Submitted:
11 April 2025
Posted:
14 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
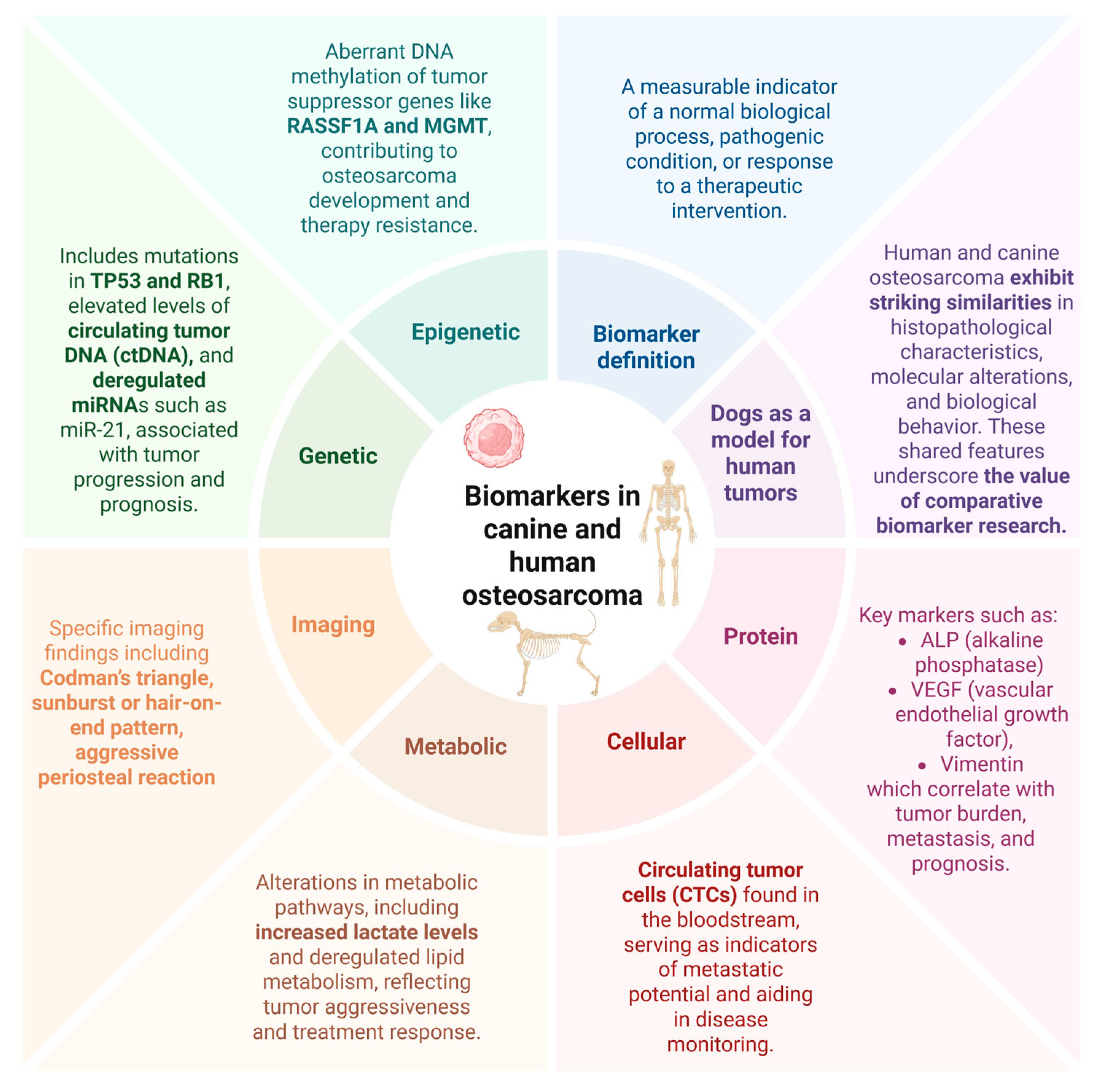
2. Biomarker: A Concise Definition
3. Osteosarcoma: Shared Features Between Humans and Canines
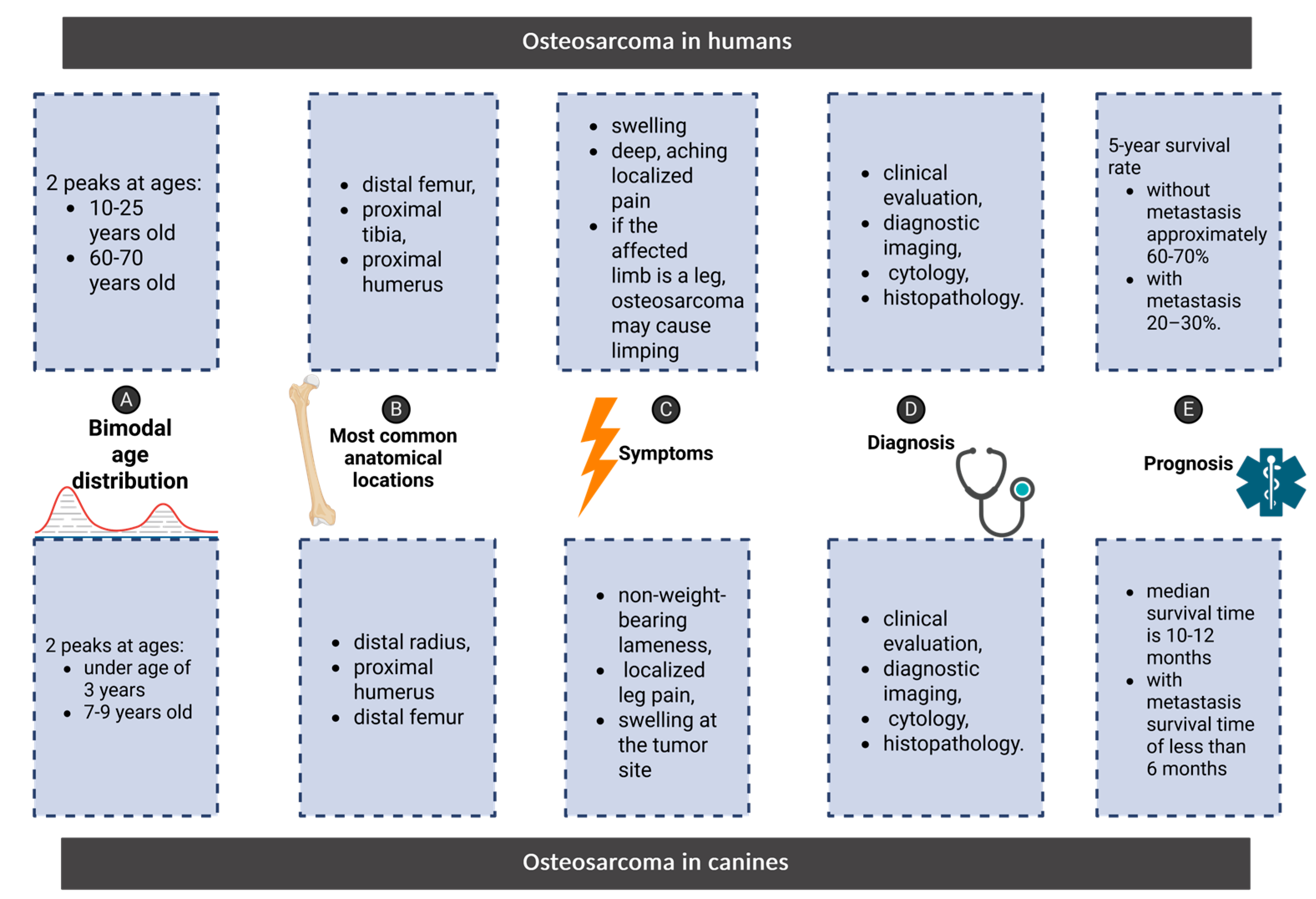
4. Parallels in Osteosarcoma: Comparing Clinical Features, Diagnosis, and Prognosis in Humans and Canines
Symptoms
Diagnosis
Prognosis
Biomarkers in Osteosarcoma: Translational Indicators of Disease and Therapeutic Response
5. Genetic and Epigenetic Biomarkers
6. Small Non-Codnig RNAs (microRNA/miRNAs)
7. DNA Methylation Alterations
8. Cell-Based Biomarkers
9. Radiological Findings in Translational Osteosarcoma Research
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| CTCs | Circulating Tumor Cells |
| ctDNA | circulating tumor DNA |
| DST | dystonin |
| EMT | Epithelial |
| Mesenchymal Transition | |
| LDH | Dehydrogenase lactate |
| miRNA | MicroRNA |
| OSA | Osteosarcoma |
| VEGF | Serum vascular endothelial growth factor |
| VIM | Vimentin |
References
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Martano, M.; Buracco, P. Biology, Diagnosis and Treatment of Canine Appendicular Osteosarcoma: Similarities and Differences with Human Osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Botter, S.M.; Neri, D.; Fuchs, B. Recent Advances in Osteosarcoma. Curr. Opin. Pharmacol. 2014, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, M.; De Maria, R.; Morello, E.M.; Della Salda, L. Editorial: Canine Osteosarcoma as a Model in Comparative Oncology: Advances and Perspective. Front. Vet. Sci. 2023, 10, 1141666. [Google Scholar] [CrossRef]
- Zamborsky, R.; Kokavec, M.; Harsanyi, S.; Danisovic, L. Identification of Prognostic and Predictive Osteosarcoma Biomarkers. Med. Sci. Basel Switz. 2019, 7, 28. [Google Scholar] [CrossRef]
- Cagney, D.N.; Sul, J.; Huang, R.Y.; Ligon, K.L.; Wen, P.Y.; Alexander, B.M. The FDA NIH Biomarkers, EndpointS, and Other Tools (BEST) Resource in Neuro-Oncology. Neuro-Oncol. 2018, 20, 1162–1172. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. Maywood NJ 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef]
- Oh, J.H.; Cho, J.-Y. Comparative Oncology: Overcoming Human Cancer through Companion Animal Studies. Exp. Mol. Med. 2023, 55, 725–734. [Google Scholar] [CrossRef]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative Review of Human and Canine Osteosarcoma: Morphology, Epidemiology, Prognosis, Treatment and Genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef]
- Tarone, L.; Barutello, G.; Iussich, S.; Giacobino, D.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. Naturally Occurring Cancers in Pet Dogs as Pre-Clinical Models for Cancer Immunotherapy. Cancer Immunol. Immunother. CII 2019, 68, 1839–1853. [Google Scholar] [CrossRef]
- Morrow, J.J.; Khanna, C. Osteosarcoma Genetics and Epigenetics: Emerging Biology and Candidate Therapies. Crit. Rev. Oncog. 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.M.; Khanna, C. Comparative Aspects of Osteosarcoma Pathogenesis in Humans and Dogs. Vet. Sci. 2015, 2, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Ren, L.; Huang, S.; Berger, E.; Bardales, K.; Mannheimer, J.; Mazcko, C.; LeBlanc, A. Canine and Murine Models of Osteosarcoma. Vet. Pathol. 2022, 59, 399–414. [Google Scholar] [CrossRef]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A Review of Diagnosis, Management, and Treatment Strategies. Clin. Adv. Hematol. Oncol. HO 2010, 8, 705–718. [Google Scholar]
- Sakthikumar, S.; Elvers, I.; Kim, J.; Arendt, M.L.; Thomas, R.; Turner-Maier, J.; Swofford, R.; Johnson, J.; Schumacher, S.E.; Alföldi, J.; et al. SETD2 Is Recurrently Mutated in Whole-Exome Sequenced Canine Osteosarcoma. Cancer Res. 2018, 78, 3421–3431. [Google Scholar] [CrossRef]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Varshney, J.; Scott, M.C.; Largaespada, D.A.; Subramanian, S. Understanding the Osteosarcoma Pathobiology: A Comparative Oncology Approach. Vet. Sci. 2016, 3, 3. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Malhotra, K.; Patel, S. Primary Osteosarcoma in the Elderly Revisited: Current Concepts in Diagnosis and Treatment. Curr. Oncol. Rep. 2018, 20, 13. [Google Scholar] [CrossRef]
- Meyers, P.A.; Gorlick, R. OSTEOSARCOMA. Pediatr. Clin. North Am. 1997, 44, 973–989. [Google Scholar] [CrossRef]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine Osteosarcoma: A Naturally Occurring Disease to Inform Pediatric Oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef]
- Imaging of Osteosarcoma: Presenting Findings, Metastatic Patterns, and Features Related to Prognosis Available online:. Available online: https://www.mdpi.com/2077-0383/13/19/5710 (accessed on 11 March 2025).
- Choi, L.E.; Healey, J.H.; Kuk, D.; Brennan, M.F. Analysis of Outcomes in Extraskeletal Osteosarcoma: A Review of Fifty-Three Cases. JBJS 2014, 96, e2. [Google Scholar] [CrossRef]
- Langenbach, A.; Anderson, M.; Dambach, D.; Sorenmo, K.; Shofer, F. Extraskeletal Osteosarcomas in Dogs: A Retrospective Study of 169 Cases (1986-1996). J. Am. Anim. Hosp. Assoc. 1998, 34, 113–120. [Google Scholar] [CrossRef]
- Widhe, B.; Widhe, T. Initial Symptoms and Clinical Features in Osteosarcoma and Ewing Sarcoma*. JBJS 2000, 82, 667. [Google Scholar] [CrossRef]
- Wallack, S.T.; Wisner, E.R.; Werner, J.A.; Walsh, P.J.; Kent, M.S.; Fairley, R.A.; Hornof, W.J. Accuracy of Magnetic Resonance Imaging for Estimating Intramedullary Osteosarcoma Extent in Pre-Operative Planning of Canine Limb-Salvage Procedures. Vet. Radiol. Ultrasound 2002, 43, 432–441. [Google Scholar] [CrossRef]
- Mueller, F.; Fuchs, B.; Kaser-Hotz, B. Comparative Biology of Human and Canine Osteosarcoma. Anticancer Res. 2007, 27, 155–164. [Google Scholar]
- Wittig, J.C.; Bickels, J.; Priebat, D.; Jelinek, J.; Kellar-Graney, K.; Shmookler, B.; Malawer, M.M. Osteosarcoma: A Multidisciplinary Approach to Diagnosis and Treatment. Am. Fam. Physician 2002, 65, 1123–1133. [Google Scholar]
- Mannheimer, J.D.; Tawa, G.; Gerhold, D.; Braisted, J.; Sayers, C.M.; McEachron, T.A.; Meltzer, P.; Mazcko, C.; Beck, J.A.; LeBlanc, A.K. Transcriptional Profiling of Canine Osteosarcoma Identifies Prognostic Gene Expression Signatures with Translational Value for Humans. Commun. Biol. 2023, 6, 1–18. [Google Scholar] [CrossRef]
- Das, S.; Idate, R.; Fowles, J.S.; Lana, S.E.; Regan, D.P.; Gustafson, D.L.; Duval, D.L. Molecular Landscape of Canine Osteosarcoma. FASEB J. 2020, 34, 1–1. [Google Scholar] [CrossRef]
- Kirpensteijn, J.; Kik, M.; Teske, E.; Rutteman, G.R. TP53 Gene Mutations in Canine Osteosarcoma. Vet. Surg. VS 2008, 37, 454–460. [Google Scholar] [CrossRef]
- Fu, H.-L.; Shao, L.; Wang, Q.; Jia, T.; Li, M.; Yang, D.-P. A Systematic Review of P53 as a Biomarker of Survival in Patients with Osteosarcoma. Tumor Biol. 2013, 34, 3817–3821. [Google Scholar] [CrossRef]
- Vimalraj, S.; Sekaran, S. RUNX Family as a Promising Biomarker and a Therapeutic Target in Bone Cancers: A Review on Its Molecular Mechanism(s) behind Tumorigenesis. Cancers 2023, 15, 3247. [Google Scholar] [CrossRef]
- Barger, A.; Baker, K.; Driskell, E.; Sander, W.; Roady, P.; Berry, M.; Schnelle, A.; Fan, T.M. The Use of Alkaline Phosphatase and Runx2 to Distinguish Osteosarcoma from Other Common Malignant Primary Bone Tumors in Dogs. Vet. Pathol. 2022, 59, 427–432. [Google Scholar] [CrossRef]
- Leonardi, L.; Manuali, E.; Bufalari, A.; Porcellato, I. Canine Soft Tissue Sarcomas: The Expression of RUNX2 and Karyopherin Alpha-2 in Extraskeletal (Soft Tissues) and Skeletal Osteosarcomas. Front. Vet. Sci. 2024, 11, 1292852. [Google Scholar] [CrossRef]
- Gupta, S.; Ito, T.; Alex, D.; Vanderbilt, C.M.; Chang, J.C.; Islamdoust, N.; Zhang, Y.; Nafa, K.; Healey, J.; Ladanyi, M.; et al. RUNX2 (6p21.1) Amplification in Osteosarcoma. Hum. Pathol. 2019, 94, 23–28. [Google Scholar] [CrossRef]
- Alegre, F.; Ormonde, A.R.; Godinez, D.R.; Illendula, A.; Bushweller, J.H.; Wittenburg, L.A. The Interaction between RUNX2 and Core Binding Factor Beta as a Potential Therapeutic Target in Canine Osteosarcoma. Vet. Comp. Oncol. 2020, 18, 52–63. [Google Scholar] [CrossRef]
- Gong, T.; Su, X.; Xia, Q.; Wang, J.; Kan, S. Expression of NF-κB and PTEN in Osteosarcoma and Its Clinical Significance. Oncol. Lett. 2017, 14, 6744–6748. [Google Scholar] [CrossRef]
- Chen, M.-W.; Wu, X.-J. SLC25A22 Promotes Proliferation and Metastasis of Osteosarcoma Cells via the PTEN Signaling Pathway. Technol. Cancer Res. Treat. 2018, 17, 1533033818811143. [Google Scholar] [CrossRef]
- Sikora, M.; Marycz, K.; Smieszek, A. Small and Long Non-Coding RNAs as Functional Regulators of Bone Homeostasis, Acting Alone or Cooperatively. Mol. Ther. Nucleic Acids 2020, 21, 792–803. [Google Scholar] [CrossRef]
- Dey, M.; Skipar, P.; Bartnik, E.; Piątkowski, J.; Sulejczak, D.; Czarnecka, A.M. MicroRNA Signatures in Osteosarcoma: Diagnostic Insights and Therapeutic Prospects. Mol. Cell. Biochem. 2024. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hornicek, F.J.; Duan, Z. MicroRNA Involvement in Osteosarcoma. Sarcoma 2012, 2012, 359739. [Google Scholar] [CrossRef]
- Llobat, L.; Gourbault, O. Role of MicroRNAs in Human Osteosarcoma: Future Perspectives. Biomedicines 2021, 9, 463. [Google Scholar] [CrossRef]
- Gally, T.B.; Aleluia, M.M.; Borges, G.F.; Kaneto, C.M. Circulating MicroRNAs as Novel Potential Diagnostic Biomarkers for Osteosarcoma: A Systematic Review. Biomolecules 2021, 11, 1432. [Google Scholar] [CrossRef]
- Leonardi, L.; Scotlandi, K.; Pettinari, I.; Benassi, M.S.; Porcellato, I.; Pazzaglia, L. MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects. Cells 2021, 10, 428. [Google Scholar] [CrossRef]
- Dailey, D.D.; Hess, A.M.; Bouma, G.J.; Duval, D.L. MicroRNA Expression Changes and Integrated Pathways Associated With Poor Outcome in Canine Osteosarcoma. Front. Vet. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Zhu, X.-B.; Zhang, Z.-C.; Han, G.-S.; Han, J.-Z.; Qiu, D.-P. Overexpression of miR-214 Promotes the Progression of Human Osteosarcoma by Regulating the Wnt/Β-catenin Signaling Pathway. Mol. Med. Rep. 2017, 15, 1884–1892. [Google Scholar] [CrossRef]
- Cai, H.; Miao, M.; Wang, Z. miR-214-3p Promotes the Proliferation, Migration and Invasion of Osteosarcoma Cells by Targeting CADM1. Oncol. Lett. 2018, 16, 2620–2628. [Google Scholar] [CrossRef]
- Rehei, A.-L.; Zhang, L.; Fu, Y.-X.; Mu, W.-B.; Yang, D.-S.; Liu, Y.; Zhou, S.-J.; Younusi, A. MicroRNA-214 Functions as an Oncogene in Human Osteosarcoma by Targeting TRAF3. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5156–5164. [Google Scholar] [CrossRef]
- MicroRNA-214 Functions as an Oncogene in Human Osteosarcoma by Targeting TRAF3.
- Ludwig, L.; Edson, M.; Treleaven, H.; Viloria-Petit, A.M.; Mutsaers, A.J.; Moorehead, R.; Foster, R.A.; Ali, A.; Wood, R.D.; Wood, G.A. Plasma microRNA Signatures Predict Prognosis in Canine Osteosarcoma Patients. PloS One 2024, 19, e0311104. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Shen, Y.; Zheng, S.; Liu, J.; Jiang, X. miR-21 Predicts Poor Prognosis in Patients with Osteosarcoma. Br. J. Biomed. Sci. 2016, 73, 158–162. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Feng, Y.; Liu, T.; He, S. Role of Exosomal miR-21 in the Tumor Microenvironment and Osteosarcoma Tumorigenesis and Progression (Review). Int. J. Oncol. 2020, 56, 1055–1063. [Google Scholar] [CrossRef]
- The Clinical Significance of miR-21 in Guiding Chemotherapy for Patients with Osteosarcoma. Available online: https://pubmed.ncbi.nlm.nih.gov/34616172/ (accessed on 7 April 2025).
- Full Article: The Clinical Significance of miR-21 in Guiding Chemotherapy for Patients with Osteosarcoma. Available online: https://www.tandfonline.com/doi/full/10.2147/PGPM.S321637 (accessed on 31 March 2025).
- Dong, J.; Liu, Y.; Liao, W.; Liu, R.; Shi, P.; Wang, L. miRNA-223 Is a Potential Diagnostic and Prognostic Marker for Osteosarcoma. J. Bone Oncol. 2016, 5, 74–79. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, W.; Liu, Y.; Guo, A.; Yang, D. Let-7b Acts as a Tumor Suppressor in Osteosarcoma via Targeting IGF1R. Oncol. Lett. 2019, 17, 1646–1654. [Google Scholar] [CrossRef]
- Wei, H.; Cui, R.; Bahr, J.; Zanesi, N.; Luo, Z.; Meng, W.; Liang, G.; Croce, C.M. miR-130a Deregulates PTEN and Stimulates Tumor Growth. Cancer Res. 2017, 77, 6168–6178. [Google Scholar] [CrossRef]
- Ludwig, L.; Vanderboon, E.N.; Treleaven, H.; Wood, R.D.; Schott, C.R.; Wood, G.A. Patient-Matched Tumours, Plasma, and Cell Lines Reveal Tumour Microenvironment- and Cell Culture-Specific microRNAs. Biol. Open 2024, 13, bio060483. [Google Scholar] [CrossRef]
- Mills, L.J.; Scott, M.C.; Shah, P.; Cunanan, A.R.; Deshpande, A.; Auch, B.; Curtin, B.; Beckman, K.B.; Spector, L.G.; Sarver, A.L.; et al. Comparative Analysis of Genome-Wide DNA Methylation Identifies Patterns That Associate with Conserved Transcriptional Programs in Osteosarcoma. Bone 2022, 158, 115716. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, P.; Engskog-Vlachos, P.; Zhang, H.; Murgoci, A.-N.; Zerdes, I.; Joseph, B. SETD2 Mutation in Renal Clear Cell Carcinoma Suppress Autophagy via Regulation of ATG12. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Xie, Y.; Sahin, M.; Sinha, S.; Wang, Y.; Nargund, A.M.; Lyu, Y.; Han, S.; Dong, Y.; Hsieh, J.J.; Leslie, C.S.; et al. SETD2 Loss Perturbs the Kidney Cancer Epigenetic Landscape to Promote Metastasis and Engenders Actionable Dependencies on Histone Chaperone Complexes. Nat. Cancer 2022, 3, 188–202. [Google Scholar] [CrossRef]
- Sakthikumar, S.; Elvers, I.; Kim, J.; Arendt, M.L.; Thomas, R.; Turner-Maier, J.; Swofford, R.; Johnson, J.; Schumacher, S.E.; Alföldi, J.; et al. SETD2 Is Recurrently Mutated in Whole-Exome Sequenced Canine Osteosarcoma. Cancer Res. 2018, 78, 3421–3431. [Google Scholar] [CrossRef]
- Suehara, Y.; Alex, D.; Bowman, A.; Middha, S.; Zehir, A.; Chakravarty, D.; Wang, L.; Jour, G.; Nafa, K.; Hayashi, T.; et al. Clinical Genomic Sequencing of Pediatric and Adult Osteosarcoma Reveals Distinct Molecular Subsets with Potentially Targetable Alterations. Clin. Cancer Res. 2019, 25, 6346–6356. [Google Scholar] [CrossRef] [PubMed]
- Espinoza Pereira, K.N.; Shan, J.; Licht, J.D.; Bennett, R.L. Histone Mutations in Cancer. Biochem. Soc. Trans. 2023, 51, 1749–1763. [Google Scholar] [CrossRef]
- Mancini, M.; Monaldi, C.; De Santis, S.; Papayannidis, C.; Rondoni, M.; Sartor, C.; Bruno, S.; Pagano, L.; Criscuolo, M.; Zanotti, R.; et al. SETD2 Non Genomic Loss of Function in Advanced Systemic Mastocytosis Is Mediated by an Aurora Kinase A/MDM2 Axis and Can Be Therapeutically Targeted. Biomark. Res. 2023, 11, 29. [Google Scholar] [CrossRef]
- Sakthikumar, S.; Elvers, I.; Kim, J.; Arendt, M.L.; Thomas, R.; Turner-Maier, J.; Swofford, R.; Johnson, J.; Schumacher, S.E.; Alföldi, J.; et al. SETD2 Is Recurrently Mutated in Whole-Exome Sequenced Canine Osteosarcoma. Cancer Res. 2018, 78, 3421–3431. [Google Scholar] [CrossRef]
- Gardner, H.L.; Sivaprakasam, K.; Briones, N.; Zismann, V.; Perdigones, N.; Drenner, K.; Facista, S.; Richholt, R.; Liang, W.; Aldrich, J.; et al. Canine Osteosarcoma Genome Sequencing Identifies Recurrent Mutations in DMD and the Histone Methyltransferase Gene SETD2. Commun. Biol. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular Endothelial Growth Factor (VEGF) Delivery Approaches in Regenerative Medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef]
- Scheidegger, P.; Weiglhofer, W.; Suarez, S.; Kaser-Hotz, B.; Steiner, R.; Ballmer-Hofer, K.; Jaussi, R. Vascular Endothelial Growth Factor (VEGF) and Its Receptors in Tumor-Bearing Dogs. Biol. Chem. 1999, 380. [Google Scholar] [CrossRef]
- Thamm, D.H.; O’Brien, M.G.; Vail, D.M. Serum Vascular Endothelial Growth Factor Concentrations and Postsurgical Outcome in Dogs with Osteosarcoma. Vet. Comp. Oncol. 2008, 6, 126–132. [Google Scholar] [CrossRef]
- Tabone, M.-D.; Brugières, L.; Piperno-Neumann, S.; Selva, M.-A.; Marec-Bérard, P.; Pacquement, H.; Lervat, C.; Corradini, N.; Gentet, J.-C.; Couderc, R.; et al. Prognostic Impact of Blood and Urinary Angiogenic Factor Levels at Diagnosis and during Treatment in Patients with Osteosarcoma: A Prospective Study. BMC Cancer 2017, 17, 419. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, J.; Jiang, T.; Zhao, H.; Gao, Y.; Zheng, C.; Shi, X. Difference in Pre- and Postchemotherapy Vascular Endothelial Growth Factor Levels as a Prognostic Indicator in Osteosarcoma. J. Int. Med. Res. 2011, 39, 1474–1482. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, K.-H.; Moon, S.-H.; Jang, J.; Kim, H.S.; Suh, J.-S.; Yang, W.-I. Reassessment of Alkaline Phosphatase as Serum Tumor Marker with High Specificity in Osteosarcoma. Cancer Med. 2017, 6, 1311–1322. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Sun, L.-L.; Li, H.-Y.; Ye, Z.-M. Prognostic Significance of Serum Alkaline Phosphatase Level in Osteosarcoma: A Meta-Analysis of Published Data. BioMed Res. Int. 2015, 2015, 160835. [Google Scholar] [CrossRef]
- Hao, H.; Chen, L.; Huang, D.; Ge, J.; Qiu, Y.; Hao, L. Meta-Analysis of Alkaline Phosphatase and Prognosis for Osteosarcoma. Eur. J. Cancer Care (Engl.) 2017, 26, e12536. [Google Scholar] [CrossRef]
- Shu, J.; Tan, A.; Li, Y.; Huang, H.; Yang, J. The Correlation between Serum Total Alkaline Phosphatase and Bone Mineral Density in Young Adults. BMC Musculoskelet. Disord. 2022, 23, 467. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Sun, L.-L.; Li, H.-Y.; Ye, Z.-M. Prognostic Significance of Serum Alkaline Phosphatase Level in Osteosarcoma: A Meta-Analysis of Published Data. BioMed Res. Int. 2015, 2015, 160835. [Google Scholar] [CrossRef]
- Gu, R.; Sun, Y. Does Serum Alkaline Phosphatase Level Really Indicate the Prognosis in Patients with Osteosarcoma? A Meta-Analysis. J. Cancer Res. Ther. 2018, 14, S468. [Google Scholar] [CrossRef]
- McKenna, R.J.; Schwinn, C.P.; Soong, K.Y.; Higinbotham, N.L. Osteogenic Sarcoma Arising in Paget’s Disease. Cancer 1964, 17, 42–66. [Google Scholar] [CrossRef]
- Ehrhart, N.; Dernell, W.S.; Hoffmann, W.E.; Weigel, R.M.; Powers, B.E.; Withrow, S.J. Prognostic Importance of Alkaline Phosphatase Activity in Serum from Dogs with Appendicular Osteosarcoma: 75 Cases (1990-1996). J. Am. Vet. Med. Assoc. 1998, 213, 1002–1006. [Google Scholar] [CrossRef]
- Garzotto, C.K.; Berg, J.; Hoffmann, W.E.; Rand, W.M. Prognostic Significance of Serum Alkaline Phosphatase Activity in Canine Appendicular Osteosarcoma. J. Vet. Intern. Med. 2000, 14, 587–592. [Google Scholar] [CrossRef]
- Walenta, S.; Mueller-Klieser, W.F. Lactate: Mirror and Motor of Tumor Malignancy. Semin. Radiat. Oncol. 2004, 14, 267–274. [Google Scholar] [CrossRef]
- Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Available online: https://www.mdpi.com/2306-7381/6/2/48 (accessed on 10 April 2025).
- Chen, J.; Sun, M.; Hua, Y.; Cai, Z. Prognostic Significance of Serum Lactate Dehydrogenase Level in Osteosarcoma: A Meta-Analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zeng, J.; Song, C.; Yu, H.; Shi, Q.; Mai, W.; Qu, G. A Retrospective Clinicopathological Study of Osteosarcoma Patients with Metachronous Metastatic Relapse. J. Cancer 2019, 10, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Ogenyi, S.I.; Madukwe, J.; Onyemelukwe, A.O.; Ngokere, A.A. Vimentin and Cytokeratin Immunostaining: The Role in Basic Diagnosis and Prognosis of Sarcomas. J. Drug Deliv. Ther. 2020, 10, 175–178. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Barger, A.; Graca, R.; Bailey, K.; Messick, J.; de Lorimier, L.-P.; Fan, T.; Hoffmann, W. Use of Alkaline Phosphatase Staining to Differentiate Canine Osteosarcoma from Other Vimentin-Positive Tumors. Vet. Pathol. 2005, 42, 161–165. [Google Scholar] [CrossRef]
- Sittiju, P.; Chaiyawat, P.; Pruksakorn, D.; Klangjorhor, J.; Wongrin, W.; Phinyo, P.; Kamolphiwong, R.; Phanphaisarn, A.; Teeyakasem, P.; Kongtawelert, P.; et al. Osteosarcoma-Specific Genes as a Diagnostic Tool and Clinical Predictor of Tumor Progression. Biology 2022, 11, 698. [Google Scholar] [CrossRef]
- Le, M.-C.N.; Smith, K.A.; Dopico, P.J.; Greer, B.; Alipanah, M.; Zhang, Y.; Siemann, D.W.; Lagmay, J.P.; Fan, Z.H. Investigating Surface Proteins and Antibody Combinations for Detecting Circulating Tumor Cells of Various Sarcomas. Sci. Rep. 2024, 14, 12374. [Google Scholar] [CrossRef]
- Mu, H.; Zuo, D.; Chen, J.; Liu, Z.; Wang, Z.; Yang, L.; Shi, Q.; Hua, Y. Detection and Surveillance of Circulating Tumor Cells in Osteosarcoma for Predicting Therapy Response and Prognosis. Cancer Biol. Med. 2022, 19, 1397–1409. [Google Scholar] [CrossRef]
- Dai, S.; Shao, X.; Wei, Q.; Du, S.; Hou, C.; Li, H.; Jin, D. Association of Circulating Tumor Cells and IMP3 Expression with Metastasis of Osteosarcoma. Front. Oncol. 2023, 13. [Google Scholar] [CrossRef]
- Wright, T.; Brisson, B.A.; Wood, G.A.; Oblak, M.; Mutsaers, A.J.; Sabine, V.; Skowronski, K.; Belanger, C.; Tiessen, A.; Bienzle, D. Flow Cytometric Detection of Circulating Osteosarcoma Cells in Dogs. Cytometry A 2019, 95, 997–1007. [Google Scholar] [CrossRef]
- Wright, T.F.; Brisson, B.A.; Belanger, C.R.; Tiessen, A.; Sabine, V.; Skowronski, K.; Wood, G.A.; Oblak, M.L.; Mutsaers, A.J.; Sears, W.; et al. Quantification of Circulating Tumour Cells over Time in Dogs with Appendicular Osteosarcoma. Vet. Comp. Oncol. 2023, 21, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Peihong, T.; Lingling, R.; Haifeng, H.; Chang, L.; Guifeng, L. Application and Progress of X-Ray, Computed Tomography, and Magnetic Resonance Imaging Radiomics in Osteosarcoma. iRADIOLOGY 2023, 1, 262–268. [Google Scholar] [CrossRef]
- Hameed, M.; Dorfman, H. Primary Malignant Bone Tumors—Recent Developments. Semin. Diagn. Pathol. 2011, 28, 86–101. [Google Scholar] [CrossRef]
- Kundu, Z.S. Classification, Imaging, Biopsy and Staging of Osteosarcoma. Indian J. Orthop. 2014, 48, 238–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




