Submitted:
22 April 2025
Posted:
22 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Animals
Color
Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, O.B.; Stewart-Knox, B.J.; Mitchell, P.C.; Thurnham, D.I. Consumer perceptions of poultry meat: a qualitative analysis. Nutr. Food Sci. 2004, 34, 122–129. [Google Scholar] [CrossRef]
- Castañeda, M.P.; Hirschler, E.M.; Sams, A.M. Skin pigmentation evaluation in broilers fed natural and synthetic pigments. Poult. Sci. 2005, 84, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Sirri, F.; Petracci, M.; Bianchi, M.; Meluzzi, A. Survey of skin pigmentation of yellow-skinned broiler chickens. Poult. Sci. 2010, 89, 1556–1561. [Google Scholar] [CrossRef]
- Torrey, S.; Mohammadigheisar, M.; Nascimento dos Santos, M.; Rothschild, D.; Dawson, L.C.; Liu, Z.; Kiarie, E.G.; Edwards, A.M.; Mandell, I.; Karrow, N.; Tulpan, D.; Widowski, T.M. In pursuit of a better broiler: growth, efficiency, and mortality of 16 strains of broiler chickens. Poult. Sci. 2021, 100, 100955. [Google Scholar] [CrossRef]
- Lusk, J.L. Consumer preferences for and beliefs about slow growth chicken. Poult. Sci. 2018, 97, 4159–4166. [Google Scholar] [CrossRef]
- Sunde, M.L. The scientific way to pigment poultry products. Poult. Sci. 1992, 71, 709–710. [Google Scholar] [CrossRef]
- Pérez-Vendrell, A.M.; Hernández, J.M.; Llauradó, L.; Schierle, J.; Brufau, J. Influence of source and ratio of xanthophyll pigments on broiler chicken pigmentation and performance. Poult. Sci. 2001, 80, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Blanch, A. Getting the color of yolk and skin right. World Poult Sci. J. 1999, 15, 32–33. [Google Scholar]
- Alonso-Alvarez, C.; García-de-Blas, E.; Mateo, R. Dietary canthaxanthin reduces xanthophyll uptake and red coloration in adult red-legged partridges. J Exp. Biol. 2018, 221, jeb185074. [Google Scholar] [CrossRef]
- Hencken, H. Chemical and physiological behavior of feed carotenoids and their effects on pigmentation. Poult. Sci. 1992, 71, 711–717. [Google Scholar] [CrossRef]
- Odunitan-Wayas, F.A.; Kolanisi, U.; Chimonyo, M.; Siwela, M. Effect of provitamin A biofortified maize inclusion on quality of meat from indigenous chickens. J. Appl. Poult. Res. 2016, 25, 581–590. [Google Scholar] [CrossRef]
- Martínez Peña, M.; Cortés Cuevas, A.; Avila González, E. Evaluation of three pigment levels of marigold petals (Tagetes erecta) on skin pigmentation of broiler chicken. Tec. Pecu. Mex. 2004, 42, 105–111. [Google Scholar]
- Muñoz-Díaz, J.I.; Fuente-Martínez, B.; Hernández-Velasco, X.; Ávila-González, E. Skin pigmentation in broiler chickens fed various levels of metabolizable energy and xanthophylls from Tagetes erecta. J. Appl. Poult. Res. 2012, 21, 788–796. [Google Scholar] [CrossRef]
- Ponsano, E.H.G.; Pinto, M.F.; Garcia-Neto, M.; Lacava, P.M. Performance and color of broilers fed diets containing Rhodocyclus gelatinosus biomass. Braz. J. Poult. Sci. 2004, 6, 237–242. [Google Scholar] [CrossRef]
- Rajput, N.; Naeem, M.; Ali, S.; Rui, Y.; Tian, W. Effect of dietary supplementation of marigold pigment on immunity, skin and meat color, and growth performance of broiler chickens. Braz. J. Poult. Sci. 2012, 14, 233–304. [Google Scholar] [CrossRef]
- Abraham, M.E.; Weimer, S.L.; Scoles, K.; Vargas, J.I.; Johnson, T.A.; Robison, C.; Hoverman, L.; Rocheford, E.; Rocheford, T.; Ortiz, D.; Karcher, D.M. Orange corn diets associated with lower severity of footpad dermatitis in broilers. Poult. Sci. 2021, 100, 101054. [Google Scholar] [CrossRef]
- C.I.E. (Commission Internationale de L’Eclairage). Colorimetry; C.I.E.: Vienna, Austria. 1986.
- Peng, Y.; Tan, H.; Liu, S.; Li, H.; Chen, Y.; Lin, J. Effects of different grain sources in both maternal and offspring diets on pigmentation and growth performance in yellow-skinned chickens. J. Poult. Sci. 2017, 54, 228–235. [Google Scholar] [CrossRef]
- Lyon, B.G.; Smith, D.P.; Lyon, C.E.; Savage, E.M. Effects of diet and feed withdrawal on the sensory descriptive and instrumental profiles of broiler breast fillets. Poult. Sci. 2004, 83, 275–281. [Google Scholar] [CrossRef]
- Ciurescu, G.; Vasilachi, A.; Idriceanu, L.; Dumitru, M. Effects of corn replacement by sorghum in broiler chickens diets on performance, blood chemistry, and meat quality. Ital. J. Anim. Sci. 2023, 22, 537–547. [Google Scholar] [CrossRef]
- Moreno, J.A.; Diaz-Gomez, J.; Fuentes-Font, L.; Angulo, E.; Gosalvez, L.F.; Sandmann, G.; Portero-Otin, M.; Capell, T.; Zhu, C.; Christou, P.; Nogareda, C. Poultry diets containing (keto)carotenoid-enriched maize improve egg yolk color and maintain quality. Anim. Feed Sci. Technol. 2020, 260, 114334. [Google Scholar] [CrossRef]
- Trono, D. Carotenoids in cereal food crops: composition and retention throughout grain storage and food processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Noboa, H.A.; Oviedo-Rondón, E.O.; Sarsour, A.H.; Barnes, J.; Ferzola, P.; Rademacher-Heilshorn, M.; Braun, U. Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic. Poultr. Sci. 2018, 97, 2479–2493. [Google Scholar] [CrossRef]
- Bai, S.; Wang, G.; Zhang, W.; Zhang, S.; Rice, B.B.; Cline, M.A.; Gilbert, E.R. Broiler chicken adipose tissue dynamics during the first two weeks post-hatch. Comp Biochem Physiol A. 2015, 189, 115–123. [Google Scholar] [CrossRef]
- Muzhingi, T.; Yeum, K.J.; Russell, R.M.; Johnson, E.L.; Qin, J.; Tang, G. Determination of carotenoids in yellow maize, the effects of saponification and food preparations. Int. J. Vitam. Nutr. Res. 2008, 78, 112–120. [Google Scholar] [CrossRef]
- Kean, E.G.; Bordenave, N.; Ejeta, G.; Hamaker, B.R.; Ferruzzi, M.G. Carotenoid bioaccessibility from whole grain and decorticated yellow endosperm sorghum porridge. J. Cereal Sci. 2011, 54, 450–459. [Google Scholar] [CrossRef]
- Branellec, J.C. La pigmentation du poulet de chair. Aliscope 1985, 85, 1–13. [Google Scholar]
- Riveros Lizana, R.; Zea Mendoza, O.; Vilchez Perales, C. Evaluation of tarsus pigmentation in chickens fed with different levels of xanthophyll pigment: A practical application of the CIELab system. Int. J. Poult. Sci. 2020, 19, 265–269. [Google Scholar] [CrossRef]
- Deming, D.M.; Erdman Jr, J.W. Mammalian carotenoid absorption and metabolism. Pure Appl. Chem. 1999, 71, 2213–2223. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Lin, Z.X.; Zhang, M.; Yang, R.; Guo, P.T.; Zhang, J.; Wang, C.K.; Jin, L. Health effects of astaxanthin in the intestinal tract of yellow-feathered broilers. Poult. Sci. 2025, 104, 104768. [Google Scholar] [CrossRef]
- Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Analysis of lutein, zeaxanthin, and meso-zeaxanthin in the organs of carotenoid-supplemented chickens. Foods 2018, 7, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Illingworth, D.R.; Connor, S.L.; Duell, P.B.; Connor, W.E. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur. J. Nutr. 2010, 49, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Abril, M.; Campo, M.M.; Önenç, A.; Sañudo, C.; Albertí, P.; Negueruela, A.I. Beef color evolution as a function of ultimate pH. Meat Sci. 2001, 58, 69–78. [Google Scholar] [CrossRef] [PubMed]

|
Starter (0-14d) Crumble |
Grower (15-41d) Pellet |
|||
| WHEAT | CORN | WHEAT | CORN | |
| Ingredients % | ||||
| Corn | 5.00 | 30.0 | 0 | 45.0 |
| Wheat | 30.0 | 15.0 | 45.1 | 0 |
| Barley | 20.0 | 6.70 | 25.0 | 22.9 |
| Soybean meal, 47% | 30.0 | 30.0 | 20.8 | 25.4 |
| Full fat soybean meal | 5.00 | 6.00 | 0 | 0 |
| Soybean oil | 0 | 0 | 2.51 | 2.25 |
| Sunflower meal, 34% | 3.00 | 4.00 | 3.00 | 1.00 |
| Tallow | 1.60 | 1.60 | 0 | 0 |
| Mucose, hydrolised | 2.00 | 2.65 | 0 | 0 |
| Dicalcium phosphate | 0.920 | 0.968 | 0.965 | 1.10 |
| Limestone | 0.786 | 0.712 | 0.981 | 0.871 |
| Vitamin-mineral premix1 | 0.500 | 0.500 | 0.500 | 0.500 |
| MHA-Ca2 | 0.447 | 0.447 | 0.327 | 0.346 |
| L-Lysine HCl | 0.278 | 0.260 | 0.293 | 0.233 |
| Salt (NaCl) | 0.168 | 0.153 | 0.167 | 0.217 |
| Sodium bicarbonate | 0.119 | 0.100 | 0.189 | 0.137 |
| L-Threonine | 0.130 | 0.115 | 0.106 | 0.071 |
| Calculated nutrients % | ||||
| Dry matter | 89.5 | 88.4 | 90.0 | 87.9 |
| ME poultry, kcal/kg | 2980 | 2980 | 3064 | 3050 |
| Crude protein | 29.0 | 23.8 | 18.7 | 18.3 |
| Lysine, total | 1.47 | 1.47 | 1.09 | 1.10 |
| Lysine, digestible | 1.31 | 1.31 | 0.970 | 0.970 |
| Crude fibre | 3.91 | 3.81 | 3.72 | 3.15 |
| Crude fat | 4.09 | 4.82 | 4.00 | 4.77 |
| Ash | 5.54 | 5.51 | 5.10 | 5.00 |
| Calcium | 0.965 | 0.950 | 1.00 | 0.995 |
| Phosphorus, total | 0.597 | 0.609 | 0.551 | 0.553 |
| Phosphorus, digestible | 0.440 | 0.440 | 0.420 | 0.420 |
| FEED | SEM | P | ||
|---|---|---|---|---|
| WHEAT | CORN | |||
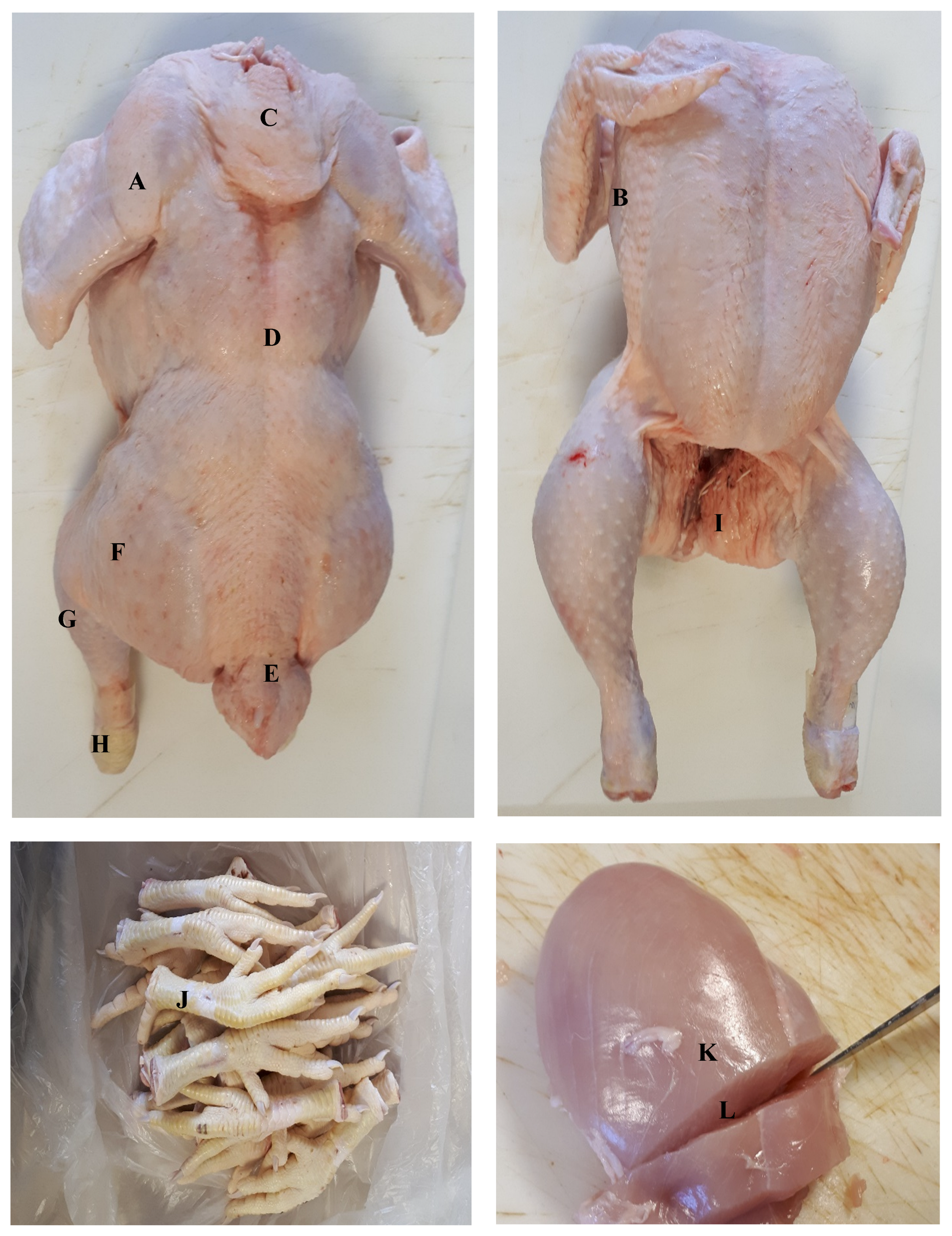
| Apterial latero-pectoral area | 74.43 cdeX | 72.28 cY | 0.47 | 0.020 |
| Back | 74.89 bcdX | 72.58 cY | 0.33 | <0.001 |
| Breast interior | 51.95 g | 51.59 f | 0.67 | 0.791 |
| Breast surface | 54.55 g | 54.50 e | 0.46 | 0.959 |
| Drumette | 72.71 deX | 71.31 cY | 0.31 | 0.021 |
| Ham | 66.05 f | 65.81 d | 0.48 | 0.805 |
| Hock | 77.49 ab | 76.19 a | 0.35 | 0.057 |
| Neck | 75.97 bcX | 73.49 bcY | 0.37 | <0.001 |
| Saddle | 73.02 de | 73.00 c | 0.34 | 0.975 |
| Shank | 78.99 aX | 75.84 abY | 0.32 | <0.001 |
| Thigh | 72.93 de | 71.57 c | 0.37 | 0.064 |
| Vent | 72.09 e | 71.93 c | 0.37 | 0.830 |
| SEM | 0.63 | 0.59 | ||
| P | <0.001 | <0.001 | ||
| FEED | SEM | P | ||
|---|---|---|---|---|
| WHEAT | CORN | |||
| Apterial latero-pectoral area | 0.78 dY | 2.05 deX | 0.30 | 0.030 |
| Back | 2.84 cY | 4.76 bcX | 0.32 | 0.001 |
| Breast interior | -0.94 eY | 0.80 efX | 0.20 | <0.001 |
| Breast surface | -0.32 deY | 1.02 efX | 0.17 | <0.001 |
| Drumette | 3.46 bcY | 5.12 bX | 0.31 | 0.005 |
| Ham | -0.43 eY | 0.25 fX | 0.12 | 0.002 |
| Hock | -0.36 deY | 2.14 deX | 0.30 | <0.001 |
| Neck | 3.19 bcY | 5.17 bX | 0.28 | <0.001 |
| Saddle | 4.31 ab | 5.08 b | 0.26 | 0.137 |
| Shank | -0.27 deY | 3.32 cdX | 0.33 | <0.001 |
| Thigh | -0.39 de | 0.15 f | 0.14 | 0.062 |
| Vent | 5.30 aY | 6.78 aX | 0.31 | 0.015 |
| SEM | 0.17 | 0.19 | ||
| P | <0.001 | <0.001 | ||
| FEED | SEM | P | ||
|---|---|---|---|---|
| WHEAT | CORN | |||
| Apterial latero-pectoral area | 7.78 deY | 11.64 efX | 0.58 | <0.001 |
| Back | 13.17 cY | 15.21 deX | 0.40 | 0.009 |
| Breast interior | 6.53 efY | 10.93 fX | 0.46 | <0.001 |
| Breast surface | 7.39 deY | 10.58 fX | 0.45 | <0.001 |
| Drumette | 14.35 bcY | 17.75 cdX | 0.45 | <0.001 |
| Ham | 4.62 f | 5.47 g | 0.42 | 0.320 |
| Hock | 16.39 abY | 21.52 bcX | 1.23 | 0.035 |
| Neck | 14.98 bcY | 19.70 bcX | 0.52 | <0.001 |
| Saddle | 16.58 abY | 18.57 cdX | 0.44 | 0.020 |
| Shank | 18.70 aY | 31.59 aX | 1.23 | <0.001 |
| Thigh | 9.50 d | 10.86 f | 0.57 | 0.237 |
| Vent | 18.65 aY | 23.03 bX | 0.58 | <0.001 |
| SEM | 0.39 | 0.56 | ||
| P | <0.001 | <0.001 | ||
| FEED | SEM | P | ||
|---|---|---|---|---|
| WHEAT | CORN | |||
| Apterial latero-pectoral area | 7.89 efY | 11.90 eX | 0.60 | <0.001 |
| Back | 13.50 dY | 16.04 dX | 0.43 | 0.002 |
| Breast interior | 6.62 fgY | 10.98 eX | 0.46 | <0.001 |
| Breast surface | 7.41 efY | 10.65 eX | 0.46 | <0.001 |
| Drumette | 14.80 cdY | 18.53 cdX | 0.49 | <0.001 |
| Ham | 4.76 g | 5.50 f | 0.40 | 0.365 |
| Hock | 16.40 bcY | 21.67 bcX | 1.24 | 0.030 |
| Neck | 15.34 cdY | 20.40 bcX | 0.55 | <0.001 |
| Saddle | 17.16 abcY | 19.29 cdX | 0.46 | 0.018 |
| Shank | 18.71 abY | 31.77 aX | 1.24 | <0.001 |
| Thigh | 9.53 e | 10.90 e | 0.57 | 0.232 |
| Vent | 19.45 aY | 24.02 bX | 0.61 | <0.001 |
| SEM | 0.40 | 0.58 | ||
| P | <0.001 | <0.001 | ||
| FEED | SEM | P | ||
|---|---|---|---|---|
| WHEAT | CORN | |||
| Apterial latero-pectoral area | 84.91 cd | 80.81 cd | 1.48 | 0.169 |
| Back | 77.93 deX | 72.55 eY | 1.12 | 0.014 |
| Breast interior | 97.58 abX | 85.91 abcY | 1.33 | <0.001 |
| Breast surface | 93.05 bcX | 84.56 bcY | 1.06 | <0.001 |
| Drumette | 76.70 de | 73.92 e | 0.86 | 0.106 |
| Ham | 102.70 aX | 88.90 abY | 3.36 | 0.037 |
| Hock | 91.29 bcX | 84.14 bcY | 0.94 | <0.001 |
| Neck | 78.06 deX | 75.25 deY | 0.71 | 0.047 |
| Saddle | 75.64 de | 74.60 e | 0.65 | 0.429 |
| Shank | 90.98 bcX | 84.06 bcY | 0.64 | <0.001 |
| Thigh | 93.04 bc | 90.74 a | 0.89 | 0.200 |
| Vent | 74.35 e | 73.64 e | 0.62 | 0.573 |
| SEM | 0.89 | 0.58 | ||
| P | <0.001 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





