Submitted:
14 April 2025
Posted:
15 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cellular Senescence in Pre-Eclampsia
3. The Roles of Autophagy in Senescence and Its Inhibition
4. Senomorphics and Senolytics
5. The Role of Nrf2 in Autophagy and Cytoprotection
6. Spermine and Spermidine as Geroprotectors
7. Ergothioneine and Cardiovascular Diseases
8. Use of Traditional Chinese Medicine in Modulating Autophagy
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aden, D., et al., 2022. Beyond covid-19 and sars-cov-2, cardiovascular outcomes of "long covid" from a pathological perspective - a look back and road ahead. Pathol Res Pract. 239, 154144.
- Aihara, S., et al., 2023. Spermidine from arginine metabolism activates nrf2 and inhibits kidney fibrosis. Commun Biol. 6, 676.
- Alamgir, K.M., et al., 2015. Production of ergothioneine by methylobacterium species. Front Microbiol. 6, 1185.
- Alon, U., 2006. An introduction to systems biology: Design principles of biological circuits Chapman and Hall/CRC: London.
- Alqrad, M.a.I., et al., 2023. Sirt1/nrf2/nf-kappab signaling mediates anti-inflammatory and anti-apoptotic activities of oleanolic acid in a mouse model of acute hepatorenal damage. Medicina (Kaunas). 59, 1351.
- Alsaleh, G., et al., 2020. Autophagy in t cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. Elife. 9, e57950.
- Alves, L.D.F., et al., 2024. The biology and biochemistry of kynurenic acid, a potential nutraceutical with multiple biological effects. Int J Mol Sci. 25, 9082.
- Ames, B.N., 2018. Prolonging healthy aging: Longevity vitamins and proteins. Proc Natl Acad Sci. 115, 10836-10844.
- An, N., et al., 2024. Celastrol alleviates diabetic vascular injury via keap1/nrf2-mediated anti-inflammation. Front Pharmacol. 15, 1360177.
- Aouache, R., et al., 2018. Oxidative stress in preeclampsia and placental diseases. Int J Med Sci 19.
- Apparoo, Y., et al., 2024. Potential role of ergothioneine rich mushroom as anti-aging candidate through elimination of neuronal senescent cells. Brain Res. 1824, 148693.
- Bansal, Y., et al., 2019. Quinolinic acid and nuclear factor erythroid 2-related factor 2 in depression: Role in neuroprogression. Front Pharmacol. 10, 452.
- Barak, O., et al., 2025. Characterization of senescence-associated transcripts in the human placenta. Placenta. 161, 31-38.
- Berardi, D., et al., 2022. Integration of mass-spectrometry-based global metabolomics and proteomics analysis to characterise different senescence induced molecular sub-phenotypes. bioRxiv. 2022.11.30.518588.
- Bernardo, V.S., et al., 2022. Potential cytoprotective and regulatory effects of ergothioneine on gene expression of proteins involved in erythroid adaptation mechanisms and redox pathways in k562 cells. Genes (Basel). 13, 2368.
- Birch, J., Gil, J., 2020. Senescence and the sasp: Many therapeutic avenues. Genes Dev. 34, 1565-1576.
- Borodina, I., et al., 2020. The biology of ergothioneine, an antioxidant nutraceutical. Nutr Res Rev. 33, 190-217.
- Brancaccio, M., et al., 2022. First evidence of dermo-protective activity of marine sulfur-containing histidine compounds. Free Radic Biol Med.
- Cao, F., et al., 2023. Celastrol treatment ameliorated acute ischemic stroke-induced brain injury by microglial injury inhibition and nrf2/ho-1 pathway activations. Biomed Res Int. 2023, 1076522.
- Cassidy, L.D., Narita, M., 2022. Autophagy at the intersection of aging, senescence, and cancer. Mol Oncol. 16, 3259-3275.
- Castellano, J.M., et al., 2013. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 62, 1791-9.
- Chaib, S., et al., 2022. Cellular senescence and senolytics: The path to the clinic. Nat Med. 28, 1556-1568.
- Chapple, S.J., et al., 2015. Keap1-nrf2 regulated redox signaling in utero: Priming of disease susceptibility in offspring. Free Radic Biol Med. 88, 212-220.
- Cheah, I.K., Halliwell, B., 2012. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta. 1822, 784-93.
- Cheah, I.K., et al., 2017. Administration of pure ergothioneine to healthy human subjects: Uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxid Redox Signal. 26, 193-206.
- Chen, S.Y., et al., 2020. Traditional chinese medicine: Role in reducing beta-amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of alzheimer’s disease. Front Pharmacol. 11, 497.
- Chen, Y., et al., 2021a. Traditional chinese medication tongxinluo attenuates lipidosis in ox-ldl-stimulated macrophages by enhancing beclin-1-induced autophagy. Front Pharmacol. 12, 673366.
- Chen, Z., et al., 2022. Ferroptosis and its emerging role in pre-eclampsia. Antioxidants (Basel). 11, 1282.
- Chen, Z., et al., 2021b. Advanced maternal age causes premature placental senescence and malformation via dysregulated alpha-klotho expression in trophoblasts. Aging Cell. 20, e13417.
- Cheng, S., et al., 2022. Hypoxia-reoxygenation impairs autophagy-lysosomal machinery in primary human trophoblasts mimicking placental pathology of early-onset preeclampsia. Int J Mol Sci. 23, 5644.
- Chigusa, Y., et al., 2012. Decreased lectin-like oxidized ldl receptor 1 (lox-1) and low nrf2 activation in placenta are involved in preeclampsia. J Clin Endocrinol Metab. 97, E1862-70.
- Choi, T.Y., et al., 2016. Concept of blood stasis in chinese medical textbooks: A systematic review. Eur J Integr Med. 8, 158-164.
- Chuang, S.Y., et al., 2014. Natural compounds and aging: Between autophagy and inflammasome. Biomed Res Int. 2014, 297293.
- Chung, S., et al., 2014. Oleanolic acid attenuates renal fibrosis in mice with unilateral ureteral obstruction via facilitating nuclear translocation of nrf2. Nutr Metab (Lond). 11, 2.
- Chuprin, A., et al., 2013. Cell fusion induced by ervwe1 or measles virus causes cellular senescence. Genes Dev. 27, 2356-66.
- Cindrova-Davies, T., et al., 2018. Evidence of oxidative stress-induced senescence in mature, post-mature and pathological human placentas. Placenta. 68, 15-22.
- Coppé, J.P., et al., 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic ras and the p53 tumor suppressor. PLoS Biol. 6, 2853-68.
- Cordell, G.A., Lamahewage, S.N.S., 2022. Ergothioneine, ovothiol a, and selenoneine-histidine-derived, biologically significant, trace global alkaloids. Molecules. 27.
- Correia-Melo, C., et al., 2016. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 35, 724-42.
- Cox, L.S., Redman, C., 2017. The role of cellular senescence in ageing of the placenta. Placenta.
- Cui, B., Yu, J.M., 2018. Autophagy: A new pathway for traditional chinese medicine. J Asian Nat Prod Res. 20, 14-26.
- D’onofrio, N., et al., 2016. Ergothioneine oxidation in the protection against high-glucose induced endothelial senescence: Involvement of sirt1 and sirt6. Free Radic Biol Med. 96, 211-22.
- Dare, A., et al., 2021. L-ergothioneine and its combination with metformin attenuates renal dysfunction in type-2 diabetic rat model by activating nrf2 antioxidant pathway. Biomed Pharmacother. 141, 111921.
- Dare, A., et al., 2022. Cardioprotective effects and in-silico antioxidant mechanism of l-ergothioneine in experimental type-2 diabetic rats. Cardiovasc Hematol Agents Med Chem. 20, 133-147.
- Datta, S., et al., 2022. Flexion of nrf2 by tea phytochemicals: A review on the chemopreventive and chemotherapeutic implications. Pharmacol Res. 182, 106319.
- Davy, P., et al., 2009. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta. 30, 539-42.
- De Magalhães, J.P., 2024. Cellular senescence in normal physiology. Science. 384, 1300-1301.
- Di Micco, R., et al., 2021. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 22, 75-95.
- Dimitriadis, E., et al., 2023. Pre-eclampsia. Nat Rev Dis Primers. 9, 8.
- Dimri, G.P., et al., 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 92, 9363-7.
- Dinkova-Kostova, A.T., Copple, I.M., 2023. Advances and challenges in therapeutic targeting of nrf2. Trends Pharmacol Sci. 44, 137-149.
- Divya, T., et al., 2016. Celastrol enhances nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem Biol Interact. 246, 52-62.
- Doherty, J., Baehrecke, E.H., 2018. Life, death and autophagy. Nat Cell Biol. 20, 1110-1117.
- Duan, B., et al., 2025. Maternal supplementation spermidine during gestation improves placental angiogenesis and reproductive performance of high prolific sows. J Nutr Biochem. 136, 109792.
- Dubost, N.J., et al., 2005. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography-mass spectroscopy. Int J Med Mush. 8, 215-222.
- Egbujor, M.C., et al., 2023. Nrf2 activation by nitrogen heterocycles: A review. Molecules. 28, 2751.
- Eggler, A.L., et al., 2008. Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by nrf2. Mol Nutr Food Res. 52 Suppl 1, S84-94.
- Eisenberg, T., et al., 2009. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 11, 1305-14.
- Erlandsson, L., et al., 2021. The roles of free iron, heme, haemoglobin, and the scavenger proteins haemopexin and alpha-1-microglobulin in preeclampsia and fetal growth restriction. J Intern Med. 290, 952-968.
- Fahey, R.C., 2013. Glutathione analogs in prokaryotes. Biochim Biophys Acta. 1830, 3182-98.
- Fakhri, S., et al., 2020. Attenuation of nrf2/keap1/are in alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules. 25, 4926.
- Fan, Z., et al., 2024. Paeoniae radix rubra: A review of ethnopharmacology, phytochemistry, pharmacological activities, therapeutic mechanism for blood stasis syndrome, and quality control. Chem Biodivers. 21, e202401119.
- Farladansky-Gershnabel, S., et al., 2019. Telomere homeostasis and senescence markers are differently expressed in placentas from pregnancies with early- versus late-onset preeclampsia. Reprod Sci. 26, 1203-1209.
- Feng, L., et al., 2019. The association between mushroom consumption and mild cognitive impairment: A community-based cross-sectional study in singapore. J Alzheimers Dis. 68, 197-203.
- Fovet, T., et al., 2022. Ergothioneine improves aerobic performance without any negative effect on early muscle recovery signaling in response to acute exercise. Front Physiol. 13, 834597.
- Fox, H., 1967. Senescence of placental villi. J Obstet Gynaecol Br Commonw. 74, 881-5.
- Fu, Y., et al., 2023. Ursolic acid reduces oxidative stress injury to ameliorate experimental autoimmune myocarditis by activating nrf2/ho-1 signaling pathway. Front Pharmacol. 14, 1189372.
- Gal, H., et al., 2019. Molecular pathways of senescence regulate placental structure and function. EMBO J. 38, e100849.
- Gao, X., et al., 2019. Huganpian, a traditional chinese medicine, inhibits liver cancer growth in vitro and in vivo by inducing autophagy and cell cycle arrest. Biomed Pharmacother. 120, 109469.
- Gao, Y., et al., 2023. Kynurenic acid inhibits macrophage pyroptosis by suppressing ros production via activation of the nrf2 pathway. Mol Med Rep. 28, 211.
- García-Prat, L., et al., 2016. Autophagy maintains stemness by preventing senescence. Nature. 529, 37-42.
- Gemechu, K.S., et al., 2020. Prevalence of hypertensive disorders of pregnancy and pregnancy outcomes in sub-saharan africa: A systematic review and meta-analysis. Womens Health (Lond). 16, 1745506520973105.
- Ghasemzadeh Rahbardar, M., Hosseinzadeh, H., 2023. A review of how the saffron (crocus sativus) petal and its main constituents interact with the nrf2 and nf-kappab signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 396, 1879-1909.
- Ghosh, I., et al., 2020. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides. 83, 102083.
- Gong, S., et al., 2018. Placental polyamine metabolism differs by fetal sex, fetal growth restriction, and preeclampsia. JCI Insight. 3, e120723.
- Grosse, L., et al., 2020. Defined p16high senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87-99 e6.
- Gründemann, D., 2012. The ergothioneine transporter controls and indicates ergothioneine activity--a review. Prev Med. 54 Suppl, S71-S74.
- Gründemann, D., et al., 2005. Discovery of the ergothioneine transporter. Proc Natl Acad Sci. 102, 5256-61.
- Gründemann, D., et al., 2022. The ergothioneine transporter (ett): Substrates and locations, an inventory. FEBS Lett. 596, 1252-1269.
- Gumilar, K.E., et al., 2023. Iron metabolism and ferroptosis: A pathway for understanding preeclampsia. Biomed Pharmacother. 167, 115565.
- Guo, F.F., et al., 2022. Spermidine inhibits lps-induced pro-inflammatory activation of macrophages by acting on nrf2 signaling but not autophagy. J Funct Foods. 94, 105115.
- Halliwell, B., et al., 2018. Ergothioneine - a diet-derived antioxidant with therapeutic potential. FEBS Lett. 592, 3357-3366.
- Halliwell, B., et al., 2023. Diet-derived antioxidants: The special case of ergothioneine. Annu Rev Food Sci Technol. 14, 323-345.
- Han, W., et al., 2023. Progress in the mechanism of autophagy and traditional chinese medicine herb involved in alcohol-related liver disease. PeerJ. 11, e15977.
- Hanna, R.A., et al., 2012. Microtubule-associated protein 1 light chain 3 (lc3) interacts with bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 287, 19094-104.
- Hayflick, L., And P. S. Moorehead, 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585-621.
- He, J., et al., 2025. The mechanism of acupuncture regulating autophagy: Progress and prospect. Biomolecules. 15, 263.
- He, L., et al., 2023. Protective role of metformin in preeclampsia via the regulation of nf-kappab/sflt-1 and nrf2/ho-1 signaling pathways by activating ampk. Placenta. 143, 91-99.
- He, P., et al., 2015. Analysis of gene expression identifies candidate markers and pathways in pre-eclampsia. J Obstet Gynaecol. 35, 578-84.
- Herbig, U., et al., 2004. Telomere shortening triggers senescence of human cells through a pathway involving atm, p53, and p21(cip1), but not p16(ink4a). Mol Cell. 14, 501-13.
- Herman-Antosiewicz, A., et al., 2006. Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 66, 5828-35.
- Higuchi, S., et al., 2019. Trophoblast type-specific expression of senescence markers in the human placenta. Placenta. 85, 56-62.
- Hireche-Chikaoui, H., et al., 2018. Nonejecting hearts on femoral veno-arterial extracorporeal membrane oxygenation: Aortic root blood stasis and thrombus formation-a case series and review of the literature. Crit Care Med. 46, e459-e464.
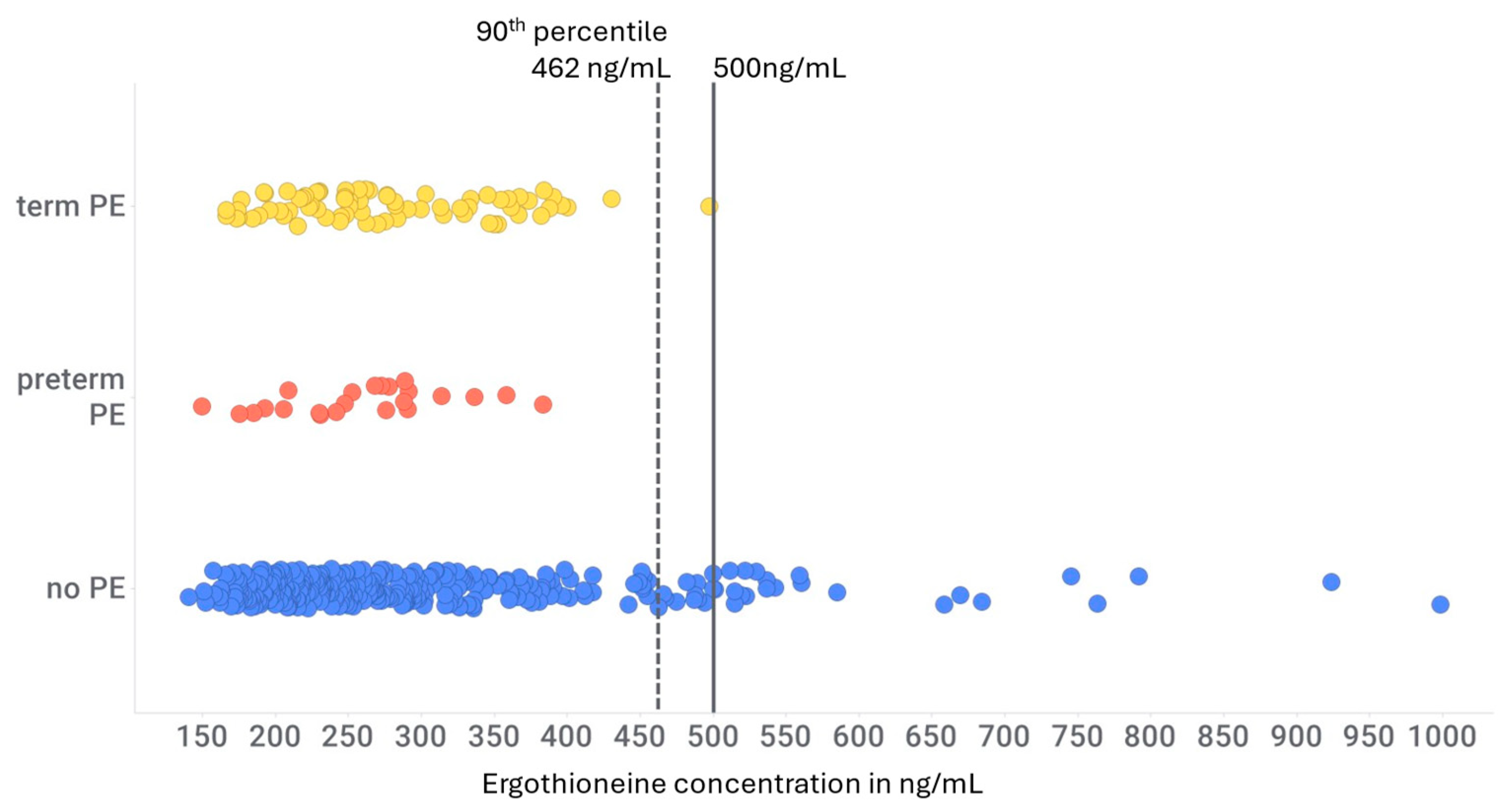
- Ho, K.M., 2023. Dose-related relationship between ergothioneine concentrations and risk of preeclampsia. Biosci Rep.
- Hofer, S.J., et al., 2022. Mechanisms of spermidine-induced autophagy and geroprotection. Nat Aging. 2, 1112-1129.
- Hood, L., 2003. Systems biology: Integrating technology, biology, and computation. Mech Ageing Dev. 124, 9-16.
- Houghton, C.A., et al., 2016. Sulforaphane and other nutrigenomic nrf2 activators: Can the clinician’s expectation be matched by the reality? Oxid Med Cell Longev. 2016, 7857186.
- Hseu, Y.C., et al., 2015. Dermato-protective properties of ergothioneine through induction of nrf2/are-mediated antioxidant genes in uva-irradiated human keratinocytes. Free Radic Biol Med. 86, 102-17.
- Hseu, Y.C., et al., 2020. The antiaging activity of ergothioneine in uva-irradiated human dermal fibroblasts via the inhibition of the ap-1 pathway and the activation of nrf2-mediated antioxidant genes. Oxid Med Cell Longev. 2020, 2576823.
- Hu, H., et al., 2022. Cyclosporin a alleviates trophoblast apoptosis and senescence by promoting autophagy in preeclampsia. Placenta. 117, 95-108.
- Hu, M., et al., 2023. Defective uterine spiral artery remodeling and placental senescence in a pregnant rat model of polycystic ovary syndrome. Am J Pathol. 193, 1916-1935.
- Huang, H., et al., 2021a. Chinese herbal medicines for promoting blood circulation and removing blood stasis for preventing deep venous thrombosis after total hip arthroplasty: A systematic review and meta-analysis. Comb Chem High Throughput Screen. 24, 893-907.
- Huang, L., et al., 2022. Human placental extract delays in vitro cellular senescence through the activation of nrf2-mediated antioxidant pathway. Antioxidants (Basel). 11, 1545.
- Huang, L., et al., 2021b. Traditional chinese medicine injection for promoting blood circulation and removing blood stasis in treating angina pectoris of coronary heart disease: A protocol for systematic review and network meta-analysis. Medicine (Baltimore). 100, e25608.
- Huang, R., Zhou, P.K., 2021. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct Target Ther. 6, 254.
- Huang, X.P., et al., 2015. Autophagy in cerebral ischemia and the effects of traditional chinese medicine. J Integr Med. 13, 289-96.
- Huang, Z., et al., 2024. Exploiting sweet relief for preeclampsia by targeting autophagy-lysosomal machinery and proteinopathy. Exp Mol Med. 56, 1206-1220.
- Imazu, N., et al., 2024. Arginase 2 attenuates ulcerative colitis by antioxidant effects of spermidine. J Gastroenterol. 59, 682-698.
- Ito, T., et al., 2011. Ergothioneine as an anti-oxidative/anti-inflammatory component in several edible mushrooms. Food Sci Technol Res. 17, 103-110.
- Jayaram, A., et al., 2021. Who said differentiating preeclampsia from covid-19 infection was easy? Pregnancy Hypertens. 26, 8-10.
- Jeong, J.Y., et al., 2023. Antioxidant effect of ergothioneine on in vitro maturation of porcine oocytes. J Vet Sci. 24, e24.
- Jiang, H., et al., 2007. Telomere shortening and ageing. Z Gerontol Geriatr. 40, 314-24.
- Jomova, K., et al., 2023. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch Toxicol. 97, 2499-2574.
- Ju, Y., et al., 2022. Combined apocyanin and aspirin treatment activates the pi3k/nrf2/ho-1 signaling pathway and ameliorates preeclampsia symptoms in rats. Hypertens Pregnancy. 41, 39-50.
- Kajdy, A., et al., 2021. Molecular pathways of cellular senescence and placental aging in late fetal growth restriction and stillbirth. Int J Mol Sci. 22, 4186.
- Kalaras, M.D., et al., 2017. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 233, 429-433.
- Kameda, M., et al., 2020. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc Natl Acad Sci U S A. 117, 9483–9489.
- Kang, H.T., et al., 2011. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS One. 6, e23367.
- Kavian, N., et al., 2018. The nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front Immunol. 9, 1896.
- Kell, D.B., 2006a. Metabolomics, modelling and machine learning in systems biology: Towards an understanding of the languages of cells. The 2005 theodor bücher lecture. FEBS J. 273, 873-894.
- Kell, D.B., 2006b. Systems biology, metabolic modelling and metabolomics in drug discovery and development. Drug Disc Today. 11, 1085-1092.
- Kell, D.B., 2009. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom. 2, 2.
- Kell, D.B., 2010. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, huntington’s, alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol. 577, 825-889.
- Kell, D.B., Kenny, L.C., 2016. A dormant microbial component in the development of pre-eclampsia. Front Med Obs Gynecol. 3, 60.
- Kell, D.B., Knowles, J.D., 2006. The role of modeling in systems biology. In: Szallasi, Z., et al. (Eds.) System modeling in cellular biology: From concepts to nuts and bolts. MIT Press: Cambridge, pp. 3-18.
- Kell, D.B., et al., 2022. A central role for amyloid fibrin microclots in long covid/pasc: Origins and therapeutic implications. Biochem J. 479, 537-559.
- Kell, D.B., Pretorius, E., 2018. No effects without causes. The iron dysregulation and dormant microbes hypothesis for chronic, inflammatory diseases. Biol Rev. 93, 1518-1557.
- Kell, D.B., Pretorius, E., 2022. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long covid and me/cfs: Evidence, mechanisms, and therapeutic implications. Biochem J. 479, 1653-1708.
- Kell, D.B., et al., 2025. A direct relationship between ‘blood stasis’ and fibrinaloid microclots in chronic, inflammatory and vascular diseases, and some traditional natural products approaches to treatment. Preprints. 2025021537. Available online: https://www.preprints.org/manuscript/202502.1537/v1.
- Kell, L., et al., 2023. The central role of DNA damage in immunosenescence. Front Aging. 4, 1202152.
- Kenny, L.C., Kell, D.B., 2018. Immunological tolerance, pregnancy and pre-eclampsia: The roles of semen microbes and the father. Front Med Obs Gynecol. 4, 239.
- Kenny, L.C., et al., 2023. Relationship between the concentration of ergothioneine in plasma and the likelihood of developing pre-eclampsia. Biosci Rep. 43, BSR20230160.
- Kenny, L.C., et al., 2020. Prediction of preeclampsia risk in first time pregnant women: Metabolite biomarkers for a clinical test. PLoS One. 15, e0244369.
- Kerley, R.N., et al., 2018. The potential therapeutic effects of ergothioneine in pre-eclampsia. Free Radic Biol Med. 117, 145-157.
- Khadir, F., et al., 2022. Nrf2 rs6721961 and oxidative stress in preeclampsia: Association with the risk of preeclampsia and early-onset preeclampsia. Int J Mol Cell Med. 11, 127-136.
- Khandia, R., et al., 2019. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: Current knowledge and prospects for disease prevention, novel drug design, and therapy. Cells. 8, 674.
- Kirkland, J.L., Tchkonia, T., 2017. Cellular senescence: A translational perspective. EBioMedicine. 21, 21-28.
- Kitano, H., 2002. Systems biology: A brief overview. Science. 295, 1662-4.
- Klipp, E., et al., 2005. Systems biology in practice: Concepts, implementation and clinical application Wiley/VCH: Berlin.
- Ko, H.J., et al., 2021. Ergothioneine alleviates senescence of fibroblasts induced by uvb damage of keratinocytes via activation of the nrf2/ho-1 pathway and hsp70 in keratinocytes. Exp Cell Res. 112516.
- Koh, S.S., et al., 2021. Effect of ergothioneine on 7-ketocholesterol-induced endothelial injury. Neuromolecular Med. 23, 184-198.
- Kovacic, J.C., et al., 2011a. Cellular senescence, vascular disease, and aging: Part 1 of a 2-part review. Circulation. 123, 1650-60.
- Kovacic, J.C., et al., 2011b. Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation. 123, 1900-10.
- Kruger, A., et al., 2025. Vascular pathogenesis in acute and long covid: Current insights and therapeutic outlook Semin Throm Hemost. 51, 256-271.
- Kryszczuk, M., Kowalczuk, O., 2022. Significance of nrf2 in physiological and pathological conditions an comprehensive review. Arch Biochem Biophys. 730, 109417.
- Kubo, E., et al., 2017. Sulforaphane reactivates cellular antioxidant defense by inducing nrf2/are/prdx6 activity during aging and oxidative stress. Sci Rep. 7, 14130.
- Kushairi, N., et al., 2020. Dietary amino acid ergothioneine protects ht22 hippocampal neurons against h2o2-induced neurotoxicity via antioxidative mechanism. Pharmanutrition. 13.
- Kweider, N., et al., 2011. Interplay between vascular endothelial growth factor (vegf) and nuclear factor erythroid 2-related factor-2 (nrf2): Implications for preeclampsia. J Biol Chem. 286, 42863-72.
- Kweider, N., et al., 2014. A possible protective role of nrf2 in preeclampsia. Ann Anat. 196, 268-77.
- Kweider, N., et al., 2012. A role for nrf2 in redox signalling of the invasive extravillous trophoblast in severe early onset iugr associated with preeclampsia. PLoS One. 7, e47055.
- Kweider, N., et al., 2013. New insights into the pathogenesis of preeclampsia - the role of nrf2 activators and their potential therapeutic impact. Geburtshilfe Frauenheilkd. 73, 1236-1240.
- Kwon, Y., et al., 2017. Autophagy is pro-senescence when seen in close-up, but anti-senescence in long-shot. Mol Cells. 40, 607-612.
- Lagoumtzi, S.M., Chondrogianni, N., 2021. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic Biol Med. 171, 169-190.
- Lee, B.Y., et al., 2006. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 5, 187-95.
- Lee, S., et al., 2022. Decreased expression of caveolin-1 have relevance to promoted senescence in preeclamptic placenta. Pregnancy Hypertens. 30, 59-67.
- Leidal, A.M., et al., 2018. Autophagy and the cell biology of age-related disease. Nat Cell Biol. 20, 1338-1348.
- Leon, L.J., et al., 2019. Preeclampsia and cardiovascular disease in a large uk pregnancy cohort of linked electronic health records: A caliber study. Circulation. 140, 1050-1060.
- Leow, D.M., et al., 2023. Protective effect of ergothioneine against 7-ketocholesterol-induced mitochondrial damage in hcmec/d3 human brain endothelial cells. Int J Mol Sci. 24.
- Li, C.P., et al., 2023a. Vitamin d may alleviate pre-eclampsia by modulating the ferroptosis signalling pathway: A hypothesis based on recent literature. J Cell Mol Med. 27, 1923-1927.
- Li, H.Q., et al., 2015. Promoting blood circulation for removing blood stasis therapy for acute intracerebral hemorrhage: A systematic review and meta-analysis. Acta Pharmacol Sin. 36, 659-75.
- Li, L., et al., 2020. Nanoparticle-mediated simultaneous downregulation of placental nrf2 and sflt1 improves maternal and fetal outcomes in a preeclampsia mouse model. ACS Biomater Sci Eng. 6, 5866-5873.
- Li, L., et al., 2013. Ursolic acid promotes the neuroprotection by activating nrf2 pathway after cerebral ischemia in mice. Brain Res. 1497, 32-9.
- Li, M., et al., 2017. Celastrol attenuates angiotensin ii mediated human umbilical vein endothelial cells damage through activation of nrf2/erk1/2/nox2 signal pathway. Eur J Pharmacol. 797, 124-133.
- Li, Q., et al., 2023b. Autophagy and senescence: The molecular mechanisms and implications in liver diseases. Int J Mol Sci. 24, 16880.
- Li, R.W.S., et al., 2014. Uptake and protective effects of ergothioneine in human endothelial cells. J Pharmacol Exp Ther. 350, 691-700.
- Li, W., et al., 2021. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the nrf2 signaling pathway: A mini review. J Biochem Mol Toxicol. 35, e22658.
- Li, Z., et al., 2022. Advanced oxidative protein products drive trophoblast cells into senescence by inhibiting the autophagy: The potential implication of preeclampsia. Front Cell Dev Biol. 10, 810282.
- Liang, X., et al., 2020. Autophagy-driven netosis is a double-edged sword - review. Biomed Pharmacother. 126, 110065.
- Liao, T., et al., 2022. Dj-1 upregulates the nrf2/gpx4 signal pathway to inhibit trophoblast ferroptosis in the pathogenesis of preeclampsia. Sci Rep. 12, 2934.
- Liu, C., et al., 2017. Traditional chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J Gastroenterol. 23, 1964-1973.
- Liu, H.X., et al., 2025a. Shenqi granules enhance recovery from cerebral ischemia-reperfusion injury by modulating tryptophan and tyrosine metabolism and activating nfe2l2/nrf2. Phytomedicine. 140, 156623.
- Liu, J., et al., 2023a. Nrf2 and fxr dual signaling pathways cooperatively regulate the effects of oleanolic acid on cholestatic liver injury. Phytomedicine. 108, 154529.
- Liu, J., et al., 2022a. Oleanolic acid alleviates anit-induced cholestatic liver injury by activating fxr and nrf2 pathways to ameliorate disordered bile acids homeostasis. Phytomedicine. 102, 154173.
- Liu, J., et al., 2008. New insights into generalized hepatoprotective effects of oleanolic acid: Key roles of metallothionein and nrf2 induction. Biochem Pharmacol. 76, 922-8.
- Liu, L., et al., 2022b. Procyanidin b2 ameliorates endothelial dysfunction and impaired angiogenesis via the nrf2/ppargamma/sflt-1 axis in preeclampsia. Pharmacol Res. 177, 106127.
- Liu, M., et al., 2024a. Activation of nrf2 by celastrol increases antioxidant functions and prevents the progression of osteoarthritis in mice. Chin J Nat Med. 22, 137-145.
- Liu, P., et al., 2019. Spermidine confers liver protection by enhancing nrf2 signaling through a map1s-mediated noncanonical mechanism. Hepatology. 70, 372-388.
- Liu, S., et al., 2023b. Autophagy: Regulator of cell death. Cell Death Dis. 14, 648.
- Liu, W., et al., 2015. Chinese patent medicine for chronic obstructive pulmonary disease based on principles of tonifying qi, promoting blood circulation by removing blood stasis, and resolving phlegm: A systematic review of randomized controlled trials. J Tradit Chin Med. 35, 1-10.
- Liu, X., et al., 2025b. 1,25-dihydroxyvitamin d(3) protects against placental inflammation by suppressing nlrp3-mediated il-1beta production via nrf2 signaling pathway in preeclampsia. Metabolism. 162, 156058.
- Liu, X.Y., et al., 2024b. Advances in research on the effectiveness and mechanism of active ingredients from traditional chinese medicine in regulating hepatic stellate cells autophagy against hepatic fibrosis. Drug Des Devel Ther. 18, 2715-2727.
- Liu, Y.T., et al., 2022c. Traditional chinese medicine formula t33 inhibits the proliferation of human colorectal cancer cells by inducing autophagy. Environ Toxicol. 37, 1007-1017.
- López-Otín, C., et al., 2023. Hallmarks of aging: An expanding universe. Cell. 186, 243-278.
- Lu, Z., et al., 2021. Inhibiting autophagy enhances sulforaphane-induced apoptosis via targeting nrf2 in esophageal squamous cell carcinoma. Acta Pharm Sin B. 11, 1246-1260.
- Luo, D., et al., 2017. Natural product celastrol suppressed macrophage m1 polarization against inflammation in diet-induced obese mice via regulating nrf2/ho-1, map kinase and nf-kappab pathways. Aging (Albany NY). 9, 2069-2082.
- Luo, X., et al., 2023. Efficacy and safety of activating blood circulation and removing blood stasis of traditional chinese medicine for managing renal fibrosis in patients with chronic kidney disease: A systematic review and meta-analysis. J Tradit Chin Med. 43, 429-440.
- Ma, J.Q., et al., 2015. Protective effects of ursolic acid in an experimental model of liver fibrosis through nrf2/are pathway. Clin Res Hepatol Gastroenterol. 39, 188-97.
- Machano, M.M., Joho, A.A., 2020. Prevalence and risk factors associated with severe pre-eclampsia among postpartum women in zanzibar: A cross-sectional study. BMC Public Health. 20, 1347.
- Madeo, F., et al., 2010. Spermidine: A novel autophagy inducer and longevity elixir. Autophagy. 6, 160-2.
- Manna, S., et al., 2019. Placental ageing in adverse pregnancy outcomes: Telomere shortening, cell senescence, and mitochondrial dysfunction. Oxid Med Cell Longev. 2019, 3095383.
- Martel, J., et al., 2020. Emerging use of senolytics and senomorphics against aging and chronic diseases. Med Res Rev. 40, 2114-2131.
- Martin, K.R., 2010. The bioactive agent ergothioneine, a key component of dietary mushrooms, inhibits monocyte binding to endothelial cells characteristic of early cardiovascular disease. J Med Food. 13, 1340-6.
- Mccord, J.M., et al., 2023. The complex genetic and epigenetic regulation of the nrf2 pathways: A review. Antioxidants (Basel). 12, 366.
- Mestres, J., et al., 2009. The topology of drug-target interaction networks: Implicit dependence on drug properties and target families. Mol Biosyst. 5, 1051-7.
- Miller, S.J., et al., 2023. Senolytic and senomorphic secondary metabolites as therapeutic agents in drosophila melanogaster models of parkinson’s disease. Front Neurol. 14, 1271941.
- Miller, W.C., et al., 2024. Cellular senescence in acute human infectious disease: A systematic review. Front Aging. 5, 1500741.
- Misztal, T., et al., 2024. Kynurenic acid modulates the expression of genes and the activity of cellular antioxidant enzymes in the hypothalamus and hippocampus in sheep. Int J Mol Sci. 25, 9428.
- Moratilla-Rivera, I., et al., 2023. Natural products as modulators of nrf2 signaling pathway in neuroprotection. Int J Mol Sci. 24, 3748.
- Moskalev, A., et al., 2017. Geroprotectors: A unified concept and screening approaches. Aging Dis. 8, 354-363.
- Muchtaridi, M., et al., 2022. Role of nuclear factor erythroid 2 (nrf2) in the recovery of long covid-19 using natural antioxidants: A systematic review. Antioxidants (Basel). 11, 1551.
- Mundal, S.B., et al., 2022. Divergent regulation of decidual oxidative-stress response by nrf2 and keap1 in preeclampsia with and without fetal growth restriction. Int J Mol Sci. 23, 1966.
- Muralimanoharan, S., et al., 2018. Redox-sensitive transcription factor nrf2 enhances trophoblast differentiation via induction of mir-1246 and aromatase. Endocrinology. 159, 2022-2033.
- Mutter, F.E., et al., 2015. Value of monitoring nrf2 activity for the detection of chemical and oxidative stress. Biochem Soc Trans. 43, 657-62.
- Nakashima, A., et al., 2017a. Autophagy regulation in preeclampsia: Pros and cons. J Reprod Immunol. 123, 17-23.
- Nakashima, A., et al., 2017b. Role of autophagy in oocytogenesis, embryogenesis, implantation, and pathophysiology of pre-eclampsia. J Obstet Gynaecol Res. 43, 633-643.
- Nakashima, A., et al., 2020a. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy. 16, 1771-1785.
- Nakashima, A., et al., 2024. Immunological regulation and the role of autophagy in preeclampsia. Am J Reprod Immunol. 91, e13835.
- Nakashima, A., et al., 2020b. Placental autophagy failure: A risk factor for preeclampsia. J Obstet Gynaecol Res. 46, 2497-2504.
- Nakashima, A., et al., 2013. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 9, 303-16.
- Negre-Salvayre, A., et al., 2022. Oxidative stress, lipid peroxidation and premature placental senescence in preeclampsia. Arch Biochem Biophys. 730, 109416.
- Nelson, G., et al., 2018. The senescent bystander effect is caused by ros-activated nf-kappab signalling. Mech Ageing Dev. 170, 30-36.
- Nelson, G., et al., 2012. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell. 11, 345-9.
- Nezu, M., et al., 2017. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci Signal. 10, eaam5711.
- Ng, S.W., et al., 2019. The impact of iron overload and ferroptosis on reproductive disorders in humans: Implications for preeclampsia. Int J Mol Sci. 20, 3283.
- Niu, C., et al., 2023. Spermidine suppresses oxidative stress and ferroptosis by nrf2/ho-1/gpx4 and akt/fhc/acsl4 pathway to alleviate ovarian damage. Life Sci. 332, 122109.
- Nunes, J.M., et al., 2023. Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (me/cfs): A role for viruses. Blood Rev. 60, 101075.
- Nunes, J.M., et al., 2024. Herpesvirus infection of endothelial cells as a systemic pathological axis in myalgic encephalomyelitis/chronic fatigue syndrome. Viruses. 16, 572.
- O’sullivan, E.A., et al., 2024. The paradox of senescent-marker positive cancer cells: Challenges and opportunities. NPJ Aging. 10, 41.
- Okuno, K., et al., 2020. Targeting molecular mechanism of vascular smooth muscle senescence induced by angiotensin ii, a potential therapy via senolytics and senomorphics. Int J Mol Sci. 21, 6579.
- Ortega, M.A., et al., 2024. Oxidative stress, lipid peroxidation and ferroptosis are major pathophysiological signatures in the placental tissue of women with late-onset preeclampsia. Antioxidants (Basel). 13, 591.
- Padron, J.G., et al., 2022. Stretch causes cell stress and the downregulation of nrf2 in primary amnion cells. Biomolecules. 12, 766.
- Palsson, B.Ø., 2006. Systems biology: Properties of reconstructed networks Cambridge University Press: Cambridge.
- Pan, M., et al., 2023. Celastrol alleviated acute kidney injury by inhibition of ferroptosis through nrf2/gpx4 pathway. Biomed Pharmacother. 166, 115333.
- Park, M.S., et al., 2023. Modern concepts and biomarkers of blood stasis in cardio- and cerebrovascular diseases from the perspectives of eastern and western medicine: A scoping review protocol. JBI Evid Synth. 21, 214-222.
- Passos, J.F., et al., 2010. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 6, 347.
- Patel, N.H., et al., 2020. The roles of autophagy and senescence in the tumor cell response to radiation. Radiat Res. 194, 103-115.
- Paul, B.D., Snyder, S.H., 2010. The unusual amino acid l-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 17, 1134-40.
- Peng, X., et al., 2025. Exploring the impact of apelin and reactive oxygen species on autophagy and cell senescence in pre-eclampsia. Free Radic Res. 59, 23-48.
- Poon, L.C., et al., 2019. The international federation of gynecology and obstetrics (figo) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 145 Suppl 1, 1-33.
- Proshkina, E., et al., 2020. Terpenoids as potential geroprotectors. Antioxidants (Basel). 9, 529.
- Puleston, D.J., Simon, A.K., 2015. New roles for autophagy and spermidine in t cells. Microb Cell. 2, 91-93.
- Puleston, D.J., et al., 2014. Autophagy is a critical regulator of memory cd8(+) t cell formation. Elife. 3, e03706.
- Qin, J.J., et al., 2019. Dual roles and therapeutic potential of keap1-nrf2 pathway in pancreatic cancer: A systematic review. Cell Commun Signal. 17, 121.
- Qin, S., et al., 2022. Phytochemical activators of nrf2: A review of therapeutic strategies in diabetes. Acta Biochim Biophys Sin (Shanghai). 55, 11-22.
- Qin, S., et al., 2023. Phytochemical activators of nrf2: A review of therapeutic strategies in diabetes. Acta Biochim Biophys Sin (Shanghai). 55, 11-22.
- Qin, S., Hou, D.X., 2016. Multiple regulations of keap1/nrf2 system by dietary phytochemicals. Mol Nutr Food Res. 60, 1731-55.
- Qing, T.L., et al., 2023. Celastrol alleviates oxidative stress induced by multi-walled carbon nanotubes through the keap1/nrf2/ho-1 signaling pathway. Ecotoxicol Environ Saf. 252, 114623.
- Raghunath, A., et al., 2018. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 17, 297-314.
- Raijmakers, M.T.M., et al., 2004. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension. 44, 374-80.
- Raijmakers, M.T.M., et al., 2005. Amino thiols, detoxification and oxidative stress in pre-eclampsia and other disorders of pregnancy. Curr Pharm Des. 11, 711-34.
- Rajendran, P., et al., 2019. Autophagy and senescence: A new insight in selected human diseases. J Cell Physiol. 234, 21485-21492.
- Raman, B., et al., 2022. Long covid: Post-acute sequelae of covid-19 with a cardiovascular focus. Eur Heart J. 43, 1157-1172.
- Rao, S.G., Jackson, J.G., 2016. Sasp: Tumor suppressor or promoter? Yes! Trends Cancer. 2, 676-687.
- Redman, C.W., et al., 2014. Ifpa senior award lecture: Making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 35 Suppl, S20-5.
- Redman, C.W., Staff, A.C., 2015. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 213, S9 e1, S9-11.
- Redman, C.W.G., 1991. Current topic: Pre-eclampsia and the placenta. Placenta. 12, 301-8.
- Redman, C.W.G., et al., 2022. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am J Obstet Gynecol. 226, S907-S927.
- Reisman, S.A., et al., 2009. Oleanolic acid activates nrf2 and protects from acetaminophen hepatotoxicity via nrf2-dependent and nrf2-independent processes. Biochem Pharmacol. 77, 1273-82.
- Roberts, I., et al., 2022. Untargeted metabolomics of covid-19 patient serum reveals potential prognostic markers of both severity and outcome. Metabolomics. 18, 6.
- Robertson, R.P., 2023. Nrf2 and antioxidant response in animal models of type 2 diabetes. Int J Mol Sci. 24, 3082.
- Robledinos-Antón, N., et al., 2019. Activators and inhibitors of nrf2: A review of their potential for clinical development. Oxid Med Cell Longev. 2019, 9372182.
- Roda, E., et al., 2023. Cognitive healthy aging in mice: Boosting memory by an ergothioneine-rich hericium erinaceus primordium extract. Biology (Basel). 12.
- Rodier, F., et al., 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 11, 973-9.
- Roh, J.D., et al., 2024. Placental senescence pathophysiology is shared between peripartum cardiomyopathy and preeclampsia in mouse and human. Sci Transl Med. 16, eadi0077.
- Rossiello, F., et al., 2022. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 24, 135-147.
- Saito, S., Nakashima, A., 2013. Impaired autophagy in extravillous trophoblast may induce poor placentation in preeclampsia. Pregnancy Hypertens. 3, 65-6.
- Saito, S., Nakashima, A., 2014. A review of the mechanism for poor placentation in early-onset preeclampsia: The role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol. 101-102, 80-88.
- Salama, S.A., et al., 2021. Ergothioneine mitigates cisplatin-evoked nephrotoxicity via targeting nrf2, nf-kappab, and apoptotic signaling and inhibiting gamma-glutamyl transpeptidase. Life Sci. 278, 119572.
- Salminen, A., et al., 2012. Emerging role of nf-kappab signaling in the induction of senescence-associated secretory phenotype (sasp). Cell Signal. 24, 835-45.
- Samuel, P., et al., 2022. Ergothioneine mitigates telomere shortening under oxidative stress conditions. J Diet Suppl. 19, 212-225.
- Santoro, L., et al., 2023. Role of endothelium in cardiovascular sequelae of long covid. Biomedicines. 11, 2239.
- Satarker, S., et al., 2024. Spermidine as an epigenetic regulator of autophagy in neurodegenerative disorders. Eur J Pharmacol. 979, 176823.
- Scaife, P.J., et al., 2021. Increased placental cell senescence and oxidative stress in women with pre-eclampsia and normotensive post-term pregnancies. Int J Mol Sci. 22, 7295.
- Sehrawat, A., et al., 2023. Dysregulated autophagy: A key player in the pathophysiology of type 2 diabetes and its complications. Biochim Biophys Acta Mol Basis Dis. 1869, 166666.
- Seo, W.Y., et al., 2011. Celastrol induces expression of heme oxygenase-1 through ros/nrf2/are signaling in the hacat cells. Biochem Biophys Res Commun. 407, 535-40.
- Shafqat, A., et al., 2024. Long covid as a disease of accelerated biological aging: An opportunity to translate geroscience interventions. Ageing Res Rev. 99, 102400.
- Shah, A., et al., 2024. Exploring sulforaphane as neurotherapeutic: Targeting nrf2-keap & nf-kb pathway crosstalk in asd. Metab Brain Dis. 39, 373-385.
- Shaikh, S.B., et al., 2024. A signaling pathway map of plasminogen activator inhibitor-1 (pai-1/serpine-1): A review of an innovative frontier in molecular aging and cellular senescence. Cell Commun Signal. 22, 544.
- Shan, Y., et al., 2023. Impact of ferroptosis on preeclampsia: A review. Biomed Pharmacother. 167, 115466.
- Shi, X., et al., 2022. Traditional chinese medicine compounds ameliorating glomerular diseases via autophagy: A mechanism review. Biomed Pharmacother. 156, 113916.
- Siddique, N., Cox, B., 2022. Computational analysis identified accelerated senescence as a significant contribution to preeclampsia pathophysiology. Placenta. 121, 70-78.
- Singh, E., et al., 2021. Management of covid-19-induced cytokine storm by keap1-nrf2 system: A review. Inflammopharmacology. 29, 1347-1355.
- Singh, V.P., Singh, P., 2024. Linking DNA damage and senescence to gestation period and lifespan in placental mammals. Front Cell Dev Biol. 12, 1480695.
- Smith, E., et al., 2020. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart. 106, 691-697.
- Sotgia, S., et al., 2014. Clinical and biochemical correlates of serum l-ergothioneine concentrations in community-dwelling middle-aged and older adults. PLoS One. 9, e84918.
- Staff, A.C., 2019. The two-stage placental model of preeclampsia: An update. J Reprod Immunol. 134-135, 1-10.
- Staff, A.C., et al., 2016. Pregnancy and long-term maternal cardiovascular health: Progress through harmonization of research cohorts and biobanks. Hypertension. 67, 251-60.
- Su, X., et al., 2018. Anticancer activity of sulforaphane: The epigenetic mechanisms and the nrf2 signaling pathway. Oxid Med Cell Longev. 2018, 5438179.
- Sugulle, M., et al., 2024. Placental senescence and the two-stage model of preeclampsia. Am J Reprod Immunol. 92, e13904.
- Sultana, Z., et al., 2018. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 218, S762-S773.
- Sun, Y., et al., 2022. The multifaceted role of the sasp in atherosclerosis: From mechanisms to therapeutic opportunities. Cell Biosci. 12, 74.
- Suvakov, S., et al., 2019. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ. 10, 49.
- Suvakov, S., et al., 2023. Impact of aging and cellular senescence in the pathophysiology of preeclampsia. Compr Physiol. 13, 5077-5114.
- Suvakov, S., et al., 2024. Women with a history of preeclampsia exhibit accelerated aging and unfavorable profiles of senescence markers. Hypertension. 81, 1550-1560.
- Tamaru, E., et al., 2024. Nrf2 induction potency of plant-derived compounds determined using an antioxidant response element luciferase reporter and conventional nad(p)h-quinone acceptor oxidoreductase 1 activity assay. BMC Res Notes. 17, 373.
- Tanida, I., et al., 2008. Lc3 and autophagy. Methods Mol Biol. 445, 77-88.
- Tantengco, O.a.G., et al., 2021a. The role of nuclear factor erythroid 2-related factor 2 (nrf2) in normal and pathological pregnancy: A systematic review. Am J Reprod Immunol. 86, e13496.
- Tantengco, O.a.G., et al., 2021b. The role of nuclear factor erythroid 2-related factor 2 (nrf2) in normal and pathological pregnancy: A systematic review. Am J Reprod Immunol. 86, e13496.
- Tao, P., et al., 2022. Progress in the mechanism of autophagy and traditional chinese medicine herb involved in dementia. Front Pharmacol. 12, 825330.
- Tao, Y., et al., 2023. Adipocyte-derived exosomal nox4-mediated oxidative damage induces premature placental senescence in obese pregnancy. Int J Nanomedicine. 18, 4705-4726.
- Tasta, O., et al., 2021. A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free Radic Biol Med. 164, 303-314.
- Tchkonia, T., et al., 2013. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J Clin Invest. 123, 966-72.
- Thagard, P., 1989. Explanatory coherence. Behav Brain Sci. 12, 435-502.
- Thagard, P., 1998. Explaining disease: Correlations, causes, and mechanisms. Minds and Machines. 8, 61-78.
- Thagard, P., 1999. How scientists explain disease Princeton University Press: Princeton, NJ.
- Thagard, P., 2007. Coherence, truth, and the development of scientific knowledge. Philosophy of Science. 74, 28-47.
- Thagard, P., 2008. Explanatory coherence. Reasoning: Studies of Human Inference and Its Foundations. 471-513.
- Thagard, P., 2012. The cognitive science of science: Explanation, discovery, and conceptual change. MIT Press: Cambridge, MA.
- Tian, R., et al., 2023a. Perturbed autophagy intervenes systemic lupus erythematosus by active ingredients of traditional chinese medicine. Front Pharmacol. 13, 1053602.
- Tian, X., et al., 2023b. Ergothioneine: An underrecognised dietary micronutrient required for healthy ageing? Br J Nutr. 129, 104-114.
- Tong, D. Tong, D., Hill, J.A., 2017. Spermidine promotes cardioprotective autophagy. Circ Res. 120, 1229-1231.
- Tossetta, G., et al., 2023. Modulation of nrf2/keap1 signaling in preeclampsia. Cells. 12, 1545.
- Tóth, F., et al., 2021. Natural molecules and neuroprotection: Kynurenic acid, pantethine and alpha-lipoic acid. Int J Mol Sci. 22, 403.
- Tseng, C.K., et al., 2017. Celastrol inhibits hepatitis c virus replication by upregulating heme oxygenase-1 via the jnk mapk/nrf2 pathway in human hepatoma cells. Antiviral Res. 146, 191-200.
- Turska, M., et al., 2022. A review of the health benefits of food enriched with kynurenic acid. Nutrients. 14, 4182.
- Uddin, M.S., et al., 2020. Emerging promise of sulforaphane-mediated nrf2 signaling cascade against neurological disorders. Sci Total Environ. 707, 135624.
- Velicky, P., et al., 2018. Genome amplification and cellular senescence are hallmarks of human placenta development. PLoS Genet. 14, e1007698.
- Vomhof-Dekrey, E.E., Picklo, M.J., Sr., 2012. The nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J Nutr Biochem. 23, 1201-6.
- Von Zglinicki, T., 2000. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 908, 99-110.
- Vriend, J., Reiter, R.J., 2015. The keap1-nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol Cell Endocrinol. 401, 213-20.
- Wai, K.W., et al., 2024. Nrf2 connects cellular autophagy and vascular senescence in atherosclerosis: A mini-review. J Lipid Atheroscler. 13, 292-305.
- Wang, C., et al., 2018. Histone methyltransferase setd7 regulates nrf2 signaling pathway by phenethyl isothiocyanate and ursolic acid in human prostate cancer cells. Mol Nutr Food Res. 62, e1700840.
- Wang, J., et al., 2015. Shuangshen ningxin capsule, a traditional chinese medicinal preparation, alleviates myocardial ischemia through autophagy regulation. Evid Based Complement Alternat Med. 2015, 581260.
- Wang, K., et al., 2020. Yishen huazhuo decoction induces autophagy to promote the clearance of abeta(1-42) in samp8 mice: Mechanism research of a traditional chinese formula against alzheimer’s disease. CNS Neurol Disord Drug Targets. 19, 276-289.
- Wang, K., et al., 2024a. Yishen huazhuo decoction regulates microglial polarization to reduce alzheimer’s disease-related neuroinflammation through trem2. Heliyon. 10, e35800.
- Wang, S.F., et al., 2016. Autophagy modulators from traditional chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J Ethnopharmacol. 194, 861-876.
- Wang, X., et al., 2013. Protective effect of oleanolic acid against beta cell dysfunction and mitochondrial apoptosis: Crucial role of erk-nrf2 signaling pathway. J Biol Regul Homeost Agents. 27, 55-67.
- Wang, X., et al., 2024b. Ursolic acid attenuates cholestasis through nrf2-mediated regulation of ugt2b7 and bsep/mrp2. Naunyn Schmiedebergs Arch Pharmacol. 397, 2257-2267.
- Wang, X., et al., 2010. Antioxidant activities of oleanolic acid in vitro: Possible role of nrf2 and map kinases. Chem Biol Interact. 184, 328-37.
- Wang, Y., et al., 2022. Sirt1 regulates trophoblast senescence in premature placental aging in preeclampsia. Placenta. 122, 56-65.
- Wang, Z., et al., 2021a. Downregulation of cd151 induces oxidative stress and apoptosis in trophoblast cells via inhibiting erk/nrf2 signaling pathway in preeclampsia. Free Radic Biol Med. 164, 249-257.
- Wang, Z.Y., et al., 2021b. Traditional chinese medicine compounds regulate autophagy for treating neurodegenerative disease: A mechanism review. Biomed Pharmacother. 133, 110968.
- Wei, G., et al., 2015. Xingnaojing, prescription of traditional chinese medicine, prevents autophagy in experimental stroke by repressing p53-dram pathway. BMC Complement Altern Med. 15, 377.
- Williamson, R.D., et al., 2020. L-(+)-ergothioneine significantly improves the clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension. 75, 561-568.
- Wu, D., et al., 2020. Plasma metabolomic and lipidomic alterations associated with covid-19. Natl Sci Rev. 7, 1157-1168.
- Wu, N., et al., 2025. Traditional chinese medication qili qiangxin capsule protects against myocardial ischemia-reperfusion injury through suppressing autophagy via the phosphoinositide 3-kinase/protein kinase b/forkhead box o3 axis. J Ethnopharmacol. 337, 118821.
- Wu, Z., et al., 2018a. Traditional chinese medicine cff-1 induced cell growth inhibition, autophagy, and apoptosis via inhibiting egfr-related pathways in prostate cancer. Cancer Med. 7, 1546-1559.
- Wu, Z., et al., 2018b. Egfr-associated pathways involved in traditional chinese medicine (tcm)-1-induced cell growth inhibition, autophagy and apoptosis in prostate cancer. Mol Med Rep. 17, 7875-7885.
- Xiao, L., et al., 2025. Targeted degradation technology based on the autophagy-lysosomal pathway: A promising strategy for treating preeclampsia. Am J Reprod Immunol. 93, e70066.
- Xin, Q.Q., et al., 2021. Correlation of platelet and coagulation function with blood stasis syndrome in coronary heart disease: A systematic review and meta-analysis. Chin J Integr Med. 27, 858-866.
- Xu, X., et al., 2024. Epigallocatechin gallate (egcg) alleviates inflammation and endothelial dysfunction and improves pregnancy outcomes in preeclampsia (pe)-like rats via enos/nrf2/ho-1 pathway. J Reprod Immunol. 164, 104263.
- Yanagisawa, M., et al., 2023. Upregulation of autotaxin by oxidative stress via nrf2 activation: A novel insight into the compensation mechanism in preeclamptic placenta. J Reprod Immunol. 160, 104153.
- Yang, F., et al., 2018. Sulforaphane induces autophagy by inhibition of hdac6-mediated pten activation in triple negative breast cancer cells. Life Sci. 213, 149-157.
- Yang, J., et al., 2021. The paradoxical role of cellular senescence in cancer. Front Cell Dev Biol. 9, 722205.
- Yang, R., et al., 2024a. Treatment of microvascular angina pectoris by activating blood circulation to remove blood stasis: A systematic review and meta-analysis. Medicine (Baltimore). 103, e40012.
- Yang, S., et al., 2020. Astragaloside iv ameliorates preeclampsia-induced oxidative stress through the nrf2/ho-1 pathway in a rat model. Am J Physiol Endocrinol Metab. 319, E904-E911.
- Yang, X., et al., 2024b. Ursolic acid inhibits the proliferation of triple-negative breast cancer stem-like cells through nrf2-mediated ferroptosis. Oncol Rep. 52, 94.
- Yarmohammadi, F., et al., 2021. Natural compounds against doxorubicin-induced cardiotoxicity: A review on the involvement of nrf2/are signaling pathway. Phytother Res. 35, 1163-1175.
- Yau, Y.F., et al., 2024. Investigating the efficacy of ergothioneine to delay cognitive decline in mild cognitively impaired subjects: A pilot study. J Alzheimers Dis. 102, 841-854.
- Yee, S.W., et al., 2020. Deorphaning a solute carrier 22 family member, slc22a15, through functional genomic studies. FASEB J. 34, 15734-15752.
- Young, A.R.J., Narita, M., 2009. Sasp reflects senescence. EMBO Rep. 10, 228-30.
- Younis, N.S., Ghanim, A.M.H., 2022. The protective role of celastrol in renal ischemia-reperfusion injury by activating nrf2/ho-1, pi3k/akt signaling pathways, modulating nf-kappab signaling pathways, and inhibiting erk phosphorylation. Cell Biochem Biophys. 80, 191-202.
- Yu, L., et al., 2019. The potentially protective role of atp-binding cassette transporters in preeclampsia via nrf2. Pregnancy Hypertens. 18, 21-28.
- Yu, Y.Y., et al., 2022. Clinical efficacy and safety of removing blood stasis and resolving phlegm in the treatment of epilepsy with cognitive impairment: A systematic review and meta-analysis. Medicine (Baltimore). 101, e30212.
- Zakeri, S., et al., 2024. The influence of nrf2 gene promoter methylation on gene expression and oxidative stress parameters in preeclampsia. BMC Med Genomics. 17, 64.
- Zalachoras, I., et al., 2020. Therapeutic potential of glutathione-enhancers in stress-related psychopathologies. Neurosci Biobehav Rev. 114, 134-155.
- Zhang, C., et al., 2021a. The nrf2-nlrp3-caspase-1 axis mediates the neuroprotective effects of celastrol in parkinson’s disease. Redox Biol. 47, 102134.
- Zhang, H., et al., 2019. Polyamines control eif5a hypusination, tfeb translation, and autophagy to reverse b cell senescence. Mol Cell. 76, 110-125 e9.
- Zhang, H., Simon, A.K., 2020. Polyamines reverse immune senescence via the translational control of autophagy. Autophagy. 16, 181-182.
- Zhang, J., et al., 2023a. Natural nrf2 inhibitors: A review of their potential for cancer treatment. Int J Biol Sci. 19, 3029-3041.
- Zhang, J.X., et al., 2017. Hemorheology index changes in a rat acute blood stasis model: A systematic review and meta-analysis. Afr J Tradit Complement Altern Med. 14, 96-107.
- Zhang, L., et al., 2023b. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 290, 1362-1383.
- Zhang, Q., et al., 2010. A systems biology perspective on nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 244, 84-97.
- Zhang, W., et al., 2021b. Novel target for treating alzheimer’s diseases: Crosstalk between the nrf2 pathway and autophagy. Ageing Res Rev. 65, 101207.
- Zhang, Y., et al., 2024a. Unveiling the network regulatory mechanism of ncrnas on the ferroptosis pathway: Implications for preeclampsia. Int J Womens Health. 16, 1633-1651.
- Zhang, Y., et al., 2024b. Single cell rna-sequencing reveals the cellular senescence of placental mesenchymal stem/stromal cell in preeclampsia. Placenta. 150, 39-51.
- Zhao, C., et al., 2021. Kynurenic acid protects against mastitis in mice by ameliorating inflammatory responses and enhancing blood-milk barrier integrity. Mol Immunol. 137, 134-144.
- Zhao, J., et al., 2023. Hotspots and future trends of autophagy in traditional chinese medicine: A bibliometric analysis. Heliyon. 9, e20142.
- Zheng, L., et al., 2024. Targeting cellular senescence in aging and age-related diseases: Challenges, considerations, and the emerging role of senolytic and senomorphic therapies. Aging Dis. 15, 2554-2594.
- Zhong, Y., et al., 2022. Tlr4 modulates senescence and paracrine action in placental mesenchymal stem cells via inhibiting hedgehog signaling pathway in preeclampsia. Oxid Med Cell Longev. 2022, 7202837.
- Zhou, Y., et al., 2019. Celastrol protects rpe cells from oxidative stress-induced cell death via activation of nrf2 signaling pathway. Curr Mol Med. 19, 172-182.
- Zhu, M.L., et al., 2017. Traditional chinese medicine ka-sai-ping suppresses the growths of gastric cancers via induction of autophagy. Oncotarget. 8, 95075-95082.
- Zhu, N., et al., 2022. Gestational exposure to no(2) aggravates placental senescence. Environ Res. 212, 113263.
- Zhu, Y., et al., 2024. Past and future directions for research on cellular senescence. Cold Spring Harb Perspect Med. 14, a041205.
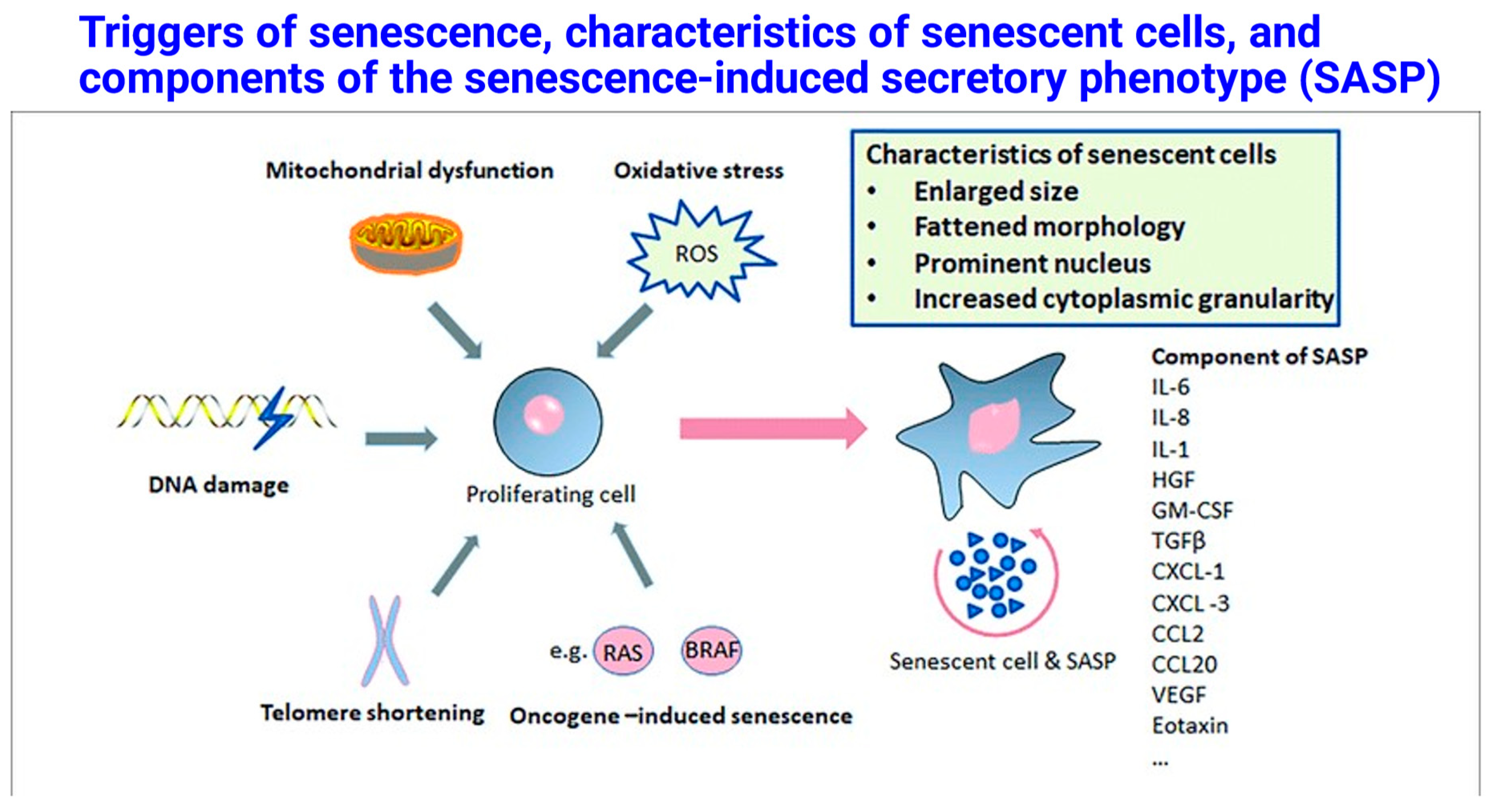
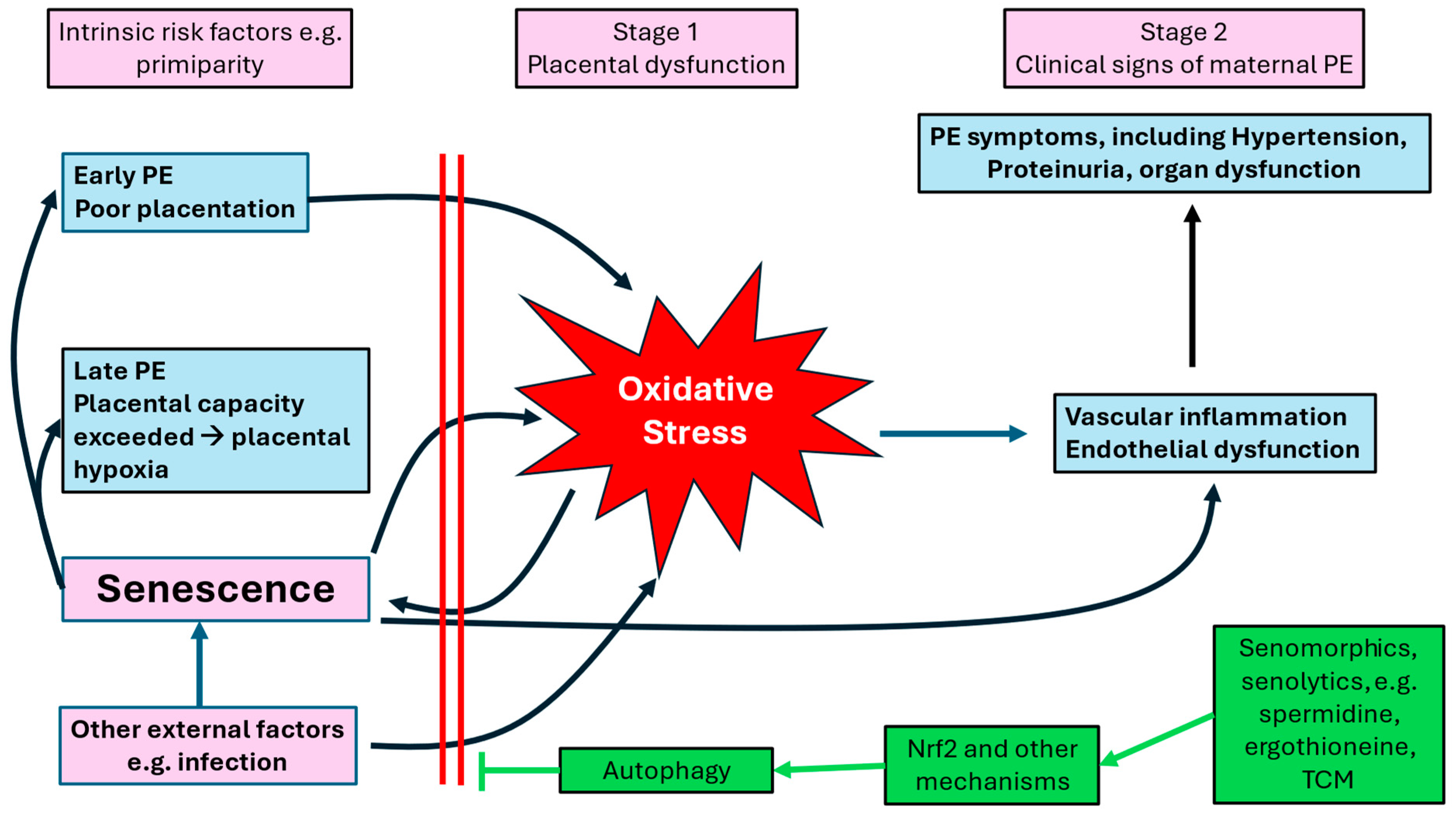
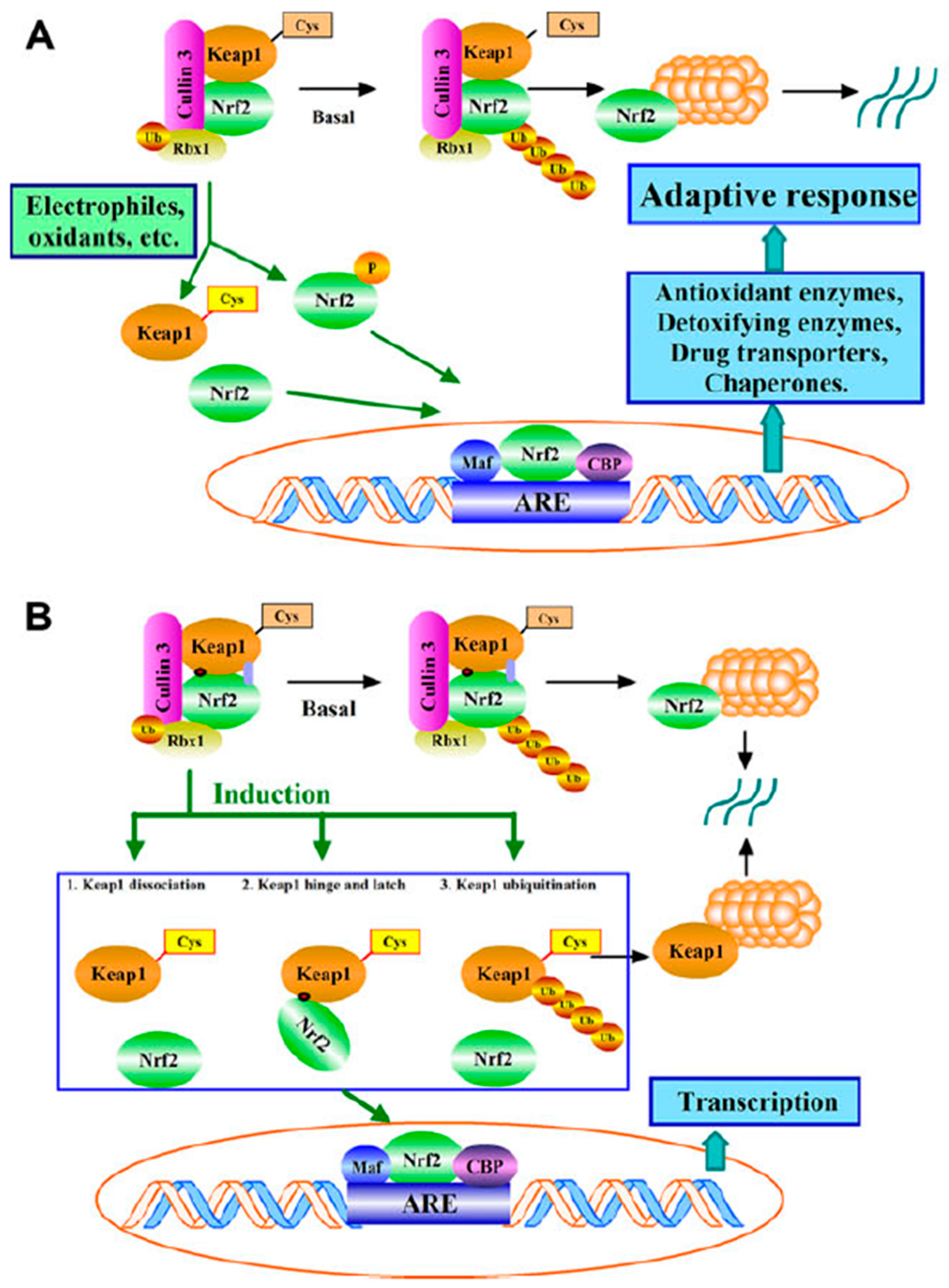
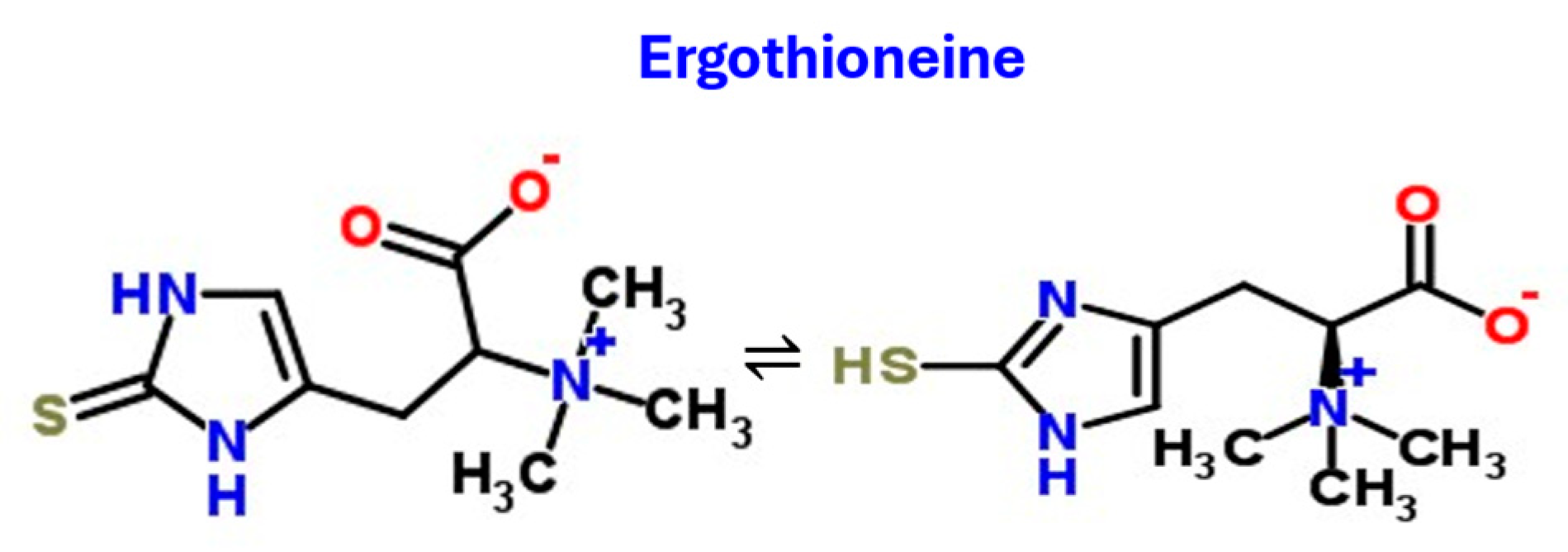
| Selected references | Senescence Biomarkers and proposals | Comments |
|---|---|---|
| (Barak et al. 2025) | Multiple, including FSTL3, VEGFA, and DKK1 | Measured via placental transcripts |
| (Chen et al. 2021b) | Reduced a-Klotho expression and increased levels of p21, p53, p16, and SAβG activity in advanced maternal age compared to placentas from young control donors | Maternal age leads to senescence and PE |
| (Cindrova-Davies et al. 2018) | PE placentas exhibited increased p21 and γH2AX levels compared to healthy control placentas. Evidence of oxidative stress-induced senescence | |
| (Cox and Redman 2017) | Review of multiple biomarkers including excessive telomere attrition in PE trophoblasts | Senescence also occurs in normal pregnancy development |
| (Davy et al. 2009) | Telomere shortening and increased p16 and p21 transcripts in foetal growth restriction placentas | Correlates with foetal growth restriction |
| (Farladansky-Gershnabel et al. 2019) | Decreased telomere length and increased levels of p16 transcripts and SAβG activity in PE compared to gestational age-matched healthy controls, particularly in early-onset PE | Telomere homeostasis worse in PE, more so in early onset PE |
| (Fox 1967) | Regressed villi seen as reflecting senescence | Early detection of senescence in placental villi |
| (Hu et al. 2022) | Nitro-L-arginine methyl ester-induced PE mouse model exhibited increased placental p53 and p21 levels; attenuated by cyclosporin A through induction of autophagy | Cyclosporin A relieves trophoblast apoptosis and senescence in mouse model of PE |
| (Hu et al. 2023) | Pregnant Rat Model of Polycystic Ovary Syndrome shows increased placental senescence (phospho-p53, p21, and γH2AX) |
Uses a Pregnant Rat Model of Polycystic Ovary Syndrome |
| (Huang et al. 2022) | Activation of Nrf2 by human placental extract helps delay replicative and oxidative stress-induced senescence in cultured human dermal fibroblasts | |
| (Kajdy et al. 2021) | Review of placental ageing (that may be considered to relate to senescence) | Includes foetal growth restriction and stillbirth |
| (Lee et al. 2022) | Decreased caveolin-1 and increased p53/p21, particularly in early compared to late-onset PE placentas | Senescence markers in PE |
| (Manna et al. 2019) | Review of multiple markers of aberrant senescence in adverse pregnancy outcomes | Relation to PE |
| (Negre-Salvayre et al. 2022) | Review, lipid oxidation products such as 4-hydroxy-2-nonenal present in severe PE and may drive oxidative stress-induced placental senescence | Stimulation of senescence |
| (Peng et al. 2025) | Various, including apelin and apoptotic markers | Apelin increases oxidative stress and senescence in PE |
| (Roh et al. 2024) | Increased SASP molecules in human PE serum and placenta, and PE placental SAβG+ and p21+ cells. Senolytic treatment with fisetin improved cardiac function in mouse model of peripartum cardiomyopathy | Assessed using serum proteomics |
| (Scaife et al. 2021) | Increased expression of p21 and levels of NOX4 and 8-OHdG (indicative of oxidative DNA damage) in PE compared to term normotensive placentae. Gestational age associated with increased placental p16 expression | Senescence biomarkers parallel oxidative stress |
| (Siddique and Cox 2022) | Gene expression analysis of placentas across several subtypes of PE show accelerated senescence | Increased downregulation of anti-senescence gene expression, e.g., CDK2 |
| (Sugulle et al. 2024) | Review of senescence and PE | Multiple senescence biomarkers summarised |
| (Sultana et al. 2018) | Review of senescence in pregnancy disorders | |
| (Suvakov et al. 2019) | Increased senescence (SAβG activity, and IL-6, IL-6, MCP-1, PAI-1, PA-2, p16, p21 mRNA expression) in mesenchymal stem cells from PE compared to normal pregnancies. Senolytic treatment of PE MSCs improved angiogenic potential | Inhibit angiogenesis in PE |
| (Suvakov et al. 2023) | Comprehensive review | |
| (Suvakov et al. 2024) | Multiple ageing markers | Related to those seen n PE and senescence |
| (Tao et al. 2023) | Increased senescence (high p53/p21, γH2AX and d-OHdG levels, and SAβG activity, low CDK2) in placentas from obese compared to non-obese pregnancies. Adipocyte-derived exosomes from obese donors contain NOX4; exposure of human trophoblasts to NOX4+ exosomes from obese human adipocytes induced senescence through oxidative damage | NOX4-mediated oxidative damage induces premature placental senescence in obese pregnancy |
| (Tasta et al. 2021) | Increased γH2AX+ DNA damaged cells with lipofuscin granules in PE compared to normal placentas. Induced by oxidative stress marker 4-hydroxy-2-nonenal | Induced by oxidative stress marker 4-hydroxy-2-nonenal |
| (Wang et al. 2022) | Multiple biomarkers (p21, p53, p16, pRb, SAβG activity) increased in PE compared to normal placentas; decrease in SIRT expression. SIRT1 activation by resveratrol decreases senescence in forskolin-activated cells | SIRT1 activation by resveratrol decreases senescence in forskolin-activated cells |
| (Zhang et al. 2024b) | Single cell sequencing shows exacerbation of senescence in placental mesenchymal stem/stromal cells from PE compared to healthy donors | Single cell sequencing shows senescence in PE |
| (Zhong et al. 2022) | Increased senescence (p16, p53, SAβG activity, decreased S-phase proliferation) in placental mesenchymal stem cells isolated from PE compared to healthy placentas. Related to increased TLR4 expression and decreased Hedgehog signalling. Suppression via LPS acting of TLR4 causing senescence as judged e.g., by SAβG activity | Suppression via LPS acting of TLR4 causing senescence as judged e.g., by SASP |
| (Zhu et al. 2022) | Gestational exposure to NO2 in mice drives reduced Sirt1 and Tert expression, leading to short telomeres and senescence | Gestational exposure to NO2 aggravates senescence |
| Literature references | Comments |
|---|---|
| (Chapple et al. 2015) | Review of the role of Nrf2-Keap1 in foetal protection in utero |
| (Chigusa et al. 2012) | Low placental Nrf2 activation in pre-eclampsia |
| (He et al. 2023) | Metformin is protective against pre-eclampsia by various mechanisms, including Nrf2 activation |
| (Ju et al. 2022) | A combined treatment of rats with apocyanin and aspirin activates the PI3K/Nrf2/HO-1 signaling pathway and is protective against pre-eclampsia |
| (Khadir et al. 2022) | Polymorphisms in the Nrf2 gene modulate the risk of pre-eclampsia |
| (Kweider et al. 2011, Kweider et al. 2012) | Interplay between VEGF and Nrf2 affects/ regulates pre-eclampsia |
| (Kweider et al. 2013, Kweider et al. 2014) | Role of the Nrf2/HO-1 pathway in preventing PE |
| (Li et al. 2020) | Here simultaneous downregulation of placental Nrf2 and sFlt1 improved maternal and fetal outcomes in a pre-eclampsia mouse model |
| (Liao et al. 2022) | Upregulating the Nrf2/GPX4 signalling pathway inhibits trophoblast ferroptosis and alleviates pre-eclampsia |
| (Liu et al. 2022b) | Use of procyanidin B2 to ameliorate dysfunction of endothelia and angiogenesis via Nrf2/PPARγ/sFlt-1 in pre-eclampsia |
| (Liu et al. 2025b) | Vitamin D3-driven foetal protection vs pre-eclampsia via Nrf2 |
| (Mundal et al. 2022) | Differences in Nrf2 between pre-eclampsia with and without Foetal Growth Restriction |
| (Muralimanoharan et al. 2018) | NRF2 promotes syncytiotrophoblast differentiation and is dysregulated in preeclampsia. |
| (Nezu et al. 2017) | Nrf2 inactivation enhances placental angiogenesis in a RAS-based mouse model of pre-eclampsia |
| (Padron et al. 2022) | Downregulation of Nrf2 in Primary Amnion Cells caused by stretch, and alleviation via Nrf2 stimulation |
| (Tantengco et al. 2021a) | Review of the role of Nrf2 in the pathophysiology of preeclampsia |
| (Tossetta et al. 2023) | Review, also discussing natural and synthetic compounds that can regulate he Nrf2/Keap1 pathway |
| (Wang et al. 2021a) | Inhibition of ERK/Nrf2 signalling pathway by lowering CD151 (a tetraspanin) induces oxidative stress in trophoblast cells in pre-eclampsia |
| (Xu et al. 2024) | Epigallocatechin gallate alleviates inflammation, endothelial dysfunction and placental ferroptosis, and improves pregnancy outcomes in PE-like rats via eNOS/Nrf2/HO-1 |
| (Yanagisawa et al. 2023) | Oxidative stress in preeclamptic placentae may activate the trophoblast ATX–LPA system via the Nrf2 pathway to effect protection |
| (Yang et al. 2020) | Astragaloside IV, a Traditional Chinese Medicine (TCM) component, ameliorates oxidative stress and pre-eclampsia via the Nrf2/HO-1 pathway in a rat model |
| (Yu et al. 2019) | The protective role of Nrf2 in PE is partially mediated via ATP-binding cassette transporters |
| (Zakeri et al. 2024) | Decreased expression of the Nrf2 gene in PE is mediated in part via epigenetic gene methylation |
| Literature Reference | Comments |
|---|---|
| (Chen et al. 2020) | Focus on role of TCM in Alzheimer’s Disease including reduction of b-amyloid via autophagy |
| (Chen et al. 2021a) | Attenuation of lipidosis in oxidised-LDL-stimulated macrophages by stimulating Beclin-1-induced autophagy |
| (Cui and Yu 2018) | Useful review of the use of TCM, especially natural products (Chuang et al. 2014), in autophagy |
| (Gao et al. 2019) | Inhibition of liver cancer growth via induction of autophagy and cell cycle arrest |
| (Han et al. 2023) | Role of autophagy, especially as stimulated by flavonoids, in ameliorating alcoholic liver disease |
| (He et al. 2025) | Acupuncture can modulate autophagy via LC3, Beclin1, p53, and autophagy-associated (ATG) protein expression. |
| (Huang et al. 2015) | Neuronal protection by autophagy in cerebral ischaemia, as stimulated by various TCM herbs |
| (Liu et al. 2017) | TCM herbal extracts inducing autophagy for treating nonalcoholic fatty liver disease |
| (Liu et al. 2022c) | Inhibition of colorectal cancer cell proliferation via autophagy induction |
| (Liu et al. 2024b) | Use of various active ingredients from TCM that modulate autophagy to reduce liver fibrosis |
| (Shi et al. 2022) | Use of various active ingredients from TCM that modulate autophagy for ameliorating glomerular diseases |
| (Tao et al. 2022) | Use of various active ingredients from TCM that modulate autophagy for ameliorating dementia |
| (Tian et al. 2023a) | Use of various active ingredients from TCM that modulate autophagy for ameliorating Systemic lupus erythematosus (‘Lupus’) |
| (Wang et al. 2015) | Use of various active ingredients from TCM that modulate autophagy for ameliorating myocardial ischaemia |
| (Wang et al. 2016) | Use of various active ingredients from TCM that modulate autophagy for ameliorating cancer and neurodegenerative diseases |
| (Wang et al. 2020, Wang et al. 2024a) | Role of Yishen Huazhuo decoction in reducing Alzheimer’s disease-related neuroinflammation and lowering Ab1-42 |
| (Wang et al. 2021b) | Role of TCM compounds in regulating autophagy for treating neurodegenerative diseases |
| (Wei et al. 2015) | Describes a formula for preventing autophagy in experimental stroke |
| (Wu et al. 2018a, Wu et al. 2018b) | TCM-induced cell growth inhibition, autophagy and apoptosis in prostate cancer via the EGFR pathway |
| (Wu et al. 2025) | The use of qili qiangxin capsule protects against myocardial ischemia-reperfusion injury via the suppression of autophagy |
| (Zhao et al. 2023) | Bibliometric analysis of 916 papers reporting on TCM and autophagy |
| (Zhu et al. 2017) | Focuses on Ka-Sai-Ping, a TCM formula that suppresses the growth of gastric cancers via induction of autophagy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





