Submitted:
27 November 2024
Posted:
28 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Case Selection
- -
- -
- neoplastic mononuclear stromal cell with mild cellular atypia (but mitoses can be numerous), together with numerous multinucleated giant cells;
- -
- scant/absent osteoid deposition. [35]
Histology, Histochemistry and Immunohistochemistry
3. Results
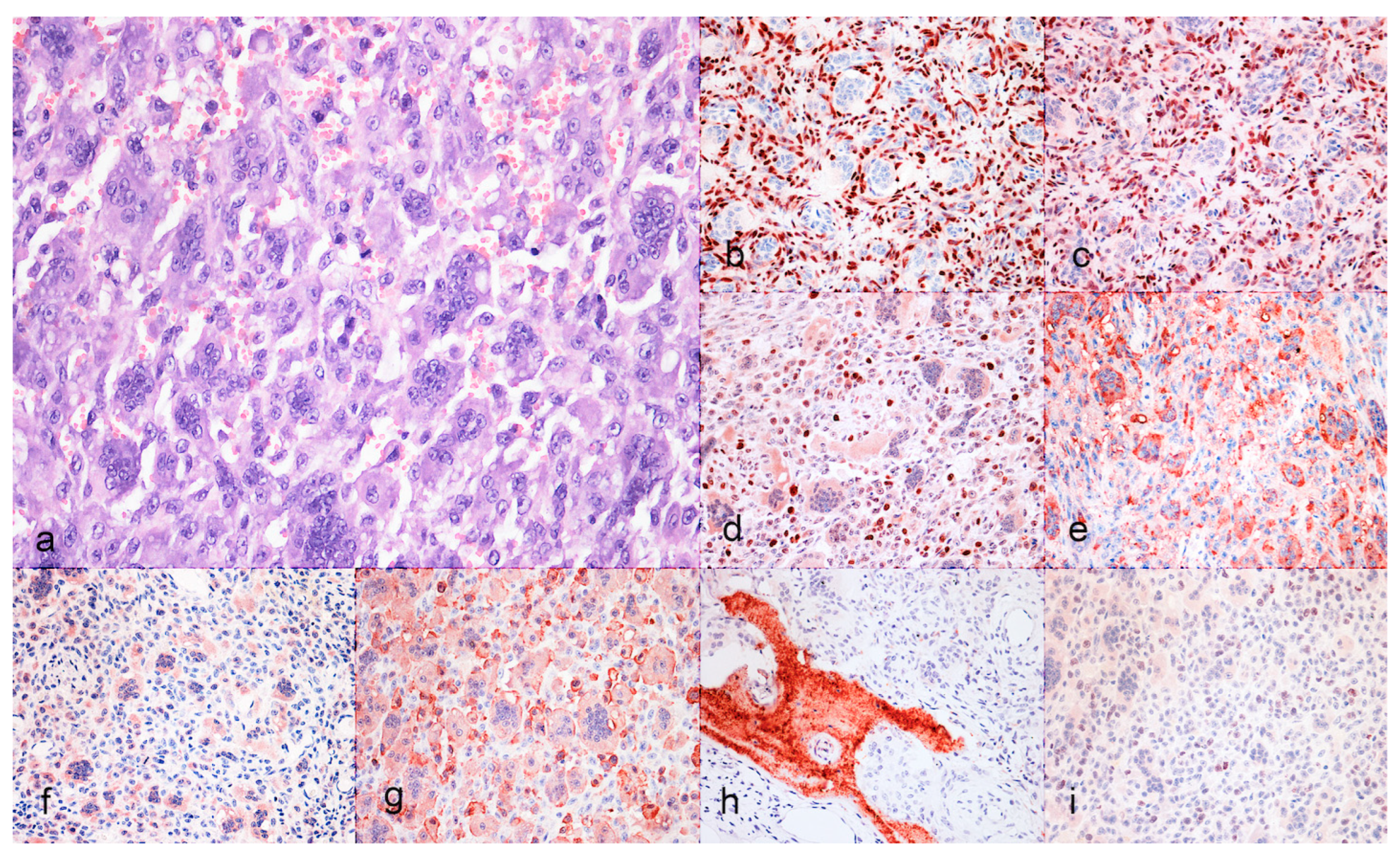
3.1. Case Selection and Histological Features
3.2. Immunohistochiemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jha, Y.; Chaudhary, K. Giant Cell Tumour of Bone: A Comprehensive Review of Pathogenesis, Diagnosis, and Treatment. Cureus 2023, 15, e46945. [Google Scholar] [CrossRef]
- Hartmann, W.; Harder, D.; Baumhoer, D. Giant Cell-Rich Tumors of Bone. Surg Pathol Clin 2021, 14, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Igoumenou, V.G.; Megaloikonomos, P.D.; Panagopoulos, G.N.; Papagelopoulos, P.J.; Soucacos, P.N. Giant Cell Tumor of Bone Revisited. SICOT J 2017, 3, 54. [Google Scholar] [CrossRef]
- Sung, H.W.; Kuo, D.P.; Shu, W.P.; Chai, Y.B.; Liu, C.C.; Li, S.M. Giant-Cell Tumor of Bone: Analysis of Two Hundred and Eight Cases in Chinese Patients. J Bone Joint Surg Am 1982, 64, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Saikia, K.C.; Bhuyan, S.K.; Borgohain, M.; Ahmed, F.; Saikia, S.P.; Bora, A. Giant Cell Tumour of Bone: An Analysis of 139 Indian Patients. Journal of Orthopaedic Science 2011, 16, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Gundavda, M.K.; Agarwal, M.G.; Reddy, R.; Wagh, Y.; Ghanate, V. Is Vitamin D Deficiency behind the Scenes for High Incidence of Giant Cell Tumor amongst the Indian Population? Unraveling the Vitamin D – RANKL Association. Medical Hypotheses 2019, 123, 67–71. [Google Scholar] [CrossRef]
- Muran, A.; Fallon, J.; Jung, B.; Dzaugis, P.; Zhang, A.; Fitzgerald, M.; Goodman, H.J.; Kenan, S.; Kenan, S. Treatment Trends of Benign Bone Lesions in a Suburban New York Healthcare System. J Family Med Prim Care 2023, 12, 1979–1983. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, L.; Zhang, H.; Yu, X.; Wang, Z.; Ye, Z.; Wu, S.; Guo, S.; Zhang, G.; Wang, J.; et al. Sex Differences in the Recurrence Rate and Risk Factors for Primary Giant Cell Tumors Around the Knee in China. Sci Rep 2016, 6, 28173. [Google Scholar] [CrossRef] [PubMed]
- Sobti, A.; Agrawal, P.; Agarwala, S.; Agarwal, M. Giant Cell Tumor of Bone - An Overview. Arch Bone Jt Surg 2016, 4, 2–9. [Google Scholar]
- Becker, R.G.; Galia, C.R.; Pestilho, J.F.C.S.; Antunes, B.P.; Baptista, A.M.; Guedes, A. GIANT CELL TUMOR OF BONE: A MULTICENTER EPIDEMIOLOGICAL STUDY IN BRAZIL. Acta ortop. bras. 2024, 32, e273066. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, A.J.; Bovée, J.V.M.G.; Mastboom, M.J.L.; Sander Dijkstra, P.D.; Van De Sande, M.A.J.; Gelderblom, H. Incidence and Demographics of Giant Cell Tumor of Bone in The Netherlands: First Nationwide Pathology Registry Study. Acta Orthop 2018, 89, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, R.E. Giant Cell Tumor of Bone. Orthopedic Clinics 2006, 37, 35–51. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Y.; Wang, L.; Nie, Z.; Zhu, J.; Yan, Q. Rare Features of Giant Cell Tumors of the Bone: A Case Report. Exp Ther Med 2024, 28, 409. [Google Scholar] [CrossRef]
- Çomunoğlu, N.; Kepil, N.; Dervişoğlu, S. Histopathology of Giant Cell Tumors of the Bone: With Special Emphasis on Fibrohistiocytic and Aneurysmal Bone Cyst like Components. Acta Orthopaedica et Traumatologica Turcica 2019, 53, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.H.; Robbins, P.; Xu, J.; Huang, L.; Wood, D.J.; Papadimitriou, J.M. The Histogenesis of Giant Cell Tumour of Bone: A Model of Interaction between Neoplastic Cells and Osteoclasts. Histology and Histopathology 2001, 16, 297–307. [Google Scholar] [PubMed]
- Kim, Y.; Nizami, S.; Goto, H.; Lee, F.Y. Modern Interpretation of Giant Cell Tumor of Bone: Predominantly Osteoclastogenic Stromal Tumor. Clin Orthop Surg 2012, 4, 107–116. [Google Scholar] [CrossRef]
- Murata, A.; Fujita, T.; Kawahara, N.; Tsuchiya, H.; Tomita, K. Osteoblast Lineage Properties in Giant Cell Tumors of Bone. J Orthop Sci 2005, 10, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Amzajerdi, A.N.; Banuelos, E.; Sassoon, A. SATB2 Expression in Locally Aggressive Giant Cell Tumor of Bone Can Be a Pitfall in Bone Pathology. American Journal of Clinical Pathology 2016, 146, 39. [Google Scholar] [CrossRef]
- Milton, S.; Prabhu, A.J.; Titus, V.T.K.; John, R.; Backianathan, S.; Madhuri, V. Special AT-Rich Sequence-Binding Protein 2 (SATB2) in the Differential Diagnosis of Osteogenic and Non-Osteogenic Bone and Soft Tissue Tumors. J Pathol Transl Med 2022, 56, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Hartmann, W.; Rókusz, A.; Leinauer, B.; von Baer, A.; Schultheiss, M.; Pablik, J.; Fritzsche, H.; Mogler, C.; Antal, I.; et al. Histomorphometric Analysis of 38 Giant Cell Tumors of Bone after Recurrence as Compared to Changes Following Denosumab Treatment. Cancers (Basel) 2023, 15, 4249. [Google Scholar] [CrossRef]
- Scotto di Carlo, F.; Divisato, G.; Iacoangeli, M.; Esposito, T.; Gianfrancesco, F. The Identification of H3F3A Mutation in Giant Cell Tumour of the Clivus and the Histological Diagnostic Algorithm of Other Clival Lesions Permit the Differential Diagnosis in This Location. BMC Cancer 2018, 18, 358. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, F.; Righi, A.; Benini, S.; Magagnoli, G.; Chiaramonte, I.; Manfrini, M.; Gasbarrini, A.; Frisoni, T.; Gambarotti, M. Giant Cell Tumor of Bone in Patients under 16 Years Old: A Single-Institution Case Series. Cancers 2021, 13, 2585. [Google Scholar] [CrossRef]
- Walsh, B.A.; Rhodes, W.H. Giant Cell Tumour of Bone in a Cat. J Small Anim Pract 1995, 36, 325–329. [Google Scholar] [CrossRef]
- Ferreras, M.C.; Fuertes, M.; Pérez, V.; Benavides, J.; García-Pariente, C.; Reyes, L.E.; García-Marín, J.F. Giant Cell Tumour of Bone in a Cat with Extraskeletal Metastases: Pathological and Immunohistochemical Study. J Vet Med A Physiol Pathol Clin Med 2005, 52, 225–229. [Google Scholar] [CrossRef]
- Caldero Carrete, J.; Tabanez, J.; Civello, A.; Rusbridge, C. Vertebral Giant Cell Tumour of Bone in a Domestic Shorthair Cat. JFMS Open Rep 2023, 9, 20551169231160227. [Google Scholar] [CrossRef]
- Leonardi, L.; Quattrini, I.; Roperto, F.; Benassi, M.S. Protease Expression in Giant Cell Tumour of Bone: A Comparative Study on Feline and Human Samples. Research in Veterinary Science 2013, 95, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, L. Bone Tumors in Domestic Animals: Comparative Clinical Pathology; Springer Nature; ISBN 978-3-030-90210-0.
- Thornburg, L.P. Giant Cell Tumor of Bone in a Cat. Vet Pathol 1979, 16, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Schneck, G.W. A Case of Giant Cell Epulis (Osteoclastoma) in a Cat. Vet Rec 1975, 97, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Blondel, M.; Gros, L.; Semin, M.-O.; Delverdier, M.; Palierne, S.; Autefage, A. A Case of Giant Cell Tumour of Bone in a Dog. VCOT Open 2019, 02, e64–e69. [Google Scholar] [CrossRef]
- Lecouteur, R.; Nimmo, J.; Price, S.M.; Pennock, P. A Case of Giant Cell Tumor of Bone (Osteoclastoma) in a Dog. Journal of The American Animal Hospital Association 1978. [Google Scholar]
- Manuali, E.; Morgante, R.A.; Maresca, C.; Leonardi, L.; Purificato, I.; Giaimo, M.D.; Giovannini, G. A Web-Based Tumor Registration System for a Regional Canine Cancer Registry in Umbria, Central Italy. Ann Ist Super Sanita 2019, 55, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Meuten, D.J. Tumors in Domestic Animals; John Wiley & Sons, 2020; ISBN 978-0-8138-2179-5.
- Dittmer, K.; Roccabianca, P.; Bell, C.; Murphy, B.; R A, F.; Scruggs, J.; Schulman, Y.; Thompson, D.; Avallone, G.; Kiupel, M. Surgical Pathology Of Tumors Of Domestic Animals. 4: Tumors of Bone, Cartilage and Other Hard Tissues; C. L. Davis Thomson Foundation, 2021; ISBN 978-1-73374-913-8.
- Maxie, M.G.; Miller, M.A. Chapter 1 - Introduction to the Diagnostic Process. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 1 (Sixth Edition); Maxie, M.G., Ed.; W.B. Saunders, 2016; pp. 1-15.e1 ISBN 978-0-7020-5317-7.
- Porcellato, I.; Sforna, M.; Lo Giudice, A.; Bossi, I.; Musi, A.; Tognoloni, A.; Chiaradia, E.; Mechelli, L.; Brachelente, C. Tumor-Associated Macrophages in Canine Oral and Cutaneous Melanomas and Melanocytomas: Phenotypic and Prognostic Assessment. Front. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Leonardi, L.; Manuali, E.; Bufalari, A.; Porcellato, I. Canine Soft Tissue Sarcomas: The Expression of RUNX2 and Karyopherin Alpha-2 in Extraskeletal (Soft Tissues) and Skeletal Osteosarcomas. Front Vet Sci 2024, 11, 1292852. [Google Scholar] [CrossRef]
- Cheng, D.; Hu, T.; Zhang, H.; Huang, J.; Yang, Q. Factors Affecting the Recurrence of Giant Cell Tumor of Bone After Surgery: A Clinicopathological Study of 80 Cases from a Single Center. Cell Physiol Biochem 2015, 36, 1961–1970. [Google Scholar] [CrossRef]
- Lujic, N.; Sopta, J.; Kovacevic, R.; Stevanovic, V.; Davidovic, R. Recurrence of Giant Cell Tumour of Bone: Role of P53, Cyclin D1, β-Catenin and Ki67. Int Orthop 2016, 40, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Pierezan, F.; Mansell, J.; Ambrus, A.; Rodrigues Hoffmann, A. Immunohistochemical Expression of Ionized Calcium Binding Adapter Molecule 1 in Cutaneous Histiocytic Proliferative, Neoplastic and Inflammatory Disorders of Dogs and Cats. J Comp Pathol 2014, 151, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Carrete, J.C.; Tabanez, J.; Civello, A.; Rusbridge, C. Vertebral Giant Cell Tumour of Bone in a Domestic Shorthair Cat. JFMS Open Reports 2023, 9, 20551169231160227. [Google Scholar] [CrossRef]
- Noguchi, T.; Sakamoto, A.; Murotani, Y.; Murata, K.; Hirata, M.; Yamada, Y.; Toguchida, J.; Matsuda, S. Inhibition of RANKL Expression in Osteocyte-like Differentiated Tumor Cells in Giant Cell Tumor of Bone After Denosumab Treatment. Journal of Histochemistry and Cytochemistry 2023, 71, 131. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.Y.; Haupt, H.M.; Kanetsky, P.A.; Donthineni-Rao, R.; Arenas-Elliott, C.; Lackman, R.D.; Martin, A.-M. Giant Cell Tumors: Inquiry into Immunohistochemical Expression of CD117 (c-Kit), Microphthalmia Transcription Factor, Tartrate-Resistant Acid Phosphatase, and HAM-56. Arch Pathol Lab Med 2005, 129, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Toda, Y.; Matsumoto, Y.; Yamamoto, H.; Yahiro, K.; Shimada, E.; Kanahori, M.; Oyama, R.; Fukushima, S.; Nakagawa, M.; et al. Nuclear β-Catenin Translocation Plays a Key Role in Osteoblast Differentiation of Giant Cell Tumor of Bone. Sci Rep 2022, 12, 13438. [Google Scholar] [CrossRef]
- Wu, P.-F.; Tang, J.; Li, K. RANK Pathway in Giant Cell Tumor of Bone: Pathogenesis and Therapeutic Aspects. Tumour Biol 2015, 36, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Amazit, L.; Meduri, G.; Guiochon-Mantel, A.; Milgrom, E.; Mariette, X. RANK (Receptor Activator of Nuclear Factor Kappa B) and RANK Ligand Are Expressed in Giant Cell Tumors of Bone. Am J Clin Pathol 2002, 117, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Roles of Runx2 in Skeletal Development. Adv Exp Med Biol 2017, 962, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Barger, A.; Baker, K.; Driskell, E.; Sander, W.; Roady, P.; Berry, M.; Schnelle, A.; Fan, T.M. The Use of Alkaline Phosphatase and Runx2 to Distinguish Osteosarcoma from Other Common Malignant Primary Bone Tumors in Dogs. Vet Pathol 2022, 59, 427–432. [Google Scholar] [CrossRef]
- Mak, I.W.Y.; Cowan, R.W.; Popovic, S.; Colterjohn, N.; Singh, G.; Ghert, M. Upregulation of MMP-13 via Runx2 in the Stromal Cell of Giant Cell Tumor of Bone. Bone 2009, 45, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Moroianu, J.; Hijikata, M.; Blobel, G.; Radu, A. Mammalian Karyopherin Alpha 1 Beta and Alpha 2 Beta Heterodimers: Alpha 1 or Alpha 2 Subunit Binds Nuclear Localization Signal and Beta Subunit Interacts with Peptide Repeat-Containing Nucleoporins. Proceedings of the National Academy of Sciences 1995, 92, 6532–6536. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, J.; Wei, Q.; Wang, Y. KPNA2 Expression Is a Potential Marker for Differential Diagnosis between Osteosarcomas and Other Malignant Bone Tumor Mimics. Diagn Pathol 2020, 15, 135. [Google Scholar] [CrossRef]
| Antibody | Manufacturer | Dilution | Antigen retrieval |
|---|---|---|---|
| Iba1 | Merck Millipore | 1:100 | HIER, Tris-EDTA buffer; pH 9.0 |
| TRAP | Santa Cruz Biotechnology | 1:50 | HIER, Tris-EDTA buffer; pH 9.0 |
| SATB2 | Cell Signaling Technology | 1:200 | HIER, Tris-EDTA buffer; pH 9.0 |
| RUNX2 | Santa Cruz Biotechnology | 1:200 | HIER, Tris-EDTA buffer; pH 9.0 |
| RANK | Santa Cruz Biotechnology | 1:50 | HIER, Tris-EDTA buffer; pH 9.0 |
| KPNA-2 | Santa Cruz Biotechnology | 1:150 | HIER, Tris-EDTA buffer; pH 9.0 |
| Osteocalcin | BioGenex LifeSciences | 1:50 | HIER, Tris-EDTA buffer; pH 9.0 |
| Ki-67 | Agilent Dako | 1:200 | HIER, Tris-EDTA buffer; pH 9.0 |
| Case | Breed | Age | Sex | Tumor Location |
|---|---|---|---|---|
| 1 | Domestic shorthair | 15 | M | Tibia |
| 2 | Domestic shorthair | 15 | F | Tibia |
| 3 | Siamese | 5 | M | Dewclaw |
| Case | Iba1 | TRAP | SATB2 | RUNX2 | RANK | KPNA-2 | Osteocalcin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | MGC | MC | MGC | MC | MGC | MC | MGC | MC | MGC | MC | MGC | MC | MGC | |
| 1 | +/- | + | +/- | + | + | - | + | - | +/- | + | - | + | +/- | - |
| 2 | +/- | + | +/- | + | + | - | + | - | +/- | + | - | + | - | - |
| 3 | +/- | + | - | + | +/- | - | + | - | +/- | + | - | + | +/- | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





