Submitted:
27 April 2025
Posted:
28 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. Total Protein Concentration
2.4. Total Antioxidant Capacity
2.5. Measurement of Malondialdehyde (MDA)
2.6. Measurement of Salivary 8-OHdG Levels by ELISA
2.7. Measurement of Thiobarbituric Acid Reactive Substances (TBARS)
2.8. Extraction of Salivary RNA
2.9. Quantitative Gene Expression (qRT-PCR)
| Gene Symbol | Sequence | Gene ID | Amplicon Size (bp) | References |
|---|---|---|---|---|
| IL-8 | F1: GAGGGTTGTGGAGAAGTTTTTG R1: CTGGCATCTTCACTGATTCTTG |
NM_000584 | 88bp | [35,36,37] |
| IL-1β | F2: GTGCTGAATGTGGACTCAATCC R2: ACCCTAAGGCAGGCAGTTG |
M15330 | 120bp | |
| GAPDH | F3: TGAAGGTCGGAGTCAACGGATTTGGT R3: CATGTGGGCCATGAGGTCCACCAC |
NM_002046.7 | 983bp | [37,38,39]. |
3. Results
| Gender Distribution | Control (n=30) Frequency, % |
Case (n=30) Frequency, % |
P value | |
|---|---|---|---|---|
| Male | 14 (46.7) | 13 (43.3) | 0.79 | |
| Female | 16 (53.3) | 17 (56.7) | ||
| Age (Mean ± SD) | 26.0 (5.33) | 26.1 (5.12) | 0.94 | |
| Smoking status | Smoker | 10 (33.3) | 12 (40) | 0.59 |
| Nonsmoker | 20 (66.7) | 18 (60) | ||
| Parameters | Control (n=30) Frequency, % |
Case (n=30) Frequency, % |
P value | |
|---|---|---|---|---|
|
Total Protein g/dl |
Mean ± SD | 1.71 (0.12) | 2.25 (1.82) | P = 0.1103 |
|
TBARS μmol/l |
0.48 (0.24) | 1.40 (0.87) | P < 0.0001 | |
|
TAC mmol/l |
0.34 (0.11) | 0.57 (0.44) | P = 0.0074 | |
|
MDA μmol/l |
0.31 (0.08) | 0.59 (0.49) | P = 0.0031 | |
|
8-OHdG ng/ml |
0.49 (0.05) | 0.83 (0.66) | P = 0.0067 | |
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| 8-OHdG | 8-Hydroxy-2'-Deoxyguanosine |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| MDA | Malondialdehyde |
| qRT-PCR | Quantitative Reverse Transcription-Polymerase Chain Reaction |
| TAC | Total Antioxidant Capacity |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Inchingolo F, Inchingolo AM, Latini G, Ferrante L, Trilli I, Del Vecchio G; et al. Oxidative stress and Natural products in Orthodontic Treatment: a systematic review. 2023, 16, 113. [CrossRef]
- Bazarova KM, Zhartybaev RA, Salymbekov BB, Iskakova MK, editors. Orthodontic problems in the treatment of patients with dental defects. Science and education: problems and innovations; 2021. [CrossRef]
- Yan M, Zhang Y, Niu W, Liu K, Xue L, Zhou KJMR; et al. Reactive oxygen species-mediated endoplasmic reticulum stress contributes to osteocyte death induced by orthodontic compressive force. 2023, 86, 1529–41. [CrossRef]
- GEÇER RBJH, -II S. OXIDATIVE STRESS AND ORTHODONTIC TREATMENT. 2024:137.
- Buczko, P.; Knaś, M.; Grycz, M.; Szarmach, I.; Zalewska, A.J.A.i.M.S. Orthodontic treatment modifies the oxidant–antioxidant balance in saliva of clinically healthy subjects. 2017, 62, 129-35. [CrossRef]
- Li Y, Jacox LA, Little SH, Ko C-CJTKjoms. Orthodontic tooth movement: The biology and clinical implications. 2018, 34, 207–14. [CrossRef]
- Menéndez López-Mateos C, Menéndez López-Mateos ML, Aguilar-Salvatierra A, Gómez-Moreno G, Carreño JC, Khaldy H; et al. Salivary Markers of Oxidative Stress in Patients Undergoing Orthodontic Treatment with Clear Aligners versus Self-Ligating Brackets: A Non-Randomized Clinical Trial. 2022, 11, 3531. [CrossRef]
- Tóthová Lu, Celec PJFip. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. 2017, 8, 1055. [CrossRef]
- Ren Y, Hazemeijer H, de Haan B, Qu N, de Vos PJJop. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. 2007, 78, 453–8. [CrossRef]
- Karimi-Afshar, M.; Torabi, M.; Abdollahi, S.; Safarian, M.S.; Farsinejad, A.J.J.o.K.U.o.M.S. A comparative study on the IL-8 expression in gingival crevicular fluid during early alignment stage of orthodontic treatment in adults and adolescents. 2021, 28, 367-73. [CrossRef]
- Rodrigues R, Mesquita CM, ALVES HBdN, Silva FG, VIEIRA WdA, AGUIAR PCSd; et al. Changes in salivary biomarkers of pain, anxiety, stress, and inflammation related to tooth movement during orthodontic treatment: a systematic review. 2024, 29, e242436. [CrossRef]
- Primožič, J.; Poljšak, B.; Jamnik, P.; Kovač, V.; Čanadi Jurešić, G.; Spalj, S.J.A. Risk assessment of oxidative stress induced by metal ions released from fixed orthodontic appliances during treatment and indications for supportive antioxidant therapy: A narrative review. 2021, 10, 1359. [CrossRef]
- Buljan, Z.I.; Ribaric, S.P.; Abram, M.; Ivankovic, A.; Spalj, S.J.T.A.O. In vitro oxidative stress induced by conventional and self-ligating brackets. 2012, 82, 340-5. [CrossRef]
- Spalj S, Zrinski MM, Spalj VT, Buljan ZIJAjoo, orthopedics d. In-vitro assessment of oxidative stress generated by orthodontic archwires. 2012, 141, 583–9. [CrossRef]
- Olteanu C, Muresan A, Daicoviciu D, Tarmure V, Olteanu I, Keularts IMJPF. VARIATIONS OF SOME SALIVA MARKERS OF THE OXIDATIVE STRESS IN PATIENTS WITH ORTHODONTIC APPLIANCES. 2009, 19.
- Atuğ Özcan SS, Ceylan İ, Özcan E, Kurt N, Dağsuyu İM, Çanakçi CFJDm. Evaluation of oxidative stress biomarkers in patients with fixed orthodontic appliances. 2014, 2014, 597892. [CrossRef]
- Vlková B, Stanko P, Minárik G, Tóthová Ľ, Szemes T, Baňasová L; et al. Salivary markers of oxidative stress in patients with oral premalignant lesions. 2012, 57, 1651–6. [CrossRef]
- Cazzolla AP, Brescia V, Lovero R, Fontana A, Giustino A, Dioguardi M; et al. Evaluation of Biomarkers of Bone Metabolism on Salivary Matrix in the Remodeling of Periodontal Tissue during Orthodontic Treatment. 2024, 12, 209. [CrossRef]
- Chairatnathrongporn, R.; Tansriratanawong, K.; Santiprabhob, J.; Boriboonhirunsarn, C.; Promsudthi, A.J.J.o.I.S.o.P.; Dentistry, C. Salivary gene expression of RANK, RANKL, and OPG in type 1 diabetes mellitus and periodontal disease patients. 2022, 12, 603-11. [CrossRef]
- Ismah, N.; Bachtiar, E.W.; Purwanegara, M.K.; Tanti, I.; Mardiati, E.J.J.o.I.S.o.P.; Dentistry, C. Evaluation of IL-1β and CRP mRNA expression levels by RT-PCR in postorthodontic treatment patients with temporomandibular joint disorders: a cross-sectional Study. 2024, 14, 98-104. [CrossRef]
- Guarnieri R, Reda R, Di Nardo D, Miccoli G, Pagnoni F, Zanza A; et al. Expression of IL-1β, IL-6, TNF-α, and a-MMP-8 in sites with healthy conditions and with periodontal and peri-implant diseases: A case-control study. 2024, 18, 135. [CrossRef]
- Alhashimi, N.; Frithiof, L.; Brudvik, P.; Bakhiet, M.J.A.J.o.O.; Orthopedics, D. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. 2001, 119, 307-12. [CrossRef]
- Başaran, G.; Özer, T.; Kaya, F.A.; Hamamci, O.J.A.J.o.O.; Orthopedics, D. Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. 2006, 130, 7. e1-7. e6. [CrossRef]
- Saloom, H.F. Evaluation of Salivary Levels of Proinflammatory Cytokines (IL-1α, IL-8 and GM-CSF) in Adult Orthodontic Patients. 2018.
- de Sousa Né YG, Lima WF, Mendes PFS, Baia-da-Silva DC, Bittencourt LO, Nascimento PC; et al. Dental caries and salivary oxidative stress: global scientific research landscape. 2023, 12, 330. [CrossRef]
- Demirci-Çekiç S, Özkan G, Avan AN, Uzunboy S, Çapanoğlu E, Apak RJJop; et al. Biomarkers of oxidative stress and antioxidant defense. 2022, 209, 114477. [CrossRef]
- Khajuria AK, Thalquotra M, Singh P, Agarwal GJEJoM, Medicine C. ASSESSMENT OF OXIDATIVE STRESS GENERATED BY ORTHODONTIC ARCHWIRES-AN IN VITRO STUDY. 2022, 9, 2092–7.
- Syed IB, Khalid S, Abbas A, Faisal Z, Abbas H, Azeem MJJOKCOD. IN-VIVO ANALYSIS OF OXIDATIVE STRESS IN AESTHETIC COATED ORTHODONTIC ARCH-WIRES & BRACKETS. 2025, 15, 44–51. [CrossRef]
- Henson, B.S.; Wong, D.T. Collection, storage, and processing of saliva samples for downstream molecular applications. Oral biology: molecular techniques and applications: Springer; 2010. p. 21-30. [CrossRef]
- Akbarnejad, A.A.; Mahjoub, S.; Tamaddoni, A.; Masrour-Roudsari, J.; Seyedmajidi, S.A.; Ghasempour, M.J.J.o.D. Salivary oxidative stress, total protein, iron and ph in children with β-thalassemia major and their correlation with dental caries. 2022, 23, 266. [CrossRef]
- Benzie IF, Strain JJJAb. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. 1996, 239, 70–6. [CrossRef]
- De Leon, J.A.D.; Borges, C.R.J.J.o.v.e.J. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. 2020(159):10.3791/61122.
- Arunachalam, R.; Reshma, A.P.; Rajeev, V.; Kurra, S.B.; Prince, M.R.J.; Syam, N.J.T.S.J.f.D.R. Salivary 8-Hydroxydeoxyguanosine–a valuable indicator for oxidative DNA damage in periodontal disease. 2015, 6, 15-20. [CrossRef]
- Celec P, Hodosy J, Celecová V, Vodrážka J, Červenka T, Halčák L; et al. Salivary thiobarbituric acid reacting substances and malondialdehyde–their relationship to reported smoking and to parodontal status described by the papillary bleeding index. 2005, 21, 133–7. [CrossRef]
- Li Y, St. John MA, Zhou X; et al. Salivary transcriptome diagnostics for oral cancer detection. 2004, 10, 8442-50. [CrossRef]
- Park NJ, Zhou X, Yu T, Brinkman BM, Zimmermann BG, Palanisamy V; et al. Characterization of salivary RNA by cDNA library analysis. 2007, 52, 30–5. [CrossRef]
- Senevirathna K, Mahakapuge TAN, Jayawardana NU, Rajapakse J, Gamage CU, Seneviratne B; et al. Diagnostic potential of salivary IL-1β, IL-8, SAT, S100P, and OAZ1 in oral squamous cell carcinoma, oral submucous fibrosis, and oral lichen planus based on findings from a Sri Lankan cohort. 2024, 14, 27226. [CrossRef]
- Uehara A, Sugawara S, Watanabe K, Echigo S, Sato M, Yamaguchi T; et al. Constitutive expression of a bacterial pattern recognition receptor, CD14, in human salivary glands and secretion as a soluble form in saliva. 2003, 10, 286–92. [CrossRef]
- Feng, P.; Tong, C.; Li, Y.; Liu, L.J.A.R. LncRNA HOTAIR: A Novel Biomarker for the Diagnosis of Asymptomatic Carotid Artery Stenosis and Prediction of the Onset of Cerebral Ischemic Events. 2024, 30, 5. [CrossRef]
- Watanabe S, Kawasaki Y, Kawai KJG, Environment. Diurnal variation of salivary oxidative stress marker 8-hydroxyguanine. 2019, 41, 1–4. [CrossRef]
- Borisenkov, M.; Erunova, L.; Lyuseva, E.; Pozdeeva, N.J.H.P. Diurnal changes in the total antioxidant activity of human saliva. 2007, 33, 375-6. [CrossRef]
- Alajbeg IZ, Lapić I, Rogić D, Vuletić L, Andabak Rogulj A, Illeš D; et al. Within-subject reliability and between-subject variability of oxidative stress markers in saliva of healthy subjects: A longitudinal pilot study. 2017, 2017, 2697464. [CrossRef]
- Kamodyová, N.; Celec, P.J.C.C.; Medicine, L. Salivary markers of oxidative stress and Salivette collection systems. 2011, 49, 1887-90.
- Dallel I, Salem IB, Merghni A, Bellalah W, Neffati F, Tobji S; et al. Influence of orthodontic appliance type on salivary parameters during treatment. 2020, 90, 532. [CrossRef]
- Teixeira, H.S.; Kaulfuss, S.M.O.; Ribeiro, J.S.; Pereira, B.d.R.; Brancher, J.A.; Camargo, E.S.J.D.P.J.o.O. Calcium, amylase, glucose, total protein concentrations, flow rate, pH and buffering capacity of saliva in patients undergoing orthodontic treatment with fixed appliances. 2012, 17, 157-61. [CrossRef]
- ANA-MĀDĀLINAR, *!!! REPLACE !!!*; Sebastian, M.; Aureliana, C.; Victoria, B. ANA-MĀDĀLINAR; Sebastian, M.; Aureliana, C.; Victoria, B. Studies regarding salivary total antioxidant activity in different types of orthodontic treatement. 2019. [CrossRef]
- Shamaa, M.S.; Mansour, M.M.J.E.D.J. Long-term assessment of the salivary oxidative stress status during orthodontic treatment with fixed appliances. 2019;65:3151-7. [CrossRef]
- Ángeles-Estrada, L.; Pérez-Soto, E.; Pérez-Vielma, N.M.; Gómez-López, M.; Sánchez-Monroy, V. Oxidative stress and genotoxicity in oral epithelial cells of subjects undergoing Fixed Orthodontic Appliances. 2023. [CrossRef]
- Yamyar, S.; Daokar, S.J.O.J.o.N. Oxidative Stress Levels in Orthodontic Patients and Efficacy of Antioxidant Supplements in Combating Its Effects-A Randomized Clinical Study. 2019, 9, 29-34.
- Pérez-Vielma, N.M.; Domínguez-Rojas, M.; Mendoza-Tapia, S.V.; Angeles-Estrada, L.; Sánchez-Monroy, V.J.R.O.M. Genotoxicity of Fixed Orthodontic Treatment. 2023, 27, 3-11. [CrossRef]
- Kovac V, Poljsak B, Perinetti G, Primozic JJBri. Systemic level of oxidative stress during orthodontic treatment with fixed appliances. 2019, 2019, 5063565. [CrossRef]
- Żukowski P, Maciejczyk M, Waszkiel DJAoOB. Sources of free radicals and oxidative stress in the oral cavity. 2018, 92, 8–17. [CrossRef]
- Kaya FA, Hamamci N, Basaran G, Dogru M, Yildirim TTJJoID, Research M. TNF-α, IL-1β AND IL-8 LEVELS IN TOOTH EARLY LEVELLING MOVEMENT ORTHODONTIC TREATMENT. 2010, 3.
- Chen, Y.; Wong, W.K.; Seneviratne, J.C.; Huang, S.; McGrath, C.; Hagg, U.J.M. Associations between salivary cytokines and periodontal and microbiological parameters in orthodontic patients. 2021, 100, e24924. [CrossRef]
- Kazan, D.; BAŞ, B.; Aksoy, A.; Atmaca, E.J.T.J.o.M.S. The evaluation of oxidative stress and inflammation markers in serum and saliva of the patients with temporomandibular disorders. 2023, 53, 1690-6. [CrossRef]
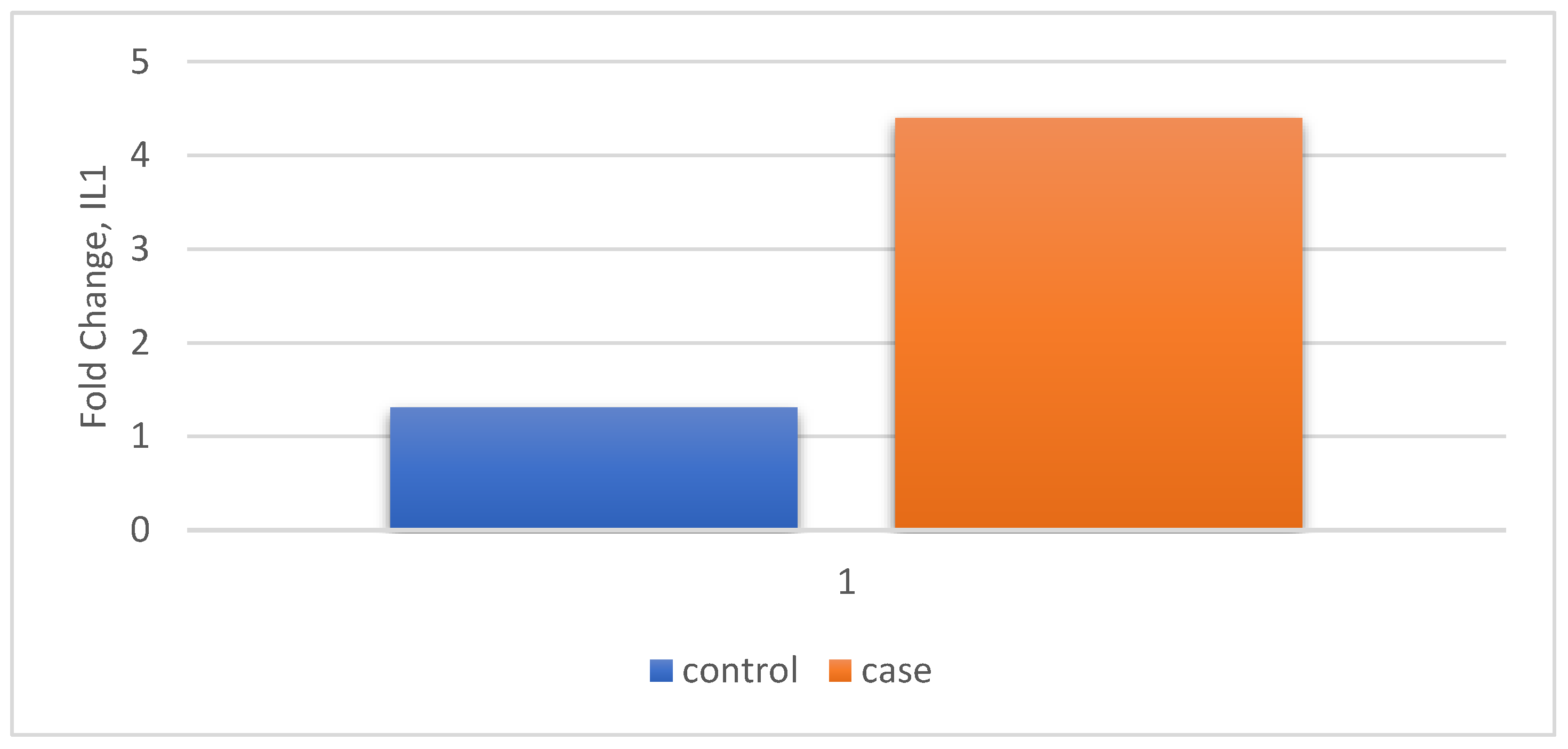
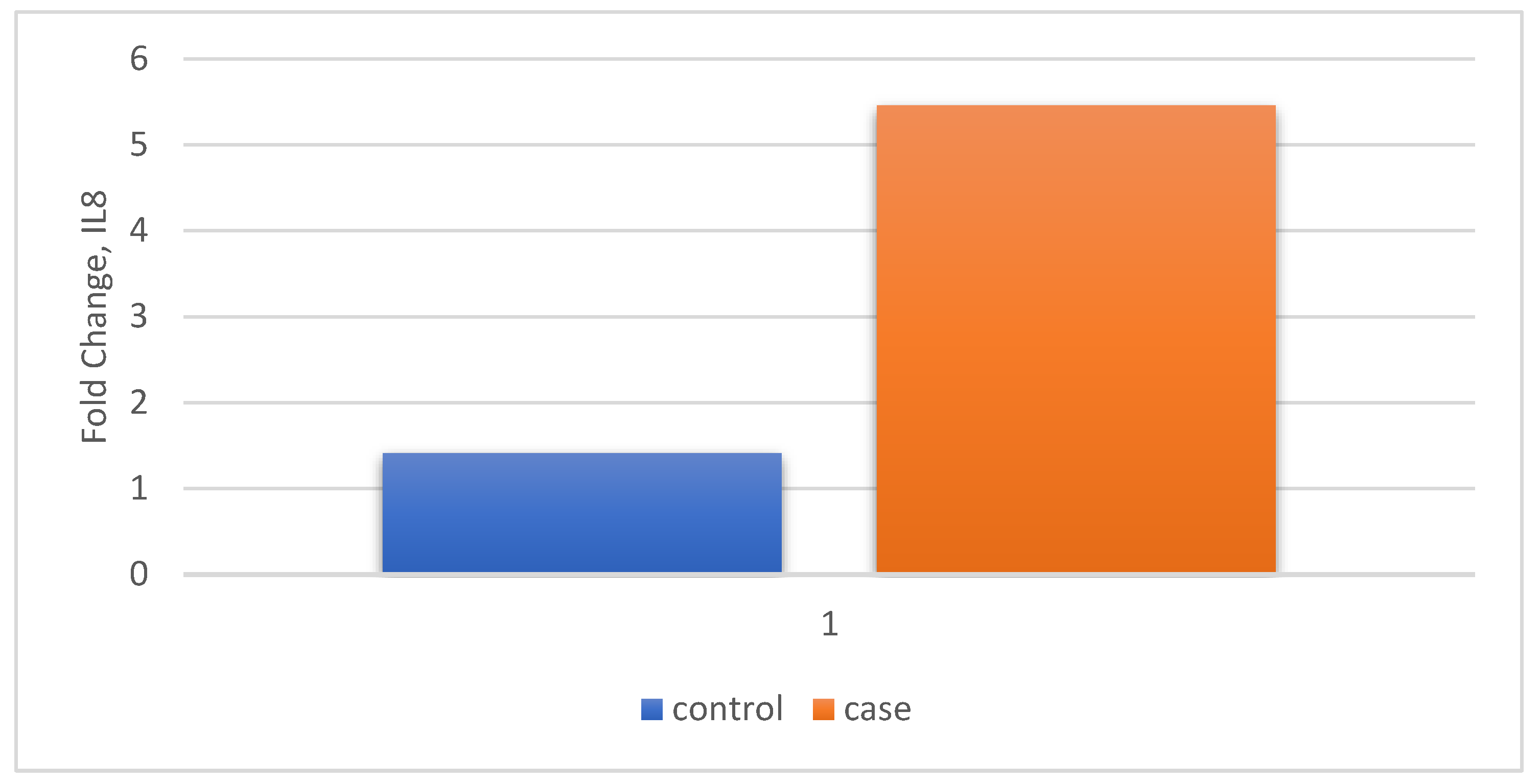
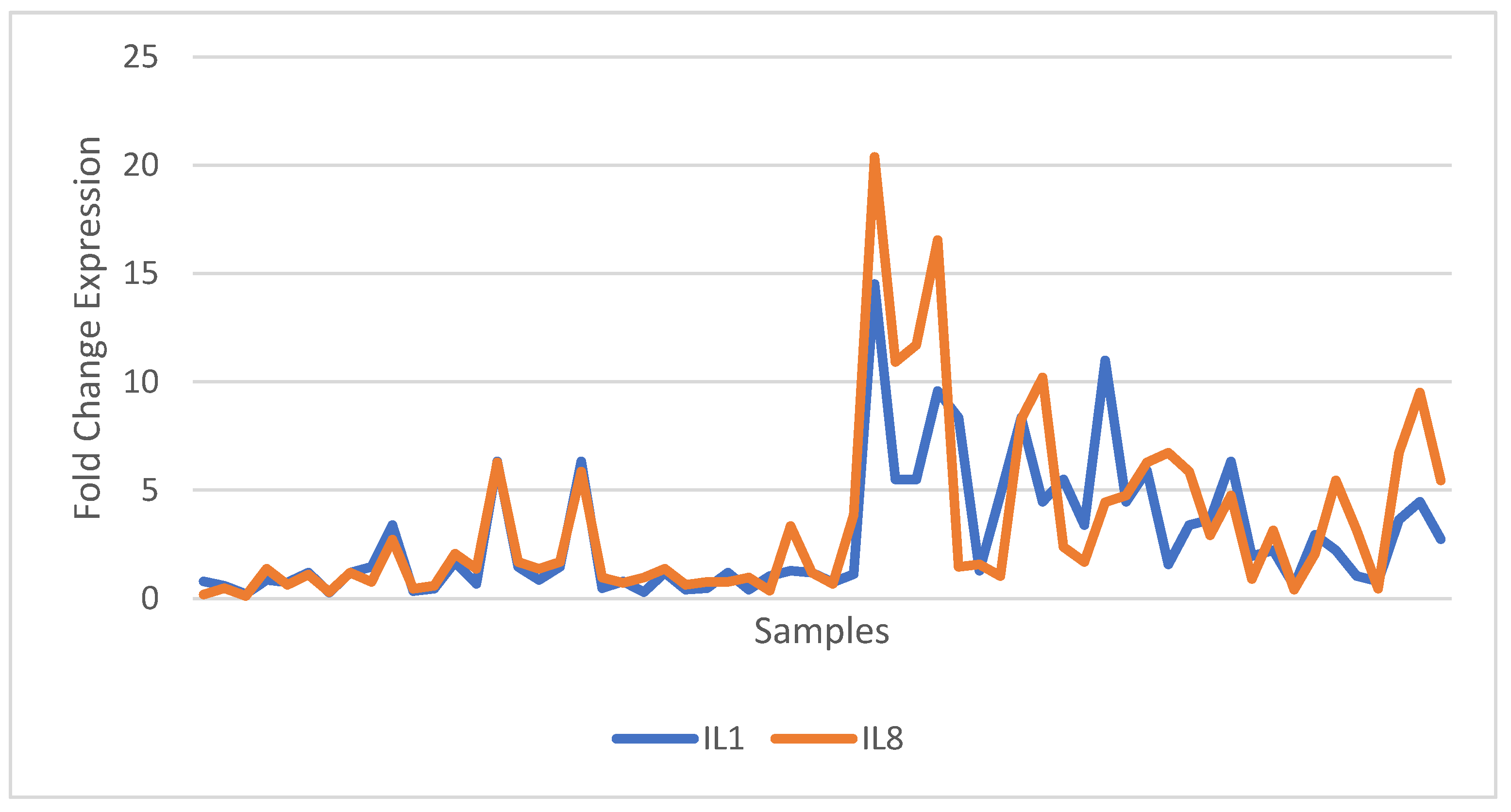
| Relative Gene Expression | Control n=30 |
Case n=30 |
P value | |
|---|---|---|---|---|
| IL1 | Fold Change (Mean ± SD) |
1.3 (1.49) | 4.4 (3.31) | P < 0.0001 t-statistic =4.678 |
| IL8 | Fold Change (Mean ± SD) |
1.41 (1.45) | 5.45 (4.79) | P < 0.0001 t-statistic =4.421 |
| Variable | Relative gene expression IL1 |
Relative gene expression IL8 |
||
| r | p | r | p | |
| Age | 0.021 | 0.873 | 0.088 | 0.503 |
| Gender | 0.086 | 0.513 | 0.010 | 0.942 |
| Smoking | 0.027 | 0.838 | 0.080 | 0.544 |
| Total Protein concentration (g/dL) | 0.264 | 0.041 | 0.234 | 0.072 |
| MDA (μmol/l) | 0.575 | 0.000 | 0.479 | 0.000 |
| TAC (mmol/l) | 0.318 | 0.013 | 0.194 | 0.138 |
| 8-OHdG (ng/ml) | 0.416 | 0.001 | 0.467 | 0.000 |
| IL1 | 1.000 | - | 0.821 | 0.000 |
| IL8 | 0.821 | 0.000 | 1.000 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





