1. Introduction
Mental illnesses are among the leading global health challenges [
1,
2], with depressive and anxiety disorders being the most prevalent [
3]. They also represent a significant burden on the population, ranking second after cardiovascular diseases in terms of disability-adjusted life years (DALY) and years lived with disability (YLD) [
4]. Depressive disorders are the most common within this group, and the true prevalence is likely underestimated due to undiagnosed cases. Projections indicate that by 2030, depression will become the leading cause of YLD worldwide [
5]. In severe cases, depression can result in suicide, which is the fourth most common cause of death among individuals aged 15 to 29 years [
6].
Depression is a multifactorial disorder, with genetic, hormonal, and biochemical factors playing a significant role in its development, along with risk factors such as childhood abuse and neglect, the presence of chronic illnesses, and living conditions [
7]. Epidemiological studies indicate that approximately one-third of the global population experiences an anxiety disorder at some point in their lives, with significantly higher prevalence rates reported in North and South America, Western and Central Europe, and Australia compared to other regions [
8]. Anxiety disorders are associated with a significant degree of disability and often occur as comorbidities with other mental illnesses.
The treatment of depressive disorders typically involves pharmacotherapy, psychotherapy, or a combination of both. In cases where these approaches prove ineffective, electroconvulsive therapy (ECT) may be considered as an alternative. Antidepressants, a cornerstone of pharmacological treatment, are classified based on their structure and mechanism of action. These include tricyclic and tetracyclic antidepressants (TCA), monoamine oxidase inhibitors (MAOI), selective serotonin reuptake inhibitors (SSRI), serotonin-norepinephrine reuptake inhibitors (SNRI), norepinephrine reuptake inhibitors (NaRI), norepinephrine-dopamine reuptake inhibitors (NDRI), serotonin antagonist and reuptake inhibitors (SARI), noradrenergic and specific serotonergic receptor antagonists (NASSA), as well as newer antidepressants, including agomelatine and vortioxetine.
SSRIs are considered a first-line pharmacological treatment for depressive disorders. In the Republic of Serbia, commonly prescribed SSRIs include fluoxetine, citalopram, paroxetine, sertraline, and escitalopram. Their therapeutic indications and pharmacokinetic profiles are presented in
Table 1 and
Table 2. The most significant adverse effects of SSRIs include activation syndrome (psychomotor agitation, restlessness, tension and irritability, insomnia, panic attacks) [
9], discontinuation syndrome (paresthesia, vertigo, lethargy, headache, sweating, insomnia and nightmares, nausea, vomiting, diarrhea, and extrapyramidal symptoms) [
10], as well as sexual dysfunction (reduced libido, delayed orgasm or anorgasmia, and delayed ejaculation) [
11,
12]. Additionally, the concurrent use of SSRIs and MAOIs is contraindicated due to the risk of serotonin syndrome, a potentially life-threatening condition characterized by restlessness, myoclonus, hyperreflexia, tremors, hyperthermia, hypertension, seizures, and death [
13].
The aim of this study is to determine the trend of SSRI consumption in Serbia from 2018 to 2022, as well as to compare this trend with SSRI consumption in other European countries.
2. Materials and Methods
For the analysis of SSRI consumption from 2018 to 2022, data on the sale of medicines for human use were obtained from the Medicines and Medical Devices Agency of Serbia (ALIMS). The ATC/DDD methodology, recommended by the World Health Organization (WHO), was applied. According to the Anatomical Therapeutic Chemical (ATC) classification, SSRIs belong to the main anatomical group of drugs affecting the nervous system, specifically within group N06 - psychoanaleptics, and subgroup N06A - antidepressants.
The consumption analysis focuses on the most commonly used oral formulations (tablets and capsules) covering five antidepressants from the SSRI group: fluoxetine, citalopram, paroxetine, sertraline, and escitalopram. The consumption indicator used is the number of defined daily doses per 1,000 inhabitants per day (DDD/TID).
The annual group consumption was calculated using the following formula:
DID= (([DDD]flu+[DDD]cit+[DDD]par)/20 + [DDD]sert/50 + [DDD]escit/10)/(s*b)
DDDescit - number of consumed DDDs of escitalopram
10 - DDD of escitalopram
DDDflu - number of consumed DDDs of fluoxetine
DDDcit - number of consumed DDDs of citalopram
DDDpar - number of consumed DDDs of paroxetine
20 - DDD of fluoxetine, citalopram, and paroxetine
DDDsert - number of consumed DDDs of sertraline
50 - DDD of sertraline
s - total population of the Republic of Serbia in the corresponding year
b - number of days in a year
The total population of the Republic of Serbia in the corresponding year was obtained from the data provided by the Statistical Office of the Republic of Serbia.
The values for drug consumption in European countries were obtained from the respective national registers of Italy (Agenzia Italiana del Farmaco), Iceland (Embætti landlæknis), Spain (Agencia Española de Medicamentos y Productos Sanitarios), Croatia (Agencija za lijekove i medicinske proizvode), Norway (Folkehelseinstituttet), the Netherlands (Zorgintituut Nederland), Estonia (Ravimiamet), Finland (Fimea), Latvia (Zāļu valsts aģentūra), Lithuania (Valstybinė vaistų kontrolės tarnyba prie LR Sveikatos Apsaugos Ministerijos), Slovenia (Nacionalni inštitut za javno zdravje), and Denmark (Sundhedsdatastyrelsen).
Data on GDP per capita were retrieved from the Eurostat database. For data analysis, Microsoft Excel and EZR software were used.
3. Results
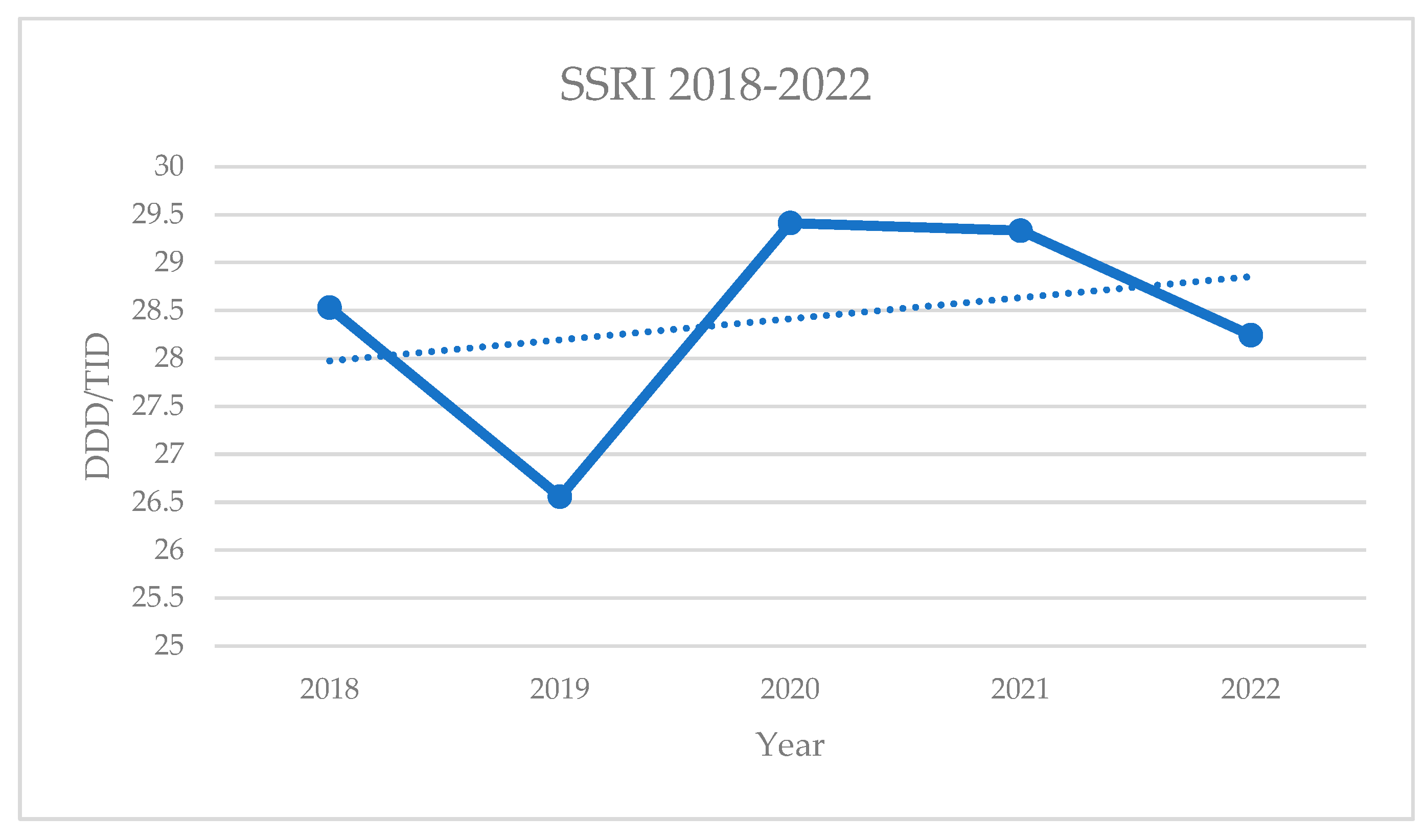
From 2018 to 2021, sertraline was the best-selling SSRI drug in the Republic of Serbia. However, its consumption showed a statistically significant decline (R² = 0.7948, p = 0.042). Over the same period, escitalopram showed a statistically significant increase (p = 0.006), becoming the best-selling SSRI in 2022. Citalopram, the least prescribed drug in this group, also experienced a statistically significant decline (p = 0.007).
The overall consumption of SSRI drugs fluctuated between 2018 and 2022, reaching its highest values in 2020. However, these changes were not statistically significant (p = 0.6223).
Table 4.
Annual group SSRI consumption in the Republic of Serbia.
Table 4.
Annual group SSRI consumption in the Republic of Serbia.
| Year |
DID SSRI |
| 2018 |
28.52887 |
| 2019 |
26.55683 |
| 2020 |
29.41245 |
| 2021 |
29.33288 |
| 2022 |
28.24096 |
Figure 2.
Annual consumption of SSRIs in the Republic of Serbia.
Figure 2.
Annual consumption of SSRIs in the Republic of Serbia.
The obtained SSRI consumption values for the Republic of Serbia were compared to the consumption values in 12 European countries (Italy, Iceland, Spain, Croatia, Norway, the Netherlands, Estonia, Finland, Latvia, Lithuania, Slovenia, and Denmark).
Table 5.
Annual group consumption of SSRIs in the Republic of Serbia and 12 European countries.
Table 5.
Annual group consumption of SSRIs in the Republic of Serbia and 12 European countries.
| Country |
DID 2018 |
DID 2019 |
DID 2020 |
DID 2021 |
DID 2022 |
| Serbia |
28.53 |
26.56 |
29.41 |
29.33 |
28.24 |
| Italy |
29.70 |
29.90 |
30.6 |
31.20 |
31.70 |
| Iceland |
103.3 |
106.3 |
111.7 |
118.9 |
116.3 |
| Spain |
49.09 |
50.27 |
52.11 |
55.07 |
57.71 |
| Croatia |
21.66 |
22.19 |
22.69 |
23.85 |
24.61 |
| Norway |
35.46 |
35.97 |
36.35 |
37.58 |
/ |
| The Netherlands |
42.35 |
44.41 |
46.5 |
48.21 |
49.71 |
| Estonia |
18.91 |
20.58 |
21.72 |
24.06 |
26.26 |
| Finland |
41.39 |
43.40 |
44.58 |
46.45 |
/ |
| Latvia |
10.42 |
11.45 |
13.17 |
14.26 |
15.76 |
| Lithuania |
20.127 |
22.50 |
23.22 |
23.86 |
25.74 |
| Slovenia |
40.30 |
41.00 |
41.30 |
42.90 |
44.20 |
| Denmark |
46.70 |
47.80 |
49.50 |
52.50 |
55.20 |
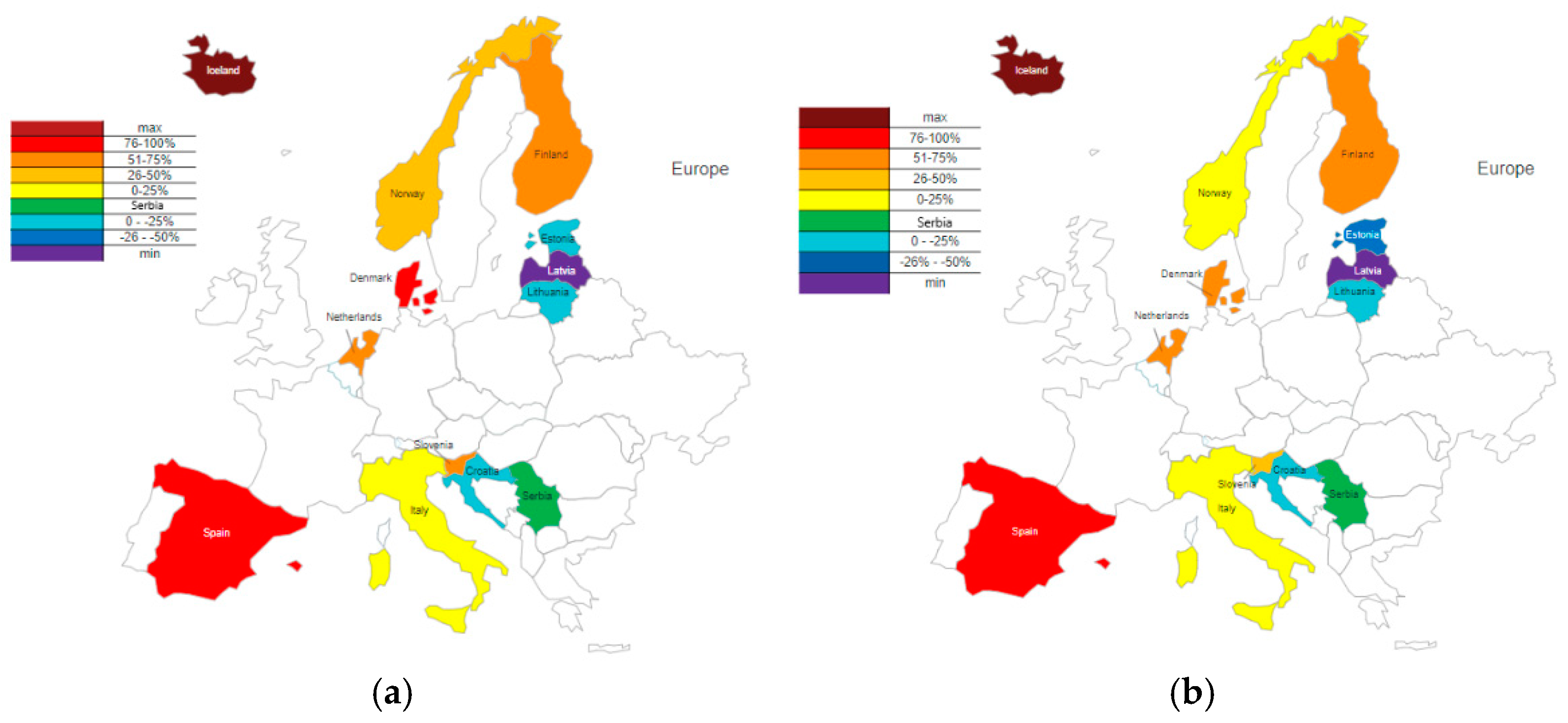
These data were compared with SSRI consumption in Serbia and expressed as percentages. The percentage differences were calculated and graphically presented for 2019, the year with the lowest consumption in Serbia, and 2020, the year with the highest consumption (
Figure 3).
The individual consumption of SSRI drugs in Serbia, Denmark, Spain, and Latvia in 2019 and 2020 is presented in
Table 7. These data were compared with drug consumption in Serbia and converted into percentages, enabling the calculation of the percentage difference for each drug within this group.
Table 6.
Individual consumption of SSRIs in Serbia, Denmark, Spain, and Latvia in 2019 and 2020.
Table 6.
Individual consumption of SSRIs in Serbia, Denmark, Spain, and Latvia in 2019 and 2020.
| Title 1 |
Serbia |
Denmark |
Spain |
Latvia |
| |
2019 |
2020 |
2019 |
2020 |
2019 |
2020 |
2019 |
2020 |
| fluoxetine |
2.82 |
3.4 |
2 |
1.8 |
6.94 |
7.06 |
0.51 |
0.63 |
| citalopram |
0.95 |
0.91 |
13.9 |
13 |
6.11 |
6.09 |
0.86 |
0.94 |
| paroxetine |
4.33 |
4.79 |
2.2 |
2.1 |
8.8 |
8.9 |
3.1 |
3.52 |
| sertraline |
11.02 |
11.85 |
25.9 |
28.7 |
14.56 |
15.78 |
1.4 |
1.63 |
| escitalopram |
7.44 |
8.46 |
4.4 |
4.4 |
13.46 |
13.88 |
5.39 |
6.24 |
Table 7.
Annual group consumption in 13 European countries and their GDP per capita in 2019 and 2020.
Table 7.
Annual group consumption in 13 European countries and their GDP per capita in 2019 and 2020.
| Country |
DID SSRI
(2019) |
GDP per capita
(2019) |
DID SSRI
(2020) |
GDP per capita
(2020) |
| Serbia |
26.56 |
9,927.1 |
29.41 |
9,809.7 |
| Italy |
29.90 |
21,336.7 |
30.60 |
19,772.8 |
| Iceland |
106.3 |
26,029.8 |
111.7 |
24,937.5 |
| Spain |
50.27 |
19,497.2 |
52.11 |
17,253.5 |
| Croatia |
22.19 |
14,168.2 |
22.69 |
14,004.4 |
| Norway |
35.97 |
27,761.2 |
36.35 |
26,073.7 |
| The Netherlands |
44.41 |
23,939.2 |
46.50 |
23,281.6 |
| Estonia |
20.58 |
15,760.0 |
21.72 |
15,771.9 |
| Finland |
43.40 |
23,237.4 |
44.58 |
23,047.9 |
| Latvia |
11.45 |
14,628.4 |
13.17 |
14,325.5 |
| Lithuania |
22.50 |
18,444.7 |
23.22 |
18,053.4 |
| Slovenia |
41 |
17,652.1 |
41.30 |
16,907.5 |
| Denmark |
47.80 |
25,277.4 |
49.50 |
25,457.2 |
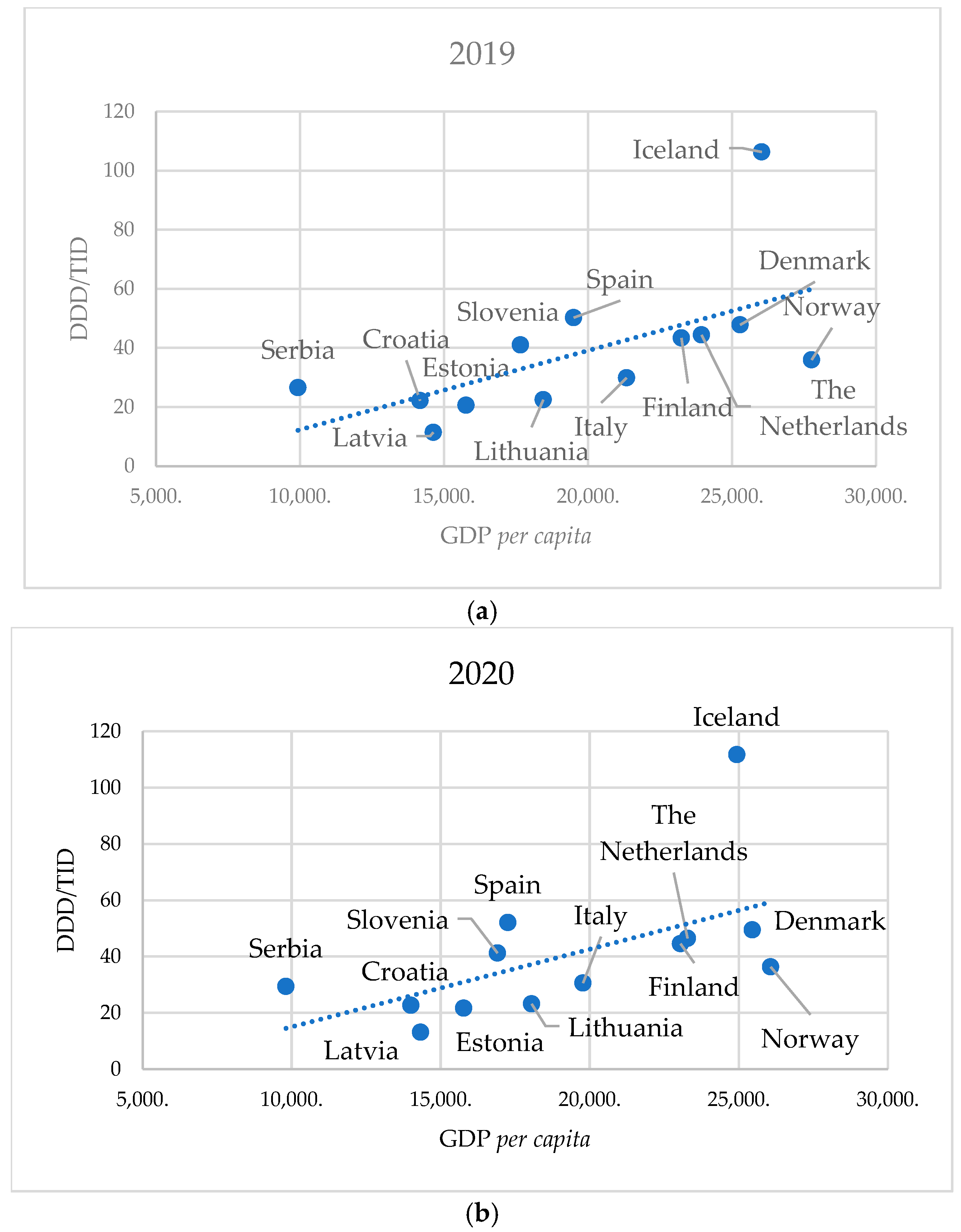
A comparison of total SSRI consumption and GDP per capita across European countries revealed a strong positive correlation in 2019 (ρ = 0.714; p = 0.0081), the year with the lowest SSRI consumption, and a moderate positive correlation in 2020 (ρ = 0.659; p = 0.0171), the year with the highest consumption.
Figure 4.
Correlation of annual SSRI group consumption in 13 European countries with their GDP per capita in 2019 (a) and 2020 (b).
Figure 4.
Correlation of annual SSRI group consumption in 13 European countries with their GDP per capita in 2019 (a) and 2020 (b).
4. Discussion
Since their discovery in the 1990s, SSRIs have become the preferred choice for treating depressive disorders due to their simpler therapeutic application, as well as their milder side effects and lower toxicity compared to tricyclic antidepressants [
14,
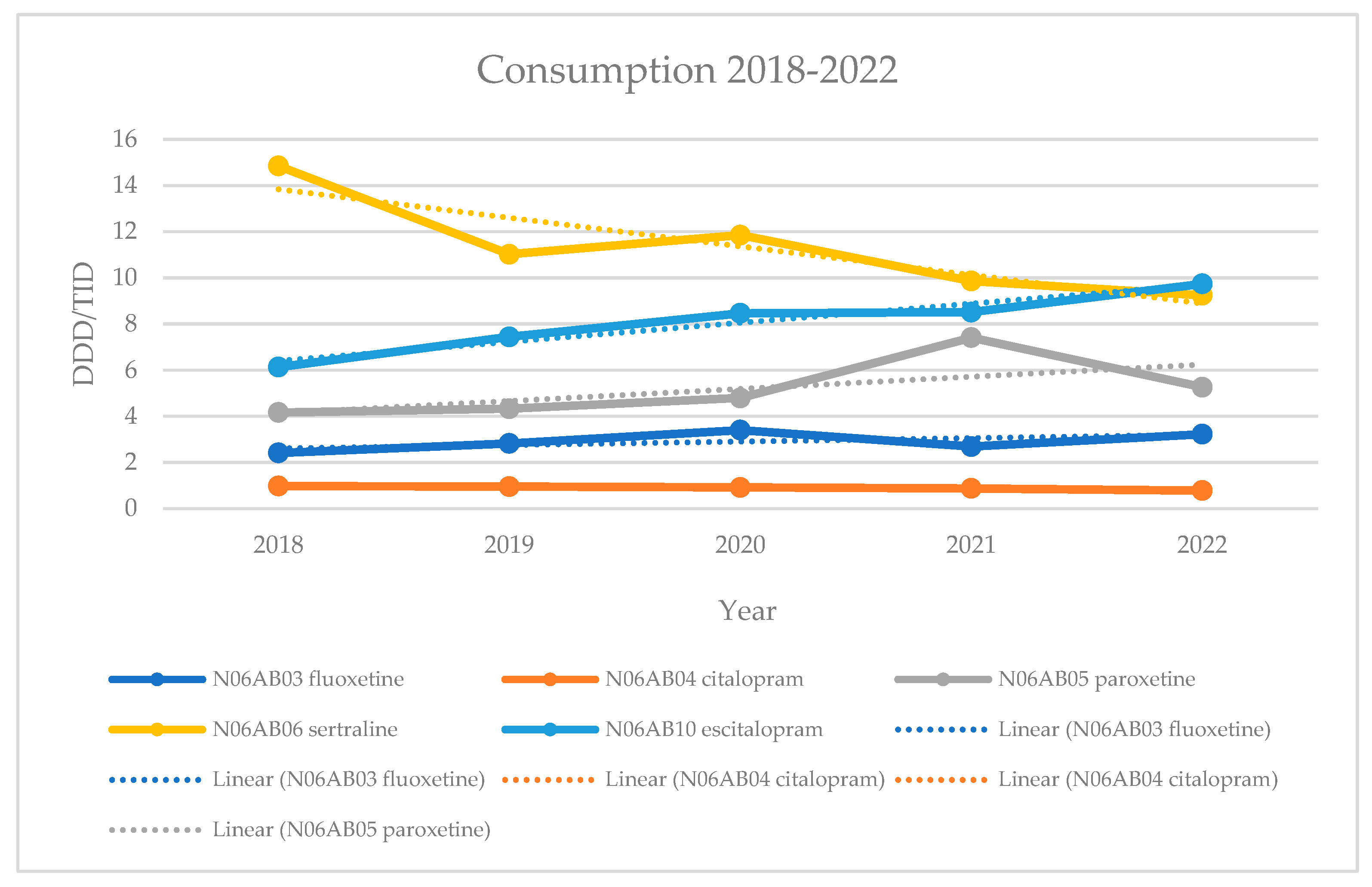
15], which were previously considered first-line treatments. Our analysis showed that sertraline experienced a decrease in consumption in 2019 compared to 2018 (20%), followed by an increase in 2020 (8%), and then a further decrease in 2021 and 2022 (17% and 6%).
Escitalopram experienced constant growth (21%, 14%, 1%, and 14%), making it the most widely used drug by 2022. Paroxetine also showed consistent growth until 2022 (4%, 11%, and 55%), but its consumption decreased by 29% in 2022. Fluoxetine had an increase in consumption in 2019 and 2020 (17% and 21%), followed by a decrease in 2021 (21%), before seeing another increase in 2022 (20%). Citalopram showed a constant decline in consumption (2%, 4%, 4%, 10%), remaining the least prescribed drug in this group. Group consumption fluctuated, with a decrease in consumption in 2019 compared to 2018 (7%), followed by an increase in 2020 (11%), and then a decline in 2021 and 2022 (0.3% and 4%). Compared to 2015 [
16], there was an increase in the consumption of sertraline (81%), escitalopram (40%), paroxetine (2%), and group SSRI consumption (38%). In contrast, the consumption of fluoxetine (17%) and citalopram (16%) decreased.
The most commonly used SSRI in Serbia from 2018-2021 was sertraline. Sertraline is recommended for patients over 60 years old due to its relatively mild interactions with other drugs compared to other SSRIs [
17]. Furthermore, since the half-life and incidence of side effects of sertraline are similar in pediatric populations and adults, it can be used in children and adolescents following the standard titration regimen for adults [
18]. Although both sertraline and its metabolite, DMS, were found in breast milk and infant plasma, their concentrations are low enough to avoid causing adverse effects in infants [
19,
20]. In patients with renal insufficiency, there are no significant differences in sertraline pharmacokinetics compared to healthy individuals [
21,
22]. However, in patients with hepatic insufficiency, clearance is reduced, and the half-life is greatly prolonged, requiring dose adjustments [
23]. Significant drug interactions include the potential for sertraline to enhance the anticoagulant effect of warfarin, necessitating the monitoring of INR. Additionally, combining sertraline with lithium may increase the risk of tremor [
24]. Increased concentrations of sertraline have also been observed in adolescents who consume marijuana [
25].
Escitalopram, which has shown a consistent increase in consumption both in Serbia and globally [
26], became the most commonly used SSRI in Serbia in 2022. As the S-enantiomer of citalopram, escitalopram is responsible for all of its therapeutic effects [
27]. No statistically significant differences in pharmacokinetics have been found in adolescents, the elderly, or patients with liver insufficiency compared to the general population [
28]. Escitalopram is a weak inhibitor of several CYP isoenzymes, including 1A2, 2C9, 2C19, 2D6, and 3A4, and has a low potential for interactions with drugs metabolized via these enzymes [
29]. However, when used in combination with omeprazole, a CYP2C19 and CYP3A4 inhibitor, or cimetidine, a CYP2D6 and CYP3A4 inhibitor, the elimination of escitalopram is reduced [
30]. A statistically but not clinically significant prolongation of the half-life of metoprolol has been observed when it is used concurrently with escitalopram [
31]. Recent studies comparing the antidepressant effects of escitalopram and psilocybin, a psychoactive substance derived from mushrooms, have indicated better effects with psilocybin, although these were not statistically significant [
32].
Paroxetine is the third most commonly consumed SSRI in Serbia. Its half-life is prolonged in the elderly and patients with renal insufficiency, so smaller initial doses are recommended for these populations [
33]. As a strong inhibitor of CYP2D6, paroxetine can interfere with both its own metabolism [
34] and the metabolism of other drugs metabolized by this enzyme, such as metoprolol [
35], clozapine [
36], desipramine [
37], and imipramine [
38]. Furthermore, paroxetine may interact with anticoagulant drugs, such as warfarin [
39]. Although paroxetine is excreted in breast milk, there is no contraindication to breastfeeding.
Fluoxetine, the first SSRI, was introduced in the literature in 1974, making it the oldest drug in this group [
40]. It is a potent inhibitor of CYP2D6 [
41,
42], which can lead to the inhibition of its own metabolism [
43], contributing to its long half-life. In patients with hepatic insufficiency, the half-life is further prolonged, necessitating dose adjustments [
33]. Since both fluoxetine and its active metabolite, norfluoxetine, are excreted in breast milk, breastfeeding should either be discontinued or conducted with minimal therapeutic doses [
44,
45]. As a potent CYP2D6 inhibitor, numerous interactions with drugs metabolized via this enzyme are possible, including beta-blockers (atenolol, bisoprolol, metoprolol), antiarrhythmics (amiodarone), antihypertensives (clonidine), antipsychotics (risperidone, haloperidol), other antidepressants (citalopram, escitalopram, amitriptyline), and cancer drugs (tamoxifen) [
46]. Additionally, fluoxetine can interact with anticoagulant drugs, such as warfarin.
Citalopram is the least frequently used antidepressant among SSRIs in Serbia. It is a racemic mixture composed of two enantiomers, S- (escitalopram) and R-citalopram, in a 1:1 ratio [
47]. The S-enantiomer is responsible for citalopram's effects in inhibiting serotonin reuptake, whereas the R-enantiomer may attenuate this effect through a pharmacodynamic interaction [
27,
47]. The half-life of citalopram is prolonged in elderly patients and individuals with hepatic insufficiency, warranting dose adjustments in these populations [
33].
Among the 12 European countries in 2019 and 2020, 8 had higher consumption than Serbia (Italy, Iceland, Spain, Norway, Netherlands, Finland, Slovenia, and Denmark), and 4 had lower consumption (Croatia, Estonia, Latvia, and Lithuania). Based on the percentage difference in SSRI consumption relative to Serbia, countries with higher usage were categorized into four groups: Group 1 (0-25%), Group 2 (25.1-50%), Group 3 (50.1-75%), and Group 4 (>75%). In Group 1, Italy remained in both years with a percentage difference of 13% and 4%. Norway, initially in Group 2 in 2019 with a 35% higher consumption, shifted to Group 1 in 2020 (24%). The Netherlands and Finland consistently belonged to Group 3, with respective differences of 67% and 58% for the Netherlands, and 63% and 52% for Finland in 2019 and 2020. Slovenia, which was also in Group 3 in 2019 (54%), transitioned to Group 2 in 2020 (40%). Spain remained stable in Group 4 (89% and 77%), while Denmark showed a decrease, moving from Group 4 to Group 3 (80% and 68%). Iceland, the country with the highest global consumption of SSRIs, reported levels approximately 300% higher than Serbia in 2019 and 280% higher in 2020. Among the countries with lower SSRI consumption than Serbia, two groups were defined: Group 1 (0-25%) and Group 2 (>25.1%). Croatia (16% and 23%) and Lithuania (15% and 21%) remained within Group 1 in both years, indicating relatively stable trends. Estonia transitioned from Group 1 (23%) to Group 2 (26%), reflecting a relative decrease. Latvia, which had the lowest SSRI consumption among the 13 selected countries, reported levels that were 57% and 55% lower.
From the 12 European countries analyzed, three were selected for detailed comparison based on their SSRI consumption relative to Serbia: two with higher consumption (Denmark and Spain) and one with lower consumption (Latvia). In Denmark, sertraline consumption was 135% higher in 2019 and 142% higher in 2020, while escitalopram use was 41% and 48% lower, respectively. Citalopram consumption in Denmark was significantly higher, with differences of 1363% in 2019 and 1329% in 2020. In Spain, sertraline usage exceeded Serbia’s by 32% and 33%, escitalopram by 81% and 64%, and citalopram by 543% and 569%, respectively. In contrast, Latvia demonstrated significantly lower consumption: sertraline use was 87% and 86% lower, escitalopram 28% and 26% lower, while citalopram was 9.5% lower in 2019 but 3% higher in 2020 compared to Serbia.
5. Conclusions
The overall consumption of antidepressants from the SSRI group in Serbia fluctuated between 2018 and 2022, without reaching statistical significance. However, significant trends were observed in the consumption patterns of individual SSRIs, particularly sertraline, escitalopram, and citalopram. Sertraline was the most frequently prescribed SSRI from 2018 to 2021, after which escitalopram use showed a steady increase, becoming the most consumed SSRI in 2022 both in Serbia and globally. This shift reflects a broader trend in prescribing practices, favoring escitalopram likely due to its favorable efficacy, tolerability profile, and lower potential for drug interactions. When compared to 12 other European countries, Serbia ranked in the mid-range, with 8 countries demonstrating higher SSRI consumption and 4 showing lower levels. Notably, Iceland had the highest SSRI consumption, while Latvia had the lowest among the selected countries. Additionally, a positive correlation was found between SSRI use and GDP per capita across 13 European countries, suggesting that economic factors may influence antidepressant consumption. Further research into regional prescribing patterns, socioeconomic factors, and clinical guidelines could help improve mental health treatment and ensure equal access to effective antidepressant therapy.
Author Contributions
All authors contributed equally to the conceptualization, writing and revision of the original draft. There is no methodology, software, investigation, data curation, project administration, funding or supervision included. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DDD |
Defined daily doses |
| DDD/TID |
Defined daily doses per 1000 inhabitants per day |
References
- Wu, Y.; Wang, L.; Tao, M.; Cao, H.; Yuan, H.; Ye, M.; Chen, X.; Wang, K.; Zhu, C. Changing Trends in the Global Burden of Mental Disorders from 1990 to 2019 and Predicted Levels in 25 Years. Epidemiol. Psychiatr. Sci. 2023, 32, e63. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Kalin, N.H. The Critical Relationship between Anxiety and Depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the True Global Burden of Mental Illness. Lancet Psychiatry 2016, 3, 171–178. [Google Scholar] [CrossRef]
- Hock, R.S.; Or, F.; Kolappa, K.; Burkey, M.D.; Surkan, P.J.; Eaton, W.W. A New Resolution for Global Mental Health. Lancet 2012, 379, 1367–1368. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Subedi, S.; Paudyal, S.; Panta, P.P. Suicidal Attempt among Psychiatry Patients Presented to the Department of Emergency of a Tertiary Care Centre: A Descriptive Cross-Sectional Study. JNMA J. Nepal Med. Assoc. 2023, 61, 442–445. [Google Scholar] [CrossRef]
- Alshaya, D.S. Genetic and Epigenetic Factors Associated with Depression: An Updated Overview. Saudi J. Biol. Sci. 2022, 29, 103311. [Google Scholar] [CrossRef]
- Javaid, S.F.; Hashim, I.J.; Hashim, M.J.; Stip, E.; Samad, M.A.; Ahbabi, A.A. Epidemiology of Anxiety Disorders: Global Burden and Sociodemographic Associations. Middle East Curr. Psychiatr. 2023, 30. [Google Scholar] [CrossRef]
- Luft, M.J.; Lamy, M.; DelBello, M.P.; McNamara, R.K.; Strawn, J.R. Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management. Curr. Probl. Pediatr. Adolesc. Health Care 2018, 48, 50–62. [Google Scholar] [CrossRef]
- Gabriel, M.; Sharma, V. Antidepressant Discontinuation Syndrome. CMAJ 2017, 189, E747. [Google Scholar] [CrossRef]
- Jing, E.; Straw-Wilson, K. Sexual Dysfunction in Selective Serotonin Reuptake Inhibitors (SSRIs) and Potential Solutions: A Narrative Literature Review. Ment. Health Clin. 2016, 6, 191–196. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Yang, L.; Zhang, K.; Li, S. Safety Profile of Selective Serotonin Reuptake Inhibitors in Real-World Settings: A Pharmacovigilance Study Based on FDA Adverse Event Reporting System. Ann. Pharmacother. 2024, 58, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, N.; Damkier, P.; Pedersen, S.A. Serotonin Syndrome-A Focused Review. Basic Clin. Pharmacol. Toxicol. 2023, 133, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Grimsley, S.R.; Jann, M.W. Paroxetine, Sertraline, and Fluvoxamine: New Selective Serotonin Reuptake Inhibitors. Clin. Pharm. 1992, 11, 930–957. [Google Scholar] [PubMed]
- Nawaz, A.; Mamoon, B.; Batool, T.E.; Khattak, M.I.; Amir, F.; Akbar, A.; Khan, S. Advances in Antidepressant Therapy: Comparing the Efficacy of Selective Serotonin Reuptake Inhibitors (SSRIs), Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), and Novel Agents. Cureus 2024, 16, e76318. [Google Scholar] [CrossRef]
- Milijašević, B., Ž.; Vlajankov, A., L.; Ubavić, M., B.; Rašković, A., L.; Martić, N., B.; Tomić, Z.S. Analysis of Antidepressant Use in Republic of Serbia from 2013 to 2015. Hosp. Pharmacol. - Int. Multidiscip. J. 2018, 5, 607–616. [Google Scholar] [CrossRef]
- Muijsers, R.B.R.; Plosker, G.L.; Noble, S. Spotlight on Sertraline in the Management of Major Depressive Disorder in Elderly Patients. CNS Drugs 2002, 16, 789–794. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Bloch, M.H. Antidepressants for Pediatric Patients. Curr. Psychiatr. 2019, 18, 26–42. [Google Scholar]
- Pinheiro, E.; Bogen, D.L.; Hoxha, D.; Ciolino, J.D.; Wisner, K.L. Sertraline and Breastfeeding: Review and Meta-Analysis. Arch. Womens. Ment. Health 2015, 18, 139–146. [Google Scholar] [CrossRef]
- Cuomo, A.; Maina, G.; Neal, S.M.; De Montis, G.; Rosso, G.; Scheggi, S.; Beccarini Crescenzi, B.; Bolognesi, S.; Goracci, A.; Coluccia, A.; Ferretti, F.; Fagiolini, A. Using Sertraline in Postpartum and Breastfeeding: Balancing Risks and Benefits. Expert Opin. Drug Saf. 2018, 17, 719–725. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.; Hicks, J.K.; Ramsey, L.B.; Strawn, J.R.; Smith, D.M.; Bobonis Babilonia, M.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Sertraline Pathway, Pharmacokinetics. Pharmacogenet. Genomics 2020, 30, 26–33. [Google Scholar] [CrossRef]
- Mauri, M.C.; Fiorentini, A.; Paletta, S.; Altamura, A.C. Pharmacokinetics of Antidepressants in Patients with Hepatic Impairment. Clin. Pharmacokinet. 2014, 53, 1069–1081. [Google Scholar] [CrossRef]
- Apseloff, G.; Wilner, K.D.; von Deutsch, D.A.; Henry, E.B.; Tremaine, L.M.; Gerber, N.; Lazar, J.D. Sertraline Does Not Alter Steady-State Concentrations or Renal Clearance of Lithium in Healthy Volunteers. J. Clin. Pharmacol. 1992, 32, 643–646. [Google Scholar] [CrossRef]
- Vaughn, S.E.; Strawn, J.R.; Poweleit, E.A.; Sarangdhar, M.; Ramsey, L.B. The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations. J. Pers. Med. 2021, 11, 615. [Google Scholar] [CrossRef]
- Cavanah, L.R.; Ray, P.K.; Goldhirsh, J.L.; Huey, L.Y.; Piper, B.J. Patterns in (Es)Citalopram Prescriptions to Medicaid and Medicare Patients in the United States: The Potential Effects of Evergreening. Front. Psychiatry 2025, 16, 1450111. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, J.; Bøgesø, K.P.; Perregaard, J.; Sánchez, C. The Pharmacological Effect of Citalopram Residues in the (S)-(+)-Enantiomer. J. Neural Transm. Gen. Sect. 1992, 88, 157–160. [Google Scholar] [CrossRef]
- Rao, N. The Clinical Pharmacokinetics of Escitalopram. Clin. Pharmacokinet. 2007, 46, 281–290. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, L.L.; Greenblatt, D.J.; Giancarlo, G.M.; Granda, B.W.; Harmatz, J.S.; Shader, R.I. Escitalopram (S-Citalopram) and Its Metabolites in Vitro: Cytochromes Mediating Biotransformation, Inhibitory Effects, and Comparison to R-Citalopram. Drug Metab. Dispos. 2001, 29, 1102–1109. [Google Scholar]
- Malling, D.; Poulsen, M.N.; Søgaard, B. The Effect of Cimetidine or Omeprazole on the Pharmacokinetics of Escitalopram in Healthy Subjects. Br. J. Clin. Pharmacol. 2005, 60, 287–290. [Google Scholar] [CrossRef]
- Molden, E.; Spigset, O. Interactions between metoprolol and antidepressants. Tidsskr. Nor. Laegeforen. 2011, 131, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Zakaraya, Z.; Abu Assab, M.; Tamimi, L.N.; Karameh, N.; Hailat, M.; Al-Omari, L.; Abu Dayyih, W.; Alasasfeh, O.; Awad, M.; Awad, R. Pharmacokinetics and Pharmacodynamics: A Comprehensive Analysis of the Absorption, Distribution, Metabolism, and Excretion of Psychiatric Drugs. Pharmaceuticals 2024, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Laine, K.; Kytölä, J.; Bertilsson, L. Severe Adverse Effects in a Newborn with Two Defective CYP2D6 Alleles after Exposure to Paroxetine during Late Pregnancy. Ther. Drug Monit. 2004, 26, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.B.; Soberman, J.E. Effects of Paroxetine on the Pharmacokinetics and Pharmacodynamics of Immediate-Release and Extended-Release Metoprolol. Pharmacotherapy 2011, 31, 630–641. [Google Scholar] [CrossRef]
- Spina, E.; Avenoso, A.; Salemi, M.; Facciolá, G.; Scordo, M.G.; Ancione, M.; Madia, A. Plasma Concentrations of Clozapine and Its Major Metabolites during Combined Treatment with Paroxetine or Sertraline. Pharmacopsychiatry 2000, 33, 213–217. [Google Scholar] [CrossRef]
- Alderman, J.; Preskorn, S.H.; Greenblatt, D.J.; Harrison, W.; Penenberg, D.; Allison, J.; Chung, M. Desipramine Pharmacokinetics When Coadministered with Paroxetine or Sertraline in Extensive Metabolizers. J. Clin. Psychopharmacol. 1997, 17, 284–291. [Google Scholar] [CrossRef]
- Albers, L.J.; Reist, C.; Helmeste, D.; Vu, R.; Tang, S.W. Paroxetine Shifts Imipramine Metabolism. Psychiatry Res. 1996, 59, 189–196. [Google Scholar] [CrossRef]
- Bannister, S.J.; Houser, V.P.; Hulse, J.D.; Kisicki, J.C.; Rasmussen, J.G. Evaluation of the Potential for Interactions of Paroxetine with Diazepam, Cimetidine, Warfarin, and Digoxin. Acta Psychiatr. Scand. Suppl. 1989, 350, 102–106. [Google Scholar] [CrossRef]
- Hillhouse, T.M.; Porter, J.H. A Brief History of the Development of Antidepressant Drugs: From Monoamines to Glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef]
- Stevens, J.C.; Wrighton, S.A. Interaction of the Enantiomers of Fluoxetine and Norfluoxetine with Human Liver Cytochromes P450. J. Pharmacol. Exp. Ther. 1993, 266, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, R.; Forti, G.C.; Raggi, M.A. Fluoxetine Metabolism and Pharmacological Interactions: The Role of Cytochrome P450. Curr. Drug Metab. 2006, 7, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, M.; Rihani, S.B.A.; Darakjian, L.; Turgeon, J.; Michaud, V. Assessing the Mechanism of Fluoxetine-Mediated CYP2D6 Inhibition. Pharmaceutics 2021, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, G.; Zandonella Callegher, R.; Butera, R.; De Santis, M.; Cavaliere, A.F.; Vecchio, S.; Lanzi, C.; Davanzo, R.; Mangili, G.; Bondi, E.; et al. Consensus Panel Recommendations for the Pharmacological Management of Breastfeeding Women with Postpartum Depression. Int. J. Environ. Res. Public Health 2024, 21, 551. [Google Scholar] [CrossRef]
- Vitale, S.G.; Laganà, A.S.; Muscatello, M.R.A.; La Rosa, V.L.; Currò, V.; Pandolfo, G.; Zoccali, R.A.; Bruno, A. Psychopharmacotherapy in Pregnancy and Breastfeeding. Obstet. Gynecol. Surv. 2016, 71, 721–733. [Google Scholar] [CrossRef]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A Review of the Important Role of CYP2D6 in Pharmacogenomics. Genes (Basel) 2020, 11, 1295. [Google Scholar] [CrossRef]
- Sánchez, C.; Bøgesø, K.P.; Ebert, B.; Reines, E.H.; Braestrup, C. Escitalopram versus Citalopram: The Surprising Role of the R-Enantiomer. Psychopharmacology (Berl.) 2004, 174, 163–176. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).