Submitted:
18 September 2025
Posted:
21 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Design, Birds and Management
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kralik, G.; Kralik, Z.; Grčević, M.; Hanžek, D. Quality of Chicken Meat. In Animal Husbandry and Nutrition, B. Yucel, & T. Taskin (Eds.); InTechOpen, 2018; pp.69-94. [CrossRef]
- Petracci, M. Current meat quality challenges for the poultry industry – a review. Anim. Sci. Pap. Rep. 2022, 40, 253–261. Available online: http://www.igbzpan.pl/uploaded/FSiBundleContentBlockBundleModelTranslatableBlockTranslatableFilesElement/filePath/2201/str253-262.pdf.
- Petracci, M.; Mudalal, S.; Babini, E.; Cavani, C. Effect of white striping on chemical composition and nutritional value of chicken breast meat. Ital. J. Anim. Sci. 2014, 13, 179–183. [Google Scholar] [CrossRef]
- Santos, M.N.; Rothschild, D.; Widowski, T.M.; Shai Barbut, S.; Elijah, G.; Kiarie, E.G.; Mandell, I.; Guerin, M.T.; Edwards, A.M.; Torrey, S. In pursuit of a better broiler: carcass traits and muscle myopathies in conventional and slower-growing strains of broiler chickens. Poult. Sci. 2021, 100, 101309. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Ricci, R.; Cullere, M.; Serva, L.; Tenti, S.; Marchesini, G. Effect of chicken genotype and white striping–wooden breast condition on breast meat proximate composition and amino acid profile. Poult. Sci. 2020a, 99, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Mudalal, S.; Soglia, F.; Cavani, C. Meat quality in fast-growing broiler chickens. World Poult. Sci. J. 2015, 71, 363–374. [Google Scholar] [CrossRef]
- Lukić, M.; Škrbić, Z.; Petričević, V.; Delić, N.; Tolimir, N.; Dosković, V.; Rakonjac, S. Genotype and breeder flock age impact on broiler performance in suboptimal conditions. Biotech. Anim. Husb. 2020, 36, 447–462. [Google Scholar] [CrossRef]
- Menchetti, L.; Birolo, M.; Mugnai, C.; Mancinelli, A.C.; Xiccato, G.; Trocino, A.; Castellini, C. Effect of genotype and nutritional and environmental challenges on growth curve dynamics of broiler chickens. Poult. Sci. 2024, 103, 104095. [Google Scholar] [CrossRef]
- Riber, A.B.; Wurtz, K.E. Impact of Growth Rate on the Welfare of Broilers. Animals 2024, 14, 3330. [Google Scholar] [CrossRef]
- Sokołowicz, Z.; Krawczyk, J.; Świątkiewicz, S. Quality of poultry meat from native chicken breeds – a review. Ann. Anim. Sci. 2016, 16, 347–368. [Google Scholar] [CrossRef]
- Sosnówka-Czajka, E.; Skomorucha, I.; Muchacka, R. Effect of organic production system on the performance and meat quality of two purebred slow-growing chicken breeds. Ann. Anim. Sci. 2017, 17, 1197–1213. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations [FAO]. Domestic Animal Diversity Information System (DAD-IS). 2025. Available online: https://www.fao.org/dad-is/browse-by-country-and-species/en/ (accessed on May 2025).
- Mean Breeding Programm of indigenous poultry breeds of RS [MBP]/Glavni odgajivački program autohtonih rasa živine RS [GOP] (in Serbian). Institut za stočarstvo, Beograd, 2024. Available online: https://istocar.bg.ac.rs/wp-content/uploads/2024/11/GOP-ZIVINARSTVO-Autohtone-rase-2025-2029.pdf.
- Food and Agriculture Organization of the United Nations [FAO]. The State of the World’s Biodiversity for Food and Agriculture. FAO Commission on Genetic Resources for Food and Agriculture Assessments, Rome, Italy, 2019. [CrossRef]
- Pavlovski, Z.; Škrbić, Z.; Lukić, M.; Vitorović, D.; Petričević, V. Naked Neck – Autochthonous Breed of Chicken in Serbia: Carcass Characteristics. Biotech. Anim. Husb. 2009, 25, 1–10. [Google Scholar] [CrossRef]
- Mitrovic, S.; Bogosavljevic-Boskovic, S.; Stanisic, G.; Djermanovic, V.; Doskovic, V.; Rakonjac, S. Carcass characteristics of two strains of native broilers (White Naked Neck and Black Svrljig) fattened under a semi-intensive system. Afr. J. Biotechnol. 2011, 10, 15813–15818. [Google Scholar] [CrossRef]
- Pavlovski, Z.; Škrbić, Z.; Stanišić, N.; Lilić, S.; Hengl, B.; Lukić, M.; Petričević, V. Differences in fatty acid composition of meat between naked neck and two commercial broiler chicken breeds. Biotech. Anim. Husb. 2013, 29, 467–476. [Google Scholar] [CrossRef]
- Pavlovski, Z.; Škrbić, Z.; Lukić, M.; Petričević, V.; Stanojković, A. Carcass quality of chickens of different conformation. Biotech. Anim. Husb. 2014, 30, 473–479. [Google Scholar] [CrossRef]
- Škrbić, Z.; Petričević, V.; Rakonjac, S.; Dosković, V.; Tolimir, N.; Bogosavljević-Bošković, S.; Lukić, M. Comparative study of the production potential of indigenous poultry breeds of Banat Naked Neck and Svrljig Hen: Reproductive parameters and egg quality. Acta Agric. Serb. 2024, 29, 97–103. [Google Scholar] [CrossRef]
- Pavlovski, Z.; Lukić, M.; Cmiljanić, R.; Škrbić, Z. Conformation measures on carcass of chickens [in Serbian]. Biotech. Anim. Husb. 2006, 22, 83–97. [Google Scholar] [CrossRef]
- European Commission EC. Commission Regulation (EC) No 543/2008 of 16 June 2008 laying down detailed rules for the application of Council Regulation (EC) No 1234/2007 as regards the marketing standards for poultrymeat. Available online: https://eur-lex.europa.eu/eli/reg/2008/543/oj.
- ISO 1442: Meat and meat products – Determination of moisture content, Switzerland: International Organization for Standardization, 1997.
- ISO 937: Meat and meat products – Determination of nitrogen content, Switzerland: International Organization for Standardization, 1978.
- ISO 1443: Meat and meat Products – Determination of total fat content, Switzerland: International Organization for Standardization, 1973.
- ISO 936: Meat and meat products – Determination of ash content, Switzerland: International Organization for Standardization, 1998.
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A Direct Method for Fatty Acid Methyl Ester Synthesis: Applica-tion to Wet Meat Tissues, Oils, and Feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef]
- Stanišić, N.; Škrbić, Z.; Petričević, V.; Milenković, D.; Petričević, M.; Gogić, M.; Lukić, M. The fatty acid and mineral composition of Cobb 500 broiler meat influenced by the nettle (Urtica dioica) dietary supplementation, broiler gender and muscle portion. Agriculture 2023, 13, 799. [Google Scholar] [CrossRef]
- Volodkevich, N.N. Apparatus for measurement of chewing resistance or tenderness of foodstuffs. Food Res. 1938, 3, 221–225. [Google Scholar] [CrossRef]
- Blagojević, M.; Pavlovski, Z.; Škrbić, Z.; Lukić, M.; Milošević, N.; Perić, L. The effect of genotype of broiler chickens on carcass quality in extensive rearing system. Acta Vet.-Beogr. 2009, 59, 91–97. [Google Scholar] [CrossRef]
- Škrbić, Z.; Pavlovski, Z.; Lukić, M.; Petričević, V. Production performance and carcass quality of coloured broilers differentiated genetic potencial for growth. Biotech. Anim. Husb. 2013, 29, 615–624. [Google Scholar] [CrossRef]
- Dosković, V.; Bogosavljević-Bošković, S.; Škrbić, Z.; Jašović, B.; Rakonjac, S.; Petričević, V.; Petrović, D.M. Carcass conformation of Master Gris broiler chickens as an indicator of carcass quality. Acta Agric. Serb. 2018, 23, 247–255. [Google Scholar] [CrossRef]
- Mosca, F.; Zaniboni, L.; Stella, S.; Kuster, C.A.; Iaffaldano, N.; Cerolini, S. Slaughter performance and meat quality of Milanino chickens reared according to a specific free-range program. Poult. Sci. 2018, 97, 1148–1154. [Google Scholar] [CrossRef]
- Padhi, M.K. Importance of indigenous breeds of chicken for rural economy and their improvements for higher production performance. Scientifica 2016, ID 2604685. [CrossRef]
- Hanusová, E.; Oravcová, M.; Hanus, A.; Hrnčár, C. Factors affecting growth in native Oravka chicken breed. Slovak J. Anim. Sci. 2017, 50, 112–117. [Google Scholar]
- Tasoniero, G.; Cullere, M.; Baldan, G.; Dalle Zotte, A. Productive performances and carcase quality of male and female Italian Padovana and Polverara slow-growing chicken breeds. Ital. J. Anim. Sci. 2017, 17, 530–539. [Google Scholar] [CrossRef]
- Oblakova, M.; Mincheva, N.; Hristakieva, P.; Ivanova, I.; Lalev, M.; Georgieva, Sv. Carcass traits and meat quality of different slow growing and fast growing broiler chickens. Agri. Sci. and Tech. 2017, 9, 351–357. [Google Scholar] [CrossRef]
- Farzana, N.; Habib, M.; Ali, M.H.; Hashem, M.A.; Ali, M.S. Comparison of meat yield and quality characteristics between indigenous chicken and commercial broiler. Bangladesh Vet. 2017, 34, 61–70. [Google Scholar] [CrossRef]
- Rizzi, C. Growth and slaughtering performance, carcase fleshiness and meat quality according to the plumage colour in Padovana male chickens slaughtered at 18 weeks of age. Ital. J. Anim. Sci. 2019, 18, 450–459. [Google Scholar] [CrossRef]
- Horsted, K.; Allesen-Holm, B.H.; Hermansen, J.E.; Kongsted, A.G. Sensory profiles of breast meat from broilers reared in an organic niche production system and conventional standard broilers. J. Sci. Food. Agric. 2012, 92, 258–265. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Gleeson, E.; Franco, D.; Cullere, M.; Lorenzo, J.M. Proximate Composition, Amino Acid Profile, and Oxidative Stability of Slow-Growing Indigenous Chickens Compared with Commercial Broiler Chickens. Foods 2020b, 9, 546. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Mattioli, S.; Twining, C.; Dal Bosco, A.; Donoghue, A.M.; Arsi, K.; Angelucci, E.; Chiattelli, D.; Castellini, C. Poultry Meat and Eggs as an Alternative Source of n-3 Long-Chain Polyunsaturated Fatty Acids for Human Nutrition. Nutrients 2022, 14, 1969. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Tasoniero, G.; Baldan, G.; Cullere, M. Meat quality of male and female Italian Padovana and Polverara slow-growing chicken breeds. Ital. J. Anim. Sci. 2019, 18, 398–404. [Google Scholar] [CrossRef]
- Pellattiero, E.; Tasoniero, G.; Cullere, M.; Gleeson, E.; Baldan, G.; Contiero, B.; Dalle Zotte, A. Are Meat Quality Traits and Sensory Attributes in Favor of Slow-Growing Chickens? Animals 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Cao, Qq.; Shaukat, A.; Zhang, C.; Huang, Sc. Insights into the evaluation, influential factors and improvement strategies for poultry meat quality: a review. npj Sci Food 2024, 8, 62. [Google Scholar] [CrossRef]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estévez, M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef]
- Baéza, E.; Guillier, L.; Petracci, M. Review: Production factors affecting poultry carcass and meat quality attributes. Animal 2022, Suppl 1, 100331. [Google Scholar] [CrossRef]
- da Silva, D.C.F.; de Arruda, A.M.V.; Goncalves, A.A. Quality characteristics of broiler chicken meat from free-range and industrial poultry system for the consumers. J. Food. Sci. Technol. 2017, 54, 1818–1826. [Google Scholar] [CrossRef]
- Faria, P.B.; Esteves, C.; Cruz, F.L.; Bruhn, F.R.P.; Souza, X.R.; Bressan, M.C. Gender, lineage, and age on muscle morphometry traits and meat tenderness of free-range chickens. Cienc. Rural 2022, 52, e20210444. [Google Scholar] [CrossRef]
- Faria, P.B.; Geraldo, A.; Oliveira, G.P.; Marçal, J.O.; Miranda, J.R.; Moreira, L.F.S.M.; de Almeida, A.A.; de Souza Camargos, R. Performance and meat quality of Label Rouge chickens at different slaughter ages. Ciênc. Rural 2023, 53, e20210616. [Google Scholar] [CrossRef]
- Owens, C.M.; Fanatico, A.C.; Pillai, P.B.; Meullenet, J.F.; Emmert, J.L. Evaluation of alternative genotypes and production systems for natural and organic poultry markets in the U.S Proceedings XII European Poultry Conference, Verona, Italy, 2006, 10730.
- Zhuang, H.; Savage, E.M. Effects of fillet weight on sensory descriptive flavor and texture profiles of broiler breast meat. Poult. Sci. 2012, 91, 1695–1702. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007, 86, 2245–2255. [Google Scholar] [CrossRef]
- Horsted, K.; Allesen-Holm, B.H.; Hermansen, J.E. The effect of breed and feed-type on the sensory profile of breast meat in male broilers reared in an organic free-range system. Br. Poult. Sci. 2010, 51, 515—524. [Google Scholar] [CrossRef]
- Deng, S.; Liu, R.; Li, C.; Xu, X.; Zhou, G. Meat quality and flavor compounds of soft-boiled chickens: effect of Chinese yellow-feathered chicken breed and slaughter age. Poult. Sci. 2022, 101, 102168. [Google Scholar] [CrossRef]
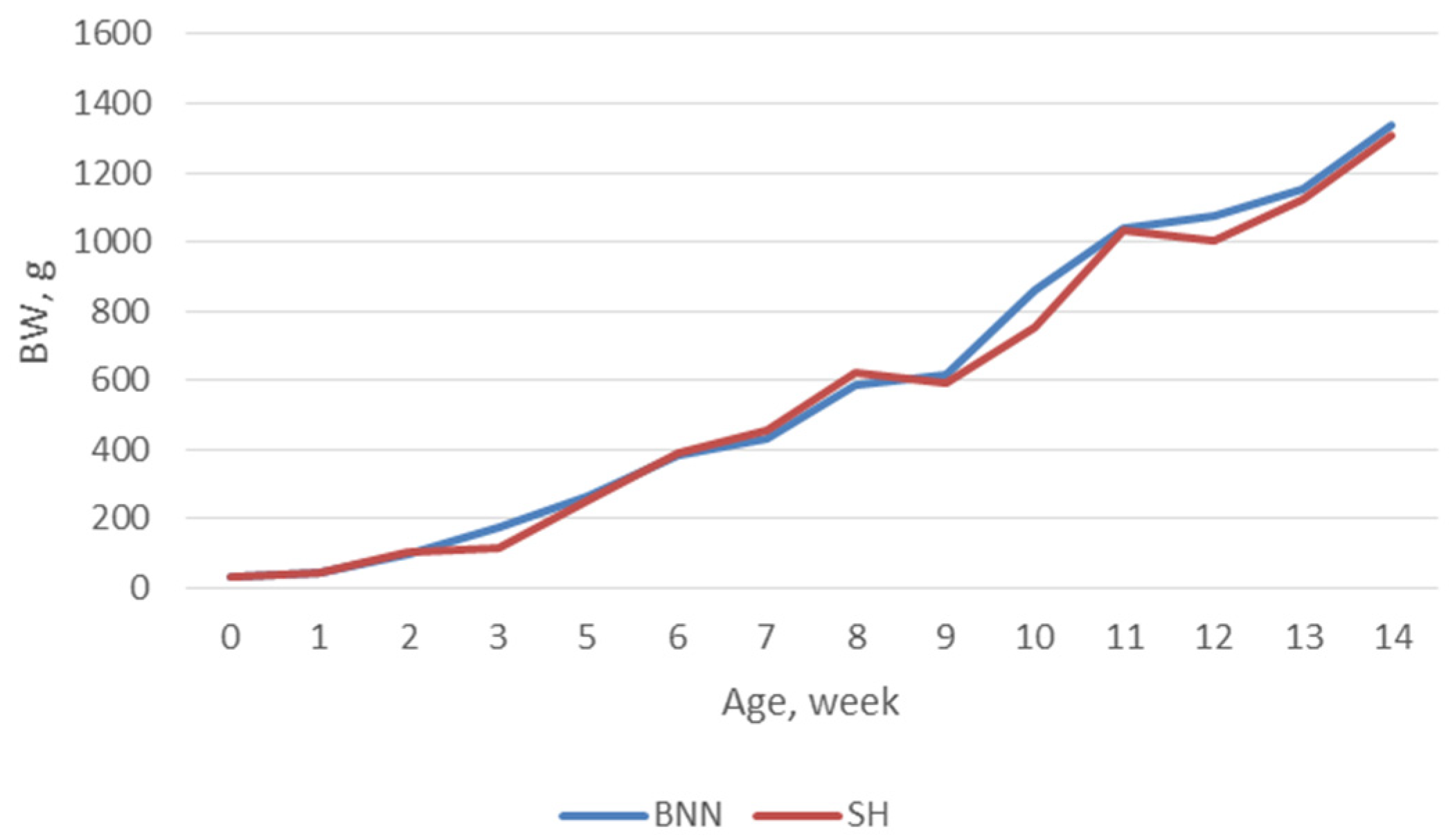
| BNN | SH | p-value | |||||
|---|---|---|---|---|---|---|---|
| 12 | 14 | 12 | 14 | Breed | Age | B x A | |
| SW | 1226.10±79.13 | 1457.10±157.93 | 1092.90±224.70 | 1461.10±206.62 | 0.254 | <0.01 | 0.227 |
| Processed carcass yield, g | |||||||
| CC | 1000.09±58.28 | 1206.51±139.47 | 806.36±194.89 | 1171.12±174.33 | 0.075 | <0.01 | 0.282 |
| RR | 902.37±51.66 | 1087.76±130.10 | 773.07±183.20 | 1052.65±159.51 | 0.072 | <0.01 | 0.295 |
| RG | 783.40±44.84 | 952.41±113.87 | 660.33±158.72 | 920.53±137.43 | 0.051 | <0.01 | 0.243 |
| Processed carcass yield, % SW | |||||||
| CC | 81.62±2.20 | 82.75±1.24 | 78.30±3.01 | 80.07±1.81 | <0.01 | 0.042 | 0.646 |
| RR | 73.66±2.10 | 74.58±1.35 | 70.20±3.42 | 71.97±2.26 | <0.01 | 0.084 | 0.582 |
| RG | 63.95±1.97 | 65.31±1.78 | 59.90±3.47 | 62.93±1.70 | <0.01 | <0.01 | 0.269 |
| Conformation measures | BNN | SH | p-value | ||||
|---|---|---|---|---|---|---|---|
| 12 | 14 | 12 | 14 | Breed | Age | B x A | |
| Absolute values | |||||||
| SL, mm | 84.0±4.85 | 91.0±5.77 | 78.2±7.93 | 90.0±5.62 | 0.089 | <0.01 | 0.225 |
| KL, mm | 104.4±4.03 | 108.2±5.61 | 99.1±7.32 | 107.5±11.25 | 0.217 | 0.015 | 0.342 |
| BD, mm | 96.4±5.40 | 99.6±8.03 | 92.8±7.80 | 103.1±7.26 | 0.982 | <0.01 | 0.128 |
| TG, mm | 112.5±4.03 | 119.3±3.40 | 106.1±8.49 | 116.5±7.96 | 0.03 | <0.01 | 0.379 |
| BA, degree | 96.1±3.96 | 101.4±6.33 | 93.2±4.71 | 94.9±5.63 | <0.01 | <0.05 | 0.284 |
| Index values | |||||||
| SW/SL, g/mm | 14.62±0.98 | 16.00±1.25 | 13.84±1.67 | 16.21±1.91 | 0.547 | <0.01 | 0.304 |
| SW/KL, g/mm | 11.74±0.58 | 13.46±0.96 | 11.00±1.93 | 13.57±1.05 | 0.420 | <0.01 | 0.280 |
| SW/BD, g/mm | 12.77±0.86 | 14.65±1.31 | 11.69±1.70 | 14.14±1.30 | 0.067 | <0.01 | 0.511 |
| SW/TG, g/mm | 10.90±0.66 | 12.21±1.23 | 10.23±1.58 | 12.50±1.15 | 0.616 | <0.01 | 0.211 |
| Carcass parts | BNN | SH | p-value | ||||
|---|---|---|---|---|---|---|---|
| 12 | 14 | 12 | 14 | Breed | Age | B x A | |
| Weight, g | |||||||
| Head | 42.62±3.51 | 51.51±4.89 | 36.52±5.32 | 46.21±5.37 | <0.01 | <0.01 | 0.795 |
| Neck | 52.50±4.96 | 59.99±8.47 | 50.74±14.46 | 70.15±13.44 | 0.236 | <0.01 | 0.096 |
| Metatarsus | 55.18±6.72 | 67.31±8.26 | 50.08±8.26 | 62.35±10.89 | 0.074 | <0.01 | 0.98 |
| Back | 102.06±11.14 | 122.60±16.94 | 86.19±19.65 | 116.15±18.86 | 0.044 | <0.01 | 0.386 |
| Pelvis | 102.28±11.70 | 125.22±16.54 | 86.09±21.53 | 122.76±21.01 | 0.112 | <0.01 | 0.238 |
| Wings | 113.18±10.18 | 134.77±12.79 | 99.52±20.94 | 129.66±18.91 | 0.77 | <0.01 | 0.412 |
| Breast | 190.78±12.82 | 225.20±29.91 | 148.39±43.42 | 219.90±35.31 | 0.026 | <0.01 | 0.078 |
| Thighs & drumstick | 265.22±19.98 | 328.86±36.01 | 233.21±58.37 | 321.11±51.03 | 0.161 | <0.01 | 0.388 |
| Heart | 6.18±1.40 | 7.03±0.93 | 5.59±1.18 | 6.55±1.74 | 0.178 | 0.031 | 0.991 |
| Liver | 25.03±4.29 | 27.36±5.69 | 21.88±3.52 | 27.73±4.13 | 0.333 | <0.01 | 0.222 |
| Stomach | 35.69±3.27 | 41.16±12.86 | 34.79±9.11 | 37.48±10.83 | 0.460 | 0.192 | 0.653 |
| % SW | |||||||
| Head | 3.76B±0.54 | 3.55B±0.29 | 4.71A±1.30 | 3.18B±0.20 | 0.214 | <0.01 | <0.01 |
| Neck | 4.28±0.30 | 4.11±0.31 | 4.57±0.48 | 4.77±0.40 | <0.01 | 0.871 | 0.124 |
| Metatarsus | 4.50±0.40 | 4.63±0.41 | 4.64±0.47 | 4.28±0.56 | 0.494 | 0.449 | 0.105 |
| Back | 8.34±0.88 | 8.39±0.46 | 7.85±0.56 | 7.96±0.76 | 0.04 | 0.71 | 0.911 |
| Pelvis | 8.34±0.81 | 8.58±0.34 | 7.85±0.74 | 8.37±0.60 | 0.1 | 0.073 | 0.5 |
| Wings | 9.23±0.60 | 9.27±0.39 | 9.09±0.58 | 8.88±0.37 | 0.1 | 0.566 | 0.428 |
| Breast | 15.60a±1.22 | 15.44a±0.91 | 13.31b±1.61 | 15.04a±0.89 | <0.01 | 0.045 | 0.017 |
| Thighs & drumstick | 21.63±0.70 | 22.59±1.00 | 21.12±1.80 | 21.94±1.02 | 0.136 | 0.024 | 0.856 |
| Heart | 0.51±0.12 | 0.49±0.05 | 0.51±0.05 | 0.44±0.07 | 0.394 | 0.094 | 0.272 |
| Liver | 2.04±0.31 | 1.87±0.28 | 2.05±0.35 | 1.90±0.18 | 0.82 | 0.096 | 0.883 |
| Stomach | 2.92±0.26 | 2.81±0.76 | 3.20±0.49 | 2.54±0.49 | 0.975 | 0.03 | 0.11 |
| BNN | SH | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| 12 | 14 | 12 | 14 | Breed | Age | BxA | ||
| Breast | ||||||||
| Muscle tissue | g | 124.07 ±10.25 | 150.06 ±21.77 | 94.19 ± 32.06 | 143.05 ±24.05 | 0.017 | <0.001 | 0.131 |
| % SW | 10.15 ± 0.98 | 10.28 ± 0.74 | 8.37 ± 1.44 | 9.78 ± 0.74 | 0.001 | 0.022 | 0.055 | |
| % CCW | 12.42a ± 1.02 | 12.42a ± 0.84 | 10.66b ± 1.62 | 12.22a ± 0.95 | 0.01 | 0.039 | 0.039 | |
| Bone tissue | g | 45.27 ± 5.32 | 51.79 ± 7.59 | 38.28 ± 8.92 | 51.08 ± 7.28 | 0.108 | <0.001 | 0.187 |
| % SW | 3.70 ± 0.47 | 3.56 ± 0.4 | 3.49 ± 0.38 | 3.50 ± 0.26 | 0.287 | 0.6 | 0.533 | |
| % CCW | 4.53 ± 0.48 | 4.30 ± 0.44 | 4.46 ± 0.45 | 4.38 ± 0.34 | 0.964 | 0.262 | 0.593 | |
| Skin | g | 14.06 ± 1.84 | 16.69 ± 2.29 | 10.85 ± 3.19 | 16.57 ± 3.82 | 0.077 | <0.001 | 0.1 |
| % SW | 1.15 ± 0.15 | 1.15 ± 0.12 | 0.99 ± 0.18 | 1.13 ± 0.18 | 0.082 | 0.169 | 0.154 | |
| % CCW | 1.41 ± 0.18 | 1.39 ± 0.14 | 1.26 ± 0.22 | 1.41 ± 0.23 | 0.333 | 0.293 | 0.163 | |
| Thighs & Drumsticks | ||||||||
| Muscle tissue | g | 164.56 ±14.14 | 209.37 ±24.46 | 143.83 ±40.38 | 205.26 ±34.37 | 0.199 | <0.001 | 0.387 |
| % SW | 13.42 ± 0.65 | 14.38 ± 0.84 | 12.94 ± 1.49 | 14.02 ± 0.85 | 0.199 | 0.003 | 0.866 | |
| % CCW | 16.44 ± 0.70 | 17.38 ± 0.97 | 16.50 ± 1.46 | 17.51 ± 0.89 | 0.785 | 0.005 | 0.918 | |
| Bone tissue | g | 64.35 ± 6.38 | 78.40 ± 10.82 | 58.41 ± 11.18 | 74.21 ± 11.67 | 0.126 | <0.001 | 0.788 |
| % SW | 5.25 ± 0.39 | 5.38 ± 0.51 | 5.37 ± 0.47 | 5.09 ± 0.44 | 0.551 | 0.597 | 0.149 | |
| % CCW | 6.43 ± 0.44 | 6.50 ± 0.59 | 6.87 ± 0.61 | 6.35 ± 0.48 | 0.406 | 0.193 | 0.086 | |
| Skin | g | 26.02 ± 3.83 | 29.72 ± 5.29 | 20.34 ± 7.39 | 29.91 ± 6.4 | 0.148 | 0.001 | 0.123 |
| % SW | 2.12 ± 0.26 | 2.05 ± 0.37 | 1.82 ± 0.42 | 2.04 ± 0.28 | 0.152 | 0.49 | 0.186 | |
| % CCW | 2.60 ±0.33 | 2.48 ± 0.45 | 2.31 ± 0.49 | 2.54 ± 0.35 | 0.402 | 0.678 | 0.181 | |
| BNN | SH | p-value | |||||
|---|---|---|---|---|---|---|---|
| 12 | 14 | 12 | 14 | Breed | Age | BxA | |
| Chemical composition, % | |||||||
| Water | 74.41b±0.60 | 74.22b±0.85 | 75.15a±0.59 | 73.96b±0.71 | 0.283 | 0.003 | 0.023 |
| Fat | 0.50±0.12 | 0.54±0.13 | 0.41±0.11 | 0.46±0.13 | 0.04 | 0.278 | 0.837 |
| Proteins | 24.10a±0.61 | 24.24a±0.76 | 23.45b±0.53 | 24.59a±0.76 | 0.495 | 0.005 | 0.024 |
| Ash | 0.78±0.41 | 1.00±0.05 | 0.88±0.29 | 0.98±0.05 | 0.625 | 0.059 | 0.464 |
| Fatty acid content | |||||||
| SFA | 40.26±3.85 | 40.33±3.79 | 41.87±3.55 | 42.21±4.12 | 0.158 | 0.866 | 0.913 |
| MUFA | 26.50±2.93 | 25.20±3.94 | 25.28±2.41 | 24.52±3.31 | 0.352 | 0.315 | 0.795 |
| PUFA | 33.13±2.33 | 34.23±3.13 | 32.74±3.63 | 33.31±4.16 | 0.540 | 0.438 | 0.805 |
| SFA/PUFA | 1.23±0.19 | 1.19±0.17 | 1.30±0.24 | 1.30±0.27 | 0.203 | 0.774 | 0.829 |
| n-6 | 31.19±2.00 | 32.84±3.09 | 30.68±3.28 | 30.76±4.27 | 0.218 | 0.405 | 0.449 |
| n-3 | 1.97±0.55 | 1.63±0.21 | 2.05±0.48 | 2.08±0.43 | 0.063 | 0.262 | 0.188 |
| n-6/n-3 | 16.66b±3.33 | 20.56a±3.50 | 15.46b±2.62 | 15.15b±2.51 | 0.001 | 0.068 | 0.035 |
| Physical properties | |||||||
| Cooking loss, % | 26.59±1.68 | 26.61±1.12 | 26.77±1.97 | 26.43±0.92 | 0.996 | 0.734 | 0.701 |
| Tenderness, kg | 3.57±1.02 | 3.11±0.37 | 3.14±0.87 | 2.88±0.44 | 0.157 | 0.129 | 0.673 |
| BNN | SH | p-value | |||||
|---|---|---|---|---|---|---|---|
| 12 | 14 | 12 | 14 | Breed | Age | BxA | |
| Chemical composition, % | |||||||
| Water | 75.58±0.78 | 75.09±0.57 | 76.32±0.93 | 75.60±0.74 | 0.014 | 0.017 | 0.637 |
| Fat | 2.60±0.28 | 2.55±0.59 | 1.66±0.69 | 2.06±0.60 | <0.001 | 0.330 | 0.212 |
| Proteins | 20.86±0.83 | 21.27±0.87 | 21.06±1.27 | 21.35±0.87 | 0.664 | 0.259 | 0.849 |
| Ash | 0.95±0.04 | 0.98±0.04 | 0.96±0.06 | 0.98±0.09 | 0.760 | 0.165 | 0.922 |
| Fatty acid content | |||||||
| SFA | 34.67±2.16 | 35.97±2.65 | 35.23±2.92 | 33.98±2.73 | 0.394 | 0.981 | 0.135 |
| MUFA | 32.68±2.05 | 30.94±3.05 | 30.06±3.61 | 30.90±5.19 | 0.257 | 0.700 | 0.273 |
| PUFA | 32.46±3.07 | 33.09±4.48 | 34.71±4.63 | 34.62±5.30 | 0.187 | 0.847 | 0.798 |
| SFA/PUFA | 1.08±0.16 | 1.11±0.21 | 1.04±0.18 | 1.01±0.21 | 0.224 | 0.984 | 0.632 |
| n-6 | 30.97±2.64 | 31.48±4.26 | 32.72±4.52 | 32.93±5.33 | 0.247 | 0.795 | 0.914 |
| n-3 | 1.68±0.16 | 1.62±0.31 | 1.99±0.30 | 1.92±0.40 | 0.003 | 0.496 | 0.947 |
| n-6/n-3 | 18.56±1.59 | 19.78±2.72 | 16.67±2.60 | 17.46±2.52 | 0.009 | 0.193 | 0.780 |
| Physical properties | |||||||
| Cooking loss, % | 28.49±1.93 | 27.77±1.20 | 29.54±1.75 | 28.86±1.22 | 0.037 | 0.165 | 0.970 |
| Tenderness, kg | 3.47±0.87 | 2.58±0.49 | 3.33±0.90 | 2.44±0.31 | 0.520 | <0.001 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





