Submitted:
17 April 2025
Posted:
21 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathophysiological Overlap Between Sepsis and Cancer and Therapeutic Implications
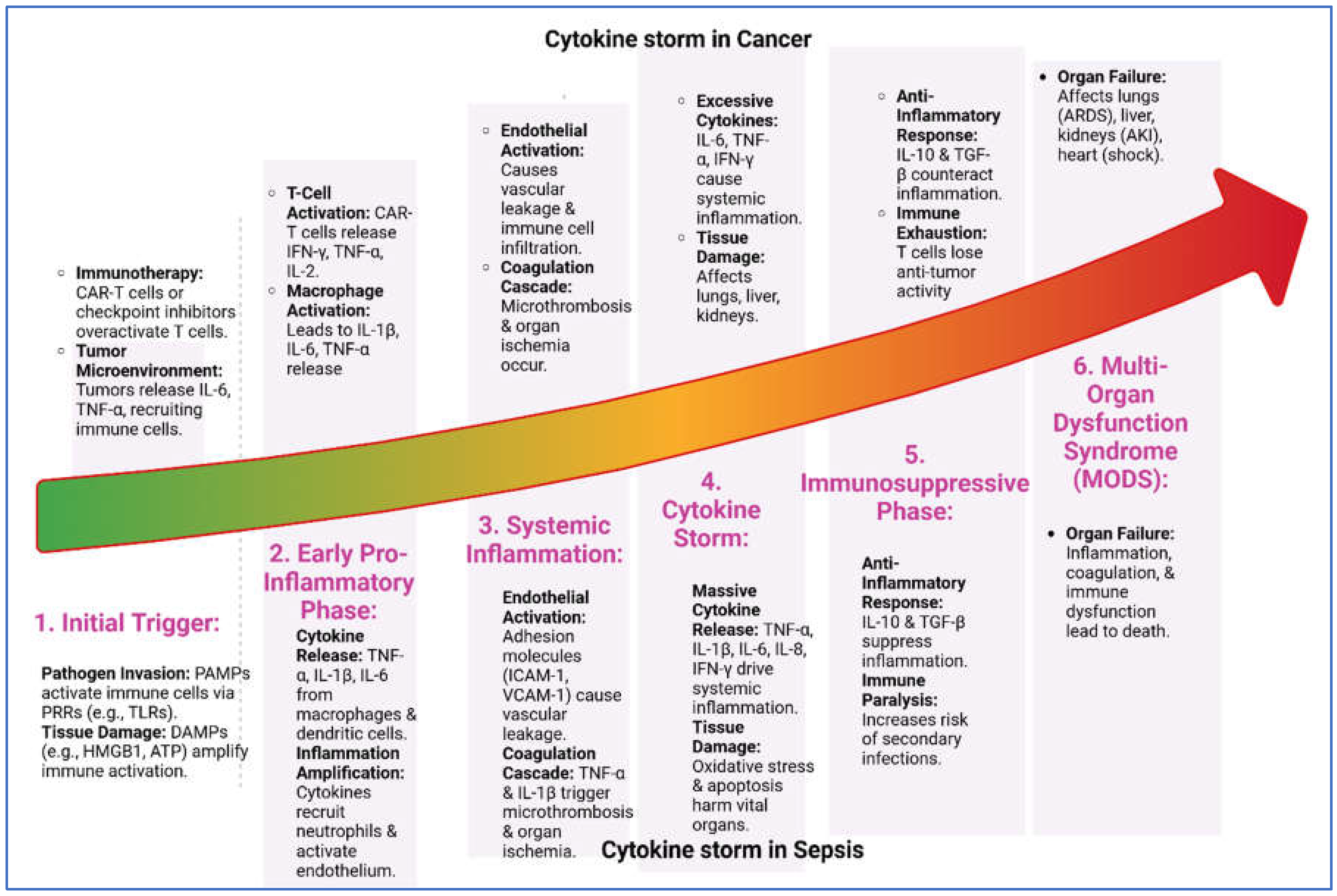
2.1. Cytokine Storm in Sepsis
2.2. Cytokines in Cancer
3. Immunosuppression in Sepsis and Cancer and Therapeutic Implications
3.1. Immune Paralysis in Sepsis
3.2. Immunosuppression in Cancer
4. Metabolic Alterations
4.1. The Warburg Effect on Cancer
4.2. Metabolic Shifts in Sepsis
4.3. Shared Metabolic Pathways and Therapeutic Implications
4.4. Glutamine Metabolism
4.5. Lipid Metabolism
6. Clinical Implications of the Sepsis-Cancer Connection
6.1. Impact of Sepsis on Tumor Microenvironment
6.2. Inflammatory Cytokines and Reactive Oxygen Species (ROS)
7. Long-Term Consequences of Sepsis in Cancer Patients
8. Therapeutic Opportunities
9. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- La Via, L., et al., The Global Burden of Sepsis and Septic Shock. Epidemiologia (Basel), 2024. 5(3): p. 456-478.
- Schlapbach, L.J., et al., World Sepsis Day: a global agenda to target a leading cause of morbidity and mortality. Am J Physiol Lung Cell Mol Physiol, 2020. 319(3): p. L518-L522. [CrossRef]
- Sung, H., et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 2021. 71(3): p. 209-249. [CrossRef]
- Staudinger, T. and F. Pene, Current insights into severe sepsis in cancer patients. Rev Bras Ter Intensiva, 2014. 26(4): p. 335-8. [CrossRef]
- Gudiol, C., et al., Understanding and Managing Sepsis in Patients With Cancer in the Era of Antimicrobial Resistance. Front Med (Lausanne), 2021. 8: p. 636547. [CrossRef]
- Said, S.A., et al., Impact of Sepsis on the Oncologic Outcomes of Advanced Epithelial Ovarian Cancer Patients: A Multicenter Observational Study. Cancers (Basel), 2023. 15(18). [CrossRef]
- Hensley, M.K., et al., Epidemiology and Outcomes of Cancer-Related Versus Non-Cancer-Related Sepsis Hospitalizations. Crit Care Med, 2019. 47(10): p. 1310-1316. [CrossRef]
- Chesney, J.A., R.A. Mitchell, and K. Yaddanapudi, Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy. J Leukoc Biol, 2017. 102(3): p. 727-740. [CrossRef]
- Chaudhry, H., et al., Role of cytokines as a double-edged sword in sepsis. In Vivo, 2013. 27(6): p. 669-84.
- van der Poll, T., et al., The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol, 2017. 17(7): p. 407-420.
- Schulte, W., J. Bernhagen, and R. Bucala, Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm, 2013. 2013: p. 165974. [CrossRef]
- Damas, P., et al., Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg, 1992. 215(4): p. 356-62.
- Kang, S., et al., IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A, 2020. 117(36): p. 22351-22356. [CrossRef]
- Liang, Y., et al., Elevated levels of plasma TNF-alpha are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-kappaB and p38 mitogen-activated protein kinase in endothelial cells. Shock, 2014. 41(4): p. 275-81. [CrossRef]
- Riedemann, N.C., et al., Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol, 2003. 170(1): p. 503-7. [CrossRef]
- Zhao, H., et al., Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther, 2021. 6(1): p. 263. [CrossRef]
- Mantovani, A., The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol, 2010. 40(12): p. 3317-20. [CrossRef]
- Raskova, M., et al., The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells, 2022. 11(22). [CrossRef]
- Coward, J., et al., Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res, 2011. 17(18): p. 6083-96.
- Yan, B., et al., Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res, 2006. 66(24): p. 11565-70.
- Balkwill, F., Tumour necrosis factor and cancer. Nat Rev Cancer, 2009. 9(5): p. 361-71.
- Du, Y., et al., Tocilizumab for Advanced Non-Small-Cell Lung Cancer With Concomitant Cachexia: An Observational Study. J Cachexia Sarcopenia Muscle, 2024. 15(6): p. 2815-2825. [CrossRef]
- Tavaci, T., et al., The impact of tocilizumab treatment on the severity of inflammation and survival rates in sepsis is significantly influence by the timing of administration. Inflammopharmacology, 2025.
- Jang, D.I., et al., The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int J Mol Sci, 2021. 22(5). [CrossRef]
- Bilal, J., et al., Risk of Infections and Cancer in Patients With Rheumatologic Diseases Receiving Interleukin Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open, 2019. 2(10): p. e1913102. [CrossRef]
- Nakamori, Y., E.J. Park, and M. Shimaoka, Immune Deregulation in Sepsis and Septic Shock: Reversing Immune Paralysis by Targeting PD-1/PD-L1 Pathway. Front Immunol, 2020. 11: p. 624279.
- Boomer, J.S., J.M. Green, and R.S. Hotchkiss, The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence, 2014. 5(1): p. 45-56.
- Jensen, I.J., et al., Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J Immunol, 2018. 200(5): p. 1543-1553. [CrossRef]
- Hotchkiss, R.S., G. Monneret, and D. Payen, Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol, 2013. 13(12): p. 862-74. [CrossRef]
- Jain, N., et al., Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A, 2010. 107(4): p. 1524-8. [CrossRef]
- Togashi, Y., K. Shitara, and H. Nishikawa, Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol, 2019. 16(6): p. 356-371. [CrossRef]
- Jimenez-Morales, S., et al., Mechanisms of Immunosuppressive Tumor Evasion: Focus on Acute Lymphoblastic Leukemia. Front Immunol, 2021. 12: p. 737340. [CrossRef]
- Topalian, S.L., C.G. Drake, and D.M. Pardoll, Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell, 2015. 27(4): p. 450-61. [CrossRef]
- Kang, S.P., et al., Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann Oncol, 2017. 28(6): p. 1388-1398. [CrossRef]
- Chang, K.C., et al., Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care, 2013. 17(3): p. R85. [CrossRef]
- Koppenol, W.H., P.L. Bounds, and C.V. Dang, Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer, 2011. 11(5): p. 325-37.
- Liberti, M.V. and J.W. Locasale, The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci, 2016. 41(3): p. 211-218.
- Cairns, R.A., I.S. Harris, and T.W. Mak, Regulation of cancer cell metabolism. Nat Rev Cancer, 2011. 11(2): p. 85-95.
- Harami-Papp, H., et al., TP53 mutation hits energy metabolism and increases glycolysis in breast cancer. Oncotarget, 2016. 7(41): p. 67183-67195. [CrossRef]
- Ferreira, B.L., et al., Glucose metabolism is upregulated in the mononuclear cell proteome during sepsis and supports endotoxin-tolerant cell function. Front Immunol, 2022. 13: p. 1051514. [CrossRef]
- Liu, J., et al., Metabolic reprogramming consequences of sepsis: adaptations and contradictions. Cell Mol Life Sci, 2022. 79(8): p. 456. [CrossRef]
- Suetrong, B. and K.R. Walley, Lactic Acidosis in Sepsis: It's Not All Anaerobic: Implications for Diagnosis and Management. Chest, 2016. 149(1): p. 252-61.
- Fan, Y., et al., Exploiting the Achilles' heel of cancer: disrupting glutamine metabolism for effective cancer treatment. Front Pharmacol, 2024. 15: p. 1345522. [CrossRef]
- Jin, J., et al., Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp Mol Med, 2023. 55(4): p. 706-715. [CrossRef]
- Jin, L., G.N. Alesi, and S. Kang, Glutaminolysis as a target for cancer therapy. Oncogene, 2016. 35(28): p. 3619-25.
- Ni, R., et al., Rethinking glutamine metabolism and the regulation of glutamine addiction by oncogenes in cancer. Front Oncol, 2023. 13: p. 1143798. [CrossRef]
- Cooper, A.J.L., et al., Metabolic Heterogeneity, Plasticity, and Adaptation to "Glutamine Addiction" in Cancer Cells: The Role of Glutaminase and the GTomegaA [Glutamine Transaminase-omega-Amidase (Glutaminase II)] Pathway. Biology (Basel), 2023. 12(8).
- Munir, R., et al., Lipid metabolism in cancer cells under metabolic stress. Br J Cancer, 2019. 120(12): p. 1090-1098. [CrossRef]
- Soula, M., et al., Glycosphingolipid synthesis mediates immune evasion in KRAS-driven cancer. Nature, 2024. 633(8029): p. 451-458. [CrossRef]
- Van Wyngene, L., et al., Hepatic PPARalpha function and lipid metabolic pathways are dysregulated in polymicrobial sepsis. EMBO Mol Med, 2020. 12(2): p. e11319.
- Ganapathy-Kanniappan, S. and J.F. Geschwind, Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer, 2013. 12: p. 152. [CrossRef]
- Wicker, C.A., et al., Glutaminase inhibition with telaglenastat (CB-839) improves treatment response in combination with ionizing radiation in head and neck squamous cell carcinoma models. Cancer Lett, 2021. 502: p. 180-188. [CrossRef]
- Jin, Z., Y.D. Chai, and S. Hu, Fatty Acid Metabolism and Cancer. Adv Exp Med Biol, 2021. 1280: p. 231-241.
- Xiao, Y. and D. Yu, Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther, 2021. 221: p. 107753. [CrossRef]
- Chen, G., et al., Role of hypoxia in the tumor microenvironment and targeted therapy. Front Oncol, 2022. 12: p. 961637. [CrossRef]
- Dong, G., et al., Intermittent hypoxia alleviates increased VEGF and pro-angiogenic potential in liver cancer cells. Oncol Lett, 2019. 18(2): p. 1831-1839. [CrossRef]
- Semenza, G.L., Hypoxia-inducible factors in physiology and medicine. Cell, 2012. 148(3): p. 399-408. [CrossRef]
- Fu, Z., et al., Tumour Hypoxia-Mediated Immunosuppression: Mechanisms and Therapeutic Approaches to Improve Cancer Immunotherapy. Cells, 2021. 10(5). [CrossRef]
- Jin, F., et al., Impairment of hypoxia-induced angiogenesis by LDL involves a HIF-centered signaling network linking inflammatory TNFalpha and angiogenic VEGF. Aging (Albany NY), 2019. 11(2): p. 328-349. [CrossRef]
- Grivennikov, S.I., F.R. Greten, and M. Karin, Immunity, inflammation, and cancer. Cell, 2010. 140(6): p. 883-99.
- Gabrilovich, D.I. and S. Nagaraj, Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol, 2009. 9(3): p. 162-74. [CrossRef]
- Soler, M.F., et al., New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J Immunother Cancer, 2023. 11(11). [CrossRef]
- Mantovani, A., et al., Cancer-related inflammation. Nature, 2008. 454(7203): p. 436-44. [CrossRef]
- Baylin, S.B. and P.A. Jones, A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer, 2011. 11(10): p. 726-34. [CrossRef]
- Verma, D.P., A.K. Tripathi, and A.K. Thakur, Innovative Strategies and Methodologies in Antimicrobial Peptide Design. J Funct Biomater, 2024. 15(11). [CrossRef]
- Tripathi, A.K., et al., Shaping the Future of Antimicrobial Therapy: Harnessing the Power of Antimicrobial Peptides in Biomedical Applications. J Funct Biomater, 2023. 14(11). [CrossRef]
- Tripathi, A.K. and J.K. Vishwanatha, Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy. Pharmaceutics, 2022. 14(12). [CrossRef]
- Kumar, A., et al., Single Amino Acid Substitutions at Specific Positions of the Heptad Repeat Sequence of Piscidin-1 Yielded Novel Analogs That Show Low Cytotoxicity and In Vitro and In Vivo Antiendotoxin Activity. Antimicrob Agents Chemother, 2016. 60(6): p. 3687-99. [CrossRef]
- Tandon, A., et al., Characterization of a Myeloid Differentiation Factor 2-Derived Peptide that Facilitates THP-1 Macrophage-Mediated Phagocytosis of Gram-Negative Bacteria. ACS Infect Dis, 2024. 10(3): p. 845-857. [CrossRef]
- Tandon, A., et al., An MD2-derived peptide promotes LPS aggregation, facilitates its internalization in THP-1 cells, and inhibits LPS-induced pro-inflammatory responses. Cell Mol Life Sci, 2018. 75(13): p. 2431-2446.
- Kumari, T., et al., 10-Residue MyD88-Peptide Adopts beta-Sheet Structure, Self-Assembles, Binds to Lipopolysaccharides, and Rescues Mice from Endotoxin-Mediated Lung-Infection and Death. ACS Chem Biol, 2022. 17(12): p. 3420-3434. [CrossRef]
- Tripathi, A.K., et al., Short peptides based on the conserved regions of MIEN1 protein exhibit anticancer activity by targeting the MIEN1 signaling pathway. J Biol Chem, 2024. 300(3): p. 105680. [CrossRef]
- Vishwanatha, J.K.F.W., TX, US), Tripathi, Amit Kumar (Fort Worth, TX, US), DESIGN AND CHARACTERIZATION OF INHIBITORY PEPTIDES (IPEPS) DERIVED FROM OF MIEN 1 PROTEIN SEQUENCE. 2024, The University of North Texas Health Sciences Cente (Fort Worth, TX, US): United States.
- Desai, P.P., et al., Combination of Small Extracellular Vesicle-Derived Annexin A2 Protein and mRNA as a Potential Predictive Biomarker for Chemotherapy Responsiveness in Aggressive Triple-Negative Breast Cancer. Cancers (Basel), 2022. 15(1). [CrossRef]
- Guo, C., et al., Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion. Cancers (Basel), 2022. 14(22). [CrossRef]
- Tripathi, A.K. and J.K. Vishwanatha, Abstract 2184: Short Peptides derived from MIEN1 and their analogs exhibit anti-cancer activity in breast and prostate cancer cells. Cancer Research, 2023. 83(7_Supplement): p. 2184-2184. [CrossRef]
- Tripathi, A.K. and J.K. Vishwanatha, Abstract LB034: First-in-class compounds targeting the MIEN1 signaling pathway: A breakthrough approach for breast and prostate cancer therapy. Cancer Research, 2024. 84(7_Supplement): p. LB034-LB034. [CrossRef]
- Tripathi, A.K., et al., Identification of GXXXXG motif in Chrysophsin-1 and its implication in the design of analogs with cell-selective antimicrobial and anti-endotoxin activities. Sci Rep, 2017. 7(1): p. 3384. [CrossRef]
- Saxena, R., et al., HIV-1 Nef CAWLEAQ motif: a regulator of monocytes invasion through ENO1 modulation. Mol Cell Biochem, 2018. 447(1-2): p. 151-164. [CrossRef]
- Tripathi, A.K., et al., Selective phenylalanine to proline substitution for improved antimicrobial and anticancer activities of peptides designed on phenylalanine heptad repeat. Acta Biomater, 2017. 57: p. 170-186. [CrossRef]
- Srivastava, S., et al., Modulation of anti-endotoxin property of Temporin L by minor amino acid substitution in identified phenylalanine zipper sequence. Biochem J, 2016. 473(21): p. 4045-4062. [CrossRef]
- Rumienczyk, I., et al., Oncology Drug Repurposing for Sepsis Treatment. Biomedicines, 2022. 10(4). [CrossRef]
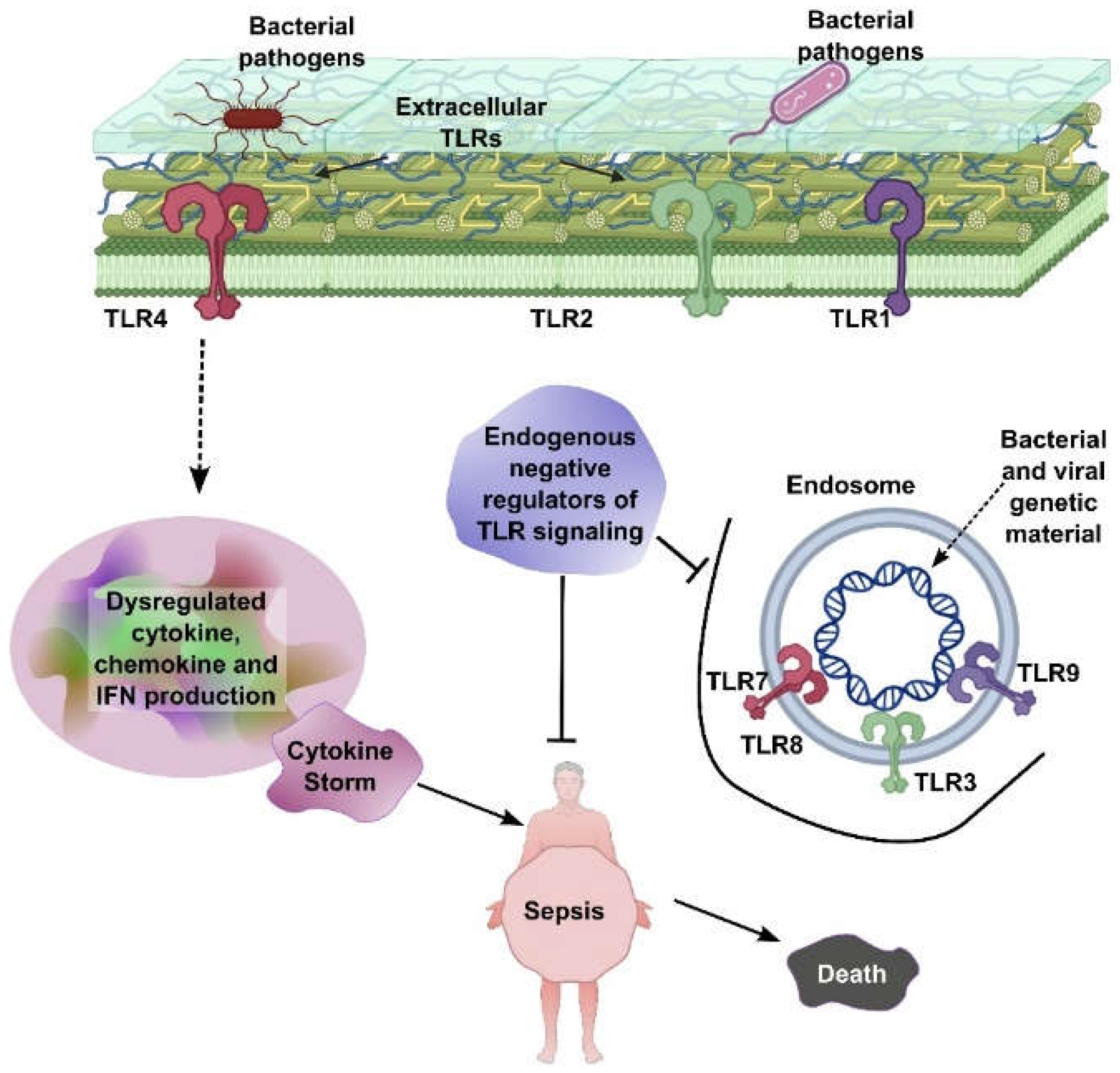
| Cytokine | Role in Sepsis | Role in Cancer |
| TNF-α | - Early pro-inflammatory mediator. - Promotes inflammation, endothelial activation, and organ dysfunction. |
- Promotes tumor progression, angiogenesis, and metastasis. - Can induce tumor cell death in high concentrations. |
| IL-1β | - Induces fever, vasodilation, and immune cell recruitment. - Contributes to tissue damage. |
- Promotes tumor growth, angiogenesis, and metastasis. - Enhances immunosuppressive microenvironment. |
| IL-6 | - Key mediator of acute-phase response. - Correlates with severity and mortality. |
- Promotes tumor growth, survival, and metastasis. - Drives chronic inflammation and immune evasion. |
| IL-8 (CXCL8) | - Chemoattractant for neutrophils. - Contributes to tissue injury. |
- Promotes angiogenesis, tumor growth, and metastasis. - Attracts immunosuppressive cells to the tumor microenvironment. |
| IL-10 | - Anti-inflammatory cytokine. - Suppresses pro-inflammatory responses. - Can lead to immunosuppression. |
- Promotes immune evasion by suppressing anti-tumor immunity. - Enhances tumor progression. |
| IL-17 | - Produced by Th17 cells. - Promotes neutrophil recruitment and inflammation. |
- Promotes tumor growth, angiogenesis, and metastasis. - Contributes to chronic inflammation. |
| IL-23 | - Promotes Th17 cell differentiation and IL-17 production. - Amplifies inflammation. |
- Promotes tumor growth and immune evasion. - Enhances chronic inflammation. |
| IFN-γ | - Activates macrophages and enhances pro-inflammatory responses. - Contributes to tissue damage. |
- Can have anti-tumor effects by activating immune cells. - May promote tumor immune evasion in chronic settings. |
| TGF-β | - Anti-inflammatory cytokine. - Promotes tissue repair and immunosuppression. |
- Promotes tumor progression, immune evasion, and metastasis. - Induces epithelial-mesenchymal transition (EMT). |
| VEGF | - Promotes vascular permeability and endothelial dysfunction. | - Drives angiogenesis, supporting tumor growth and metastasis. |
| HMGB1 | - Late-phase mediator of sepsis. - Sustains inflammation and organ damage. |
- Promotes tumor growth, metastasis, and immune evasion. - Acts as a damage-associated molecular pattern (DAMP). |
| PD-1/PD-L1 | - Contributes to T-cell exhaustion and immunosuppression in sepsis. | - Key immune checkpoint in cancer. - Promotes immune evasion and tumor progression. |
| G-CSF | - Stimulates neutrophil production and mobilization. | - Promotes tumor growth and metastasis. - Enhances myeloid-derived suppressor cells (MDSCs). |
| MCP-1 (CCL2) | - Recruits monocytes and macrophages to sites of inflammation. | - Recruits tumor-associated macrophages (TAMs), promoting tumor progression and immune evasion. |
| Aspect | Sepsis | Cancer | Clinical Implications |
| Glucose Metabolism | - Hyperglycemia: Insulin resistance and increased gluconeogenesis. - Warburg Effect: Increased glycolysis. |
- Warburg Effect: Aerobic glycolysis for rapid ATP production. - Increased Glucose Uptake: Enhanced by GLUT transporters. |
- Targeting Glycolysis: Inhibitors like 2-DG may help in both conditions. - Glucose Control: Tight glucose management improves outcomes in sepsis. |
| Lactate Production | - Lactic Acidosis: Excessive glycolysis leads to lactate accumulation. | - High Lactate Levels: Lactate contributes to tumor microenvironment acidosis. | - Lactate as a Biomarker: High lactate levels correlate with poor prognosis in both conditions. |
| Lipid Metabolism | - Lipolysis: Increased breakdown of fats for energy. - Hyperlipidemia: Elevated free fatty acids. |
- Lipid Synthesis: Increased de novo lipogenesis. - Fatty Acid Oxidation: Some cancers rely on fatty acids for energy. |
- Lipid-Targeting Therapies: Inhibitors of lipogenesis (e.g., FASN inhibitors) are explored in cancer. |
| Protein Metabolism | - Protein Catabolism: Muscle breakdown for gluconeogenesis. - Negative Nitrogen Balance. |
- Increased Protein Synthesis: Supports cell proliferation. - Amino Acid Dependency: Reliance on glutamine. |
- Nutritional Support: Glutamine supplementation may benefit both conditions. |
| Glutamine Metabolism | - Glutamine Utilization: Supports immune cell function and energy production. | - Glutamine Addiction: Used for anaplerosis and nucleotide synthesis. | - Glutaminase Inhibitors: CB-839 is being tested in cancer and may have potential in sepsis. |
| Mitochondrial Dysfunction | - Impaired Oxidative Phosphorylation: Reduced ATP production. - ROS Production: Contributes to tissue damage. |
- Altered Mitochondrial Function: Dysfunction or upregulation depending on cancer type. - ROS Signaling: Promotes tumor growth. |
- Antioxidant Therapies: May help mitigate ROS-induced damage in both conditions. |
| Ketone Body Metabolism | - Increased Ketogenesis: In response to energy demands. | - Ketone Utilization: Some cancers use ketone bodies as an energy source. | - Ketogenic Diets: May benefit cancer patients and potentially sepsis patients. |
| Immune Cell Metabolism | - Metabolic Reprogramming: Immune cells shift to glycolysis. - Immunosuppression: M2 macrophages rely on oxidative metabolism. |
- Tumor-Associated Immune Cells: TAMs and Tregs exhibit metabolic changes supporting tumor growth. | - Immunometabolism Targeting: Modulating immune cell metabolism may improve outcomes. |
| Hypoxia Response | - HIF-1α Activation: Promotes glycolysis and angiogenesis. | - HIF-1α Activation: Drives angiogenesis and tumor progression. | - HIF-1α Inhibitors: Potential therapeutic target in both conditions. |
| Insulin Resistance | - Peripheral Insulin Resistance: Reduces glucose uptake in muscle and adipose tissue. | - Altered Insulin Signaling: Some cancers exhibit insulin resistance or upregulate insulin/IGF-1 signaling. | - Insulin Sensitizers: May improve outcomes in sepsis and certain cancers. |
| Acidosis | - Metabolic Acidosis: Due to lactate accumulation and impaired renal function. | - Tumor Microenvironment Acidosis: Results from high lactate production. | - pH Modulation: Alkalinizing agents may help mitigate acidosis in both conditions. |
| Energy Demand | - Increased Energy Demand: To support hypermetabolic state and immune responses. | - Increased Energy Demand: To support rapid cell proliferation and tumor growth. | - Nutritional Support: High-calorie diets may benefit patients in both conditions. |
| Drug Name | Cancer Indication | Sepsis Application |
| Irinotecan | Ovarian, small cell lung, cervical cancer | Bacterial-induced sepsis models [82] |
| Topotecan | Ovarian, small cell lung, cervical cancer | LPS and S. aureus infection models [82] |
| Olaparib | Ovarian, breast, prostate, pancreatic cancer | CLP and LPS sepsis models [82] |
| Trametinib | Melanoma | CLP and LPS sepsis models [82] |
| SCH772984 | Melanoma, colon cancer | CLP and LPS sepsis models [82] |
| Ceritinib | Non-small-cell lung cancer | CLP and LPS sepsis models [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





