1. Introduction
Culicoides spp. (Diptera: Ceratopogonidae) are biting midges that are the world’s smallest blood-feeding flies measuring only 1-3 mm in size (Mellor et al., 2000). Many of their distinguishing morphological features can only be examined under a microscope. Currently, over 1,400 Culicoides species are recognized within the genus and they occur globally with the exceptions of New Zealand, Patagonia, Hawaii and Antarctica (Mellor et al., 2000). They can vector more than 50 arboviruses, including some important and severe pathogens affecting livestock and horses, such as bluetongue virus, African horse sickness virus, Akabane virus, and bovine ephemeral fever virus (Kampen & Warner 2023, Mellor et al., 2000; Venter et al., 2009).
Although most flight ranges of Culicoides are very short distances, they are capable of being dispersed passively over much longer distances by wind (Sellers & Pedgley, 1985; Sellers & Maarouf, 1991; Braverman & Chechik, 1996). Windborne dispersal has attributed to outbreaks of bluetongue virus in the Mediterranean, North Africa as well as northern Europe, African horse sickness in the Middle East and epizootic haemorrhagic disease in Israel (Sellers et al., 1977; Braverman & Chechik, 1996; Ducheyne et al., 2007; Gloster et al., 2007; Agren et al., 2010; García-Lastra et al., 2012). It is also believed that the introduction of novel serotype of bluetongue virus into Australia was via windborne dispersal from regional neighbours, such as Indonesia or other islands of Timor, across the Timor Sea (Daniels et al., 2004; Eagles et al., 2012).
New Zealand’s current freedom from Culicoides species and subsequent arboviruses has been possible due to its geographical isolation and robust biosecurity system. Maintaining a Culicoides-free status minimizes the risk of introducing these diseases and protects the health and productivity of New Zealand's livestock. However, substantial evidence suggests that many insect species (e.g., moths and butterflies), fungal spores, pollen and seeds were introduced in New Zealand via windborne dispersal (Close et al., 1978; Fox, 1978; Hoare, 2001; Withers, 2001). Thus, the risk of a windblown Culicoides incursion from Australia and other neighbouring countries is considered possible, which has resulted in the implementation of a national surveillance programme for both the vector and its arboviruses (Ryan et al., 1991).
Over the years, traditional pest surveillance tools have been improved. For example, light traps combined with a green LED, octenol, and carbon dioxide attractants have been effective in attracting various midge species (Kline et al., 1994). However, the small size of the midges trapped and other by-catch is time consuming and labour-intensive screening process under the microscope, and taxonomic expertise and resources required to identify each individual has been a significant challenge in the current surveillance programme (Douglas et al., 2012; Bista et al., 2018). On average in a typical year 15,000-20,000 insects are collected from the light traps.
To mitigate this challenge, molecular approaches, such as DNA metabarcoding in combination with high-throughput sequencing can offer a more efficient, cost-effective, reliable alternative option to identify the insect species from the insect trap samples. This could be achieved using mixed insect bulk samples as well as liquid medium from the trap containing the shed environmental DNA (eDNA) (Darling & Mahon, 2011; Hajibabaei et al., 2011; Ji et al., 2013; Gibson et al., 2015, Taberlet et al., 2012). Recently, eDNA metabarcoding approach showed inconsistency in identifying Culicoides specimens at species level from the light traps filled with saturated salt solution (Milián-García et al. 2021). It is also noteworthy that the high sensitivity of DNA metabarcoding is capable to detect rare species in environmental samples to reduce false negatives (Ficetola et al., 2015). This approach has been widely implemented for detection of a variety of invasive species of flying insects, such as mosquito, stink bugs, from various insect traps, leaf surfaces or water samples (Zenker et al. 2020; Schneider et al., 2016; Valentin et al., 2018).
The main objective of this study was to explore the insect biodiversity in the trap samples and detection of Ceratopogonidae species while assessing the effectiveness of DNA metabarcoding using homogenized insect bulk samples and environmental DNA (eDNA) metabarcoding using liquid samples collected in insect traps in Culicoides surveillance programme in New Zealand in 2020.
2. Materials and Methods
2.1. Study Sites and Sample Collection
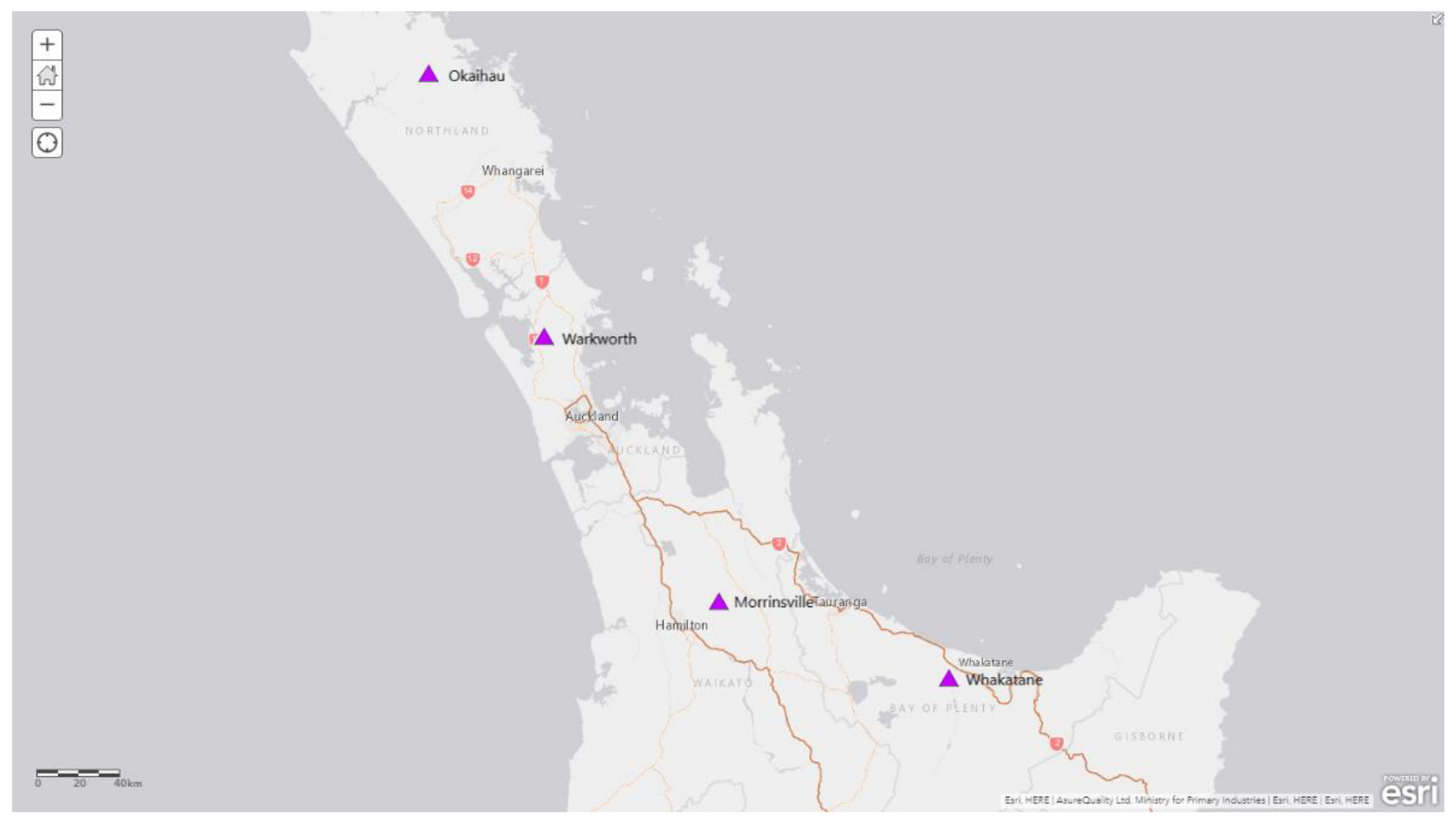
A total of four cattle farms with predominantly high cattle numbers from four districts (i.e., Morrinsville, Okaihau, Warkworth and Whakatane) from the 2020 surveillance programme were selected for this study (
Figure 1). The surveillance traps were deployed at those sites from the start of February until the beginning of April. The green LED light traps incorporated with CO
2 and octenol attractant were deployed on three consecutive nights for over nine or ten weeks (Okaihau and Whakatane for nine weeks, and Morrinsville and Warkworth for ten weeks) and insect traps samples were collected every week.
The preservative fluid used in the trap is ethanol-based (70% alcohol), and all the trap contents, with their by-catch, were transported to the laboratory for morphological identification and eDNA extraction. The insect trap liquid was filtered into a sterilized sampling bottle by a sterilized 0.2 µM filter. The insect specimens derived from the sample were inspected using morphological characteristics under the microscope (Khalaf, 1954; Chang et al. 2017). For each trap, the total number of insects and the prevalence of Culicoides species were recorded.
As Culicoides species are absent in New Zealand, the prevalence of New Zealand’s native species of Ceratopogonidae, which belong to the same family as exotic Culicoides species, was used as a proxy to examine the detection accuracy of DNA metabarcoding approaches.
Both the samples of ethanol fluid from traps and insect body bulk after inspection were stored at 4 0C until further processing. Overall, a total of 38 insect trap samples (2 sites x 10 weeks + 2 sites x 9 weeks = 38 samples) were collected for two sample types.
2.2. eDNA Capture and Extraction
To avoid DNA degradation and increase the detection probability (Barnes et al. 2014), within 48 hours after sample inspection, insect trap liquid samples were filtered through 0.2 μM sterile nitrocellulose filters (Ahlstrom-Munksjo, Finland) with a vacuum pump using filter holder (Rocker Scientific, China). To prevent cross-contamination, after each sample, the filter holder was cleaned to destroy the residual DNA by soaking for 30 min in a 10% bleach solution and then rinsing with distilled water three times to prevent build-up of bleach salts. The nitrocellulose filters were replaced with a new one after filtering each sample. The samples were then extracted and purified using the DNeasy Blood & Tissue kit (Qiagen, UK) following the manufacturer’s protocol. DNA was finally eluted in 100 μl AE buffer. Prior to filtration of the field samples, a negative control (sterile water) was used to examine for possible cross-contamination.
Additionally, each bulk sample of insect bodies was placed on one bacterial culture plate to air-dry the rest of ethanol. Then, the bulk sample was placed into a grinding extraction bag and 10 ml CTAB buffer (containing antifoam) was added into the bag. After thoroughly grinding the insects using the Homex grinder (Bioreba, Switzerland), 1 ml of the ground mixture along with 50 μl of Proteinase K were pipetted into 3 x 1.5 ml tubes (triplicates for each sample). Those samples were then incubated at 65 OC for 20-30 min in a thermomixer. Finally, after centrifugation at ≥10,000 RPM for 2 min to pellet the debris, eDNA was extracted from all samples using the Kingfisher mL workstation (Thermo Fisher, USA) with an InviMag Plant Kit (Invitek GmbH, Germany) following the manufacturer’s protocol.
The quality of the extracted DNA for both insect trap fluids and insect bulk samples was assessed using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, USA) and quantified using Quant-iT Picogreen dsDNA assay kit (Thermo Fisher Scientific, USA).
2.3. PCR and Metabarcoding Sequencing
For both DNA sample types, DNA amplification within the COI subunit region was performed with two sets of primer pairs LCO1490/HCO2198 (Folmer et al. 1994) and mlCOIintF/jgHCO2198 (Leray et al. 2013), which were both modified to include Illumina adapter sequences required for downstream sequencing processes based on the standard Illumina protocol (Illumina, 2013). PCR reactions for both primer pairs contained 5.4 μl sterile water, 10 μl Platinum™ SuperFi II PCR Master Mix (Thermofisher, USA), 1 μl each of the forward and reverse primers (5 μM concentration), 0.6 μl 50 mM MgCl2, 1 μl BSA and 1 μl DNA template. Reactions for the primer pair LCO1490/HCO2198 were held at 94 °C for 5 min, before 40 cycles at 94 °C for 15 s, 50 °C for 40 s and 72 °C for 45 s, and finally extension at 72 °C for 7 min. The amplification protocol for the primer pair mlCOIintF/jgHCO2198 was as follows: 95 °C for 3 min, 40 cycles of 95 °C for 30 s, 50 °C for 30 s and 72 °C for 1 min, and then final extension 72 °C for 10 min.
After PCR run, all amplified PCR products were checked for DNA integrity on 1.5% agarose gels in TAE buffer stained with SYBR safe (Life Technologies, USA) and visualised using a Gel Doc Software system (BioRad, USA). All negative controls for both DNA extraction and PCR amplification were negative, indicating that no cross-contamination occurred. Triplicate DNA samples from each insect bulk sample were pooled for the following processes to reduce the number of sequencing samples and to ensure insect trap liquid samples and insect bulk samples are comparable in terms of the sampling size.
After PCR amplification, each PCR product was individually purified using AMPure XP reagents (Beckman Coulter, USA), according to the manufacturer’s instructions. DNA concentration of those amplified PCR products was also quantified using a Qubit® dsDNA HS Assay Kit (Life technologies, USA). During the library preparation stage, a combination of Nextera XT A and B barcode dual indices (Illumina Inc., USA) was attached to the DNA from each sample. This approach ensured that the DNA from each sample could be identified by its unique DNA barcode. Amplicons from all the samples were composited together in equimolar concentrations and sequenced on an Illumina MiSeq instrument with 2 × 300 bp paired-end sequencing chemistry at the Auckland Genomics Facility, New Zealand. Raw sequencing data has been deposited to NCBI SRA (Sequence Read Archive) database with the BioProject ID PRJNA1249277.
2.4. In-House Reference Database of Ceratopogonidae Species
Over the past 6 years, a total of 90 COI sequences of six different genera (i.e., Leptoconops, Atrichopogon, Austrohelea, Dasyhelea, Forcipomyia and Paradasyhelea) belonging to Ceratopogonidae family were sequenced in-house (unpublished data). Using these sequences, an in-house COI barcoding database was generated, containing all known Ceratopogonidae species from New Zealand. This customized reference database was used for DNA-based taxonomic identification of Ceratopogonidae sequences.
2.5. OTU Analysis and Taxonomic Assignment
Bioinformatics analysis was conducted to process demultiplexed raw HTS sequences using the USEARCH tool (Edgar 2013). Briefly, paired-end reads were merged and filtered for quality using default parameters. Because the fragment of PCR product of the primer pair LCO1490/HCO2198 is about 650 bp, the 2 × 300 bp paired-end sequencing couldn’t recover the whole length of the fragment. Thus, only the forward reads for the LCO1490/HCO2198 primer pair were used, whereas only reads that merged both the forward and reverse reads and those that had a minimal length of 300 bp for the primer pair mlCOIintF/jgHCO2198 were used for further analysis.
After the removal of replicate (i.e., dereplication) and singleton sequences, chimeric sequences were removed and DNA sequence reads were de novo clustered into operational taxonomic units (OTUs) at 97% similarity using the UPARSE algorithm. Reads were then mapped to the final list of OTUs to assign abundances to each OTU and generate the OTU table for each primer pair.
Taxa was assigned to each OTU using the USEARCH global alignment algorithm by aligning the OTU sequences against the in-house customized COI reference database of Ceratopogonidae. All hits above threshold for minimum sequence identity of 95% and minimum alignment length of 200 bp were collected. Meanwhile, if no reference sequence with identity > 95% was available for the OTU sequence, those sequences were queried again against the NCBI nr database (downloaded in August 2019) using the Basic Local Alignment Search Tool (i.e., BLAST) algorithm, with the following parameters: maximum e-value of 0.05 and minimum sequence identity of 95%. In both USEARCH and BLAST approaches, a match with the highest sequence identity and the longest alignment length was selected as the best hit and the corresponding taxa was assigned to that OTU.
2.6. Data Analysis and Visualization
To evaluate the accuracy and detectability of metabarcoding-based approach, the prevalence of Ceratopogonidae species identified via the molecular approach was compared with the results obtained from traditional morphological screening Detection accuracy for each combination of sample type (insect trap fluid versus insect bulk) and primer pair (LCO1490/HCO2198 versus MlCOIintF/jgHCO2198) was quantified and visualised using pie charts.
Then, to further determine the relationship between the detectability and performance of eDNA approach and the relative abundance of Ceratopogonidae species within the total insect bulk, balloon plots were used to visualize the data of the detectability of the eDNA approach and ratio of the number of Ceratopogonidae species versus total number of insects identified at each sampling site across ten weeks. The performance of two COI universal primer pairs was visualized in pie charts using the number of OTU reads belonging to different common insect types, order and family. Finally, to visualize and assess variations in the composition of insect communities derived from Insect trap liquid and insect bulk samples amplified by two COI primer pairs, HTS data were plotted using nMDS (Non-metric multidimensional scaling) analysis of Bray-Curtis dissimilarity matrix derived from those data. All of the abovementioned figures were generated using the ‘ggplot2’ and ‘vegan’ packages in R (Dixon 2003, Wickham 2009).
Finally, to visualize and assess variations in the composition of insect communities derived from insect trap liquid and insect bulk samples amplified by two COI primer pairs, HTS data were plotted using nMDS (Non-metric multidimensional scaling) analysis of Bray-Curtis dissimilarity matrix derived from those data. All of the abovementioned figures were generated using the ‘ggplot2’ and ‘vegan’ packages in R (Dixon 2003, Wickham 2009).
3. Results
3.1. Morphological Identification of Insect Trap Samples
Under the microscope, a total 45,745 insects were found in 38 surveillance trap samples. No exotic Culicoides species were identified in any of the trap samples. However, native Ceratopogonidae species were found in 58% of the trap samples (22 out of 38 traps) (
Table 1). Only 0.25% of trap catches (n=114) were identified as belonging to the Ceratopogoniade family, with 83% of those individuals detected from the Whakatane district in the Bay of Plenty, where Ceratopogonodae species were found in all 9 surveillance trap samples. All individual insects belonging to the Ceratopogonidae family were sent to an external taxonomic expert for further indentifiction at genus and species level (data not available).
3.2. Detection Accuracy of eDNA Metabarcoding Approach
In practical applications, no single approach can accurately or consistently identify true positives or true negatives of the target species from mixed environemtal samples. Morphological identification, DNA metabarcoding of bulk samples, and eDNA metabarcoding complement each other in detecting and identifying target species in mixed environment samples (Martoni et al. 2023, Macher et al. 2018). However, in this study, detection accuracy was defined as how well the molecular methods detected and identified the target species found via traditional morphological methods, with congruence to morphological outcomes considered as true postives or negatives.
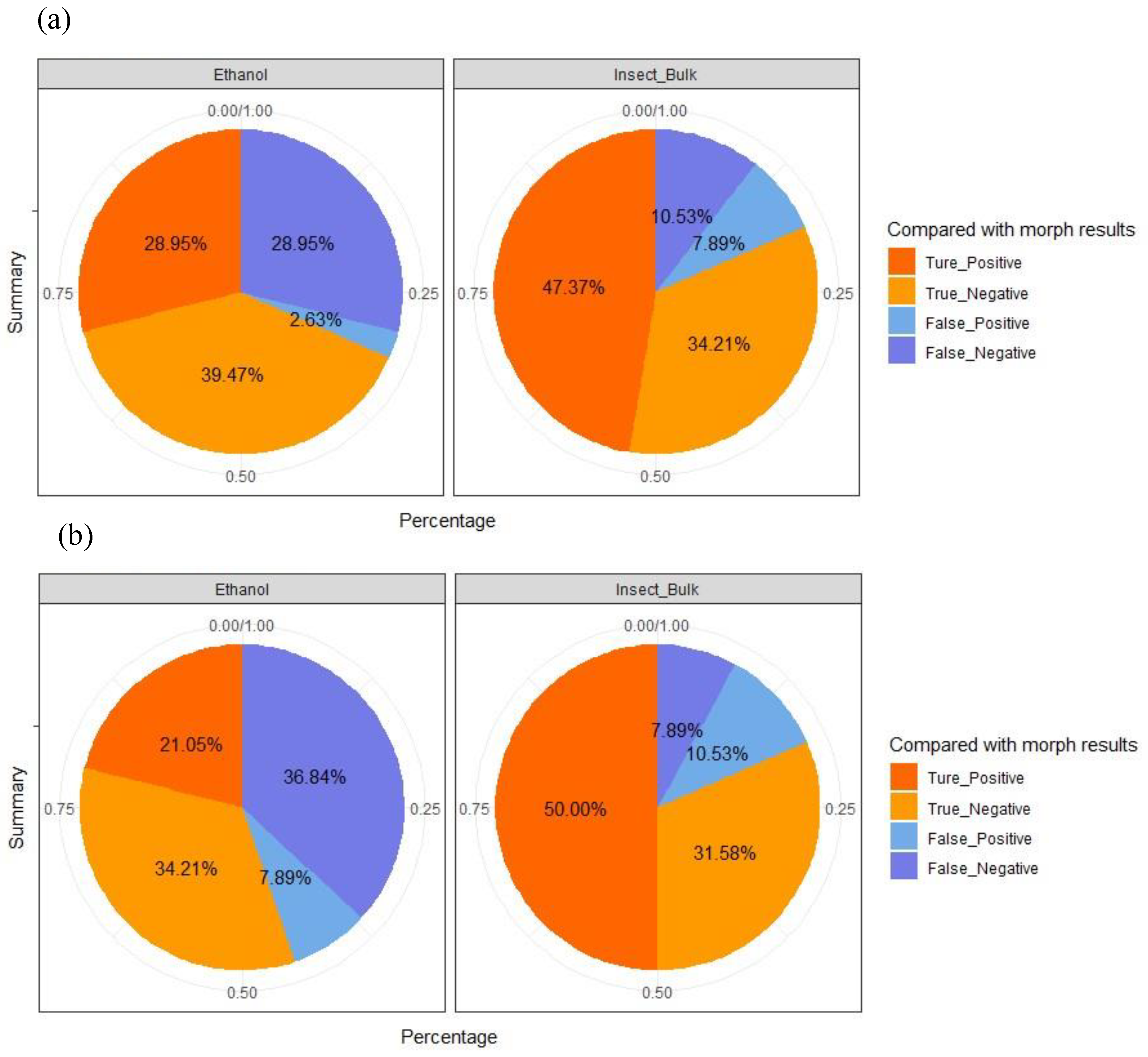
DNA extracted from insect trap samples was run on Illumina MiSeq high-throuhput DNA sequencing platform. In total, 4.6 million reads by primer pair LCO1490/HCO2198 and 6 million reads by the primer pair mlCOIintF/jgHCO2198, which passed the quality filtering could be assigned to a sample. In that dataset, 557 and 6,078 distinct unique operational taxonomic units (OTUs) with 97% DNA sequence similarity were indentifed for the primer LCO1490/HCO2198 and mlCOIintF/ jgHCO2198, respectively. Among these, 486 and 1,247 OTUs, respectively, could be assigned to taxonomic groups at family level with 95% similarity to the COI reference sequences derived from in-house database and the public databases. Taxonomic assignment of OTUs to Ceratopogonidae species were compared to morphologocal identifications to determine the detection accuracy rate of the molecular approches via two different primers. Based on OTU counts from DNA metabarcoding sequences of trap ethanol fluids and homogenized insect bulk samples, the detection accuracy rates (i.e, true postive rates + true negative rates) for the primer pair LCO1490/HCO2198 (
Figure 2a) were 68.42%, (n=11+15) and 81.94% (n=18+13), respectively.
In contrast, overall detection accuracy rates using the primer pair mlCOIintF/jgHCO2198 (
Figure 2b), were 55.26%, (n=8+13) and 81.58%, (n=19+12), respectively, for trap ethanol fluids and insect bulk samples, respectively. The results indicated that regardless of the primer sets used for amplification, in general, DNA metabarcoding approach using insect bulk correctly identified the presence of native Ceratopogonidiae species in over 81% of insect traps samples (n=31 samples out of 38) compared to the eDNA metabarcoding approach using insect trap ethanol fluid samples (overall accuracy rate: 68.42% and 55.26% using two separate primers). Overall, these findings suggest that DNA-metabarcoding approach using insect bulk could detect the presence or absence of target species (Ceratopogonidae species in this case) from pest surveillance trap samples in majority of the cases, while the detectability and accuracy of such approach via eDNA metabarcoding using the liquid samples from insect trap requires further improvement.
3.3. Effect of Target Species Abundance on eDNA Detection
Due to the variation in the detection accuracy rate of the DNA metabarcoding approach observed from different sample types (trap ethanol fluid versus insect bulk) and different primer sets used for PCR amplifications, it was further determined whether the relative abundance of target species (Ceratopogonidae species as the indicator in this case) in the total insect communities obtained from the trap collection would impact the eDNA detection accuracy.
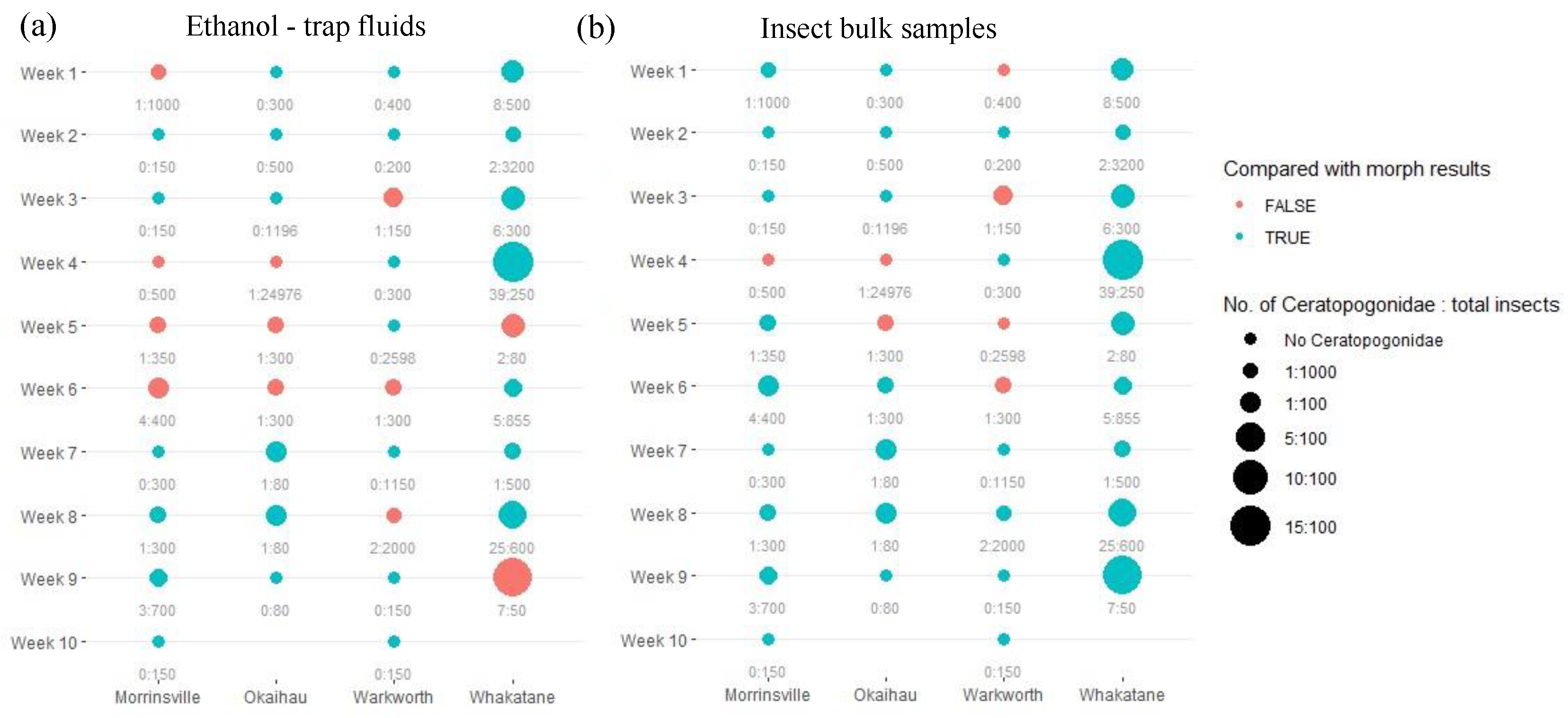
For the primer pair LCO1490/HCO2198, detection (presence/absence) of Ceratopogonidae species failed via eDNA metabarcoding using trap fluid samples compared to morphological findings in 12 out of 38 trap samples (31.6%) (
Figure 3a). In contrast, DNA metabarcoding using insect bulk samples had fewer detection failure cases (7 out of 38 trap samples; 18.4%) (
Figure 3b). In insect bulk samples, false negatives (i.e., detected under the microscope but not via metabarcoding) and false positives (i.e., detected via metabarcoding but not under the microscope) contributed almost equally to the detection failures, unlike trap liquids where detection failures occurred mainly due to false negatives. However, a trend of fewer detection failures was observed with an increase in the relative abundance of target species within the community. Notably, although classified as false positives when compared to trap catches under the microscopes, metabarcoding approaches detected the presence of Ceratopogonodiae species in one liquid trap sample and three insect bulk samples that morphological appoach failed to detect.
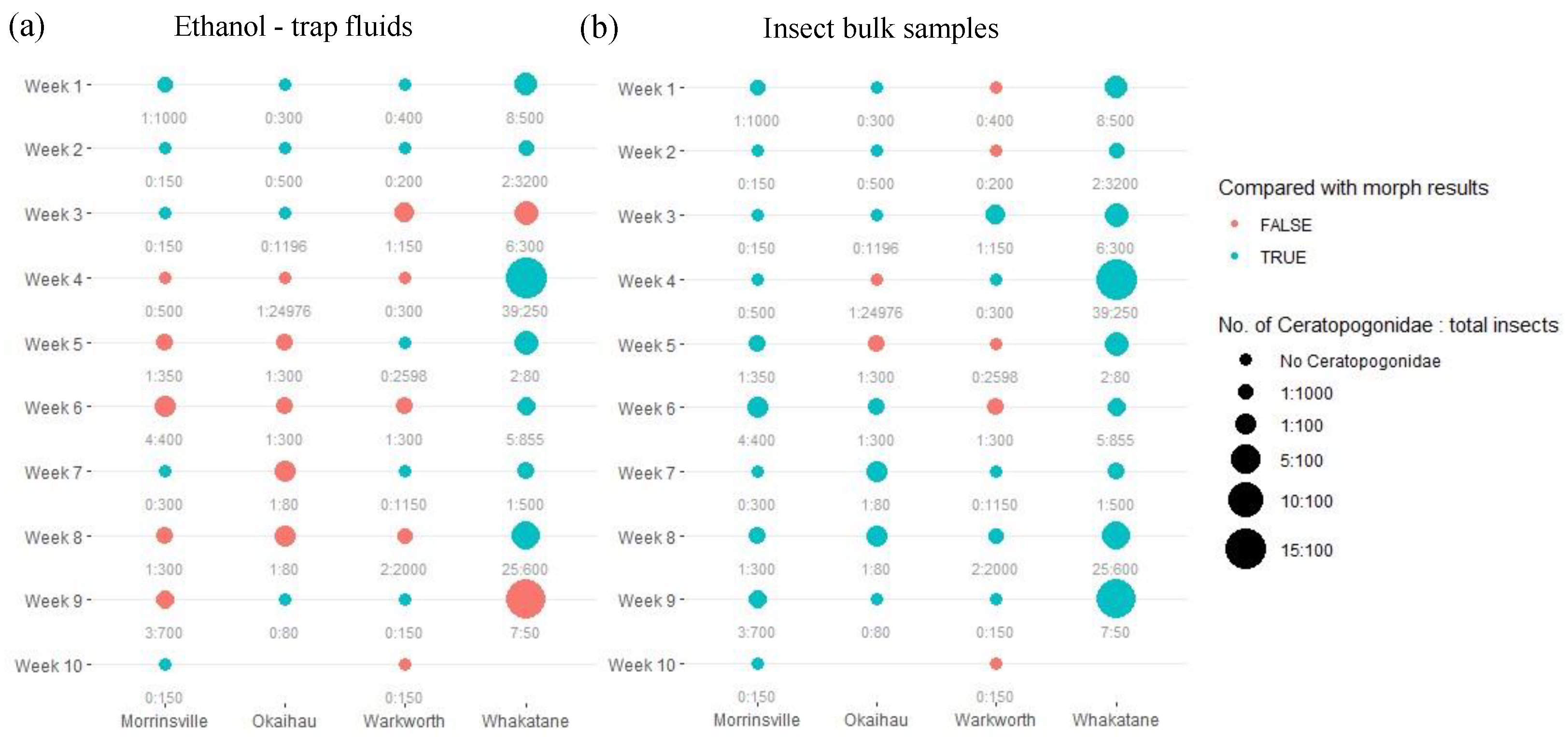
In addition, regarding the observations made from the trap ethanol liquids (
Figure 4a), the primer pair mlCOIintF/jgHCO2198 performed worse than those observed from the primer LCO1490/HCO2198 (
Figure 3a). Similar to the previous observation, the eDNA metabarcoding approach using insect trap fluids was unable to replicate the outcomes achieved under the microscope, and DNA metabarcoding approach using insect bulk samples (
Figure 4a). However, the choice of different primers did not affect the detection accuracy of DNA metabarcoding on the insect bulk samples (
Figure 3b, 4b), as a similarly accurate observation was made for mlCOIintF/jgHCO2198 in comparison with the one derived from LCO1490/HCO2198.
3.4. Impact of Primer Choices and Sample Types on the Recovery of Overall Taxonomic Diversity
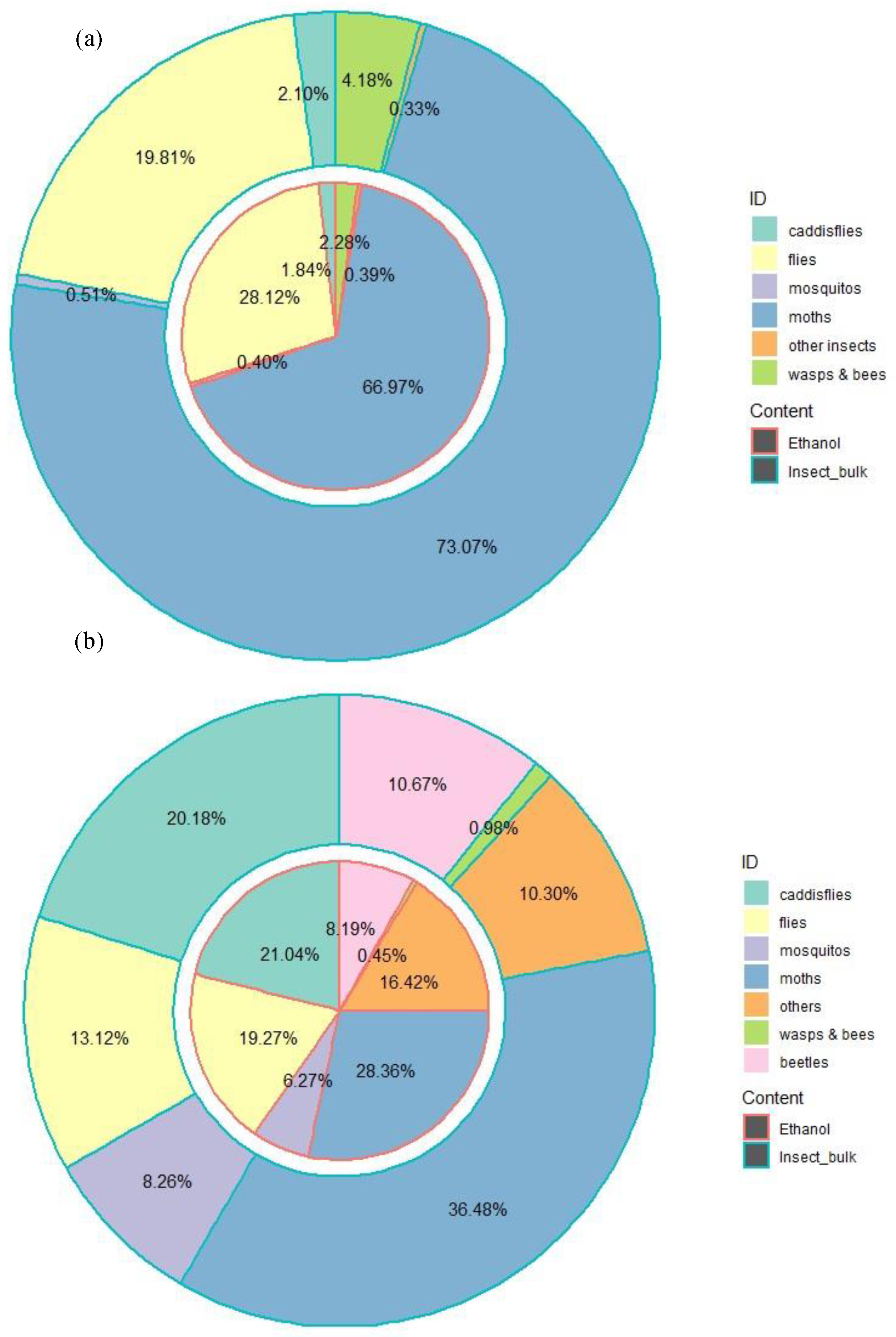
Besides species belonging to Ceratopogonidae family, both primer pairs LCO1490/HCO2198 and mlCOIintF/jgHCO2198 identified a variety of other insect species (
Figure 5 & S1). In general, the significant variation in the diversity and relative abundance of different insects recovered from the trap sample is mainly caused by the primer used, independent of the difference in the sample types (trap ethanol fluid or insect bulk). For example, a substantial proportion of the LCO1490/HCO2198 COI OTUs were taxonomically assigned to moths (i.e., order Lepidoptera); specifically, 73.07% and 66.97% of OTUs were derived from insect bulk and ethanol fluid, respectively.
However, based on the OTUs derived from mlCOIintF/jgHCO2198, that proportion drops to 36.48% and 28.36% for insect bulk and trap ethanol fluid, respectively. Moreover, flies (Diptera) were the second most abundant insect observed in both the trap ethanol fluid (28.12%) and insect bulk (19.81%) samples based on the OTUs derived from LCO1490/HCO2198. In the mlCOIintF/jgHCO2198 OTU dataset, that proportion only reached 19.27% and 13.12% for the trap ethanol fluid and insect bulk samples, respectively, making flies the third most abundant insect following the caddisflies (Trichoptera; 21.04% for ethanol fluid and 20.18% for insect bulk). More importantly, instead of one prevalent insect type acting as the most dominant order group observed in the dataset of LCO1490/HCO2198 (
Figure 5a), more diverse taxonomic groups with a similar proportion for each insect type were observed from the mlCOIintF/jgHCO2198 dataset (
Figure 5b). Such a difference in the taxonomic diversity derived from different primer choices is more distinct at the family level (
Figure S2).
3.5. Similar Insect Community Composition Recovered by Ethanol Fluid and Insect Bulk Samples
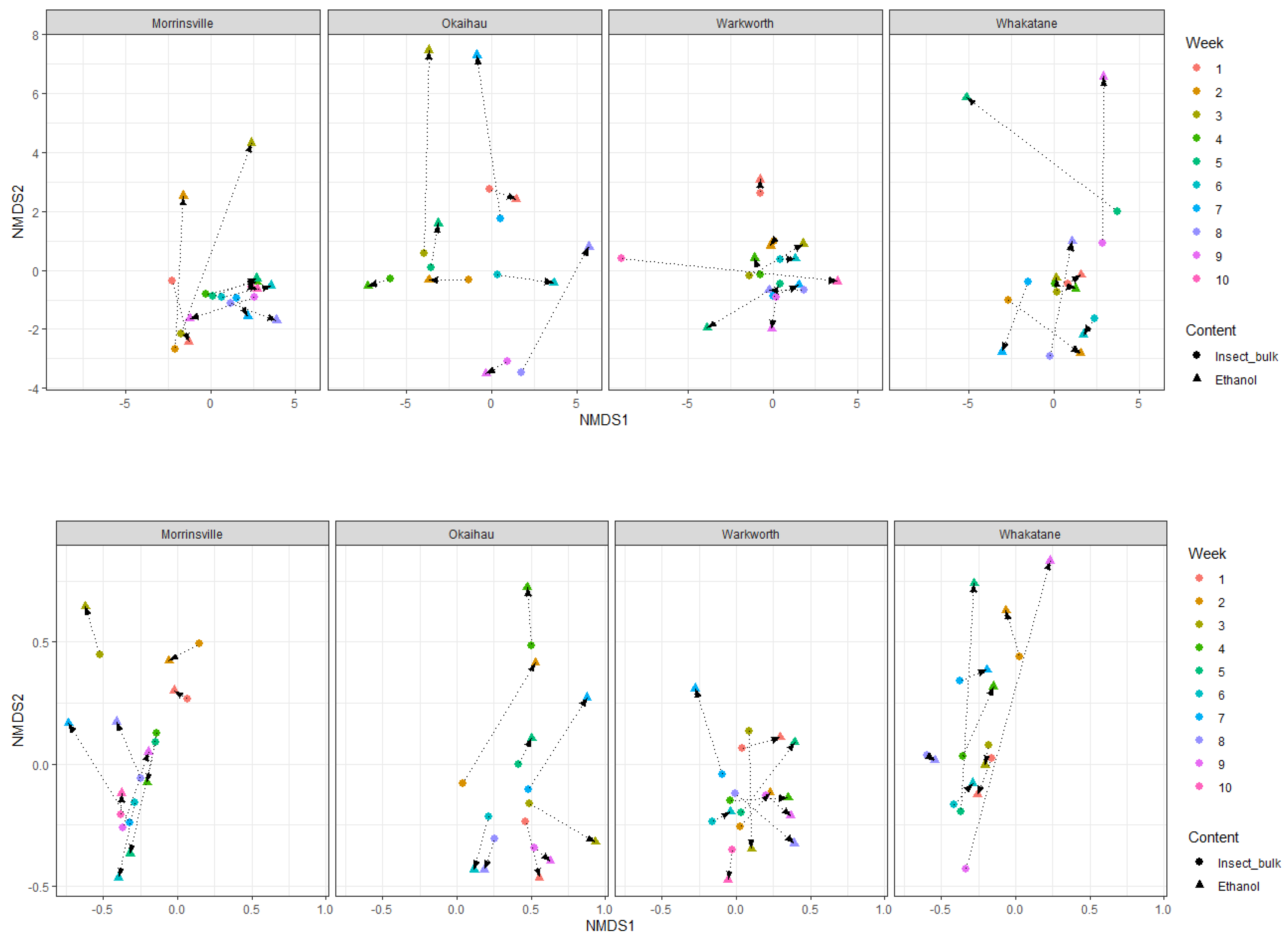
In general, the insect community composition recovered from the trap samples did not significantly differ between different sample types (trap ethanol fluids versus insect bulks) or sampling sites (
Figure S3), as the majority of them overlapped between the OTUs recovered from ethanol fluid and insect bulk samples. A closer look revealed that for most trap samples, their community composition recovered from ethanol fluid and insect bulk were greatly similar when comparing the samples collected from the same site at same collection time (
Figure 6).
Furthermore, a greater Bray-Curtis dissimilarity distances between the same sample derived from ethanol fluid and insect bulk was observed, based on their OTUs amplified by mlCOIintF/jgHCO2198, than those amplified by LCO1490/HCO2198, suggesting a greater influence of the primer selection on OTUs obtained from the same sample, in terms of the taxonomic diversity and composition of the whole insect community, compared with the sample types.
4. Discussion
In the current study, the detection accuracy of the DNA metabarcoding approaches in the surveillance of biting midges (Ceratopogonidae) were assessed using temporal samples collected over ten weeks from light traps at four different surveillance sites. The study compared DNA metabarcoding with morphological findings to determine if it could accurately detect target species and recover the taxonomic diversity of insect communities using different sample types (trap ethanol fluid versus insect bulk samples) and two COI barcoding regions for DNA metabarcoding approaches. Results indicated that sample types impacted the detection accuracy of target species of Ceratopogonidae. DNA metabarcoding using homogenized insect bulk samples correctly identified the target species observed via morphology in over 81% of trap samples, while the accuracy using eDNA from ethanol fluids was only 55-68% with two different primer sets. However, the recovery of overall taxonomic diversity and insect community composition was influenced by primer choices rather than sample types. These findings highlight opportunities to optimise diagnostics using insect bulk samples and eDNA from trap fluids for detecting unwanted insect pests and monitoring biodiversity in pest surveillance traps.
4.1. Detection of Target Species Is Greatly Impacted by Sample Types and Primer Selection
Using mixed homogenized insect bulk from trap samples can result in the loss of individual insects, leaving no sample for morphological examination after the extraction. While DNA extraction from homogenized insect tissues is used to study community composition (Emilson et al. 2017, Kocher et al. 2017, Blackman et al. 2019), some other studies have suggested that eDNA extraction from preservative mediums like ethanol or saturated salt (NaCl) solution can be an alternative while preserving intact specimens for further taxonomic work (Hajibabaei et al. 2012, Erdozain et al. 2019, Zizka et al. 2019, Milián-García et al. 2021).
However, metabarcoding results from preservative trap fluid may differ from those of homogenized tissues due to the disproportionate DNA contribution from larger organisms in comparison to smaller ones, subsequently biasing the proportion of HTS reads per specimen in the sample (Krehenwinkel et al. 2017, Bista et al. 2018). To address this, this study compares the consistency and accuracy of metabarcoding results between homogenized insect bulk and ethanol fluid samples using two COI barcoding markers, alongside morphological findings. The eDNA approach from ethanol fluid offers a non-destructive method to detect target species and investigate biodiversity.
The study of Hajibabaei et al. (2012) pioneered the use of eDNA extracted from the preservative ethanol for high-throughput barcoding, achieving an ~89% recovery rate of taxa identified from insect tissue homogenate. Since then, there has been growing interest in using preservative ethanol for metabarcoding in biodiversity monitoring surveys (Linard et al., 2016; Erdozain et al., 2019; Marquina et al., 2019; Zizka et al., 2019; Persaud et al. 2021). However, its effectiveness in biosecurity surveillance, specifically targeting unwanted species, remains largely unexplored. To our knowledge, this study is among the first to compare DNA metabarcoding results from both preservative ethanol and insect tissue homogenate with morphological findings in a biosecurity surveillance context with targeted species.
We found that the detection and identification of the presence of the indicator species, Ceratopogonidae species in surveillance trap samples recovered from eDNA metabarcoding of preservative ethanol fluid was significantly less sensitive, compared with those recovered from homogenized insect body bulk sample. In this case, eDNA approach was unable to recover the DNA of target species from the ethanol fluid was the major reason causing the failure of eDNA-based detection, regardless the relative abundance of the target species. Notably, our study is not the first to report the detection failure (e.g., false negative) of DNA of the specific insect species from the fluid samples (e.g., water). For example, the study of Schneider et al. (2016) achieved false negatives results through metabarcoding and it was likely due to the low specimen numbers within the whole community. Similarly, Krol et al. (2019) reported that only a subset of the species discovered from the trap was identified by eDNA extracted from environmental water. Similar observations were also made in other eDNA field studies targeting other organisms, such as fish (Curtis & Larson, 2020) and other macroinvertebrates (Fernández et al., 2018). The heterogeneous distribution of eDNA in the fluid body as well as PCR-biases could contribute together to the detection failures we observed in the current study.
Since eDNA of the specific target is not evenly distributed in the fluid body (Nathan et al., 2014; Dunker et al., 2016), after the insect is captured and dead in the preservative ethanol in the surveillance trap, its eDNA is more likely flow around its body. In addition, Culicoides and Ceratopogonidae species are well-known as their small body size, measuring only 1-3 mm (Mellor et al., 2000). In consequence, they release less DNA into the ethanol, therefore, representing only a small fraction of the total biomass, as well as of the eDNA in the sample (Elbrecht et al., 2017). Together, those impacts reduce the possibility of eDNA capture and extraction of small insects, like Ceratopogonidae species from the ethanol.
Furthermore, during the downstream metabarcoding amplification, such uneven distribution of eDNA derived from the insect with different body sizes within the community could be exacerbated by PCR-biases (Ficetola et al., 2010; Deagle et al., 2014; Cowart et al., 2015), possibly leading to underestimate or under-detection of our small-body sized target species. This could also explain why the eDNA metabarcoding conducted by the primer set mlCOIintF/jgHCO2198 produced more detection failures (i.e. false negatives), compared with those achieved using the primer set LCO1490/HCO2198.
In contrast, the detection accuracy of DNA metabarcoding using the homogenized insect body bulk samples were largely consistent between the amplicon sequencing conducted by different primer sets, both of which, in general, greatly recovered the DNA of species belong to Ceratopogonidae family from the traps. This could explain very low false negatives produced via this approach.
Besides the false negatives likely caused by the same reasons as discussed above for eDNA derived from ethanol, a few false positives produced by metabarcoding could possibly caused by the ‘cross-talk’, the incorrect assignment of reads to the wrong samples (Edgar, 2018). Similar observation was previously reported by Mata et al. (2020), suggesting that the DNA of some insect species, which could only be caught during late summer and early autumn, was detected from DNA extracted from the homogenized insect bulk caught by the trap placed in late spring. In aquatic environment, similar observation was made where bulk-sample metabarcoding identified more taxa with higher taxonomic resolution than eDNA metabarcoding in freshwater biomonitoring (Macher at al. 2018; Murria et al. 2024; Doloiras-Laraño et al. 2023).
While morphological identification served as the reference standard for comparing DNA metabarcoding outcomes across two sample types, it is important to acknowledge that some false positives (i.e., detection via metabarcoding but not by morphology) could be attributed to inherent limitations in the morphological approach's ability to detect all target species. This was evident in some samples where metabarcoding detected the presence of Ceratopogonidae species in one liquid trap sample and three insect bulk samples that the morphological screening failed to identify. This discrepancy could be explained by the potential for small or fragmented body parts, possibly missed during microscopic examination, to be captured and detected through the homogenized bulk samples and trap liquid samples used for metabarcoding.
4.2. Consistent Insect Community Composition Data in Ethanol Versus Homogenized Samples
Although a variable number of Ceratopogonidae species catches were observed across the four surveillance sites, and within samples from the same site, detection failures were minimal when the relative abundance of the target species within the community increased. This suggests a higher probability of detecting shed eDNA in the trap liquid or recovering DNA from homogenized insect bulk samples when multiple individuals of the same species are present in a trap, compared to the presence of a single individual.
While targeted species detection varied between ethanol and homogenized samples, overall taxonomic diversity and insect community composition were consistent. This aligns with most recent studies (Erdozain et al., 2019; Martins et al., 2019; Zizka et al., 2019), though other studies (Marquina et al. 2019, Persaud et al. 2021) reported divergent estimates of community composition recovered from ethanol eDNA, compared with those derived from homogenate bulk samples, likely due to their use of heavily sclerotized terrestrial insects.
Our findings, supported by Mata et al. (2020), which focused on nocturnal flying insect communities using light traps (similar with our study using light traps to attract nocturnal Culicoides and Ceratopogonidae species), found highly similar community composition between morphology and metabarcoding approach derived from homogenized insect bulk samples. This suggests that insect type influences eDNA recovery from trap ethanol fluid. Further research is required to determine how trapping methods affect diversity recovery from trap fluid samples. Collectively, further experimental work is required to address whether different trapping methods, such as light traps and Malaise traps, would have influence on the diversity recovery of a non-destructive extraction protocol using the preservative ethanol from the insect trap samples.
5. Conclusions
In conclusion, we demonstrate that using a COI-based metabarcoding approach, homogenized insect bulk samples collected from surveillance traps can correctly detect the presence or absence of the target species identified morphologically in most trap samples (i.e., over 81%). In contrast, the preservative ethanol fluid derived from the trap provided weaker detection accuracy (i.e., 55%-68%). This difference between destructive (i.e. homogenization of insect bulk samples) and non-destructive (i.e. eDNA shed from trap fluids) approaches was previously observed (Marquina et al., 2019; Martins et al., 2019), likely due to variations in the amount of DNA of target species leaking into the ethanol or homogenized from insect bulk, based on their biomass size and sclerotization.
Therefore, the utility of more sensitive and specific diagnosis techniques, such as quantitative PCR (qPCR), targeting only the specific unwanted species could potentially increase the detection accuracy of the non-destructive eDNA approach. However, similar insect community composition observed from both approaches suggests that the implementation of eDNA metabarcoding using preservative ethanol fluid provides a convenient approach for rapid and non-destructive monitoring of spatio-temporal variations in the diversity and composition of complex insect communities. This approach offers the ability to process hundreds to thousands of insect samples at high taxonomic resolution in a relatively short time frame, which is hardly achieved by conventional morphological approaches.
Overall, DNA metabarcoding using either destructive or non-destructive protocols shows the potential of improving and complementing the efficiency and early detection of invasive species in traditional surveillance. Meanwhile, this approach also demonstrates its capability to reliably recover the true insect community composition for biodiversity monitoring.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Pie charts showing the overall taxonomic range at order level across the study sites; Figure S2: Pie charts showing the overall taxonomic range at family level across the study sites; Figure S3: Non-metric multidimensional scaling (nMDS) plots showing variations in the composition of eDNA sequencing data.
Author Contributions
Conceptualization, C.P., L.P., and S.G.; methodology, C.P., and J.W.; software, J.W. and C.P.; validation, J.W., C.P., and D.L.; formal analysis, J.W.; investigation, J.W.; resources, S.G, and C.P..; data curation, J.W., D.L, R.B; writing—original draft preparation, J.W; writing—review and editing, C.P.; visualization, J.W.; supervision, C.P., D.L; project administration, C.P.; funding acquisition, C.P., and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Operational Research Programme of the Ministry for Primary Industries (MPI), New Zealand, grant number 405732.
Data Availability Statement
All relevant data are included in the article. Raw sequencing data has been deposited to NCBI SRA (Sequence Read Archive) database with the BioProject ID PRJNA1249277. The in-house customized COI reference database of Ceratopogonidae species are available on request.
Acknowledgments
We would like to thank Ben Boyd, Yan Chen, and Stacey Lamont for filtering the trap samples for eDNA work. We thank Mosquito Consulting Services, Wellington for conducting the field work and collecting surveillance trap samples. Sequencing was conducted at the GENEWIZ laboratory, China and Auckland Genomics at the University of Auckland, New Zealand. We would also like to thank two internal reviewers for their feedback on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| eDNA |
Environmental DNA |
| COI |
Cytochrome Oxidase I |
| HTS |
High-Throughput Sequencing |
| OTU |
Operational Taxonomic Unit |
| MPI |
Ministry for Primary Industries |
| NCBI |
National Centre for Biotechnology Information |
| NMDS |
Non-metric multidimensional scaling |
| PHEL |
Plant Health and Environment Laboratory |
| SRA |
Sequence Read Archive |
References
- Agren, E.C.; Burgin, L.; Lewerin, S.S.; Gloster, J.; Elvander, M. Possible means of introduction of bluetongue virus serotype 8 (BTV-8) to Sweden in August 2008: comparison of results from two models for atmospheric transport of the Culicoides vector. Vet Rec 2010, 167, 484–488. [Google Scholar] [CrossRef]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H. Oropouche virus: a new human disease agent from Trinidad, West Indies. Am J Trop Med Hyg 1961, 10, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.A.; Turner, C.R.; Jerde, C.L.; Renshaw, M.A.; Chadderton, W.L.; Lodge, D.M. Environmental conditions influence eDNA persistence in aquatic systems. Environ Sci Technol 2014, 48, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Prasad, G.; Srivastava, R. Culicoides (Ceratopogonidae: Diptera) as vector of bluetongue virus. Annals of Biology 1994, 10, 179–179. [Google Scholar]
- Bishop, A.L.; Bellis, G.A.; McKenzie, H.J.; Spohr, L.J.; Worrall, R.J.; Harris, A.M.; Melville, L. Light trapping of biting midges Culicoides spp.(Diptera: Ceratopogonidae) with green light-emitting diodes. Aust J Entomol 2006, 45, 202–205. [Google Scholar] [CrossRef]
- Bista, I.; Carvalho, G.R.; Tang, M.; Walsh, K.; Zhou, X.; Hajibabaei, M.; Shokralla, S.; Seymour, M.; Bradley, D.; Liu, S. Performance of amplicon and shotgun sequencing for accurate biomass estimation in invertebrate community samples. Molecular Ecology Resources 2018, 18, 1020–1034. [Google Scholar] [CrossRef]
- Blackman, R.C.; Mächler, E.; Altermatt, F.; Arnold, A.; Beja, P.; Boets, P.; Egeter, B.; Elbrecht, V.; Filipe, A.F.; Jones, J.I. Advancing the use of molecular methods for routine freshwater macroinvertebrate biomonitoring–the need for calibration experiments. Metabarcoding and Metagenomics 2019, 3, 49–57. [Google Scholar] [CrossRef]
- Braverman, R.J. Species composition and blood-meal identification in samples of Culicoides (Diptera: Ceratopogonidae) collected near Salisbury, Zimbabwe in 1976-77. Journal of the Entomological Society of Southern Africa 1981, 44, 315–323. [Google Scholar]
- Braverman, Y.; Chechik, F. Air streams and the introduction of animal diseases borne on Culicoides (Diptera, Ceratopogonidae) into Israel. Revue Scientifique et Technique-Office International des Epizooties 1996, 15, 1037–1052. [Google Scholar] [CrossRef]
- Chang, Q.Q.; Jiang, X.H.; Liu, G.P.; Li, X.F.; Hou, X.H. A species checklist of the subgenus Culicoides (Avaritia) in China, with a description of a new species (Diptera, Ceratopogonidae). ZooKeys 2017, 706, 117. [Google Scholar] [CrossRef]
- Close, R.C.; Moar, N.; Tomlinson, A.; Lowe, A. Aerial dispersal of biological material from Australia to New Zealand. Int J Biometeorol 1978, 22, 1–19. [Google Scholar] [CrossRef]
- Cowart, D.A.; Pinheiro, M.; Mouchel, O.; Maguer, M.; Grall, J.; Miné, J.; Arnaud-Haond, S. Metabarcoding is powerful yet still blind: a comparative analysis of morphological and molecular surveys of seagrass communities. PloS One 2015, 10, e0117562. [Google Scholar] [CrossRef]
- Curtis, A.N.; Larson, E.R. No evidence that crayfish carcasses produce detectable environmental DNA (eDNA) in a stream enclosure experiment. PeerJ 2020, 8, e9333. [Google Scholar] [CrossRef] [PubMed]
- Daniels, P.W.; Sendow, I.; Pritchard, L.I.; Sukarsih; Eaton, B.T. Regional overview of bluetongue viruses in South-East Asia: viruses, vectors and surveillance. Vet Ital 2004, 40, 94–100. [Google Scholar] [PubMed]
- Darling, J.A.; Mahon, A.R. From molecules to management: adopting DNA-based methods for monitoring biological invasions in aquatic environments. Environ Res 2011, 111, 978–988. [Google Scholar] [CrossRef]
- Deagle, B.E.; Jarman, S.N.; Coissac, E.; Pompanon, F.; Taberlet, P. DNA metabarcoding and the cytochrome c oxidase subunit I marker: not a perfect match. Biol Lett 2014, 10. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Doloiras-Laraño, A.D.; Yaegashi, S.; Serrana, J.M.; Ishitani, N.; Watanabe, K. Comparison of eDNA, bulk-sample metabarcoding, and morphological approaches: A case study of riverine benthic macroinvertebrate communities. bioRxiv 2023. 2023.05.30.542510. [Google Scholar] [CrossRef]
- Ducheyne, E.; De Deken, R.; Bécu, S.; Codina, B.; Nomikou, K.; Mangana-Vougiaki, O.; Georgiev, G.; Purse, B.; Hendrickx, G. Quantifying the wind dispersal of Culicoides species in Greece and Bulgaria. Geospatial Health 2007, 177-189. [Google Scholar] [CrossRef]
- Dunker, K.J.; Sepulveda, A.J.; Massengill, R.L.; Olsen, J.B.; Russ, O.L.; Wenburg, J.K.; Antonovich, A. Potential of environmental DNA to evaluate northern pike (Esox lucius) eradication efforts: an experimental test and case study. PLoS One 2016, 11, e0162277. [Google Scholar] [CrossRef]
- Eagles, D.; Deveson, T.; Walker, P.; Zalucki, M.; Durr, P. Evaluation of long-distance dispersal of Culicoides midges into northern Australia using a migration model. Med Vet Entomol 2012, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UNCROSS2: identification of cross-talk in 16S rRNA OTU tables. bioRxiv 2018, 400762. [Google Scholar]
- Elbrecht, V.; Peinert, B.; Leese, F. Sorting things out: Assessing effects of unequal specimen biomass on DNA metabarcoding. Ecology and Evolution 2017, 7, 6918–6926. [Google Scholar] [CrossRef]
- Emilson, C.E.; Thompson, D.G.; Venier, L.A.; Porter, T.M.; Swystun, T.; Chartrand, D.; Capell, S.; Hajibabaei, M. DNA metabarcoding and morphological macroinvertebrate metrics reveal the same changes in boreal watersheds across an environmental gradient. Scientific Reports 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Erdozain, M.; Thompson, D.G.; Porter, T.M.; Kidd, K.A.; Kreutzweiser, D.P.; Sibley, P.K.; Swystun, T.; Chartrand, D.; Hajibabaei, M. Metabarcoding of storage ethanol vs. conventional morphometric identification in relation to the use of stream macroinvertebrates as ecological indicators in forest management. Ecol Ind 2019, 101, 173–184. [Google Scholar] [CrossRef]
- Fernández, S.; Rodríguez, S.; Martínez, J.L.; Borrell, Y.J.; Ardura, A.; García-Vázquez, E. Evaluating freshwater macroinvertebrates from eDNA metabarcoding: A river Nalón case study. PLoS One 2018, 13, e0201741. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Coissac, E.; Zundel, S.; Riaz, T.; Shehzad, W.; Bessière, J.; Taberlet, P.; Pompanon, F. An in silico approach for the evaluation of DNA barcodes. BMC Genomics 2010, 11, 434. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 1994, 3, 294–9. [Google Scholar] [PubMed]
- Fox, K. The transoceanic migration of Lepidoptera to New Zealand—a history and a hypothesis on colonisation. N Z Entomol 1978, 6, 368–380. [Google Scholar] [CrossRef]
- García-Lastra, R.; Leginagoikoa, I.; Plazaola, J.M.; Ocabo, B.; Aduriz, G.; Nunes, T.; Juste, R.A. Bluetongue virus serotype 1 outbreak in the Basque Country (Northern Spain) 2007–2008. Data support a primary vector windborne transport. PLoS One 2012, 7, e34421. [Google Scholar] [CrossRef]
- Gerry, A.C.; Monteys, V.S.I.; Vidal, J.M.; Francino, O.; Mullens, B.A. Biting rates of Culicoides midges (Diptera: Ceratopogonidae) on sheep in northeastern Spain in relation to midge capture using UV light and carbon dioxide-baited traps. J Med Entomol 2009, 46, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.F.; Shokralla, S.; Curry, C.; Baird, D.J.; Monk, W.A.; King, I.; Hajibabaei, M. Large-scale biomonitoring of remote and threatened ecosystems via high-throughput sequencing. PLoS One 2015, 10, e0138432. [Google Scholar] [CrossRef]
- Gloster, J.; Mellor, P.; Manning, A.; Webster, H.; Hort, M. Assessing the risk of windborne spread of bluetongue in the 2006 outbreak of disease in northern Europe. Vet Rec 2007, 160, 54. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaei, M.; Shokralla, S.; Zhou, X.; Singer, G.A.; Baird, D.J. Environmental barcoding: a next-generation sequencing approach for biomonitoring applications using river benthos. PLoS One 2011, 6, e17497. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Spall, J.L.; Shokralla, S.; van Konynenburg, S. Assessing biodiversity of a freshwater benthic macroinvertebrate community through non-destructive environmental barcoding of DNA from preservative ethanol. BMC Ecology 2012, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Hoare, R.J. Adventive species of Lepidoptera recorded for the first time in New Zealand since 1988. N Z Entomol 2001, 24, 23–47. [Google Scholar] [CrossRef]
- Illumina. 16S Metagenomic Sequencing Library Preparation. 2013. Available at http://support.illumina.com/downloads/ 16s_metagenomic_sequencing_library_preparation.ilmn.
- Ji, Y.; Ashton, L.; Pedley, S.M.; Edwards, D.P.; Tang, Y.; Nakamura, A.; Kitching, R.; Dolman, P.M.; Woodcock, P.; Edwards, F.A. Reliable, verifiable and efficient monitoring of biodiversity via metabarcoding. Ecol Lett 2013, 16, 1245–1257. [Google Scholar] [CrossRef]
- Kampen, H.; Werner, D. Biting Midges (Diptera: Ceratopogonidae) as Vectors of Viruses. Microorganisms 2023, 11, 2706. [Google Scholar] [CrossRef]
- Kettle, D. Ceratopogonidae (Biting Midges). Chapter 8. In Medical and Veterinary Entomology; John Wiley and Sons: New York, 1984. [Google Scholar]
- Kline, D.L.; Hagan, D.V.; Wood, J.R. Culicoides responses to 1-octen-3-ol and carbon dioxide in salt marshes near Sea Island, Georgia, USA. Med Vet Entomol 1994, 8, 25–30. [Google Scholar] [CrossRef]
- Khalaf, K. The speciation of the genus Culicoides (Diptera, Heleidae). Annals of the Entomological Society of America 1954, 47, 34–51. [Google Scholar] [CrossRef]
- Kocher, A.; Gantier, J.; Gaborit, P.; Zinger, L.; Holota, H.; Valiere, S.; Dusfour, I.; Girod, R.; Bañuls, A.; Murienne, J. Vector soup: High-throughput identification of Neotropical phlebotomine sand flies using metabarcoding. Molecular Ecology Resources 2017, 17, 172–182. [Google Scholar] [CrossRef]
- Krehenwinkel, H.; Wolf, M.; Lim, J.Y.; Rominger, A.J.; Simison, W.B.; Gillespie, R.G. Estimating and mitigating amplification bias in qualitative and quantitative arthropod metabarcoding. Scientific Reports 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krol, L.; Van der Hoorn, B.; Gorsich, E.E.; Trimbos, K.; Bodegom, P.V.; Schrama, M. How does eDNA compare to traditional trapping? Detecting mosquito communities in South-African freshwater ponds. Frontiers in Ecology and Evolution 2019, 7, 260. [Google Scholar] [CrossRef]
- Leray, M.; Yang, J.Y.; Meyer, C.P.; et al. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Front Zool 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Lillie, T.H.; Marquardt, W.C.; Jones, R.H. The flight range of Culicoides variipennis (Diptera: Ceratopogonidae). The Canadian Entomologist 1981, 113, 419–426. [Google Scholar] [CrossRef]
- Linard, B.; Arribas, P.; Andújar, C.; Crampton-Platt, A.; Vogler, A.P. Lessons from genome skimming of arthropod-preserving ethanol. Molecular Ecology Resources 2016, 16, 1365–1377. [Google Scholar] [CrossRef]
- Linley, J.R. Biting midges (Diptera: Ceratopogonidae) as vectors of nonviral animal pathogens. J Med Entomol 1985, 22, 589–599. [Google Scholar] [CrossRef]
- Macher, J.-N.; Vivancos, A.; Piggott, J.J.; Centeno, F.C.; Matthaei, C.D.; Leese, F. Comparison of environmental DNA and bulk-sample metabarcoding using highly degenerate cytochrome c oxidase I primers. Molecular Ecology Resources 2018, 18, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Marquina, D.; Esparza-Salas, R.; Roslin, T.; Ronquist, F. Establishing arthropod community composition using metabarcoding: Surprising inconsistencies between soil samples and preservative ethanol and homogenate from Malaise trap catches. Molecular Ecology Resources 2019, 19, 1516–1530. [Google Scholar] [CrossRef]
- Martins, F.M.; Galhardo, M.; Filipe, A.F.; Teixeira, A.; Pinheiro, P.; Paupério, J.; Alves, P.C.; Beja, P. Have the cake and eat it: Optimizing nondestructive DNA metabarcoding of macroinvertebrate samples for freshwater biomonitoring. Molecular Ecology Resources 2019, 19, 863–876. [Google Scholar] [CrossRef]
- Martoni, F.; Smith, R.L.; Piper, A.M.; Nancarrow, N.; Aftab, M.; Trebicki, P.; Kimber, R.B.E.; Rodoni, B.C.; Blacket, M.J. Non-destructive insect metabarcoding as a surveillance tool for the Australian grains industry: a first trial for the iMapPESTS smart trap. Metabarcoding and Metagenomics 2023, 7, e95650. [Google Scholar] [CrossRef]
- Mata, V.A.; Ferreira, S.; Campos, R.M.; da Silva, L.P.; Veríssimo, J.; Corley, M.F.; Beja, P. Efficient assessment of nocturnal flying insect communities by combining automatic light traps and DNA metabarcoding. bioRxiv 2020. [Google Scholar] [CrossRef]
- Meiswinkel, R.; Van Rijn, P.; Leijs, P.; Goffredo, M. Potential new Culicoides vector of bluetongue virus in northern Europe. Vet Rec 2007, 161, 564. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Mellor, P.S.; Carpenter, S.; Harrup, L.; Baylis, M.; Mertens, P.P. Bluetongue in Europe and the Mediterranean Basin: history of occurrence prior to 2006. Prev Vet Med 2008, 87, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.R.; Castillo-Pizango, M.J. Changes in relative species compositions of biting midges (Diptera: Ceratopogonidae) and an outbreak of Oropouche virus in Iquitos, Peru. J Med Entomol 2005, 42, 554–558. [Google Scholar] [CrossRef]
- Múrria, C.; Wangensteen, O. S.; Somma, S.; Väisänen, L.; Fortuño, P.; Arnedo, M. A.; Prat, N. Taxonomic accuracy and complementarity between bulk and eDNA metabarcoding provides an alternative to morphology for biological assessment of freshwater macroinvertebrates. Science of The Total Environment 2020, 713, 136589. [Google Scholar] [CrossRef]
- Nathan, L.M.; Simmons, M.; Wegleitner, B.J.; Jerde, C.L.; Mahon, A.R. Quantifying environmental DNA signals for aquatic invasive species across multiple detection platforms. Environ Sci Technol 2014, 48, 12800–12806. [Google Scholar] [CrossRef]
- Persaud, S.F.; Cottenie, K.; Gleason, J.E. Ethanol eDNA Reveals Unique Community Composition of Aquatic Macroinvertebrates Compared to Bulk Tissue Metabarcoding in a Biomonitoring Sampling Scheme. Diversity 2021, 13, 34. [Google Scholar] [CrossRef]
- Phillips, C.; Townsend, H.; Vink, C. Blown in the wind or border slippage. What natural dispersal of exotic species to New Zealand has to do with biosecurity. Biosecurity 2006, 69, 14–15. [Google Scholar]
- Pinheiro, F.; Travassos da Rosa, A.; Travassos da Rosa, J.; Bensabath, G. An outbreak of Oropouche virus disease in the vicinity of Santarem, Para, Brazil. Tropenmed Parasitol 1976, 27, 213–223. [Google Scholar] [PubMed]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.P.; Baylis, M. Climate change and the recent emergence of bluetongue in Europe. Nature Reviews Microbiology 2005, 3, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.; Frampton, E.; Motha, M.; Horner, G. Arbovirus and arbovirus vector surveillance in New Zealand. Surveillance 1991, 18, 24–26. [Google Scholar]
- Schneider, J.; Valentini, A.; Dejean, T.; Montarsi, F.; Taberlet, P.; Glaizot, O.; Fumagalli, L. Detection of invasive mosquito vectors using environmental DNA (eDNA) from water samples. PloS one 2016, 11, e0162493. [Google Scholar] [CrossRef]
- Sellers, R.F.; Pedgley, D.E. Possible windborne spread to western Turkey of bluetongue virus in 1977 and of Akabane virus in 1979. Epidemiology & Infection 1985, 95, 149–158. [Google Scholar]
- Sellers, R.F.; Pedgley, D.E.; Tucker, M.R. Possible spread of African horse sickness on the wind. Epidemiology & Infection 1977, 79, 279–298. [Google Scholar]
- Sellers, R.F.; Maarouf, A.R. Possible introduction of epizootic hemorrhagic disease of deer virus (serotype 2) and bluetongue virus (serotype 11) into British Columbia in 1987 and 1988 by infected Culicoides carried on the wind. Can J Vet Res 1991, 55, 367–370. [Google Scholar]
- Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides biting midges—underestimated vectors for arboviruses of public health and veterinary importance. Viruses 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Coissac, E.; Hajibabaei, M.; Rieseberg, L.H. Environmental DNA. Mol Ecol 2012, 21, 1789–1793. [Google Scholar] [CrossRef]
- Veiga, J.; Martínez-de la Puente, J.; Václav, R.; Figuerola, J.; Valera, F. Culicoides paolae and C. circumscriptus as potential vectors of avian haemosporidians in an arid ecosystem. Parasites & Vectors 2018, 11, 1–10. [Google Scholar]
- Venter, G.; Labuschagne, K.; Hermanides, K.; Boikanyo, S.; Majatladi, D.; Morey, L. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet Parasitol 2009, 166, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Viennet, E.; Garros, C.; Lancelot, R.; Allène, X.; Gardès, L.; Rakotoarivony, I.; Crochet, D.; Delécolle, J.; Moulia, C.; Baldet, T. Assessment of vector/host contact: comparison of animal-baited traps and UV-light/suction trap for collecting Culicoides biting midges (Diptera: Ceratopogonidae), vectors of Orbiviruses. Parasites & Vectors 2011, 4, 119. [Google Scholar]
- Watts, D.M.; Phillips, I.; Callahan, J.D.; Griebenow, W.; Hyams, K.C.; Hayes, C.G. Oropouche virus transmission in the Amazon River basin of Peru. Am J Trop Med Hyg 1997, 56, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Elegant graphics for data analysis (ggplot2). 2009.
- Withers, T. Colonization of eucalypts in New Zealand by Australian insects. Austral Ecol 2001, 26, 467–476. [Google Scholar] [CrossRef]
- Yates, J.A.; Lowrie Jr, R.C.; Eberhard, M.L. Development of Tetrapetalonema llewellyni to the infective stage in Culicoides hollensis. J Parasitol 1982, 293-296. [Google Scholar] [CrossRef]
- Zizka, V.M.; Leese, F.; Peinert, B.; Geiger, M.F. DNA metabarcoding from sample fixative as a quick and voucher-preserving biodiversity assessment method. Genome 2019, 62, 122–136. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).