Submitted:
10 April 2025
Posted:
16 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Reverse Transcription-Polymerase Chain Reaction
2.3. Clinical Assessment
2.4. Statistical Analysis
3. Results
3.1. Associations between CD133 Expression and Clinicopathologic Features
3.2. Association between CD133 Expression in CTCs and Survival Rate
3.3. Multivariate Cox Analysis for DSS, Incorporating CD133 Expression
3.4. Association between CEA Levels and Survival Rates
3.5. Multivariate Cox Analysis for DSS, Incorporating CEA Levels
3.6. Impact of Combined CD133 Expression and CEA Levels on DSS in Stage I–III Cases
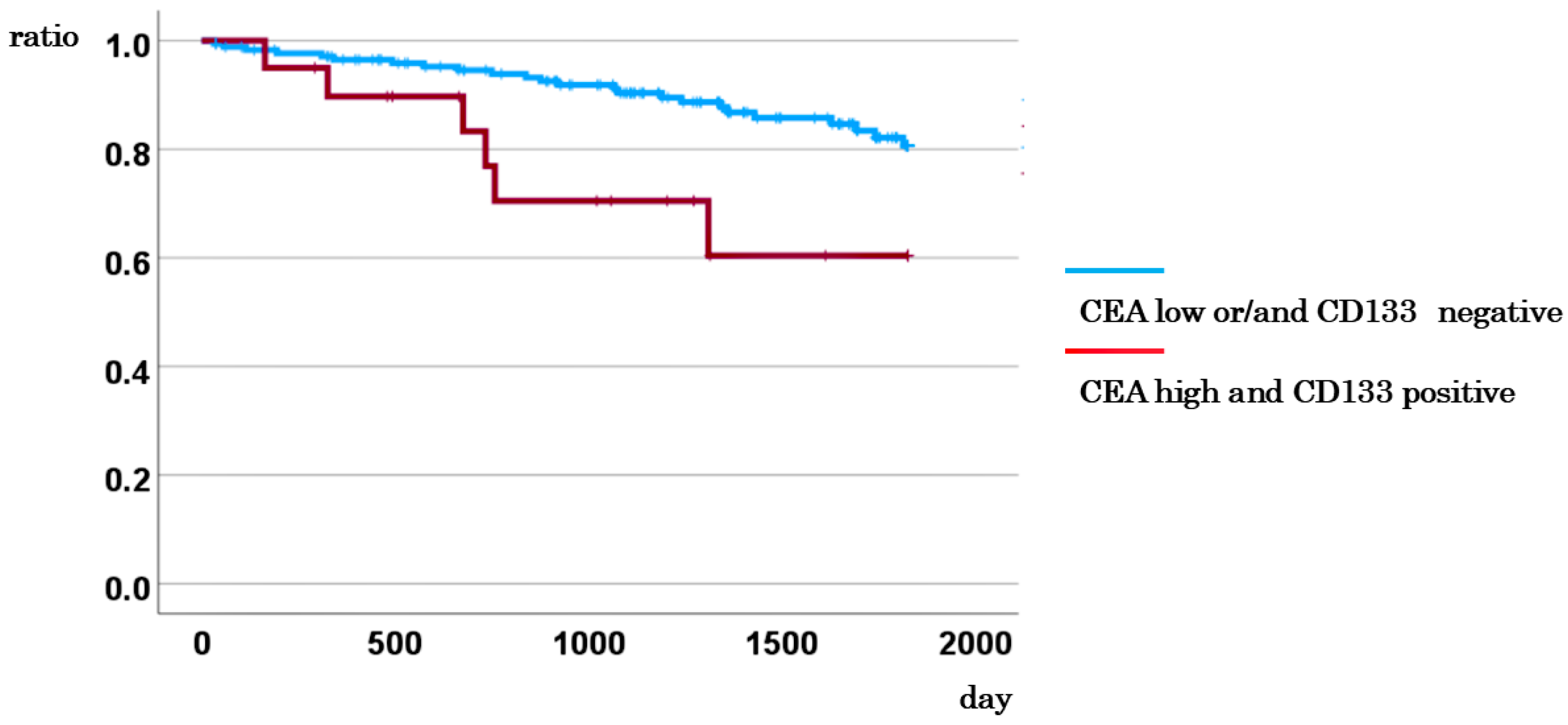
3.7. Comparison of DSS between Cases with CD133-Positive CTCs and High CEA Levels and Other Cases
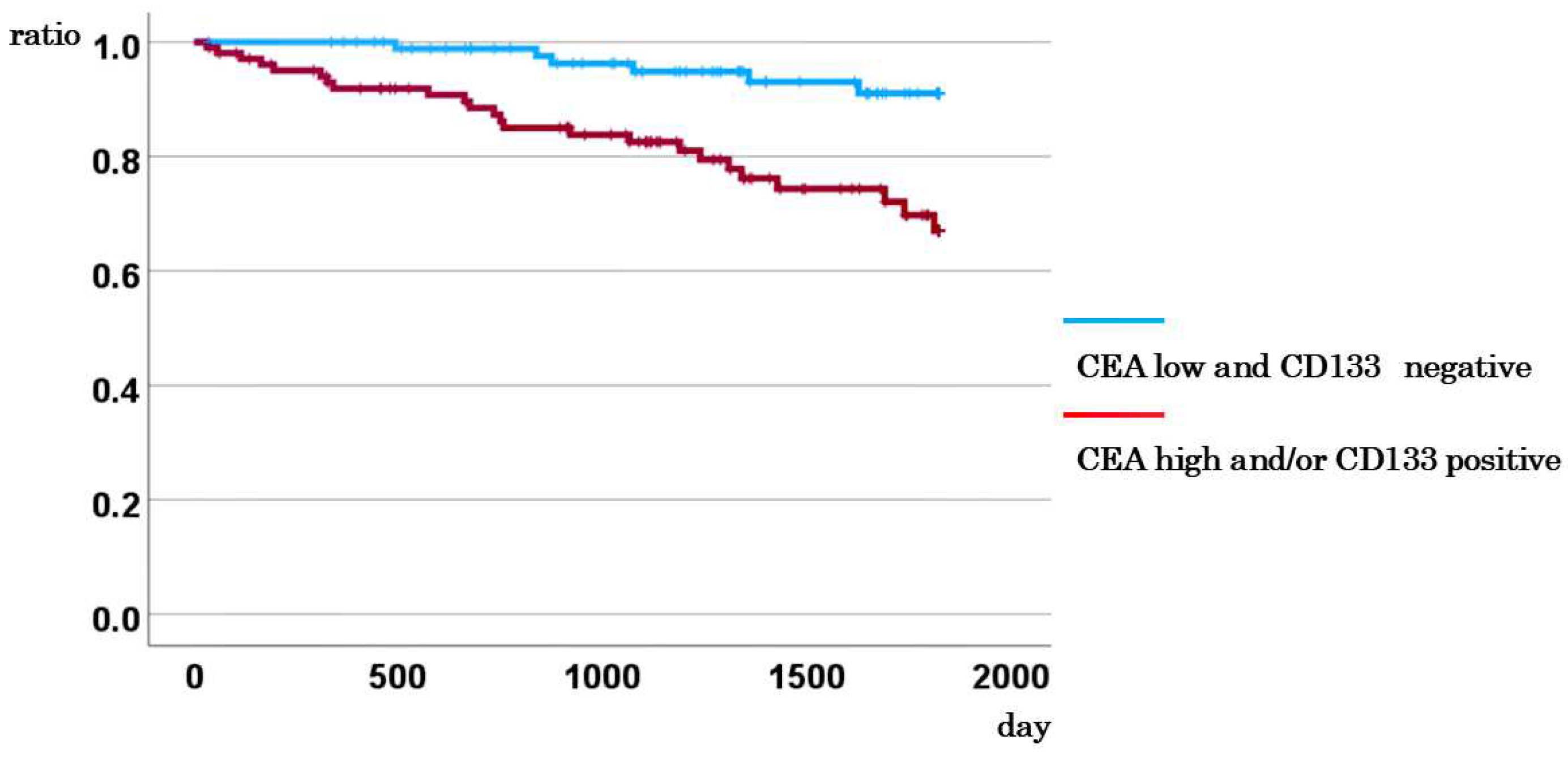
3.8. Comparison of DSS between Cases with CD133-Negative CTCs and Low CEA, and Other Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Iinuma, H.; Watanabe, T.; Mimori, K.; Adachi, M.; Hayashi, N.; Tamura, J.; Matsuda, K.; Fukushima, R.; Okinaga, K.; Sasako, M.; et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with dukes’ stage b and c colorectal cancer. J. Clin. Oncol. 2011, 29, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; van Dalen, A.; Haglund, C.; Hansson, L.; Holinski-Feder, E.; Klapdor, R.; Lamerz, R.; Peltomaki, P.; Sturgeon, C.; Topolcan, O. Tumour markers in colorectal cancer: European group on tumour markers (egtm) guidelines for clinical use. Eur. J. Cancer 2007, 43, 1348–1360. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C. Asco 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon cancer, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Ward, U.; Primrose, J.N.; Finan, P.J.; Perren, T.J.; Selby, P.; Purves, D.A.; Cooper, E.H. The use of tumour markers cea, ca-195 and ca-242 in evaluating the response to chemotherapy in patients with advanced colorectal cancer. Br. J. Cancer 1993, 67, 1132–1135. [Google Scholar] [CrossRef]
- Preketes, A.P.; King, J.; Caplehorn, J.R.M.; Clingan, P.R.; Ross, W.B.; Morris, D.L. Cea reduction after cryotherapy for liver metastases from colon-cancer predicts survival. Aust. N. Z. J. Surg. 1994, 64, 612–614. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the united states, National Cancer Institute of canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Kim, G.; Jung, E.J.; Ryu, C.G.; Hwang, D.Y. Usefulness of carcinoembryonic antigen for monitoring tumor progression during palliative chemotherapy in metastatic colorectal cancer. Yonsei Med. J. 2013, 54, 116–122. [Google Scholar] [CrossRef]
- Hisae Iinuma, T.W.; Mimori, K.; Adachi, M.; Hayashi, N.; Tamura, J.; Matsuda, R.F.K.; Okinaga, K.; Sasako, M.; Mori, M. Clinical Significance of Circulating Tumor Cells, Including Cancer Stem-Like Cells, in Peripheral Blood for Recurrence and Prognosis in Patients with Dukes’ Stage b and c Colorectal Cancer.
- Kim, I.H.; Lee, J.E.; Yang, J.H.; Jeong, J.W.; Ro, S.; Oh, S.T.; Kim, J.G.; Choi, M.H.; Lee, M.A. Clinical significance of discordance between carcinoembryonic antigen levels and recist in metastatic colorectal cancer. Cancer Res. Treat. 2018, 50, 283–292. [Google Scholar] [CrossRef]
- de Haas, R.J.; Wicherts, D.A.; Flores, E.; Ducreux, M.; Lévi, F.; Paule, B.; Azoulay, D.; Castaing, D.; Lemoine, A.; Adam, R. Tumor marker evolution: Comparison with imaging for assessment of response to chemotherapy in patients with colorectal liver metastases. Ann. Surg. Oncol. 2010, 17, 1010–1023. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Aggarwal, C. Relationship among circulating tumor cells, cea and overall survival in patients with metastatic colorectal cancer. Ann. Oncol. 2013, 24, 2708–2710. [Google Scholar] [CrossRef] [PubMed]
- Bork, U.; Rahbari, N.N.; Schölch, S.; Reissfelder, C.; Kahlert, C.; Büchler, M.W.; Weitz, J.; Koch, M. Circulating tumour cells and outcome in non-metastatic colorectal cancer: A prospective study. Br. J. Cancer 2015, 112, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E.; Miller, M.C.; et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann. Oncol. 2009, 20, 1223–1229. [Google Scholar] [CrossRef]
- Netterberg, I.; Karlsson, M.O.; Terstappen, L.W.M.M.; Koopman, M.; Punt, C.J.A.; Friberg, L.E. Comparing circulating tumor cell counts with dynamic tumor size changes as predictor of overall survival: A quantitative modeling framework. Clin. Cancer Res. 2020, 26, 4892–4900. [Google Scholar] [CrossRef]
- Weiss, L. Metastatic inefficiency. Adv. Cancer Res. 1990, 54, 159–211. [Google Scholar] [CrossRef]
- Sipos, F.; Műzes, G. Interconnection of CD133 stem cell marker with autophagy and apoptosis in colorectal cancer. Int. J. Mol. Sci. 2024, 25, 11201. [Google Scholar] [CrossRef]
- Zhou, F.; Mu, Y.D.; Liang, J.; Liu, Z.X.; Chen, H.S.; Zhang, J.F. Expression and prognostic value of tumor stem cell markers aldh1 and CD133 in colorectal carcinoma. Oncol. Lett. 2014, 7, 507–512. [Google Scholar] [CrossRef]
- Abdul Khalek, F.J.; Gallicano, G.I.; Mishra, L. Colon cancer stem cells. Gastrointest. Cancer Res. 2010, (Supplement 1), S16–23. [Google Scholar]
- Dalerba, P.; Dylla, S.J.; Park, I.K.; Liu, R.; Wang, X.H.; Cho, R.W.; Hoey, T.; Gurney, A.; Huang, E.H.; Simeone, D.M.; et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. U S A 2007, 104, 10158–10163. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611-U119. [Google Scholar] [CrossRef]
- Kazama, S.; Kishikawa, J.; Kiyomatsu, T.; Kawai, K.; Nozawa, H.; Ishihara, S.; Watanabe, T. Expression of the stem cell marker CD133 is related to tumor development in colorectal carcinogenesis. Asian J. Surg. 2018, 41, 274–278. [Google Scholar] [CrossRef]
- Corbeil, D.; Röper, K.; Hellwig, A.; Tavian, M.; Miraglia, S.; Watt, S.M.; Simmons, P.J.; Peault, B.; Buck, D.W.; Huttner, W.B. The human ac133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J. Biol. Chem. 2000, 275, 5512–5520. [Google Scholar] [CrossRef]
- Ren, F.; Sheng, W.Q.; Du, X. CD133: A cancer stem cells marker, is used in colorectal cancers. World J. Gastroenterol. 2013, 19, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ohuchida, K.; Mizumoto, K.; Moriyama, T.; Onimaru, M.; Nakata, K.; Nabae, T.; Ueki, T.; Sato, N.; Tominaga, Y.; et al. Prospectively isolated cancer-associated CD10(+) fibroblasts have stronger interactions with CD133(+) colon cancer cells than with CD133(-) cancer cells. PLoS ONE 2010, 5, e12121. [Google Scholar] [CrossRef]
- Chao, C.; Carmical, J.R.; Ives, K.L.; Wood, T.G.; Aronson, J.F.; Gomez, G.A.; Djukom, C.D.; Hellmich, M.R. CD133+ colon cancer cells are more interactive with the tumor microenvironment than CD133- cells. Lab. Invest. 2012, 92, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kawai, K.; Sonoda, H.; Shiratori, H.; Kishikawa, J.; Nagata, H.; Nozawa, H.; Sasaki, K.; Kaneko, M.; Murono, K.; et al. Epithelial-mesenchymal transition and metastatic ability of CD133+ colorectal cancer stem-like cells under hypoxia. Oncol. Lett. 2021, 21, 19. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Huang, R.; Mo, D.; Wu, J.; Ai, H.; Lu, Y. CD133 expression correlates with clinicopathologic features and poor prognosis of colorectal cancer patients an updated meta-analysis of 37 studies. Medicine 2018, 97, e10446. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Song, X.; Chen, Z.; Li, X.; Li, M.; Liu, H.; Li, J. CD133 expression and the prognosis of colorectal cancer: A systematic review and meta-analysis. PLoS ONE 2013, 8, e56380. [Google Scholar] [CrossRef]
- Wang, K.; Xu, J.; Zhang, J.; Huang, J. Prognostic role of CD133 expression in colorectal cancer: A meta-analysis. BMC Cancer 2012, 12, 573. [Google Scholar] [CrossRef]
- Akbari, M.; Shanehbandi, D.; Asadi, M.; Shomali, N.; Faraji, A.; Khaze, V.; Pakdel, A.; Mokhtarzadeh, A.; Ebrahimi, A.A.; Shabani, A.; et al. Effects of CD133 silencing on survival and migration of ht-29 colorectal cancer cells. Iran. J. Immunol. 2019, 16, 246–257. [Google Scholar] [CrossRef]
- Masuda, T.; Hayashi, N.; Iguchi, T.; Ito, S.; Eguchi, H.; Mimori, K. Clinical and biological significance of circulating tumor cells in cancer. Mol. Oncol. 2016, 10, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Del Fiore, P.; Guarnieri, L.; Scalerta, R.; Foletto, M.; Chiarion, V.; Pilati, P.; Nitti, D.; Lise, M.; Rossi, C.R. Molecular detection of circulating tumor cells is an independent prognostic factor in patients with high-risk cutaneous melanoma. Int. J. Cancer 2004, 111, 741–745. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer New York: New York, NY, 2000. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Siskind, V. Quantification of completeness of follow-up. In Lancet 2002, 360, 724. [Google Scholar] [CrossRef]
- Harel, O.; Zhou, X.H. Multiple imputation: Review of theory, implementation and software. Stat. Med. 2007, 26, 3057–3077. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, M.; Pruvot, F.R.; Romano, O.; Triboulet, J.P.; de Gramont, A. Integration of neoadjuvant and adjuvant chemotherapy in patients with resectable liver metastases from colorectal cancer. Cancer Treat. Rev. 2009, 35, 668–675. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsieh, J.C.H.; Wu, T.M.H.; Yeh, T.S.; Wang, H.M.; Lin, Y.C.; Chen, J.S.; Lee, C.L.; Huang, W.K.; Hung, T.M.; et al. Baseline circulating stem-like cells predict survival in patients with metastatic breast cancer. BMC Cancer 2019, 19, 1167. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Wang, Q.; Chang, K.; Zhang, J.; Ye, D.; Kong, Y.; Dai, B. Presence of CD133-positive circulating tumor cells predicts worse progression-free survival in patients with metastatic castration-sensitive prostate cancer. Int. J. Urol. 2022, 29, 383–389. [Google Scholar] [CrossRef]
- Sawai, K.; Goi, T.; Kimura, Y.; Koneri, K. Presence of cd44v9-expressing cancer stem cells in circulating tumor cells and effects of carcinoembryonic antigen levels on the prognosis of colorectal cancer. Cancers 2024, 16, 1556. [Google Scholar] [CrossRef]
- Pennacchietti, S.; Michieli, P.; Galluzzo, M.; Mazzone, M.; Giordano, S.; Comoglio, P.M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003, 3, 347–361. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting hif-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Yun, Z.; Lin, Q. Hypoxia and regulation of cancer cell stemness. Adv. Exp. Med. Biol. 2014, 772, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Oshi, M.; Endo, I.; Takabe, K. Clinical relevance of stem cell surface markers CD133, CD24, and CD44 in colorectal cancer. Am. J. Cancer Res. 2021, 11, 5141–5154. [Google Scholar] [PubMed]
- Kojima, M.; Ishii, G.; Atsumi, N.; Fujii, S.; Saito, N.; Ochiai, A. Immunohistochemical detection of CD133 expression in colorectal cancer: A clinicopathological study. Cancer Sci. 2008, 99, 1578–1583. [Google Scholar] [CrossRef]
- Jao, S.W.; Chen, S.F.; Lin, Y.S.; Chang, Y.C.; Lee, T.Y.; Wu, C.C.; Jin, J.S.; Nieh, S. Cytoplasmic CD133 expression is a reliable prognostic indicator of tumor regression after neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Ann. Surg. Oncol. 2012, 19, 3432–3440. [Google Scholar] [CrossRef]
- Pilati, P.; Mocellin, S.; Bertazza, L.; Galdi, F.; Briarava, M.; Mammano, E.; Tessari, E.; Zavagno, G.; Nitti, D. Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Ann. Surg. Oncol. 2012, 19, 402–408. [Google Scholar] [CrossRef]
- Lin, E.H.; Hassan, M.; Li, Y.N.; Zhao, H.; Nooka, A.; Sorenson, E.; Xie, K.P.; Champlin, R.; Wu, X.F.; Li, D.H. Elevated circulating endothelial progenitor marker CD133 messenger RNA levels predict colon cancer recurrence. Cancer 2007, 110, 534–542. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, H.; Xiang, F.; Yang, X.; Li, R.; Zeng, Y.; Liu, Z. Correlation analysis of the expression of mesenchymal circulating tumor cells and CD133 with the prognosis of colorectal cancer. Am. J. Transl. Res. 2023, 15, 3489–3499. [Google Scholar]
- Konishi, T.; Shimada, Y.; Hsu, M.; Tufts, L.; Jimenez-Rodriguez, R.; Cercek, A.; Yaeger, R.; Saltz, L.; Smith, J.J.; Nash, G.M.; et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018, 4, 309–315. [Google Scholar] [CrossRef]
- Chu, H.Y.; Lu, L.S.; Cho, W.; Wu, S.Y.; Chang, Y.C.; Lin, C.P.; Yang, C.Y.; Lin, C.H.; Jiang, J.K.; Tseng, F.G. Enumerating circulating tumor cells with a self-assembled cell array (saca) chip: A feasibility study in patients with colorectal cancer. Cancers (Basel) 2019, 11, 56. [Google Scholar] [CrossRef]
- Zahran, A.M.; Rayan, A.; Fakhry, H.; Attia, A.M.; Ashmawy, A.M.; Soliman, A.; Elkady, A.; Hetta, H.F. Pretreatment detection of circulating and tissue CD133(+) CD44(+) cancer stem cells as a prognostic factor affecting the outcomes in Egyptian patients with colorectal cancer. Cancer Manag. Res. 2019, 11, 1237–1248. [Google Scholar] [CrossRef]
- Fang, C.; Fan, C.W.; Wang, C.; Huang, Q.R.; Meng, W.T.; Yu, Y.Y.; Yang, L.; Hu, J.K.; Li, Y.; Mo, X.M.; et al. Prognostic value of CD133+cd54+cd44+ circulating tumor cells in colorectal cancer with liver metastasis. Cancer Med. 2017, 6, 2850–2857. [Google Scholar] [CrossRef]
- Wu, X.S.; Xi, H.Q.; Chen, L. Lgr5 is a potential marker of colorectal carcinoma stem cells that correlates with patient survival. World J. Surg. Oncol. 2012, 10, 244. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Li, W.M.; Ji, Y.Q.; Cao, H.Z.; Zheng, P. Prognostic value of lgr5 in colorectal cancer: A meta-analysis. PLoS ONE 2014, 9, e107013. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xue, X.; Jiang, M.; Guo, X.; Li, P.; Liu, F.; Yuan, B.; Shen, Y.; Guo, X.; Zhi, Q.; et al. Lgr5, a relevant marker of cancer stem cells, indicates a poor prognosis in colorectal cancer patients: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hongo, K.; Kazama, S.; Sunami, E.; Tsuno, N.H.; Takahashi, K.; Nagawa, H.; Kitayama, J. Immunohistochemical detection of CD133 is associated with tumor regression grade after chemoradiotherapy in rectal cancer. Med. Oncol. 2012, 29, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Ieta, K.; Tanaka, F.; Haraguchi, N.; Kita, Y.; Sakashita, H.; Mimori, K.; Matsumoto, T.; Inoue, H.; Kuwano, H.; Mori, M. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann. Surg. Oncol. 2008, 15, 638–648. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Yuan, J.; Xiao, X.; Tang, N.; Hao, J.; Wang, H.; Bian, X.; Deng, Y.; Ding, Y. CD133(+) single cell-derived progenies of colorectal cancer cell line sw480 with different invasive and metastatic potential. Clin. Exp. Metastasis 2010, 27, 517–527. [Google Scholar] [CrossRef]
- Gao, W.; Chen, L.; Ma, Z.; Du, Z.; Zhao, Z.; Hu, Z.; Li, Q. Isolation and phenotypic characterization of colorectal cancer stem cells with organ-specific metastatic potential. Gastroenterology 2013, 145, 636–46.e5. [Google Scholar] [CrossRef]
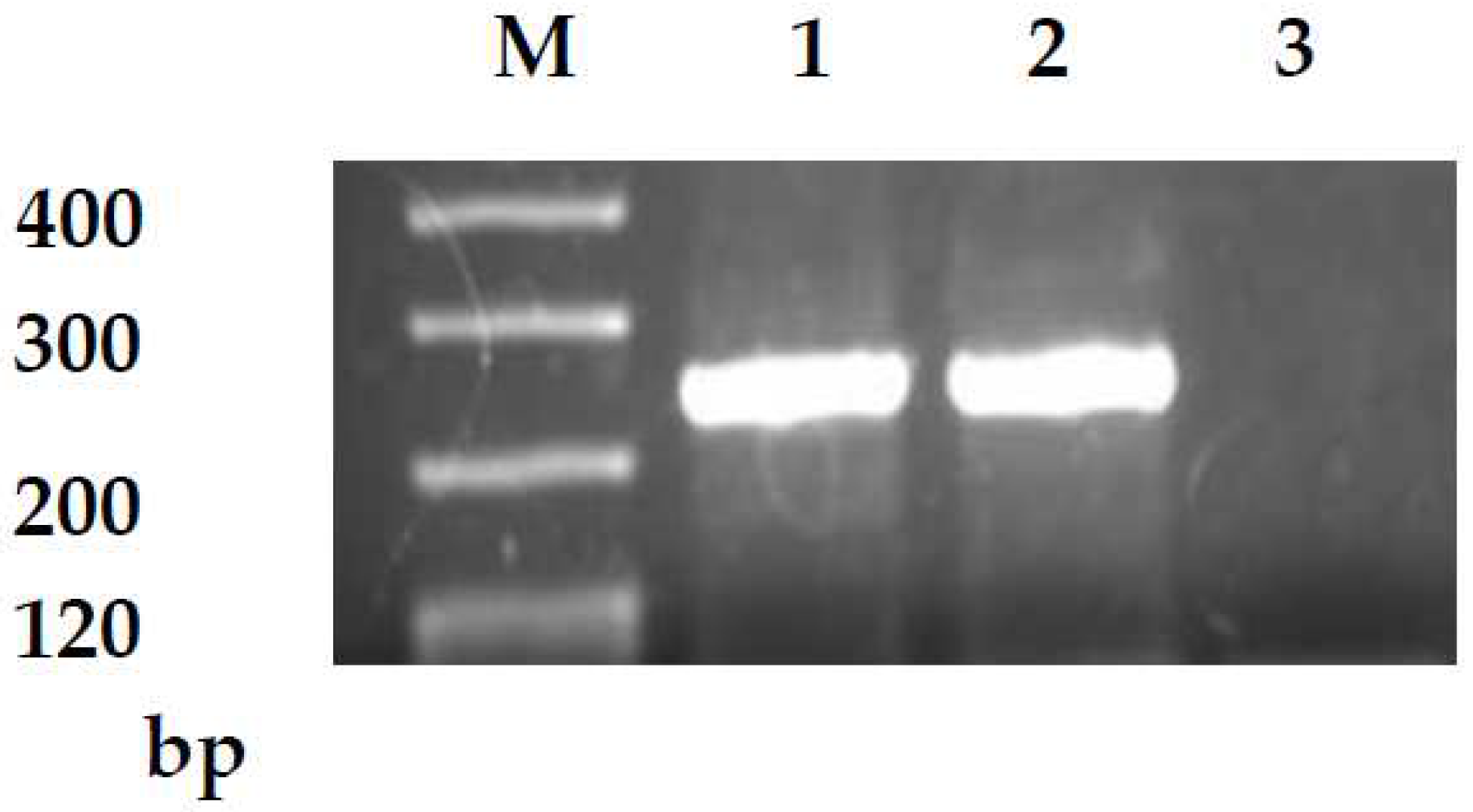
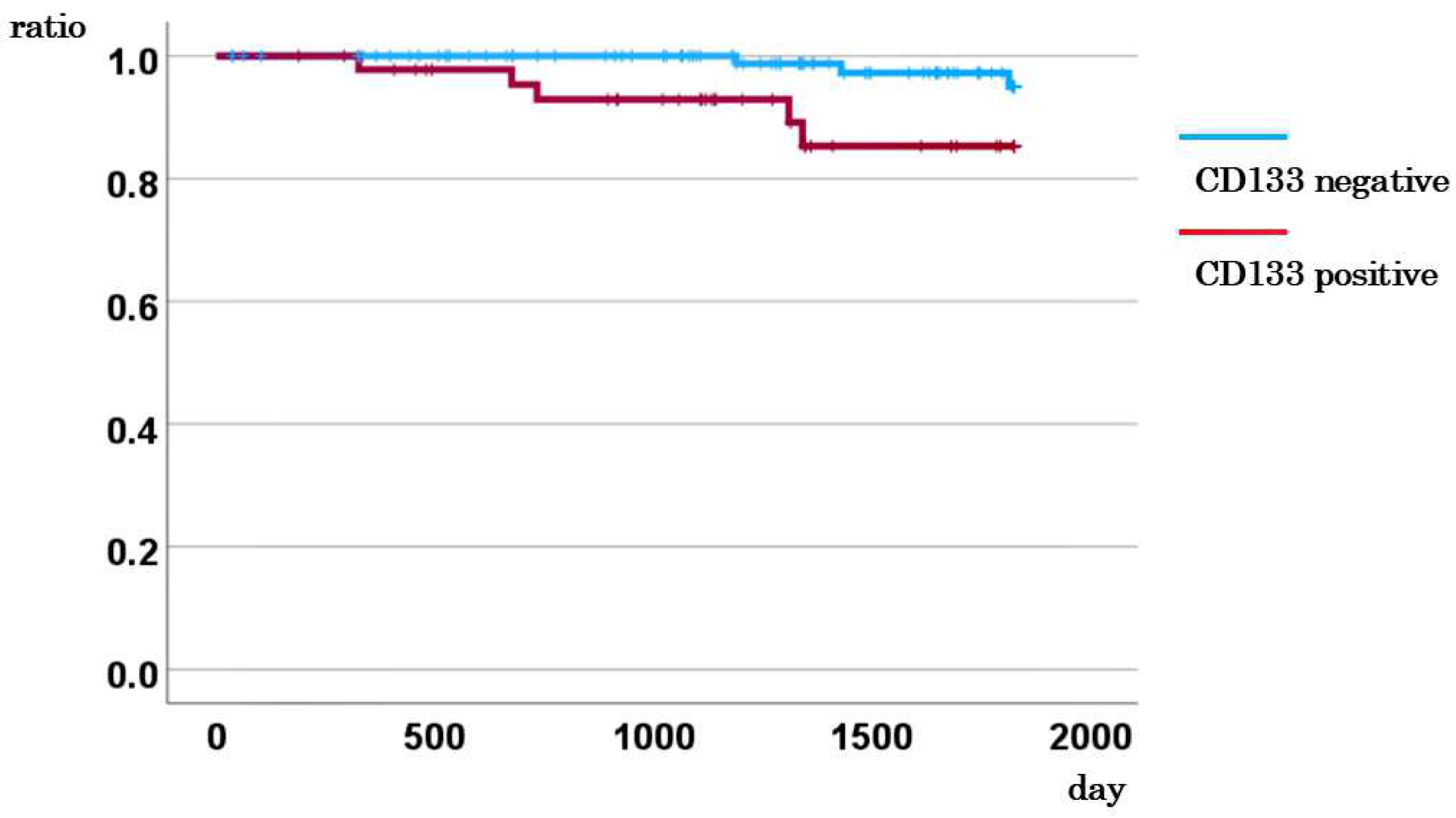
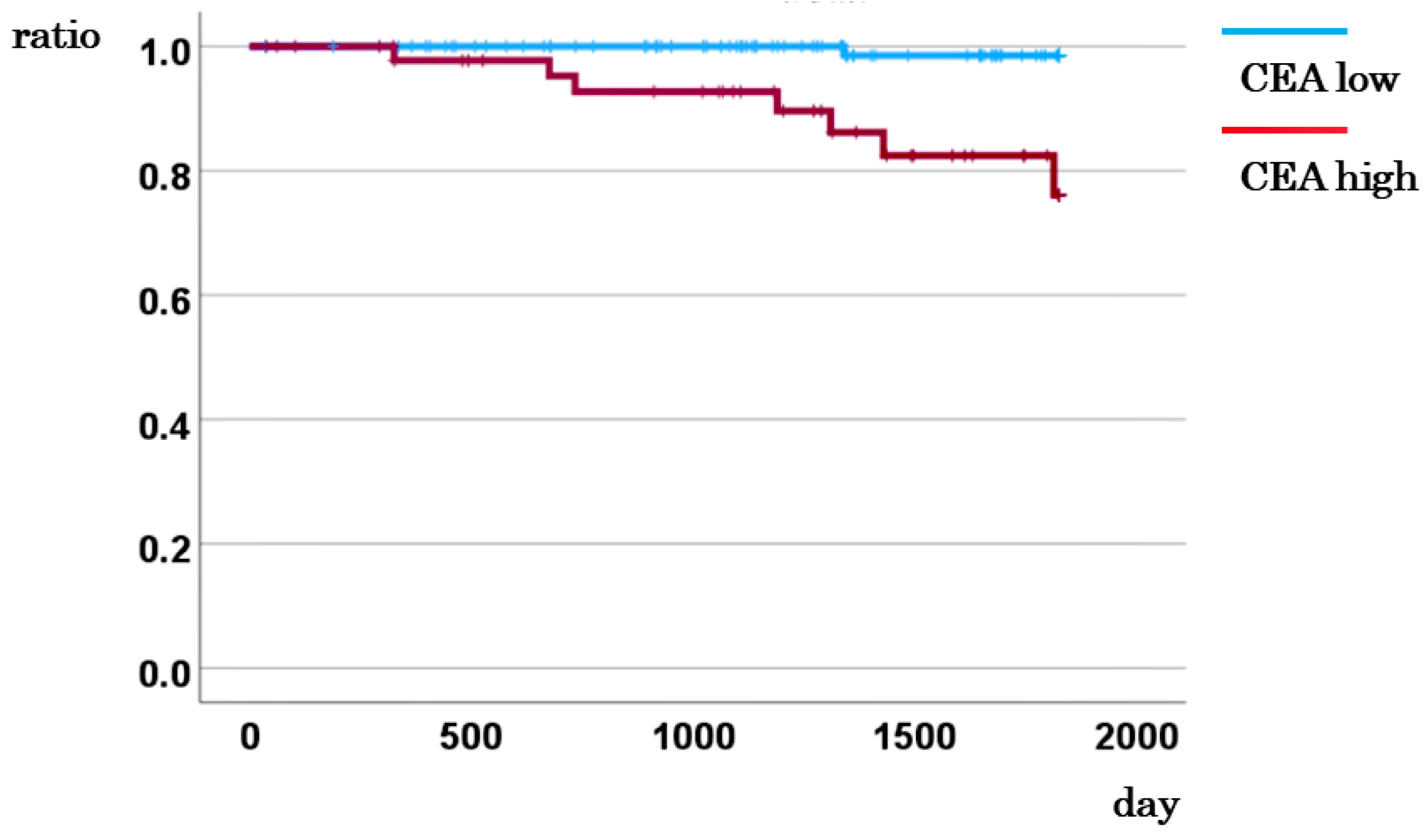
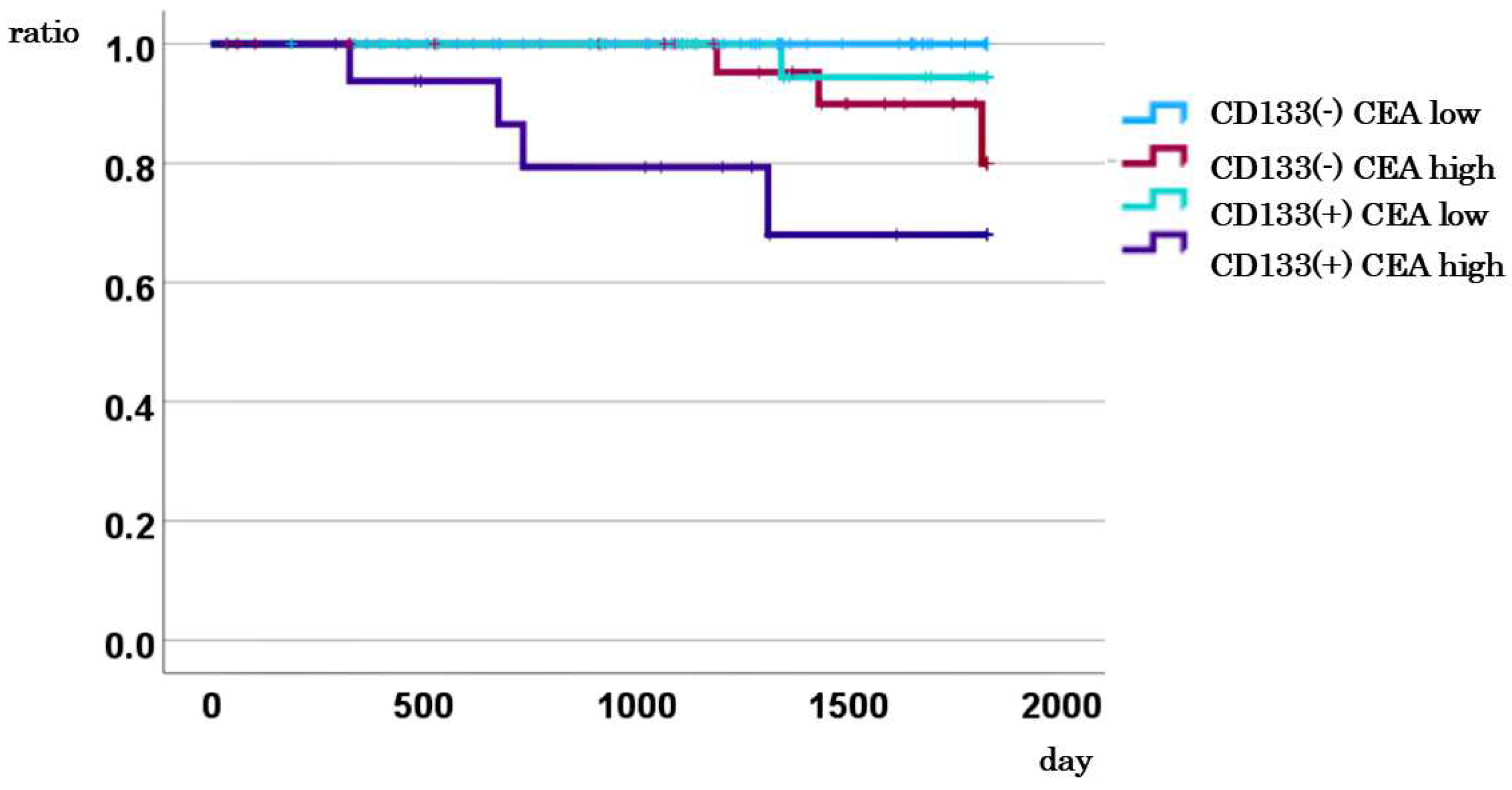
| CD133 mRNA | |||||
| No. of cases | Negative cases (%) | Positive cases (%) | P-value | ||
| All cases (%) | 195 | 142 (72.8) | 53 (27.2) | ||
| Age (median years) | 71.0 | 70.0 | 0.900 | ||
| Sex | Male | 105 | 76 (72.4) | 29 (27.6) | 0.882 |
| Female | 90 | 66 (73.3) | 24 (26.7) | ||
| Location | Right colon | 74 | 53 (71.6) | 21 (28.4) | 0.769 |
| Left colon | 121 | 89 (73.6) | 32 (26.4) | ||
| Size (average mm) | < 50 | 113 | 88(77.9) | 25(22.1) | 0.062 |
| ≧50 | 82 | 54(65.9) | 28(34.1) | ||
| Histological type | Differentiated | 184 | 135 (73.4) | 49 (26.6) | 0.481 |
| Undifferentiated | 11 | 7 (63.6) | 4 (36.4) | ||
| Serosal involvement | Negative | 52 | 44 (84.6) | 8 (15.4) | 0.026 |
| Positive | 143 | 98 (68.5) | 45 (31.5) | ||
| Lymph node metastasis | Negative | 113 | 82 (72.6) | 31 (27.4) | 0.925 |
| Positive | 82 | 60 (73.2) | 22 (26.8) | ||
| Distant metastasis | Negative | 163 | 116 (71.2) | 47 (28.8) | 0.241 |
| Positive | 32 | 26 (81.2) | 6(18.8) | ||
| Stage | I, II | 108 | 78 (72.2) | 30 (27.8) | 0.834 |
| III, IV | 87 | 64 (73.6) | 23 (26.4) | ||
| CEA | 5.0> | 125 | 92 (73.6) | 33 (26.4) | 0.744 |
| 5.0≦ | 70 | 50 (71.4) | 20 (28.6) | ||
| Multivariate | ||||
| Variable | Odds ration | 95% CI | P-value | |
| Age | 0.997 | 0.967-1.028 | 0.847 | |
| Sex | Male vs Female | 0.961 | 0.493-1.874 | 0.907 |
| Serosal invasion | Negative vs Positive | 3.000 | 1.225-7.345 | 0.016 |
| Lymph node metastasis | Negative vs Positive | 0.850 | 0.412-1.754 | 0.660 |
| Distant metastasis | Negative vs Positive | 0.472 | 0.165-1.353 | 0.162 |
| CEA | 5.0> vs 5.0≦ | 1.074 | 0.532-2.167 | 0.842 |
| Multivariate | ||||
| Variable | HR | 95% CI | P-value | |
| Distant metastasis | Negative vs. Positive | 35.713 | 14.520-87.838 | < 0.001 |
| CD133 expression | Negative vs. Positive | 3.057 | 1.298-7.196 | 0.011 |
| Multivariate | ||||
| Variable | HR | 95% CI | P-value | |
| Distant metastasis | Negative vs. Positive | 19.715 | 8.296-46.853 | < 0.001 |
| CEA level | CEA< 5 vs. CEA≧5 | 2.309 | 1.017-5.244 | 0.046 |
| Multivariate | ||||
| Variable | HR | 95% CI | P-value | |
| Distant metastasis | Negative vs. Positive | 33.112 | 13.797-79.468 | < 0.001 |
| CD133 and CEA | Others vs. CD133 (positive) and CEA≧5 | 5.948 | 2.210-16.007 | <0.001 |
| Multivariate | ||||
| Variable | HR | 95% CI | P-value | |
| Distant metastasis | Negative vs. Positive | 22.126 | 9.549-51.266 | < 0.001 |
| CD133 and CEA | CD133 (negative) and CEA<5 vs. others | 2.600 | 1.049-6.441 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





