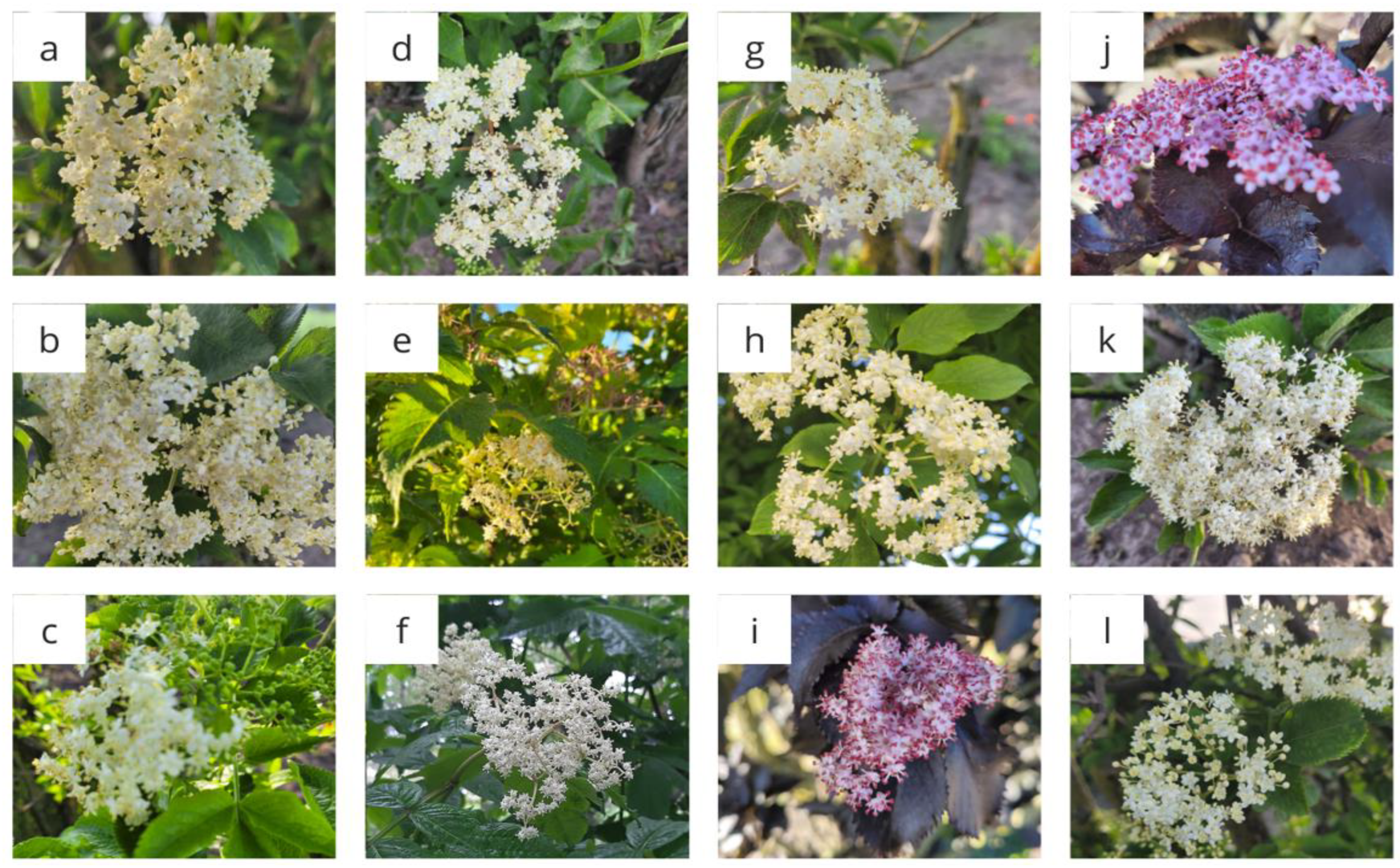
The appearance of the flowers of the tested varieties is shown in
Figure 7.
3.3. Optimization of the Extraction Process
The Box-Behnken statistical design was used to develop the model and to investigate and evaluate the influence of input factors on the experimental result using Statistica 13.3 software (TIBCO Software Inc.). The input data for Sambuci flos extraction were the influence of three variables – the influence of process time, methanol concentration and the ratio of plant material mass to solvent (expressed in the experimental plan as the volume of solvent used). The output data were the content of polyphenols in the extracts assessed by Folin-Ciocalteu method. The following constant parameters were assumed: 1) plant material weight: 500 mg, 2) extraction temperature: 50°C, 3) number of extraction repetitions: 3.
Taking these basics into account, the experimental plan was generated and placed in
Table 4. Fifteen extracts (S1-S15) were obtained.
The experiments were performed on the pharmacopoeial plant material Sambuci flos (from Wild elderberry). The weighed portions together with the solvent were placed in falcons sealed with parafilm. The extraction was performed in an ultrasonic bath, then the extracts were filtered through cotton wool into a beaker, in which the filtrate from each repetition stage was combined. Due to the threefold repetition of the extraction, the following sample volumes were obtained: 30 mL, 60 mL and 150 mL.
In the next stage, the volume of the extracts was equalized to 100 mL. For this purpose, the 30 mL and 60 mL samples were supplemented with the appropriate solvent concentration for the given sample. The obtained 150 mL samples were concentrated using an evaporator to a volume of 90 mL and then supplemented with the appropriate solvent.
In order to test the content of polyphenols in the obtained extracts, 1 mL samples were taken into 2 mL Eppendorf bottles. Samples were evaporated and then dissolved in 1 mL of DMSO, which prevented precipitation during analysis.
3.3.1. Determination of Total Polyphenol Content (TPC) Using the Folin-Ciocalteu Reagent
The analysis was performed following the method described by Studzińska-Sroka et al. [
42]. In short, 25.0 µL of the tested extract or standard, 200.0 µL of distilled water, 15.0 µL of Folin-Ciocalteu reagent, and 60.0 µL of a 20% calcium carbonate solution were added sequentially to the wells. A blank sample, consisting of the reagents without the tested extract or reference compound, was used as a control. The plate was shaken for 5 minutes at 600 rpm (at room temperature and in the dark), followed by a 25-minute incubation. The absorbance was measured at a wavelength of 760 nm using a microplate reader (Multiskan GOx1510 from Thermo-Scientific, Waltham, MA, USA). The results were the average from n= 6 measurements and were expressed as milligrams of gallic acid equivalent (GAE)/g dry plant material ± standard deviation (SD).
3.4. Extraction and Preparation of Extracts for Testing
Due to the results of the extraction process optimization, the following extraction conditions were adopted for further studies:
1) Plant material weight: 500 mg
2) Extraction temperature: 50°C
3) Methanol concentration: 45%
4) Process time: 15 minutes
5) Solvent volume: 10 mL
6) Number of extraction repetitions: 5
The weights together with the solvent were placed in conical flasks and extracted in an ultrasonic bath. The extracts were then filtered through cotton wool, and the filtrate from each repetition was collected in round-bottom flasks. The extracts obtained were concentrated on an evaporator to a volume of approx. 10 mL, transferred to 10 mL measuring flasks, made up to commensurability using a 45% methanol solution and then divided into 6 Eppendorf flasks. The extracts were frozen at -20°C and stored for further studies.
3.5. Phytochemical Studies of the Extracts
3.5.1. Standardization of the Extract Using High-Performance Liquid Chromatography (HPLC)
Using high-performance liquid chromatography, the presence and content of chlorogenic acid, rutin and isoquercetin were detected and determined in extracts from flowers of different varieties of black elderberry. The analysis was carried out using the method described by Paczkowska-Walendowska et al. [
43]. To ensure the reliability of the obtained results, the method was validated. Process parameters: Detection wavelength: 325 nm (chlorogenic acid), 360 nm (rutin, isoquercetin); Mobile phase flow: 1 mL/min; Column temperature: 40 °C; Mobile phase: A- 0.1% formic acid, B-acetonitrile, according to the gradient: 0-35 min: 2-20% B; 35-55 min: 20 - 70% B; 55 min: 2% B; 55-60 min: 2% B.
Analysis procedure: Solutions of standard substances were prepared by weighing and dissolving aliquots in HPLC grade methanol (chlorogenic acid 1 mg/mL and 0.1 mg/mL, rutin 0.5 mg/mL, isoquercetin 0.5 mg/mL). The obtained solutions were filtered through a membrane filter (0.45 μL). The following injection volumes were made: chlorogenic acid 1 mg/mL: 12, 10, 8, 6, 4, 2, 1 and 0.1 mg/mL: 8, 6, 4 μL; rutin 0.5 mg/mL: 80, 60, 40, 20, 10, 5 μL; Isoquercetin 0.5 mg/mL: 12, 8, 6, 4, 2, 1 µL. The standard curve was determined, and the method was validated by determining the parameters, i.e. linearity, indirect and direct precision, limit of quantification (LOQ) and limit of detection (LOD).
3.5.2. Determination of Total Polyphenol Content (TPC)
Total polyphenol content was determined according to the procedure described in Chapter 3.3.1, except the extracts were prepared at a concentration of 1.5625 mg/mL.
3.5.3. Determination of Total Flavonoid Content (TFC) Using Aluminum Chloride
The analysis was performed following the method described by Studzińska-Sroka et al. [
44]. In short, 100.0 µL of the tested extract or standard was mixed with 100.0 µL of 2% methanolic solution of aluminum chloride. The absorbance was read at 415 nm using the microplate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). The blank contained extract and methanol instead of 2% methanolic solution of aluminum chloride. The results were the average from n= 6 measurements and were expressed as milligrams of quercetin equivalent (QE)/g dry plant material ± standard deviation (SD).
3.6. Studies of Biological Activity of Extracts
3.6.1. Antioxidant Activity Assay
The antioxidant activity of the extracts was determined as the ability to reduce the activity of the DPPH radical and the ability to reduce copper ions using the CUPRAC method. Results are expressed in Trolox equivalent (TE mg/g).
3.6.1.1. DPPH Assay
A previously described DPPH analysis was used to determine the antiradical activity [
42]. Briefly, 25.0 μL of the tested extract, prepared in concentrations 0.78125 mg of dry plant material per mL, was combined with 175.0 μL of DPPH solution (3.9 mg/50 mL of methanol). The mixture was shaken in the dark at 500 rpm for 30 minutes at room temperature. Absorbance was measured at 517 nm using a plate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). Each test was conducted in duplicate, and the final result was calculated as the average of six measurements (n = 6). Trolox, at concentrations ranging from 0.125 to 0.0078125 mg/mL, served as the positive control.
3.6.1.2. CUPRAC Assay
The CUPRAC analysis was conducted using the procedure described previously [
42]. The CUPRAC reagent was prepared by mixing equal volumes of neocuproine solution (7.5 mM), copper (II) chloride solution (10 mM), and ammonium acetate buffer (1 M, pH = 7.0). Then, 50 μL of the tested extract (1.5625 mg of dry plant material per mL) and 150 μL of the CUPRAC reagent were mixed. The absorbance was measured after a 30 min incubation in a dark place and at room temperature, at a wavelength of λ = 450 nm. Blank was an attempt to replace 50.0 μL of the tested extract with 50.0 μL of extraction solvent. The assay was repeated twice, and the result represent the average of seven determinations (n = 7). Trolox, at concentrations ranging from 0.2 to 0.00625 mg/mL, served as the positive control.
3.6.2. α-Glucosidase Inhibition Assay
The inhibition of α-glucosidase by the extracts was conducted based on the method by Studzińska-Sroka et al. [
44], with some modifications. In brief, 50.0 μL of the sample solution (3.125 mg dry plant material/mL) or positive control (acarbose at 0.78125 – 25 mg/mL) were pre-incubated with 50.0 μL of 0.1 M phosphate buffer (pH 6.8) and 30.0 µL of α-glucosidase solution (0.5 U/mL) in 96-well plates at 37 °C for 15 min. Then, 20.0 μL of 5 mM p-nitrophenyl-α-D-glucopyranoside (pNPG) in 0.1 M phosphate buffer was added and incubated at 37 °C for 20 min. The reaction was stopped by adding 100.0 µL of 0.2 M sodium carbonate, and the absorbance was measured at 405 nm after 2 min using the microplate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). The control absorbance was measured without extracts/acarbose, and the absorbance of extract/compound solution without enzyme was used as the blank for the tested sample. The results were the average from n= 7 measurements. The enzyme inhibition rate was expressed as a percentage of inhibition based on the final concentration of the substance in the enzymatic reaction.
3.6.3. α-Amylase Inhibition Assay
The inhibition of α-glucosidase by the extracts was conducted based on the method by Studzińska-Sroka et al. [
44], with some modifications. In brief, 50.0 μL of the sample solution (100 mg dry plant material/mL) or positive control (acarbose at 0.005 – 1.25 mg/mL) were preincubated with 20 µL of α-amylase solution prepared by dissolving in phosphate buffer with pH = 6.9 (2.0 U/mL) at 37 °C for 20 min. Then, 20.0 μL of amylase solution (0.5% in the buffer) was added and the plate was incubated again at 37 °C for 20 min. Then, 60 µL of colour reagent (96 mM 3,5-dinitrosalicylic acid solution, 5.31 M potassium sodium tartrate solution in 2 M sodium hydroxide, and deionized water) was added, and the plate was placed in an oven heated to 90 °C. After 45 minutes, 100 µL of distilled water was added to the samples, and their absorbance was measured at a wavelength of λ = 540 nm using the microplate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). The control sample consisted of a mixture of 20 µL of distilled water and 20 µL of amylase solution, while the blank consisted of a mixture of 20 µL of distilled water and 20 µL of phosphate buffer. The results were the average from n= 6 measurements. The enzyme inhibition rate was expressed as a percentage of inhibition based on the final concentration of the substance in the enzymatic reaction.
3.6.4. Anti-Inflammatory Activity Assay
3.6.4.1. Hyaluronidase Inhibition Assay
The anti-hyaluronidase activity was performed according to the previously described method [
42]. Briefly, 25.0 µL of incubation buffer, 25.0 µL of enzyme solution (30 U/mL), 10.0 µL of the tested extract (250 mg/mL), and 15.0 µL of acetate buffer were mixed in the well. After 15 min. of incubation (37 °C) with shaking, 25.0 µL of hyaluronic acid (HA) solution was added and incubated for 45 min with shaking (37 °C). After this time, 200.0 µL of CTAB solution in 2% sodium hydroxide was added. After 10 min of incubation without shaking (at room temperature), the absorbance (λ = 600 nm) was measured using a plate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). The composition of blanks was previously described [
43]. The determination was repeated twice and calculated from four results (n = 4) for samples and three (n = 3) for reference (β-escin). The results are expressed as percentage of inhibition ± SD. β-Escin in the concentration range of 10.0–5.0 mg/mL was used as a positive control.
3.6.4.2. Determination of Anti-Inflammatory Potential in RAW 264.7 Model
Murine RAW 264.7 macrophages were grown in DMEM high glucose, supplemented with 10% fetal bovine serum and 1% antibiotics solution. Before the main experiment, the cytotoxic potential of the tested samples to RAW264.7 macrophages was assessed using the MTT assay, in the concentration range of 10-100 μg/mL, as described previously [
45]. For the anti-inflammatory assay, the cells were seeded onto 96-well plates (1.5 × 105 cells/well) and pre-treated with the tested samples (at the concentration range indicated above) or dexamethasone (0.5 μg/mL), as a reference drug, for 1 h. Cells treated with LPS alone were used as a positive control, and untreated cells were included in the experiment. Subsequently, 10 ng/mL of LPS was added to induce inflammation, as previously described [
45] and the incubation was performed for 24 h. Cell culture supernatants were used for the determination of nitric oxide level, using Griess Reagent Kit (Promega Corporation, Madison, Winooski, VT, USA), according to the manufacturer’s protocol. The results were expressed as a percentage of the LPS control.
3.7. Statistical Analysis
The data that was gathered was expressed using the means ± SD. The statistical methodology employed in this study was one-way analysis of variance (ANOVA), and statistical differences were computed using Duncan's post hoc tests or post hoc Tukey’s test (anti-inflammatory activity) and a significance threshold of p < 0.05 using Statistica 13.3 software (Statsoft, Krakow, Poland). Principal component analysis (PCA) was used to analyze correlations using Statistica 13.1 and PQStat Software version 1.8.4.142.
4. Conclusions
This study highlights the promising antidiabetic potential of black elderberry (Sambucus nigra L.) flower extracts, specifically targeting their ability to inhibit enzymes involved in carbohydrate digestion, such as α-glucosidase and α-amylase. Optimized extraction conditions, determined through the Box-Behnken model, maximized the yields of bioactive compounds, particularly polyphenols like chlorogenic acid and rutin. Among the tested varieties, Black Beauty, Obelisk, and Haschberg exhibited the highest levels of these compounds, correlating with significant anti-diabetic enzyme inhibition and antioxidant activity. Additionally, among these varieties, Black Beauty was the most active as an anti-inflammatory agent, as evidenced by both cell-based and cell-free tests.
The PCA analysis findings suggest that chlorogenic acid plays a pivotal role in the antidiabetic potential of elderberry flower extracts, with high content linked to effective α-glucosidase inhibition and antioxidant activity. While most extracts displayed limited α-amylase inhibition, the potent α-glucosidase inhibition aligns with the goal of managing postprandial blood glucose levels. Additionally, the strong antioxidant activity further supports these extracts’ potential role in mitigating oxidative stress—a key factor in diabetes complications, and the anti-inflammatory potential may have a protective effect against diabetes complications.
Overall, black elderberry flower extracts, especially those from specific varieties, show potential as natural agents for blood glucose regulation, offering a basis for further investigation into their integration into antidiabetic treatments. Further research should focus on in vivo studies to validate these in vitro findings and explore the practical applications of elderberry flower extracts, especially of Black Beauty variety in diabetes management.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, E.S.-S., and J.C.-P.; methodology, E.S.-S., A.Ga, M.P.-W. and A.Go.; software, M.P.-W., A.Go.; validation, M.P.-W., and E.S.-S.; formal analysis, J.K., E.S.-S., A.Ga., M.P.-W.; investigation, J.K., E.S.-S., A.Ga., and M.P.-W.; resources, P.S., J.C.-P., K.K.; data curation, J.K., E.S.-S. M.P.-W., A.Ga.; writing—original draft preparation, M.P.-W., E.S.-S.; writing—review and editing, E.S.-S., M.P.-W., A.Ga., K.K., and P.S.; visualization, M.P.-W., E.S.-S., and A.Go.; supervision, E.S.-S.; project administration, E.S.-S.; funding acquisition K.K. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting reported results can be found within the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Association, A. D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32 (Suppl 1), S62. [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K. B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. International Journal of Molecular Sciences 2020, 21 (17), 6275. [CrossRef]
- Oguntibeju, O. O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. International Journal of Physiology, Pathophysiology and Pharmacology 2019, 11 (3), 45.
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M. P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; Monda, M.; Giordano, A.; Sasso, F. C. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Current Issues in Molecular Biology 2023, 45 (8), 6651–6666. [CrossRef]
- Sugandh, F. N. U.; Chandio, M.; Raveena, F. N. U.; Kumar, L.; Karishma, F. N. U.; Khuwaja, S.; Memon, U. A.; Bai, K.; Kashif, M.; Varrassi, G.; Khatri, M.; Kumar, S. Advances in the Management of Diabetes Mellitus: A Focus on Personalized Medicine. Cureus 2023, 15 (8), e43697. [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S. K.; Wadhwa, P.; Kaur, P.; Sahu, S. K. Alpha-Amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chemical Biology & Drug Design 2021, 98 (4), 539–560. [CrossRef]
- Kashtoh, H.; Baek, K.-H. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants 2023, 12 (16), 2944. [CrossRef]
- Przeor, M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals 2022, 15 (1), 65. [CrossRef]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12 (11), 1692. [CrossRef]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus Nigra L. Plant Foods Hum Nutr 2017, 72 (1), 82–87. [CrossRef]
- Stępień, A. E.; Trojniak, J.; Tabarkiewicz, J. Health-Promoting Properties: Anti-Inflammatory and Anticancer Properties of Sambucus Nigra L. Flowers and Fruits. Molecules 2023, 28 (17), 6235. [CrossRef]
- Osman, A. G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A. G.; Khan, I. A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28 (7), 3148. [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G. P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus Nigra L. Molecules 2020, 25 (4), 876. [CrossRef]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes 2022, 10 (11), 2288. [CrossRef]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Tomović, V.; Šojić, B.; Đurović, S.; Radojković, M. Elderberry ( Sambucus Nigra L.) Juice as a Novel Functional Product Rich in Health-Promoting Compounds. RSC Advances 2020, 10 (73), 44805–44814. [CrossRef]
- Li, J.; Zhang, S.; Zhang, M.; Sun, B. Novel Approach for Extraction of Grape Skin Antioxidants by Accelerated Solvent Extraction: Box–Behnken Design Optimization. J Food Sci Technol 2019, 56 (11), 4879–4890. [CrossRef]
- Hosni, S.; Gani, S. S. A.; Orsat, V.; Hassan, M.; Abdullah, S. Ultrasound-Assisted Extraction of Antioxidants from Melastoma Malabathricum Linn.: Modeling and Optimization Using Box–Behnken Design. Molecules 2023, 28 (2), 487. [CrossRef]
- Gościniak, A.; Bazan-Woźniak, A.; Pietrzak, R.; Cielecka-Piontek, J. Pomegranate Flower Extract—The Health-Promoting Properties Optimized by Application of the Box–Behnken Design. Molecules 2022, 27 (19), 6616. [CrossRef]
- Tai, N. V.; Linh, M. N.; Thuy, N. M. Optimization of Extraction Conditions of Phytochemical Compounds in “Xiem” Banana Peel Powder Using Response Surface Methodology. J App Biol Biotech 2021, 9 (6), 56–62. [CrossRef]
- Oziembłowski, M.; Nawirska-Olszańska, A.; Maksimowski, D. Optimization of Chlorogenic Acid in Ethanol Extracts from Elderberry Flowers (Sambucus Nigra L.) under Different Conditions: Response Surface Methodology. Applied Sciences 2023, 13 (5), 3201. [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P. E. S.; Lorenzo, J. M. Elderberry (Sambucus Nigra L.) as Potential Source of Antioxidants. Characterization, Optimization of Extraction Parameters and Bioactive Properties. Food Chemistry 2020, 330, 127266. [CrossRef]
- Zawiślak, A.; Francik, R.; Francik, S.; Knapczyk, A. Impact of Drying Conditions on Antioxidant Activity of Red Clover (Trifolium Pratense), Sweet Violet (Viola Odorata) and Elderberry Flowers (Sambucus Nigra). Materials 2022, 15 (9), 3317. [CrossRef]
- Dangles, O. Anthocyanins as Natural Food Colorings: The Chemistry Behind and Challenges Still Ahead. J. Agric. Food Chem. 2024, 72 (22), 12356–12372. [CrossRef]
- Matłok, N.; Kapusta, I.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Characterisation of Some Phytochemicals Extracted from Black Elder (Sambucus Nigra L.) Flowers Subjected to Ozone Treatment. Molecules 2021, 26 (18), 5548. [CrossRef]
- Tundis, R.; Ursino, C.; Bonesi, M.; Loizzo, M. R.; Sicari, V.; Pellicanò, T.; Manfredi, I. L.; Figoli, A.; Cassano, A. Flower and Leaf Extracts of Sambucus Nigra L.: Application of Membrane Processes to Obtain Fractions with Antioxidant and Antityrosinase Properties. Membranes 2019, 9 (10), 127. [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N. V.; Zucca, P.; Varoni, E. M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P. V.; Azzini, E.; Peluso, I.; Prakash Mishra, A.; Nigam, M.; El Rayess, Y.; Beyrouthy, M. E.; Polito, L.; Iriti, M.; Martins, N.; Martorell, M.; Docea, A. O.; Setzer, W. N.; Calina, D.; Cho, W. C.; Sharifi-Rad, J. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11. [CrossRef]
- Chen, X.; Xie, N.; Feng, L.; Huang, Y.; Wu, Y.; Zhu, H.; Tang, J.; Zhang, Y. Oxidative Stress in Diabetes Mellitus and Its Complications: From Pathophysiology to Therapeutic Strategies. Chinese Medical Journal 10.1097/CM9.0000000000003230. [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S. T.; Bin Asad, M. H. H. Enzymes Inhibitors from Natural Sources with Antidiabetic Activity: A Review. Phytotherapy Research 2019, 33 (1), 41–54. [CrossRef]
- Assefa, S. T.; Yang, E.-Y.; Chae, S.-Y.; Song, M.; Lee, J.; Cho, M.-C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2020, 9 (1), 2. [CrossRef]
- Oboh, G.; Agunloye, O. M.; Adefegha, S. A.; Akinyemi, A. J.; Ademiluyi, A. O. Caffeic and Chlorogenic Acids Inhibit Key Enzymes Linked to Type 2 Diabetes (in Vitro): A Comparative Study. J Basic Clin Physiol Pharmacol 2015, 26 (2), 165–170. [CrossRef]
- Bljajić, K.; Brajković, A.; Čačić, A.; Vujić, L.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical Composition, Antioxidant, and α-Glucosidase-Inhibiting Activity of Aqueous and Hydroethanolic Extracts of Traditional Antidiabetics from Croatian Ethnomedicine. Horticulturae 2021, 7 (2), 15. [CrossRef]
- Ferreira, S. S.; Silva, P.; Silva, A. M.; Nunes, F. M. Effect of Harvesting Year and Elderberry Cultivar on the Chemical Composition and Potential Bioactivity: A Three-Year Study. Food Chemistry 2020, 302, 125366. [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of Porcine Pancreatic α-Amylase Activity by Chlorogenic Acid. Journal of Functional Foods 2020, 64, 103587. [CrossRef]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic Enzyme Inhibitory and Antiglycation Potential of Rutin. Future Journal of Pharmaceutical Sciences 2017, 3 (2), 158–162. [CrossRef]
- Bennett, J. M.; Reeves, G.; Billman, G. E.; Sturmberg, J. P. Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5. [CrossRef]
- Forrester, J. V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11. [CrossRef]
- Jung, H. Hyaluronidase: An Overview of Its Properties, Applications, and Side Effects. Arch Plast Surg 2020, 47 (04), 297–300. [CrossRef]
- Rayahin, J. E.; Buhrman, J. S.; Zhang, Y.; Koh, T. J.; Gemeinhart, R. A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1 (7), 481–493. [CrossRef]
- Kim, M.-Y.; Kim, Y.-C.; Chung, S.-K. Identification and in Vitro Biological Activities of Flavonols in Garlic Leaf and Shoot: Inhibition of Soybean Lipoxygenase and Hyaluronidase Activities and Scavenging of Free Radicals. Journal of the Science of Food and Agriculture 2005, 85 (4), 633–640. [CrossRef]
- Lee, J.-H.; Kim, G.-H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J Food Sci 2010, 75 (7), H212-217. [CrossRef]
- Ho, G. T. T.; Wangensteen, H.; Barsett, H. Elderberry and Elderflower Extracts, Phenolic Compounds, and Metabolites and Their Effect on Complement, RAW 264.7 Macrophages and Dendritic Cells. International Journal of Molecular Sciences 2017, 18 (3), 584. [CrossRef]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Woźna, Z.; Plech, T.; Szulc, P.; Cielecka-Piontek, J. Elderberry Leaves with Antioxidant and Anti-Inflammatory Properties as a Valuable Plant Material for Wound Healing. Pharmaceuticals (Basel) 2024, 17 (5), 618. [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10 (12), 1945. [CrossRef]
- Studzińska-Sroka, E.; Galanty, A.; Gościniak, A.; Wieczorek, M.; Kłaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal Infusions as a Valuable Functional Food. Nutrients 2021, 13 (11), 4051. [CrossRef]
- Galanty, A.; Juncewicz, P.; Podolak, I.; Grabowska, K.; Służały, P.; Paśko, P. Comparative Analysis of Polyphenolic Profile and Chemopreventive Potential of Hemp Sprouts, Leaves, and Flowers of the Sofia Variety. Plants 2024, 13 (15), 2023. [CrossRef]
Figure 1.
Total polyphenol content in the tested Sambucus nigra samples.
Figure 1.
Total polyphenol content in the tested Sambucus nigra samples.
Figure 2.
Pareto chart of standardized effects of Box-Behnken experimental analysis for total polyphenol content (TPC) in the extracts.
Figure 2.
Pareto chart of standardized effects of Box-Behnken experimental analysis for total polyphenol content (TPC) in the extracts.
Figure 3.
Response surface curve illustrating the effect of methanol content and plant material to solvent ratio (a), methanol content and time (b) and plant material to solvent ratio and time (c) on TPC.
Figure 3.
Response surface curve illustrating the effect of methanol content and plant material to solvent ratio (a), methanol content and time (b) and plant material to solvent ratio and time (c) on TPC.
Figure 4.
Results of the hyaluronidase inhibition test (na – not active at 25 mg/mL). Mean values with the same letter are not significantly different at p = 0.05 using Duncan’s test.
Figure 4.
Results of the hyaluronidase inhibition test (na – not active at 25 mg/mL). Mean values with the same letter are not significantly different at p = 0.05 using Duncan’s test.
Figure 5.
The effect of flower extracts from Black Tower and Black Beauty variety of elderberry on nitric oxide release in LPS-stimulated RAW 264.7 macrophages. Significant differences between the doses were marked by upper black line and * (p < 0.05).
Figure 5.
The effect of flower extracts from Black Tower and Black Beauty variety of elderberry on nitric oxide release in LPS-stimulated RAW 264.7 macrophages. Significant differences between the doses were marked by upper black line and * (p < 0.05).
Figure 6.
Principal component analysis (PCA) showing the factor loading plot considering active components contents (TPC, TFC, CGA, Rutin, Isoquer), and antioxidant (DPPH, CUPRAC) and anti-inflammatory (Hyal) and others (Gluc.I.) activities.
Figure 6.
Principal component analysis (PCA) showing the factor loading plot considering active components contents (TPC, TFC, CGA, Rutin, Isoquer), and antioxidant (DPPH, CUPRAC) and anti-inflammatory (Hyal) and others (Gluc.I.) activities.
Figure 7.
Flowers of black elderberry varieties: Samyl (a), Samyl 1 (b), Obelisk (c), Sambo (d), Golden Hybrid (e), Bez koralowy (f), Haschberg (g), Sampo (h), Black Tower (i), Black Beauty (j), Haschberg 1 (k), Bez dwubarwny (l).
Figure 7.
Flowers of black elderberry varieties: Samyl (a), Samyl 1 (b), Obelisk (c), Sambo (d), Golden Hybrid (e), Bez koralowy (f), Haschberg (g), Sampo (h), Black Tower (i), Black Beauty (j), Haschberg 1 (k), Bez dwubarwny (l).
Table 1.
Content of chlorogenic acid, rutin and isoquercetin, TPC and TFC in flower extracts of elderberry varieties.
Table 1.
Content of chlorogenic acid, rutin and isoquercetin, TPC and TFC in flower extracts of elderberry varieties.
| Variety |
Content [mg of standard/g of plant material] |
TPC
[mg GAE/g] |
TFC
[mg QE/g] |
| Chlorogenic acid |
Rutin |
Isoquercetin |
| Samyl |
19.35 ± 0.16 c
|
25.70 ± 0.11 c
|
0.16 ± 0.01 h
|
33.00 ± 0.49 e
|
12.52 ± 0.23 g
|
| Samyl 1 |
19.40 ± 0.07 c
|
14.35 ± 0.22 g
|
2.13 ± 0.04 c
|
36.00 ± 0.77 d
|
13.90 ± 0.48 e
|
| Obelisk |
21.03 ± 0.00 b
|
30.12 ± 0.18 b
|
1.91 ± 0.11 d
|
36.48 ± 1.09 d
|
18.65 ± 0.25 b
|
| Sambo |
19.46 ± 0.00 c
|
10.63 ± 0.06 k
|
4.69 ± 0.00 a |
38.47 ± 1.75 c
|
13.44 ± 0.31 f
|
| Golden Hybrid |
16.00 ± 0.02 g
|
4.01 ± 0.01 l
|
0.53 ± 0.02 g
|
32.14 ± 1.38 e,f
|
16.68 ± 0.35 c,d
|
| Bez koralowy |
9.39 ± 0.05 j
|
22.41 ± 0.27 d
|
0.22 ± 0.01 h
|
25.05 ± 1.12 h
|
13.15 ± 0.14 f
|
| Haschberg |
18.57 ± 0.44 d
|
38.37 ± 0.01 a |
0.09 ± 0.03 h
|
30.80 ± 0.89 f
|
20.29 ± 0.20 a |
| Sampo |
16.78 ± 0.05 f
|
11.33 ± 0.15 j
|
1.78 ± 0.11 d,e
|
32.40 ± 0.67 e,f
|
11.68 ± 0.17 h
|
| Black Tower |
17.30 ± 0.02 e
|
16.58 ± 0.03 f
|
1.72 ± 0.00 e
|
40.48 ± 1.04 b
|
16.28 ± 0.09 d
|
| Black Beauty |
23.73 ± 0.01 a |
12.75 ± 0.01 i
|
1.10 ± 0.21 f
|
44.97 ± 1.15 a |
14.27 ± 0.18 e
|
| Haschberg 1 |
18.46 ± 0.06 d
|
10.51 ± 0.00 k
|
4.46 ± 0.05 b
|
34.95 ± 1.23 d
|
12.72 ± 0.12 g
|
| Bez dwubarwny |
14.78 ± 0.15 i
|
20.09 ± 0.30 e
|
1.84 ± 0.12 d,e
|
32.09 ± 0.61 e,f
|
16.84 ± 0.23 c
|
| Wild elderberry |
15.39 ± 0.10 h
|
13.33 ± 0.15 h
|
1.76 ± 0.09 d,e
|
28.57 ± 0.77 g
|
11.46 ± 0.23 h
|
Table 2.
Antioxidant activity results for the tested varieties expressed as trolox equivalents (TE mg/g), determined by the DPPH and CUPRAC methods, and the main compound measured as references.
Table 2.
Antioxidant activity results for the tested varieties expressed as trolox equivalents (TE mg/g), determined by the DPPH and CUPRAC methods, and the main compound measured as references.
| Variety |
DPPH
[mg TE/g] |
CUPRAC
[mg TE/g] |
| Samyl |
38.30 ± 4.64 c,d
|
55.10 ± 0.92 g
|
| Samyl 1 |
39.61 ± 1.80 c,d
|
70.32 ± 0.70 b
|
| Obelisk |
49.92 ± 2.40 a
|
68.25 ± 1.00 c
|
| Sambo |
40.09 ± 1.82 c
|
67.80 ± 2.06 c
|
| Golden Hybrid |
33.22 ± 1.03 e,f
|
62.46 ± 1.73 e
|
| Bez koralowy |
19.23 ± 0.64 h
|
42.32 ± 0.99 j
|
| Haschberg |
45.01 ± 1.12 b
|
65.71 ± 0.80 d
|
| Sampo |
31.57 ± 1.29 f,g
|
59.16 ± 1.41 f
|
| Black Tower |
38.18 ± 2.16 c,d
|
67.71 ± 1.09 c
|
| Black Beauty |
53.15 ± 0.85 a |
77.19 ± 1.21 a |
|
| Haschberg 1 |
41.16 ± 2.41 c
|
62.71 ± 1.00 e
|
| Bez dwubarwny |
35.92 ± 2.00 d,e
|
52.70 ± 1.11 h
|
| Wild elderberry |
29.34 ± 2.47 g
|
50.47 ± 0.21 i
|
| Chlorogenic acid |
0.150 ± 0.011
IC50 [mg/mL] |
25.70 ± 0.52
IC0.5 [mg/mL] |
| Rutin |
0.148 ± 0.000
IC50 [mg/mL] |
39.86 ± 0.21
IC0.5 [mg/mL] |
Table 3.
Results of the α-glucosidase and α-amylase inhibition test.
Table 3.
Results of the α-glucosidase and α-amylase inhibition test.
| Variety |
α-glucosidase
inhibition [%]
|
α-amylase
inhibition [%]
|
|
| Samyl |
42.31 ± 1.67 e
|
nd |
|
| Samyl 1 |
50.96 ± 1.79 b
|
nd |
|
| Obelisk |
49.61 ± 1.36 b,c
|
33.95 ± 3.48 a
|
|
| Sambo |
48.34 ± 1.70 b,c,d
|
13.48 ± 1.45 c
|
|
| Golden Hybrid |
46.72 ± 1.11 c,d
|
22.89 ± 2.77 b
|
|
| Bez koralowy |
34.30 ± 3.52 f
|
nd |
|
| Haschberg |
55.58 ± 2.42 a |
nd |
|
| Sampo |
45.25 ± 1.25 d,e
|
nd |
|
| Black Tower |
51.39 ± 0.83 b
|
nd |
|
| Black Beauty |
55.89 ± 1.51 a |
nd |
|
| Haschberg 1 |
46.83 ± 1.51 c,d
|
13.70 ± 2.28 c
|
|
| Bez dwubarwny |
42.24 ± 1.51 e
|
22.70 ± 2.34 b
|
|
| Wild elderberry |
29.74 ± 1.21g
|
nt |
|
| Chlorogenic acid |
0.509 ± 0.021
IC50 [mg/mL] |
nt |
|
| Rutin |
0.390 ± 0.011
IC50 [mg/mL] |
nt |
|
| Acarbose 3.125 |
27.85 ± 1.70 |
nt |
|
| Acarbose |
8.24 ± 0.55
IC50 [mg/mL] |
0.011 ± 0.001
IC50 [mg/mL] |
|
Table 4.
Extraction process experiment plan.
Table 4.
Extraction process experiment plan.
| Lp. |
Methanol content [%] |
Time [min] |
Solvent volume [mL] |
| S1 |
50 |
15 |
50 |
| S2 |
100 |
52.5 |
10 |
| S3 |
0 |
52.5 |
50 |
| S4 |
100 |
52.5 |
50 |
| S5 |
0 |
52.5 |
10 |
| S6 |
50 |
52.5 |
30 |
| S7 |
100 |
15 |
30 |
| S8 |
50 |
15 |
10 |
| S9 |
0 |
15 |
30 |
| S10 |
100 |
90 |
30 |
| S11 |
0 |
90 |
30 |
| S12 |
50 |
90 |
50 |
| S13 |
50 |
90 |
10 |
| S14 |
50 |
52.5 |
30 |
| S15 |
50 |
52.5 |
30 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).