1. Introduction
Oral health significantly influences overall health and quality of life [
1]. Among oral conditions, dental caries and periodontal diseases are the most common chronic diseases, primarily caused by dental plaque, a biofilm formed on tooth surfaces [
2]. A biofilm consists of structured microbial communities that firmly adhere to tooth surfaces and are difficult to remove by routine oral hygiene practices, such as toothbrushing alone [
3]. Over time, as these biofilms mature, the proportion of pathogenic bacteria increases, resulting in acid production, which in turn causes tooth demineralization and gum inflammation, ultimately leading to dental caries and periodontitis [
4]. Therefore, early and accurate detection and effective management of dental plaque are critical steps in preventing oral diseases [
5].
In clinical dental practice, plaque-disclosing agents are frequently used to visually identify dental plaque, which is difficult to observe with the naked eye. These agents are essential tools in oral hygiene education as they provide clear visual feedback for patients [
6]. Traditionally used plaque-disclosing agents contain chemically synthesized dyes, particularly erythrosine (Red No. 3). Erythrosine, an organoiodine compound, is widely used clinically due to its strong staining capabilities and excellent plaque visualization properties [
7]. Additionally, fluorescein, which fluoresces under ultraviolet light, is employed in some plaque-disclosing agents, and two-tone dye systems combining erythrosine with Fast Green or Brilliant Blue are commonly used clinically [
8].
Despite its effective staining properties due to strong binding to oral tissues, erythrosine has recently raised concerns regarding its potential cytotoxicity to oral epithelial cells . For instance, a study by Jung et al. reported that erythrosine could induce damage and apoptosis in human oral epithelial cells [
9]. Furthermore, the U.S. Food and Drug Administration (FDA) has restricted the use of erythrosine in cosmetics and topical applications based on animal studies that linked it to potential thyroid dysfunction and carcinogenicity [
10]. Similarly, in Korea, plaque-disclosing agents were reclassified as quasi-drugs in 2014, leading to stricter standards for safety and toxicity assessment, subsequently causing many erythrosine-based products to be discontinued from production and distribution. These issues emphasize the urgent need to develop safer and more biocompatible natural dye-based plaque-disclosing agents to replace synthetic dyes.
Recently, natural dye-based oral care products have gained increased attention, particularly focusing on the potential of Gardenia Blue pigment derived from Gardenia jasminoides. Various toxicity studies have demonstrated that Gardenia Blue has negligible biological toxicity and is harmless to tissues even upon prolonged use, differentiating it significantly from synthetic dyes [
11]. For example, chronic toxicity and carcinogenicity studies using Sprague-Dawley rats fed with up to 5% Gardenia Blue for two years showed no carcinogenic effects or tissue abnormalities [
12]. Likewise, a six-month carcinogenicity study using rasH2 mice, which are sensitive to carcinogenic substances, reported no tumor formation, confirming the high biocompatibility and safety of Gardenia Blue [
13]. These findings strongly suggest Gardenia Blue as a promising candidate for overcoming the toxicity and safety concerns associated with synthetic dyes.
Nevertheless, previous research on natural dye-based plaque-disclosing agents has predominantly been limited to laboratory conditions or small-scale clinical trials. There is currently insufficient objective comparative research conducted under clinical conditions evaluating the efficacy and safety of natural dyes versus conventional synthetic dye-based products. Therefore, this randomized crossover clinical trial aimed to objectively assess whether the newly developed natural Gardenia Blue-based plaque-disclosing agent is equivalent to conventional erythrosine-based products regarding staining effectiveness, superior ease of removal, and high biocompatibility in clinical settings. The findings from this study are expected to provide robust evidence supporting the clinical use of natural dye-based plaque-disclosing agents in both dental practice and oral health education.
2. Results
2.1. Evaluation of Staining Effect and Completion Time
Both the conventional and natural dye-based plaque-disclosing agents effectively stained dental plaque. The conventional agent tended to stain slightly larger areas than the natural agent across most tooth surfaces. Mean PHP scores were generally similar between the two agents, but the conventional agent consistently produced slightly higher scores, with mean differences ranging from 0.07 to 0.26. Specifically, the largest mean difference was observed at the mandibular first molar lingual surface (36L), with scores of 2.30 ± 1.10 for the conventional agent and 2.04 ± 1.12 for the natural agent. Similar patterns were also observed on tooth surfaces 26B, 31B, and 46L (
Table 1). All observed differences were statistically significant (p < 0.001). Pearson correlation coefficients between the two agents ranged from 0.75 to 0.97, indicating high spatial concordance in plaque staining. These results suggest that although the conventional agent generally stained broader areas, the natural agent consistently identified the same plaque regions, demonstrating clinically reliable reproducibility regarding plaque localization.
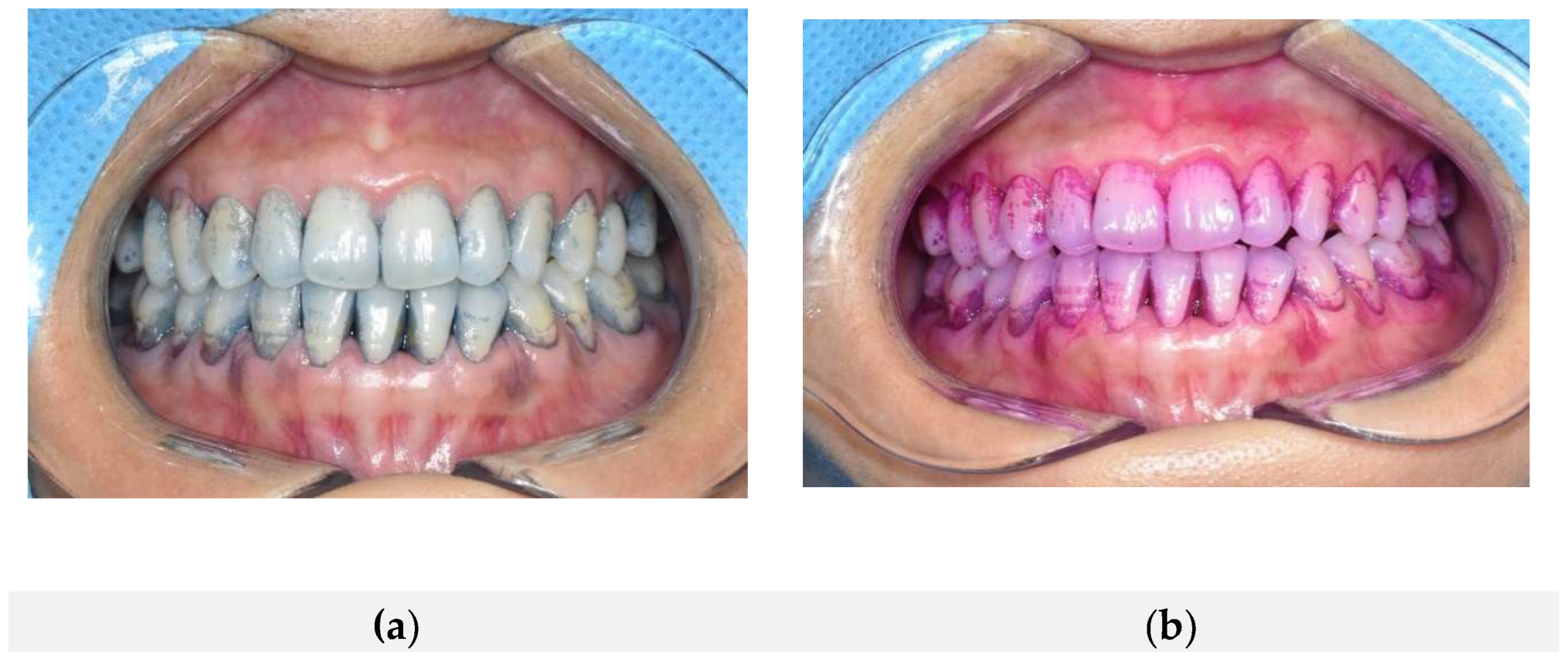
Figure 2 shows the plaque staining results after sequential application of the natural dye-based disclosing agent followed by the conventional dye-based disclosing agent. Both agents consistently stained identical regions of dental plaque, including buccal surfaces of the maxillary and mandibular anterior teeth, gingival margins, and proximal (mesial and distal) areas, demonstrating complete overlap in staining patterns. These results suggest that both dye-based agents accurately detect the same plaque locations regardless of application order. Thus, these findings qualitatively confirm that the natural dye-based disclosing agent possesses equivalent clinical efficacy compared to the conventional product in terms of spatial accuracy and reproducibility of plaque staining.
2.2. Evaluation of Staining Effect and Completion Time
Sequential application of the natural dye-based disclosing agent followed by the conventional erythrosine-based disclosing agent on the same tooth surfaces resulted in clear plaque staining by both agents, demonstrating high spatial concordance in the locations of stained plaque areas. Overall, the conventional agent exhibited slightly higher average PHP scores compared to the natural agent, with mean differences ranging from –0.06 to –0.19. These small differences suggest that the conventional dye-based agent tends to stain slightly broader plaque areas in some regions, although the differences were minor. For example, on the buccal surface of the first molar (16B), the natural agent scored 1.98 while the conventional agent scored 2.04, resulting in a mean difference of 0.06. On the buccal surface of the second molar (26B), the natural agent scored 1.79 and the conventional agent scored 1.98, with a mean difference of 0.19. Cohen’s d values ranged from 0.497 to 0.761, representing moderate effect sizes, and these differences were statistically significant across all tooth surfaces (p < .001). Additionally, Pearson correlation coefficients ranged from 0.795 to 0.916, indicating strong positive correlations between the plaque-staining locations identified by the two agents (
Table 2). These findings support the spatial concordance between the two disclosing agents, confirming that while the natural dye-based agent may stain slightly smaller areas compared to the conventional agent, it reliably reproduces and effectively identifies plaque locations, thus demonstrating adequate clinical reliability.
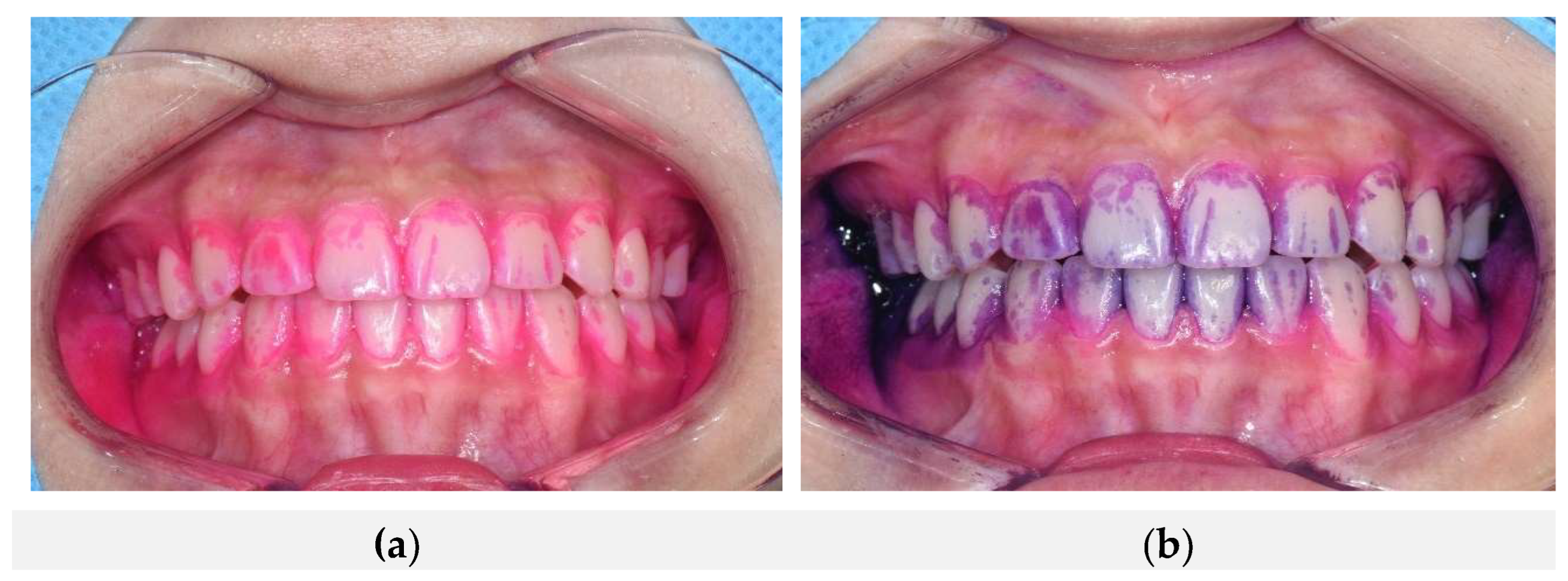
Figure 3 shows intraoral comparative photographs of the same participant, first after applying the conventional plaque-disclosing agent, followed by the natural dye-based plaque-disclosing agent. Panel (a) illustrates the staining pattern after the conventional disclosing agent application, and panel (b) shows the result following subsequent application of the natural dye-based agent. Both agents effectively stained nearly identical regions of dental plaque, including the buccal surfaces of maxillary and mandibular anterior teeth, gingival margins, and proximal (mesial and distal) surfaces. This demonstrates the high spatial concordance and effectiveness of both agents in identifying the same plaque locations.
2.3. Evaluation of Stain Retention (Durability)
Assessment of staining status 10 minutes after application showed that both the conventional and the natural dye-based disclosing agents exhibited excellent stain retention overall. The average PHP scores for both agents decreased slightly by 0.1–0.3 points compared to immediately after application, with staining clearly maintained on tooth surfaces where plaque accumulation was prevalent, such as the buccal and lingual surfaces. For the natural dye-based agent, the average PHP score decreased slightly from 1.87 (±1.47) immediately after application to 1.66 (±1.43) after 10 minutes, with correlation coefficients (r) ranging from 0.96 (16B) to 0.64 (46L), indicating a high level of stability and concordance at most sites. Particularly high spatial stability was observed on the central incisor buccal surface (11B, r = 0.966) and lateral incisor buccal surface (31B, r = 0.891).
These findings suggest that both plaque-disclosing agents can reliably maintain stable staining effects for a clinically adequate duration. Moreover, the natural dye-based agent demonstrated comparable stain retention capability to the conventional product (
Table 3).
2.4. Evaluation of Ease of Stain Removal
Ease of stain removal was assessed in two stages. Initially, participants were instructed to rinse lightly with water once after staining. Results showed that most of the stained plaque areas remained intact after this single rinse, indicating that a simple water rinse was insufficient to remove the stains, confirming a stable binding of both disclosing agents to the plaque surface.
When comparing stain intensity scores immediately after application (Stage 4) and after water rinsing (Stage 5), a statistically significant reduction in stain scores was observed across all tooth surfaces, suggesting that while water rinsing alone did not completely remove the stained plaque, it partially reduced stain intensity. Mean reductions in staining scores ranged from 0.21 to 0.36 points across different tooth surfaces, with the greatest reduction (mean difference = 0.362, Cohen’s d = 0.770) occurring on the mandibular first molar lingual surface (46L). Despite these reductions, Pearson correlation coefficients (r = 0.77–0.91) remained high for all surfaces, indicating that the spatial pattern of stained plaque remained consistent. Thus, while water rinsing partially decreased stain intensity and coverage, the overall spatial correlation and plaque location consistency were strongly maintained (
Table 4)
2.5. Effectiveness of Toothbrushing in Removing Disclosed Plaque Stains
After plaque disclosure and a single water rinse, most stained plaque areas remained visibly intact. However, when participants performed standard toothbrushing, there was a substantial decrease in stained plaque scores across all tooth surfaces. The mean reduction in plaque staining ranged from 0.51 to 1.17, with all differences being statistically significant (p < .001). The effect sizes (Cohen’s d) were consistently large (0.78–1.40), indicating that brushing was highly effective in removing disclosed plaque. Notably, the greatest stain removal was observed on the lateral incisor buccal surface (31B) with Cohen’s d = 1.40, followed by the central incisor buccal surface (11B). These results confirm that while a simple water rinse leaves significant plaque residue, brushing can reliably and substantially eliminate stained biofilm. This demonstrates the utility of the disclosing agent as a tool for providing visible feedback for oral hygiene effectiveness, especially when integrated into behavior change interventions (
Table 8).
3. Discussion
This study comprehensively evaluated the clinical efficacy, retention, ease of removal, and safety of a natural dye-based plaque disclosing agent derived from Gardenia blue, comparing it against a conventional erythrosine-based agent. The natural disclosing agent demonstrated comparable plaque-staining efficacy to the conventional product, as evidenced by similar PHP index scores and consistently high spatial concordance (Pearson correlation coefficient > 0.9) observed across tooth surfaces. This strong spatial concordance was maintained irrespective of the application order (natural-first or conventional-first), highlighting that the natural dye-based agent provides equivalent reliability and reproducibility in plaque visualization, supporting its clinical utility as a visual feedback tool for oral hygiene.
Regarding stain retention, the natural dye-based disclosing agent exhibited excellent stability in both intensity and surface area coverage after 10 minutes in the oral environment, despite natural salivary flow and moisture exposure. High Pearson correlation coefficients (r ≥ 0.85) confirmed consistent and stable plaque-staining patterns over time. This characteristic is particularly beneficial in clinical and educational settings, allowing extended visual feedback to patients, thereby promoting improved self-management of oral hygiene practices.
The evaluation of stain removal revealed that the natural dye-based plaque-disclosing agent was not easily removed by simple water rinsing alone, with a significant amount of initial plaque staining remaining intact. This indicates that the natural dye-based agent effectively adheres to dental plaque, providing stable staining even under the oral environment. However, following standard toothbrushing, the disclosed plaque was rapidly and effectively eliminated from all tooth surfaces. Post-brushing PHP scores sharply decreased, approaching near zero levels, and these reductions were statistically significant with large effect sizes (Cohen’s d ≥ 1.0). These findings highlight the clinical practicality of the natural dye-based disclosing agent as an effective visual feedback tool in clinical and oral hygiene educational settings, promoting improved oral hygiene behaviors while minimizing patient discomfort.
From a safety perspective, the natural dye-based disclosing agent, primarily composed of natural anthocyanins, effectively addresses concerns regarding the potential cytotoxicity associated with synthetic dyes and tar-based colorants. No adverse reactions, mucosal irritation, or alterations in taste sensation were reported among study participants, emphasizing its broad safety profile and suitability for diverse patient populations, including children and sensitive or high-risk groups.
Our findings align well with previous research emphasizing the effectiveness and user acceptance of natural dye-based plaque disclosing agents. Laela et al. reported comparable staining effectiveness and lower mucosal irritation with mixed Mulberry and Red Beetroot natural colorants compared to synthetic dyes, highlighting their acceptability [
14]. Additionally, a patent by Steinberg described beetroot-derived natural dyes capable of selectively staining plaque while avoiding mucosal staining and facilitating easy removal by simple rinsing, features corroborated by our current results with Gardenia blue [
15].
Historically, plaque-disclosing agents have served primarily as diagnostic tools, but recently their role as educational instruments to facilitate oral hygiene behavior changes has gained recognition. Maya et al. (2018) demonstrated that visual feedback from plaque-disclosing agents positively influenced the maintenance of oral hygiene behaviors, particularly in children and adolescents [
16]. Given its low irritancy and user-friendly characteristics, the natural dye-based agent evaluated in this study possesses significant potential for application in community-based and educational oral health programs.
A notable methodological strength of this study is the randomized crossover design, ensuring reliable and unbiased comparative evaluation between products. By alternating the order of application, the study minimized potential biases and provided robust scientific evidence supporting the clinical efficacy and advantages of the natural dye-based agent.
Nevertheless, certain limitations exist. Firstly, the narrow range of participant ages and oral health statuses restricts the generalizability of our findings. Further studies involving broader demographics and various oral health conditions are necessary. Additionally, this study was confined to Gardenia blue as a natural dye; future research should explore diverse natural dyes, such as beetroot and turmeric, to assess broader efficacy and application potential. Establishing standardized manufacturing protocols and quality control measures to ensure stability and consistency of natural products also remains an essential future objective.
In conclusion, this study clearly demonstrated that the natural Gardenia blue-based plaque-disclosing agent possesses staining efficacy, retention, ease of removal, and safety equal to or surpassing that of conventional synthetic agents. Its favorable clinical characteristics position it as an effective alternative to conventional products, particularly valuable as a visual feedback tool in oral hygiene education. Further research with broader clinical conditions and patient populations will likely enhance the scope and impact of natural dye-based plaque-disclosing agents in clinical practice and public oral health initiatives. The findings presented here provide compelling scientific evidence supporting the transition toward safer, more biocompatible oral health management tools, significantly contributing to clinical practice and dental public health.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
This randomized crossover clinical trial demonstrated that a natural dye-based plaque disclosing agent derived from Gardenia blue has comparable plaque-staining efficacy and retention to conventional erythrosine-based agents, while providing superior ease of stain removal and improved biocompatibility. These findings support the adoption of the natural dye-based agent as an effective, safe, and patient-friendly alternative in clinical dental practice and oral health education programs. Further studies involving broader patient populations and various natural dye formulations are recommended to expand the clinical applicability and validate the long-term effectiveness of natural dye-based disclosing agents.
Author Contributions
Conceptualization, J.-N. L. and S.-H. S. and J.-Y. L.; methodology, J.-N. L. and S.-H. S. and J.-Y. L.; validation, J.-Y. L.; formal analysis, J.-N. L. and S.-H. S. and J.-Y. L.; investigation, J.-N. L.; resources J.-N. L. and S.-H. S. and J.-Y. L.; data curation, J.-N. L. and J.-Y. L.; writing—original draft preparation J.-N. L. and S.-H. S. and J.-Y. L.; writing—review and editing, J.-N. L. and S.-H. S. and J.-Y. L.; visualization, J.-N. L. and S.-H. S. and J.-Y. L.; supervision, J.-Y. L.; project administration, J.-Y. L.; funding acquisition, J.-Y. L.. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Dankook University (IRB File No. DKU 2024-02-005-003, approval date: 2024-03-27).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request
Acknowledgments
This research was supported by the Technology Innovation Program for Startups (TIPS Program) funded by the Ministry of SMEs and Startups (Project Number: RS-2024-00437832), Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dörfer, C.; Benz, C.; Aida, J.; Campard, G. The relationship of oral health with general health and NCDs: A brief review. Int. Dent. J. 2017, 67, 14–18. [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community–Implications for health and disease. BMC Oral Health 2006, 6(Suppl 1), S14. [CrossRef]
- Jakubovics, N.S.; Kolenbrander, P.E. The road to ruin: The formation of disease-associated oral biofilms. Oral Dis. 2010, 16, 729–739. [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [CrossRef]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [CrossRef]
- Peng, Y.; Wu, R.; Qu, W.; Wu, W.; Chen, J.; Fang, J.; Yangxi, C.; Mauro, F.; Li, M. Effect of visual method vs plaque disclosure in enhancing oral hygiene in adolescents and young adults: A single-blind randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 280–286. [CrossRef]
- Zoya, C.; Ranjana, M.; Vandana, S.; Rohit, R.; Aruna, D. Disclosing agents in periodontics: An update. J. Dent. Coll. Azamgarh. 2015, 1, 103–110.
- FDA. Red Dye’s Reluctant Regulator; Partial Ban Points to Limitations of 30-Year-Old Delaney Clause. The Washington Post, 7 February 1990. Available online: https://www.washingtonpost.com/archive (accessed on 20 April 2024).
- Jung, J.Y.; Yeon, K.H.; Song, H.R.; Hwang, Y.S. Cytotoxicity of dental disclosing solution on gingival epithelial cells in vitro. Clin. Exp. Dent. Res. 2020, 6, 669–676. [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Safety Evaluation and Regulatory Update on Plaque Disclosing Agents in Korea; MFDS: Osong, Republic of Korea, 2014. Available online: https://www.mfds.go.kr (accessed on 20 April 2024).
- Jung, I. H., & Hwang, Y. S. (2022). Evaluation of the suitability of Gardenia blue pigment derived from Gardenia jasminoides Ellis (Rubiaceae) as a dental plaque disclosant. Clinical and Experimental Dental Research, 8(5), 1035-1039.
- Maronpot, R.; Ramot, Y.; Nyska, A.; Sproul, C.; Moore, R.; Koyanagi, M.; Hayashi, S.M. Chronic toxicity and carcinogenicity study of dietary gardenia blue in Sprague Dawley rats. Food Chem. Toxicol. 2023, 176, 113734. [CrossRef]
- Mahapatra, D., Nishino, M., & Iniwa, M. (2023). Gardenia blue is not carcinogenic in the rasH2 mouse. Toxicology Research and Application, 7, 23978473231173093.
- Laela, D. S., Mulyanti, S., & Mulyo, G. P. (2022). The effectiveness of mulberry and red beetroot as plaque coloring on Streptococcus mutans glycoprotein. Journal of International Dental & Medical Research, 15(4), 1462–1466.
- Steinberg, L. M. (1984). Dental plaque disclosing compositions and methods. U.S. Patent No. 4,431,628. U.S. Patent and Trademark Office.
- Maya, M. A., Kahabuka, F., & Mbawalla, H. (2018). Effectiveness of supervised tooth-brushing and use of plaque disclosing agent on children’s tooth-brushing skills and oral hygiene: A cluster randomized trial. EC Dental Science, 17(11), 1929–1938.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).