1. Introduction
Diabetic ketoacidosis (DKA) is a life-threatening hyperglycemic emergency, characterized by the triad of hyperglycemia, increased blood or urine ketone concentration and metabolic acidosis [
1,
2]. This pathology accounts for 14% of all hospital admissions and 16% of all the deaths in patients with known diabetes [
3]. Patients are at risk of developing DKA regardless of their diabetes mellitus (DM) type [
4], even though it is more frequent in patients with type 1 DM [
5].
Spontaneous pneumomediastinum (SPM) is a rare, usually benign, condition defined as the presence of free air within the mediastinum, in the absence of identifiable tracheobronchial trauma [
6,
7,
8,
9]. Pneumomediastinum has a reported prevalence of less than 1 out of 44,000 emergency department (ED) visits [
10], being more frequent in young males [
11]. Mortality associated with SPM is usually low and associated with the development of mediastinitis [
12]. The association between SPM and DKA has been previously documented in the literature [
13,
14,
15,
16,
17,
18,
19,
20], with the first mention being made by Hamman in 1939 [
20]. The largest study on DKA complicated with SPM was published by Zhang et al.[
16], encompassing 79 patients. Out of those, 5 patients associated epidural pneumatosis and 3 of them spontaneous pneumothorax (SP). The reported mortality was low, with the authors documenting it in only two cases. In another case series of 10 patients with SPM complicating DKA, described by Xu et al. [
18], 3 patients did not have a medical history of DM and no deaths were reported.
Acute pancreatitis (AP) represents the inflammation of the pancreas, with a potentially life-threating evolution [
21,
22]. The reported age standardized incidence rate of AP varies significantly across different regions, between 1990 and 2019 varying from 8.7 to 82 cases per 100,000 individuals (higher rates in Eastern Europe) [
23]. Mild cases have a low mortality rate of less than 1%, while severe acute pancreatitis can have mortality rates ranging from 10% to 30%, particularly in the presence of infected pancreatic necrosis or persistent organ failure [
24]. In a 6-year multicentric study, Khan et al. [
25] analysed the outcomes of the patients with DKA with coexistent AP. The reported incidence of AP was 9%. In this cohort, the association of DKA and AP significantly increased the median length of hospital admissions (2.4 vs 4.8 days) and the rate of ICU admission (23.1% vs 39.3%), with no remarkable effect on mortality, readmission and DKA recurrence.
This paper presents a rare and complex case of inaugural diabetic ketoacidosis complicated by spontaneous pneumomediastinum, spontaneous pneumothorax, and acute pancreatitis. To our knowledge, the simultaneous occurrence of all three complications in the context of DKA has not been previously reported. This case aims to raise awareness among clinicians about this unusual triad, emphasizing the importance of early recognition and appropriate management to avoid misdiagnosis and unnecessary interventions.
2. Case Presentation
A 60-year-old Caucasian woman with no documented pre-existing medical conditions, body mass index 25.1 kg/m2, presented to the emergency department (ED) with fatigue, nausea, a single episode of vomiting, loss of appetite, unintentional weight loss, upper-level abdominal pain and obtundation. Clinical examination revealed warm peripheries, confusion, and peri-umbilical and epigastric pain on superficial palpation. Vital signs on admission were: blood pressure of 140/95 mmHg, T=36.1oC, heart rate of 151 beats per minute and respiratory rate of 28 breaths per minute.
After ABCDE assessment, a localized area of subcutaneous crepitus was noted in the lateral cervical region, without history or clinical signs of trauma. Chest expansion was symmetrical bilaterally, pulmonary percussion demonstrated normal resonance, and auscultation revealed a normally transmitted vesicular breath sound without any rales. Peripheral oxygen saturation on room air was 93%. These findings raised concerns about a possible small SP, and a thoracic computed tomography (CT) scan was planned.
Two large-bore intravenous lines were placed, blood specimens were collected and fluid resuscitation was initiated. The abdomen exhibited respiratory mobility, with no postoperative scars, and was tender on deep palpation. The Glasgow coma scale was 13.
The initial investigations performed were the acid/base status, electrolyte and metabolites, which revealed the following findings: pH 7.06, pressure of O2 18.4 mmHg, pressure of CO2 28.2, base excess −19.5 mmol/L, bicarbonate (HCO₃⁻) 8.4 mmol/L, anion gap 33.1 mmol/L, blood glucose 559 mg/dL, lactate 2.44 mmol/L, sodium (Na+) 140.7 mmol/l, potassium (K+) 3.76 mmol/L, calcium (Ca2+) 1.29 mmol/l, chlorine (Cl-) 103 mmol/l. Based on these results, a diagnosis of diabetic ketoacidosis was considered the most probable A urine sample was collected after urinary catheterization was performed, and treatment for the suspected condition was initiated promptly, in accordance with the DKA guidelines. A twelve-lead electrocardiogram revealed sinus tachycardia with a ventricular rate of approximately 166 beats per minute, without evidence of ischemic changes.
The initial blood works (taken in the ED) and the following values taken on the ward are depicted in Table 1. The urine exam revealed glucosuria (>1000 mg/ml) and ketonuria (>160 mg/dl). Given the high values of lipase (increased by almost 10 times) accompanied by epigastric pain, acute pancreatitis was also suspected.
Table 1.
Serial relevant laboratory analysis of the patient.
Table 1.
Serial relevant laboratory analysis of the patient.
| Bloodwork |
Reference range and measure unit |
Initial |
7 days |
Discharge |
| White blood cells |
4-10 x 103/ µL |
29.55 |
23.35 |
7.26 |
| Neutrophiles |
2-7 x 103/ µL |
24.19 |
19.34 |
4.74 |
| Hemoglobin |
12-15 g/dl |
16.09 |
14.25 |
12.32 |
| Platelets |
150-410 x 103/ µL |
473.2 |
230.5 |
454 |
| Potassium |
3.5-5.1 mmol/L |
3.5 |
2.3 |
4.3 |
| Sodium |
136-145 mmol/L |
137 |
133 |
135 |
| Creatinine |
0.55-1.02 mg/dl |
1.58 |
0.6 |
0.6 |
| Urea |
15-39 mg/dl |
90 |
27 |
6 |
| Glycemia |
74-106 mg/dl |
569 |
390 |
110 |
| Lipase |
16-77 U/l |
687 |
421 |
213 |
| ALT |
14-59 U/l |
47 |
25 |
20 |
| AST |
15-37 U/l |
15 |
18 |
18 |
| Total Bilirubin |
0.2-1.00 mg/dl |
0.66 |
0.9 |
0.5 |
| C reactive protein |
0-5 mg/l |
34.4 |
73 |
34.3 |
| Procalcitonin |
0.1-0.25 ng/ml |
0.14 |
- |
- |
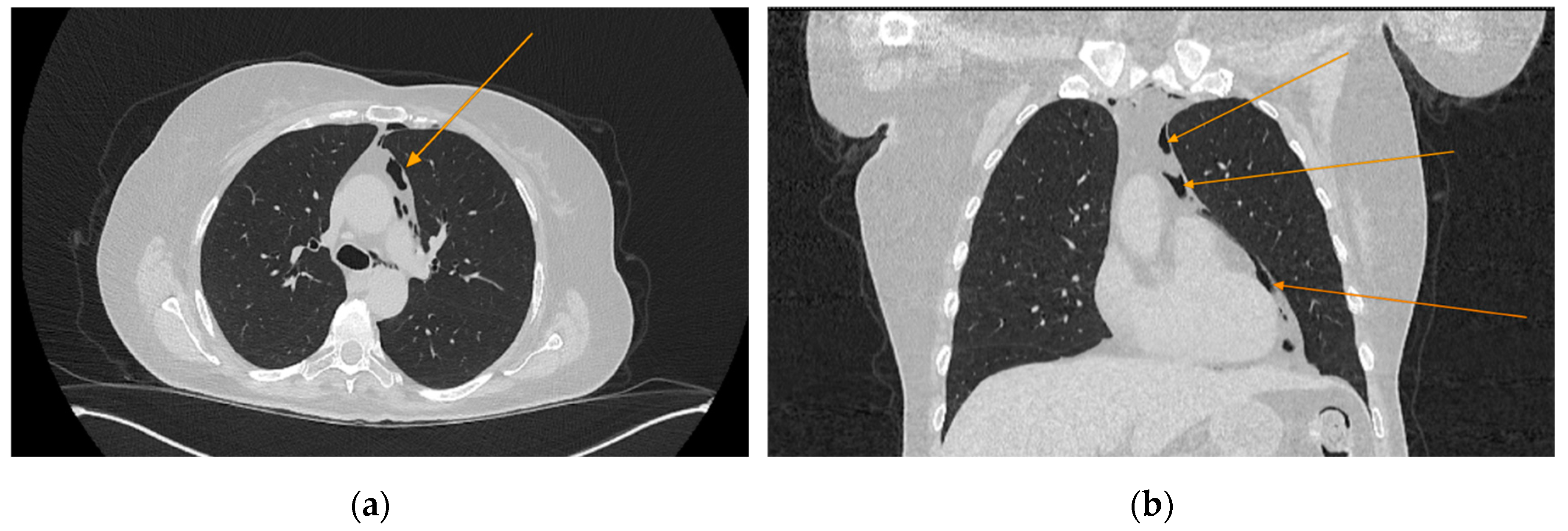
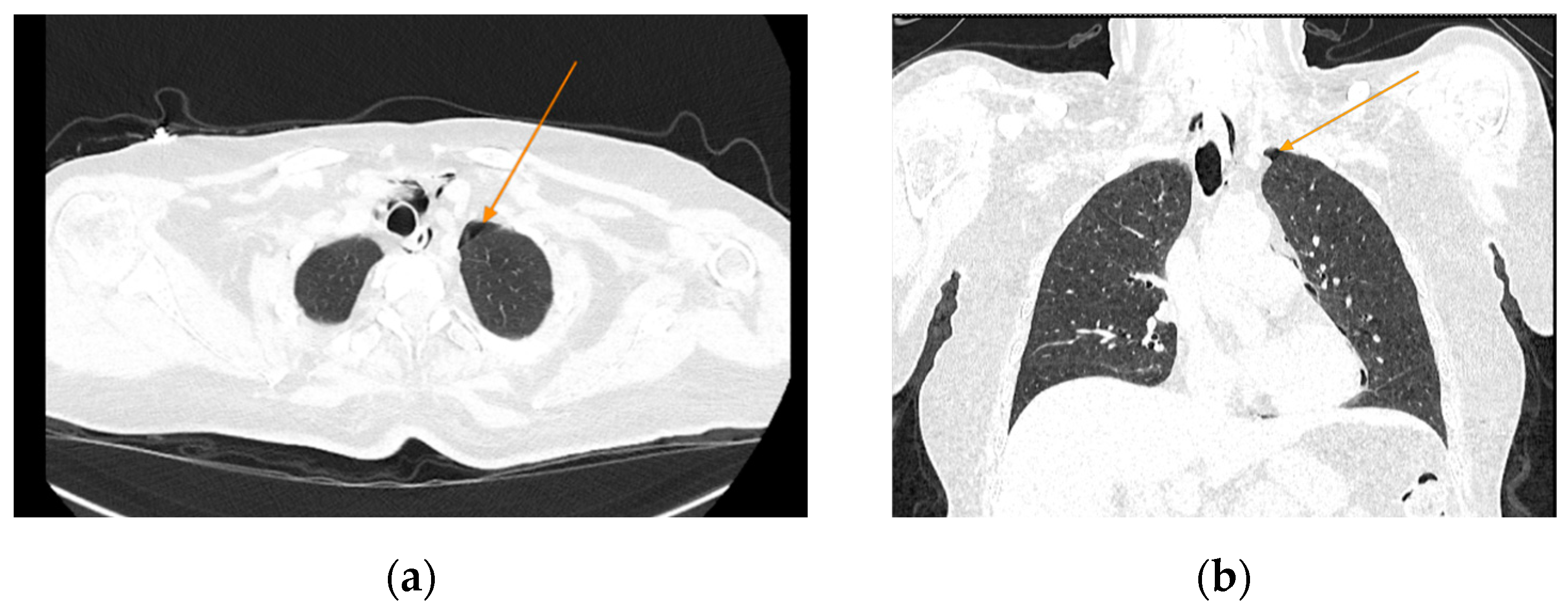
The chest CT scan performed in the emergency department (
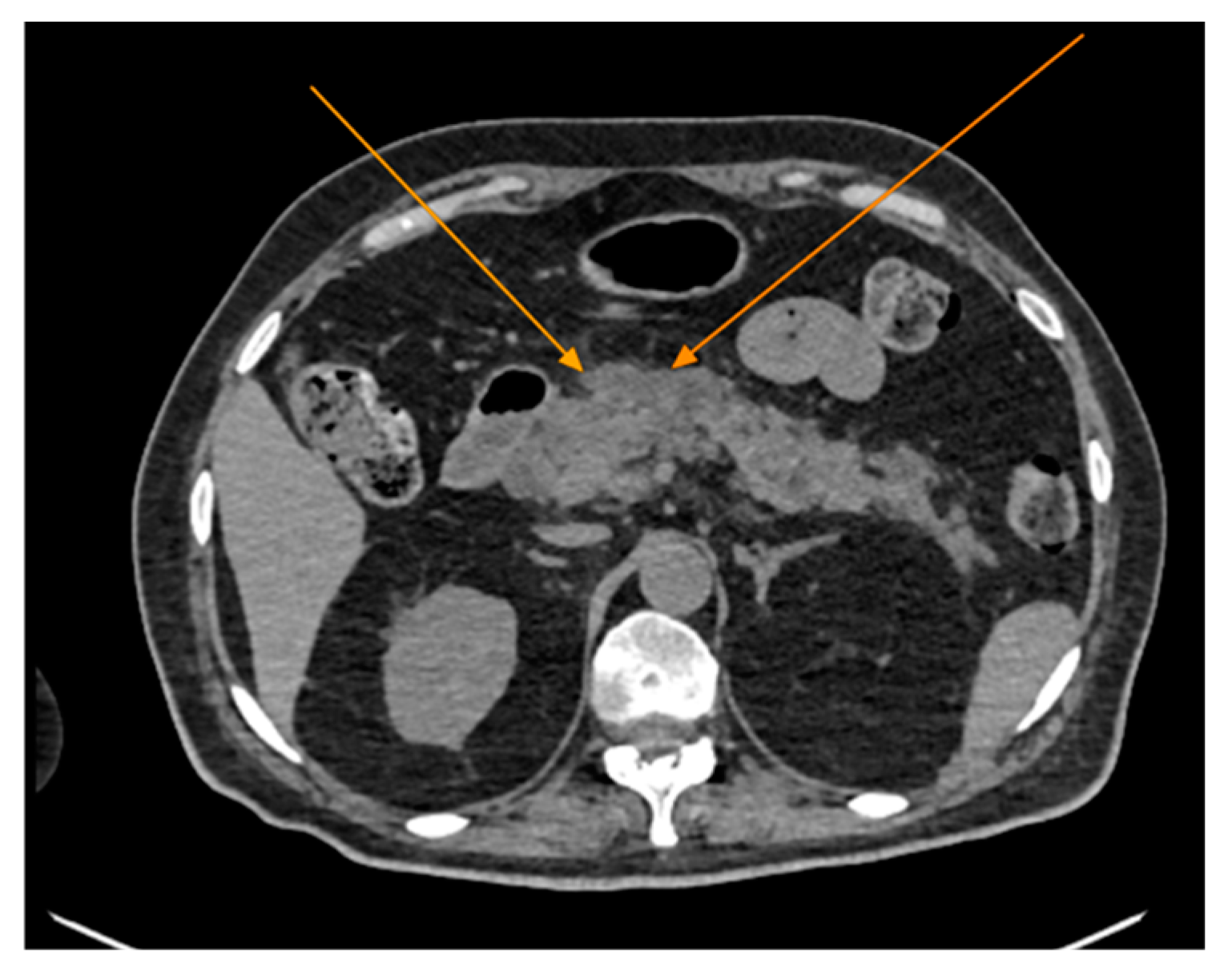
Figure 1) revealed the presence of pneumomediastinum. CT scans of the abdomen and pelvis were also performed, giving the high lipase and epigastric pain, revealing findings consistent with acute pancreatitis (with peripancreatic fluid collection, consistent with moderate acute pancreatitis per the modified CT severity index), as well as a solitary biliary stone measuring approximately 20 mm in diameter, without intra or extrahepatic biliary ducts enlargement (
Figure 2).
Figure 1.
Initial chest CT, arrows indicate pneumomediastinum (arrows): (a) axial view; (b) coronal view.
Figure 1.
Initial chest CT, arrows indicate pneumomediastinum (arrows): (a) axial view; (b) coronal view.
Figure 2.
Abdominal CT scan showing acute pancreatitis (arrows), axial view.
Figure 2.
Abdominal CT scan showing acute pancreatitis (arrows), axial view.
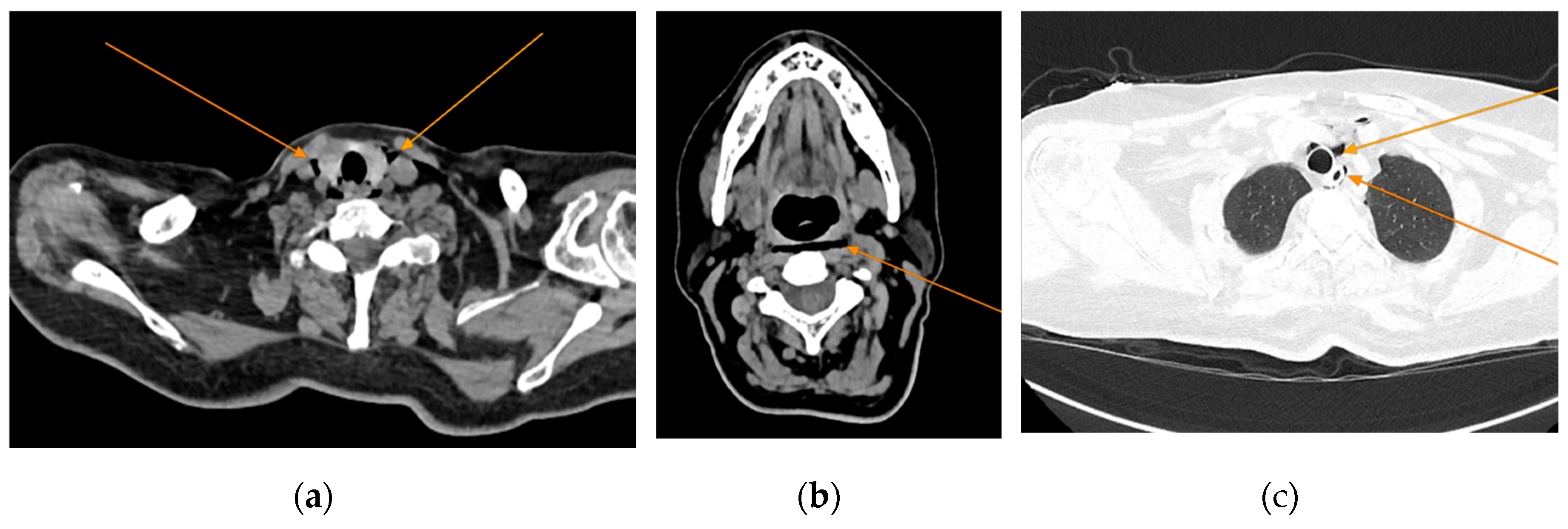
Given the patient’s history of vomiting, and consulting with the thoracic surgeon, a second CT scan with oral contrast was performed approximately 3 hours later, after initial stabilization of the patient, to rule out an oesophageal rupture. The subsequent imaging revealed the progression of the pneumomediastinum, with increased extension into the cervical region, including the peritracheoesophageal, perithyroidal, and retropharyngeal spaces (Figure 3), without evidence of oesophageal rupture (
Figure 4). Oxygenotherapy and empiric antibiotherapy were initiated in the ED as initial treatments of this condition.
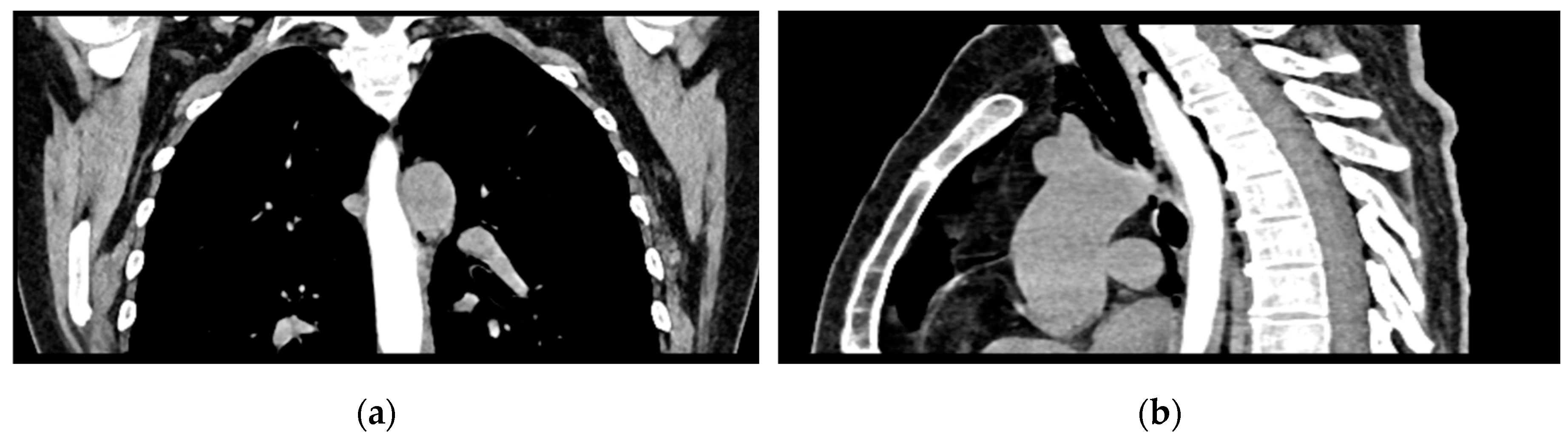
The patient was referred to diabetology in order to be admitted to hospital. Although the patient presented a good clinical and biological evolution, her condition deteriorated after about 12 hours, therefore another chest CT scan was performed, revealing extension of the pneumomediastinum in comparison to the prior scan and the presence of an additional left pneumothorax measuring approximately 7 mm (
Figure 5).
Figure 3.
Repeated chest CT showcasing extension of the pneumomediastinum (arrows), axial views.
Figure 3.
Repeated chest CT showcasing extension of the pneumomediastinum (arrows), axial views.
Figure 4.
Oral contrast chest CT with no extravasation of the contrast substance: (a) coronal view; (b) sagittal view.
Figure 4.
Oral contrast chest CT with no extravasation of the contrast substance: (a) coronal view; (b) sagittal view.
Figure 5.
Chest CT showing the presence of pneumothorax: (a) axial view; (b) coronal view.
Figure 5.
Chest CT showing the presence of pneumothorax: (a) axial view; (b) coronal view.
The evolution was favourable, with the general state of the patient improving during the admission. Supplementary investigations performed consist of serial blood glucose measurements (with a 24h mean glycemia of 117 mg/dl), HbA1C (10.4%), C peptide (2.650 ng/ml, normal range 0.810 – 3.850 ng/ml), FT3 (3.13 pmol/ml).
The patient was discharged after 28 days of hospitalization, presenting no residual symptoms and complete resolution of the pneumomediastinum. A pancreatic pseudocyst, which developed during the hospital stay, was the only remaining finding. Discharge recommendations included oral antidiabetic therapy and adherence to a dietary regimen.
4. Discussion
The current paper presented the case of a 60-year-old woman, with not known medical history, who presented in the ED with classic signs of severe DKA. Additionally, the patient presented SPM, SP and AP associated with the ketoacidosis. The symptomatology developed about 2 weeks prior to the ED presentation. Furthermore, the patient presented HbA1C levels of 10.4% suggesting a prolonged evolution of the disease. Type 2 DM was confirmed using the C peptide levels.
Inaugural DKA in Adult Patients
DKA represents about 38% of the total number of hospital admissions for hyperglycemic crisis in diabetic patients, with a peak incidence in adults aged 18-44 years with type 1 DM [
1]. Infections, particularly pneumonias and urinary tract infections, are widely recognized as the most common precipitating factors for DKA [
26,
27,
28]. Inaugural DKA represents the development of DKA in a patient with no previous diagnosis of DM. In a recently published study, Sall et. al. reported a prevalence of inaugural diabetic ketoacidosis of 17.1% in adult patients [
5], while other studies reported rates of up to 20% [
26]. In this case report, the patient was a 60-year-old-woman, with classic signs of diabetic ketoacidosis. Even though she was not previously diagnosed with DM, the patient presented HbA1C levels of 10.4% suggesting a prolonged evolution of the disease.
DKA is the result of a reduced or absent function of pancreatic beta cells to secrete insulin, along with an exaggerated response of the liver to compensate for this energy deficit. As a consequence, fatty acid metabolism is increased, leading to the accumulation of ketoacids (acetoacetate and β-hydroxybutyrate) [
29].
Severity of the disease is defined by the American Diabetes Association taking into account the degree of acidosis and level of consciousness, the severe diseases being characterized by a pH below 7.0, bicarbonate levels below 10, a positive urine or serum nitroprusside test, an anion gap above 12 and presence of either stupor or coma [
30]. The patient from the current case report respects all the criteria, excepting the pH values (which were slightly higher, 7.08).
Ketosis-prone diabetes (sometimes referred to as Flatbush disease) is a syndrome characterized by inaugural diabetic ketoacidosis in patients without typical characteristics of autoimmune type 1 DM [
31]. It is highly prevalent in African and Hispanic people [
32,
33] and accounts for about one third of inaugural DKA or new onset ketosis in adults [
34]. The underlying mechanism is believed to be the reversible, transient, destruction of the alpha and beta pancreatic cells [
32]. Most patients with ketosis-prone diabetes become insulin independent and their pathology can be managed utilizing diet alone or in association with oral antidiabetics [
35]. Older patients with obesity and a family history of type 2 DM, who present with DKA, must raise suspicion of ketosis-prone diabetes [
32]. The pathology puts patients at risk of recurrent episodes of DKA, therefore supplemental attention is needed to diagnose and observe atypical forms of DM. Considering that hyperglycemic events related visits in the ED are in a permanent growth, supplemental attention is required to prevent these complications. Benoit et al. observed an annual percentage change of 13.5% of adult patients presenting with DKA in the ED [
36]. Proper diagnosis of this pathology is required in order to ensure adequate follow-up, avoiding unnecessary acute hyperglycemic crisis in this patient population.
Acute Pancreatitis and DKA
Coexisting acute pancreatitis and DKA may be present in 10% to 15% of the cases [
49]. Acute pancreatitis has been reported as both a cause and a complication of DKA [
50]. The increased degree of lipolysis in the adipose tissue promoted by DKA causes severe hypertriglyceridemia, which is known to precipitate acute pancreatitis usually when surpassing 1000 mg/dl [
51]. Acidosis (which promotes trypsinogen activations) and hyperviscosity are other incriminated factors in developing acute pancreatitis [
52]. Tollard et al. [
53] presented the case of a 32-year-old obese female patient who developed acute pancreatitis as a complication of DKA probably precipitated by SARS-CoV-2 infection. In this particular case, the underlying mechanism was hypothesized to have been the pancreatic cellular damage caused by the virus, as other causes of acute pancreatitis were excluded.
Alternatively, stress hyperglycemia in acute pancreatitis triggers a cascade of neuroendocrine and inflammatory responses that sharply elevate glucose levels and can precipitate DKA in insulin-intolerant individuals [
51]. In this case, the only possible cause of acute pancreatitis identified was the presence of a biliary stone. Yet, the risk of developing acute pancreatitis is increased by small stones and multiple stones [
54,
55], rather than a large solitary stone.
It is worth mentioning that nonspecific elevations of amylase and lipase may occur in the context of DKA, maybe due to the reduced glomerular filtration rate present in hyperglycemic crises [
49].
5. Conclusions
Diabetic ketoacidosis as an inaugural presentation of type 2 diabetes mellitus is relatively uncommon and often challenging to manage. However, this case proved to be significantly more complex when imaging diagnostics revealed three additional, potentially fatal, diagnoses: SPM, SP and AP. This case report aims to highlight the necessity of promptly corelating clinical, biological, and imaging data. Timely diagnosis and targeted interventions can significantly improve outcomes, as demonstrated by the favorable evolution of this case. However, given the limited reports on this topic in older patients, the long-term evolution of such a complex pathology remains uncertain, underlining the need for further clinical observation and research.
Author Contributions
Conceptualization, A.C.C.; methodology, A.C.C. and A.M.M..; software, A.C.C., A.A.A.; validation, M.M.M., A.P. and O.A.M.; investigation, A.C.C., A.M.M., C.G.W, and A.P.; resources, A.C.C. and A.M.M.; data curation, A.C.C. and A.M.M.; writing—original draft preparation, A.C.C., A.M.M., A.M.B. and C.G.W.; writing—review and editing, A.C.C., C.G.W., A.M.B., A.M.M., A.P., M.M.M., A.A.A., O.A.M.; visualization, A.M.B.; supervision, O.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge Victor Babes University of Medicine and Pharmacy Timisoara for their support in covering the costs of publication for this research paper.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Emergency Clinical Municipal Hospital from Timisoara, Romania (number E-1668/11.04.2025).
Informed Consent Statement
Informed consent was obtained from the patient at admission in the Emergency Clinical Municipal Hospital from Timisoara.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Umpierrez, G.E.; Davis, G.M.; ElSayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycemic Crises in Adults With Diabetes: A Consensus Report. Diabetes Care 2024, 47, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Singh, S.; Syed, M.H.; Dave, H.; Hasnain, M.; Zahid, D.; Haider, M.; Jilani, S.M.A.; Mirza, M.A.; Kiran, N.; et al. Temporal Trends in the Prevalence of Diabetes Decompensation (Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State) Among Adult Patients Hospitalized with Diabetes Mellitus: A Nationwide Analysis Stratified by Age, Gender, and Race. Cureus 2019. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; David, J.A.; Udoyen, A.-O.; Egbunu, E.O.; Ogbuiyi-Chima, I.C.; Unakalamba, L.O.; Temitope, A.I.; Ibhiedu, J.O.; Ibhiedu, A.O.; Nwosu, P.U.; et al. Comprehensive Review of Diabetic Ketoacidosis: An Update. Annals of Medicine & Surgery 2023, 85, 2802–2807. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, J.; Wang, G.; Zhong, S.; Su, X.; Huang, Z.; Chen, G.; Zhang, J.; Hou, X.; Yu, X.; et al. Clinical Profile of Diabetic Ketoacidosis in Tertiary Hospitals in China: A Multicentre, Clinic-based Study. Diabetic Medicine 2016, 33, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sall, S.A.B.; Ndiaye, N.; Diack, N.D.; Leye, M.Y.; Leye, P.A.; Diallo, V.M.P.C.; Leye, A. Diagnostic and Evolutionary Aspects of Inaugural Diabetic Ketoacidosis in the Dakar Hospital Setting: A Descriptive and Analytical Cross-Sectional Study in the Endocrinology-Metabolism-Nutrition Department of CHN de Pikine. Open J Endocr Metab Dis 2025, 15, 8–21. [Google Scholar] [CrossRef]
- Susai, C.J.; Banks, K.C.; Alcasid, N.J.; Velotta, J.B. A Clinical Review of Spontaneous Pneumomediastinum. Mediastinum 2024, 8, 4–4. [Google Scholar] [CrossRef]
- Caceres, M.; Ali, S.Z.; Braud, R.; Weiman, D.; Garrett, H.E. Spontaneous Pneumomediastinum: A Comparative Study and Review of the Literature. Ann Thorac Surg 2008, 86, 962–966. [Google Scholar] [CrossRef]
- Talwar, A.; Rajeev, A.; Rachapudi, S.; Khan, S.; Singh, V.; Talwar, A. Spontaneous Pneumomediastinum: A Comprehensive Review of Diagnosis and Management. Intractable Rare Dis Res 2024, 13, 2024.01020. [Google Scholar] [CrossRef]
- Marza, A.M.; Cindrea, A.C.; Petrica, A.; Stanciugelu, A.V.; Barsac, C.; Mocanu, A.; Critu, R.; Botea, M.O.; Trebuian, C.I.; Lungeanu, D. Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with COVID-19: Three-Year Debriefing across Five Pandemic Waves. J Pers Med 2023, 13, 1497. [Google Scholar] [CrossRef]
- Talwar, A.; Rajeev, A.; Rachapudi, S.; Khan, S.; Singh, V.; Talwar, A. Spontaneous Pneumomediastinum: A Comprehensive Review of Diagnosis and Management. Intractable Rare Dis Res 2024, 13, 2024.01020. [Google Scholar] [CrossRef]
- Al-Mufarrej, F.; Badar, J.; Gharagozloo, F.; Tempesta, B.; Strother, E.; Margolis, M. Spontaneous Pneumomediastinum: Diagnostic and Therapeutic Interventions. J Cardiothorac Surg 2008, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Dirol, H.; Keskin, H. Risk Factors for Mediastinitis and Mortality in Pneumomediastinum. J Cardiovasc Thorac Res 2022, 14, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Yonezawa, N.; Fukasawa, M.; Fujisawa, M. Extensive Epidural Pneumatosis and Pneumomediastinum Combined with Diabetic Ketoacidosis. Clin Case Rep 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Alkhuja, S.; Gazizov, N.; Charles, G. Pneumomediastinum Complicating Diabetic Ketoacidosis and Boerhaave’s Syndrome. Case Rep Med 2013, 2013. [Google Scholar] [CrossRef]
- Murayama, S. Spontaneous Pneumomediastinum and Macklin Effect: Overview and Appearance on Computed Tomography. World J Radiol 2014, 6, 850. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Wu, X.; Chen, L.; Wei, J.; Xue, M.; Liang, Q. Analysing the Clinical Features of Pneumomediastinum Associated with Diabetic Ketoacidosis in 79 Cases. Diabetes, Metabolic Syndrome and Obesity 2020, 13, 405–412. [Google Scholar] [CrossRef]
- Usman, A.; Angelopoulos, J.; Kumar, R. Spontaneous Pneumomediastinum and Subcutaneous Emphysema Associated With Diabetic Ketoacidosis: A Case Report. Cureus 2024. [Google Scholar] [CrossRef]
- Xu, W.L.; Sun, L.C.; Zang, X.X.; Wang, H.; Li, W. Spontaneous Pneumomediastinum in Diabetic Ketoacidosis: A Case Series of 10 Patients. World J Emerg Med 2022, 13, 141–143. [Google Scholar] [CrossRef]
- Pain, A.R.; Pomroy, J.; Benjamin, A. Hamman’s Syndrome in Diabetic Ketoacidosis. Endocrinol Diabetes Metab Case Rep 2017, 2017. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chang, C.-T.; Chen, C.-C.; Wang, T.-Y. Diabetic Ketoacidosis Complicated by Pneumomediastinum and Mallory-Weiss Tear in a Young Boy with New Onset Type 2 Diabetes. South Med J 2008, 101, 769–770. [Google Scholar] [CrossRef]
- Hu, J.-X.; Zhao, C.-F.; Wang, S.-L.; Tu, X.-Y.; Huang, W.-B.; Chen, J.-N.; Xie, Y.; Chen, C.-R. Acute Pancreatitis: A Review of Diagnosis, Severity Prediction and Prognosis Assessment from Imaging Technology, Scoring System and Artificial Intelligence. World J Gastroenterol 2023, 29, 5268–5291. [Google Scholar] [CrossRef] [PubMed]
- Zerem, E.; Kurtcehajic, A.; Kunosić, S.; Zerem Malkočević, D.; Zerem, O. Current Trends in Acute Pancreatitis: Diagnostic and Therapeutic Challenges. World J Gastroenterol 2023, 29, 2747–2763. [Google Scholar] [CrossRef]
- Wen, Y.; Luo, Y.; Huang, Y.; Zhang, Z.; Xiong, L.; Wang, Y. Global, Regional, and National Pancreatitis Burden and Health Inequality of Pancreatitis from 1990 to 2019 with a Prediction from 2020 to 2034. BMC Public Health 2024, 24, 3329. [Google Scholar] [CrossRef]
- Carroll, J.K.; Herrick, B.; Gipson, T.; Lee, S.P. Acute Pancreatitis: Diagnosis, Prognosis, and Treatment. Am Fam Physician 2007, 75, 1513–1520. [Google Scholar]
- Khan, A.A.; Ata, F.; Yousaf, Z.; Aljafar, M.S.; Seijari, M.N.; Matarneh, A.; Dakkak, B.; Halabiya, M.; Muthanna, B.; Maliyakkal, A.M.; et al. A Retrospective Study on Comparison of Clinical Characteristics and Outcomes of Diabetic Ketoacidosis Patients with and without Acute Pancreatitis. Sci Rep 2023, 13, 4347. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Murphy, M.B.; Kitabchi, A.E. Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Syndrome. Diabetes Spectrum 2002, 15, 28–36. [Google Scholar] [CrossRef]
- Shahid, W.; Khan, F.; Makda, A.; Kumar, V.; Memon, S.; Rizwan, A. Diabetic Ketoacidosis: Clinical Characteristics and Precipitating Factors. Cureus 2020. [Google Scholar] [CrossRef]
- Ahmad, R.; Narwaria, M.; Singh, A.; Kumar, S.; Haque, M. Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools. Diagnostics 2023, 13, 2441. [Google Scholar] [CrossRef]
- El-Remessy, A. Diabetic Ketoacidosis Management: Updates and Challenges for Specific Patient Population. Endocrines 2022, 3, 801–812. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic Ketoacidosis. Nat Rev Dis Primers 2020, 6, 40. [Google Scholar] [CrossRef]
- Kikani, N.; Balasubramanyam, A. Remission in Ketosis-Prone Diabetes. Endocrinol Metab Clin North Am 2023, 52, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Makahleh, L.; Othman, A.; Vedantam, V.; Vedantam, N. Ketosis-Prone Type 2 Diabetes Mellitus: An Unusual Presentation. Cureus 2022. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, Å. Ketosis-Prone Type 2 Diabetes: A Case Series. Front Endocrinol (Lausanne) 2019, 10. [Google Scholar] [CrossRef]
- Kovacs, A.; Bunduc, S.; Veres, D.S.; Palinkas, D.; Gagyi, E.B.; Hegyi, P.J.; Eross, B.; Mihaly, E.; Hegyi, P.; Hosszufalusi, N. One Third of Cases of New-onset Diabetic Ketosis in Adults Are Associated with Ketosis-prone Type 2 Diabetes—A Systematic Review and Meta-analysis. Diabetes Metab Res Rev 2024, 40. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E.; Banerji, M.A. Ketosis-Prone Diabetes (Flatbush Diabetes): An Emerging Worldwide Clinically Important Entity. Curr Diab Rep 2018, 18, 120. [Google Scholar] [CrossRef]
- Benoit, S.R.; Hora, I.; Pasquel, F.J.; Gregg, E.W.; Albright, A.L.; Imperatore, G. Trends in Emergency Department Visits and Inpatient Admissions for Hyperglycemic Crises in Adults With Diabetes in the U.S., 2006–2015. Diabetes Care 2020, 43, 1057–1064. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jin, S.W.; Jang, S.H.; Jang, Y.S.; Lee, E.K.; Kim, Y.J.; Lee, M.Y.; Park, J.C.; Rho, T.H.; Kim, J.H.; et al. A Case of Spontaneous Pneumomediastinum and Pneumopericardium in a Young Adult. Korean J Intern Med 2001, 16, 205–209. [Google Scholar] [CrossRef]
- Moraes, A.G. de; Surani, S. Effects of Diabetic Ketoacidosis in the Respiratory System. World J Diabetes 2019, 10, 16–22. [Google Scholar] [CrossRef]
- Deshwal, H.; Elkhapery, A.; Ramanathan, R.; Nair, D.; Singh, I.; Sinha, A.; Vashisht, R.; Mukherjee, V. Patient-Self Inflicted Lung Injury (P-SILI): An Insight into the Pathophysiology of Lung Injury and Management. J Clin Med 2025, 14, 1632. [Google Scholar] [CrossRef]
- Carteaux, G.; Parfait, M.; Combet, M.; Haudebourg, A.-F.; Tuffet, S.; Mekontso Dessap, A. Patient-Self Inflicted Lung Injury: A Practical Review. J Clin Med 2021, 10, 2738. [Google Scholar] [CrossRef]
- Marongiu, I.; Slobod, D.; Leali, M.; Spinelli, E.; Mauri, T. Clinical and Experimental Evidence for Patient Self-Inflicted Lung Injury (P-SILI) and Bedside Monitoring. J Clin Med 2024, 13, 4018. [Google Scholar] [CrossRef] [PubMed]
- Sklienka, P.; Frelich, M.; Burša, F. Patient Self-Inflicted Lung Injury—A Narrative Review of Pathophysiology, Early Recognition, and Management Options. J Pers Med 2023, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Hongo, T.; Nojima, T.; Yumoto, T.; Nakao, A.; Naito, H. Hamman’s Syndrome Accompanied by Diabetic Ketoacidosis; a Case Report. Arch Acad Emerg Med 2022, 10, e68. [Google Scholar] [CrossRef]
- Garcipérez de Vargas, F.J.; Gómez Barrado, J.J.; Moyano Calvente, S.L.; Amaya García, M.J.; Marcos, G. Pneumopericardium and Pneumomediastinum in a Diabetic and Cocaine User Patient. Endocrinología y Nutrición (English Edition) 2013, 60, e3–e4. [Google Scholar] [CrossRef]
- Pooyan, P.; Puruckherr, M.; Summers, J.A.; Byrd, R.P.; Roy, T.M. Pneumomediastinum, Pneumopericardium, and Epidural Pneumatosis in DKA. J Diabetes Complications 2004, 18, 242–247. [Google Scholar] [CrossRef]
- Sato, M.; Toyoshima, K.; Tamura, Y.; Araki, A. Spontaneous Pneumoperitoneum and Diabetic Ketoacidosis in Fulminant Type 1 Diabetes: A Case Report. Oxf Med Case Reports 2023, 2023. [Google Scholar] [CrossRef]
- Marchon, K.A.; Nunn, M.O.; Chakera, A.J. Images of the Month: An Incidental Finding of Spontaneous Pneumomediastinum (Hamman’s Syndrome) Secondary to Diabetic Ketoacidosis during the Coronavirus Pandemic. Clinical Medicine 2020, 20, e275–e277. [Google Scholar] [CrossRef]
- Pauw, R.G.; van der Werf, T.S.; van Dullemen, H.M.; Dullaart, R.P.F. Mediastinal Emphysema Complicating Diabetic Ketoacidosis: Plea for Conservative Diagnostic Approach. Neth J Med 2007, 65, 368–371. [Google Scholar]
- Gaglia, J.L.; Wyckoff, J.; Abrahamson, M.J. Acute Hyperglycemic Crisis in the Elderly. Medical Clinics of North America 2004, 88, 1063–1084. [Google Scholar] [CrossRef]
- Ma, L.P.; Liu, X.; Cui, B.C.; Liu, Y.; Wang, C.; Zhao, B. Diabetic Ketoacidosis With Acute Pancreatitis in Patients With Type 2 Diabetes in the Emergency Department: A Retrospective Study. Front Med (Lausanne) 2022, 9. [Google Scholar] [CrossRef]
- Imburgio, S.; Vedire, A.; Sanekommu, H.; Johal, A.; Taj, S.; Lesniak, C.; Mushtaq, A. Acute Pancreatitis as an Unusual Culprit of Diabetic Ketoacidosis in a Nondiabetic: A Case-Based Review. Case Rep Endocrinol 2023, 2023, 1–5. [Google Scholar] [CrossRef] [PubMed]
- de Pretis, N.; Amodio, A.; Frulloni, L. Hypertriglyceridemic Pancreatitis: Epidemiology, Pathophysiology and Clinical Management. United European Gastroenterol J 2018, 6, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Tollard, C.; Champenois, V.; Delemer, B.; Carsin-Vu, A.; Barraud, S. An Inaugural Diabetic Ketoacidosis with Acute Pancreatitis during COVID-19. Acta Diabetol 2021, 58, 389–391. [Google Scholar] [CrossRef]
- Benjaminov, F.; Yassin, S.; Stein, A.; Naftali, T.; Konikoff, F.M. Presence of Small and Multiple Gallstones Increases the Risk of Biliary Complications. International Journal of Gastrointestinal Intervention 2024, 13, 37–40. [Google Scholar] [CrossRef]
- Diehl, A.K. Gallstone Size and Risk of Pancreatitis. Arch Intern Med 1997, 157, 1674. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).