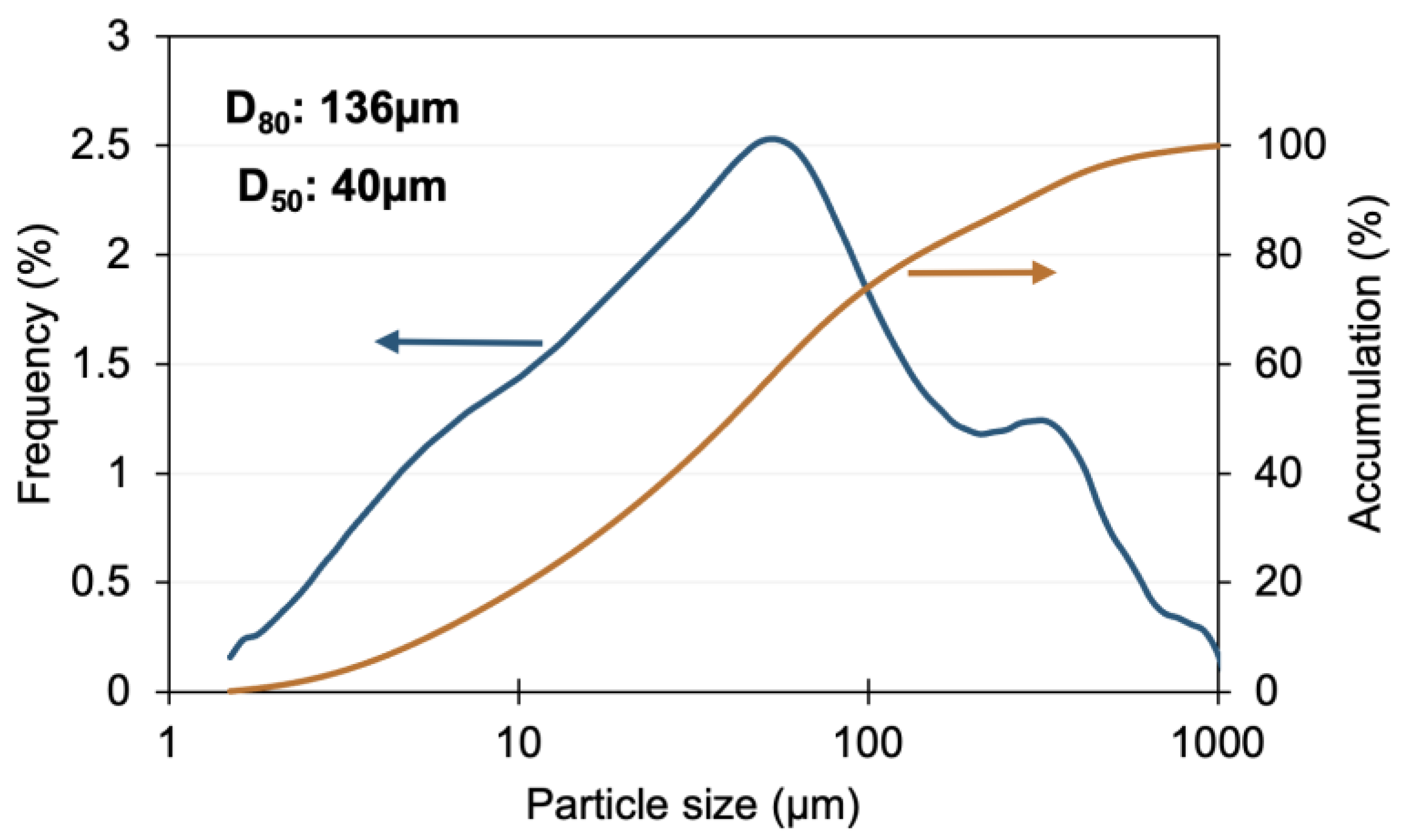
3.1. Leaching of Gold from Flotation Concentrate
Thiosulfate-only leaching of gold in alkaline or neutral is a very slow process in the presence of oxygen (1). Moreover, studies have demonstrated that in the absence of copper-ammonia, thiosulfate decomposes on the surface of gold, forming a sulfur coating that leads to gold passivation [
15,
17,
18,
19,
20,
21]. The effective method for gold dissolution involves the combination of thiosulfate and Cu
2+ ions as an oxidant, with ammonia serving as a stabilizer [
22,
23].
Previous studies on ammonia-copper-thiosulfate leaching have demonstrated varying levels of gold recovery depending on the ore type and leaching conditions. For instance, Jeon et al. (2020) reported a gold extraction efficiency of over 99 % from printed circuit boards using 1 M Na
2S
2O
3, 10 mM CuSO
4, and 1 M ammonium at 24 hours under oxygen presence, while Yener Yazıcı et al. (2011) achieved ~62 % gold extraction from a copper-rich gold ore using 0.5 M S
2O
32-, 25 mM Cu
2+, and 0.5 mM NH
3 under similar leaching conditions. In contrast, this study achieved 88 % gold extraction under optimized conditions of 0.5 M thiosulfate, 1.0 M ammonia, and 0.1 M Cu
2+, at pH 12, with a leaching duration of 2 hours. The significant improvement in gold recovery observed in this study, compared to Yener Yazıcı et al. (2011), can be attributed to the optimized pH conditions and controlled reagent concentrations, which minimized thiosulfate decomposition. Importantly, our results align with findings by Oraby et al. 2014, who demonstrated that silver content significantly enhances gold dissolution in thiosulfate solutions, with dissolution rates for gold increasing in the presence of higher silver contents [
24]. However, the slightly lower recovery compared to Jeon et al. (2020) suggests that electronic waste materials may have different leaching efficiencies due to the absence of certain sulfide minerals that contribute to reagent consumption in natural ores.
The comparisons highlight that while thiosulfate leaching provides a viable alternative to cyanide-based extraction, the efficiency of the system is highly dependent on process optimization. The integration of various additives in this study further reduces reagent consumption, making the process both environmentally and economically sustainable.
Initially, the cupric-tetraamonia complex is formed during the preparation of the lixiviant (2), followed by continuous electrochemical reactions as shown in the Equations (3-7).
At the anode, gold is oxidized to form
ions. These ions then react with ammonia to form a gold-ammonia complex,
, which subsequently reacts with thiosulfate to produce a stable gold-thiosuflate complex,
, while releasing ammonia back into solution. At the cathode, the cupric-ammonia complex,
, reacts with thiosulfate and gains an electron to form a copper-thiosulfate complex,
, while releasing ammonia. The copper-thiosulfate complex is then regenerated into the cupric-ammonia complex in the presence of oxygen, water, and ammonia, producing hydroxide ions and additional thiosulfate. The overall dissolution reaction for gold in copper-ammonia-thiosulfate solutions can be expressed as reaction (8):
To increase gold extraction, optimum operating conditions for leach control variables such as temperature, pH, pulp density, stirring rate, and time were individually tested. Since too much or too little of each reagent can either hinder or enhance the gold leaching process, it was therefore paramount to experimentally determine the optimum dosage for each reagent.
3.1.1. Effect of Thiosulfate on Metals Extraction
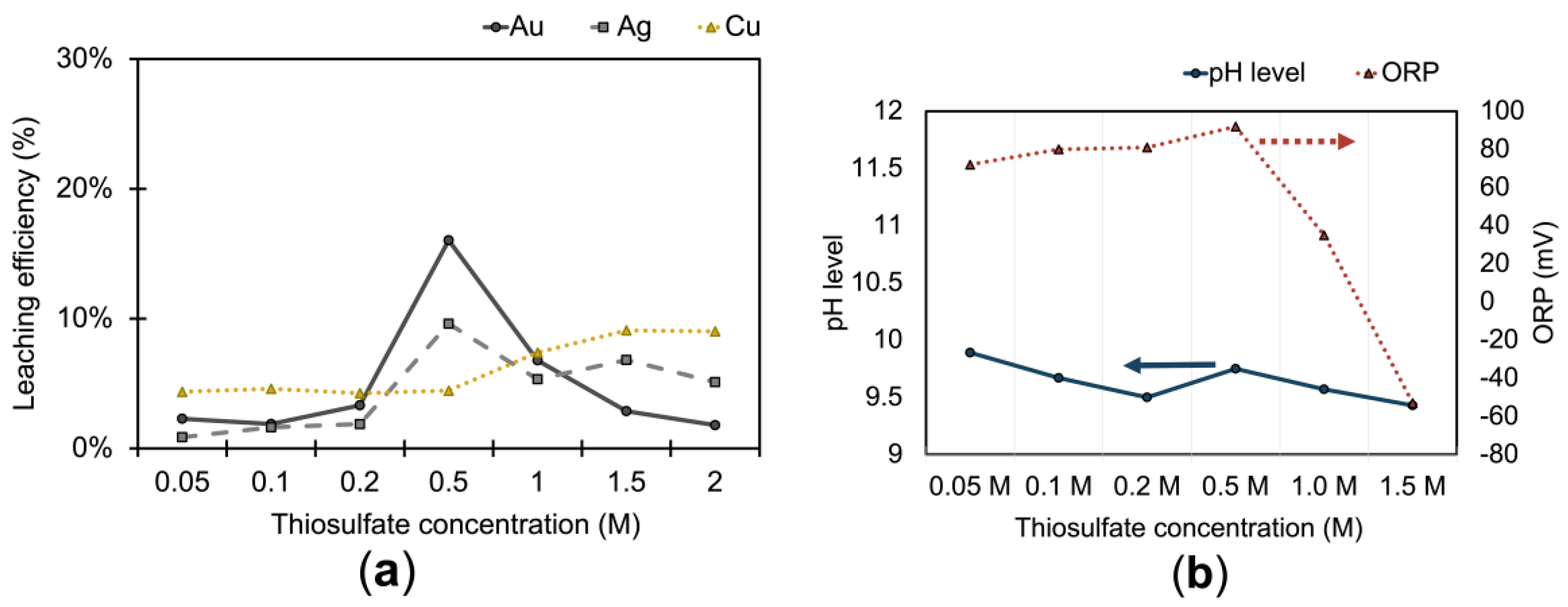
The effect of thiosulfate on leaching is shown in
Figure 4(a). Leaching experiments were conducted within a concentration range of 0.05 M to 2 M, while maintaining constant parameters: 0.1 M Cu
2+, 0.05 M NH
3, a stirring rate of 400 rpm, a pulp density of 20 %, a leaching time of 1 hour, a temperature of 25 ˚C, and a pH of 10. The efficiency of leaching for all target metals was below one-fifth. The gold and silver extraction increased with an increase in thiosulfate concentration of 0.5 M, then decreased with a further increase in thiosulfate concentration. Conversely, the leaching of copper exhibited a slight increase with the rising thiosulfate concentration, stabilizing at less than 10 % at a thiosulfate concentration of 2 M. At a concentration higher than 0.5 M, Au and Ag dissolution is comparatively reduced, possibly due to the formation of undesirable products such as sulfite
) and dithionate
ions. This is supported by the Eh-pH diagram for the S-H
2O system, which indicates that these species are thermodinamically stable within the corresponding pH and redox potential (
Figure 4(b)) regions [
15].
Moreover, high concentrations of thiosulfate tend to stabilize copper, widen the stability region for Cu(S
2O
3)
3- instead of Cu(NH
3)
42+, and even precipitate Cu
2+ thus hindering the role of copper as an oxidant and restraining gold dissolution [
22,
25]. The relatively higher concentration of thiosulfate within the shorter leaching time used in this study also aligns with trends observed in similar systems [
22,
26,
27,
28], where the concentrate used in this study, 0.5 M thiosulfate was ideal for gold dissolution in the conditions used.
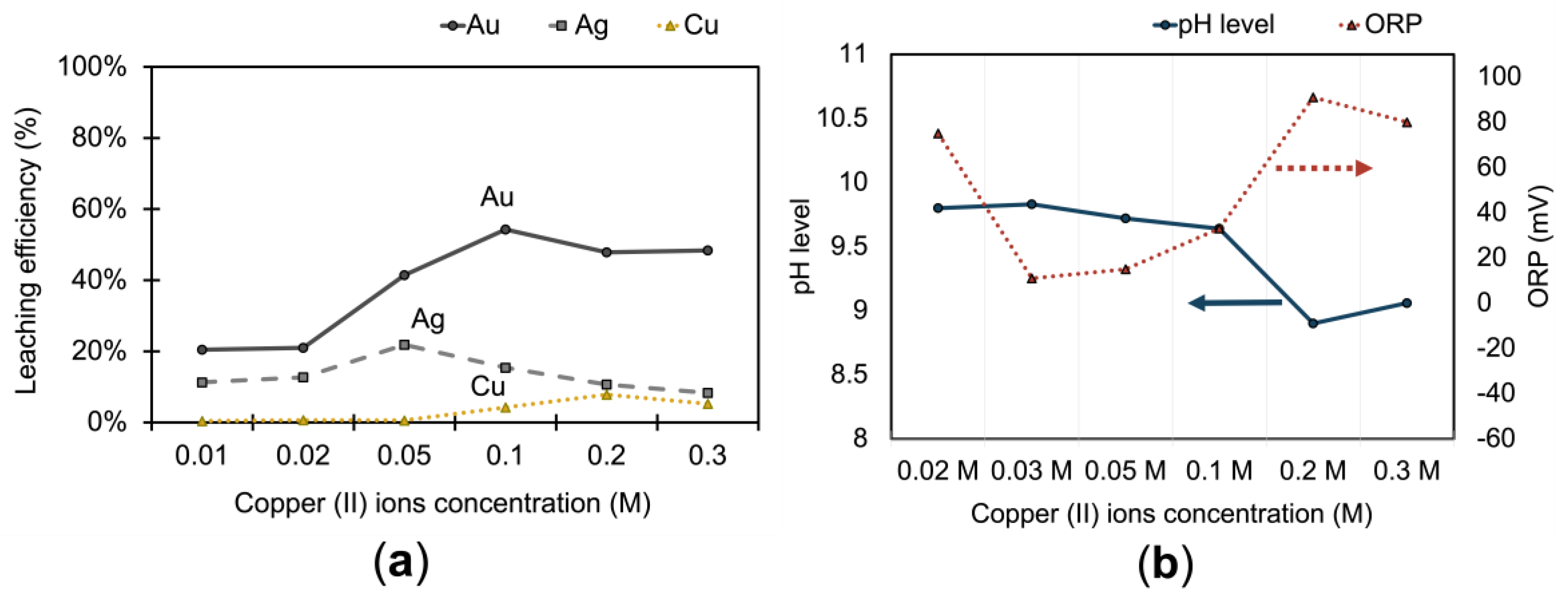
3.1.2. Effect of Copper (II) Ions on Metals Extraction
Leaching experiments to determine the optimum copper ion concentration were conducted under the following conditions: 0.5 M
0.05 M NH
3, a stirring rate of 400 rpm, a pulp density of 20 %, a leaching time of 1 hour, a temperature of 25 ˚C, and a pH of 10. Gold dissolution in thiosulfate solution in the presence of air is very slow but copper (II) has been reported as a better oxidant to dissolve gold at a faster rate by 10-fold [
14]. However, not only gold but also silver and copper are affected by the presence of copper (II) ions. As shown in the results in
Figure 5(a), the leaching efficiency of gold increases with the concentration of Cu(II) up to 0.1 M and then slightly decreases, which is a trend similarly observed for silver and copper. The leaching efficiency for silver shows significant improvement at a Cu(II) concentration of 0.2 M, reaching a peak, but then slightly diminishes as the concentration of copper increases further. Copper extraction is quite low at smaller concentrations of Cu(II), but sees a substantial increase at 0.1 M, following which it again decreases, suggesting an optimal Cu(II) concentration for the leaching process of these metals.
This pattern for all three metals—gold, silver, and copper— is due to an increase in copper concentration, which narrows the region of stability for Cu(NH
3)
42+ and Cu(S
2O
3)
5- and expands the stability region for CuO, Cu
2O, and Cu
2S [
15,
26]. Additionally, as Cu (II) ions oxidizing properties is high, in higher concentration can lead to degradation of
into some by-products, such as tetrathionate
, trithionate
, sulfate
, cyclo-
and copper sulfide Cu
2S (Eqs. 9-13).
High concentrations of copper (II) may therefore not only hinder the extraction of gold but also the leaching efficiency of silver and copper due to the formation of these precipitates [
19,
23,
26,
29].
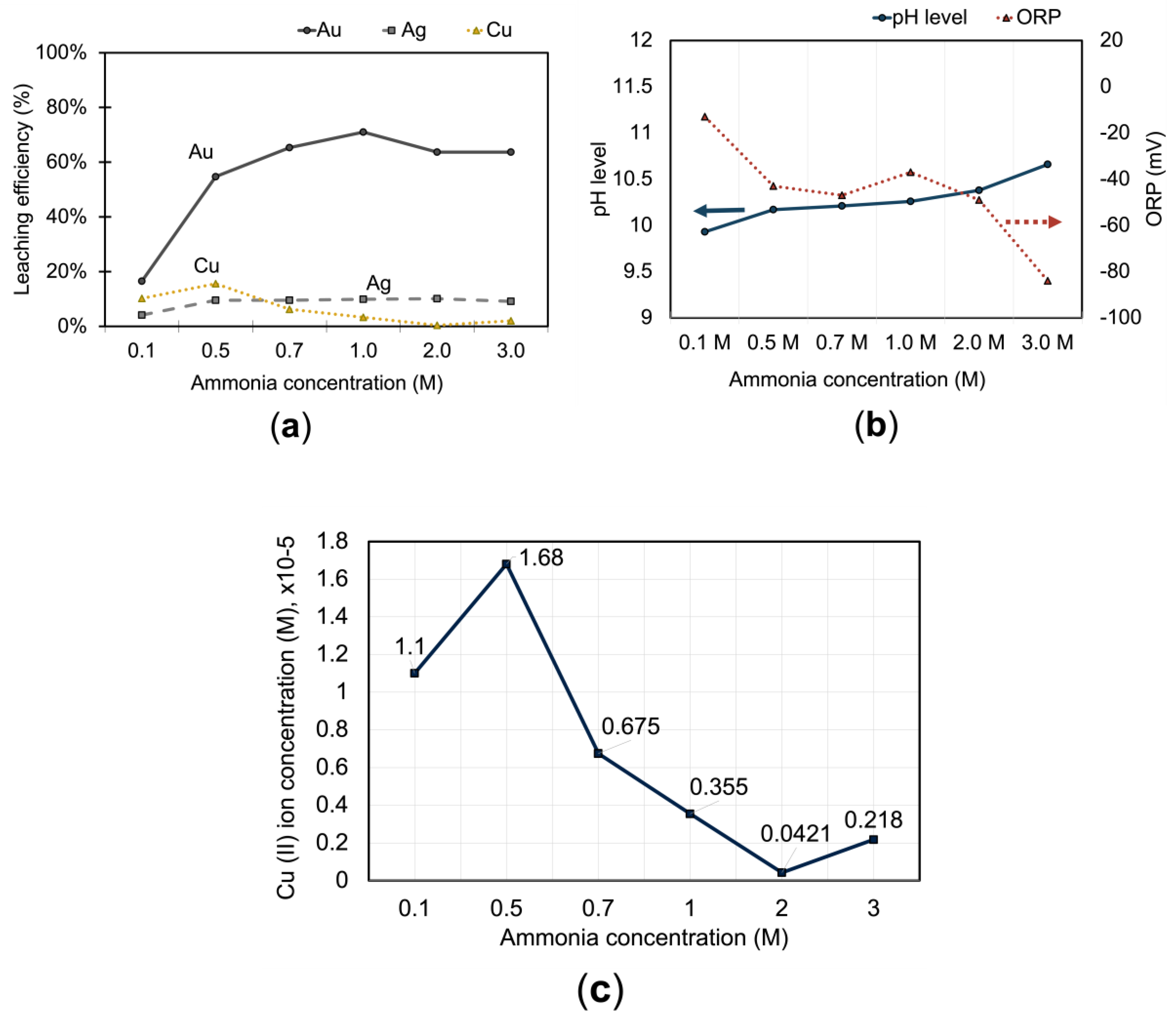
3.1.3. Effect of Ammonia on Metals Extraction
The use of ammonia in the copper-thiosulfate leaching system serves several purposes: it minimizes the decomposition of thiosulfate into polythionates [
30], stabilizes the copper (II) complexes [
31], helps to maintain a stable pH, and improves the leaching kinetics [
25]. The impact of ammonia on the dissolution of gold, silver, and copper was examined by varying the concentrations of ammonia from 0.1 M to 3 M, while keeping other parameters constant: 0.5 M
0.1 M Cu
2+, a stirring rate of 400 rpm, a pulp density of 20 %, a leaching time of 1 hour, a temperature of 25 ˚C, and a pH of 10. For gold, leaching efficiency improved progressively with rising ammonia concentration up to 1.0 M, achieving its highest observed recovery, followed by a slight decline. This aligns with prior studies [
13,
28], where moderate ammonia levels enhance gold dissolution by stabilizing the Au(S₂O₃)₂³⁻ complex.
However, the extraction rates for all three metals—gold, silver, and copper—experienced a decline at higher ammonia concentrations (more than 1.0 M). The concentration of Cu (II) ions in the solution, representative of the oxidizing agent (Cu(NH
3)
42+ ) for gold, started to decrease from 1.68 × 10
-5 M to 2.18 × 10
-6 M with increasing ammonia concentrations (
Figure 6c). This decline could be attributed to increased pH level as well which reduces the thermodynamic stability of Cu(NH
3)
42+ and Cu(S
2O
3)
3- while expanding the stability regions of solid copper species such as Cu
2S, CuO and Cu
2O [
15,
26]. Such precipitation would hinder the catalytic effect of copper on the leaching process for gold and also affect the leaching efficiencies of silver and copper. This indicates that there is an optimal ammonia concentration above which the benefits of stabilization give way to negative effects due to the over-stabilization and subsequent precipitation of copper.
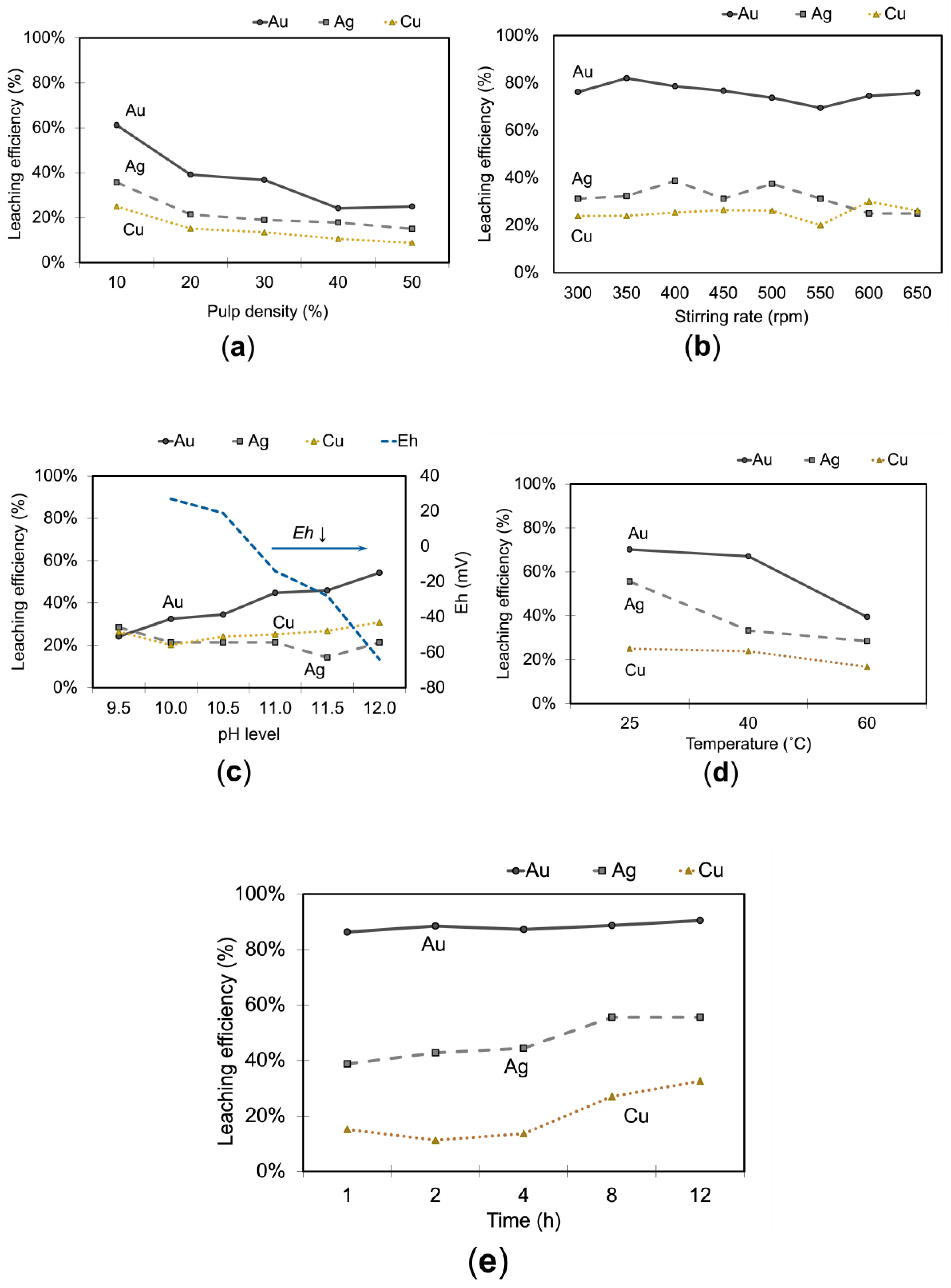
3.1.4. Effect of Solid-to-liquid Ratio on Metals Extraction
Solid-liquid ratio (w/v) ranging from 10 – 50 % was investigated. The results in
Figure 7(a) showed higher extraction at low pulp density due to easy exposure of the solids to the leach solution. Gold dissolution for dense slurry suffers partly due to hindered contact between leach reagents and gold surface because of particle mass transfer limitations. In addition, the concentration of reagents may have been insufficient for higher pulp densities [
19]. Silver and copper also showed higher extraction rates at this low pulp density, with 36 % and 25 % respectively.
3.1.5. Effect of Stirring Rate on Metals Extraction
Stirring is essential for agitating and mixing solids with the leach solution, aerating the mixture, and intensifying the leaching reaction.
Figure 7(b) presents the outcomes of metal extraction under various stirring speeds. In general, leaching efficiency decreased with higher stirring rates because excessive swirling of the slurry led to excessive oxygen dissolution in the solution. This behavior differs from cyanide leaching systems, which require air or oxygen. Furthermore, high stirring rates exacerbate undesirable side effects, such as the simultaneous decomposition of thiosulfate and the formation of Cu(I) ions [
32]. Based on
Figure 7(b), an optimal stirring rate of 350 rpm was determined.
3.1.6. Effect of pH Level on Metals Extraction
A pH above 9 is commonly maintained in this system because thiosulfate tends to decompose quickly at pH levels below 9. In this study, a range of pH levels between 9.5 and 12.0 were tested individually to determine the optimum condition for leaching the concentrate. Contrary to previous reports suggesting a stable pH of 10 is necessary [
25], our current results indicate that a pH of 12 is more favorable, resulting in higher gold dissolution rates as seen in
Figure 7(c). The favorable outcome at a pH of 12 can be attributed to the availability of a broader copper ammine complex region at high reagent concentrations, making this condition particularly advantageous for the process [
15]. Silver and copper dissolve differently depending on pH. Silver recovery varies unpredictably, peaking at pH 9.5 but dropping at mid-range values. In contrast, copper dissolution steadily increases with pH, reaching its highest level at pH 12.0. This difference suggests that copper benefits from stable ammonia complexes at higher pH, while silver is affected by a sharp drop in redox potential (Eh) at pH 11.0 or higher, reducing its ability to dissolve. These findings highlight copper’s resistance to alkaline conditions compared to silver’s sensitivity to redox changes.
3.1.7. Effect of Temperature on Metals Extraction
In general, leaching efficiency increases with higher temperatures [
27,
33,
34]. To verify whether this behavior holds the same in our system, the effects of temperature on thiosulfate leaching were investigated. The results on
Figure 7(d) showed that [
29] at high-temperature gold dissolution declines rapidly because the solution becomes unstable due to the (a) loss of ammonia (i.e., evaporation) and (b) decomposition of thiosulfate (Eqs. 14 and 15 [
23,
26,
27,
35], while the formation of cupric sulfide escalates (Eq. 16) [
26]. Silver and copper follow a similar trend, with their highest dissolution rates at 25 °C (55.56 % for silver and 25.04 % for copper) and a noticeable decline as the temperature increases. Although Sitando et al. and Bae et al. have reported high gold dissolution at high temperatures (60 °C) [
23,
26,
27], it could be surmised from Abbruzzese et al. that such a result is possible for a short time when high dosages of reagents are used [
26].
3.1.8. Effect of Time on Metals Extraction
Figure 7(e) presents the results for leaching over 12 hours. Rapid gold dissolution occurred in the first hour wherein 80 % of gold was extracted, then increased by 10 % in the second hour and plateaued at 90 %. Bas et al. reported a similar trend but with a lower gold extraction of 70 % [
31]. Extending the time to 12 hours of leaching only increased both silver and copper extraction increased by 15 %. Leaching beyond 2 hours would be uneconomical since it only encourages Cu dissolution, which is detrimental to the downstream Au recovery process. A leaching time of 2 hours was deemed ideal for the concentrate used in the present study.
The increase in gold leaching to over 80% was due to the synergistic effects of optimized reagent concentrations and controlled physicochemical parameters, which enhanced the stability and reactivity of the gold-thiosulfate complex, thereby improving the kinetics of the gold dissolution process. The optimized leaching condition was: 0.5 M S2O32-, 1.0 M NH3, 0.1M Cu2+, 350 rpm, pH 12, 10 % solids, 25 ˚C and 2 hours. Under the optimized condition, extractions for gold, silver, and copper were 88 %, 43 %, and 11 % respectively.
3.2. Effects of Additives on Thiosulfate Decomposition
To further refine and enhance the leaching process, additives were introduced in subsequent tests. The primary objectives of incorporating additives are: (1) to reduce the decomposition of thiosulfate, thereby preserving its concentration and effectiveness throughout the leaching process, and (2) to increase the leaching efficiency, particularly for gold, but potentially also benefiting the extraction of silver and copper. The precise impact of the additives, whether in improving the overall extraction yields or in facilitating more selective leaching of gold, is a focal point of the ensuing evaluations and analyses.
The chosen concentration of 0.1 M additives in this study was selected to be equal to the copper ion concentration (0.1 M Cu2+) to maintain a balanced chemical environment and ensure effective interactions between copper and the stabilizing additives. This approach aimed to optimize gold dissolution while preventing excessive reagent consumption and maintaining solution stability.
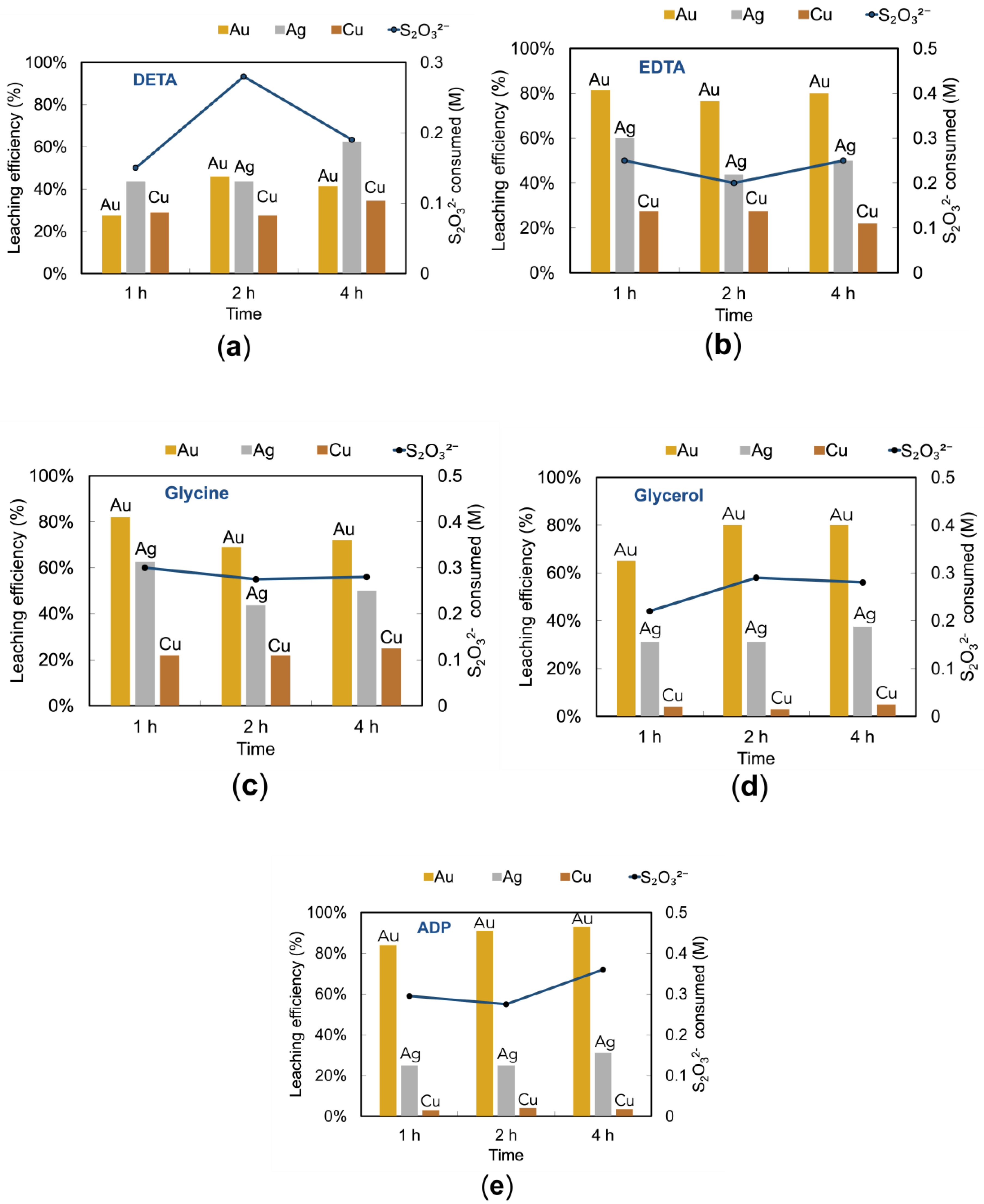
DETA is known for its strong chelating properties, which can help in stabilizing copper ions in solution, thereby preventing unwanted side reactions that consume thiosulfate. Additionally, its ability to form stable complexes with transition metals enhances the overall selectivity of the leaching process, reducing the consumption of thiosulfate and improving the efficiency of gold extraction.
Figure 8 (a) shows the results for metal extraction and thiosulfate consumption as a function of time when DETA was added. Gold extraction initially increased, followed by a subsequent reduction. Silver and copper extraction gradually increased overall. Thiosulfate consumption increased initially and then decreased, which may reflect the changing stability of copper-ammonia complexes over time.
The observed fluctuations in gold extraction could be linked to the role of DETA in altering the surface chemistry of pyrite and other sulfide minerals [
36]. Additionally, while DETA’s ability to form stable complexes with copper ions improves the leaching process and initially increases gold extraction, it may also hinder gold leaching over time. This is due to the ability of Cu (II) catalytic oxidation is inhibited gradually, because some or all of the
will be converted to Cu (II)-DETA. This explanation aligns with the initial increase and then a decline in gold extractions over time. Furthermore, DETA’s impact on the leaching environment may extend to the stability of thiosulfate itself, which is crucial for gold dissolution.
EDTA is a powerful chelating agent that effectively binds to metal ions, reducing their reactivity and preventing precipitation or unwanted side reactions. Its ability to stabilize Cu(II) ions is particularly advantageous in thiosulfate leaching systems, as it helps maintain the concentration of active thiosulfate species, reducing overall decomposition. Moreover, EDTA’s formation of stable metal complexes can enhance the selectivity of the leaching process, particularly benefiting the extraction of precious metals like gold and silver.
Figure 8(b) shows the effects of EDTA additive on Au, Ag, and Cu extraction with its thiosulfate consumption. As shown in the figure, approximately 80 % of Au, 50 – 60 % of Ag, and 20 – 30 % of Cu leaching efficiency were obtained with half of the thiosulfate (0.25 M) decomposition rate. In comparison to DETA system, the results showed that higher extractions were obtained, and this can be attributed to following reasons: EDTA can stabilize copper (II), which plays an important role in thiosulfate leaching system, prevent reaction with sulfide minerals [
37], and favorably form complexes with metals including Cu or lead (Pb) facilitating the high dissolution of precious metal [
18,
38]. The stability constants of
and Cu (II)-EDTA are 4.35 and 12.28, respectively, indicating that EDTA stabilizes the cupric ion significantly more than ammonia. This stronger stabilization by EDTA reduces the availability of free Cu (II) ions to form
complexes, thereby influencing the overall concentration of
in the system. The preference for Cu (II)-EDTA complex formation ensures that the Cu (II) species remain more stable and active within the leaching system, further enhancing the efficiency of gold dissolution through the reactions described (Eqs. 17, 18, 19 and 20). However, it is important to consider that the high dissolution of copper, facilitated by EDTA, may not always favor downstream gold recovery, as it could complicate the subsequent separation process.
Glycine, an amino acid, serves as an effective complexing agent that stabilizes metal ions in solution, reducing the formation of unwanted precipitates. Its role in forming stable gold-glycine complexes ensures a higher initial rate of gold extraction. Furthermore, glycine helps to maintain the pH and prevent the decomposition of thiosulfate by minimizing side reactions. This dual functionality not only enhances gold leaching efficiency but also promotes a more sustainable use of thiosulfate.
The effect of glycine
Figure 8 (c) revealed significant insights into the leaching behavior and thiosulfate consumption over time. In the initial hour, the gold dissolution reached approximately 80 %, indicating a rapid leaching process. However, a notable decrease in gold leaching efficiency was observed over the subsequent hours. The initial gold dissolution rate can be attributed to the formation of a stable gold-thiosulfate complex, facilitated by the presence of glycine. Glycine acts as a stabilizing agent for the gold complex, forming a more stable gold-glycine complex as indicated by the reaction mechanism (Eqs. 21, 22) described in the study by Godigamuwa et al. 2024:
In the glycine-thiosulfate system, the initial stage involves the rapid formation of the gold-thiosulfate complex, which is stabilized by glycine, converting it to a more stable gold-glycine complex:
The decrease in gold leaching efficiency observed after the initial hour could be due to the partial decomposition or instability of the gold-glycine complex under prolonged exposure. This phenomenon aligns with the findings of Godigamuwa et al. 2024, where the stability of the leached gold was enhanced initially but showed fluctuations over time due to the dynamic nature of the complex formation and dissolution processes [
39].
Glycine functions like NH
3 by stabilizing Cu(II) and oxidizing gold [
35,
40], hence gold extraction also showed about 80 % at 1 h of leaching time together with Ag (63 %) and Cu extractions (20 %). Oraby and Eksteen reported on glycine as a lixiviant for gold [
35]. Their work revealed that a higher concentration of glycine could enhance the gold dissolution, but the kinetics can be very slow. Above pH 12 and in the presence of glycine, copper forms passivating layers of tenorite (CuO) and cuprite (Cu
2O) [
40], consequently hindering gold dissolution.
This study also noted a decrease in thiosulfate consumption from 0.3 M to 0.28 M over the period. This reduction suggests that glycine not only aids in the stabilization of the gold complex but also improves the efficiency of thiosulfate usage. Glycine’s presence likely reduces the formation of side reactions that consume thiosulfate, thereby optimizing the leaching process.
Glycerol, a trihydroxy alcohol, acts as an effective stabilizing agent in leaching systems due to its ability to form hydrogen bonds, which can help maintain the integrity of thiosulfate in solution. Its use in minimizing thiosulfate decomposition is critical, as it reduces the need for replenishment of the leaching agent, thereby cutting operational costs. Moreover, glycerol’s properties as an emulsifier and stabilizer can prevent the formation of precipitates, ensuring a more efficient leaching process.
Glycerol, sometimes known as glycerin/glycerine or 1,2,3-propanetriol, is an alcohol that has three hydroxyl (OH) groups. Glycerol remains stable though can be reacted as an alcohol via the three (3) hydroxyl groups in its structure. Among its many properties, glycerol is an emulsifier and has been used in many everyday products such as food and ointments [
41]. However, the use of glycerol in the processing of ores/concentrates is rare. The reagent was explored for its potential in cyanide-based gold leaching process to enhance extraction efficiency, stabilize pH, and mitigate environmental impact. Its application however has not been investigated in thiosulfate systems, making it a novel topic addressed for the first time. In this present study, three (3) features have been discovered by using glycerol as an additive. As shown in
Figure 8 (d), the presence of glycerol helps to minimize thiosulfate decomposition, hinders copper dissolution, and enhances Au and Ag dissolution. The results showed that about 80 % of Au, 31 % of Ag, and less than 2 % of Cu was extracted from the ores in 2 hrs.
The incorporation of ammonium dihydrogen phosphate (ADP) into the thiosulfate leaching system has demonstrated significant improvements in both leaching efficiency and chemical stability. ADP, known for its buffering capacity, plays a crucial role in maintaining pH stability, which is essential for optimizing the thiosulfate-based gold extraction process. This stabilization effect is particularly important in preventing the rapid decomposition of thiosulfate, especially at lower pH levels, thereby reducing overall reagent consumption.
As illustrated in
Figure 8 (e), gold leaching efficiency remains high over time, with 93 % recovery after 4 hours, accompanied by a 31 % increase in silver dissolution and suppressing copper dissolution, while thiosulfate consumption stays relatively stable. This consistency underscores ADP’s role in sustaining an optimal leaching environment, minimizing the need for frequent reagent replenishment. Further analysis in
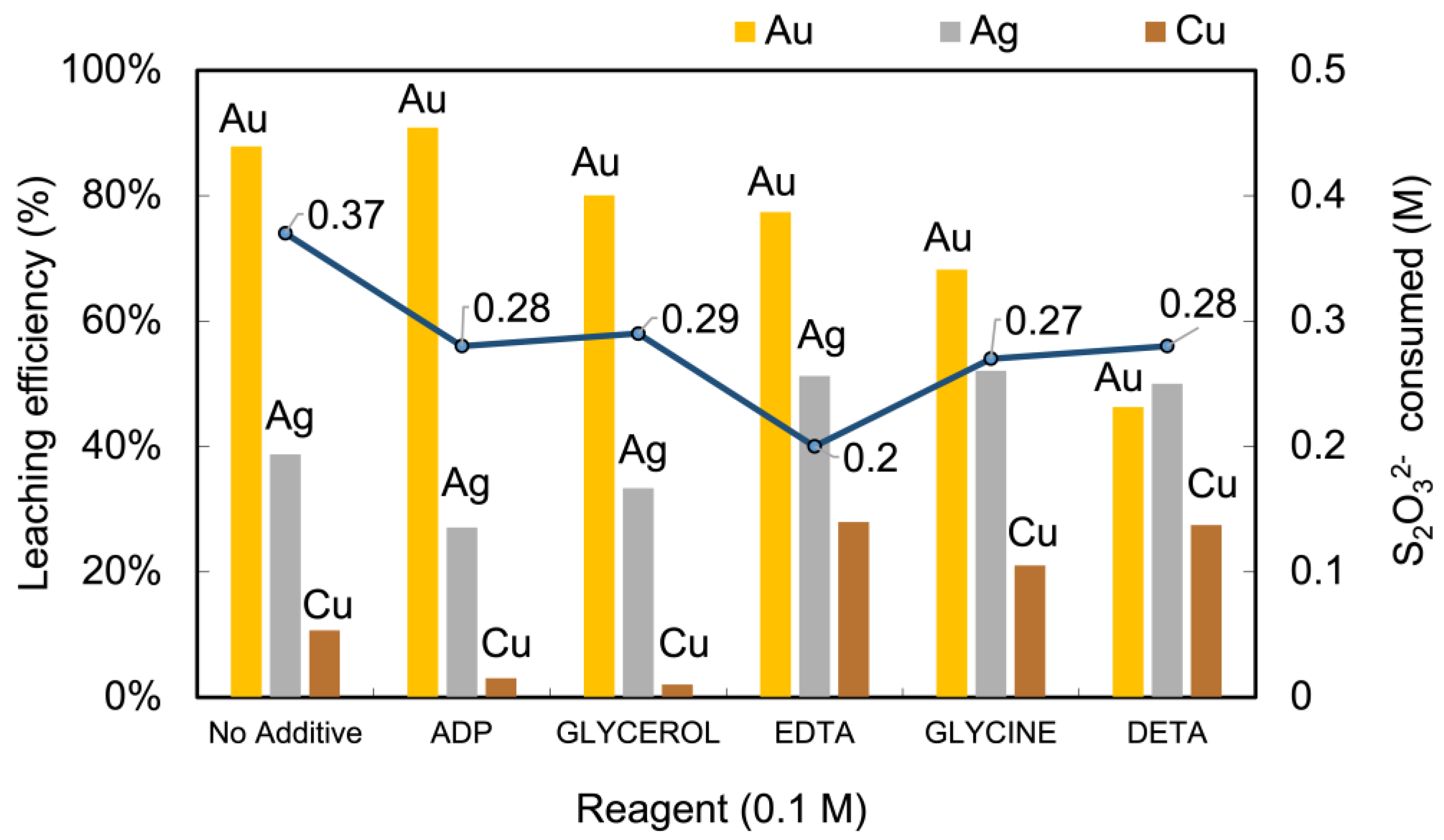
Figure 9 shows that ADP increases gold (Au) leaching efficiency to approximately 91 %, compared to 88 % without additives, while also reducing thiosulfate consumption from 0.37 M to 0.28 M. These improvements highlight ADP’s effectiveness in making the process both cost-effective and environmentally friendly.
Chemically, ADP dissociates in water to release phosphate ions (H
2PO
4-) and ammonia (NH
4+). The phosphate ions interact with copper ions (Cu
2+), stabilizing them and preventing excessive oxidation and precipitation, which is crucial for the catalytic role of copper in the thiosulfate leaching of gold. Moreover, these phosphate ions help to de-passivate the gold surface, thereby inhibiting the formation of detrimental species such as tetrathionates and polythionates that could otherwise hinder the leaching process [
18,
42]. The ammonium ions further contribute to the stability of the leaching solution by forming stable ammonium-thiosulfate complexes, reducing the rate of thiosulfate decomposition.
In conclusion, the addition of ADP creates a controlled and stable environment in the thiosulfate leaching system, enhancing gold extraction efficiency while significantly reducing thiosulfate consumption. These characteristics highlight ADP’s potential as a key additive for promoting sustainable and economically viable gold extraction, with implications for broader applications in other hydrometallurgical processes.
The summarized results using various additives (i.e., diethylenetriamine (DETA), glycine, ethylenediaminetetraacetic acid (EDTA), glycerol, and ammonia dihydrogen phosphate (ADP) are shown in
Figure 9. The significant effect of the additives was the reduction in the amount of S
2O
32- consumed. In the absence of the additives, the S
2O
32- consumption was 0.37 M (90 %), but in the presence of additives, the decomposition rate decreased to 0.2 – 0.3 M ( 40 – 50 %).
DETA, Glycine, and EDTA enhanced copper dissolution by stabilizing the cupric ion and reducing foreign ion interference, but lowered gold dissolution at excessive dosages due to the formation of more stable complexes that hinder the leaching process. Under carefully controlled conditions, EDTA, glycine, and DETA have the potential to leach gold, silver, and copper. Moreover, the presence of EDTA can decrease the formation of the S layers generated from the thiosulfate decomposition [
43]. The results showed that thiosulfate decomposition also decreased from about 0.4 M to 0.2 M. Some studies have shown the potential of glycine as a complexing agent (Eqs. 12 and 13) [
39,
44].
Unfortunately, as a complexing agent in an alkaline environment glycine, enables the effective leaching of copper, but yields a decreased leaching rate of gold under specific conditions such as higher pH levels. However, the use of glycine has made it possible to adsorb gold and silver glycinate complexes onto activated carbon effectively, facilitating the recovery process of these metals [
45,
46].
The results of ADP addition were quite similar to those in the absence of additive, but a thiosulfate decomposition rate was much lower [
43], implying that, glycerol is a potential reagent for application in processes where copper suppression is required.
The effects of various additives, such as diethylenetriamine (DETA), glycine, ethylenediaminetetraacetic acid (EDTA), glycerol, and ammonia dihydrogen phosphate (ADP), were examined. The additives were found to reduce the consumption of thiosulfate, resulting in increased gold leaching. However, DETA, glycine, and EDTA enhanced copper dissolution while lowering gold dissolution. Glycerol showed potential for copper suppression but caused a slight drop in gold extraction. EDTA was found to stabilize copper (II) and prevent its reaction with sulfide minerals. Glycerol was identified as a potential additive for ore processing, minimizing thiosulfate depletion, hindering copper dissolution, and promoting silver dissolution. ADP improved gold extraction and silver dissolution while suppressing copper, highlighting its potential applicability in optimizing precious metal recovery processes.
3.3. Kinetic Analysis of Gold Leaching
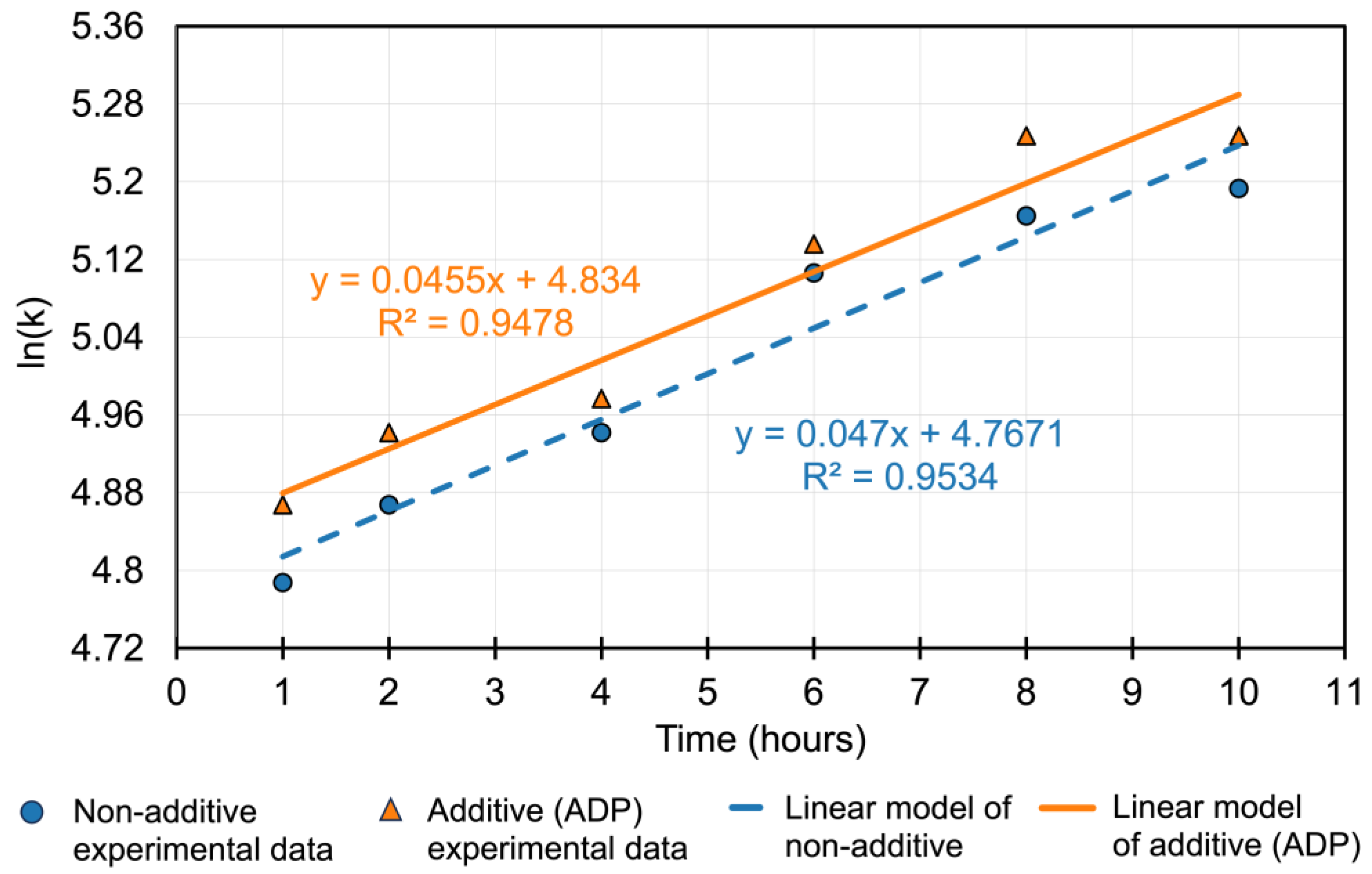
The incorporation of ammonium dihydrogen phosphate (ADP) into the thiosulfate leaching system significantly influences both the leaching kinetics and the solution chemistry, improving gold extraction while reducing reagent degradation. To further understand the leaching behavior, kinetic modeling was conducted using a pseudo-first-order kinetic approach, where ln(Cₜ) was plotted against time (t) to derive reaction rate constants (k) from the slope of the trendlines. The kinetic equations derived from experimental data (
Figure 10) are:
Here, y represents ln(Cₜ), x is time in hours, and the slope corresponds to the rate constant k. These results confirm a significantly enhanced reaction rate in the presence of ADP (k = 0.0455 hr⁻¹) compared to the non-additive system (k = 0.047 hr⁻¹), indicating improved gold dissolution efficiency due to thiosulfate stabilization.
This modeling supports the trend observed in the original kinetic data (
Figure 10), where a separate analysis showed that the linear regression for the additive system (R² = 0.9478) exhibited a steeper slope (0.0455) than the non-additive system (0.047, R² = 0.9534), both evaluated as ln(k) versus time. Both modeling approaches consistently demonstrate that the presence of ADP accelerates gold leaching kinetics. This improvement aligns with previous findings that orthophosphate compounds promote gold dissolution by stabilizing thiosulfate against oxidative degradation through copper(II) complexation [
18].
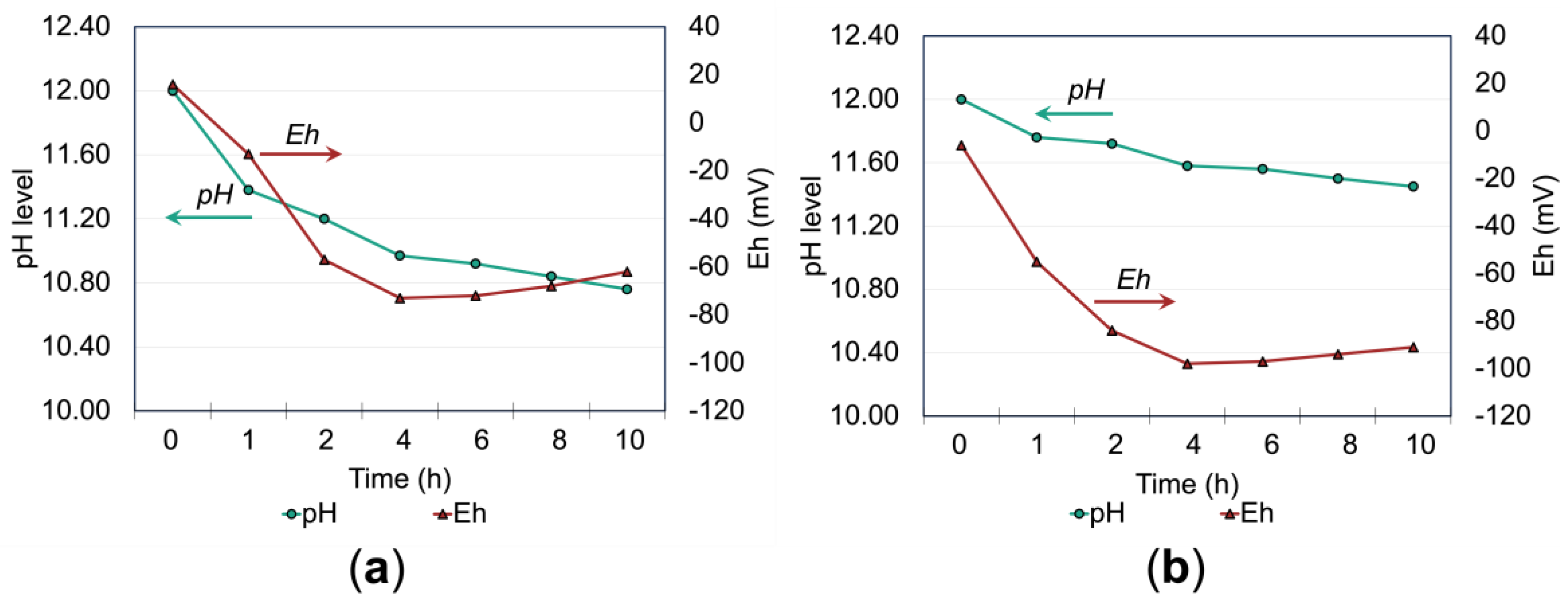
Figure 11a,b further highlight the buffering role of ADP in maintaining more stable pH and redox (Eh) conditions during the leaching process. In the non-additive system (
Figure 11a), pH declines rapidly within the first 4 hours, accompanied by a sharp drop in Eh, suggesting increased thiosulfate decomposition due to acidification. In contrast, the ADP-stabilized system (
Figure 11b) maintains a more gradual and stable pH decline with a correspondingly less consistent Eh behavior. This buffering effect minimizes conditions favorable for thiosulfate degradation and the formation of detrimental species such as tetrathionates and polythionates, which can passivate the gold surface and hinder leaching [
42].
The dissociation of ADP releases phosphate ions (H
2PO
4-) and ammonium (NH
4+), both of which contribute to solution stability. Phosphate ions complex with Cu
2+ ions, stabilizing them in solution and preventing their precipitation as copper hydroxides or phosphates. This stabilization ensures the continued catalytic role of copper without excessive consumption, a behavior similarly observed with orthophosphate and hexametaphosphate additives [
18]. Moreover, these phosphate species reduce the interaction between thiosulfate and sulfide minerals, as seen in sulfide ore systems where orthophosphate enhances leaching kinetics and suppresses side reactions [
18].
The low copper leaching efficiency in the ADP-enhanced thiosulfate system is primarily due to the formation of stable copper-phosphate complexes, which reduce free Cu
2+ availability and limit its dissolution. At higher ADP concentrations, phosphate ions precipitate copper as colloidal copper (II) phosphate species [
42], removing it from solution and decreasing its overall mobility. This behavior, consistent with the effects observed for orthophosphate and hexametaphosphate, diminishes copper’s catalytic role in thiosulfate oxidation and contributes to reduced copper leaching while stabilizing thiosulfate and enhancing precious metal selectivity.