Submitted:
18 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Is there a GLP-1 analogues’ misusing issue?
2. Results
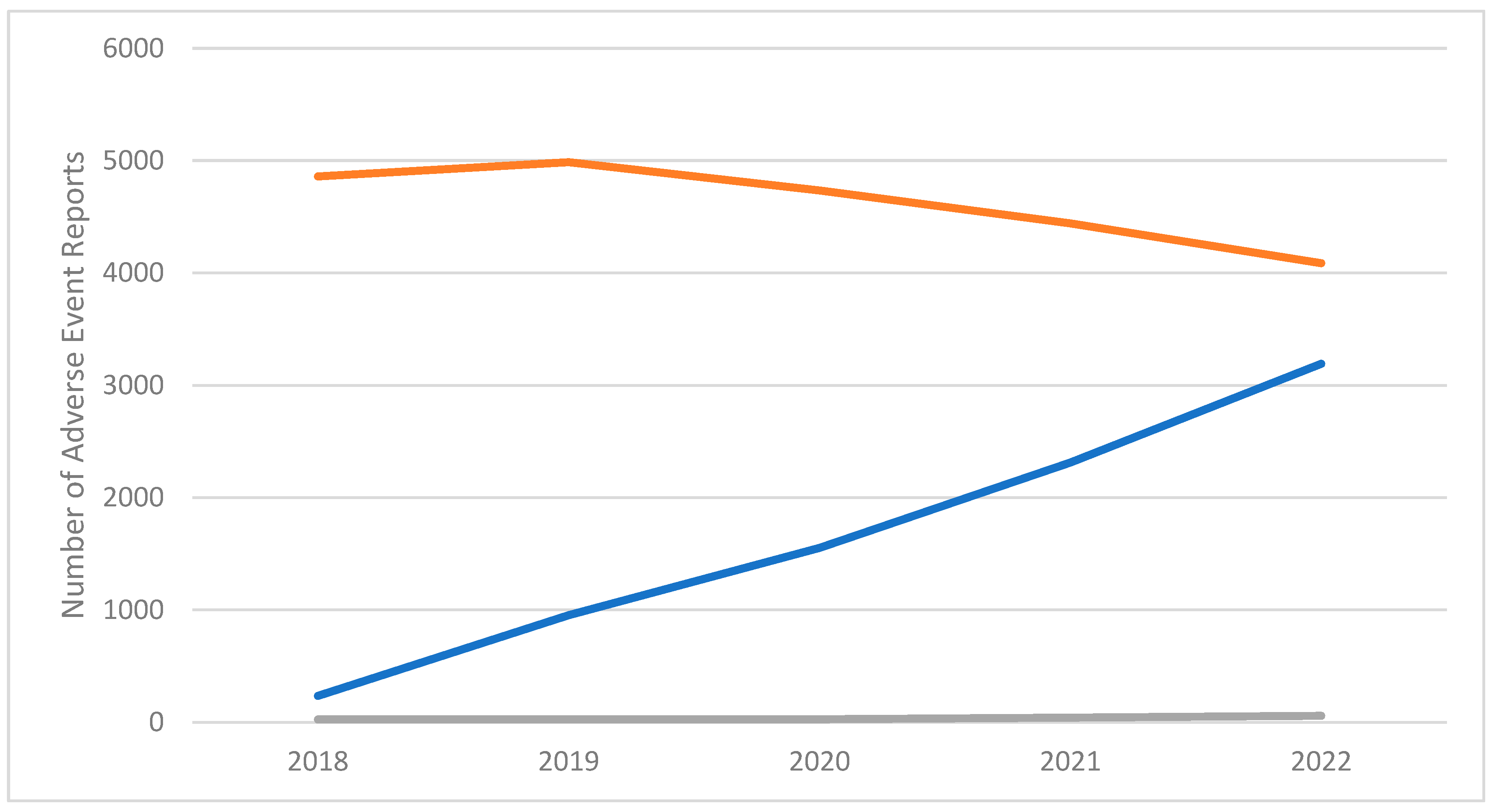
2.1. Pharmacovigilance signals
3. Discussion
Semaglutide and GLP-1RA as image- and performance-enhancing drugs (IPED)
Semaglutide and GLP-1RAs as molecules acting on the reward system?
The potential use of GLP-1 RA in neurology
Limitations
4. Materials and Methods
4.1. Data source
Data analysis
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gettman, L. New Drug: Tirzepatide (Mounjaro(™)).Sr Care Pharm. 2023 Feb 1;38(2):50-62. [CrossRef]
- Rendell, MS. Obesity and diabetes: the final frontier. Expert Rev Endocrinol Metab. 2023 Jan;18(1):81-94. [CrossRef]
- Novograd J, Mullally JA, Frishman WH. Tirzepatide for Weight Loss: Can Medical Therapy “Outweigh” Bariatric Surgery? Cardiol Rev. 2023 Jan 23. [CrossRef]
- Slahor, L. [CME: Metformin – Dos and Don’ts]. Praxis (Bern 1994). 2021;110(16):939-945. [CrossRef]
- Haddad F, Dokmak G, Bader M, Karaman R. A Comprehensive Review on Weight Loss Associated with Anti-Diabetic Medications. Life (Basel). 2023 Apr 14;13(4):1012. [CrossRef]
- Azuri J, Hammerman A, Aboalhasan E, Sluckis B, Arbel R. Tirzepatide versus emaglutide for weight loss in patients with type 2 diabetes mellitus: A value for money analysis. Diabetes Obes Metab. 2023 Apr;25(4):961-964. [CrossRef]
- Ryan DH, Deanfield JE, Jacob S. Prioritizing obesity treatment: expanding the role of cardiologists to improve cardiovascular health and outcomes. Cardiovasc Endocrinol Metab. 2023 Feb 7;12(1):e0279. [CrossRef]
- Mishra R, Raj R, Elshimy G, Zapata I, Kannan L, Majety P, Edem D, Correa R. Adverse Events Related to Tirzepatide. J Endocr Soc. 2023 Jan 26;7(4):bvad016. [CrossRef]
- Scheen, AJ. Dual GIP/GLP-1 receptor agonists: New advances for treating type-2 diabetes. Ann Endocrinol (Paris). 2023 Jan 10:S0003-4266(23)00004-5. [CrossRef]
- Neuville MF, Paquot N, Scheen AJ. [A new era for glucagon-like peptide-1 receptor agonists]. Rev Med Liege. 2023 Jan;78(1):40-45.
- Bhusal, A. Advent of tirzepatide: boon for diabetic and obese? Ann Med Surg (Lond). 2023 Feb 7;85(2):71-72. [CrossRef]
- Sinha R, Papamargaritis D, Sargeant JA, Davies MJ. Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management. J Obes Metab Syndr. 2023 Feb 8. [CrossRef]
- Ebell, MH. Tirzepatide Helps Adults With Obesity Without Diabetes Lose 15% to 21% of Their Body Weight Over 72 Weeks. Am Fam Physician. 2023 Jan;107(1):99.
- Alkhezi OS, Alahmed AA, Alfayez OM, Alzuman OA, Almutairi AR, Almohammed OA. Comparative effectiveness of glucagon-like peptide-1 receptor agonists for the management of obesity in adults without diabetes: A network meta-analysis of randomized clinical trials. Obes Rev. 2023 Mar;24(3):e13543. [CrossRef]
- Tan Q, Akindehin SE, Orsso CE, Waldner RC, DiMarchi RD, Müller TD and Haqq AM (2022) Recent Advances in Incretin-Based Pharmacotherapies for the Treatment of Obesity and Diabetes. Front. Endocrinol. 13:838410. [CrossRef]
- Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EclinicalMedicine. 2023 Mar 20;58:101882. [CrossRef]
- BBC News. Weight loss drug emaglutide approved for NHS use. https://www.bbc.com/news/health-64874243. Accessed , 2023. 08 March.
- Li A, Su X, Hu S, Wang Y. Efficacy and safety of oral emaglutide in type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2023 Apr;198:110605. [CrossRef]
- Rodríguez JE, Campbell KM. Past, Present, and Future of Pharmacologic Therapy in Obesity. Prim Care. 2016 Mar;43(1):61-7, viii. [CrossRef]
- Douglas JG, Munro JF. Drug treatment and obesity. Pharmacol Ther. 1982;18(3):351-73. [CrossRef]
- Cosentino G, Conrad AO, Uwaifo GI. Phentermine and topiramate for the management of obesity: a review. Drug Des Devel Ther. 2011;7:267-278. Published 2011 Apr 5. [CrossRef]
- Alfaris N, Minnick AM, Hopkins CM, Berkowitz RI, Wadden TA. Combination phentermine and topiramate extended release in the management of obesity. Expert Opin Pharmacother. 2015 Jun;16(8):1263-74. [CrossRef]
- Makówka A, Zawiasa A, Nowicki M. Prescription-medication sharing among family members: an unrecognized cause of a serious drug adverse event in a patient with impaired renal function. Clin Nephrol. 2015 Mar;83(3):196-200. [CrossRef]
- Song XB, Shao XT, Liu SY, Tan DQ, Wang Z, Wang DG. Assessment of metformin, nicotine, caffeine, and methamphetamine use during Chinese public holidays. Chemosphere. 2020 Nov;258:127354. [CrossRef]
- Burtscher, M. Metformin for high-altitude performance? Clin Exp Pharmacol Physiol. 2017 Aug;44(8):903. [CrossRef]
- Geer B, Gibson D, Grayeb D, Benabe J, Victory S, Mehler S, Mehler P. Metformin abuse: A novel and dangerous purging behavior in anorexia nervosa. Int J Eat Disord. 2019 Mar;52(3):319-321. [CrossRef]
- The Independent. Jameela Jamil calls out ‘extreme’ weight loss at Oscars amid ozempic controversy. https://www.independent.co.uk/life-style/ozempic-weight-loss-jameela-jamil-oscars-b2300525.html. Accessed on , 2023. 14 March.
- Le Monde, 2023. https://www.lemonde.fr/en/health/article/2023/03/02/ozempic-french-authorities-issue-alert-for-anti-diabetic-drug-misused-for-weight-loss_6017913_14.html#:~:text=While%20misuse%20of%20Ozempic%20appears,them%20of%20this%20essential%20treatment.%22. Accessed on , 2023. 08 April.
- Alvarez-Mon MA, Llavero-Valero M, Asunsolo Del Barco A, et al. Areas of Interest and Attitudes Toward Antiobesity Drugs: Thematic and Quantitative Analysis Using Twitter. J Med Internet Res. 2021 Oct 26;23(10):e24336. [CrossRef]
- The Guardian, 2023. https://www.theguardian.com/australia-news/2023/jan/06/tga-investigates-influencers-after-diabetes-drug-ozempic-promoted-as-weight-loss-treatment. Accessed on , 2023. 08 April.
- Valdesolo, F. What You Need to Know About Ozempic: The Diabetes Drug Fuelling Hollywood’s Harmful Weight-Loss Obsession; 10 February 2023. https: //www.vogue.co.uk/beauty/article/what-is-ozempic. Accessed on April 08, 2023. [Google Scholar]
- Orsolini L, Francesconi G, Papanti D, Giorgetti A, Schifano F. Profiling online recreational/prescription drugs’ customers and overview of drug vending virtual marketplaces. Hum Psychopharmacol. 2015 Jul;30(4):302-18. [CrossRef]
- Zaprutko T, Kopciuch D, Paczkowska A, et al. Facebook as a source of access to medicines. PloS One. 2022 Oct 13;17(10):e0275272. [CrossRef]
- Chiappini S, Vickers-Smith R, Guirguis A, et al. Pharmacovigilance Signals of the Opioid Epidemic over 10 Years: Data Mining Methods in the Analysis of Pharmacovigilance Datasets Collecting Adverse Drug Reactions (ADRs) Reported to EudraVigilance (EV) and the FDA Adverse Event Reporting System (FAERS). Pharmaceuticals (Basel). 2022 ;15(6):675. 27 May. [CrossRef]
- Schifano N, Capogrosso P, Boeri L, Fallara G, Cakir OO, Castiglione F, Alnajjar HM, Muneer A, Deho’ F, Schifano F, Montorsi F, Salonia A. Medications mostly associated with priapism events: assessment of the 2015-2020 Food and Drug Administration (FDA) pharmacovigilance database entries. Int J Impot Res. 2022. 21 May. [CrossRef]
- Dahlén AD, Dashi G, Maslov I, Attwood MM, Jonsson J, Trukhan V and Schiöth HB (2022) Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 12:807548. [CrossRef]
- Engler C, Leo M, Pfeifer B, Juchum M, Chen-Koenig D, Poelzl K, Schoenherr H, Vill D, Oberdanner J, Eisendle E, Middeldorf K, Heindl B, Gaenzer H, Bode G, Kirchmeyr K, Ladner G, Rieger L, Koellensperger U, Schwaiger A, Stoeckl F, Zangerl G, Lechleitner M, Delmarko I, Oberaigner W, Rissbacher C, Tilg H, Ebenbichler C. Long-term trends in the prescription of antidiabetic drugs: realworld evidence from the Diabetes Registry Tyrol 2012-2018. BMJ Open Diabetes Res Care. 2020 Sep;8(1):e001279. [CrossRef]
- Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab. 2021 Apr;46:101102. [CrossRef]
- Yamamoto-Honda R, Takahashi Y, Mori Y, et al. Changes in Antidiabetic Drug Prescription and Glycemic Control Trends in Elderly Patients with Type 2 Diabetes Mellitus from 2005-2013: An Analysis of the National Center Diabetes Database (NCDD-03). Intern Med. 2018 ;57(9):1229-1240. 1 May. [CrossRef]
- Liu L, Chen J, Wang L, Chen C, Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol (Lausanne). 2022 Dec 7;13:1043789. [CrossRef]
- Sarayani A, Hampp C, Brown JD, Donahoo WT, Winterstein AG. Topiramate Utilization After Phentermine/Topiramate Approval for Obesity Management: Risk Minimization in the Era of Drug Repurposing. Drug Saf. 2022 Dec;45(12):1517-1527. [CrossRef]
- Sharma D, Verma S, Vaidya S, Kalia K, Tiwari V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed Pharmacother. 2018 Dec;108:952-962. [CrossRef]
- Smits MM, Van Raalte DH. Safety of Semaglutide. Front Endocrinol (Lausanne). 2021 Jul 7;12:645563. [CrossRef]
- Cigrovski Berkovic M, Strollo F. Semaglutide-eye-catching results. World J Diabetes. 2023 Apr 15;14(4):424-434. [CrossRef]
- EMCDDA, 2020. Health and social responses to problems associated with the use of performance- and image-enhancing drugs A background paper for the updated European Responses Guide. https://www.emcdda.europa.eu/system/files/media/attachments/documents/14197/ERG2021_BackgroundPaper_FINAL.pdf. Accessed on , 2023. 06 May.
- Bruening AB, Perez M, Ohrt TK. Exploring weight control as motivation for illicit stimulant use. Eat Behav. 2018 Aug;30:72-75. [CrossRef]
- Milano G, Chiappini S, Mattioli F, Martelli A, Schifano F. β-2 Agonists as Misusing Drugs? Assessment of both Clenbuterol- and Salbutamol-related European Medicines Agency Pharmacovigilance Database Reports. Basic Clin Pharmacol Toxicol. 2018 Aug;123(2):182-187. [CrossRef]
- Dakanalis A, Colmegna F, Zanetti MA, Di Giacomo E, Riva G, Clerici M. Evaluation of the DSM-5 Severity Specifier for Bulimia Nervosa in Treatment-Seeking Youth. Child Psychiatry Hum Dev. 2018 Feb;49(1):137-145. [CrossRef]
- Potts AJ, Bowman NJ, Seger DL, Thomas SHL. Toxicoepidemiology and pre-dictors of death in 2,4-dinitrophenol (DNP) toxicity. Clin Toxicol (Phila). 2021 Jun;59(6):515-520. [CrossRef]
- Corazza O, Bersani FS, Brunoro R, Valeriani G, Martinotti G, Schifano F. The diffusion of performance and image-enhancing drugs (PIEDs) on the internet: the abuse of the cognitive enhancer piracetam. Subst Use Misuse. 2014 Dec;49(14):1849-56. [CrossRef]
- Hendricks, EJ. Off-label drugs for weight management. Diabetes Metab Syndr Obes. 2017 Jun 10;10:223-234. [CrossRef]
- Lee S, Kim J, In S, Choi H, Chung H, Chung KH. Detection of phentermine in hair samples from drug suspects. Forensic Sci Int. 2011 Apr 15;207(1-3):e5-7. [CrossRef]
- Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotec-tive effects of glucagon-like peptide-1 receptor agonists and other incretin re-ceptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepa-tol. 2023 Feb;8(2):179-191. [CrossRef]
- Reiner DJ, Leon RM, McGrath LE, Koch-Laskowski K, Hahn JD, Kanoski SE, Mietlicki-Baase EG, Hayes MR. Glucagon-Like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance. Neuropsychopharmacology. 2018 Feb;43(3):627-637. [CrossRef]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999 Jun 29;877:461-85. [CrossRef]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012 Apr 4;32(14):4812-20. [CrossRef]
- Eren-Yazicioglu CY, Yigit A, Dogruoz RE, Yapici-Eser H. Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front Behav Neurosci. 2021 Jan 18;14:614884. [CrossRef]
- Listos J, Listos P, Baranowska-Bosiacka I, et al. Linagliptin, a Selective Dipeptidyl Peptidase-4 Inhibitor, Reduces Physical and Behavioral Effects of Morphine Withdrawal. Molecules. 2022 Apr 12;27(8):2478. [CrossRef]
- Jerlhag, E. The therapeutic potential of glucagon-like peptide-1 for persons with addictions based on findings from preclinical and clinical studies. Front Pharmacol. 2023 Mar 30;14:1063033. [CrossRef]
- New York Times, 2023. https://www.nytimes.com/2023/02/03/well/live/ozempic-wegovy-weight-loss.html. Accessed on , 2023. 06 May.
- Wilding JPH, Batterham RL, Davies M, et al. Weight regain and cardiometabolic effects after withdrawal of emaglutide: The STEP 1 trial extension. Diabetes Obes Metab. 2022 Aug;24(8):1553-1564. [CrossRef]
- van Bloemendaal L, Ijzerman RG, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014 Dec;63(12):4186-96. [CrossRef]
- Urbanik LA, Acharya NK, Grigson PS. Acute treatment with the glucagon-like peptide-1 receptor agonist, liraglutide, reduces cue- and drug-induced fentanyl seeking in rats. Brain Res Bull. 2022 Oct 15;189:155-162. [CrossRef]
- Douton JE, Acharya NK, Stoltzfus B, Sun D, Grigson PS, Nyland JE. Acute glucagon-like peptide-1 receptor agonist liraglutide prevents cue-, stress-, and drug-induced heroin-seeking in rats. Behav Pharmacol. 2022 Aug 1;33(5):364-378. [CrossRef]
- Colvin KJ, Killen HS, Kanter MJ, et al. Differential effects of intra-ventral tegmental area ghrelin and glucagon-like peptide-1 on the stimulatory action of D-amphetamine and cocaine-induced ethanol intake in male Sprague Dawley rats. Behav Brain Res. 2022 Mar 12;421:113726. [CrossRef]
- Douton JE, Augusto C, Stoltzfus B, Carkaci-Salli N, Vrana KE, Grigson PS. Glucagon-like peptide-1 receptor agonist, exendin-4, reduces reinstatement of heroin-seeking behavior in rats. Behav Pharmacol. 2021 Jun 1;32(4):265-277. [CrossRef]
- Marty VN, Farokhnia M, Munier JJ, Mulpuri Y, Leggio L, Spigelman I. Long-Acting Glucagon-Like Peptide-1 Receptor Agonists Suppress Voluntary Alcohol Intake in Male Wistar Rats. Front Neurosci. 2020 Dec 23;14:599646. [CrossRef]
- Yammine L, Green CE, Kosten TR, de Dios C, Suchting R, Lane SD, Verrico CD, Schmitz JM. Exenatide Adjunct to Nicotine Patch Facilitates Smoking Cessation and May Reduce Post-Cessation Weight Gain: A Pilot Randomized Controlled Trial. Nicotine Tob Res. 2021 Aug 29;23(10):1682-1690. [CrossRef]
- Klausen MK, Jensen ME, Møller M, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight. 2022 Oct 10;7(19):e159863. [CrossRef]
- Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 2008; 5: 19.
- Hölscher, C. Protective properties of GLP-1 and associated peptide hormones in neurodegenerative disorders. Br J Pharmacol. 2022 Feb;179(4):695-714. [CrossRef]
- Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Oct 7;390(10103):1664-1675. [CrossRef]
- Athauda D, Foltynie T. Protective effects of the GLP-1 mimetic exendin-4 in Parkinson's disease. Neuropharmacology. 2018 Jul 1;136(Pt B):260-270. [CrossRef]
- Bomba M, Granzotto A, Castelli V, Massetti N, Silvestri E, Canzoniero LMT, Cimini A, Sensi SL. Exenatide exerts cognitive effects by modulating the BDNF-TrkB neurotrophic axis in adult mice. Neurobiol Aging. 2018 Apr;64:33-43. [CrossRef]
- Food & Drug Administration (FDA, 2021). FDA Adverse Event Reporting System (FAERS) Public Dashboard. U.S. Food & Drug Administration. 2021. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-eventreporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed on , 2023. 08 April.
- Schifano, F. Coming Off Prescribed Psychotropic Medications: Insights from Their Use as Recreational Drugs. Psychother Psychosom. 2020;89(5):274-282. [CrossRef]
- ICH. ‘MedDRA ® TERM SELECTION : POINTS TO CONSIDER. ICH-Endorsed Guide for MedDRA Users’. London Release 4.21. 21. https://alt.meddra.org/files_acrobat/000571_termselptc_r4_21_mar2021.pdf. Accessed on April 08, 2023. 20 March.
- Ahmed I, Poncet A. PhViD: An R Package for PharmacoVigilance Signal Detection. R Package Version 1.0.8., , 2022. https://cran.r-project.org/web/packages/PhViD/PhViD.pdf. Accessed on April 08, 2023. 12 October.
- Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data Mining Techniques in Pharmacovigilance: Analysis of the Publicly Accessible FDA Adverse Event Reporting System (AERS). In Data Mining Applications in Engineering and Medicine; IntechOpen: London, UK, 2012. [Google Scholar]
- Subeesh V, Maheswari E, Saraswathy GR, Swaroop AM, Minnikanti SSA. Comparative Study of Data Mining Algorithms Used for Signal Detection in FDA AERS Database. J. Young Pharm. 2018, 10, 444–449. [CrossRef]
- Ahmed I, Thiessard F, Miremont-Salam G, et al. Early Detection of Pharmacovigilance Signals with Automated Methods Based on False Discovery Rates: A Comparative Study. Drug Saf. 2012, 35, 495–506. [CrossRef] [PubMed]
- Suling M, Pigeot I. Signal detection and monitoring based on longitudinal healthcare data. Pharmaceutics 2012, 4, 607–640. [CrossRef] [PubMed]
- van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Orre R, Egberts ACG. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [CrossRef] [PubMed]
- Evans SJW, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486 https://Doiorg/101002/pds677. [CrossRef] [PubMed]
- Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RM. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [CrossRef] [PubMed]
- Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002, 25, 381–392 https://Doiorg/102165/00002018. [CrossRef] [PubMed]
| Number of AER (%) | Overall | Semaglutide | Phentermine-Topiramate | Other GLP-1 analogues* |
|---|---|---|---|---|
| Mean Age, years (SD) | 61.0 (19.2) | 60.2 (13.7) | 49.9 (14.7) | 61.4 (20.8) |
| Females | 16559 (53%) | 4470 (54%) | 156 (85%) | 11933 (52%) |
| Males | 12986 (41%) | 3449 (42%) | 22 (12%) | 9515 (41%) |
| Concomitant substances (%) | ||||
| Alcohol | 23 (0.1%) | 2 (0.0%) | 0 (0.0%) | 21 (0.0%) |
| Cannabis | 33 (0.1%) | 13 (0.2%) | 0 (0.0%) | 20 (0.0%) |
| Cocaine | 0 (0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Opioids | 1712 (5.4%) | 249 (3.0%) | 16 (8.7%) | 1447 (8.7%) |
| Amphetamines | 25 (0.1%) | 9 (0.1%) | 1 (0.0%) | 16 (0.1%) |
| Benzodiazepines | 1550 (4.9%) | 238 (2.9%) | 17 (9.3%) | 1295 (5.6%) |
| Country of origin | USA 19664(62.0%) | USA 5016 (71.0%) | USA 173 (95%) | USA 14475 (62.0%) |
| France 1729 (6.0%) | Canada 825 (10.0%) | Korea 9 (5.0%) | France 1449 (6.0%) | |
| Canada 1562 (5.0%) | United Kingdom 360 (4.0%) | Not specified (0.0%) | Japan 1078 (5.0%) |
| SEMAGLUTIDE | PHENTERMINE-TOPIRAMATE | OTHER GLP-1 analogues* | |||
|---|---|---|---|---|---|
| Preferred Term | # AER (%) | Preferred Term | # AER (%) | Preferred Term | # AER (%) |
| Nausea | 1,047 (13%) | Nephrolithiasis | 14 (8%) | Nausea | 1,843 (8%) |
| Vomiting | 921 (11%) | Headache | 11 (6%) | Blood glucose increased | 1,604 (7%) |
| Diarrhoea | 699 (8%) | Weight increased | 10 (5%) | Vomiting | 1,586 (7%) |
| Pancreatitis | 492 (6%) | Angle closure glaucoma | 9 (5%) | Pancreatitis | 1,459 (6%) |
| Off label use | 483 (6%) | Blurred vision | 9 (5%) | Diarrhoea | 1,426 (6%) |
| Weight decreased | 465 (6%) | Suicidal ideation | 8 (4%) | Acute kidney injury | 1,112 (5%) |
| Blood glucose increased | 424 (5%) | Chronic kidney disease | 7 (4%) | Weight decrease | 1,082 (5%) |
| Decreased appetite | 387 (5%) | Hypoesthesia | 7 (4%) | Fatigue | 794 (3%) |
| Fatigue | 357 (4%) | Breast cancer | 6 (3%) | Decreased appetite | 711 (3%) |
| Dehydration | 352 (4%) | Paraesthesia | 6 (3%) | Chronic kidney disease | 689 (3%) |
| Semaglutide | Phentermine-topiramate | Other GLP-1 analogues* | |||
|---|---|---|---|---|---|
| Outcome | # AER (%) | Outcome | # AER (%) | Outcome | # AER (%) |
| Other outcomes | 5418 (66%) | Other outcomes | 154 (84%) | Other outcomes | 14206 (61%) |
| Hospitalized | 3479 (42%) | Hospitalized | 46 (25%) | Hospitalized | 10287 (45%) |
| Life threatening | 306 (4%) | Disabled | 14 (8%) | Died | 1705 (7%) |
| Disabled | 299 (4%) | Life threatening | 3 (2%) | Life threatening | 1103 (5%) |
| Died | 273 (3%) | Died | 1 (1%) | Disabled | 671 (3%) |
| Required intervention | 67 (1%) | Required intervention | 1 (1%) | Required intervention | 76 (<1%) |
| SEMAGLUTIDE VS. OTHER GLP-1 analogues | SEMAGLUTIDE VS. PHENTERMINE-TOPIRAMATE | |||||||
|---|---|---|---|---|---|---|---|---|
| PT (MedDRA) | PRR (FDR) | ROR (FDR) | IC025 (FDR) | EB05 (FDR) | PRR (FDR) | ROR (FDR) | IC025 (FDR) | EB05 (FDR) |
| Accidental overdose | 0.59 (0.60) | 0.59 (0.60) | -1.62 (0.34) | 0.50 (0.41) | Inf (<0.01) | Inf (<0.01) | -1.41 (0.50) | 0.99 (0.52) |
| Drug abuse | 4.05 (<0.01) | 4.05 (<0.01) | -0.63 (0.16) | 0.80 (0.12) | Inf (<0.01) | Inf (<0.01) | -1.74 (0.52) | 0.99 (0.53) |
| Drug level increased | 0.85 (0.46) | 0.85 (0.46) | -1.12 (0.27) | 0.62 (0.29) | Inf (<0.01) | Inf (<0.01) | -1.21 (0.49) | 0.99 (0.52) |
| Drug withdrawal syndrome | 4.05 (<0.01) | 4.05 (<0.01) | -0.63 (0.16) | 0.80 (0.12) | Inf (<0.01) | Inf (<0.01) | -1.74 (0.52) | 0.99 (0.53) |
| Incorrect route of product administration | 0.55 (0.61) | 0.55 (0.61) | -1.65 (0.34) | 0.48 (0.42) | Inf (<0.01) | Inf (<0.01) | -1.34 (0.50) | 0.99 (0.52) |
| Intentional product misuse | 0.42 (0.64) | 0.42 (0.64) | -1.68 (0.35) | 0.40 (0.45) | 0.32 (<0.01) | 0.32 (<0.01) | -1.01 (0.48) | 0.99 (0.53) |
| Intentional product use issue | 1.80 (<0.01) | 1.80 (<0.01) | 0.08 (<0.01) | 1.11 (<0.01) | Inf (<0.01) | Inf (<0.01) | -0.54 (0.41) | 0.99 (0.50) |
| Overdose | 0.92 (0.46) | 0.92 (0.46) | -0.66 (0.17) | 0.72 (0.19) | Inf (<0.01) | Inf (<0.01) | -0.71 (0.44) | 0.99 (0.51) |
| Prescription drug used without a prescription | 3.60 (<0.01) | 3.60 (<0.01) | -0.42 (0.10) | 0.85 (0.08) | Inf (<0.01) | Inf (<0.01) | -1.50 (0.51) | 0.99 (0.53) |
| Substance use | Inf (0.70) | Inf (0.70) | -0.29 (0.06) | 0.91 (0.04) | Inf (0.04) | Inf (0.04) | -1.74 (0.53) | 0.99 (0.53) |
| # Reports with AE of interest | # Reports without AE of interest | |
|---|---|---|
| # Reports with drug of interest | A | b |
| # Reports without drug of interest | C | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





