Submitted:
17 April 2025
Posted:
17 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Pristine LaFeO3 Particles
2.3. Preparation of Ag Nanoparticles Modified LaFeO3 Particles
2.4. Characterization
2.5. PEC Electrode Assembly and Measurements
3. Results and Discussion
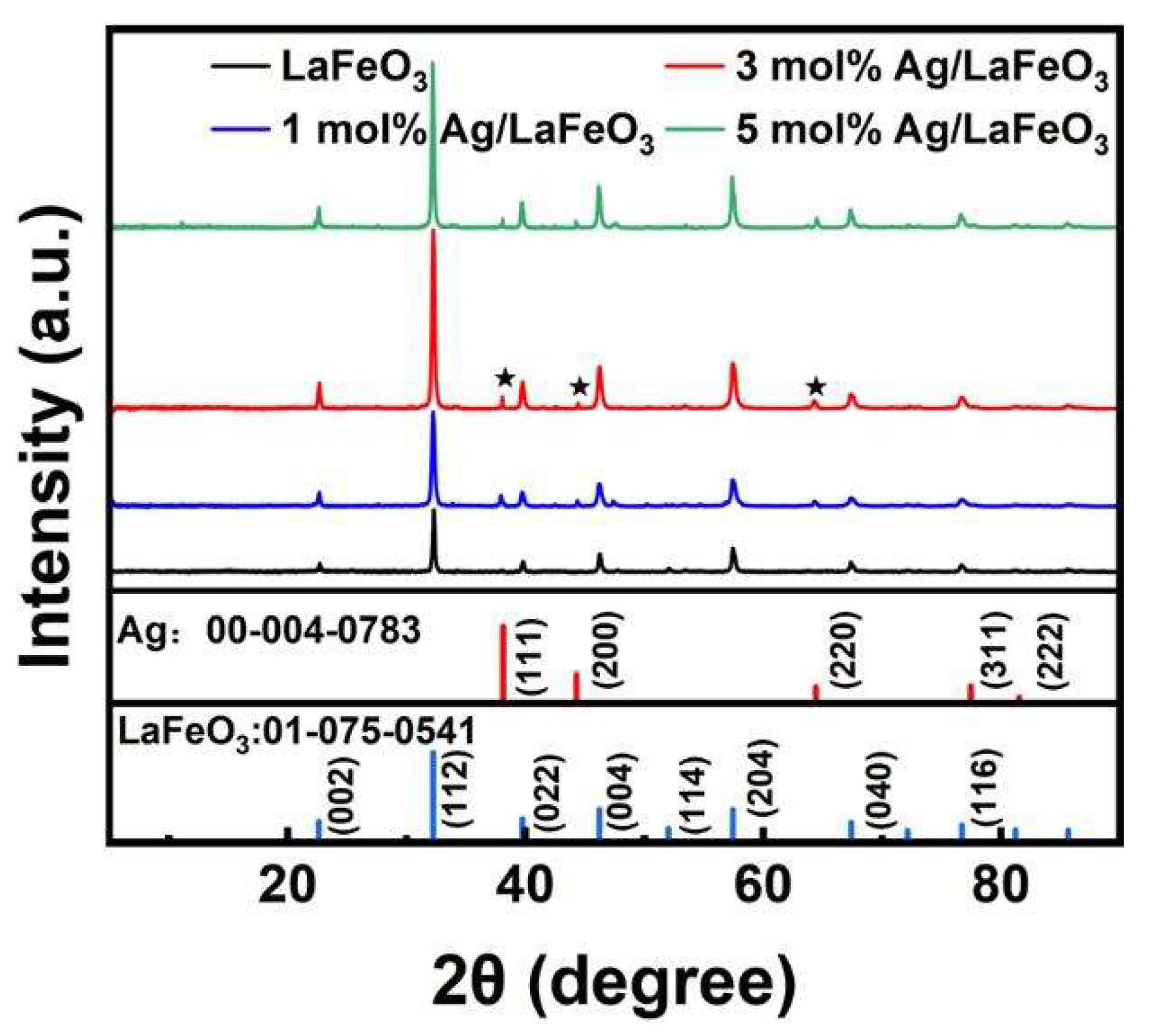
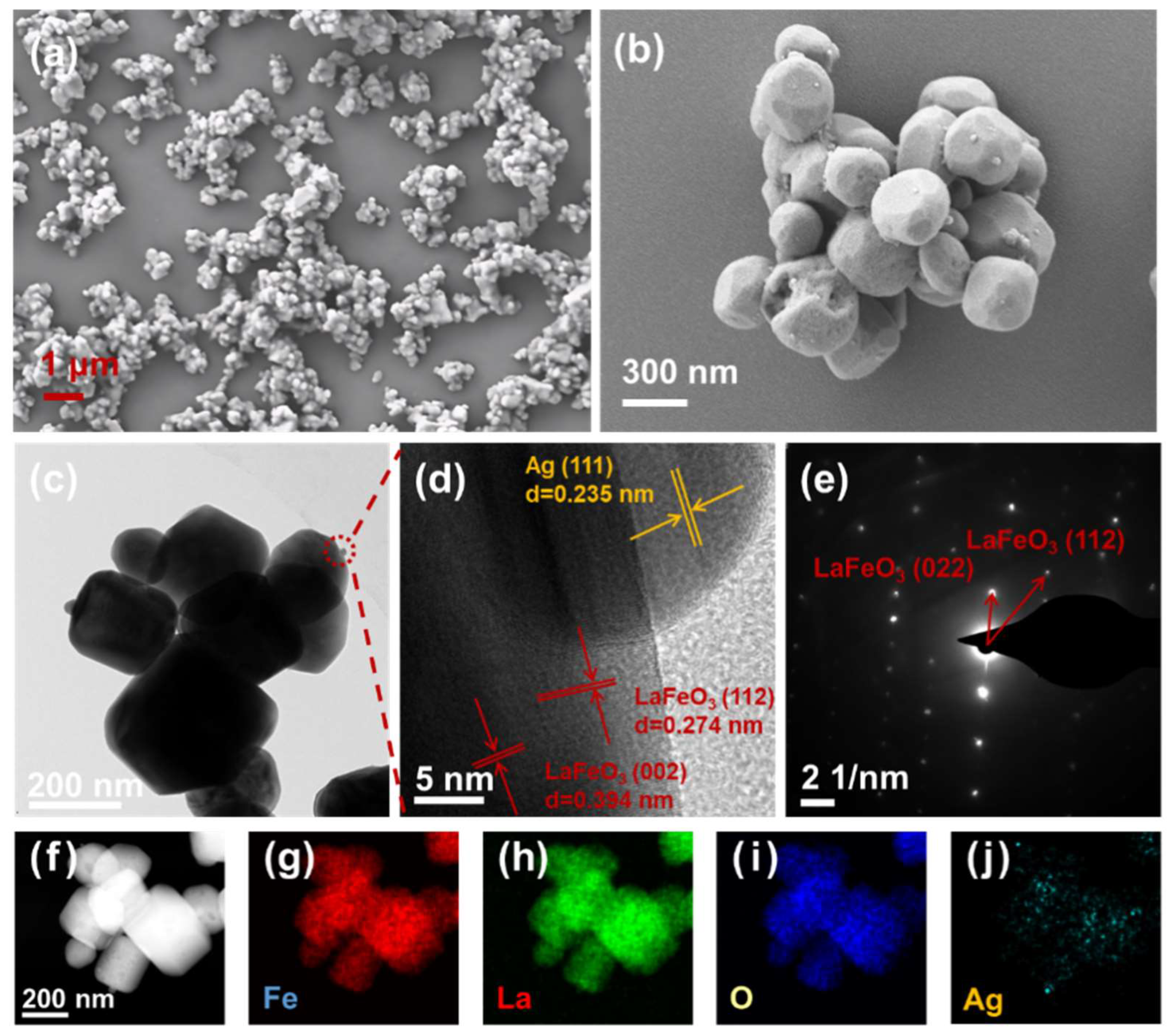
3.1. Morphology and Structure Characterization
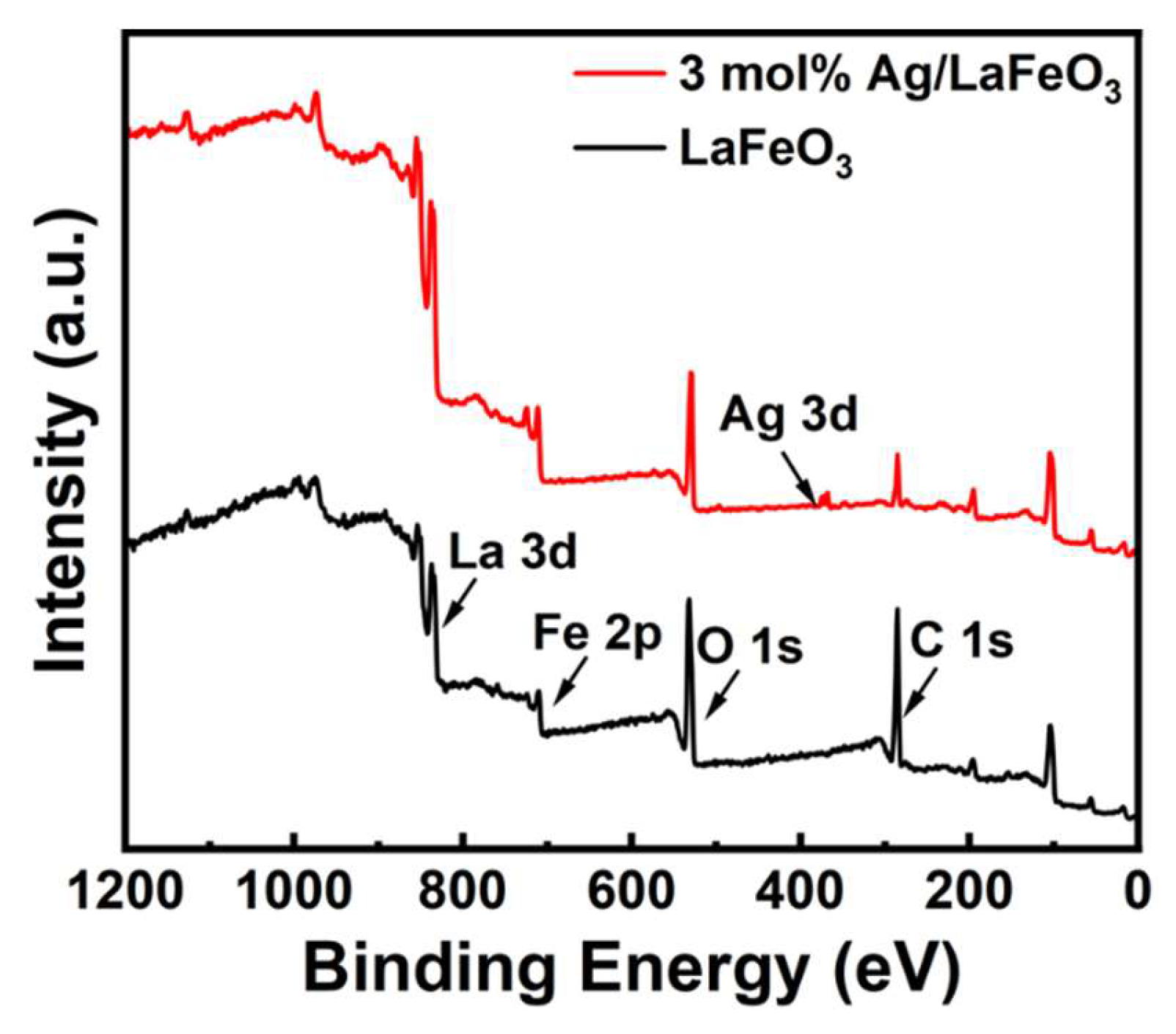
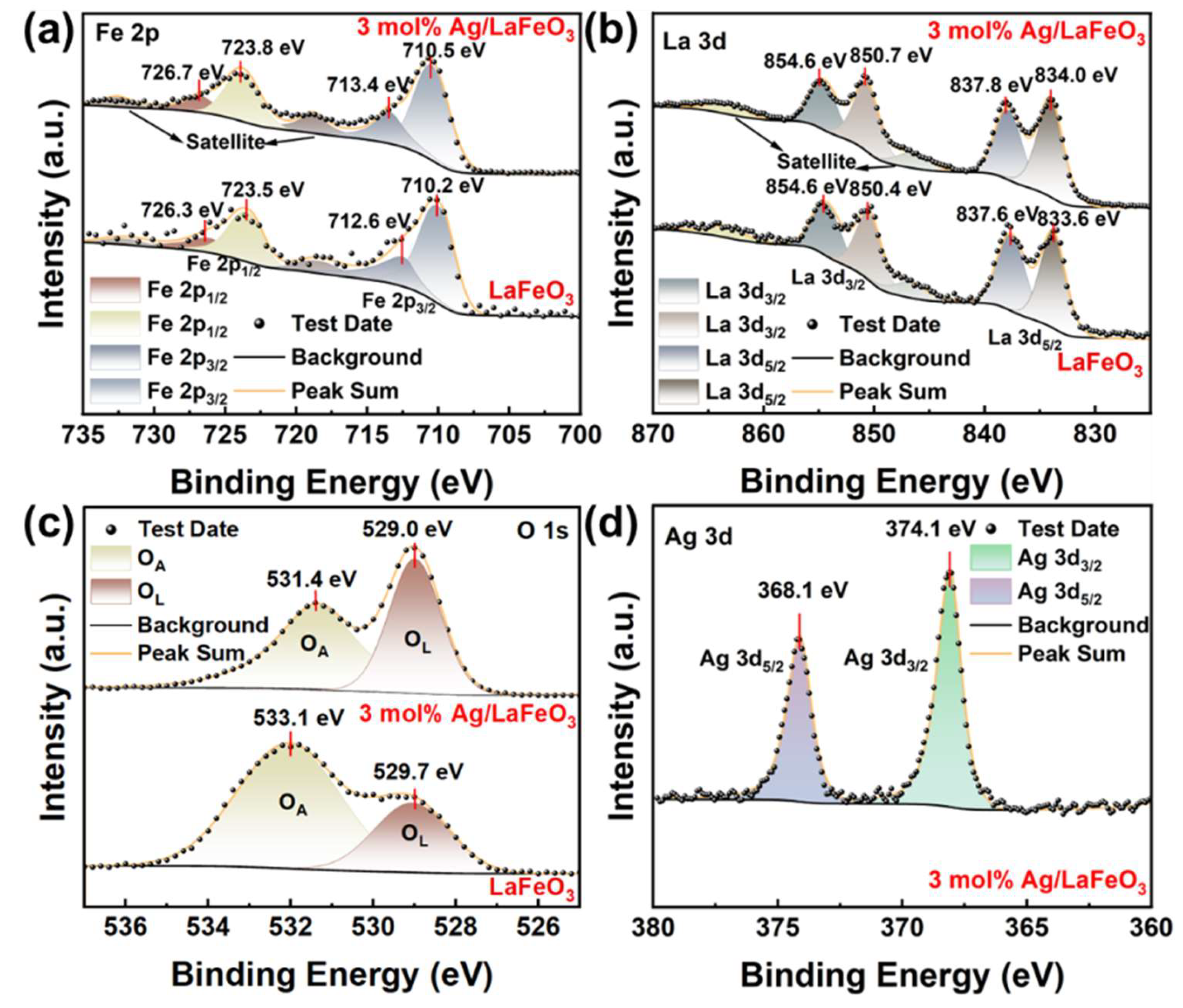
3.2. Elemental Analysis
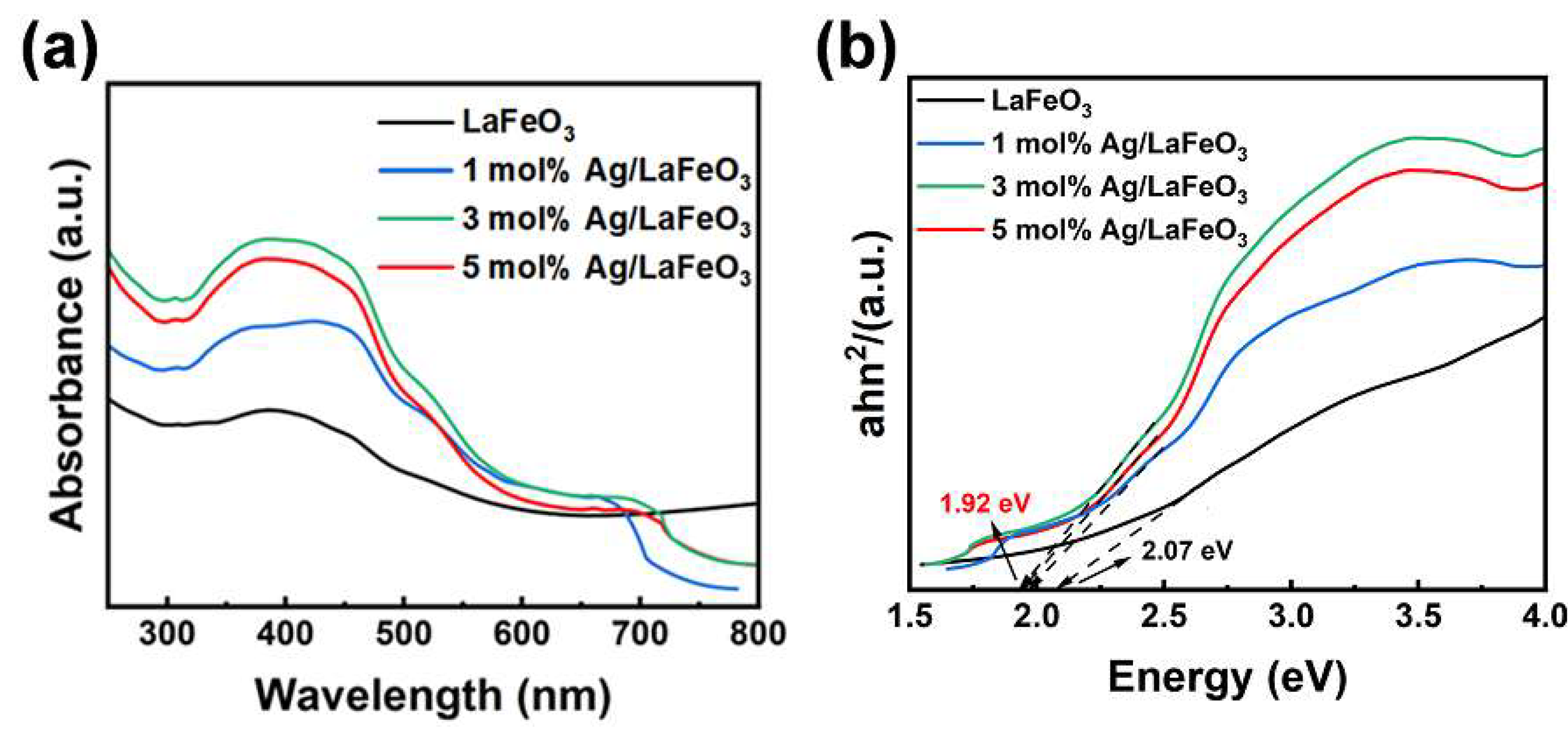
3.3. Optical Absorption Properties of Ag/LaFeO₃
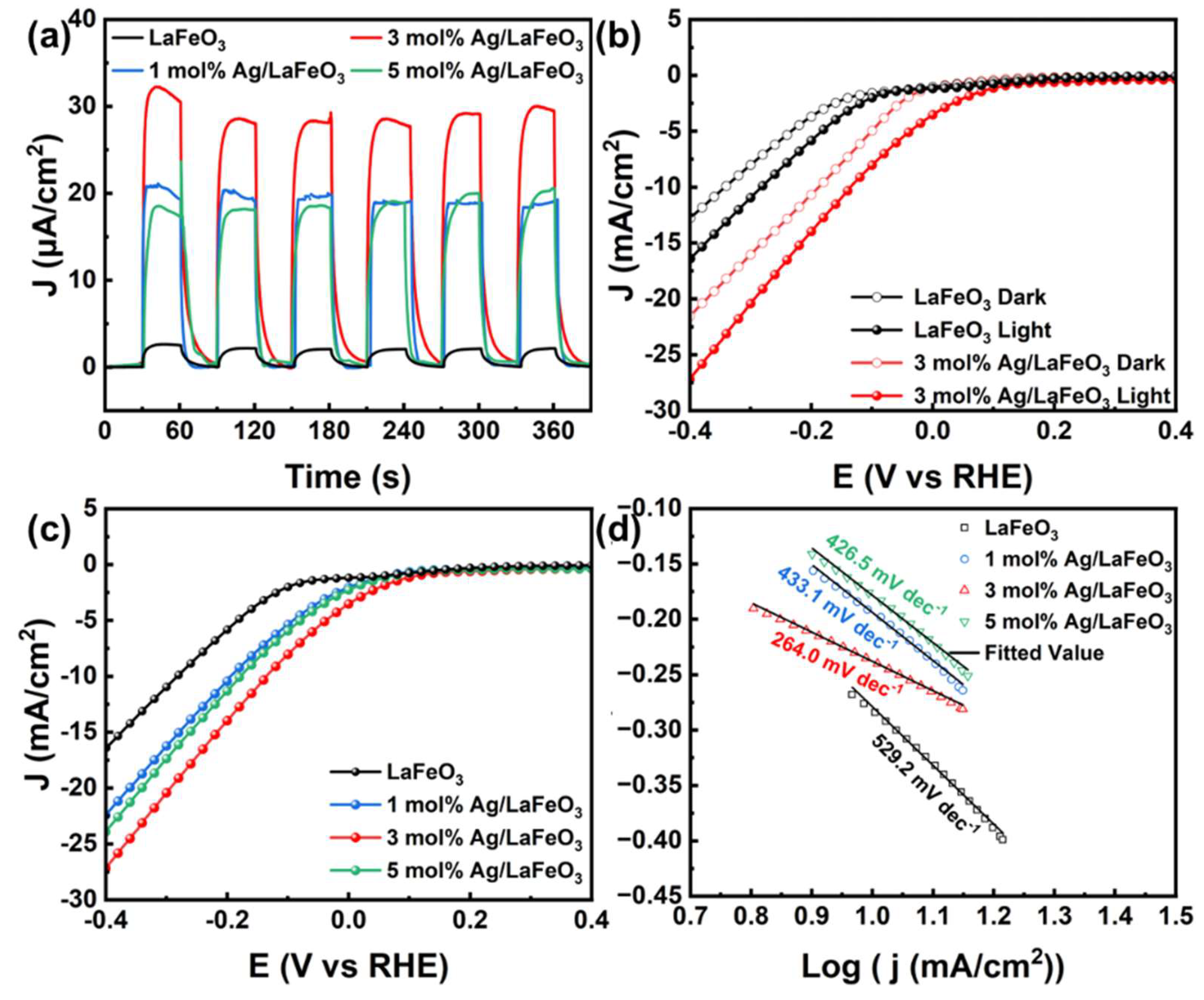
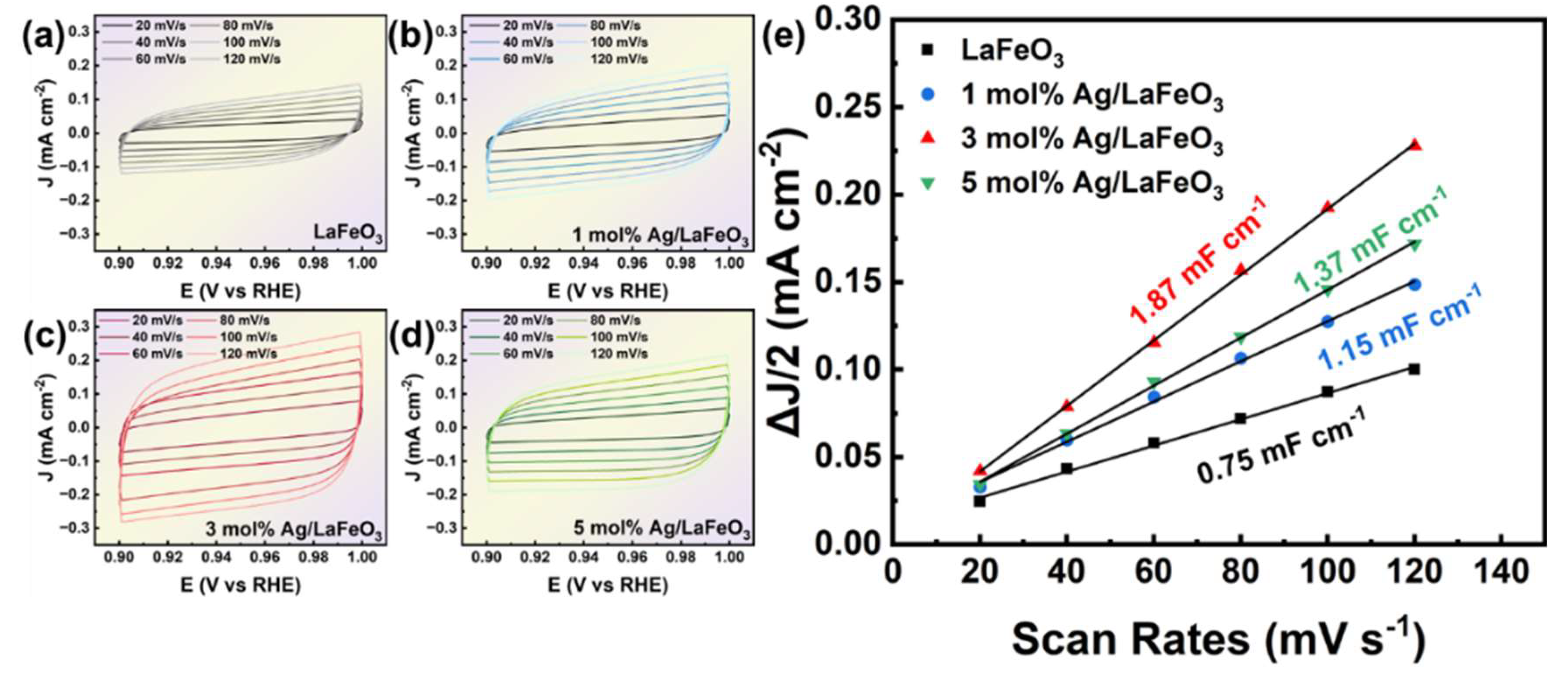
3.4. PEC Performance and Charge Transfer of the Ag/LaFeO₃ Photoelectrode
3.5. Photo-Electrocatalytic Mechanism
4. Conclusion
Acknowledgments
References
- Reddy N M, Kothandan D, Reddy V P, et al. Collating the structural, vibrational, and photocatalysis properties of LaFeO3 rare-earth orthoferrite nanoparticles synthesized by the sol-gel method. J Sol-Gel Sci Technol 2025, 113, 322–330. [Google Scholar] [CrossRef]
- Sukumar M, Simon D, Kumar A, et al. A comparative study of structural, optical, and magnetic properties of LaFeO3 and La2CuO4 perovskite nanoparticles. J Mater Sci-Mater Electron 2024, 35, 10. [Google Scholar]
- Hamdani, I.R.; Bhaskarwar, A.N. Recent progress in material selection and device designs for photoelectrochemical water-splitting. Renew. Sust. Energ. Rev. 2021, 138, 20. [Google Scholar] [CrossRef]
- Aslam, S.; Awais, M.; Ahmed, S.; Safdar, M.; Buksh, A.A.; Haroone, M.S. Photoelectrochemical Water Splitting by Using Nanomaterials: A Review. J. Electron. Mater. 2024, 53, 1–15. [Google Scholar] [CrossRef]
- Wu, W.H.; Nie, Z.F.; Huang, P.A.; Zhang, M.M.; Zhang, S.N.; Zhu, X.R.; Wang, L.J.; Zhu, L.P. Enhanced photoelectrochemical performance of BiVO<sub>4</sub> nanoparticle-modified TiO<sub>2</sub> nanorod arrays. Surf. Interfaces 2024, 55, 11. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, Z.H.; Gu, Z.X.; Li, Z.; Su, H.N.; Shen, X.Z.; Xu, Q. Construction of Fe<sub>2</sub>O<sub>3</sub>-CuO Heterojunction Photoelectrode for Enhanced Efficiency of Solar Redox Flow Batteries. Processes 2024, 12, 13. [Google Scholar] [CrossRef]
- Dong, F.L.; Zhang, P.F.; Cheng, J.Y.; Chen, J.N.; Liu, T.; Ma, X.Y.; Song, S.; Nie, S.X. Triboelectric-photocatalytic coupling enhanced photogenerated electrons and holes utilization for efficient algal inactivation. Nano Energy 2023, 118, 10. [Google Scholar] [CrossRef]
- Abdullah, A.; Tariq, F.; Kulkarni, M.A.; Thaalbi, H.; Din, H.U.; Kang, S.H.; Ha, J.S.; Ryu, S.W. Cost-effective and efficient CuS/GaN/n-Si photoanode engineered for water splitting through extended light absorption and optimized charge transport. Mater. Today Energy 2025, 49, 9. [Google Scholar] [CrossRef]
- Cao, Y.F.; Liu, D.Y.; Ni, X.; Meng, X.R.; Zhou, Y.; Sun, Z.F.; Kuang, Y.B. Better Charge Separation in CuO Nanowire Array Photocathodes: Micro-/Nanostructure Regulation for Photoelectrochemical Reaction. ACS Appl. Energ. Mater. 2020, 3, 6334–6343. [Google Scholar] [CrossRef]
- Yang, H.M.; Xu, M.L.; Li, Z.L.; Ge, S.G.; Zhang, L.N.; Zhu, P.H.; Yu, J.H. Dual-photocathode array propelled lab-on-paper ratiometric photoelectrochemical sensing platform for ultrasensitive microRNA bioassay. Sens. Actuator B-Chem. 2020, 316, 9. [Google Scholar] [CrossRef]
- Garvey, S.; Holmes, J.D.; Kim, Y.S.; Long, B. Vapor-Phase Passivation of Chlorine-Terminated Ge(100) Using Self-Assembled Monolayers of Hexanethiol. ACS Appl. Mater. Interfaces 2020, 12, 29899–29907. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.M.; Plis, E.; Amtout, A.; Bhattacharya, P.; Krishna, S. Investigation of surface passivation in InAs/GaSb strained-layer-superlattices using picosecond excitation correlation measurement and variable-area diode array surface recombination velocity measurement. In Proceedings of the Symposium on Progress in Semiconductor Materials V held at the 2005 MRS Fall Meeting, Boston, MA, 2005, Nov 28-Dec 01; p. 37.
- Cao, S.Y.; Zhang, Z.; Liao, Q.L.; Kang, Z.; Zhang, Y. Interface Engineering for High-Performance Photoelectrochemical Cells via Atomic Layer Deposition Technique. Energy Technol. 2021, 9, 14. [Google Scholar] [CrossRef]
- Liu, J.B.; Zhang, H.; Navarro-Pardo, F.; Selopal, G.S.; Sun, S.H.; Wang, Z.M.M.; Zhao, H.G.; Rosei, F. Hybrid surface passivation of PbS/CdS quantum dots for efficient photoelectrochemical hydrogen generation. Appl. Surf. Sci. 2020, 530, 9. [Google Scholar] [CrossRef]
- Zhao, Z.R.; Wang, M.Y.; Zheng, S.Y.; Zhang, Q.Q.; Han, L.; Li, X.B. Synergistic enhancement of photoelectrocatalytic activity and photostability in CdS photoanodes by ultrathin polydopamine layer. Sci. Technol. Energ. Transit 2024, 79, 9. [Google Scholar] [CrossRef]
- Xu, D.D.; Fu, Z.W.; Wang, D.J.; Lin, Y.H.; Sun, Y.J.; Meng, D.D.; Xie, T.F. A Ni(OH)<sub>2</sub>-modified Ti-doped α-Fe<sub>2</sub>O<sub>3</sub> photoanode for improved photoelectrochemical oxidation of urea: the role of Ni(OH)<sub>2</sub> as a cocatalyst. Phys. Chem. Chem. Phys. 2015, 17, 23924–23930. [Google Scholar] [CrossRef]
- Chen, D.S.; Duan, M.N.; Wang, M.; Ma, W.; Zhang, X.L.; Wu, X.F. Nanostructured Fe<sub>2</sub>TiO<sub>5</sub> photoanode with enhanced photoelectrochemical water splitting performance by Zn<SUP>2+</SUP> doping and FeNi(OH)<sub><i>x</i></sub> cocatalyst deposition. Crystengcomm 2024, 26, 5820–5825. [Google Scholar] [CrossRef]
- Sun, Q.; Cheng, T.; Liu, Z.R.; Qi, L.M. A cobalt silicate modified BiVO<sub>4</sub> photoanode for efficient solar water oxidation. Appl. Catal. B-Environ. 2020, 277, 9. [Google Scholar] [CrossRef]
- Andrei, F.; Ion, V.; Bîrjega, R.; Dinescu, M.; Enea, N.; Pantelica, D.; Mihai, M.D.; Maraloiu, V.A.; Teodorescu, V.S.; Marcu, I.C.; et al. Thickness-Dependent Photoelectrochemical Water Splitting Properties of Self-Assembled Nanostructured LaFeO<sub>3</sub> Perovskite Thin Films. Nanomaterials 2021, 11, 17. [Google Scholar] [CrossRef]
- Chertkova, V.P.; Iskortseva, A.N.; Pazhetnov, E.M.; Arkharova, N.A.; Ryazantsev, S.V.; Levin, E.E.; Nikitina, V.A. Evaluation of the Efficiency of Photoelectrochemical Activity Enhancement for the Nanostructured LaFeO<sub>3</sub> Photocathode by Surface Passivation and Co-Catalyst Deposition. Nanomaterials 2022, 12, 16. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Palma, V. Enhanced photocatalytic hydrogen production from glucose aqueous matrices on Ru-doped LaFeO<sub>3</sub>. Appl. Catal. B-Environ. 2017, 207, 182–194. [Google Scholar] [CrossRef]
- Aissa, B.; Ali, A. Piezo inkjet formation of Ag nanoparticles from microdots arrays for surface plasmonic resonance. Sci Rep 2024, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Wang, J.P.; Zhou, Y.; Chen, Y.; Xia, L.Y. Photoelectrochemical biosensor with Au@PTCA Schottky junction and multiple sandwich structures for Hg2+sensitive detection. Microchem J. 2024, 196, 10. [Google Scholar] [CrossRef]
- Michalska, M.; Matejka, V.; Pavlovsky, J.; Praus, P.; Ritz, M.; Serencísová, J.; Gembalová, L.; Kormunda, M.; Foniok, K.; Reli, M.; et al. Effect of Ag modification on TiO<sub>2</sub> and melem/g-C<sub>3</sub>N<sub>4</sub> composite on photocatalytic performances. Sci Rep 2023, 13, 20. [Google Scholar] [CrossRef]
- Pleshanov, I.M.; Marasanov, D.; Sgibnev, Y.M.; Gets, D.S.; Kuzmenko, N.K.; Belorus, A.O. Influence of silver nanoparticles in the ion-exchange layer of photo-thermo-refractive porous glass on the spectral-luminescent properties of CsPbBr3 perovskite nanocrystals. Chem. Phys. Lett. 2023, 823, 6. [Google Scholar] [CrossRef]
- Li, Y.M.; Fan, L.; Shui, X.L.; Fan, J.; Feng, X.A.; Tao, T. Boosted photocatalytic activity of LaFeO<sub>3</sub>/Ag<sub>3</sub>PO<sub>4</sub> heterojunction via carbon quantum dots: Higher conductivity, stability, and dispersivity br. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 652, 12. [Google Scholar] [CrossRef]
- Jia, D.S.; Pan, J.X.; Zhang, D.L.; Wang, M.T.; Li, Y. Construction of novel spherical ZnIn<sub>2</sub>S<sub>4</sub>-Ag-LaFeO<sub>3</sub> heterostructures for enhancing photocatalytic efficiency. J. Rare Earths 2025, 43, 295–303. [Google Scholar] [CrossRef]
- Humayun, M.; Bahadur, A.; Khan, A.; Bououdina, M. Exceptional Photocatalytic Performance of the LaFeO<sub>3</sub>/g-C<sub>3</sub>N<sub>4</sub> Z-Scheme Heterojunction for Water Splitting and Organic Dyes Degradation. Catalysts 2023, 13, 14. [Google Scholar] [CrossRef]
- Abbas, N.H.; Rasuli, R.; Panahi, P.N. Decorated titanium oxide with Ag nanoparticles as an efficient photocatalyst under visible light: a novel synthesis approach. Sci Rep 2025, 15, 17. [Google Scholar] [CrossRef]
- Sekrafi, H.E.; Costa, D.S.; Proença, M.; Meira, D.I.; Vaz, F.; Borges, J. Experimental and Theoretical Studies on Ag Nanoparticles with Enhanced Plasmonic Response, Formed Within Al<sub>2</sub>O<sub>3</sub> Thin Films Deposited by Magnetron Sputtering. Plasmonics 2024, 19, 3177–3188. [Google Scholar] [CrossRef]
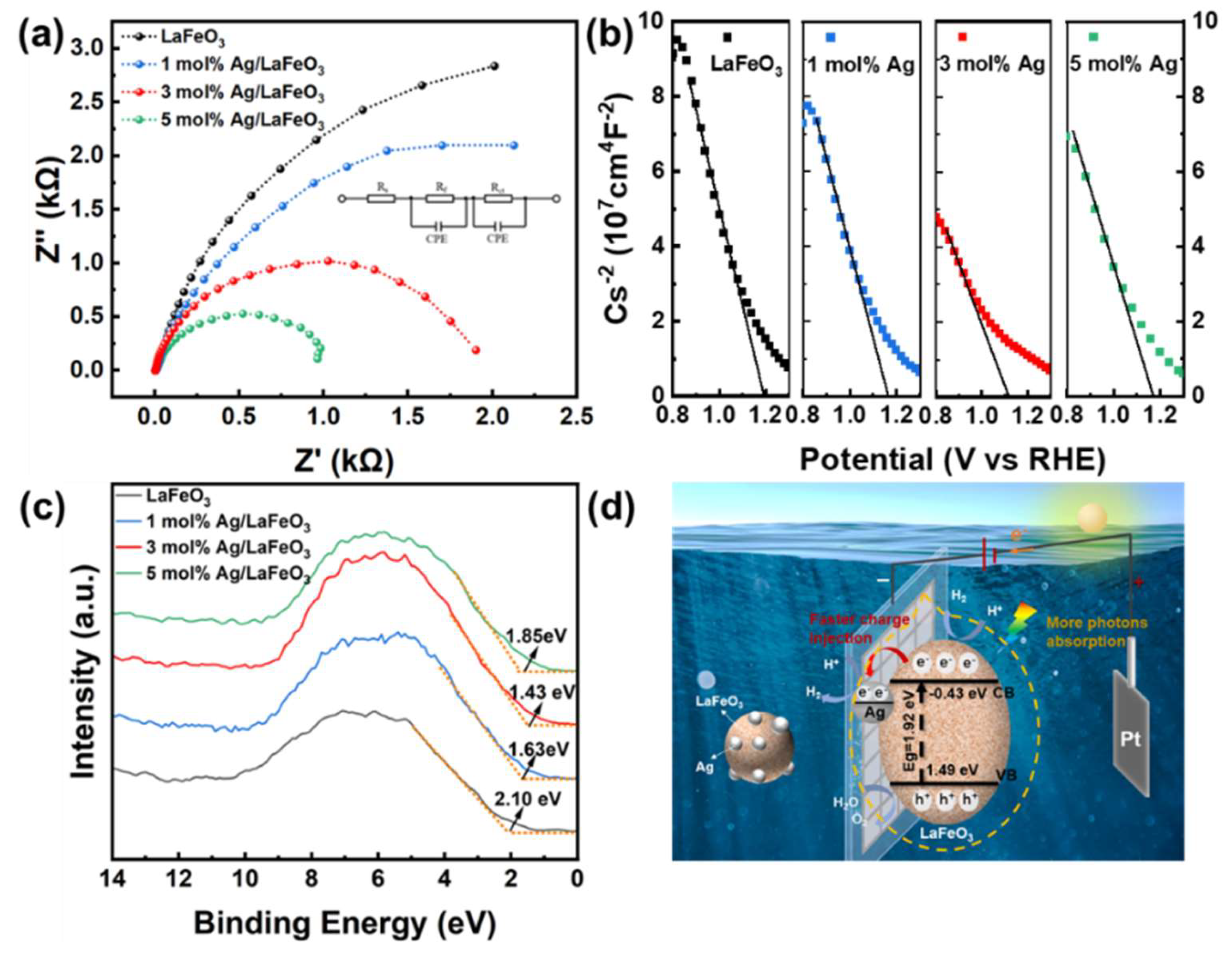
| Materials | Rs (Ω) | Rf (Ω) | CPE1-T (Ω) | CPE1-P (Ω) | Rct (Ω) | CPE2-T (Ω) | CPE2-P (Ω) |
|---|---|---|---|---|---|---|---|
| LaFeO3 | 0.62 | 3940 | 0.55×10-2 | 1.15 | 3390 | 6.44×10-3 | 1.15 |
| 1% Ag/LaFeO3 | 0.35 | 2865 | 0.55×10-2 | 1.27 | 1115 | 6.28×10-3 | 1.27 |
| 3% Ag/LaFeO3 | 0.47 | 1959 | 0.47×10-2 | 1.05 | 984 | 5.87×10-3 | 1.05 |
| 5% Ag/LaFeO3 | 0.58 | 889 | 0.43×10-2 | 1.29 | 345 | 5.73×10-3 | 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





