Submitted:
19 February 2025
Posted:
20 February 2025
Read the latest preprint version here
Abstract
Keywords:

Introduction
Preamble, Audience, and Scope
A Note on Systems and Personalised Medicine
Blood Stasis
Fibrinaloid Microclots
Diseases Involving Blood Stasis Where Fibrinaloid Microclots Are Yet to Be Measured Directly
Cancer and Classical Amyloidoses
- (i)
- Cancer, particularly pancreatic cancer (PDAC), is strongly linked to thromboembolic states 369-386, with thrombotic events often leading to a worse outcome.
- (ii)
- Cancer is strongly associated (as is Long COVID) with fatigue 387-394
- (iii)
- Unsurprisingly, therefore, cancers are strongly associated with blood stasis 62, 65, 71, 300, 395-402
Amyloid Nature of the Blood Clots in Blood Stasis
A focus on Xue Fu Zhu Yu
Mechanism(s) of Action of Xuefu Zhuyu Decoction
Summarising and Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ko, K.M.; Leung; H.Y. The Essential Role of Zheng Qi in Promoting Health: From the Perspective of Chinese Medicine and Modern Medicine, Chinese Med 2024, 15, 27-33.
- Williams, R.J.; Individuality, B.; Wiley, J.; York, N. 1956.
- Henney, A.M. The promise and challenge of personalized medicine: aging populations, complex diseases, and unmet medical need, Croat Med J 2012, 53, 207-210.
- Mastrangelo, A.; Armitage, E.G.; Garcia, A.; Barbas, C. Metabolomics as a tool for drug discovery and personalised medicine. A review, Curr Top Med Chem 2015, 14, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Superchi, C.; Bouvier, F.B.; Gerardi, C.; Carmona, M.; Miguel, L.S.; Sanchez-Gomez, L.M.; Imaz-Iglesia, I.; Garcia, P.; Demotes, J.; Banzi, R. R. Porcher and PERMIT Group, Study designs for clinical trials applied to personalised medicine: a scoping review, BMJ Open 2022, 12, e052926.
- Kitano, H. ; Systems biology: a brief overview; Science 2002, 295, 1662-1664.
- Hood, L. ; Systems biology: integrating technology; biology; computation, Mech Ageing Dev 2003, 124, 9-16.
- Klipp, E.; Herwig, R.; Kowald, A.; Wierling, C.; Lehrach, H. Systems biology in practice: concepts, implementation and clinical application, Wiley/VCH, Berlin, 2005.
- Alon, U. An introduction to systems biology: design principles of biological circuits, Chapman and Hall/CRC, London, 2006.
- Noble, D. The music of life: biology beyond genes, Oxford University Press, Oxford, 2006.
- Palsson, B.Ø. Systems biology: properties of reconstructed networks, Cambridge University Press, Cambridge, 2006.
- Park, M.S.; Kim, J.; Kim, K.H.; Yoo, H.R.; Chae, I.; Lee, J.; Joo, I.H.; Kim, D.H. Modern concepts and biomarkers of blood stasis in cardio- and cerebrovascular diseases from the perspectives of Eastern and Western medicine: a scoping review protocol, JBI Evid Synth 2023, 21, 214-222.
- Auffray, C.; Chen, Z.; Hood, L. Systems medicine: the future of medical genomics and healthcare, Genome med 2009, 1, 2.
- Hood, L.; Flores, M. A personal view on systems medicine and the emergence of proactive P4 medicine: predictive, preventive, personalized and participatory, New Biotechnology 2012, 29, 613-624.
- Achenbach, J.; Tiikkainen, P.; Franke, L.; Proschak, E. Computational tools for polypharmacology and repurposing, Future Med Chem 2011, 3, 961-968.
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and Opportunities in Drug Discovery, J Med Chem 2014, 57, 7874-7887.
- Boran, A.D.W.; Iyengar, R. Systems approaches to polypharmacology and drug discovery, Curr Opin Drug Discov Devel 2010, 13, 297-309.
- Lavecchia, A.; Cerchia, C. In silico methods to address polypharmacology: current status, applications and future perspectives, Drug Discov Today 2016, 21, 288-298.
- Peters, J.U.; Friend, P.-F.O. J Med Chem 2013, 56, 8955-8971.
- Plake, C.; Schroeder, M. Computational polypharmacology with text mining and ontologies, Curr Pharm Biotechnol 2011, 12, 449-457.
- Reddy, A.S.; Zhang, S. Polypharmacology: drug discovery for the future, Expert Rev Clin Pharmacol 2013, 6, 41-47.
- Salentin, S.; Haupt, V.J.; Daminelli, S.; Schroeder, M. Polypharmacology rescored: Protein-ligand interaction profiles for remote binding site similarity assessment, Prog Biophys Mol Biol 2014, 116, 174-186.
- Xie, L.; Xie, L.; Kinnings, S.L.; Bourne, P.E. Novel computational approaches to polypharmacology as a means to define responses to individual drugs, Annu Rev Pharmacol Toxicol 2012, 52, 361-379.
- Chand, J.; Panda, S.R.; Jain, S.; Murty, U.S.N.; Das, A.M.; Kumar, G.J.; Naidu, V.G.M. Phytochemistry and polypharmacology of cleome species: A comprehensive Ethnopharmacological review of the medicinal plants, J Ethnopharmacol 2022, 282, 114600.
- Stefan, S.M.; Rafehi, M. Medicinal polypharmacology-a scientific glossary of terminology and concepts, Front Pharmacol 2024, 15, 1419110.
- Manzoni, O.J.; Manduca, A.; Trezza, V. Therapeutic potential of cannabidiol polypharmacology in neuropsychiatric disorders, Trends Pharmacol Sci 2025, 46, 145-162.
- Hopkins, A.L. Network pharmacology: the next paradigm in drug discovery, Nat Chem Biol 2008, 4, 682-690.
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Mäkelä, S.; Aittokallio, T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products, Nat Prod Rep 2015, 32, 1249-1266.
- Dagar, N.; Kale, A.; Jadhav, H.R.; Gaikwad, A.B. Nutraceuticals and network pharmacology approach for acute kidney injury: A review from the drug discovery aspect, Fitoterapia 2023, 168, 105563.
- Duan, X.; Wang, N.; Peng, D. Application of network pharmacology in synergistic action of Chinese herbal compounds, Theory Biosci 2024, 143, 195-203.
- Kacser, H.; Burns, J.A. The control of flux, in Rate Control of Biological Processes. Symposium of the Society for Experimental Biology Vol 27, ed. D. D. Davies, Cambridge University Press, Cambridge 1973, pp. 65-104.
- Heinrich, R.; Rapoport, T.A. Linear theory of enzymatic chains: its application for the analysis of the crossover theorem and of the glycolysis of human erythrocytes, Acta Biologica et Medica Germanica 1973, 31, 479-494.
- Heinrich, R.; Rapoport, T.A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength, Eur. J. Biochem. 1974, 42, 89–95. [Google Scholar] [PubMed]
- Kell, D.B.; Westerhoff, H.V. Metabolic control theory: its role in microbiology and biotechnology. FEMS Microbiol. Rev. 1986, 39, 305–320. [Google Scholar] [CrossRef]
- Fell, D.A. Metabolic Control Analysis - a survey of its theoretical and experimental development, Biochem. J. 1992, 286, 313–330. [Google Scholar]
- Cornish-Bowden, A.; Hofmeyr, J.-H.S.; Cárdenas, M.L. Strategies for manipulating metabolic fluxes in biotechnology, Bioorg Chem 1995, 23, 439-449.
- Heinrich, R.; Schuster, S. The regulation of cellular systems. Chapman & Hall, New York, 1996.
- Fell, D.A.; Saavedra, E.; Rohwer, J. 50 years of Metabolic Control Analysis: Its past and current influence in the biological sciences, Biosystems 2024, 235, 105086.
- Kitano, H. ; Biological robustness, Nat Rev Genet 2004, 5, 826-837.
- Milo, R.; Shen-Orr, S.; Itzkovitz, S.; Kashtan, N.; Chklovskii, D.; Alon, U. Network motifs: simple building blocks of complex networks, Science 2002, 298, 824-827.
- Small, B.G.; McColl, B.W.; Allmendinger, R.; Pahle, R.; Lopez-Castejon, G.; Rothwell, N.J.; Knowles, J.; Mendes, P.; Brough, D.; Kell, D.B. Efficient discovery of anti-inflammatory small molecule combinations using evolutionary computing Nature Chem Biol 2011, 7, 902-908.
- Ihekwaba, A.E.C.; Broomhead, D.S.; Grimley, R.; Benson, N.; White, M.R.H.; Kell, D.B. Synergistic control of oscillations in the NF-kB signalling pathway, IEE Systems Biology 2005, 152, 153-160.
- Yao, Y.; Zhang, X.; Wang, Z.; Zheng, C.; Li, P.; Huang, C.; Tao, W.; Xiao, W.; Wang, Y.; Huang, L.; Yang, L. Deciphering the combination principles of Traditional Chinese Medicine from a systems pharmacology perspective based on Ma-huang Decoction, J Ethnopharmacol 2013, 150, 619-638.
- Zhao, X.; Zheng, X.; Fan, T.-P.; Li, Z.; Zhang, Y.; Zhang, J. A novel drug discovery strategy inspired by traditional medicine philosophies, Science 2015, 347, S38-S40.
- Zhong, Y.; Luo, J.; Tang, T.; Li, P.; Liu, T.; Cui, H.; Wang, Y.; Huang, Z. Exploring Pharmacological Mechanisms of Xuefu Zhuyu Decoction in the Treatment of Traumatic Brain Injury via a Network Pharmacology Approach, Evid Based Complement Alternat Med 2018, 2018, 8916938.
- Zhao, M.; Chen, Y.; Wang, C.; Xiao, W.; Chen, S.; Zhang, S.; Yang, L.; Li, Y. Systems Pharmacology Dissection of Multi-Scale Mechanisms of Action of Huo-Xiang-Zheng-Qi Formula for the Treatment of Gastrointestinal Diseases, Front Pharmacol 2018, 9, 1448.
- Chen, Y.; Zheng, J.-N.; Fang, J.; Li, S.-G.; Guan, J.-L.; Yang, P. ; Application of “Monarch, Minister, Assistant and Envoy” Principle to Rheumatoid Arthritis Management, J Rheum Arth Dis 2017, 2, 1-4.
- Dobson, P.D.; Kell, D.B. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? , Nat Rev Drug Disc 2008, 7, 205–220. [Google Scholar] [CrossRef]
- Kell, D.B.; Dobson, P.D.; Oliver, S.G. Pharmaceutical drug transport: the issues and the implications that it is essentially carrier-mediated only. Drug Disc Today 2011, 16, 704–714. [Google Scholar] [CrossRef]
- Kell, D.B.; Oliver, S.G. How drugs get into cells: tested and testable predictions to help discriminate between transporter-mediated uptake and lipoidal bilayer diffusion, Front Pharmacol 2014, 5, 231.
- Grixti, J.; S. O'Hagan; Day, P.J.; Kell, D.B. Enhancing drug efficacy and therapeutic index through cheminformatics-based selection of small molecule binary weapons that improve transporter-mediated targeting: a cytotoxicity system based on gemcitabine, Front Pharmacol 2017, 8, 155.
- Kell, D.B. The transporter-mediated cellular uptake and efflux of pharmaceutical drugs and biotechnology products: how and why phospholipid bilayer transport is negligible in real biomembranes. Molecules 2021, 26, 5629. [Google Scholar] [CrossRef]
- O'Hagan; Kell, D.B. Consensus rank orderings of molecular fingerprints illustrate the ‘most genuine’ similarities between marketed drugs and small endogenous human metabolites, but highlight exogenous natural products as the most important ‘natural’ drug transporter substrates. ADMET & DMPK 2017, 5, 85–125.
- Mestres, J.; Gregori-Puigjané, E.; Valverde, S.; Solé, R.V. The topology of drug-target interaction networks: implicit dependence on drug properties and target families, Mol Biosyst 2009, 5, 1051-1057.
- Li, S.; Zhang, B.; Jiang, D.; Wei, Y.; Zhang, N. Herb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulae, BMC Bioinformatics 2010, 11 Suppl 11, S6.
- Su, X.; Yao, Z.; Li, S.; Sun, H. Synergism of Chinese Herbal Medicine: Illustrated by Danshen Compound, Evid Based Complement Alternat Med 2016, 2016, 7279361.
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis, Fitoterapia 2014, 92, 133-147.
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research, Front Pharmacol 2016, 7, 201.
- Rigby, S.P. Uses of Molecular Docking Simulations in Elucidating Synergistic, Additive, and/or Multi-Target (SAM) Effects of Herbal Medicines, Molecules 2024, 29.
- Yan, D.-X. Aging and blood stasis: a new TCM approach to geriatrics, Blue Poppy Press, Boulder, CO, 2015.
- Chen, K.J. Blood stasis syndrome and its treatment with activating blood circulation to remove blood stasis therapy, Chin J Integr Med 2012, 18, 891-896.
- Choi, T.Y.; Jun, J.H.; Lee, J.A.; Park, B.; You, S.; Jung, J.; Lee, M.S. Expert opinions on the concept of blood stasis in China: An interview study, Chin J Integr Med 2016, 22, 823-831.
- Li, S.M.; Xu, H.; Chen, K.J. The diagnostic criteria of blood-stasis syndrome: considerations for standardization of pattern identification, Chin J Integr Med 2014, 20, 483-489.
- Park, B.; You, S.; Jung, J.; Lee, J.A.; Yun, K.J.; Lee, M.S. Korean studies on blood stasis: an overview, Evid Based Complement Alternat Med 2015, 2015, 316872.
- Hao, Q.-Y.; Jiang, Z.-X.; Wang, X.; Lu, H.-P.; Zhu, Y.; Liu, Y.-L.; Li, K.; Xie, Y.-Q. Studies on blood stasis in the traditional medicine of the Li Culture, Hist Philos Med 2021, 3, 18.
- You, S.; Kang, B.K.; Kim, J.; Lee, H.; Shim, E.H.; Ko, M.M.; Choi, J.; Choi, T.Y.; Jun, J.H.; Jung, J.; Cha, M.; Lee, J.A.; Lee, M.S. Four Subgroups of Blood Stasis Syndrome Are Identified by Manifestation Cluster Analysis in Males, Evid-Based Compl Alt 2019, 2019.
- Choi, T.Y.; Jun, J.H.; Park, B.; Lee, J.A.; You, S.S.; Jung, J.Y.; Lee, M.S. Concept of blood stasis in Chinese medical textbooks: A systematic review, Eur J Integr Med 2016, 8, 158-164.
- Liu, L.; Liang, Y.-B.; Liu, X.-L.; Wang, H.-Q.; Qi, Y.-F.; Wang, M.; Chen, B.-X.; Zhou, Q.-B.; Tong, W.-X.; Zhang, Y. Untargeted metabolomics combined with pseudotargeted lipidomics revealed the metabolite profiles of blood-stasis syndrome in type 2 diabetes mellitus, Heliyon 2024, 10, e39554.
- Sun, Z.; Ping, P.; Li, Y.; Feng, L.; Liu, F.; Zhao, Y.; Yao, Y.; Zhang, P.; Fu, S. Relationships Between Traditional Chinese Medicine Constitution and Age-Related Cognitive Decline in Chinese Centenarians, Front Aging Neurosci 2022, 14, 870442.
- Luo, X.; Xie, J.; Huang, L.; Gan, W.; Chen, M. Efficacy and safety of activating blood circulation and removing blood stasis of Traditional Chinese Medicine for managing renal fibrosis in patients with chronic kidney disease: a systematic review and Meta-analysis, J Tradit Chin Med 2023, 43, 429-440.
- Liao, J.; Wang, J.; Liu, Y.; Li, J.; Duan, L.; Chen, G.; Hu, J. Modern researches on Blood Stasis syndrome 1989-2015: A bibliometric analysis, Medicine (Baltimore) 2016, 95, e5533.
- Yan, J.; Dong, Y.; Niu, L.; Cai, J.; Jiang, L.; Wang, C.; Xing, J. Clinical effect of Chinese herbal medicine for removing blood stasis combined with acupuncture on sequelae of cerebral infarction, Am J Transl Res 2021, 13, 10843-10849.
- Rosenthal, L.; Hernandez, P.; Vaamonde, D.; Chinese medicine, T. ; Ayurveda; fertility Fertility; Pregnancy; Wellness 2022, 209-247.
- Birch, S.; Alraek, T.; Lee, M.S.; Lee, J.A.; Kim, T.-H. Understanding blood stasis in traditional East Asian medicine: a comparison of Asian and Western sources, Eur J Integr Med 2021, 44, 101341.
- Zhai, X.; Wang, X.; Wang, L.; Xiu, L.; Wang, W.; Pang, X. Treating Different Diseases With the Same Method-A Traditional Chinese Medicine Concept Analyzed for Its Biological Basis, Front Pharmacol 2020, 11, 946.
- Terasawa, K.; Shinoda, H.; Imadaya, A.; Tosa, H.; Bandoh, M.; Satoh, N. ; The presentation of diagnostic criteria for “Yuxie” (stagnated blood) conformation, Int J Oriental Med 1989, 14, 194–213.
- Matsumoto, C.; Kojima, T.; Ogawa, K.; Kamegai, S.; Oyama, T.; Shibagaki, Y.; Kawasaki, T.; Fujinaga, H.; Takahashi, K.; Hikiami, H.; Goto, H.; Kiga, C.; Koizumi, K.; Sakurai, H.; Muramoto, H.; Shimada, Y.; Yamamoto, M.; Terasawa, K.; Takeda, S.; Saiki, I. A Proteomic Approach for the Diagnosis of 'Oketsu' (blood stasis), a Pathophysiologic Concept of Japanese Traditional (Kampo) Medicine, Evid Based Complement Alternat Med 2008, 5, 463-474.
- Wang, S.; Wang, J.; Li, J. Relationship between the Gensini Score of Blood-Stasis Syndrome in Coronary Heart Disease and VEGF, World Sci Technol 2010, 12, 355-357.
- Cho, K.H.; Kim, K.P.; Woo, B.C.; Kim, Y.J.; Park, J.Y.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Park, J.M.; Moon, S.K. Relationship between Blood Stasis Syndrome Score and Cardioankle Vascular Index in Stroke Patients, Evid Based Complement Alternat Med 2012, 2012, 696983.
- Goto, H. Blood stasis syndrome in Japan and its molecular biological analysis, Chin J Integr Med 2014, 20, 490-495.
- Liao, J.; Liu, Y.; Wang, J. Identification of more objective biomarkers for Blood-Stasis syndrome diagnosis, BMC Complement Altern Med 2016, 16, 371.
- Kang, B.K.; Park, T.Y.; Jung, J.; Ko, M.; Lee, M.S.; Lee, J.A. The Optimal Cut-Off Value of Blood Stasis Syndrome Score in BSS Diagnosis in Korea, Evid Based Complement Alternat Med 2017, 2017, 8049481.
- Zhou, X.; Li, X.T.; Liu, X.Q.; Wang, B.; Fang, G. Assessment of Intermingled Phlegm and Blood Stasis Syndrome in Coronary Heart Disease: Development of a Diagnostic Scale, Evid Based Complement Alternat Med 2018, 2018, 4683431.
- Zhao, Y.; Peng, H.; Wang, S.; Liu, J. Clinical analysis of acute coronary syndrome patients with Qi-blood syndromes: establishment of a diagnostic prediction model for syndrome differentiation, Ann Palliat Med 2020, 9, 2096-2110.
- Morita, A.; Murakami, A.; Noguchi, K.; Watanabe, Y.; Nakaguchi, T.; Ochi, S.; Okudaira, K.; Hirasaki, Y.; Namiki, T. Combination Image Analysis of Tongue Color and Sublingual Vein Improves the Diagnostic Accuracy of Oketsu (Blood Stasis) in Kampo Medicine, Front Med (Lausanne) 2021, 8, 790542.
- Chen, K.-J.; Xu, H. International Diagnostic Guidelines for Blood-Stasis Syndrome, Chinese J Integr Med 2022, 28, 297-303.
- Morita, A.; Murakami, A.; Watanabe, Y.; Tamura, Y.; Suganami, A.; Shiko, Y.; Kawasaki, Y.; Nakaguchi, T.; Ochi, S.; Okudaira, K.; Hirasaki, Y.; Namik, T. The association in Kampo medicine between Oketsu (blood stasis) and sublingual vein width of the tongue on a tongue image analyzing system, Tradit Kampo Med 2020, 7, 108-112.
- Mchedlishvili, G. Disturbed blood flow structuring as critical factor of hemorheological disorders in microcirculation, Clin Hemorheol Microcirc 1998, 19, 315-325.
- Zhang, J.X.; Feng, Y.; Zhang, Y.; Liu, Y.; Li, S.D.; Yang, M.H. Hemorheology Index Changes in a Rat Acute Blood Stasis Model: A Systematic Review and Meta-Analysis, Afr J Tradit Complement Altern Med 2017, 14, 96-107.
- Dalton, C.F.; de Oliveira, M.I.R.; Stafford, P.; Peake, N.; Kane, B.; Higham, A.; Singh, D.; Jackson, N.; Davies, H.; Price, D.; Duncan, R.; Tattersall, N.; Barnes, A.; Smith, D.P. Increased fibrinaloid microclot counts in platelet-poor plasma are associated with Long COVID, medRxiv 2024, 2024.2004.2004.24305318.
- Bagot, C.N.; Arya, R. Virchow and his triad: a question of attribution, Br J Haematol 2008, 143, 180-190.
- Mehta, J.L.; Calcaterra, G.; Bassareo, P.P.; COVID-19; thromboembolic risk, and Virchow's triad: Lesson from the past, Clin Cardiol 2020, 43, 1362-1367.
- Ahmed, S.; Zimba, O.; Gasparyan, A.Y. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad, Clin Rheumatol 2020, 39, 2529-2543.
- Wolberg, A.S.; Aleman, M.M.; Leiderman, K.; Machlus, K.R. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited, Anesth Analg 2012, 114, 275-285.
- Gonzalez-Gonzalez, F.J.; Ziccardi, M.R.; McCauley, M.D. Virchow's Triad and the Role of Thrombosis in COVID-Related Stroke, Front Physiol 2021, 12, 769254.
- Ząbczyk, M.; Stachowicz, A.; Natorska, J.; Olszanecki, R.; Wiśniewski, J.R.; Undas, A. Plasma fibrin clot proteomics in healthy subjects: Relation to clot permeability and lysis time, J Proteomics 2019, 208, 103487.
- Kell, D.B.; Pretorius, E. Proteomic evidence for amyloidogenic cross-seeding in fibrinaloid microclots, bioRxiv 2024, 2024.2007.2016.603837.
- Swanepoel, A.C.; Lindeque, B.G.; Swart, P.J.; Abdool, Z.; Pretorius, E. Estrogen causes ultrastructural changes of fibrin networks during the menstrual cycle: a qualitative investigation, Microsc Res Tech 2014, 77, 594-601.
- Swanepoel, A.C.; Lindeque, B.G.; Swart, P.J.; Abdool, Z.; Pretorius, E. Ultrastructural changes of fibrin networks during three phases of pregnancy: a qualitative investigation, Microsc Res Tech 2014, 77, 602-608.
- Jankun, J.; Landeta, P.; Pretorius, E.; Skrzypczak-Jankun, E.; Lipinski, B. Unusual clotting dynamics of plasma supplemented with iron(III), Int J Mol Med 2014, 33, 367-372.
- Lipinski, B.; Pretorius, E.; Oberholzer, H.M.; Van Der Spuy, W.J. Iron enhances generation of fibrin fibers in human blood: Implications for pathogenesis of stroke, Microsc Res Tech 2012, 75, 1185-1190.
- Lipinski, B.; Pretorius, E. Novel pathway of iron-induced blood coagulation: implications for diabetes mellitus and its complications, Pol Arch Med Wewn 2012, 122, 115-122.
- Pretorius, E.; Vermeulen, N.; Bester, J.; Lipinski, B.; Kell, D.B. A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy, Toxicol Mech Methods 2013, 23, 352-359.
- Pretorius, E.; Bester, J.; Vermeulen, N.; Lipinski, B.; Gericke, G.S.; Kell, D.B. Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents, PLoS One 2014, 9, e85271.
- Pretorius, E.; Kell, D.B. Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases, Integrative Biol 2014, 6, 486-510.
- Pretorius, E.; Vermeulen, N.; Bester, J.; Lipinski, B. Novel use of scanning electron microscopy for detection of iron-induced morphological changes in human blood, Microsc Res Tech 2013, 76, 268-271.
- Pretorius, E.; Lipinski, B. Differences in morphology of fibrin clots induced with thrombin and ferric ions and its pathophysiological consequences, Heart Lung Circ 2013, 22, 447-449.
- Swanepoel, A.C.; Visagie, A.; de Lange, Z.; Emmerson, O.; Nielsen, V.G.; Pretorius, E. The clinical relevance of altered fibrinogen packaging in the presence of 17beta-estradiol and progesterone, Thromb Res 2016, 146, 23-34.
- Kell, D.B.; Pretorius, E. Potential roles of fibrinaloid microclots in fibromyalgia syndrome, OSF preprint 2024, https://osf.io/9e2y5/.
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils, Biochim Biophys Acta 2010, 1804, 1405-1412.
- Amdursky, N.; Erez, Y.; Huppert, D. Molecular rotors: what lies behind the high sensitivity of the thioflavin-T fluorescent marker, Acc Chem Res 2012, 45, 1548-1557.
- Malmos, K.G.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: a primer on the use of thioflavin T to investigate amyloid formation, Amyloid 2017, 24, 1-16.
- Pretorius, E.; Mbotwe, S.; Bester, J.; Robinson, C.J.; Kell, D.B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide, J R Soc Interface 2016, 123, 20160539.
- Pretorius, E.; Mbotwe, S.; Kell, D.B. Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular comorbidities, Sci Rep 2017, 7, 9680.
- Kell, D.B.; Pretorius, E. No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases, Biol Rev 2018, 93, 1518–1557. [Google Scholar] [PubMed]
- Kell, D.B.; Pretorius, E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting, Progr Biophys Mol Biol 2017, 123, 16–41. [Google Scholar]
- Kell, D.B.; Pretorius, E. Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases? , Biochem J 2023, 480, 1217–1240. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Page, M.J.; Hendricks, L.; Nkosi, N.B.; Benson, S.R.; Kell, D.B. Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains, J R Soc Interface 2018, 15, 20170941.
- Huang, H.; Hou, J.; Li, M.; Wei, F.; Liao, Y.; Xi, B. Microplastics in the bloodstream can induce cerebral thrombosis by causing cell obstruction and lead to neurobehavioral abnormalities, Sci Adv 2025, 11, eadr8243.
- Mallapaty, S. Microplastics block blood flow in the brain, mouse study reveals, Nature 2025, 638, 20.
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications, Biochem J 2022, 479, 537-559.
- Götz, J.; Ittner, L.M.; Lim, Y.A. Common features between diabetes mellitus and Alzheimer's disease, Cell Mol Life Sci 2009, 66, 1321-1325.
- Janson, J.; Laedtke, T.; Parisi, J.E. ; P. O'Brien; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease, Diabetes 2004, 53, 474–481. [Google Scholar]
- Li, L.; Hölscher, C. Common pathological processes in Alzheimer disease and type 2 diabetes: A review, Brain Research Reviews 2007, 56, 384-402.
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer's disease: from epidemiology to mechanism and treatment, Clin Interv Aging 2015, 10, 549-560.
- Luchsinger, J.A.; Gustafson, D.R. ; Adiposity; type; Alzheimer's disease, J Alzheimers Dis 2009, 16, 693-704.
- Mascitelli, L.; Pezzetta, F.; Goldstein, M.R. ; Iron; type; Alzheimer's disease, Cell Mol Life Sci 2009, 66, 2943.
- Miklossy, J.; McGeer, P.L. Common mechanisms involved in Alzheimer's disease and type 2 diabetes: a key role of chronic bacterial infection and inflammation, Aging (Albany NY) 2016, 8, 575-588.
- Mittal, K.; Katare, D.P. Shared links between type 2 diabetes mellitus and Alzheimer's disease: A review, Diabetes Metab Syndr 2016, 10, S144-149.
- Moroz, N.; Tong, M.; Longato, L.; Xu, H.; de la Monte, S.M. Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus, J Alzheimers Dis 2008, 15, 29-44.
- Oskarsson, M.E.; Paulsson, J.F.; Schultz, S.W.; Ingelsson, M.; Westermark, P.; Westermark, G.T. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease, Am J Pathol 2015, 185, 834-846.
- Taguchi, A. Vascular factors in diabetes and Alzheimer's disease, J Alzheimers Dis 2009, 16, 859-864.
- Toro, P.; Schonknecht, P.; Schroder, J. Type II diabetes in mild cognitive impairment and Alzheimer's disease: results from a prospective population-based study in Germany, J Alzheimers Dis 2009, 16, 687-691.
- Vignini, A.; Giulietti, A.; Nanetti, L.; Raffaelli, F.; Giusti, L.; Mazzanti, L.; Provinciali, L. Alzheimer's disease and diabetes: new insights and unifying therapies, Current diabetes reviews 2013, 9, 218-227.
- Xu, W.L.; von Strauss, E.; Qiu, C.X.; Winblad, B.; Fratiglioni, L. Uncontrolled diabetes increases the risk of Alzheimer's disease: a population-based cohort study, Diabetologia 2009, 52, 1031-1039.
- Zhao, W.Q.; Townsend, M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer's disease, Biochim Biophys Acta 2009, 1792, 482-496.
- Zhang, C.-J.; Zhang, X.-J.; Chen, L.-F.; Lai, S.-G.; Wu, Q. The mechanism of different diseases treated by the same way of acupuncture at Chanqqianq for cognitive disorder, World J Acupunc Moxibust 2016, 26, 24-29.
- Zhang, X.; Zhou, L.; Qian, X.; Mechanism of, T. “Treating Different Diseases with the Same Treatment” by Qiangji Jianpi Decoction in AnkylosingSpondylitis Combined with Inflammatory Bowel Disease: A Comprehensive Analysis of Multiple Methods, Gastroenterology Research and Practice 2024, 2024, 9709260.
- Fu, S.F.; Yi, W.; Ai, J.Q.; Bah, A.J.; Gao, X.M. Clinical application of "treating different diseases with the same method" - Xuefu Zhuyu Capsule (a traditional Chinese patent medicine) for blood stasis syndrome, Journal of the American College of Cardiology 2014, 64, C208-C209.
- Bester, J.; Soma, P.; Kell, D.B.; Pretorius, E. Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer-type dementia, and the possible role of bacterial lipopolysaccharides (LPS), Oncotarget Gerontology 2015, 6, 35284-35303.
- de Waal, G.M.; Engelbrecht, L.; Davis, T.; de Villiers, W.J.S.; Kell, D.B.; Pretorius, E. Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus, Sci Rep 2018, 8, 16798.
- Pretorius, E.; Bester, J.; Kell, D.B. A bacterial component to Alzheimer-type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease J Alzheimers Dis 2016, 53, 1237-1256.
- Pretorius, E.; Bester, J.; Page, M.J.; Kell, D.B. The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia, Frontiers Aging Neurosci 2018, 10, 257.
- Yeh, C.W.; Liu, H.K.; Lin, L.C.; Liou, K.T.; Huang, Y.C.; Lin, C.H.; Tzeng, T.T.; Shie, F.S.; Tsay, H.J.; Shiao, Y.J. Xuefu Zhuyu decoction ameliorates obesity, hepatic steatosis, neuroinflammation, amyloid deposition and cognition impairment in metabolically stressed APPswe/PS1dE9 mice, J Ethnopharmacol 2017, 209, 50-61.
- Tao, P.; Xu, W.; Gu, S.; Shi, H.; Wang, Q.; Xu, Y. Traditional Chinese medicine promotes the control and treatment of dementia, Front Pharmacol 2022, 13, 1015966.
- Tao, P.; Ji, J.; Gu, S.; Wang, Q.; Xu, Y. Progress in the Mechanism of Autophagy and Traditional Chinese Medicine Herb Involved in Dementia, Front Pharmacol 2022, 12, 825330.
- Grobbelaar, L.M.; Venter, C.; Vlok, M.; Ngoepe, M.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19, Biosci Rep 2021, 41, BSR20210611.
- Grobbelaar, L.M.; Kruger, A.; Venter, C.; Burger, E.M.; Laubscher, G.J.; Maponga, T.G.; Kotze, M.J.; Kwaan, H.C.; Miller, J.B.; Fulkerson, D.; Huff, W.; Chang, E.; Wiarda, G.; Bunch, C.M.; Walsh, M.M.; Raza, S.; Zamlut, M.; Moore, H.B.; Moore, E.E.; Neal, M.D.; Kell, D.B.; Pretorius, E. Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Haemost 2022, 48, 858–868. [Google Scholar] [CrossRef]
- Laubscher, G.J.; Lourens, P.J.; Venter, C.; Kell, D.B.; Pretorius, E. ; TEG®, Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy, J Clin Med 2021, 10, 5381.
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B. Prevalence of amyloid blood clots in COVID-19 plasma, medRxiv 2020, 2020.2007.2028.20163543v20163541.
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B. Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: A preliminary report, Cardiovasc Diabetol 2020, 19, 193.
- Guo, H.; Zheng, J.; Huang, G.; Xiang, Y.; Lang, C.; Li, B.; Huang, D.; Sun, Q.; Luo, Y.; Zhang, Y.; Huang, L.; Fang, W.; Zheng, Y.; Wan, S. Xuebijing injection in the treatment of COVID-19: a retrospective case-control study, Ann Palliat Med 2020, 9, 3235-3248.
- Wu, D.Y.; Hou, X.T.; Xia, Z.S.; Hao, E.W.; Xie, J.L.; Liang, J.Y.; Liang, Q.M.; Du, Z.C.; Deng, J.G. Analysis on oral medication rules of traditional Chinese medicine prescriptions for prevention of COVID-19, Chin Herb Med 2021, 13, 502-517.
- Ling, L.; Wang, X.; Zhang, Y.; Yin, F.; Zhang, Z.; Lyu, X. Efficacy of Qingfei Paidu Granules combined with non-drug traditional Chinese medicine therapy in the treatment of patients with asymptomatic coronavirus disease: A retrospective study, Medicine (Baltimore) 2023, 102, e34868.
- Ruan, J.; Chen, S.; Ho, Y.S.; Wong, V.T.; Lam, M.Y.; Tsang, H.W.H.; Cheng, I.H.; Yeung, W.F. Chinese medicine practitioners’ consensus on traditional Chinese medicine diagnostic patterns, symptoms, and herbal formulas for COVID-19 survivors: A Delphi study, Eur J Chinese Med 2024, 66, 102339.
- Zhang, H.; Liu, Y.; Shang, X.; Cao, Y.; Li, J.; Chen, G.; Ji, X.; Zhang, L.; Fan, Y.; Ma, Y. Status and hotspot analysis of Qingfei Paidu Decoction for the prevention and treatment of COVID-19 based on bibliometric analysis, Front Pharmacol 2024, 15, 1422773.
- Zhang, Q.; Liang, Z.; Wang, X.; Zhang, S.; Yang, Z. Exploring the potential mechanisms of Danshen against COVID-19 via network pharmacology analysis and molecular docking, Sci Rep 2024, 14, 12780.
- Zhou, X.; Cheng, Z.; Hu, Y. COVID-19 and Venous Thromboembolism: From Pathological Mechanisms to Clinical Management, J Pers Med 2021, 11, 1328.
- Yang, Z.; Liu, Y.; Wang, L.; Lin, S.; Dai, X.; Yan, H.; Ge, Z.; Ren, Q.; Wang, H.; Zhu, F.; Wang, S. Traditional Chinese medicine against COVID-19: Role of the gut microbiota, Biomed Pharmacother 2022, 149, 112787.
- Pretorius, E.; Oberholzer, H.M.; van der Spuy, W.J.; Swanepoel, A.C.; Soma, P. Qualitative scanning electron microscopy analysis of fibrin networks and platelet abnormalities in diabetes, Blood Coagul Fibrinol 2011, 22, 463-467.
- Pretorius, E.; Bester, J.; Vermeulen, N.; Alummoottil, S.; Soma, P.; Buys, A.V.; Kell, D.B. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics, Cardiovasc Diabetol 2015, 134, 30.
- Pretorius, E.; Page, M.J.; Engelbrecht, L.; Ellis, G.C.; Kell, D.B. Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains, Cardiovasc Diabetol 2017, 16, 141.
- Wei, J.; Wu, R.; Zhao, D. Analysis on traditional Chinese medicine syndrome elements and relevant factors for senile diabetes, J Tradit Chin Med 2013, 33, 473-478.
- Xu, J.; Bai, L.; Ma, E.; Guo, Q.; Wang, Y.; Zhang, M.; Chen, Z. Correlativity between blood measures related to blood stasis blocking collaterals and gene expression of angiotensin-converting enzyme of renal cortex in diabetic rats and effect of stasis removing and collaterals dredging, J Tradit Chin Med 2014, 34, 597-603.
- Wang, J.; Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, T.; Wang, C.; Wang, T.; Zhao, B. Research progress on Traditional Chinese Medicine syndromes of diabetes mellitus, Biomed Pharmacother 2020, 121, 109565.
- Tanaka, K.; Chiba, K.; Nara, K. A Review on the Mechanism and Application of Keishibukuryogan, Front Nutr 2021, 8, 760918.
- Kell, D.B.; Pretorius, E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: evidence, mechanisms, and therapeutic implications, Biochem J 2022, 479, 1653-1708.
- Kruger, A.; Vlok, M.; Turner, S.; Venter, C.; Laubscher, G.J.; Kell, D.B.; Pretorius, E. Proteomics of fibrin amyloid microclots in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system, Cardiovasc Diabetol 2022, 21, 190.
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin, Cardiovasc Diabetol 2021, 20, 172.
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. ; Prevalence of symptoms; comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc Diabetol 2022, 21, 148.
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: pathophysiological factors and abnormal coagulation, Trends Endocrinol Metab 2023, 34, 321-344.
- Turner, S.; Naidoo, C.A.; Usher, T.J.; Kruger, A.; Venter, C.; Laubscher, G.J.; Khan, M.A.; Kell, D.B.; Pretorius, E. Increased Levels of Inflammatory and Endothelial Biomarkers in Blood of Long COVID Patients Point to Thrombotic Endothelialitis, Semin Thromb Hemost, 2023.
- Turner, S.; Laubscher, G.J.; Khan, M.A.; Kell, D.B.; Pretorius, E. Accelerating discovery: A novel flow cytometric method for detecting fibrin(ogen) amyloid microclots using long COVID as a model Heliyon 2023, 9, e19605.
- Ono, R.; Arita, R.; Takayama, S.; Kanno, T.; Kikuchi, A.; Suzuki, S.; Ohsawa, M.; Saito, N.; Abe, M.; Onodera, K.; Akaishi, T.; Tadano, Y.; Ishii, T.; Progress; treatment of “long COVID”in non-hospitalized patients: A single-centerretrospective cohort study, Trad Kampo Med 2023, 10, 150-158.
- Hu, L.Y.; Cai, A.Q.; Li, B.; Li, Z.; Liu, J.P.; Cao, H.J. Chinese herbal medicine for post-viral fatigue: A systematic review of randomized controlled trials, PLoS One 2024, 19, e0300896.
- de Villiers, S.; Bester, J.; Kell, D.B.; Pretorius, E. Erythrocyte health and the possible role of amyloidogenic blood clotting in the evolving haemodynamics of female migraine-with-aura pathophysiology: Results from a pilot study, Frontiers Neurol 2019, 10, 1262.
- Shan, C.S.; Xu, Q.Q.; Shi, Y.H.; Wang, Y.; He, Z.X.; Zheng, G.Q. Chuanxiong Formulae for Migraine: A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials, Front Pharmacol 2018, 9, 589.
- Nunes, J.M.; Kruger, A.; Proal, A.; Kell, D.B.; Pretorius, E. The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), Pharmaceuticals (Basel) 2022, 15, 931.
- Nunes, J.M.; Kell, D.B.; Pretorius, E. Cardiovascular and haematological pathology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a role for Viruses, Blood Rev 2023, 60, 101075.
- Li, Y.; Yang, J.; Chau, C.I.; Shi, J.; Chen, X.; Hu, H.; Ung, C.O.L. Is there a role for traditional and complementary medicines in managing chronic fatigue? a systematic review of randomized controlled trials, Front Pharmacol 2023, 14, 1266803. [Google Scholar]
- Zhang, Y.; Jin, F.; Wei, X.; Jin, Q.; Xie, J.; Pan, Y.; Shen, W. Chinese herbal medicine for the treatment of chronic fatigue syndrome: A systematic review and meta-analysis, Front Pharmacol 2022, 13, 958005.
- Liu, Z.; Lv, Z.; Zhou, X.; Shi, J.; Hong, S.; Huang, H.; Lv, L. Efficacy of traditional Chinese exercises in patients with post-COVID-19 chronic fatigue syndrome: A protocol for systematic review and meta-analysis, Medicine (Baltimore) 2022, 101, e31450.
- Liu, T.; Sun, W.; Guo, S.; Chen, T.; Zhu, M.; Yuan, Z.; Li, B.; Lu, J.; Shao, Y.; Qu, Y.; Sun, Z.; Feng, C.; Yang, T. Research progress on pathogenesis of chronic fatigue syndrome and treatment of traditional Chinese and Western medicine, Auton Neurosci 2024, 255, 103198.
- Wirth, K.J.; Löhn, M. Microvascular Capillary and Precapillary Cardiovascular Disturbances Strongly Interact to Severely Affect Tissue Perfusion and Mitochondrial Function in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Evolving from the Post COVID-19 Syndrome, Medicina (Kaunas) 2024, 60, 194.
- Adams, B.; Nunes, J.M.; Page, M.J.; Roberts, T.; Carr, J.; Nell, T.A.; Kell, D.B.; Pretorius, E. Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens, Front Ag Neurosci 2019, 11, 210.
- Pretorius, E.; Page, M.J.; Mbotwe, S.; Kell, D.B. Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease, PlosOne 2018, 13, e0192121.
- van Vuuren, M.J.; Nell, T.A.; Carr, J.A.; Kell, D.B.; Pretorius, E. Iron dysregulation and inflammagens related to oral and gut health are central to the development of Parkinson’s disease, Biomolecules 2021, 11, 30.
- Chen, P.; Zhang, J.; Wang, C.; Chai, Y.H.; Wu, A.G.; Huang, N.Y.; Wang, L. The pathogenesis and treatment mechanism of Parkinson's disease from the perspective of traditional Chinese medicine, Phytomedicine 2022, 100, 154044.
- Huo, M.; Peng, S.; Li, J.; Cao, Y.; Chen, Z.; Zhang, Y.; Qiao, Y. Comparison of the clinical effect features of Han-Ku-Gan and Wen-Xin-Gan based on the efficacy of promoting blood circulation and removing blood stasis, J Trad Chinese Med Sci 2022, 9, 237-245.
- Wang, L.; An, H.; Yu, F.; Yang, J.; Ding, H.; Bao, Y.; Xie, H.; Huang, D. The neuroprotective effects of paeoniflorin against MPP(+)-induced damage to dopaminergic neurons via the Akt/Nrf2/GPX4 pathway, J Chem Neuroanat 2022, 122, 102103.
- Pretorius, E.; Oberholzer, H.M.; van der Spuy, W.J.; Swanepoel, A.C.; Soma, P. Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis, Rheumatol Int 2012, 32, 1611-1615.
- Pretorius, E.; Akeredolu, O.-O.; Soma, P.; Kell, D.B. Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability, Exp Biol Med 2017, 242, 355-373.
- Nozaki, K.; Hikiami, H.; Goto, H.; Nakagawa, T.; Shibahara, N.; Shimada, Y. ; Keishibukuryogan (gui-zhi-fu-ling-wan); a Kampo formula, decreases disease activity and soluble vascular adhesion molecule-1 in patients with rheumatoid arthritis, Evid Based Complement Alternat Med 2006, 3, 359-364.
- Hou, W.; Xu, G.; Wang, H. Overview of Chinese Medicine and Autoimmune Diseases, and the Role of Yin Deficiency, in Treating Autoimmune Disease with Chinese Medicine, ed. W. Hou, G. Xu and H. Wang, Elsevier, Amsterdam 2011, pp. 13-52.
- Schofield, J.; Abrams, S.T.; Jenkins, R.; Lane, S.; Wang, G.; Toh, C.H. ; Microclots, as defined by amyloid-fibrinogen aggregates, predict risks of disseminated intravascular coagulation and mortality, Blood Adv 2024, 8, 2499-2508.
- Meng, F.; Lai, H.; Luo, Z.; Liu, Y.; Huang, X.; Chen, J.; Liu, B.; Guo, Y.; Cai, Y.; Huang, Q. Effect of Xuefu Zhuyu Decoction Pretreatment on Myocardium in Sepsis Rats, Evid Based Complement Alternat Med 2018, 2018, 2939307.
- Bi, C.F.; Liu, J.; Hao, S.W.; Xu, Z.X.; Ma, X.; Kang, X.F.; Yang, L.S.; Zhang, J.F. Xuebijing injection protects against sepsis-induced myocardial injury by regulating apoptosis and autophagy via mediation of PI3K/AKT/mTOR signaling pathway in rats, Aging (Albany NY) 2023, 15, 4374-4390.
- Sun, J.; Xue, Q.; Guo, L.; Cui, L.; Wang, J. Xuebijing protects against lipopolysaccharide-induced lung injury in rabbits, Exp Lung Res 2010, 36, 211-218.
- Wang, J.; Zhu, J.; Guo, J.; Wang, Q. Could Xuebijing Injection Reduce the Mortality of Severe Pneumonia Patients? A Systematic Review and Meta-Analysis, Evid Based Complement Alternat Med 2020, 2020, 9605793. [Google Scholar]
- Chen, G.; Gao, Y.; Jiang, Y.; Yang, F.; Li, S.; Tan, D.; Ma, Q. Efficacy and Safety of Xuebijing Injection Combined With Ulinastatin as Adjunctive Therapy on Sepsis: A Systematic Review and Meta-Analysis, Front Pharmacol 2018, 9, 743.
- He, X.D.; Wang, Y.; Wu, Q.; Wang, H.X.; Chen, Z.D.; Zheng, R.S.; Wang, Z.S.; Wang, J.B.; Yang, Y. Xuebijing Protects Rats from Sepsis Challenged with Acinetobacter baumannii by Promoting Annexin A1 Expression and Inhibiting Proinflammatory Cytokines Secretion, Evid Based Complement Alternat Med 2013, 2013, 804940.
- Hou, S.Y.; Feng, X.H.; Lin, C.L.; Tan, Y.F. Efficacy of Xuebijing for coagulopathy in patients with sepsis, Saudi Med J 2015, 36, 164-169.
- Li, C.; Wang, P.; Zhang, L.; Li, M.; Lei, X.; Liu, S.; Feng, Z.; Yao, Y.; Chang, B.; Liu, B.; Shang, H. Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: A meta-analysis of randomized controlled trials, J Ethnopharmacol 2018, 224, 512-521.
- Xiao, S.H.; Luo, L.; Liu, X.H.; Zhou, Y.M.; Liu, H.M.; Huang, Z.F. Curative efficacy and safety of traditional Chinese medicine xuebijing injections combined with ulinastatin for treating sepsis in the Chinese population: A meta-analysis, Medicine (Baltimore) 2018, 97, e10971.
- Zheng, J.; Xiang, X.; Xiao, B.; Li, H.; Gong, X.; Yao, S.; Yuan, T. Xuebijing combined with ulinastation benefits patients with sepsis: A meta-analysis, Am J Emerg Med 2018, 36, 480-487.
- Song, Y.; Yao, C.; Yao, Y.; Han, H.; Zhao, X.; Yu, K.; Liu, L.; Xu, Y.; Liu, Z.; Zhou, Q.; Wang, Y.; Ma, Z.; Zheng, Y.; Wu, D.; Tang, Z.; Zhang, M.; Pan, S.; Chai, Y.; Song, Y.; Zhang, J.; Pan, L.; Liu, Y.; Yu, H.; Yu, X.; Zhang, H.; Wang, X.; Du, Z.; Wan, X.; Tang, Y.; Tian, Y.; Zhu, Y.; Wang, H.; Yan, X.; Liu, Z.; Zhang, B.; Zhong, N.; Shang, H.; Bai, C. XueBiJing Injection Versus Placebo for Critically Ill Patients With Severe Community-Acquired Pneumonia: A Randomized Controlled Trial, Crit Care Med 2019, 47, e735-e743.
- Cheng, C.; Yu, X. Research Progress in Chinese Herbal Medicines for Treatment of Sepsis: Pharmacological Action, Phytochemistry, and Pharmacokinetics, Int J Mol Sci 2021, 22.
- Li, C.; Wang, P.; Li, M.; Zheng, R.; Chen, S.; Liu, S.; Feng, Z.; Yao, Y.; Shang, H. The current evidence for the treatment of sepsis with Xuebijing injection: Bioactive constituents, findings of clinical studies and potential mechanisms, J Ethnopharmacol 2021, 265, 113301.
- Wu, Q.; Yin, C.H.; Li, Y.; Cai, J.Q.; Yang, H.Y.; Huang, Y.Y.; Zheng, Y.X.; Xiong, K.; Yu, H.L.; Lu, A.P.; Wang, K.X.; Guan, D.G.; Chen, Y.P. Detecting Critical Functional Ingredients Group and Mechanism of Xuebijing Injection in Treating Sepsis, Front Pharmacol 2021, 12, 769190.
- Lv, J.; Guo, X.; Zhao, H.; Zhou, G.; An, Y. Xuebijing Administration Alleviates Pulmonary Endothelial Inflammation and Coagulation Dysregulation in the Early Phase of Sepsis in Rats, J Clin Med 2022, 11, 6696.
- Shang, T.; Zhang, Z.S.; Wang, X.T.; Chang, J.; Zhou, M.E.; Lyu, M.; He, S.; Yang, J.; Chang, Y.X.; Wang, Y.; Li, M.C.; Gao, X.; Zhu, Y.; Feng, Y. Xuebijing injection inhibited neutrophil extracellular traps to reverse lung injury in sepsis mice via reducing Gasdermin D, Front Pharmacol 2022, 13, 1054176.
- Yu, X.; Niu, W.; Wang, Y.Y.; Olaleye, O.E.; Wang, J.N.; Duan, M.Y.; Yang, J.L.; He, R.R.; Chu, Z.X.; Dong, K.; Zhang, G.P.; Liu, C.X.; Cheng, C.; Li, C. Novel assays for quality evaluation of XueBiJing: Quality variability of a Chinese herbal injection for sepsis management, J Pharm Anal 2022, 12, 664-682.
- Chen, F.; Yan, S.; Xu, J.; Jiang, Y.; Wang, J.; Deng, H.; Wang, J.; Zou, L.; Liu, Y.; Zhu, Y. Exploring the potential mechanism of Xuebijing injection against sepsis based on metabolomics and network pharmacology, Anal Biochem 2023, 682, 115332.
- Liao, X.; Rello, J. Efficacy of Xuebijing Injection for Sepsis (EXIT-SEP): Lost in Translation, Anaesth Crit Care Pain Med 2023, 42, 101257.
- Liu, S.; Yao, C.; Xie, J.; Liu, H.; Wang, H.; Lin, Z.; Qin, B.; Wang, D.; Lu, W.; Ma, X.; Liu, Y.; Liu, L.; Zhang, C.; Xu, L.; Zheng, R.; Zhou, F.; Liu, Z.; Zhang, G.; Zhou, L.; Liu, J.; Fei, A.; Zhang, G.; Zhu, Y.; Qian, K.; Wang, R.; Liang, Y.; Duan, M.; Wu, D.; Sun, R.; Wang, Y.; Zhang, X.; Cao, Q.; Yang, M.; Jin, M.; Song, Y.; Huang, L.; Zhou, F.; Chen, D.; Liang, Q.; Qian, C.; Tang, Z.; Zhang, Z.; Feng, Q.; Peng, Z.; Sun, R.; Song, Z.; Sun, Y.; Chai, Y.; Zhou, L.; Cheng, C.; Li, L.; Yan, X.; Zhang, J.; Huang, Y.; Guo, F.; Li, C.; Yang, Y.; Shang, H.; Qiu, H.; Investigators, E.-S. Effect of an Herbal-Based Injection on 28-Day Mortality in Patients With Sepsis: The EXIT-SEP Randomized Clinical Trial, JAMA Intern Med 2023, 183, 647-655.
- Zhang, M.; Zheng, R.; Liu, W.J.; Hou, J.L.; Yang, Y.L.; Shang, H.C.; Xuebijing injection, a Chinese patent medicine, against severe pneumonia: Current research progress and future perspectives, J Integr Med 2023, 21, 413-422.
- Kang, X.F.; Lu, X.L.; Bi, C.F.; Hu, X.D.; Li, Y.; Li, J.K.; Yang, L.S.; Liu, J.; Ma, L.; Zhang, J.F. Xuebijing injection protects sepsis induced myocardial injury by mediating TLR4/NF-kappaB/IKKalpha and JAK2/STAT3 signaling pathways, Aging (Albany NY) 2023, 15, 8501-8517.
- Zhang, C.; Chen, X.; Wei, T.; Song, J.; Tang, X.; Bi, J.; Chen, C.; Zhou, J.; Su, X.; Song, Y. Xuebijing alleviates LPS-induced acute lung injury by downregulating pro-inflammatory cytokine production and inhibiting gasdermin-E-mediated pyroptosis of alveolar epithelial cells, Chin J Nat Med 2023, 21, 576-588.
- Cheng, C.; Ren, C.; Li, M.Z.; Liu, Y.H.; Yao, R.Q.; Yu, Y.; Yu, X.; Wang, J.L.; Wang, L.X.; Leng, Y.C.; Zhang, H.; Du, F.F.; Dong, N.; Wang, F.Q.; Wu, Y.; Xu, F.; Zhu, X.M.; Zhang, G.P.; Dong, K.; Liu, S.; Yao, X.Q.; Li, C.; Yao, Y.M. Pharmacologically significant constituents collectively responsible for anti-sepsis action of XueBiJing, a Chinese herb-based intravenous formulation, Acta Pharmacol Sin 2024, 45, 1077-1092.
- Wang, J.; Luo, C.; Luo, M.; Zhou, S.; Kuang, G. Targets and Mechanisms of Xuebijing in the Treatment of Acute Kidney Injury Associated with Sepsis: A Network Pharmacology-based Study, Curr Comput Aided Drug Des 2024, 20, 752-763.
- Zhou, X.; Zhong, M.; Xi, X.; Li, J.; Tang, G. Efficacy and safety of Chinese herbal medicine as adjunctive therapy in sepsis patients with bloodstream infection: a propensity-matched analysis, J Tradit Chin Med 2024, 44, 197-204.
- Zou, F.; Zou, J.; Du, Q.; Liu, L.; Li, D.; Zhao, L.; Tang, M.; Zuo, L.; Sun, Z. XueBiJing injection improves the symptoms of sepsis-induced acute lung injury by mitigating oxidative stress and ferroptosis, J Ethnopharmacol 2025, 337, 118732.
- Wu, W.; Jiang, R.L.; Wang, L.C.; Lei, S.; Xing, X.; Zhi, Y.H.; Wu, J.N.; Wu, Y.C.; Zhu, M.F.; Huang, L.Q. Effect of Shenfu injection on intestinal mucosal barrier in a rat model of sepsis, Am J Emerg Med 2015, 33, 1237-1243.
- Zhang, N.; Liu, J.; Qiu, Z.; Ye, Y.; Zhang, J.; Lou, T. Shenfu injection for improving cellular immunity and clinical outcome in patients with sepsis or septic shock, Am J Emerg Med 2017, 35, 1-6.
- Jin, S.; Jiang, R.; Lei, S.; Jin, L.; Zhu, C.; Feng, W.; Shen, Y. Shenfu injection prolongs survival and protects the intestinal mucosa in rats with sepsis by modulating immune response, Turk J Gastroenterol 2019, 30, 364-371.
- Liu, J.; Liu, F.; Liang, T.; Cao, P.; Li, J.; Zhang, Y.; Liu, Y. Efficacy of Shenfu decoction on sepsis in rats with condition induced by cecal ligation and puncture, J Tradit Chin Med 2020, 40, 621-628.
- Xu, P.; Zhang, W.Q.; Xie, J.; Wen, Y.S.; Zhang, G.X.; Lu, S.Q. Shenfu injection prevents sepsis-induced myocardial injury by inhibiting mitochondrial apoptosis, J Ethnopharmacol 2020, 261, 113068.
- Luo, S.; Gou, L.; Liu, S.; Cao, X. Efficacy and safety of Shenfu injection in the treatment of sepsis: A protocol for systematic review and meta-analysis, Medicine (Baltimore) 2021, 100, e27196.
- Xu, C.; Xia, Y.; Jia, Z.; Wang, S.; Zhao, T.; Wu, L. The curative effect of Shenfu-injection in the treatment of burn sepsis and its effect on the patient's immune function, HMGB, and vWF, Am J Transl Res 2022, 14, 2428-2435.
- Huang, P.; Guo, Y.; Hu, X.; Fang, X.; Xu, X.; Liu, Q. Mechanism of Shenfu injection in suppressing inflammation and preventing sepsis-induced apoptosis in murine cardiomyocytes based on network pharmacology and experimental validation, J Ethnopharmacol 2024, 322, 117599.
- Liao, J.; Qin, C.; Wang, Z.; Gao, L.; Zhang, S.; Feng, Y.; Liu, J.; Tao, L. Effect of shenfu injection in patients with septic shock: A systemic review and meta-analysis for randomized clinical trials, J Ethnopharmacol 2024, 320, 117431.
- Liu, D.; Pan, T.; Li, X.; Zhu, D.; Li, Y.; He, H.; Wu, F.; Jiang, L.; Chen, Y.; Wang, X.; Liu, J.; Tan, R.; Qu, H. Effectiveness and safety of Shenfu injection in septic patients with hypoperfusion: A multi-center, open-label, randomized, controlled trial, J Intensive Med 2024, 4, 484-490.
- Xiao, L.; Niu, L.; Xu, X.; Zhao, Y.; Yue, L.; Liu, X.; Li, G. Comparative Efficacy of Tonic Chinese Herbal Injections for Treating Sepsis or Septic Shock: A Systematic Review and Bayesian Network Meta-Analysis of Randomized Controlled Trials, Front Pharmacol 2022, 13, 830030.
- Yuan, H.; Liu, Y.; Huang, K.; Hao, H.; Xue, Y.T. Therapeutic Mechanism and Key Active Ingredients of Shenfu Injection in Sepsis: A Network Pharmacology and Molecular Docking Approach, Evid Based Complement Alternat Med 2022, 2022, 9686149.
- Yuan, H.J.; Xiang, G.H.; Liu, Y.; Li, Y.; Liu, W.L.; Wei, J.X.; Xue, Y.T.; Hao, H. Exploration and verification of the therapeutic mechanism of shenfu injection in sepsis-induced myocardial injury, PLoS One 2025, 20, e0317738.
- Grixti, J.M.; Chandran, A.; Pretorius, J.-H.; Walker, M.; Sekhar, A.; Pretorius, E.; Kell, D.B. The clots removed from ischaemic stroke patients by mechanical thrombectomy are amyloid in nature, medRxiv 2024, 10.1101/2024.1111.1101.24316555v24316551.
- Xu, M.; Wu, R.X.; Li, X.L.; Zeng, Y.S.; Liang, J.Y.; Fu, K.; Liang, Y.; Wang, Z. Traditional medicine in China for ischemic stroke: bioactive components, pharmacology, and mechanisms, J Integr Neurosci 2022, 21, 26.
- Hsu, L.W.; Shiao, W.C.; Chang, N.C.; Yu, M.C.; Yen, T.L.; Thomas, P.A.; Jayakumar, T.; Sheu, J.R. The neuroprotective effects of Tao-Ren-Cheng-Qi Tang against embolic stroke in rats, Chin Med 2017, 12, 7.
- Zhang, H.; Ouyang, H.; Zhang, J.; Lin, L.; Wei, M.; Lu, B.; Ji, L. Exploring the efficacy and mechanism of Glycyrrhizae Radix et Rhizoma in improving collagen-induced arthritis in mice, J Ethnopharmacol 2024, 322, 117554.
- Shaw, L.H.; Chen, W.M.; Tsai, T.H. Identification of multiple ingredients for a Traditional Chinese Medicine preparation (bu-yang-huan-wu-tang) by liquid chromatography coupled with tandem mass spectrometry, Molecules 2013, 18, 11281-11298.
- Wang, H.W.; Liou, K.T.; Wang, Y.H.; Lu, C.K.; Lin, Y.L.; Lee, I.J.; Huang, S.T.; Tsai, Y.H.; Cheng, Y.C.; Lin, H.J.; Shen, Y.C. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice, J Ethnopharmacol 2011, 138, 22-33.
- Chen, Z.Z.; Gong, X.; Guo, Q.; Zhao, H.; Wang, L. Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 alpha, VEGF and promotion beta-ENaC expression, J Ethnopharmacol 2019, 228, 70-81.
- Chen, K.Y.; Wu, K.C.; Hueng, D.Y.; Huang, K.F.; Pang, C.Y. Anti-inflammatory effects of powdered product of Bu Yang Huan Wu decoction: Possible role in protecting against Transient Focal Cerebral Ischemia, Int J Med Sci 2020, 17, 1854-1863.
- Peng, J.W.; Liu, Y.; Meng, G.; Zhang, J.Y.; Yu, L.F. Effects of salvianolic acid on cerebral perfusion in patients after acute stroke: A single-center randomized controlled trial, Exp Ther Med 2018, 16, 2600-2614.
- Lyu, J.; Xie, Y.; Wang, Z.; Wang, L. Salvianolic Acids for Injection Combined with Conventional Treatment for Patients with Acute Cerebral Infarction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Med Sci Monit 2019, 25, 7914-7927.
- Liu, C.D.; Liu, N.N.; Zhang, S.; Ma, G.D.; Yang, H.G.; Kong, L.L.; Du, G.H. Salvianolic acid A prevented cerebrovascular endothelial injury caused by acute ischemic stroke through inhibiting the Src signaling pathway, Acta Pharmacol Sin 2021, 42, 370-381.
- Yang, Y.; He, Y.; Wei, X.; Wan, H.; Ding, Z.; Yang, J.; Zhou, H. Network Pharmacology and Molecular Docking-Based Mechanism Study to Reveal the Protective Effect of Salvianolic Acid C in a Rat Model of Ischemic Stroke, Front Pharmacol 2021, 12, 799448.
- Zhang, Q.; Zhang, L.; Liu, Y.; Tian, X.; Li, X.; Han, B.; Zhang, Y.; Wu, Z.; Yu, H.; Zhao, H.; Wang, S.; Ma, K.; Wang, Y. Research progress on the pharmacological effect and clinical application of Tongqiao Huoxue Decoction in the treatment of ischaemic stroke, Biomed Pharmacother 2021, 138, 111460.
- Lee, H.G.; Kwon, S.; Moon, S.K.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Park, J.M.; Ko, C.N.; Cho, K.H. Neuroprotective Effects of Geopung-Chunghyuldan Based on Its Salvianolic Acid B Content Using an In Vivo Stroke Model, Curr Issues Mol Biol 2023, 45, 1613-1626.
- Wang, L.; Liu, Y.; Wei, J.; Liang, X.; Zhang, Y. Effects of intravenous thrombolysis with and without salvianolic acids for injection on the functional recovery of patients with acute ischemic stroke: A systematic review, meta-analysis, and trial sequential analysis, Phytother Res 2023, 37, 2513-2530.
- Ren, H.; Yang, A.H.; Cai, Y.S.; Qin, Y.; Luo, T.Y. Study on correlation between Chinese medicine syndromes in stroke and neurological deficits during recovery phase: Perspective, Medicine (Baltimore) 2024, 103, e39600.
- Wang, J.; Su, P.; Wan, C.; Xu, Y.; Huang, J.; Niu, J.; Jin, Z. Role of salvianolic acid B in the treatment of acute ischemic stroke: a systematic review and meta-analysis of animal models, Front Pharmacol 2024, 15, 1479765.
- Yang, H.; Ibrahim, M.M.; Zhang, S.; Sun, Y.; Chang, J.; Qi, H.; Yang, S. Targeting post-stroke neuroinflammation with Salvianolic acid A: molecular mechanisms and preclinical evidence, Front Immunol 2024, 15, 1433590.
- Yang, M.Y.; Liu, Y.; Yu, Y.W.; Gong, B.F.; Ruan, J.; Fan, H.Y. Application of targeted liposomes-based salvianolic acid A for the treatment of ischemic stroke, Neurotherapeutics 2024, 21, e00342.
- Yang, R.; Hu, N.; Liu, T.Y.; Qu, Y.; Liu, J.; Wang, J.H.; Yang, B.F.; Li, C.L. Salvianolic acid A provides neuroprotective effects on cerebral ischemia-reperfusion injury in rats via PKA/CREB/c-Fos signaling pathway, Phytomedicine 2024, 124, 155326.
- Zhong, D.Y.; Li, H.Y.; Li, L.; Ma, R.M.; Jiang, C.T.; Li, D.X.; Deng, Y.H. Effect of Tongqiao Huoxue Decoction Combined with Western Medicine on Ischemic Stroke: A Systematic Review, Evid Based Complement Alternat Med 2020, 2020, 8877998.
- Hou, F.; Liu, H.; Gong, Q.; Xu, H. Network pharmacology of Huatan Tongluo decoction and clinical effects of its combination with acupuncture in the treatment of stroke, Am J Transl Res 2022, 14, 8215-8224.
- Kell, D.B.; Pretorius, E. Proteomic evidence for amyloidogenic cross-seeding in fibrinaloid microclots, Int J Mol Sci 2024, 25, 10809.
- Kell, D.B.; Pretorius, E. The proteome content of blood clots observed under different conditions: successful role in predicting clot amyloid(ogenicity), Molecules 2025, 30, 668.
- Bondarev, S.A.; Antonets, K.S.; Kajava, A.V.; Nizhnikov, A.A.; Zhouravleva, G.A. Protein Co-Aggregation Related to Amyloids: Methods of Investigation, Diversity, and Classification, Int J Mol Sci 2018, 19, 2292.
- Cao, W.; Liao, S.; Zhang, Y.; Zhou, L.; Li, G.; Ouyang, W.; Wen, Z. Effectiveness and safety of Xuefu Zhuyu oral liquid on -stagnation and blood-stasis pattern in patients with stable angina, tension-type headache and primary dysmenorrhea: rationale and design of a master protocol, J Tradit Chin Med 2023, 43, 815-823.
- Chen, J.; Lu, P.; Zuo, X.; Shi, Q.; Zhao, H.; Luo, L.; Yi, J.; Zheng, C.; Yang, Y.; Wang, W. Clinical data mining of phenotypic network in angina pectoris of coronary heart disease, Evid Based Complement Alternat Med 2012, 2012, 546230.
- Chu, F.Y.; Wang, J.; Yao, K.W.; Li, Z.Z. Effect of Xuefu Zhuyu Capsule (血府逐瘀胶囊) on the symptoms and signs and health-related quality of life in the unstable angina patients with blood-stasis syndrome after percutaneous coronary intervention: A Randomized controlled trial, Chin J Integr Med 2010, 16, 399-405.
- Huang, P.; Li, Z.; Chen, L.; Zeng, J.; Zhao, S.; Tang, Y.; Huang, B.; Guan, H.; Chen, Y.; Feng, Y.; Lei, S.; Wu, Q.; Zhang, H.; Huang, X.; Zeng, L.; Liu, Y.; Zeng, Z.; Chen, B. The comparative effects of oral Chinese patent medicines combined with western medicine in stable angina: A systematic review and network meta-analysis of 179 trials, Front Pharmacol 2022, 13, 918689.
- Li, J.; Zhang, P.; Zhang, Y.; Wang, H.; Wu, L.; Zhao, J.; Liu, Y.; Zeng, W.; Guo, R.; Mei, J.; Xu, F. A Randomized Controlled Trial on the Efficacy of Xinnaoning Capsule in the Treatment of CSAP Complicated With Qi Stagnation and Blood Stasis Syndrome, Front Cardiovasc Med 2022, 9, 859956.
- Li, Y.; Zhao, H.; Du, J.; Jiao, Z.; Shen, D.; Gao, S.; Zheng, Y.; Li, Z.; Li, L.; Wang, Y.; Yu, C. Clinical metabolomic analysis of Danlou tablets with antioxidant effects for treating stable angina pectoris, J Pharm Biomed Anal 2022, 219, 114922.
- Liu, W.; Zhou, L.; Feng, L.; Zhang, D.; Zhang, C.; Gao, Y.; B. G. behalf of the, BuqiTongluo Granule for Ischemic Stroke, Stable Angina Pectoris, Diabetic Peripheral Neuropathy with Qi Deficiency and Blood Stasis Syndrome: Rationale and Novel Basket Design, Front Pharmacol 2021, 12, 764669.
- Wang, J.; Yang, X.; Chu, F.; Chen, J.; He, Q.; Yao, K.; Teng, F.; Gao, Y.; Xing, Y.; Wu, A.; Xing, Y. The effects of xuefu zhuyu and shengmai on the evolution of syndromes and inflammatory markers in patients with unstable angina pectoris after percutaneous coronary intervention: a randomised controlled clinical trial, Evid Based Complement Alternat Med 2013, 2013, 896467.
- Wang, J.; Yu, G. A Systems Biology Approach to Characterize Biomarkers for Blood Stasis Syndrome of Unstable Angina Patients by Integrating MicroRNA and Messenger RNA Expression Profiling, Evid Based Complement Alternat Med 2013, 2013, 510208.
- Wang, X.R.; Song, D.D.; Tao, T.Q.; He, T.; Wu, X.D.; Li, X.M.; Liu, X.H. Qi-Regulating and Blood Circulation-Promoting Therapy Improves Health Status of Stable Angina Pectoris Patients with Depressive Symptoms, Evid Based Complement Alternat Med 2021, 2021, 7319417.
- Wang, X.; Xing, X.; Huang, P.; Zhang, Z.; Zhou, Z.; Liang, L.; Yao, R.; Wu, X.; Yang, L. A Chinese classical prescription Xuefu Zhuyu decoction in the treatment of coronary heart disease: An overview, Heliyon 2024, 10, e28919.
- Weng, J.H.; Hou, F.G.; Wang, X.; Wang, Z.Y.; Wu, M.P. A Clinical Study on the Efficacy of the Yangxin Huoxue Formula in Treating Stable Angina Pectoris (Qi Deficiency and Blood Stasis Syndrome) with Concurrent Anxiety and Depression Disorders, J Multidiscip Healthc 2024, 17, 5317-5327.
- Yang, R.; Bai, Z.; Liang, C.; Qi, F. Treatment of microvascular angina pectoris by activating blood circulation to remove blood stasis: A systematic review and meta-analysis, Medicine (Baltimore) 2024, 103, e40012.
- Yao, K.W.; Wang, J.; Zhu, C.L.; Wu, J.T.; Fang, J.Z. Results of different quantitative diagnosis analysis on the symptoms and signs of blood stasis syndrome in coronary heart disease, World Sci Technol 2009, 11, 684–688.
- Yao, K.W.; He, Q.Y.; Teng, F.; Wang, J. Logistic regression analysis of syndrome essential factors in patients with unstable angina pectoris, J Tradit Chin Med 2011, 31, 273-276.
- Sun, Z.; Zhong, D.; Zhang, J.; Wang, Q.; Li, C.; Yuan, T.; Dai, X.; Duan, J.; Yao, K. Tongxinshu capsules in the treatment of stable angina pectoris due to qi deficiency and blood stasis in coronary heart disease: A multicenter, randomized, double-blind, placebo-controlled trial, J Ethnopharmacol 2025, 343, 119437.
- Qi, G.P.; Zhang, A.J.; Xu, Z.; Li, Z.H.; Zeng, W.B.; Liu, X.; Ma, J.M.; Zheng, X.S.; Li, Z.J. Application of a Complex Network Modeling Approach to Explore the Material Basis and Mechanisms of Traditional Chinese Medicine: A Case Study of Xuefu Zhuyu Decoction for the Treatment of Two Types of Angina Pectoris, Ieee Access 2022, 10, 114103-114117.
- Bai, D.; Song, J. Plasma metabolic biomarkers for syndrome of phlegm and blood stasis in hyperlipidemia and atherosclerosis, J Tradit Chin Med 2012, 32, 578-583.
- Liu, Z.; Yang, H.; Zhang, M.; Cai, J.; Huang, Z. The Interaction Effect between Blood Stasis Constitution and Atherosclerotic Factors on Cognitive Impairment in Elderly People, Evid Based Complement Alternat Med 2018, 2018, 8914090.
- Morita, A.; Namiki, T.; Nakaguchi, T.; Murai, K.; Watanabe, Y.; Nakamura, M.; Kawasaki, Y.; Shiko, Y.; Tamura, Y.; Suganami, A.; Murakami, A.; Yagi, A.; Okamoto, H.; Hirasaki, Y. Role of Blood Stasis Syndrome of Kampo Medicine in the Early Pathogenic Stage of Atherosclerosis: A Retrospective Cross-Sectional Study, Evid Based Complement Alternat Med 2021, 2021, 5557392.
- Zuo, H.L.; Linghu, K.G.; Wang, Y.L.; Liu, K.M.; Gao, Y.; Yu, H.; Yang, F.Q.; Hu, Y.J. Interactions of antithrombotic herbal medicines with Western cardiovascular drugs, Pharmacol Res 2020, 159, 104963.
- Wei, X.; Gao, M.; Sheng, N.; Yao, W.; Bao, B.; Cheng, F.; Cao, Y.; Yan, H.; Zhang, L.; Shan, M.; Chen, P. Mechanism investigation of Shi-Xiao-San in treating blood stasis syndrome based on network pharmacology, molecular docking and in vitro/vivo pharmacological validation, J Ethnopharmacol 2023, 301, 115746.
- Zhi, W.; Liu, Y.; Wang, X.; Zhang, H. Recent advances of traditional Chinese medicine for the prevention and treatment of atherosclerosis, J Ethnopharmacol 2023, 301, 115749.
- Maione, F.; Mascolo, N. Danshen and the Cardiovascular System: New Advances for an Old Remedy, Semin Thromb Hemost 2016, 42, 321-322.
- Ammash, N.; Konik, E.A.; McBane, R.D.; Chen, D.; Tange, J.I.; Grill, D.E.; Herges, R.M.; McLeod, T.G.; Friedman, P.A.; Wysokinski, W.E. Left atrial blood stasis and Von Willebrand factor-ADAMTS13 homeostasis in atrial fibrillation, Arterioscler Thromb Vasc Biol 2011, 31, 2760-2766.
- Bäck, S.; Skoda, I.; Lantz, J.; Henriksson, L.; Karlsson, L.O.; Persson, A.; Carlhäll, C.J.; Ebbers, T. Elevated atrial blood stasis in paroxysmal atrial fibrillation during sinus rhythm: a patient-specific computational fluid dynamics study, Front Cardiovasc Med 2023, 10, 1219021.
- Kell, D.B.; Lip, G.Y.H.; Pretorius, E. Fibrinaloid Microclots and Atrial Fibrillation, Biomedicines 2024, 12, 891.
- Ni, X.; Zhang-James, Y.; Han, X.; Lei, S.; Sun, J.; Zhou, R. Traditional Chinese medicine in the treatment of ADHD: a review, Child Adolesc Psychiatr Clin N Am 2014, 23, 853-881.
- Zhao, S.; Deng, Y.; Chen, Z.; Wang, S. How does traditional chinese medicine treat attention deficit hyperactivity disorder - A different understanding and treatment strategy, Open J Orthop Rheumatol 2023, 8, 013-017.
- Zhang, Y.Y.; Li, Y.P. Progress of traditional Chinese Medicine on ADHD Treatment Based on Syndrome Differentiation, Drug Combin THerapy 2021, 3, 11.
- Sun, R.; Yuan, H.; Wang, J.; Zhu, K.; Xiong, Y.; Zheng, Y.; Ni, X.; Huang, M. Rehmanniae Radix Preparata ameliorates behavioral deficits and hippocampal neurodevelopmental abnormalities in ADHD rat model, Front Neurosci 2024, 18, 1402056.
- Shen, H.S.; Hsu, C.Y.; Yip, H.T.; Lin, I.H. Lower risk of ischemic stroke among patients with chronic kidney disease using chinese herbal medicine as add-on therapy: A real-world nationwide cohort study, Front Pharmacol 2022, 13, 883148.
- Song, C.Q.; Zhu, Z.Y.; Liu, M.; Yang, W.B.; Bai, X.T.; Nan, Z. Clinical efficacy and safety of Xuefu Zhuyu decoction in the treatment of diabetic kidney disease: A protocol for systematic review and meta-analysis, Medicine 2022, 101.
- Liu, W.; Yang, S.; Fu, M.; Li, J.; Song, Y.; Wei, B.; Liu, E.; Sun, Z. Chinese patent medicine for chronic obstructive pulmonary disease based on principles of tonifying Qi, promoting blood circulation by removing blood stasis, and resolving phlegm: a systematic review of randomized controlled trials, J Tradit Chin Med 2015, 35, 1-10.
- Hu, Y.; Lan, Y.; Ran, Q.; Gan, Q.; Huang, W. Analysis of the Clinical Efficacy and Molecular Mechanism of Xuefu Zhuyu Decoction in the Treatment of COPD Based on Meta-Analysis and Network Pharmacology, Comput Math Methods Med 2022, 2022, 2615580.
- Qin, S.; Tan, P.; Xie, J.; Zhou, Y.; Zhao, J. A systematic review of the research progress of traditional Chinese medicine against pulmonary fibrosis: from a pharmacological perspective, Chin Med 2023, 18, 96.
- Yang, Q.; Yin, D.; Wang, H.; Gao, Y.; Wang, X.; Wu, D.; Tong, J.; Wang, C.; Li, Z. Uncovering the action mechanism of Shenqi Tiaoshen formula in the treatment of chronic obstructive pulmonary disease through network pharmacology, molecular docking, and experimental verification, J Tradit Chin Med 2024, 44, 770-783.
- Yu, X.; Qin, W.; Cai, H.; Ren, C.; Huang, S.; Lin, X.; Tang, L.; Shan, Z.; Al-Ameer, W.H.A.; Wang, L.; Yan, H.; Chen, M. Analyzing the molecular mechanism of xuefuzhuyu decoction in the treatment of pulmonary hypertension with network pharmacology and bioinformatics and verifying molecular docking, Comput Biol Med 2024, 169, 107863.
- Zhou, W.; Wang, Y. A network-based analysis of the types of coronary artery disease from traditional Chinese medicine perspective: potential for therapeutics and drug discovery, J Ethnopharmacol 2014, 151, 66-77.
- Chen, K.J.; Shi, D.Z.; Fu, C.G.; Gao, Z.Y.; Xu, H.; Lv, S.Z.; You, S.J.; Huang, L. Diagnostic criterion of blood stasis syndrome for coronary heart disease : Activating Blood Circulation Committee of Chinese Association of Integrative Medicine, Chin J Integr Med 2016, 22, 803-804.
- Zhang, S.; Chen, Z.L.; Tang, Y.P.; Duan, J.L.; Yao, K.W. Efficacy and Safety of Xue-Fu-Zhu-Yu Decoction for Patients with Coronary Heart Disease: A Systematic Review and Meta-Analysis, Evid Based Complement Alternat Med 2021, 2021, 9931826.
- Xin, Q.Q.; Chen, X.; Yuan, R.; Yuan, Y.H.; Hui, J.Q.; Miao, Y.; Cong, W.H.; Chen, K.J. Correlation of Platelet and Coagulation Function with Blood Stasis Syndrome in Coronary Heart Disease: A Systematic Review and Meta-Analysis, Chin J Integr Med 2021, 27, 858-866.
- Shi, H.; Tang, Z.; Liu, T.; Zhang, X.; Wang, Y.; Li, J.; Dong, C.; Chen, W.; Hou, R.; Si, G.; Liu, Y. The Effect and Safety of Xuefu Zhuoyue Prescription for Coronary Heart Disease: An Overview of Systematic Reviews and Meta-Analyses, Evid Based Complement Alternat Med 2022, 2022, 9096940.
- Yang, G.; Zhou, S.; He, H.; Shen, Z.; Liu, Y.; Hu, J.; Wang, J. Exploring the "gene-protein-metabolite" network of coronary heart disease with phlegm and blood stasis syndrome by integrated multi-omics strategy, Front Pharmacol 2022, 13, 1022627.
- Yu, G.; Wang, J. Susceptible gene polymorphisms for blood stasis syndrome of coronary heart disease, Chin J Integr Med, 2016.
- Lyu, M.; Yan, C.L.; Liu, H.X.; Wang, T.Y.; Shi, X.H.; Liu, J.P.; Orgah, J.; Fan, G.W.; Han, J.H.; Wang, X.Y.; Zhu, Y. Network pharmacology exploration reveals endothelial inflammation as a common mechanism for stroke and coronary artery disease treatment of Danhong injection, Sci Rep 2017, 7, 15427.
- Kui, F.; Gu, W.; Gao, F.; Niu, Y.; Li, W.; Zhang, Y.; Guo, L.; Wang, J.; Guo, Z.; Cen, S.; Du, G. Research on Effect and Mechanism of Xuefu Zhuyu Decoction on CHD Based on Meta-Analysis and Network Pharmacology, Evid Based Complement Alternat Med 2021, 2021, 9473531.
- Tao, T.Q.; He, T.; Wang, X.R.; Liu, X.H. Metabolic Profiling Analysis of Patients With Coronary Heart Disease Undergoing Xuefu Zhuyu Decoction Treatment, Frontiers in Pharmacology 2019, 10.
- Qian, H.; Chen, B.B.; Zhang, M.; Zeng, S.J.; Jia, Z.Z.; Wang, S.; Gao, S.; Shi, A.H.; Xie, J. Network pharmacology and molecular docking approach to explore the potential mechanisms of Xuefu Zhuyu Capsule in coronary heart disease, Medicine 2025, 104.
- Wang, Y.; Wang, J.; Lv, W.; Chen, H.; Yang, Q.; Zhang, Y.; Guo, R.; Ma, X.L.; Zhang, Q.Y. Clinical intervention effect of Xuefu Zhuyu decoction on chronic heart failure complicated with depression, World J Psychiatr 2024, 14.
- Lu, X.Y.; Xu, H.; Zhao, T.; Li, G. Study of Serum Metabonomics and Formula-Pattern Correspondence in Coronary Heart Disease Patients Diagnosed as Phlegm or Blood Stasis Pattern Based on Ultra Performance Liquid Chromatography Mass Spectrometry, Chin J Integr Med 2018, 24, 905-911.
- Liang, B.; Xiang, Y.; Zhang, X.; Wang, C.; Jin, B.; Zhao, Y.; Zheng, F. Systematic Pharmacology and GEO Database Mining Revealed the Therapeutic Mechanism of Xuefu Zhuyu Decoration for Atherosclerosis Cardiovascular Disease, Front Cardiovasc Med 2020, 7, 592201.
- Han, J.; Miao, Y.; Song, L.; Zhou, X.; Liu, Y.; Wang, L.; Zhu, K.; Ma, H.; Ma, Y.; Li, Q.; Han, D. Xuefu Zhuyu Decoction improves hyperlipidemia through the MAPK/NF-kappaB and MAPK/PPARalpha/CPT-1A signaling pathway, FASEB J 2025, 39, e70363.
- Zhu, S.; Song, Y.; Chen, X.; Qian, W. Traditional Chinese and western medicine for the prevention of deep venous thrombosis after lower extremity orthopedic surgery: a meta-analysis of randomized controlled trials, J Orthop Surg Res 2018, 13, 79.
- Yan, S.T.; Gao, F.; Dong, T.W.; Fan, H.; Xi, M.M.; Miao, F.; Wei, P.F. Meta-Analysis of Randomized Controlled Trials of Xueshuantong Injection in Prevention of Deep Venous Thrombosis of Lower Extremity after Orthopedic Surgery, Evid Based Complement Alternat Med 2020, 2020, 8877791.
- Huang, B.; Tang, P.; Liu, Y.; Liu, F.; Zheng, Y.; Yang, X.; Zhang, X.; Xie, H.; Lin, L.; Lin, B.; Lin, B. Xuefu Zhuyu decoction alleviates deep vein thrombosis through inhibiting the activation of platelets and neutrophils via sirtuin 1/nuclear factor kappa-B pathway, J Ethnopharmacol 2024, 333, 118485.
- Jo, J.; Leem, J.; Lee, J.M.; Park, K.S. Herbal medicine (Hyeolbuchukeo-tang or Xuefu Zhuyu decoction) for treating primary dysmenorrhoea: protocol for a systematic review of randomised controlled trials, BMJ Open 2017, 7, e015056.
- Jung, J.; Ko, M.M.; Lee, M.S.; Lee, S.M.; Lee, J.A. Diagnostic Indicators for Blood Stasis Syndrome Patients with Gynaecological Diseases, Chin J Integr Med 2018, 24, 752-757.
- Leem, J.; Jo, J.; Kwon, C.Y.; Lee, H.; Park, K.S.; Lee, J.M. Herbal medicine (Hyeolbuchukeo-tang or Xuefu Zhuyu decoction) for treating primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials, Medicine (Baltimore) 2019, 98, e14170.
- Li, G.; Zhang, Z.; Zhou, L.; Liao, S.; Sun, J.; Liu, Y.; Wang, X.; Wen, Z. Chinese herbal formula Xuefu Zhuyu for primary dysmenorrhea patients (CheruPDYS): a study protocol for a randomized placebo-controlled trial, Trials 2021, 22, 95.
- Tran, M.N.; Jun, H.J.; Lee, S. Identifying the molecular mechanism of blood stasis syndrome through the symptom phenotype-genotype association approach, Medicine (Baltimore) 2024, 103, e40717.
- Li, X.L.; Jin, Y.; Gao, R.; Zhou, Q.X.; Huang, F.; Liu, L. Wenjing decoction: Mechanism in the treatment of dysmenorrhea with blood stasis syndrome through network pharmacology and experimental verification, J Ethnopharmacol 2025, 337, 118818.
- Chen, R.; Moriya, J.; Yamakawa, J.; Takahashi, T.; Kanda, T. Traditional chinese medicine for chronic fatigue syndrome, Evid Based Complement Alternat Med 2010, 7, 3-10.
- Shi, Q.; Zhao, H.; Chen, J.; Li, Y.; Li, Z.; Wang, J.; Wang, W. Study on qi deficiency syndrome identification modes of coronary heart disease based on metabolomic biomarkers, Evid Based Complement Alternat Med 2014, 2014, 281829.
- Luo, L.; Chen, J.; Guo, S.; Wang, J.; Gao, K.; Zhang, P.; Chen, C.; Zhao, H.; Wang, W. Chinese Herbal Medicine in the Treatment of Chronic Heart Failure: Three-Stage Study Protocol for a Randomized Controlled Trial, Evid Based Complement Alternat Med 2015, 2015, 927160.
- Gao, K.; Zhao, H.; Gao, J.; Wen, B.; Jia, C.; Wang, Z.; Zhang, F.; Wang, J.; Xie, H.; Wang, J.; Wang, W.; Chen, J. Mechanism of Chinese Medicine Herbs Effects on Chronic Heart Failure Based on Metabolic Profiling, Front Pharmacol 2017, 8, 864.
- Lin, Y.; Han, Y.; Wang, Y. Traditional Chinese medicine for cardiovascular disease: efficacy and safety, Front Cardiovasc Med 2024, 11, 1419169.
- Kang, B.K.; Jang, S.; Ko, M.M.; Jung, J. A Study on the Development of a Korean Metabolic Syndrome Questionnaire Using Blood Stasis Clinical Data, Evid Based Complement Alternat Med 2019, 2019, 8761417.
- Ko, M.M.; Jang, S.; Jung, J. An observational study on diagnosis index of metabolic disease with blood-stasis, Medicine (Baltimore) 2020, 99, e21140.
- Bae, J.C. No More NAFLD: The Term Is Now MASLD, Endocrinol Metab (Seoul) 2024, 39, 92-94.
- Liu, J.; Dong, B.; Yang, L.; Huang, W.; Tang, S. Xuefu Zhuyu decoction for nonalcoholic fatty liver disease: A protocol for systematic review and meta-analysis, Medicine (Baltimore) 2021, 100, e25358.
- Zheng, S.; Xue, C.; Li, S.; Zao, X.; Li, X.; Liu, Q.; Cao, X.; Wang, W.; Qi, W.; Zhang, P.; Ye, Y. Chinese medicine in the treatment of non-alcoholic fatty liver disease based on network pharmacology: a review, Front Pharmacol 2024, 15, 1381712.
- M. T. El Hussein and A. Hewko, Management of Postural Orthostatic Tachycardia Syndrome: A Canadian Approach, J Nurs Pract 2025, 21, 105258.
- Kell, D.B.; Khan, M.A.; Kane, B.; Lip, G.Y.H.; Pretorius, E. Possible Role of Fibrinaloid Microclots in Postural Orthostatic Tachycardia Syndrome (POTS): Focus on Long COVID, J Pers Med 2024, 14, 170.
- Wirth, K.J.; Löhn, M. Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect? , Medicina (Kaunas) 2022, 58, 1807. [Google Scholar] [CrossRef]
- Wang, X.; Wei, W.; Qi, Y.; Dong, L.; Zhang, Y. Clinical Effects of Integrated Traditional Chinese and Western Medicine in Treating Severe Preeclampsia and Its Influence on Maternal and Infant Outcomes after Cesarean Section under Combined Lumbar and Epidural Anesthesia, Evid Based Complement Alternat Med 2021, 2021, 6366914.
- Dong, H.; Song, J.; Jia, Y.; Cui, H.; Chen, X. Research on the Improvement of Endothelial Cell Function and Trophoblast Apoptosis in Rats with Preeclampsia by Tanshinone IIA, Pharmmacognosy Mag 2025, 21, 116-123.
- Zhang, X.; Li, J.; Zhou, P.; Luo, Q.; Xiang, Z.; Wu, H. Effect of Salvia Miltiorrhiza Injection on Blood Pressure and Cardiac Function in Rats with Gestational Hypertension and Preeclampsia, J Biosci Med 2023, 11, 152-160.
- Chen, Y.; Liu, Y.; Liang, M.; Wang, Y.; Zhang, H.; Huang, L.; Ye, J. Effects of magnesium sulfate combined with compound Danshen injection on pregnancy outcome, vascular endothelia function, liver and kidney function in patients with EOSPE, Int J Clin Med 2020, 13, 3703-3709.
- Kell, D.B.; Kenny, L.C. A dormant microbial component in the development of pre-eclampsia. Front Med Obs Gynecol 2016, 3, 60. [Google Scholar]
- Kenny, L.C.; Kell, D.B. ; Immunological tolerance, pregnancy and pre-eclampsia: the roles of semen microbes and the father, Front Med Obs Gynecol 2018, 4, 239.
- Han, L.; Liu, X.; Li, H.; Zou, J.; Yang, Z.; Han, J.; Huang, W.; Yu, L.; Zheng, Y.; Li, L. Blood coagulation parameters and platelet indices: changes in normal and preeclamptic pregnancies and predictive values for preeclampsia, PLoS One 2014, 9, e114488.
- Glazier, C.R.; Baciewicz, F.A. Jr. ; Epidemiology; Etiology, and Pathophysiology of Pulmonary Embolism, Int J Angiol 2024, 33, 76–81. [PubMed]
- Li, W.; Wang, R.; Huang, W.; Shen, Y.; Du, J.; Tian, Y. BuyangHuanwu Decoction attenuates cerebral vasospasm caused by subarachnoid hemorrhage in rats via PI3K/AKT/eNOS axis, Open Life Sci 2022, 17, 735-743.
- Zhou, J.; Guo, P.; Guo, Z.; Sun, X.; Chen, Y.; Feng, H. Fluid metabolic pathways after subarachnoid hemorrhage, J Neurochem 2022, 160, 13-33.
- McMahon, C.J.; Hopkins, S.; Vail, A.; King, A.T.; Smith, D.; Illingworth, K.J.; Clark, S.; Rothwell, N.J.; Tyrrell, P.J. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage, J Neurointerv Surg 2013, 5, 512-517.
- Tang, X.; Liu, X.; Zhao, M.S.M.H.; Zhang, Y. Traditional Chinese medicine in the treatment of high incidence diseases in cold areas: the thrombotic diseases, Frigid Zone Med 2021, 23-44.
- Yin, Q.; Zhang, X.; Liao, S.; Huang, X.; Wan, C.C.; Wang, Y. Potential anticoagulant of traditional chinese medicine and novel targets for anticoagulant drugs, Phytomedicine 2023, 116, 154880.
- Yu, H.; Chai, X.; Geng, W.C.; Zhang, L.; Ding, F.; Guo, D.S.; Wang, Y. Facile and label-free fluorescence strategy for evaluating the influence of bioactive ingredients on FMO3 activity via supramolecular host-guest reporter pair, Biosens Bioelectron 2021, 192, 113488.
- Sha, R.N.; Tang, L.; Du, Y.W.; Wu, S.X.; Shi, H.W.; Zou, H.X.; Zhang, X.R.; Dong, X.L.; Zhou, L. Effectiveness and safety of Ginkgo biloba extract (GBE50) in the treatment of dizziness caused by cerebral arteriosclerosis: a multi-center, double-blind, randomized controlled trial, J Tradit Chin Med 2022, 42, 83-89.
- Ahmed, S.H.; Waseem, S.; Shaikh, T.G.; Qadir, N.A.; Siddiqui, S.A.; Ullah, I.; Waris, A.; Yousaf, Z. ; SARS-CoV-2 vaccine-associated-tinnitus: A review, Ann Med Surg (Lond) 2022, 75, 103293.
- Figueiredo, R.R.; de O, N.; de Azevedo, A.A.; de Oliveira, P.M.; de Siqueira, A.G.; Figueiredo, G.M.R.; Schlee, W.; Langguth, B. Tinnitus emerging in the context of a COVID-19 infection seems not to differ in its characteristics from tinnitus unrelated to COVID-19, Front Neurol 2022, 13, 974179.
- Wang, W.; Yellamsetty, A.; Edmonds, R.M.; Barcavage, S.R.; Bao, S. COVID-19 vaccination-related tinnitus is associated with pre-vaccination metabolic disorders, Front Pharmacol 2024, 15, 1374320.
- Yao, Q.; Zhang, L.; Zhou, J.; Li, M.; Jing, W.; Li, X.; Han, J.; He, L.; Zhang, Y. Imaging Diagnosis of Transient Ischemic Attack in Clinic and Traditional Chinese Medicine, Biomed Res Int 2019, 2019, 5094842.
- Kang, Y.; Yang, Z.; Zhou, K.; Zhou, Y. Treating transient ischemic attack from the evil of Phlegm and blood stasis based on abnormal lipid metabolism, Acad J Med Health Sci 2023, 4, 51-55.
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; Lennon, O.; Meschia, J.F.; Nguyen, T.N.; Pollak, P.M.; Santangeli, P.; Sharrief, A.Z.; Smith, S.C. Jr..; Turan, T.N.; Williams, L.S. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association, Stroke 2021, 52, e364-e467.
- Jin, X.; Shen, G.; Gao, F.; Zheng, X.; Xu, X.; Shen, F.; Li, G.; Gong, J.; Wen, L.; Yang, X.; Bie, X. Traditional Chinese drug ShuXueTong facilitates angiogenesis during wound healing following traumatic brain injury, J Ethnopharmacol 2008, 117, 473-477.
- Liu, Z.K.; Ng, C.F.; Shiu, H.T.; Wong, H.L.; Chin, W.C.; Zhang, J.F.; Lam, P.K.; Poon, W.S.; Lau, C.B.; Leung, P.C.; Ko, C.H. Neuroprotective effect of Da Chuanxiong Formula against cognitive and motor deficits in a rat controlled cortical impact model of traumatic brain injury, J Ethnopharmacol 2018, 217, 11-22.
- Kwon, C.Y.; Lee, B.; Leem, J.; Chung, S.Y.; Kim, J.W. Korean medicine treatments including blood stasis-removing therapy and auriculotherapy for persistent headache after traumatic brain injury: A case report, Explore (NY) 2019, 15, 419-424.
- Lee, B.; Leem, J.; Kim, H.; Jo, H.G.; Yoon, S.H.; Shin, A.; Sul, J.U.; Choi, Y.Y.; Yun, Y.; Kwon, C.Y. Herbal medicine for acute management and rehabilitation of traumatic brain injury: A protocol for a systematic review, Medicine (Baltimore) 2019, 98, e14145.
- Li, T.; Hu, E.; Li, P.; Yang, Z.; Wu, Y.; Ding, R.; Zhu, X.; Tang, T.; Wang, Y. Metabolomics Deciphers Potential Targets of Xuefu Zhuyu Decoction Against Traumatic Brain Injury in Rat, Front Pharmacol 2020, 11, 559618.
- Yang, Z.Y.; Tang, T.; Li, P.F.; Li, X.X.; Wu, Y.; Feng, D.D.; Hu, M.R.; Dai, F.; Zheng, F.; Zhang, W.; Wang, Y. Systematic analysis of tRNA-derived small RNAs reveals therapeutic targets of Xuefu Zhuyu decoction in the cortexes of experimental traumatic brain injury, Phytomedicine 2022, 102, 154168.
- Wei, C.; Wang, J.; Yu, J.; Tang, Q.; Liu, X.; Zhang, Y.; Cui, D.; Zhu, Y.; Mei, Y.; Wang, Y.; Wang, W. Therapy of traumatic brain injury by modern agents and traditional Chinese medicine, Chin Med 2023, 18, 25.
- Maegele, M.; Schöchl, H.; Menovsky, T.; Maréchal, H.; Marklund, N.; Buki, A.; Stanworth, S. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management, Lancet Neurol 2017, 16, 630-647.
- Jung, J.; Ko, M.M.; Lee, J.A.; Lee, M.S. Recognition of Association Between Blood Stasis Syndrome and Traumatic Injury among Doctors of Korean Medicine: A Cross-Sectional Observation Study, Chin J Integr Med 2018, 24, 254-259.
- Law, S.; Gillmore, J.D. When to Suspect and How to Approach a Diagnosis of Amyloidosis, Am J Med 2022, 135 Suppl 1, S2-S8.
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; Kumar, S.K.; Gertz, M.A. SyJung; J.; Cha; M.H.; Lee; H.; Ko; M.M.; Lee; J.A.; Lee; S.; Lee; M.S. M.; Frasconi, C.; Mege, D.; Panicot-Dubois, L.; Boiron, L.; Dahan, L.; Debourdeau, P.; Dubois, C.; Farge, D.; Sielezneff, I.emic amyloidosis from A (AA) to T (ATTR): a review, J Intern Med 2021, 289, 268-292.
- Jung; J.; Cha; M.H.; Lee; H.; Ko; M.M.; Lee; J.A.; Lee; S.; Lee; M.S. The association of C-reactive protein and serum amyloid P with blood stasis syndrome in Traditional Korean Medicine for general disease conditions: A cross-sectional, observational study, Eur J Integr Med 2016, 8, 432-437.
- Noble; S.; Pasi, Epidemiology and pathophysiology of cancer-associated thrombosis, Br J Cancer 2010, 102 Suppl 1, S2-9.
- Leiva; O.; Newcomb; R.; Connors; J.M.; Al-Samkari; Cancerrombosis: new insights to an old problem, J Med Vasc 2020, 45, 6S8-6S16.
- Leiva; O. ; AbdelHameid; D.; Connors; J.M.; Cannon; C.P.; Bhatt; D.L.ommon Pathophysiology in Cancer, Atrial Fibrillation, Atherosclerosis, and Thrombosis: JACC: CardioOncology State-of-the-Art Review, JACC CardioOncol 2021, 3, 619–634.
- Khorana; A. A.; Fine; R.L. Pancreatic cancer and thromboembolic disease, Lancet Oncol 2004, 5, 655–663.
- Epstein; A. S.; Soff; G.A.; Capanu; M.; Crosbie; C.; Shah; M.A.; Kelsen; D.P.; Denton; B.; Gardos; S.; E. M. O'Reilly; Analysis of incidence and clinical outcomes in patients with thromboembolic events and invasive exocrine pancreatic cancer, Cancer 2012, 118, 3053–3061.
- Timp; J. F.; Braekkan; S.K.; Versteeg; H.H.; Cannegieter; S.C.; Epidemiology of cancer-associated venous thrombosis; Blood 2013, 122, 1712–1723.
- Ansari; D. ; Ansari; D.; Andersson; R.; Andren-Sandberg, Pancreatic cancer and thromboembolic disease, 150 years after Trousseau, Hepatobiliary Surg Nutr 2015, 4, 325–335.
- Ouaïssi, M.; Frasconi, C.; Mege, D.; Panicot-Dubois, L.; Boiron, L.; Dahan, L.; Debourdeau, P.; Dubois, C.; Farge, D.; Sielezneff, I. Impact of venous thromboembolism on the natural history of pancreatic adenocarcinoma, Hepatobiliary Pancreat Dis Int 2015, 14, 436-442.
- Berger, A.K.; Singh, H.M.; Werft, W.; Muckenhuber, A.; Sprick, M.R.; Trumpp, A.; Weichert, W.; Jager, D.; Springfeld, C. High prevalence of incidental and symptomatic venous thromboembolic events in patients with advanced pancreatic cancer under palliative chemotherapy: A retrospective cohort study, Pancreatology 2017, 17, 629-634.
- Ishigaki, K.; Nakai, Y.; Isayama, H.; Saito, K.; Hamada, T.; Takahara, N.; Mizuno, S.; Mohri, D.; Kogure, H.; Matsubara, S.; Yamamoto, N.; Tada, M.; Koike, K. Thromboembolisms in Advanced Pancreatic Cancer: A Retrospective Analysis of 475 Patients, Pancreas 2017, 46, 1069-1075.
- Mege, D.; Crescence, L.; Ouaissi, M.; Sielezneff, I.; Guieu, R.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Fibrin-bearing microparticles: marker of thrombo-embolic events in pancreatic and colorectal cancers, Oncotarget 2017, 8, 97394-97406.
- Bosch, F.T.M.; Mulder, F.I.; Kamphuisen, P.W.; Middeldorp, S.; Bossuyt, P.M.; Buller, H.R.; van Es, N. Primary thromboprophylaxis in ambulatory cancer patients with a high Khorana score: a systematic review and meta-analysis, Blood Adv 2020, 4, 5215-5225.
- Fuentes, H.E.; Oramas, D.M.; Paz, L.H.; Wang, Y.; Andrade, X.A.; Tafur, A.J. Venous Thromboembolism Is an Independent Predictor of Mortality Among Patients with Gastric Cancer, J Gastrointest Cancer 2018, 49, 415-421.
- Quintero, L.A.D.; Fuentes, H.E.; Tafur, A.J.; Majmudar, K.; Adum, J.P.S.; Golemi, I.; Paz, L.H.; Stocker, S.; Talamonti, M. Pancreatic cancer thromboembolic outcomes: rate of thrombosis after adenocarcinoma and non-adenocarcinoma pancreatic cancer surgery, Int Angiol 2019, 38, 194-200.
- Godinho, J.; Casa-Nova, M.; Moreira-Pinto, J.; SimoeButt, Z.; Rosenbloom, S.K.; Abernethy, A.P.; Beaumont, J.L.; Paul, D.; Hampton, D.; Jacobsen, P.B.; Syrjala, K.L.s, P.; Branco, F.P.; Leal-Costa, L.; Faria, A.; Lopes, F.; Teixeira, J.A.; Passos-Coelho, J.L. ONKOTEV Score as a Predictive Tool for Thromboembolic Events in Pancreatic Cancer-A Retrospective Analysis, Oncologist 2020, 25, e284-e290.
- Hanna-Sawires; R.G.; Groen; J.V.; Hamming; A.; Tollenaar; R.A.E.M.; Mesker; W.E.; Luelmo; S.A.C.; Vahrmeijer; A.L.; Bonsing; B.A.; Versteeg; H.H.; Klok; F.A.; Mieog; J.S.D.; Incidence, timing and risk factors of venous thromboembolic events in patients with pancreatic cancer, Thromb Res 2021, 207, 134-139.
- Heffley; J.; Ganguly; E.; Tompkins; B.J.; Ades; S.; Holmes; C.E.; Zubarik, Venous thromboembolism in patients with pancreatic adenocarcinoma: Disease burden and initiation of ambulatory thromboprophylaxis, Pancreatology 2024, 24, 894-898.
- Falanga; A.; Schieppati; F.; Russo, Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer, Semin Thromb Hemost 2015, 41, 756-764.
- Bower; J.E.; Cancer-related fatigue--mechanisms; risk factors; treatments, Nat Rev Clin Oncol 2014, 11, 597-609.
- Butt, Z.; Rosenbloom, S.K.; Abernethy, A.P.; Beaumont, J.L.; Paul, D.; Hampton, D.; Jacobsen, P.B.; Syrjala, K.L. J. H. Von Roenn and D. Cella, Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy, J Natl Compr Canc Netw 2008, 6, 448-455.
- Narayanan, V.; Koshy, C. Fatigue in cancer: a review of literature, Indian J Palliat Care 2009, 15, 19-25.
- Song, Y.; Sun, X.; Shen, L.; Qu, Z.; Yin, J.; Wang, Z.; Zhang, H. ; Genes of cancer-related fatigue: a scoping review; Oncol, F. 2024, 14, 1446321.
- Thong, M.S.Y.; van Noorden, C.J.F.; Steindorf, K.; Arndt, V. Cancer-Related Fatigue: Causes and Current Treatment Options, Curr Treat Options Oncol 2020, 21, 17.
- Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis, Cells 2019, 8.
- Yang, J.; Li, Y.; Chau, C.I.; Shi, J.; Chen, X.; Hu, H.; Ung, C.O.L. Efficacy and safety of traditional Chinese medicine for cancer-related fatigue: a systematic literature review of randomized controlled trials, Chin Med 2023, 18, 142.
- Zeng, J.; Wu, Q.; Meng, X.D.; Wang, J. Systematic review of Buzhong Yiqi method in alleviating cancer-related fatigue: a meta-analysis and exploratory network pharmacology approach, Front Pharmacol 2024, 15, 1451773.
- Liang, F.; Sun, J.; Li, Q.; Li, C.H.; Fan, Z.-Z. Analysis of clinical syndromes in 47 patients with pancreatic cancer at late stage, J Tradit Chin Med 2011, 31, 182-184.
- Liu, Z.; Yu, Z.; Ouyang, X.; Du, J.; Lan, X.; Zhao, M. Applied research on serum protein fingerprints for prediction of Qi deficiency syndrome and phlegm and blood stasis in patients with non-small cell lung cancer, J Tradit Chin Med 2012, 32, 350-354.
- Guo, Z.; Lou, Y.; Kong, M.; Luo, Q.; Liu, Z.; Wu, J. A Systematic Review of Phytochemistry, Pharmacology and Pharmacokinetics on Astragali Radix: Implications for Astragali Radix as a Personalized Medicine, Int J Mol Sci 2019, 20, 1463.
- Huang, Y.X.; Xu, D.Q.; Yue, S.J.; Chen, Y.Y.; Tao, H.J.; Fu, R.J.; Xing, L.M.; Wang, T.; Ma, Y.L.; Wang, B.A.; Tang, Y.P.; Duan, J.A. Deciphering the Active Compounds and Mechanisms of Qixuehe Capsule on Qi Stagnation and Blood Stasis Syndrome: A Network Pharmacology Study, Evid Based Complement Alternat Med 2020, 2020, 5053914.
- Kim, B.S.; Jin, S.; Park, J.Y.; Kim, S.Y. Scoping review of the medicinal effects of Eupolyphaga sinensis Walker and the underlying mechanisms, J Ethnopharmacol 2022, 296, 115454.
- Zhang, F.; Ganesan, K.; Liu, Q.; Chen, J. A Review of the Pharmacological Potential of Spatholobus suberectus Dunn on Cancer, Cells 2022, 11, 2885.
- Zhang, Y.; Xu, H.; Li, Y.; Sun, Y.; Peng, X. Advances in the treatment of pancreatic cancer with traditional Chinese medicine, Front Pharmacol 2023, 14, 1089245.
- Jin, L.; Tang, B.; Liu, X.; Mao, W.; Xia, L.; Du, Y.; Ji, B.; Shou, Q.; Fu, H. Blood Stasis Syndrome Accelerates the Growth and Metastasis of Breast Cancer by Promoting Hypoxia and Immunosuppressive Microenvironment in Mice, J Immunol Res 2022, 2022, 7222638.
- Kell, D.B.; Pretorius, E. The proteome content of blood clots observed under different conditions: successful role in predicting clot amyloid(ogenicity), bioRxiv 2024, 2024.2011.2029.626062.
- Aguzzi, A.; Lakkaraju, A.K.K. Cell Biology of Prions and Prionoids: A Status Report, Trends Cell Biol 2016, 26, 40-51.
- Ashe, K.H.; Aguzzi, A. ; Prions, prionoids and pathogenic proteins in Alzheimer disease, Prion 2013, 7, 55-59.
- Bratkovič, I.H. ; Prions, prionoid complexes and amyloids: the bad, the good and something in between, Swiss Med Wkly 2017, 147, w14424.
- Liberski, P.P. ; Prion, prionoids and infectious amyloid, Parkinsonism Relat Disord 2014, 20 Suppl 1, S80-84.
- Scheckel, C.; Aguzzi, A. ; Prions, prionoids and protein misfolding disorders, Nat Rev Genet 2018, 19, 405-418.
- Verma, A. ; Prions; prion-like prionoids; neurodegenerative disorders, Ann Indian Acad Neurol 2016, 19, 169-174.
- Wells, C.; Brennan, S.E.; Keon, M.; Saksena, N.K. Prionoid Proteins in the Pathogenesis of Neurodegenerative Diseases, Front Mol Neurosci 2019, 12, 271.
- Kell, D.B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases, BMC Med Genom 2009, 2, 2.
- Yi, M.; Li, Q.; Zhao, Y.; Nie, S.; Wu, N.; Wang, D. Metabolomics study on the therapeutic effect of traditional Chinese medicine Xue-Fu-Zhu-Yu decoction in coronary heart disease based on LC-Q-TOF/MS and GC-MS analysis, Drug Metab Pharmacokinet 2019, 34, 340-349.
- Zhao, Y.; Nie, S.; Yi, M.; Wu, N.; Wang, W.; Zhang, Z.; Yao, Y.; Wang, D. UPLC-QTOF/MS-based metabolomics analysis of plasma reveals an effect of Xue-Fu-Zhu-Yu capsules on blood-stasis syndrome in CHD rats, J Ethnopharmacol 2019, 241, 111908.
- He, H.; Chen, G.; Gao, J.; Liu, Y.; Zhang, C.; Liu, C.; Li, H.; He, Q.; Li, J.; Wang, J. Xue-Fu-Zhu-Yu capsule in the treatment of qi stagnation and blood stasis syndrome: a study protocol for a randomised controlled pilot and feasibility trial, Trials 2018, 19, 515.
- Chen, S.; Wu, X.; Li, T.; Cheng, W.; Han, X.; Li, Y.; Wang, B.; Teng, Y.; Zhao, M.; Wang, Y. The Improvement of Cardiac and Endothelial Functions of Xue-Fu-Zhu-Yu Decoction for Patients with Acute Coronary Syndrome: A Meta-Analysis of Randomized Controlled Trials, Evid Based Complement Alternat Med 2022, 2022, 2671343.
- Xue, D.J.; Zhen, Z.; Wang, K.X.; Zhao, J.L.; Gao, Y.; Chen, Y.P.; Shen, Y.B.; Peng, Z.Z.; Guan, D.G.; Huang, T. Uncovering the potential mechanism of Xue Fu Zhu Yu Decoction in the treatment of intracerebral hemorrhage, BMC Complement Med Ther 2022, 22, 103.
- Kuo, C.E.; Hsu, S.F.; Chen, C.C.; Wu, S.Y.; Hung, Y.C.; Hsu, C.Y.; Tsai, I.J.; Hu, W.L. Prescription characteristics of Xue-Fu-Zhu-Yu-Tang in pain management: a population-based study using the National Health Insurance Research Database in Taiwan, Front Pharmacol 2023, 14, 1233156.
- Wang, D.; Wang, P.; Zhang, R.; Xi, X. Efficacy and safety of Xuefu Zhuyu decoction combined with Western medicine for angina pectoris in coronary heart disease: A protocol for systematic review and meta-analysis, Medicine (Baltimore) 2020, 99, e23195.
- Liao, S.; Zhang, Z.; Li, G.; Zhou, L.; Jiang, J.; Zhang, N.; Wang, Y.; Du, Y.; Wen, Z. Chinese Herbal Formula Xuefu Zhuyu for Stable Angina (CheruSA): Study Protocol for a Multicenter Randomized Controlled Trial, Evid Based Complement Alternat Med 2020, 2020, 7612721.
- Huang, Q.; Qiao, X.; Xu, X. Potential synergism and inhibitors to multiple target enzymes of Xuefu Zhuyu Decoction in cardiac disease therapeutics: a computational approach, Bioorg Med Chem Lett 2007, 17, 1779-1783.
- Zhang, G.; Yang, G.; Deng, Y.; Zhao, X.; Yang, Y.; Rao, J.; Wang, W.; Liu, X.; He, J.; Lv, L. Ameliorative effects of Xue-Fu-Zhu-Yu decoction, Tian-Ma-Gou-Teng-Yin and Wen-Dan decoction on myocardial fibrosis in a hypertensive rat mode, BMC Complement Altern Med 2016, 16, 56.
- Chen, G.; He, H.; Hu, K.; Gao, J.; Li, J.; Han, M.; Wang, J. Sensitive Biomarker Analysis of Xue-Fu-Zhu-Yu Capsule for Patients with Qi Stagnation and Blood Stasis Pattern: A Nested Case-Control Study, Evid Based Complement Alternat Med 2019, 2019, 7182865.
- Zhao, L.; Qiu, X.; Wang, R.; Wang, D. (1)H NMR-based metabolomics study of the dynamic effect of Xue-Fu-Zhu-Yu capsules on coronary heart disease rats induced by high-fat diet, coronary artery ligation, J Pharm Biomed Anal 2021, 195, 113869.
- Yi, G.Z.; Qiu, Y.Q.; Xiao, Y.; Yuan, L.X. The usefulness of xuefu zhuyu tang for patients with angina pectoris: a meta-analysis and systematic review, Evid Based Complement Alternat Med 2014, 2014, 521602.
- Yang, X.; Xiong, X.; Yang, G.; Wang, J. Chinese patent medicine Xuefu Zhuyu capsule for the treatment of unstable angina pectoris: A systematic review of randomized controlled trials, Complement Ther Med 2014, 22, 391-399.
- Yang, T.; Li, X.; Lu, Z.; Han, X.; Zhao, M. Effectiveness and safety of Xuefu Zhuyu decoction for treating coronary heart disease angina: A systematic review and meta-analysis, Medicine (Baltimore) 2019, 98, e14708.
- Qi, G.; Jiang, K.; Qu, J.; Zhang, A.; Xu, Z.; Li, Z.; Zheng, X.; Li, Z. The Material Basis and Mechanism of Xuefu Zhuyu Decoction in Treating Stable Angina Pectoris and Unstable Angina Pectoris, Evid Based Complement Alternat Med 2022, 2022, 3741027.
- Li, Y.; Liu, B.; Liu, L.; Xu, Q.; Shen, Q.; Li, W.; Zhao, J. Potential active compounds and molecular mechanism of Xuefu Zhuyu decoction for atherosclerosis, based on network pharmacology and molecular docking, Medicine (Baltimore) 2022, 101, e29654.
- Liu, D.; Zeng, Y.; Liang, P.; Jiang, Y.; An, S.; Ren, P. Efficacy and safety of Xuefu Zhuyu Granules combined with western medicine in the treatment of angina pectoris of coronary heart disease: A study protocol of a randomized, double-blind, placebo-controlled clinical trial, Medicine (Baltimore) 2022, 101, e31235.
- Yuan, J.; Yan, F.; Li, W.; Yuan, G. Network pharmacological analysis of Xuefu Zhuyu decoction in the treatment of atherosclerosis, Front Pharmacol 2022, 13, 1069704.
- Yi, T.; Gao, P.; Hou, M.; Lv, H.; Huang, M.; Gao, S.; He, J.; Yang, D.; Chen, W.; Zhu, T.; Yu, C.; Liu, F.; Yin, H.; Jin, S. The mechanisms underlying the actions of Xuefu Zhuyu decoction pretreatment against neurological deficits after ischemic stroke in mice: The mediation of glymphatic function by aquaporin-4 and its anchoring proteins, Front Pharmacol 2022, 13, 1053253.
- Duan, J.; Lin, J.; Zhang, N.; Wang, Q.; Li, N.; Yao, K. Effect of Xuefu Zhuyu Capsule on Myocardial Infarction: Network Pharmacology and Experimental Verification, Evid Based Complement Alternat Med 2023, 2023, 5652276.
- Yang, Y.; Su, C.; Zhang, X.Z.; Li, J.; Huang, S.C.; Kuang, H.F.; Zhang, Q.Y. Mechanisms of Xuefu Zhuyu Decoction in the treatment of coronary heart disease based on integrated metabolomics and network pharmacology approach, J Chromatogr B Analyt Technol Biomed Life Sci 2023, 1223, 123712.
- Jia, D.; Zhao, M.; Zhang, X.; Cheng, X.; Wei, Q.; Lou, L.; Zhao, Y.; Jin, Q.; Chen, M.; Zhang, D. Transcriptomic analysis reveals the critical role of chemokine signaling in the anti-atherosclerosis effect of Xuefu Zhuyu decoction, J Ethnopharmacol 2024, 332, 118245.
- Hung, I.L.; Chung, C.J.; Hu, W.L.; Liao, Y.N.; Hsu, C.Y.; Chiang, J.H.; Hung, Y.C. Chinese Herbal Medicine as an Adjunctive Therapy Improves the Survival Rate of Patients with Ischemic Heart Disease: A Nationwide Population-Based Cohort Study, Evid Based Complement Alternat Med 2022, 2022, 5596829.
- Xing, Z.; Xia, Z.; Peng, W.; Li, J.; Zhang, C.; Fu, C.; Tang, T.; Luo, J.; Zou, Y.; Fan, R.; Liu, W.; Xiong, X.; Huang, W.; Sheng, C.; Gan, P.; Wang, Y.; Zhuyu decoction, X. a traditional Chinese medicine, provides neuroprotection in a rat model of traumatic brain injury via an anti-inflammatory pathway, Sci Rep 2016, 6, 20040.
- Zhou, J.; Liu, T.; Cui, H.; Fan, R.; Zhang, C.; Peng, W.; Yang, A.; Zhu, L.; Wang, Y.; Tang, T. Xuefu zhuyu decoction improves cognitive impairment in experimental traumatic brain injury via synaptic regulation, Oncotarget 2017, 8, 72069-72081.
- Feng, D.; Xia, Z.; Zhou, J.; Lu, H.; Zhang, C.; Fan, R.; Xiong, X.; Cui, H.; Gan, P.; Huang, W.; Peng, W.; He, F.; Wang, Z.; Wang, Y.; Tang, T. Metabolomics reveals the effect of Xuefu Zhuyu Decoction on plasma metabolism in rats with acute traumatic brain injury, Oncotarget 2017, 8, 94692-94710.
- Zhu, L.; Tang, T.; Fan, R.; Luo, J.K.; Cui, H.J.; Zhang, C.H.; Peng, W.J.; Sun, P.; Xiong, X.G.; Wang, Y. Xuefu Zhuyu decoction improves neurological dysfunction by increasing synapsin expression after traumatic brain injury, Neural Regen Res 2018, 13, 1417-1424.
- Yang, Z.Y.; Wu, Y.; Li, X.; Tang, T.; Wang, Y.; Huang, Z.B.; Fan, R. Bioinformatics Analysis of miRNAs and mRNAs Network-Xuefu Zhuyu Decoction Exerts Neuroprotection of Traumatic Brain Injury Mice in the Subacute Phase, Front Pharmacol 2022, 13, 772680.
- Dai, F.; Tang, T.; Lu, R.; Li, P.; Feng, D.; Hu, M.; Wang, Y.; Gan, P. Systematic Analysis of tRNA-Derived Small RNAs Reveals the Effects of Xuefu-Zhuyu Decoction on the Hippocampi of Rats after Traumatic Brain Injury, Evid Based Complement Alternat Med 2022, 2022, 5748719.
- Hu, E.; Tang, T.; Li, Y.M.; Li, T.; Zhu, L.; Ding, R.Q.; Wu, Y.; Huang, Q.; Zhang, W.; Wu, Q.; Wang, Y. Spatial amine metabolomics and histopathology reveal localized brain alterations in subacute traumatic brain injury and the underlying mechanism of herbal treatment, CNS Neurosci Ther 2024, 30, e14231.
- Feng, D.; Li, P.; Xiao, W.; Pei, Z.; Chen, P.; Hu, M.; Yang, Z.; Li, T.; Xia, Z.; Cui, H.; Li, H.; Huang, Q.; Zhang, W.; Tang, T.; Wang, Y. N(6)-methyladenosine profiling reveals that Xuefu Zhuyu decoction upregulates METTL14 and BDNF in a rat model of traumatic brain injury, J Ethnopharmacol 2023, 317, 116823.
- Wang, Y.; Yan, Q.J.; Hu, E.; Wu, Y.; Ding, R.Q.; Chen, Q.; Cheng, M.H.; Yang, X.Y.; Tang, T.; Li, T. Xuefu Zhuyu Decoction Improves Blood-Brain Barrier Integrity in Acute Traumatic Brain Injury Rats via Regulating Adenosine, Chin J Integr Med, 2025.
- Li, T.; Zhang, L.; Cheng, M.; Hu, E.; Yan, Q.; Wu, Y.; Luo, W.; Su, H.; Yu, Z.; Guo, X.; Chen, Q.; Zheng, F.; Li, H.; Zhang, W.; Tang, T.; Luo, J.; Wang, Y. Metabolomics integrated with network pharmacology of blood-entry constituents reveals the bioactive component of Xuefu Zhuyu decoction and its angiogenic effects in treating traumatic brain injury, Chin Med 2024, 19, 131.
- Pei, Z.; Guo, X.; Zheng, F.; Yang, Z.; Li, T.; Yu, Z.; Li, X.; Guo, X.; Chen, Q.; Fu, C.; Tang, T.; Feng, D.; Wang, Y. Xuefu Zhuyu decoction promotes synaptic plasticity by targeting miR-191a-5p/BDNF-TrkB axis in severe traumatic brain injury, Phytomedicine 2024, 129, 155566.
- Fu, C.; Wu, Q.; Zhang, Z.; Xia, Z.; Ji, H.; Lu, H.; Wang, Y. UPLC-ESI-IT-TOF-MS metabolomic study of the therapeutic effect of Xuefu Zhuyu decoction on rats with traumatic brain injury, J Ethnopharmacol 2019, 245, 112149.
- Tang, H.; Wang, J.; Fang, Y.; Yin, Y.; Liu, W.; Hu, Y.; Peng, J. Hepatic transcriptome discloses the potential targets of Xuefu Zhuyu Decoction ameliorating non-alcoholic fatty liver disease induced by high-fat diet, J Tradit Complement Med 2024, 14, 135-147.
- Zhang, H.; Hua, H.; Liu, J.; Wang, C.; Zhu, C.; Xia, Q.; Jiang, W.; Cheng, X.; Hu, X.; Zhang, Y. Integrative analysis of the efficacy and pharmacological mechanism of Xuefu Zhuyu decoction in idiopathic pulmonary fibrosis via evidence-based medicine, bioinformatics, and experimental verification, Heliyon 2024, 10, e38122.
- Xu, A.; Wen, Z.H.; Su, S.X.; Chen, Y.P.; Liu, W.C.; Guo, S.Q.; Li, X.F.; Zhang, X.; Li, R.; Xu, N.B.; Wang, K.X.; Li, W.X.; Guan, D.G.; Duan, C.Z. Elucidating the Synergistic Effect of Multiple Chinese Herbal Prescriptions in the Treatment of Post-stroke Neurological Damage, Front Pharmacol 2022, 13, 784242.
- Ning, B.; Zhu, X.; Wu, X.; Zhu, W.; Wang, R.; Qi, C.; Li, M. Efficacy of different traditional Chinese medicine decoctions in the treatment of ischemic stroke: a network meta-analysis, Front Pharmacol 2024, 15, 1486458.
- Wang, P.; Xiong, X.; Li, S. Efficacy and Safety of a Traditional Chinese Herbal Formula Xuefu Zhuyu Decoction for Hypertension: A Systematic Review and Meta-Analysis, Medicine (Baltimore) 2015, 94, e1850.
- Wang, S.; Wu, C.; Li, Y.; Ye, B.; Wang, S.; Li, G.; Wu, J.; Liu, S.; Zhang, M.; Jia, Y.; Cao, H.; Jiang, C.; Wu, F. Analysis of the Anti-Tumour Effect of Xuefu Zhuyu Decoction Based on Network Pharmacology and Experimental Verification in Drosophila, Front Pharmacol 2022, 13, 922457.
- Wang, X.; Zhang, D.Y.; Yin, S.J.; Jiang, H.; Lu, M.; Yang, F.Q.; Hu, Y.J. Screening of Potential Thrombin and Factor Xa Inhibitors from the Danshen-Chuanxiong Herbal Pair through a Spectrum-Effect Relationship Analysis, Molecules 2021, 26, 7293.
- Pang, H.Q.; Guo, J.X.; Yang, Y.; Xu, L.; Wang, J.; Yang, F.; Xu, Z.B.; Huang, Y.F.; Shi, W.; Lu, X.; Ibrahim, M.E.H.; Hu, W.C.; Yan, B.C.; Liu, L. Elucidating the chemical interaction effects of herb pair Danshen-Chuanxiong and its anti-ischemic stroke activities evaluation, J Ethnopharmacol 2024, 318, 117058.
- Zhao, C.; Bai, X.; Ding, Y.; Wen, A.; Fu, Q. ; Combining systems pharmacology; metabolomics, and transcriptomics to reveal the mechanism of Salvia miltiorrhiza-Cortex moutan herb pair for the treatment of ischemic stroke, Front Pharmacol 2024, 15, 1431692.
- Liu, Y.; Yin, H.J.; Shi, D.Z.; Chen, K.J. Chinese herb and formulas for promoting blood circulation and removing blood stasis and antiplatelet therapies, Evid Based Complement Alternat Med 2012, 2012, 184503.
- Li, J.; Zhou, X.; Zheng, C.; Lai, L.; Li, L. Comparison of mechanisms and efficacies of five formulas for improving blood circulation and removing blood stasis. Digital Chinese Med 2021, 4, 144–158. [Google Scholar]
- Lee, J.J.; Hsu, W.H.; Yen, T.L.; Chang, N.C.; Luo, Y.J.; Hsiao, G.; Sheu, J.R.; Chinese medicine, T. ; Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats, J Ethnopharmacol 2011, 134, 824-830.
- Zhang, L.; Zhu, L.; Wang, Y.; Jiang, Z.; Chai, X.; Zhu, Y.; Gao, X.; Qi, A. Characterization and quantification of major constituents of Xue Fu Zhu Yu by UPLC-DAD-MS/MS, J Pharm Biomed Anal 2012, 62, 203-209.
- Fu, C.; Xia, Z.; Liu, Y.; Lu, H.; Zhang, Z.; Wang, Y.; Fan, X. Qualitative analysis of major constituents from Xue Fu Zhu Yu Decoction using ultra high performance liquid chromatography with hybrid ion trap time-of-flight mass spectrometry, J Sep Sci 2016, 39, 3457-3468.
- Sun, Y.; Guo, R.; Geng, Y.; Shang, H.; Guo, X.; Wu, Y.; Wang, Y.; Li, L.; Li, X.; Zhang, S.; Xu, N.; Li, X. Longitudinal Distribution Map of the Active Components and Endophytic Fungi in Angelica sinensis (Oliv. ) Diels Root and Their Potential Correlations, Metabolites 2024, 14, 48. [Google Scholar]
- Yang, C.; Li, X.; Rong, J. Amygdalin isolated from Semen Persicae (Tao Ren) extracts induces the expression of follistatin in HepG2 and C2C12 cell lines, Chin Med 2014, 9, 23.
- Yang, C.; Zhao, J.; Cheng, Y.; Li, X.; Rong, J. Bioactivity-guided fractionation identifies amygdalin as a potent neurotrophic agent from herbal medicine Semen Persicae extract, Biomed Res Int 2014, 2014, 306857. 2014.
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review, J Ethnopharmacol 2014, 151, 27–43. [Google Scholar]
- Zhang, Y.; Song, L.; Pan, R.; Gao, J.; Zang, B.X.; Jin, M. Hydroxysafflor Yellow A Alleviates Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome in Mice, Biol Pharm Bull 2017, 40, 135-144.
- Bai, X.; Wang, W.X.; Fu, R.J.; Yue, S.J.; Gao, H.; Chen, Y.Y.; Tang, Y.P. Therapeutic Potential of Hydroxysafflor Yellow A on Cardio-Cerebrovascular Diseases, Front Pharmacol 2020, 11, 01265.
- Yu, L.; Jin, Z.; Li, M.; Liu, H.; Tao, J.; Xu, C.; Wang, L.; Zhang, Q. Protective potential of hydroxysafflor yellow A in cerebral ischemia and reperfusion injury: An overview of evidence from experimental studies, Front Pharmacol 2022, 13, 1063035.
- Xue, X.; Deng, Y.; Wang, J.; Zhou, M.; Liao, L.; Wang, C.; Peng, C.; Li, Y. ; Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis, Phytomedicine 2021, 91, 153694.
- Zhang, X.; Shen, D.; Feng, Y.; Li, Y.; Liao, H.; Actions, P.; Mechanisms, M.; Progressions, P. and Clinical Applications of Hydroxysafflor Yellow A in Antidiabetic Research, J Immunol Res 2021, 2021, 4560012. 2021.
- Asgarpanah, J.; Kazemivash, N. ; Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L, Chin J Integr Med 2013, 19, 153-159. 19.
- Yu, G.; Luo, Z.; Zhou, Y.; Zhang, L.; Wu, Y.; Ding, L.; Shi, Y. Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach, Biomed Pharmacother 2019, 117, 109094.
- Liang, Y.; Wang, L. Carthamus tinctorius L.: A natural neuroprotective source for anti-Alzheimer's disease drugs, J Ethnopharmacol 2022, 298, 115656.
- Ao, H.; Feng, W.; Peng, C. Hydroxysafflor Yellow A: A Promising Therapeutic Agent for a Broad Spectrum of Diseases, Evid Based Complement Alternat Med 2018, 2018, 8259280.
- Ge, C.; Meng, D.; Peng, Y.; Huang, P.; Wang, N.; Zhou, X.; Chang, D. The activation of the HIF-1alpha-VEGFA-Notch1 signaling pathway by Hydroxysafflor yellow A promotes angiogenesis and reduces myocardial ischemia-reperfusion injury, Int Immunopharmacol 2024, 142, 113097.
- Ruan, J.; Wang, L.; Wang, N.; Huang, P.; Chang, D.; Zhou, X.; Seto, S.; Li, D.; Hou, J. Hydroxysafflor Yellow A promotes angiogenesis of brain microvascular endothelial cells from ischemia/reperfusion injury via glycolysis pathway in vitro, J Stroke Cerebrovasc Dis 2025, 34, 108107.
- Tan, Y.Q.; Chen, H.W.; Li, J.; Wu, Q.J.; Efficacy; Constituents, C. and Pharmacological Actions of Radix Paeoniae Rubra and Radix Paeoniae Alba, Front Pharmacol 2020, 11, 1054.
- Xin, Q.; Yuan, R.; Shi, W.; Zhu, Z.; Wang, Y.; Cong, W. A review for the anti-inflammatory effects of paeoniflorin in inflammatory disorders, Life Sci 2019, 237, 116925.
- Zhou, Y.X.; Gong, X.H.; Zhang, H.; Peng, C. A review on the pharmacokinetics of paeoniflorin and its anti-inflammatory and immunomodulatory effects, Biomed Pharmacother 2020, 130, 110505.
- Lu, Y.; Yin, L.; Yang, W.; Wu, Z.; Niu, J. Antioxidant effects of Paeoniflorin and relevant molecular mechanisms as related to a variety of diseases: A review, Biomed Pharmacother 2024, 176, 116772.
- Ma, X.; Zhang, W.; Jiang, Y.; Wen, J.; Wei, S.; Zhao, Y. ; Paeoniflorin, a Natural Product With Multiple Targets in Liver Diseases-A Mini Review, Front Pharmacol 2020, 11, 531.
- Zhang, X.E.; Pang, Y.B.; Bo, Q.; Hu, S.Y.; Xiang, J.Y.; Yang, Z.R.; Zhang, X.M.; Chen, A.J.; Zeng, J.H.; Ma, X.; Guo, J. Protective effect of paeoniflorin in diabetic nephropathy: A preclinical systematic review revealing the mechanism of action, PLoS One 2023, 18, e0282275.
- Yang, J.; Ren, Y.; Lou, Z.G.; Wan, X.; Weng, G.B.; Cen, D. Paeoniflorin inhibits the growth of bladder carcinoma via deactivation of STAT3, Acta Pharm 2018, 68, 211-222.
- Vellasamy, S.; Murugan, D.; Abas, R.; Alias, A.; Seng, W.Y.; Woon, C.K. Biological Activities of Paeonol in Cardiovascular Diseases: A Review, Molecules 2021, 26, 4976.
- Yu, W.; Ilyas, I.; Aktar, N.; Xu, S. A review on therapeutical potential of paeonol in atherosclerosis, Front Pharmacol 2022, 13, 950337.
- Yang, C.; Cheng, J.; Zhu, Q.; Pan, Q.; Ji, K.; Li, J. Review of the Protective Mechanism of Paeonol on Cardiovascular Disease, Drug Des Devel Ther 2023, 17, 2193-2208.
- Chen, Z.; Zhang, C.; Gao, F.; Fu, Q.; Fu, C.; He, Y.; Zhang, J. A systematic review on the rhizome of Ligusticum chuanxiong Hort. (Chuanxiong), Food Chem Toxicol 2018, 119, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, F.; Tang, Z.; Ren, H.; Wang, Q.; Shen, N.; Lin, W.; Xiao, Y.; Yuan, M.; Chen, H.; Bu, T.; Li, Q.; Huang, L. Ligusticum chuanxiong Hort as a medicinal and edible plant foods: Antioxidant, anti-aging and neuroprotective properties in Caenorhabditis elegans, Front Pharmacol 2022, 13, 1049890.
- Hu, Y.; Wang, A.; Chen, J.; Chen, H. Ligustrazine: A Review of Its Role and Mechanism in the Treatment of Obstetrical and Gynecological Diseases, Clin Exp ObsGynecol 2023, 50, 164.
- Huang, S.; Chen, J.; Liu, X.; Xing, C.; Zhao, L.; Chan, K.; Lu, G. Evaluation of the Pharmaceutical Activities of Chuanxiong, a Key Medicinal Material in Traditional Chinese Medicine, Pharmaceuticals (Basel) 2024, 17.
- Yuan, X.; Han, B.; Feng, Z.M.; Jiang, J.S.; Yang, Y.N.; Zhang, P.C. Chemical constituents of Ligusticum chuanxiong and their anti-inflammation and hepatoprotective activities, Bioorg Chem 2020, 101, 104016.
- Li, J.-S.; Wang, H.-F.; Bai, Y.-P.; Li, S.-Y.; Yu, X.-Q.; Li, Y. Ligustrazine injection for chronic pulmonary heart disease: a systematic review and meta-analysis, Evid Based Complement Alternat Med 2012, 2012, 792726.
- Shao, H.; Zhao, L.; Chen, F.; Zeng, S.; Liu, S.; Li, J. Efficacy of Ligustrazine Injection as Adjunctive Therapy for Angina Pectoris: A Systematic Review and Meta-Analysis, Med Sci Monit 2015, 21, 3704-3715.
- Zhuang, Z.; Wang, Z.H.; Huang, Y.Y.; Zheng, Q.; Pan, X.D. Protective effect and possible mechanisms of ligustrazine isolated from Ligusticum wallichii on nephropathy in rats with diabetes: A preclinical systematic review and meta-analysis, J Ethnopharmacol 2020, 252, 112568.
- Xie, Q.; Zhang, L.; Xie, L.; Zheng, Y.; Liu, K.; Tang, H.; Liao, Y.; Li, X. Z-ligustilide: A review of its pharmacokinetics and pharmacology, Phytother Res 2020, 34, 1966-1991.
- Shen, L.; Tian, Q.; Ran, Q.; Gan, Q.; Hu, Y.; Du, D.; Qin, Z.; Duan, X.; Zhu, X.; Huang, W. Z-Ligustilide: A Potential Therapeutic Agent for Atherosclerosis Complicating Cerebrovascular Disease, Biomolecules 2024, 14, 1624.
- Qi, H.; Siu, S.O.; Chen, Y.; Han, Y.; Chu, I.K.; Tong, Y.; Lau, A.S.; Rong, J. Senkyunolides reduce hydrogen peroxide-induced oxidative damage in human liver HepG2 cells via induction of heme oxygenase-1, Chem Biol Interact 2010, 183, 380-389.
- Wang, M.; Hayashi, H.; Horinokita, I.; Asada, M.; Iwatani, Y.; Liu, J.X.; Takagi, N. Neuroprotective effects of Senkyunolide I against glutamate-induced cells death by attenuating JNK/caspase-3 activation and apoptosis, Biomed Pharmacother 2021, 140, 111696.
- Huang, Y.; Wu, Y.; Yin, H.; Du, L.; Chen, C. Senkyunolide I: A Review of Its Phytochemistry, Pharmacology, Pharmacokinetics, and Drug-Likeness, Molecules 2023, 28.
- Li, P.; Tang, W.; Wen, H.; Zhou, S.; Cao, H. Senkyunolide I prevent chondrocytes from oxidative stress through Nrf2/HO-1 signaling pathway, Naunyn Schmiedebergs Arch Pharmacol, 2025.
- Li, Q.; Sheng, J.; Baruscotti, M.; Liu, Z.; Wang, Y.; Zhao, L. Identification of Senkyunolide I as a novel modulator of hepatic steatosis and PPARalpha signaling in zebrafish and hamster models, J Ethnopharmacol 2025, 336, 118743.
- Wei, Q.; He, F.; Rao, J.; Xiang, X.; Li, L.; Qi, H. Targeting non-classical autophagy-dependent ferroptosis and the subsequent HMGB1/TfR1 feedback loop accounts for alleviating solar dermatitis by senkyunolide I, Free Radic Biol Med 2024, 223, 263-280.
- Zha, Y.F.; Xie, J.; Ding, P.; Zhu, C.L.; Li, P.; Zhao, Z.Z.; Li, Y.H.; Wang, J.F. Senkyunolide I protect against lung injury via inhibiting formation of neutrophil extracellular trap in a murine model of cecal ligation and puncture, Int Immunopharmacol 2021, 99, 107922.
- Hu, Y.; Duan, M.; Liang, S.; Wang, Y.; Feng, Y. Senkyunolide I protects rat brain against focal cerebral ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3, Brain Res 2015, 1605, 39-48.
- Hu, Y.Y.; Wang, Y.; Liang, S.; Yu, X.L.; Zhang, L.; Feng, L.Y.; Feng, Y. Senkyunolide I attenuates oxygen-glucose deprivation/reoxygenation-induced inflammation in microglial cells, Brain Res 2016, 1649, 123-131.
- Zhu, Y.L.; Huang, J.; Chen, X.Y.; Xie, J.; Yang, Q.; Wang, J.F.; Deng, X.M. Senkyunolide I alleviates renal Ischemia-Reperfusion injury by inhibiting oxidative stress, endoplasmic reticulum stress and apoptosis, Int Immunopharmacol 2022, 102, 108393.
- Yang, Q.; Zhao, Z.Z.; Xie, J.; Wang, Y.P.; Yang, K.; Guo, Y.; Wang, J.F.; Deng, X.M. Senkyunolide I attenuates hepatic ischemia/reperfusion injury in mice via anti-oxidative, anti-inflammatory and anti-apoptotic pathways, Int Immunopharmacol 2021, 97, 107717.
- Yi, T.; Fang, J.Y.; Zhu, L.; Tang, Y.N.; Ji, H.; Zhang, Y.Z.; Yu, J.C.; Zhang, X.J.; Yu, Z.L.; Zhao, Z.Z.; Chen, H.B. The variation in the major constituents of the dried rhizome of Ligusticum chuanxiong (Chuanxiong) after herbal processing, Chin Med 2016, 11, 26.
- Li, J.Q.; Wang, J.F.; Li, J.; Zhang, S.H.; He, D.; Tong, R.S.; She, S.Y. A validated LC-MS/MS method for the determination of senkyunolide I in dog plasma and its application to a pharmacokinetic and bioavailability studies, Biomed Chromatogr 2018, 32, e4182.
- Chen, X.P.; Li, W.; Xiao, X.F.; Zhang, L.L.; Liu, C.X. Phytochemical and pharmacological studies on Radix Angelica sinensis, Chin J Nat Med 2013, 11, 577-587.
- Huang, C.; Zhao, Y.; Huang, S.; Li, L.; Yuan, Z.; Xu, G. Screening of anti-thrombin active components from Ligusticum chuanxiong by affinity-ultrafiltration coupled with HPLC-Q-Orbitrap-MS(n), Phytochem Anal 2023, 34, 443-452.
- Tang, S.Q.; Chen, Y.H.; Chen, X.P.; Zhang, X.D.; Huang, W. In Vivo Effect of Guiding-Herb Radix Platycodonis and Radix Cyathulae on Paeoniflorin Pharmacokinetics of Xuefu Zhuyu Tang in Rats, Afr J Tradit Complement Altern Med 2017, 14, 289-296.
- Huang, Y.; Wang, S.; Liu, L.; Peng, W.; Wang, J.; Song, Y.; Yuan, Q.; Yuan, X.; Wu, C. Review of traditional uses, botany, chemistry, pharmacology, pharmacokinetics, and toxicology of Radix Cyathulae, Chin Med 2019, 14, 17.
- Zhang, X.; Sun, Z.; Sun, W.; Li, Y.; Gao, F.; Teng, F.; Han, Z.; Lu, Y.; Zhang, S.; Li, L. Bioinformatics Analysis and Experimental Findings Reveal the Therapeutic Actions and Targets of Cyathulae Radix Against Type 2 Diabetes Mellitus, J Diabetes Res 2024, 2024, 5521114.
- Kim, S.H.; Yook, T.H.; Kim, J.U.; Radix, R. an Effective Treatment for Patients with Various Inflammatory and Metabolic Diseases: Results from a Review of Korean Publications, J Pharmacopuncture 2017, 20, 81-88.
- Liu, C.; Ma, R.; Wang, L.; Zhu, R.; Liu, H.; Guo, Y.; Zhao, B.; Zhao, S.; Tang, J.; Li, Y.; Niu, J.; Fu, M.; Zhang, D.; Gao, S. Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology, J Ethnopharmacol 2017, 198, 351-362.
- Jia, J.; Chen, J.; Wang, G.; Li, M.; Zheng, Q.; Li, D. Progress of research into the pharmacological effect and clinical application of the traditional Chinese medicine Rehmanniae Radix, Biomed Pharmacother 2023, 168, 115809.
- Matsumoto, Y.; Sekimizu, K. A hyperglycemic silkworm model for evaluating hypoglycemic activity of Rehmanniae Radix, an herbal medicine, Drug Discov Ther 2016, 10, 14-18.
- Li, Y.; Chen, Q.; Sun, H.J.; Zhang, J.H.; Liu, X. The Active Ingredient Catalpol in Rehmannia glutinosa Reduces Blood Glucose in Diabetic Rats via the AMPK Pathway, Diabetes Metab Syndr Obes 2024, 17, 1761-1767.
- Zhang, M.F.; Wang, J.H.; Sun, S.; Xu, Y.T.; Wan, D.; Feng, S.; Tian, Z.; Zhu, H.F. Catalpol attenuates ischemic stroke by promoting neurogenesis and angiogenesis via the SDF-1alpha/CXCR4 pathway, Phytomedicine 2024, 128, 155362.
- Wu, C.; Shi, Z.; Ge, Q.; Xu, H.; Wu, Z.; Tong, P.; Jin, H. Catalpol promotes articular cartilage repair by enhancing the recruitment of endogenous mesenchymal stem cells, J Cell Mol Med 2024, 28, e18242.
- Lang, X.; Xu, L.; Li, L.; Feng, X. The Mechanism of Catalpol to Improve Oxidative Damage of Dermal Fibroblasts Based on Nrf2/HO-1 Signaling Pathway, Drug Des Devel Ther 2024, 18, 2287-2297.
- Liu, Z.H.; Xu, Q.Y.; Wang, Y.; Gao, H.X.; Min, Y.H.; Jiang, X.W.; Yu, W.H. Catalpol from Rehmannia glutinosa Targets Nrf2/NF-kappaB Signaling Pathway to Improve Renal Anemia and Fibrosis, Am J Chin Med 2024, 52, 1451-1485.
- Zhang, Z.; Dai, Y.; Xiao, Y.; Liu, Q. Protective effects of catalpol on cardio-cerebrovascular diseases: A comprehensive review, J Pharm Anal 2023, 13, 1089-1101.
- Fu, Z.; Su, X.; Zhou, Q.; Feng, H.; Ding, R.; Ye, H. Protective effects and possible mechanisms of catalpol against diabetic nephropathy in animal models: a systematic review and meta-analysis, Front Pharmacol 2023, 14, 1192694.
- Bai, Y.; Zhu, R.; Tian, Y.; Li, R.; Chen, B.; Zhang, H.; Xia, B.; Zhao, D.; Mo, F.; Zhang, D.; Gao, S. Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety, Molecules 2019, 24.
- Mei, C.; Wang, X.; Meng, F.; Zhang, X.; Chen, L.; Yan, S.; Xue, J.; Sun, X.; Wang, Y. Aucuboside Inhibits the Generation of Th17 Cells in Mice Colitis, Front Pharmacol 2021, 12, 696599.
- Gao, L.; Wang, D.; Ren, J.; Tan, X.; Chen, J.; Kong, Z.; Nie, Y.; Yan, M. Acteoside ameliorates learning and memory impairment in APP/PS1 transgenic mice by increasing Abeta degradation and inhibiting tau hyperphosphorylation, Phytother Res 2024, 38, 1735-1744.
- Jia, K.; Zhang, Y.; Luo, R.; Liu, R.; Li, Y.; Wu, J.; Xie, K.; Liu, J.; Li, S.; Zhou, F.; Li, X. Acteoside ameliorates hepatic ischemia-reperfusion injury via reversing the senescent fate of liver sinusoidal endothelial cells and restoring compromised sinusoidal networks, Int J Biol Sci 2023, 19, 4967-4988.
- Xiao, Y.; Ren, Q.; Wu, L. The pharmacokinetic property and pharmacological activity of acteoside: A review, Biomed Pharmacother 2022, 153, 113296.
- Khan, R.A.; Hossain, R.; Roy, P.; Jain, D.; Saikat, A.S.M.; Shuvo, A.P.R.; Akram, M.; Elbossaty, W.F.; Khan, I.N.; Painuli, S.; Semwal, P.; Rauf, A.; Islam, M.T.; Khan, H. Anticancer effects of acteoside: Mechanistic insights and therapeutic status, Eur J Pharmacol 2022, 916, 174699.
- Wu, H.; Huang, Q.; Chao, S.; Yu, J.; Xu, S.; Wang, F.; Shang, X.; Zhu, Y. Determination of Ferulic Acid in Angelica sinensis by Temperature-Controlled Hydrophobic Ionic Liquids-Based Ultrasound/Heating-Assisted Extraction Coupled with High Performance Liquid Chromatography, Molecules 2020, 25, 3356.
- Bunel, V.; Antoine, M.H.; Nortier, J.; Duez, P.; Stévigny, C. Nephroprotective effects of ferulic acid, Z-ligustilide and E-ligustilide isolated from Angelica sinensis against cisplatin toxicity in vitro, Toxicol In Vitro 2015, 29, 458-467.
- Lu, J.; Wang, C. Ferulic acid from Angelica sinensis (Oliv.) Diels ameliorates lipid metabolism in alcoholic liver disease via AMPK/ACC and PI3K/AKT pathways, J Ethnopharmacol 2025, 338, 119118.
- Zhang, L.L.; Huang, M.Y.; Yang, Y.; Huang, M.Q.; Shi, J.J.; Zou, L.; Lu, J.J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics, Food Chem 2020, 327, 127029.
- Zhang, L.; Wang, X.; Zhang, J.; Liu, D.; Bai, G.; Ethnopharmacology; phytochemistry, pharmacology and product application of Platycodon grandiflorum: A review, Chin Herb Med 2024, 16, 327-343.
- Khan, M.; Maryam, A.; Zhang, H.; Mehmood, T.; Ma, T. Killing cancer with platycodin D through multiple mechanisms, J Cell Mol Med 2016, 20, 389-402.
- Xie, L.; Zhao, Y.X.; Zheng, Y.; Li, X.F. The pharmacology and mechanisms of platycodin D, an active triterpenoid saponin from Platycodon grandiflorus, Front Pharmacol 2023, 14, 1148853.
- Ji, R.; Wang, S.; Chen, X.; Yang, Z.; Zhang, Z.; Bao, S.; Xiao, Z.; Zhang, Y.; Yin, T.; Yang, J. Platycodin D ameliorates polycystic ovary syndrome-induced ovarian damage by upregulating CD44 to attenuate ferroptosis, Free Radic Biol Med 2024, 224, 707-722.
- Kwon, O.G.; Ku, S.K.; An, H.D.; Lee, Y.J. The Effects of Platycodin D, a Saponin Purified from Platycodi Radix, on Collagen-Induced DBA/1J Mouse Rheumatoid Arthritis, Evid Based Complement Alternat Med 2014, 2014, 954508.
- Kato, H.; Suzuki, S.; Nakao, K.; Lee, E.B.; Takagi, K. Vasodilating effect of crude platycodin in anesthetized dogs, Jpn J Pharmacol 1973, 23, 709-716.
- Kim, T.Y.; Jeon, S.; Jang, Y.; Gotina, L.; Won, J.; Ju, Y.H.; Kim, S.; Jang, M.W.; Won, W.; Park, M.G.; Pae, A.N.; Han, S.; Kim, S.; Lee, C.J. ; Platycodin D, a natural component of Platycodon grandiflorum, prevents both lysosome- and TMPRSS2-driven SARS-CoV-2 infection by hindering membrane fusion, Exp Mol Med 2021, 53, 956-972.
- Wu, J.; Huang, G.; Li, Y.; Li, X. Flavonoids from Aurantii Fructus Immaturus and Aurantii Fructus: promising phytomedicines for the treatment of liver diseases, Chin Med 2020, 15, 89.
- Zhu, J.; Luo, Y.; Tong, H.; Zhong, L.; Gong, Q.; Wang, Y.; Yang, M.; Song, Q. "Drying effect" of fructus aurantii components and the mechanism of action based on network pharmacology and in vitro pharmacodynamic validation, Front Pharmacol 2023, 14, 1114010.
- Suolinna, E.M.; Lang, D.R.; Racker, E. ; Quercetin, an artificial regulator of the high aerobic glycolysis of tumor cells, J Natl Cancer Inst 1974, 53, 1515-1519.
- Racker, E. Effect of quercetin on ATP-driven pumps and glycolysis, Prog Clin Biol Res 1986, 213, 257-271.
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix Bupleuri: A Review of Traditional Uses, Botany, Phytochemistry, Pharmacology, and Toxicology, Biomed Res Int 2017, 2017, 7597596.
- Yuan, B.; Yang, R.; Ma, Y.; Zhou, S.; Zhang, X.; Liu, Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications, Pharm Biol 2017, 55, 620-635.
- Jiang, H.; Yang, L.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Zheng, S.; Yu, H.; Wang, X.; Yang, B.; Wang, Q.; Kuang, H.; Botany; traditional uses; phytochemistry; analytical methods; processing, pharmacology and pharmacokinetics of Bupleuri Radix: A systematic review, Biomed Pharmacother 2020, 131, 110679.
- Zhong, Y.; Li, J.; Zhu, X.; Huang, N.; Liu, R.; Sun, R. A comprehensive review of bupleuri radix and its bioactive components: with a major focus on treating chronic liver diseases, J Ethnopharmacol 2024, 330, 118244.
- Liang, O.H.; Meng, C.E.; Mohamad, C.; Nasir, N.F.M.; Jian, T.X.; You, B.C.; Tarmizi, E.Z.M.; Yee, L.K.; Yeow, Y.K.; Fhan, K.S.; Fey, L.K.; Keong, T.K. M. R. Mohd Roslan and S. A. Baharuddin, Frequency-dependent dielectric spectroscopic analysis on phytochemical and antioxidant activities in Radix Glycyrrhizae extract, Heliyon 2024, 10, e37077.
- Ramalingam, M.; Kim, H.; Lee, Y.; Lee, Y.I. Phytochemical and Pharmacological Role of Liquiritigenin and Isoliquiritigenin From Radix Glycyrrhizae in Human Health and Disease Models, Front Aging Neurosci 2018, 10, 348.
- Zhang, Y.; Lu, J.; Chang, T.; Tang, X.; Wang, Q.; Pan, D.; Wang, J.; Nan, H.; Zhang, W.; Liu, L.; Qi, B. A bibliometric review of Glycyrrhizae Radix et Rhizoma (licorice) research: Insights and future directions, J Ethnopharmacol 2024, 321, 117409.
- Li, F.; Liu, B.; Li, T.; Wu, Q.; Xu, Z.; Gu, Y.; Li, W.; Wang, P.; Ma, T.; Lei, H. Review of Constituents and Biological Activities of Triterpene Saponins from Glycyrrhizae Radix et Rhizoma and Its Solubilization Characteristics, Molecules 2020, 25, 3904.
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds, Phytother Res 2008, 22, 709-724.
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Ther 2020, 214, 107618. [Google Scholar] [CrossRef]
- Chrzanowski, J.; Chrzanowska, A.; Grabon, W. Glycyrrhizin: An old weapon against a novel coronavirus, Phytother Res 2021, 35, 629-636.
- Banerjee, S.; Baidya, S.K.; Adhikari, N.; Ghosh, B.; Jha, T. Glycyrrhizin as a promising kryptonite against SARS-CoV-2: Clinical, experimental, and theoretical evidences, J Mol Struct 2023, 1275, 134642.
- Yi, Y.; Li, W.; Liu, K.; Xue, H.; Yu, R.; Zhang, M.; Bao, Y.O.; Lai, X.; Fan, J.; Huang, Y.; Wang, J.; Shi, X.; Li, J.; Wei, H.; Xiang, K.; Li, L.; Zhang, R.; Zhao, X.; Qiao, X.; Yang, H.; Ye, M. Licorice-saponin A3 is a broad-spectrum inhibitor for COVID-19 by targeting viral spike and anti-inflammation, J Pharm Anal 2023, 14, 115-127.
- Fatima, S.W.; Alam, S.; Khare, S.K. Molecular and structural insights of beta-boswellic acid and glycyrrhizic acid as potent SARS-CoV-2 Envelope protein inhibitors, Phytomed Plus 2022, 2, 100241.
- Zuo, J.; Meng, T.; Wang, Y.; Tang, W. A Review of the Antiviral Activities of Glycyrrhizic Acid, Glycyrrhetinic Acid and Glycyrrhetinic Acid Monoglucuronide, Pharmaceuticals (Basel) 2023, 16, 641.
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza spp. and Its Bioactive Constituents: Update and Review, Phytother Res 2015, 29, 1868-1886.
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives, Med Res Rev 2018, 38, 951-976.
- Huan, C.; Xu, Y.; Zhang, W.; Guo, T.; Pan, H.; Gao, S. Research Progress on the Antiviral Activity of Glycyrrhizin and its Derivatives in Liquorice, Front Pharmacol 2021, 12, 680674.
- Liu, Y.; Yang, L.; Wang, H.; Xiong, Y. Recent Advances in Antiviral Activities of Triterpenoids, Pharmaceuticals (Basel) 2022, 15.
- Kell, D.B. Scientific discovery as a combinatorial optimisation problem: how best to navigate the landscape of possible experiments? Bioessays 2012, 34, 236–244. [Google Scholar] [CrossRef]
- Breinbauer, R.; Vetter, I.R.; Waldmann, H. From protein domains to drug candidates-natural products as guiding principles in the design and synthesis of compound libraries, Angew Chem Int Ed Engl 2002, 41, 2879-2890.
- Young, R.J.; Flitsch, S.L.; Grigalunas, M.; Leeson, P.D.; Quinn, R.J.; Turner, N.J.; Waldmann, H. The Time and Place for Nature in Drug Discovery, JACS Au 2022, 2, 2400-2416.
- Domingo-Fernández, D.; Gadiya, Y.; Preto, A.J.; Krettler, C.A.; Mubeen, S.; Allen, A.; Healey, D.; Colluru, V. Natural Products Have Increased Rates of Clinical Trial Success throughout the Drug Development Process, J Nat Prod 2024, 87, 1844-1851.
- Singh, K.; Gupta, J.K.; Chanchal, D.K.; Shinde, M.G.; Kumar, S.; Jain, D.; Almarhoon, Z.M.; Alshahrani, A.M.; Calina, D.; Sharifi-Rad, J.; Tripathi, A. Natural products as drug leads: exploring their potential in drug discovery and development, Naunyn Schmiedebergs Arch Pharmacol, 2024.
- Wang, Y.; Wang, F.; Liu, W.; Geng, Y.; Shi, Y.; Tian, Y.; Zhang, B.; Luo, Y.; Sun, X. New drug discovery and development from natural products: Advances and strategies, Pharmacol Ther 2024, 264, 108752.
- Goddard, Z.R.; Searcey, M.; Osbourn, A. Advances in triterpene drug discovery, Trends Pharmacol Sci 2024, 45, 964-968.
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications, Curr Med Chem 2013, 20, 908-931.
- Hamid, K.; Alqahtani, A.; Kim, M.S.; Cho, J.L.; Cui, P.H.; Li, C.G.; Groundwater, P.W.; Li, G.Q. Tetracyclic triterpenoids in herbal medicines and their activities in diabetes and its complications, Curr Top Med Chem 2015, 15, 2406-2430.
- Akihisa, T.; Zhang, J.; Tokuda, H. Potentially Chemopreventive Triterpenoids and Other Secondary Metabolites From Plants and Fungi, Stud Nat Prod Chem 2016, 21, 1-50.
- Thimmappa, R.; Geisler, K.; Louveau, T. ; P. O'Maille; Osbourn, A. Triterpene biosynthesis in plants, Annu Rev Plant Biol 2014, 65, 225–257. [Google Scholar]
- Stephenson, M.J.; Field, R.A.; Osbourn, A. The protosteryl and dammarenyl cation dichotomy in polycyclic triterpene biosynthesis revisited: has this 'rule' finally been broken? , Nat Prod Rep 2019, 36, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Gong, L.H.; Zheng, F.Y.; Cheng, K.J.; Chen, Z.S.; Shi, Z. Triterpenoids as reversal agents for anticancer drug resistance treatment, Drug Discov Today 2014, 19, 482-488.
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A Review of Ganoderma Triterpenoids and Their Bioactivities, Biomolecules 2022, 13, 24.
- Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: molecular mechanisms and therapeutic perspectives, Curr Med Chem 2015, 22, 1414-1425.
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; M. M. L; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies, Molecules 2017, 22, 400Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; M. M. L; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies, Molecules 2017, 22, 400.
- Luo, Z.; Zhou, W.; Xie, T.; Xu, W.; Shi, C.; Xiao, Z.; Si, Y.; Ma, Y.; Ren, Q.; Di, L.; Shan, J. The role of botanical triterpenoids and steroids in bile acid metabolism, transport, and signaling: Pharmacological and toxicological implications, Acta Pharm Sin B 2024, 14, 3385-3415.
- Won, T.H.; Arifuzzaman, M.; Parkhurst, C.N.; Miranda, I.C.; Zhang, B.; Hu, E.; Kashyap, S.; Letourneau, J.; Jin, W.B.; Fu, Y.; Guzior, D.V.; Bank, J.R.I.L.C.; Quinn, R.A.; Guo, C.J.; David, L.A.; Artis, D.; Schroeder, F.C. Host metabolism balances microbial regulation of bile acid signalling, Nature 2025, 638, 216-224.
- da Silva, T.C.; Polli, J.E.; Swaan, P.W. The solute carrier family 10 (SLC10): Beyond bile acid transport, Mol Aspects Med 2013, 34, 252-269.
- Chen, F.; Eckman, E.A.; Eckman, C.B. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides, FASEB J 2006, 20, 1269-1271.
- Paris, D.; Ganey, N.J.; Laporte, V.; Patel, N.S.; Beaulieu-Abdelahad, D.; Bachmeier, C.; March, A.; Ait-Ghezala, G.; Mullan, M.J. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease, J Neuroinflammation 2010, 7, 17.
- Patil, S.P.; Maki, S.; Khedkar, S.A.; Rigby, A.C.; Chan, C. Withanolide A and asiatic acid modulate multiple targets associated with amyloid-beta precursor protein processing and amyloid-beta protein clearance, J Nat Prod 2010, 73, 1196-1202.
- Ruszkowski, P.; Bobkiewicz-Kozlowska, T. Natural Triterpenoids and their Derivatives with Pharmacological Activity Against Neurodegenerative Disorders, Mini Rev Org Chem 2014, 11, 307-315.
- Chen, F.; Wang, Y.; Yang, M.; Yin, J.; Meng, Q.; Bu, F.; Sun, D.; Liu, J. Interaction of the ginsenosides with kappa-casein and their effects on amyloid fibril formation by the protein: Multi-spectroscopic approaches, J Photochem Photobiol B 2016, 160, 306-317.
- Fujihara, K.; Koike, S.; Ogasawara, Y.; Takahashi, K.; Koyama, K.; Kinoshita, K. Inhibition of amyloid beta aggregation and protective effect on SH-SY5Y cells by triterpenoid saponins from the cactus Polaskia chichipe, Bioorg Med Chem 2017, 25, 3377-3383.
- Dubey, S.; Kallubai, M.; Subramanyam, R. Improving the inhibition of beta-amyloid aggregation by withanolide and withanoside derivatives, Int J Biol Macromol 2021, 173, 56-65.
- Fujihara, K.; Shimoyama, T.; Kawazu, R.; Sasaki, H.; Koyama, K.; Takahashi, K.; Kinoshita, K. Amyloid beta aggregation inhibitory activity of triterpene saponins from the cactus Stenocereus pruinosus, J Nat Med 2021, 75, 284-298.
- Zheng, T.; Wang, Y.; Zhao, C.; Xu, J.; Huang, X.; Du, W. Triterpenoids impede the fibrillation and cytotoxicity of human islet amyloid polypeptide, Int J Biol Macromol 2022, 199, 189-200.
- Bankar, A.A.; Nagulwar, V.P.; Kotagale, N.R.; Inamdar, N.N. Neuroprotective prospectives of triterpenoids, Expl Neurosci 2024, 3, 231-254.
- Qiu, J.; Li, W.; Feng, S.H.; Wang, M.; He, Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway, Genet Mol Res 2014, 13, 3586-3598.
- Kim, H.J.; Jung, S.W.; Kim, S.Y.; Cho, I.H.; Kim, H.C.; Rhim, H.; Kim, M.; Nah, S.Y. Panax ginseng as an adjuvant treatment for Alzheimer's disease, J Ginseng Res 2018, 42, 401-411.
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer's disease (Review), Mol Med Rep 2019, 19, 2975-2998.
- Wang, L.; Jin, G.; Yu, H.; Li, Q.; Yang, H. Protective effect of Tenuifolin against Alzheimer's disease, Neurosci Lett 2019, 705, 195-201.
- Planchard, M.S.; Samel, M.A.; Kumar, A.; Rangachari, V. The natural product betulinic acid rapidly promotes amyloid-beta fibril formation at the expense of soluble oligomers, ACS Chem Neurosci 2012, 3, 900-908.
- Tyler, S.E.B.; Tyler, L.D.K. Therapeutic roles of plants for 15 hypothesised causal bases of Alzheimer's disease, Nat Prod Bioprospect 2022, 12, 34.
- Lima, E.; Medeiros, J. Terpenes as Potential Anti-Alzheimer’s Disease Agents, Appl Sci 2024, 14, 3898.
- Wei, C.; Fan, J.; Sun, X.; Yao, J.; Guo, Y.; Zhou, B.; Shang, Y. Acetyl-11-keto-beta-boswellic acid ameliorates cognitive deficits and reduces amyloid-beta levels in APPswe/PS1dE9 mice through antioxidant and anti-inflammatory pathways, Free Radic Biol Med 2020, 150, 96-108.
- Bednarikova, Z.; Gancar, M.; Wang, R.; Zheng, L.; Tang, Y.; Luo, Y.; Huang, Y.; Spodniakova, B.; Ma, L.; Gazova, Z. Extracts from Chinese herbs with anti-amyloid and neuroprotective activities, Int J Biol Macromol 2021, 179, 475-484.
- Mustafa, N.H.; Sekar, M.; Fuloria, S.; Begum, M.Y.; Gan, S.H.; Rani, N.; Ravi, S.; Chidambaram, K.; Subramaniyan, V.; Sathasivam, K.V.; Jeyabalan, S.; Uthirapathy, S.; Ponnusankar, S.; Lum, P.T.; Bhalla, V.; Fuloria, N.K. ; Chemistry, Biosynthesis and Pharmacology of Sarsasapogenin: A Potential Natural Steroid Molecule for New Drug Design, Development and Therapy, Molecules 2022, 27, 2032.
- Du, Y.; Fu, M.; Wang, Y.T.; Dong, Z. Neuroprotective Effects of Ginsenoside Rf on Amyloid-beta-Induced Neurotoxicity in vitro and in vivo, J Alzheimers Dis 2018, 64, 309-322.
- Wang, N.; Wang, E.; Wang, R.; Muhammad, F.; Li, T.; Yue, J.; Zhou, Y.; Zhi, D.; Li, H. Ursolic acid ameliorates amyloid beta-induced pathological symptoms in Caenorhabditis elegans by activating the proteasome, Neurotoxicology 2022, 88, 231-240.
- Iwasa, K.; Yagishita, S.; Yagishita-Kyo, N.; Yamagishi, A.; Yamamoto, S.; Yamashina, K.; Haruta, C.; Asai, M.; Maruyama, K.; Shimizu, K.; Yoshikawa, K. Long term administration of loquat leaves and their major component, ursolic acid, attenuated endogenous amyloid-beta burden and memory impairment, Sci Rep 2023, 13, 16770.
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid beta-based therapy for Alzheimer's disease: challenges, successes and future, Signal Transduct Target Ther 2023, 8, 248.
- Zhao, J.; Yang, J.; Ding, L.; Wang, F.; Lin, L. A Review of the Pathogenesis and Chinese Medicine Intervention of Alzheimer's Disease, J Integr Neurosci 2022, 22, 2.
- Meeran, M.F.N.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K.; Properties, P.; Mechanisms, M. and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise, Front Pharmacol 2018, 9, 892.
- Mook-Jung, I.; Shin, J.E.; Yun, S.H.; Huh, K.; Koh, J.Y.; Park, H.K.; Jew, S.S.; Jung, M.W. Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity, J Neurosci Res 1999, 58, 417-425.
- Jew, S.S.; Yoo, C.H.; Lim, D.Y.; Kim, H.; Mook-Jung, I.; Jung, M.W.; Choi, H.; Jung, Y.H.; Kim, H.; Park, H.G. Structure-activity relationship study of asiatic acid derivatives against beta amyloid (A beta)-induced neurotoxicity, Bioorg Med Chem Lett 2000, 10, 119-121.
- Hossain, S.; Hashimoto, M.; Katakura, M. A. Al Mamun and O. Shido, Medicinal value of asiaticoside for Alzheimer's disease as assessed using single-molecule-detection fluorescence correlation spectroscopy, laser-scanning microscopy, transmission electron microscopy, and in silico docking, BMC Complement Altern Med 2015, 15, 118.
- Chen, C.L.; Tsai, W.H.; Chen, C.J.; Pan, T.M. Centella asiatica extract protects against amyloid beta(1-40)-induced neurotoxicity in neuronal cells by activating the antioxidative defence system, J Tradit Complement Med 2016, 6, 362-369.
- Gray, N.E.; Zweig, J.A.; Caruso, M.; Zhu, J.Y.; Wright, K.M.; Quinn, J.F.; Soumyanath, A. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in beta-amyloid overexpressing mice, Mol Cell Neurosci 2018, 93, 1-9.
- Gray, N.E.; Magana, A.A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica - Phytochemistry and mechanisms of neuroprotection and cognitive enhancement, Phytochem Rev 2018, 17, 161-194.
- Wang, M.; Chen, M.; Ding, Y.; Zhu, Z.; Zhang, Y.; Wei, P.; Wang, J.; Qiao, Y.; Li, L.; Li, Y.; Wen, A. Pretreatment with beta-Boswellic Acid Improves Blood Stasis Induced Endothelial Dysfunction: Role of eNOS Activation, Sci Rep 2015, 5, 15357.
- Huang, L.; Xu, R.; Huang, X.; Wang, Y.; Wang, J.; Liu, Y.; Liu, Z. Traditional Chinese medicine injection for promoting blood circulation and removing blood stasis in treating angina pectoris of coronary heart disease: A protocol for systematic review and network meta-analysis, Medicine (Baltimore) 2021, 100, e25608.
- Chen, J.; Cao, D.; Jiang, S.; Liu, X.; Pan, W.; Cui, H.; Yang, W.; Liu, Z.; Jin, J.; Zhao, Z. Triterpenoid saponins from Ilex pubescens promote blood circulation in blood stasis syndrome by regulating sphingolipid metabolism and the PI3K/AKT/eNOS signaling pathway, Phytomedicine 2022, 104, 154242.
- Wei, B.; Sun, C.; Wan, H.; Shou, Q.; Han, B.; Sheng, M.; Li, L.; Kai, G. Bioactive components and molecular mechanisms of Salvia miltiorrhiza Bunge in promoting blood circulation to remove blood stasis, J Ethnopharmacol 2023, 317, 116697.
- Oku, H.; Iwaoka, E.; Shinga, M.; Yamamoto, E.; Iinuma, M.; Ishigur, K. Effect of the Dried Flowers of Campsis grandiflora on Stagnant Blood Syndrome, Nat Prod Commun 2019, 14, 9.
- Dobson, P.D.; Patel, Y.; Kell, D.B. "Metabolite-likeness" as a criterion in the design and selection of pharmaceutical drug libraries, Drug Disc Today 2009, 14, 31-40.
- S. O'Hagan; Kell, D.B. Analysing and navigating natural products space for generating small, diverse, but representative chemical libraries, Biotechnol J 2018, 13, 1700503.
- S. O'Hagan; Kell, D.B. Generation of a small library of natural products designed to cover chemical space inexpensively, Pharm Front 2019, 1, e190005.
- S. O'Hagan; Kell, D.B. Structural similarities between some common fluorophores used in biology, marketed drugs, endogenous metabolites, and natural products, Marine Drugs 2020, 18, 582.
- Capecchi, A.; Reymond, J.L. Classifying natural products from plants, fungi or bacteria using the COCONUT database and machine learning, J Cheminform 2021, 13, 82.
- Ertl, P.; Schuffenhauer, A. Cheminformatics analysis of natural products: lessons from nature inspiring the design of new drugs, Prog Drug Res 2008, 66, 217, 219-235.
- Medina-Franco, J.L.; Saldívar-González, F.I. Cheminformatics to Characterize Pharmacologically Active Natural Products, Biomolecules 2020, 10, 1566.
- Prieto-Martínez, F.D.; Norinder, U.; Medina-Franco, J.L. Cheminformatics Explorations of Natural Products, Prog Chem Org Nat Prod 2019, 110, 1-35.
- Sorokina, M.; Steinbeck, C. Review on natural products databases: where to find data in 2020, J Cheminform 2020, 12, 20.
- Wetzel, S.; Schuffenhauer, A.; Roggo, S.; Ertl, P.; Waldmann, H. Cheminformatic analysis of natural products and their chemical space, Chimia 2007, 61, 355-360.
- He, X.; Duan, X.; Liu, J.; Sha, X.; Gong, Y.; Lu, W.; Li, Z.; Chen, X.; Li, Y.; Shen, Z. The antiinflammatory effects of Xuefu Zhuyu decoction on C3H/HeJ mice with alopecia areata, Phytomedicine 2021, 81, 153423.
- Gao, L.; Zhong, L.; Feng, T.; Yue, J.; Lu, Q.; Li, L.; Wu, A.; Lin, G.; He, Q.; Liu, K.; Cao, G.; Meng, Z.; Nie, L.; Zang, H. An AI-driven strategy for active compounds discovery and non-destructive quality control in traditional Chinese medicine: A case of Xuefu Zhuyu Oral Liquid, Talanta 2025, 287, 127627.
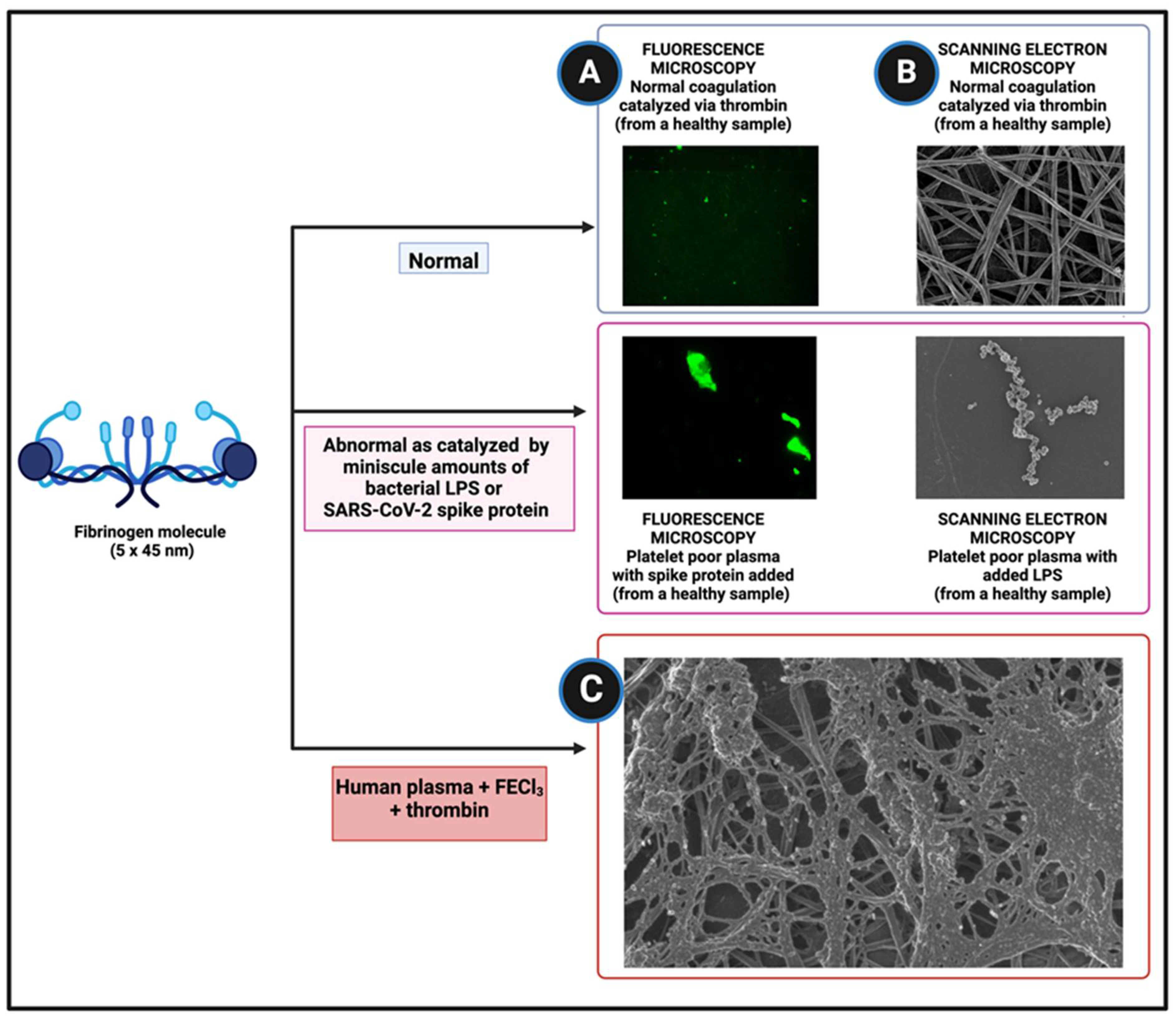
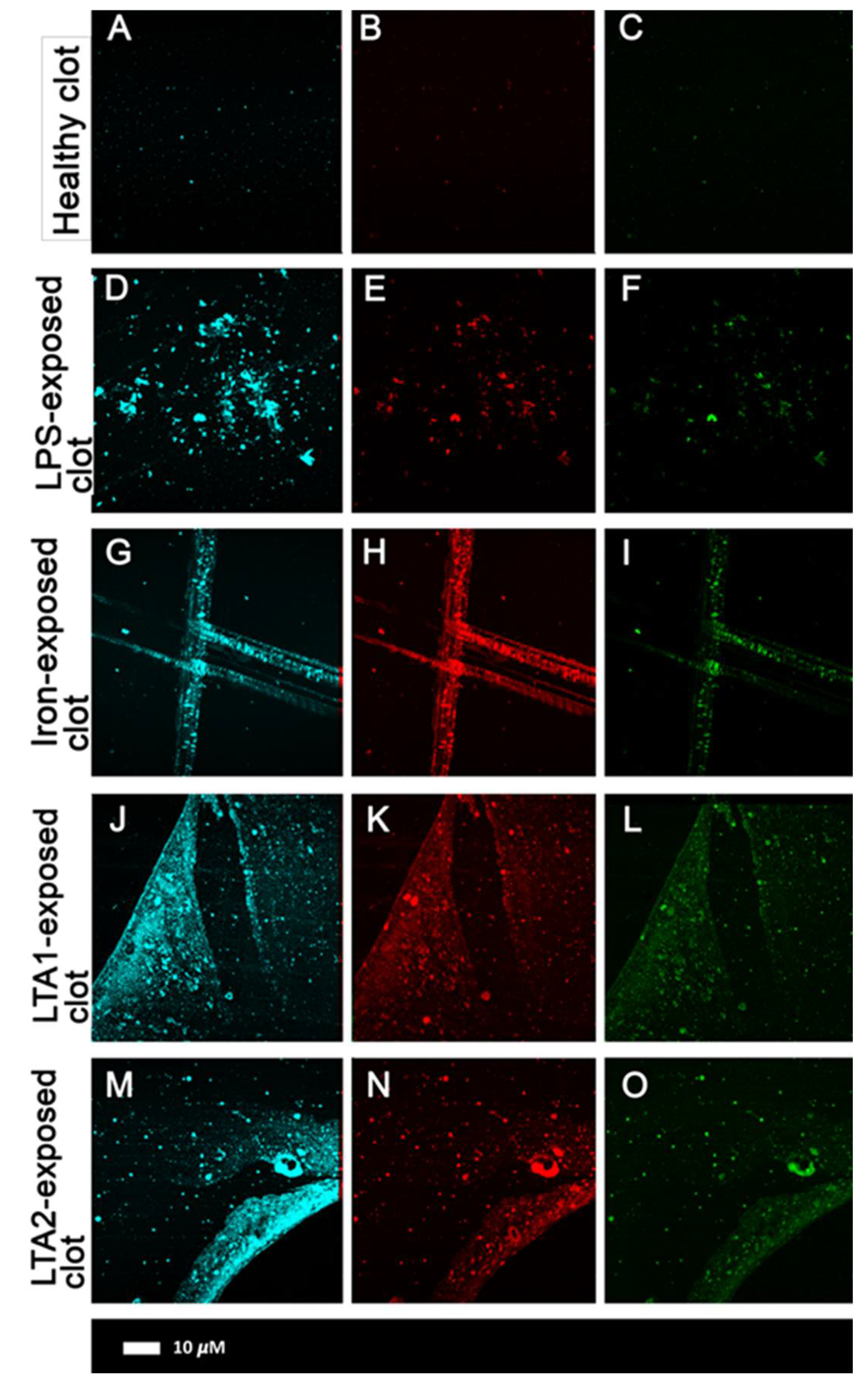
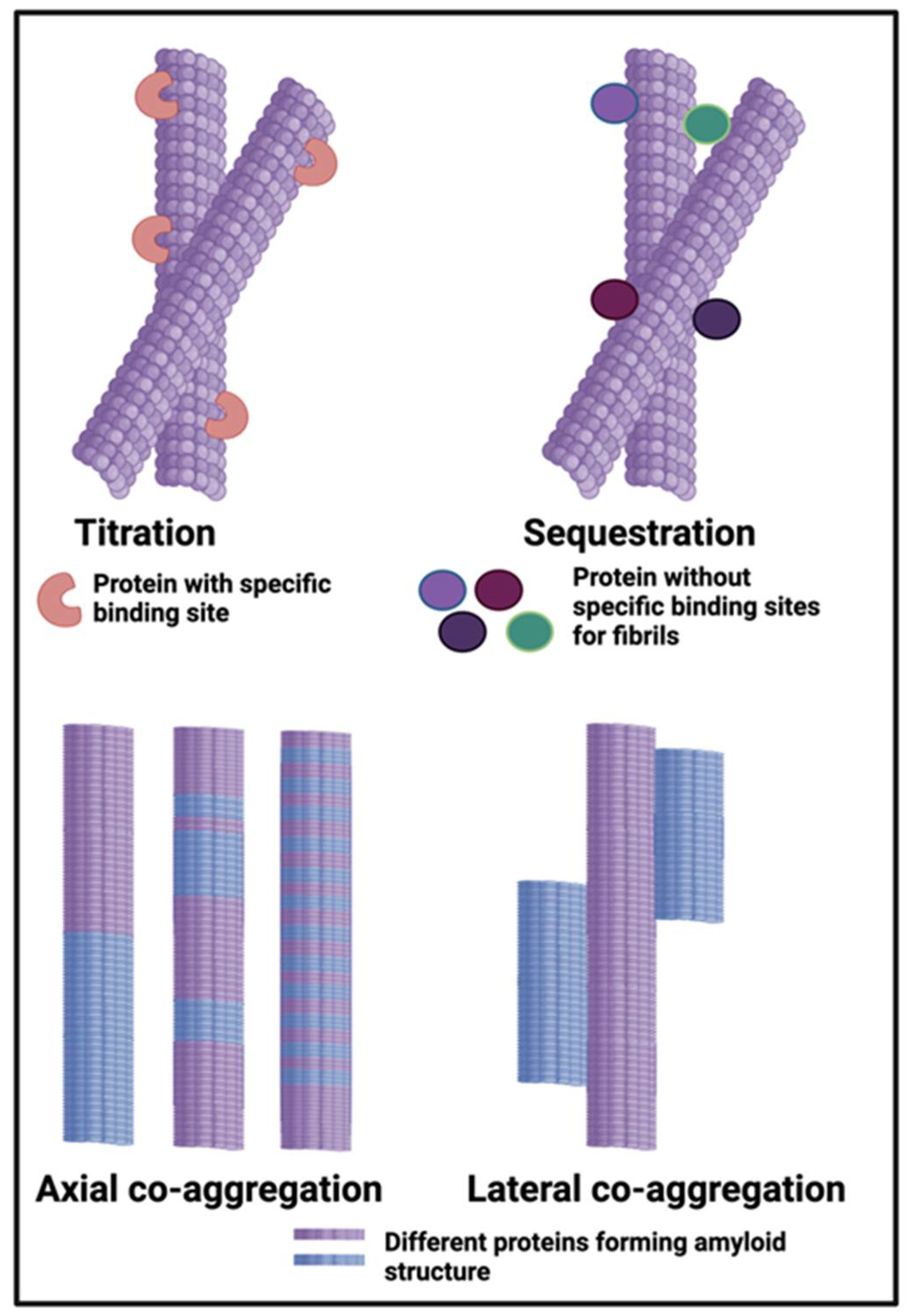
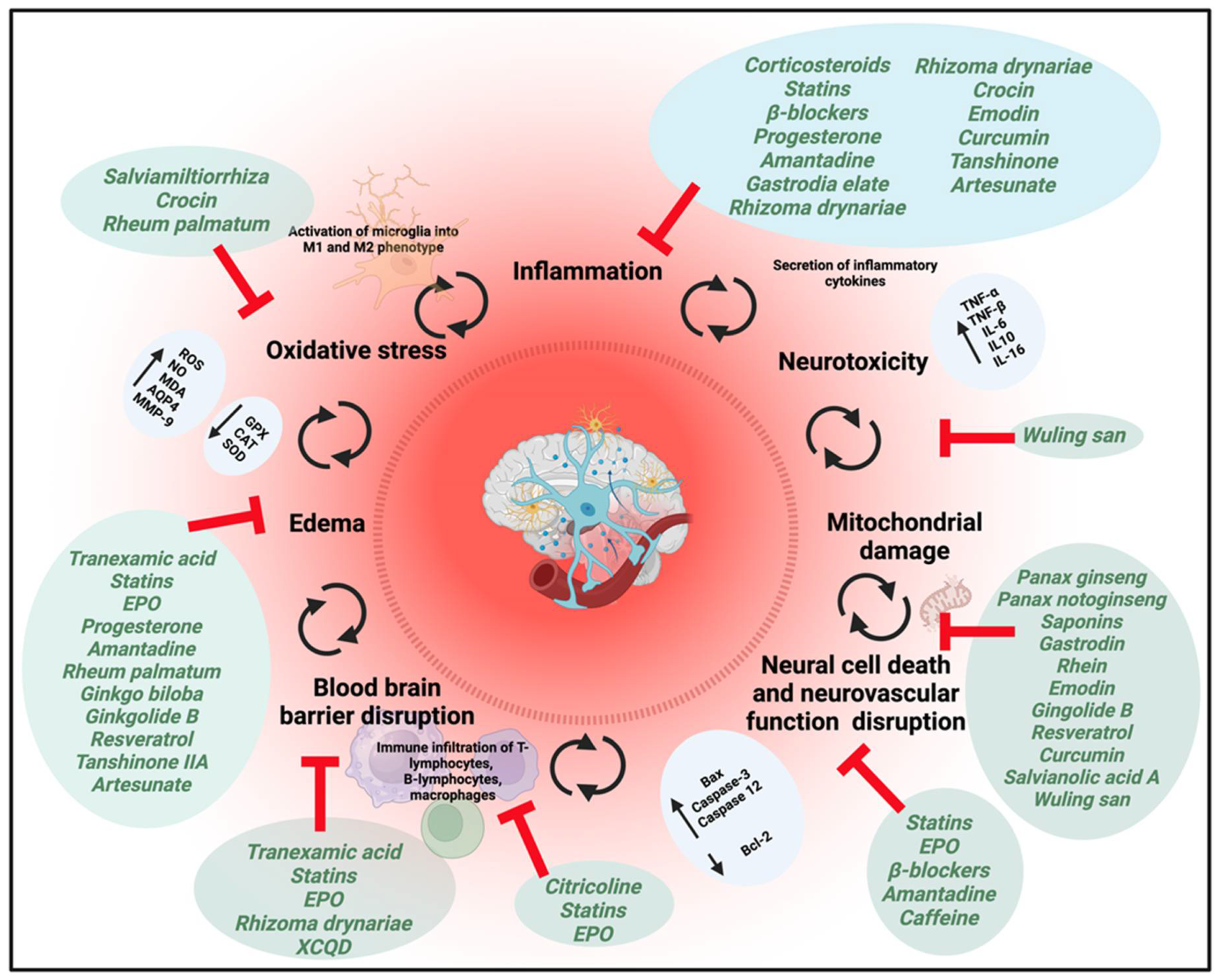
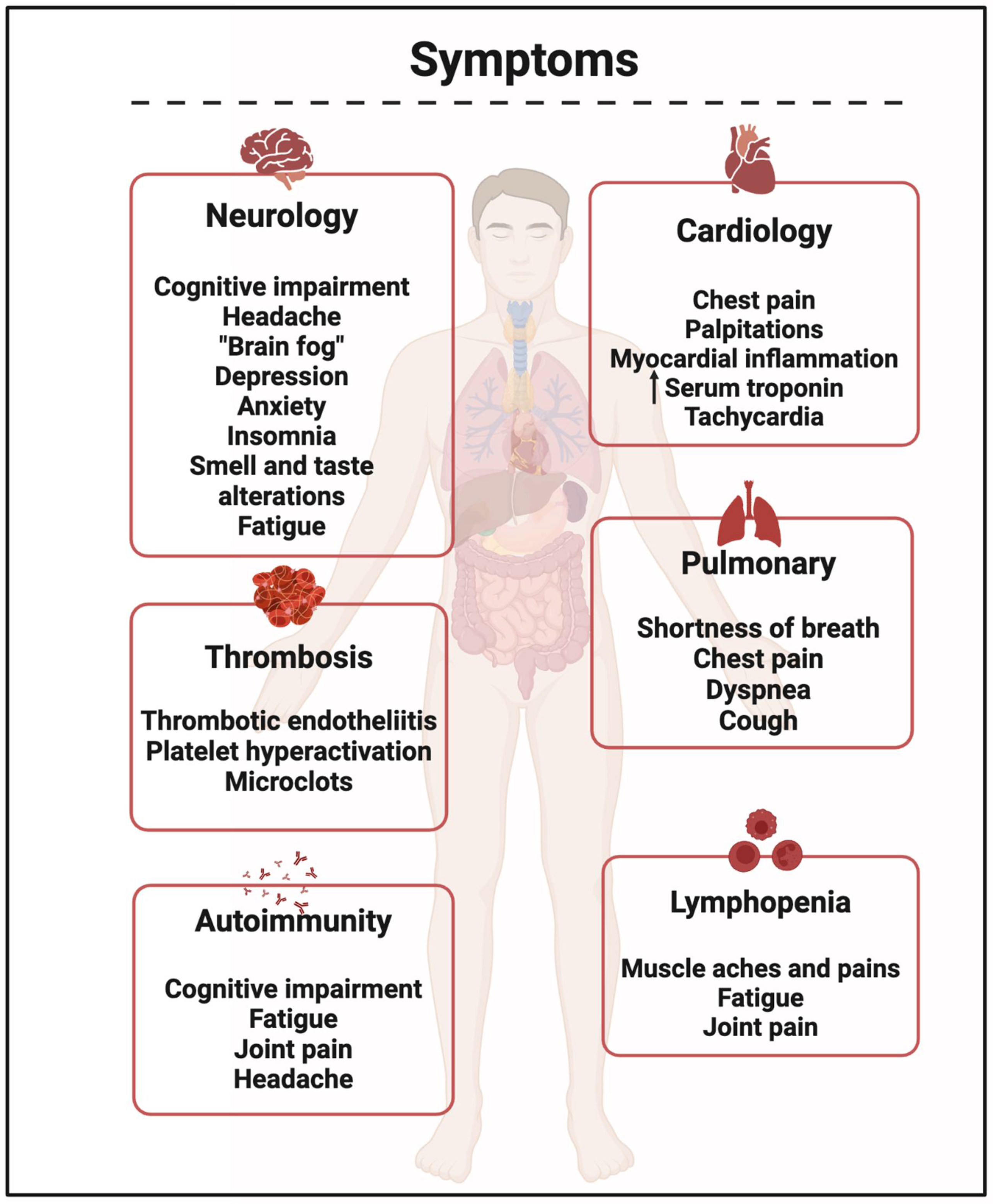
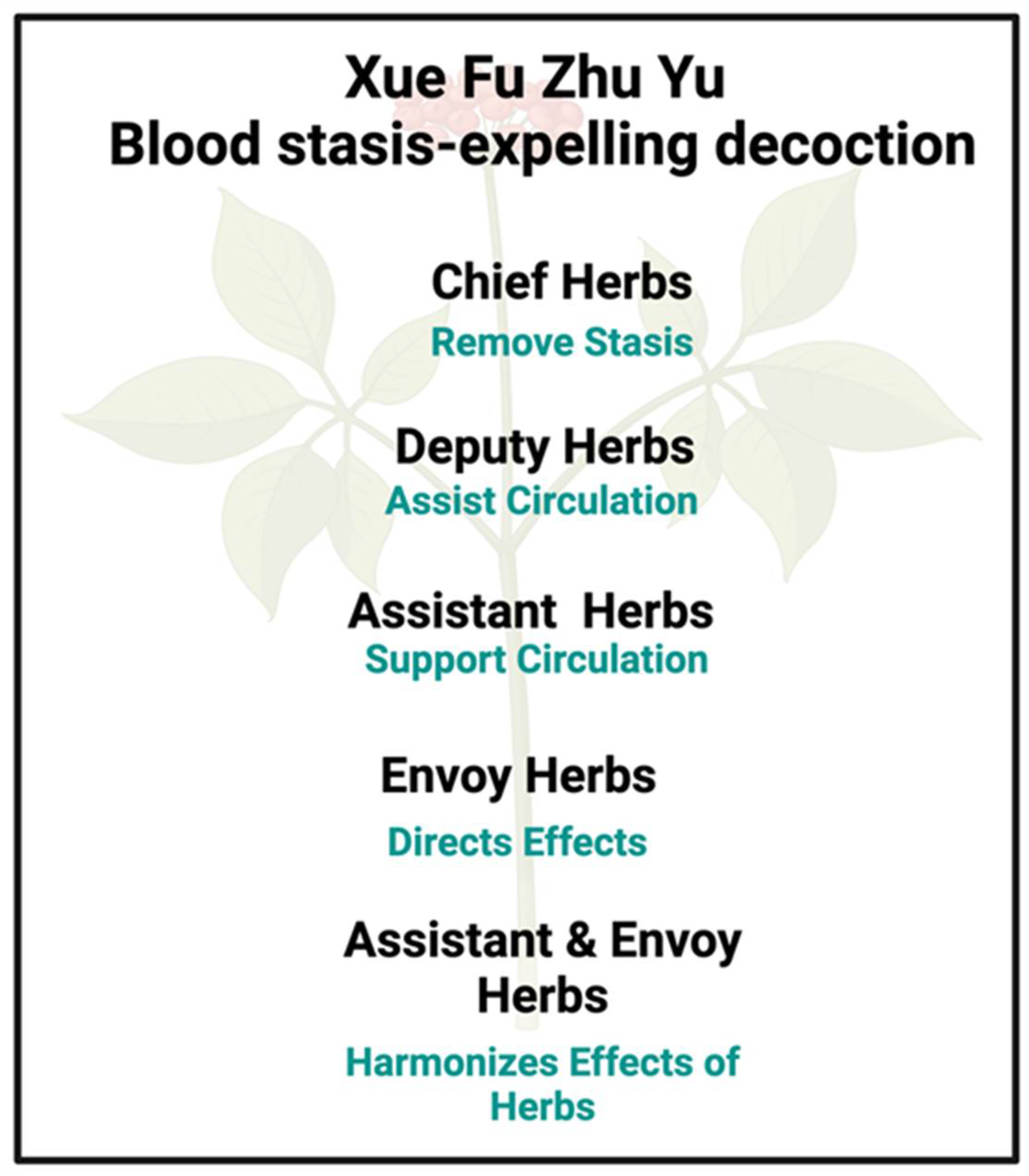
| Disease or Syndrome | Selected References Showing Microclot Formation | Selected References Relating the Disease to Blood Stasis |
|---|---|---|
| Alzheimer’s disease | 140-143 | 71, 144-146 |
| (Acute) COVID-19 infection | 147-151 | 152-159 |
| Diabetes type 2 | 141, 151, 160-162 | 68, 71, 74, 163-166 |
| Long COVID | 117, 121, 167-173 | 155, 174, 175 |
| Migraines | 176 | 71 177 |
| Myalgic Encephalopathy/ Chronic Fatigue Syndrome | 178, 179 | 175, 180-183 (see also 184) |
| Parkinson’s disease | 141, 185-187 | 61, 188-190 |
| Rheumatoid arthritis | 167, 191, 192 | 71, 166, 193, 194 |
| Sepsis | 195 | 196, 197 , 198-216, 217 , 218-222 |
| Septic shock | 195 | 223-235 |
| Stroke | 236 | 75, 79, 237-257 |
| Disease or Syndrome | Selected References Relating the Disease to Blood Stasis | Comments |
|---|---|---|
| Angina pectoris | 71, 261-277 | A very obvious example, as vasodilators are the main treatment. The tightening of the chest and shortness of breath are easily explained by microclots blocking capillaries. |
| Atherosclerosis | 278-283 | Another very obvious example of fibrinaloid microclots resisting fibrinolysis contributing to atherosclerotic plaques (and later to stroke 236). The pairing Danshen-Chuanxiong is often used 284 281. |
| Atrial Fibrillation (AF) | 12 | At one level, atrial blood stasis is seen as synonymous as an effect of AF 285, 286. Evidence that fibrinaloid microclots are more a cause than an effect of AF was summarised in 287 |
| Attention deficit hyperactivity disorder (ADHD) | 288-291 | Plausibly due to decreased blood flow caused by microclots. |
| Chronic kidney disease | 70, 292, 293 | Less likelihood of kidneys excreting microclots if diseased. |
| Chronic obstructive pulmonary disease (COPD) | 294-298 | Strong hint in the term ‘obstructive’ |
| Coronary heart disease | 83, 84, 271, 274, 299-307 308-310 | Xue Fu Zhu Yu (a formula to overcome blood stasis) helps 301, 303, 311-313. |
| Deep vein thrombosis (DVT) | 314-316 | TCM classifies DVT as blood stasis in the category of “pulse closed” and “femoral swelling”. Xue Fu Zhu Yu helps, and there is evidence 259 that the thromboses are likely to be amyloid in nature. |
| Dysmenorrhoea | 317-322 | Note that 17-b-oestradiol was one of the first small molecules discovered to induce anomalous clotting 98 |
| Fibromyalgia syndrome (FMS) | 180, 323 | We consider it likely that FMS (and fibrosis 296) is caused by the deposition of fibrin caused by fibrinaloid microclots 109. There is little actual work on BSS here. |
| Heart failure and Ischaemic heart disease | 324-327 | Various formulas used for this kind of blood stasis |
| Metabolic syndrome (MS) | 71, 80, 328, 329 | MS covers a variety of different syndromes; at this stage we do not seek to deconvolve it. |
| Non-alcoholic fatty liver disease (NAFLD) (since mid-2023 it is called metabolic dysfunction-associated steatotic liver disease (MASLD) 330) | 166, 331, 332 | |
| Postural Orthostatic Tachycardia Syndrome (POTS) | 333 | Capillary blocking by fibrinaloid microclots provides a ready explanation for POTS 334 (see also 335) |
| Pre-eclampsia (PE) | 336-339 | PE has a microbial origin 340, 341 and is significantly prothrombotic 342 |
| Pulmonary embolism | 91, 343 | |
| Sub-arachnoid haemorrhage | 344, 345 | The only predictor of a later stroke 346 was ESR, a measure of blood stasis |
| Thrombotic diseases generally | 347-349 | High likelihood of the clots involved being amyloid in nature 259 |
| Tinnitus | 74, 350 | A common accompaniment to diseases (such as Long COVID) where microclots are involved and where both can be induced by spike protein (cf. 147 and 351-353). |
| Transient ischaemic attack (TIA) | 248, 354, 355 | TIA is of course a common precursor to ischaemic stroke 356, where amyloid clotting has been demonstrated 236 |
| Traumatic brain injury (TBI) | 357-363 | Blood stasis is seen as a core component of (the sequelae of) TBI, that include coagulopathy 364. Most significantly, Xue Fu Zhu Yu ameliorated neurological deficiencies without impairing blood coagulation in a rat model 361. |
| Traumatic injury generally | 365 65 | |
| Herb | Some Known Bioactive Molecules Therein | Some Known Targets or General Properties |
|---|---|---|
| Tao Ren; Semen persicae; peach kernel (Emperor) | Amygdalin 463, 464 | Follistatin induction 463; ERK1/2 activation 464 |
| Hong Hua; Flos carthami; Carthamus tinctorius L.; safflower (Emperor) | (Hydroxy)safflor yellow A 465-470 | Antithrombotic, angiogenic, anticoagulant, antiplatelet. Reviews: 471-476 |
| Kaempferol, quercetin | Antioxidants/ anti inflammatory 465 | |
| Endothelial cell protection | Enhances HIF1-a 465 | |
| Chi Shao; Radix paeoniae rubra; Paeonia lactiflora Pall; red peony root (Minister) | Oxypaeoniflorin | Anti-thrombin 348 |
| Paeoniflorin 477 | Anti-inflammatory 478, 479 and antioxidant 480; blocks TGF1b signalling, ERK1/2, JNK1/2, NF-kB, etc 477, 481, 482; deactivation of STAT3 483; Akt/Nrf2/GPX4 190 | |
| Paeonol | Anti-inflammatory, antioxidant, protects endothelium 484; endothelium-protecting and antiplatelet 485; antioxidant and anti-inflammatory 486 | |
| Rhizoma chuanxiong (= Ligusticum chuanxiong); Szechaun lovage roots (Minister) | Reviews 487-490 | |
| Ligustrazine | Anti-inflammatory 491 492. Dilates blood vessels, inhibits platelet aggregation and prevents thrombopoiesis 492. Anti-anginal 493. Multiple roles 494. |
|
| Ligustilide (also in Dang Gui and Niu Xi) | Anti-inflammatory and anti-oxidant 495. Improves lipid metabolism, antioxidant and anti-inflammatory, protects vascular endothelium, inhibits vascular endothelial fibrosis 496. Cannabinoid receptor 2 activation |
|
| Sekyunolide I (SEI) | Reviews on antioxidant and anti-inflammatory properties 497-499. Other targets of SEI include Nrf2 500, activity vs NAFLD 501, UVB damage 502, NET formation 503, ischaemia-reperfusion injury 504-507, It occurs in appreciable concentrations in both ChuanXiong 508, 509. and Angelica sinensis (Danggui) 510. | |
| Ferulic acid | Anti-thrombin activity 511 | |
| Radix Achyranthis Bidentata (Niu Xi), also Cyathulae Radix (Minister ; may also be a Courier 512) | Achyranthine, but no real stand-outs | Seemingly not well understood 513, 514 |
| Radix rehmanniae (Di Huang or Sheng Di) (Assistant) | Reviews: 515-517 | Multiple effects, including anti-inflammation, antioxidation, anti-tumor, immunomodulation, cardiovascular and cerebrovascular regulation 517. Hypoglycaemic 518 |
| iridoid glycosides (such as catalpol and aucuboside), | Catalpol blocks AMPK signalling 519 and promotes angiogenesis 520 and cell migration 521. Antioxidant via Nrf2/HO-1 522 and NF-kB 523, Many other references, reviewed in 524-526. Aucuboside is an immunomodulator 527. | |
| phenylpropanoid glycosides (such as acteoside), | Acteoside e.g. stimulates amyloid degradation 528 and ameliorates ischaemia-reperfusion injury 529, and has many other effects 530 including anticancer 531. | |
| Radix Angelicae sinensis (Chinese angelica) (Dang Gui) (Assistant) | ||
| Z-lingustilide (see above) | ||
| Ferulic acid | Antioxidant and anti-inflammatory 532. Nephroprotective 533. Ameliorates lipid metabolism via AMPK/ACC and PI3K/AKT pathways 534. |
|
| Sekyunolide I (see above) | ||
| Radix platycodonis (Balloonflower root) (Jie Geng) (Assistant; may also be a Courier 512) | Platycodins 535 536 (triterpenoid saponins) | Many activities 537, 538 including anti-inflammatory and antioxidant 535, 539, 540, vasodilatory541, antiviral 542, |
| Fructus aurantia (Citrus aurantium L.) Bitter orange (Zhi Qiao) (Assistant) | Flavones and flavonoids, including sinensetin, tangeretin, 5-demethylnobiletin and chrysin. | Antioxidant, anti-inflammatory 543 and other activities via JAK-STAT3n and PI3K-AKT signalling 544 |
| Radix Bupleuri (Chinese Thorawax Root) (Chai Hu) (Assistant) | Quercetin 271 | Antioxidant and other properties (some not at all newly discovered 545, 546) |
| Saikosaponins (triterpenoid saponins) | Many activities including antioxidant and anti-inflammatory 547-550 | |
| Radix glycyrrhizae (Glycyrrhiza uralensis Fisch; Chinese licorice) (Gan Cao) (Courier) | Flavanones Liquiritigenin and Isoliquiritigenin | Anti-oxidant 551, also anti-amyloid 552 and transporter inducers. Reviews 553, 554. |
| Triterpene saponins including glycyrrhizin | Antiviral and other 555-561 562-565 |
| Molecule | Structure |
|---|---|
| Acteoside |  |
| Amygdalin |  |
| Aucuboside | 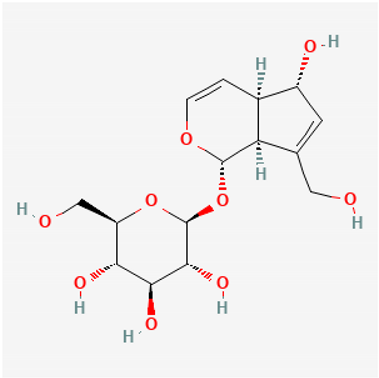 |
| Catalpol | 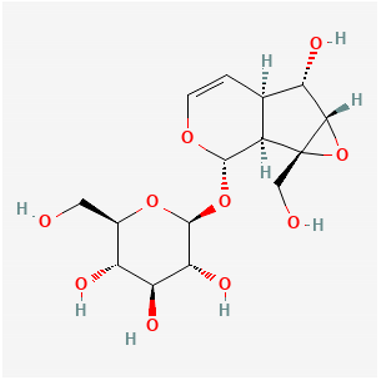 |
| Glycyrrhizin | 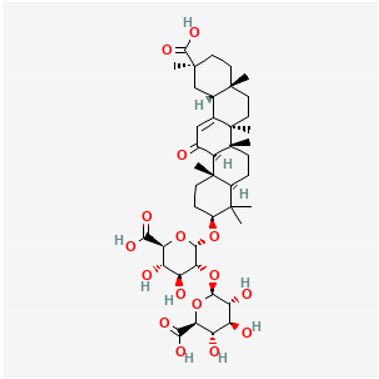 |
| Hydroxysafflor yellow A | 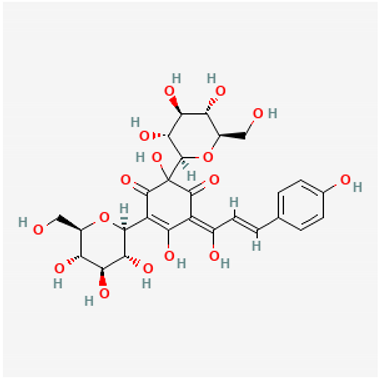 |
| Isoliquiritigenin (2',4,4'-Trihydroxychalcone) |
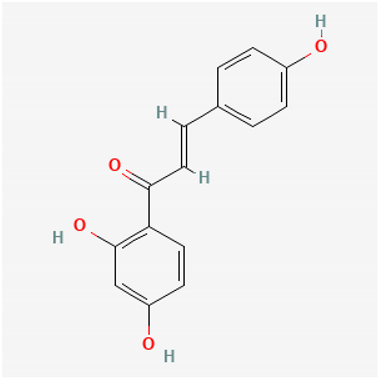 |
| (Z-)Ligustilide |  |
| Ligustrazine (2,3,5,6-tetramethylpyrazine) |  |
| Liquiritigenin 4’, 7- dihydroxyflavanone | 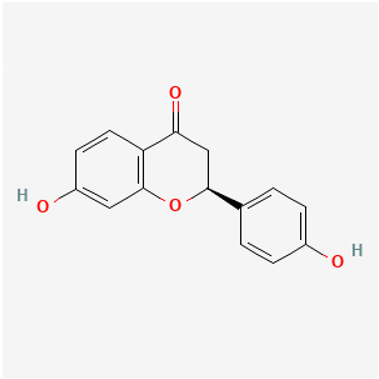 |
| Oxypaeoniflorin | 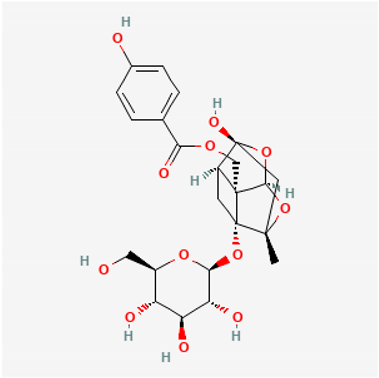 |
| Paeniflorin | 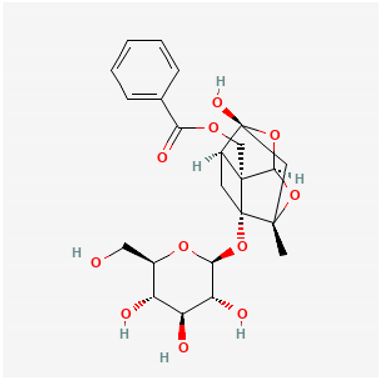 |
| Paeonol | 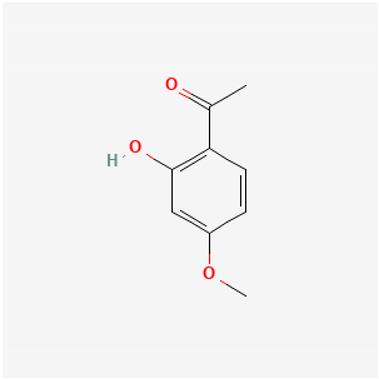 |
| Platycodins: variants of structure at right. One example is below, in which R1 is glucose and R2 is arabinose-rhamnose-xylose-apifuranosyl | 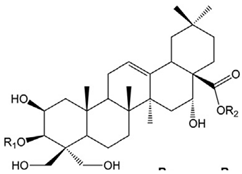 |
| Platycodin D |  |
| Quercetin |  |
| Safflor yellow A |  |
| Saikosaponin nucleus |  |
| Saikosaponin A | As above, R1 = b-OH, R2 = CH2OH |
| Sekyunolide I |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





