Submitted:
25 April 2025
Posted:
28 April 2025
You are already at the latest version
Abstract
Young vegetative quinoa (YVQ) has gained attention as a high-protein leafy crop for human consumption with potential for cultivation in Mediterranean and semiarid regions. We investigated the effects of inter-row spacing and genotype on YVQ fresh and dry matter (DM) yield, protein content (PC), and protein yield during summer cultivation in northern Israel in two separate, independent, randomized field experiments over two consecutive years (2020–2021). We hypothesized that row spacing and genotypic differences would significantly impact yield and PC. Inter-row spacing significantly affected plant density, ranging from 55–366 plants m–2. Fresh and DM yields ranged from 4,957–28,469 kg ha–1 and 661–3,737 kg DM ha–1, respectively. PC ranged from 20.5%–26.6% and was not significantly influenced by row spacing. Total protein yield ranged from 147–884 kg ha–1. Among the five tested genotypes, no significant differences were observed in fresh (7,477–17,776 kg ha–1) or dry (1,122–2,199 kg DM ha–1) biomass, PC (21.2%–26.5%), or protein yield (260–579 kg ha–1), suggesting limited genetic differentiation under the specific environmental and agronomic conditions tested. Amino acid analysis confirmed the presence of all nine essential amino acids, fulfilling over 30% of the recommended daily intake per 100 g DM. These findings highlight YVQ as a promising, sustainable, and protein-rich leafy crop for Mediterranean agriculture. Further research should explore multi-harvest potential, mechanical weeding, and optimized agronomic practices for commercial-scale production.
Keywords:
1. Introduction
2. Materials and Methods
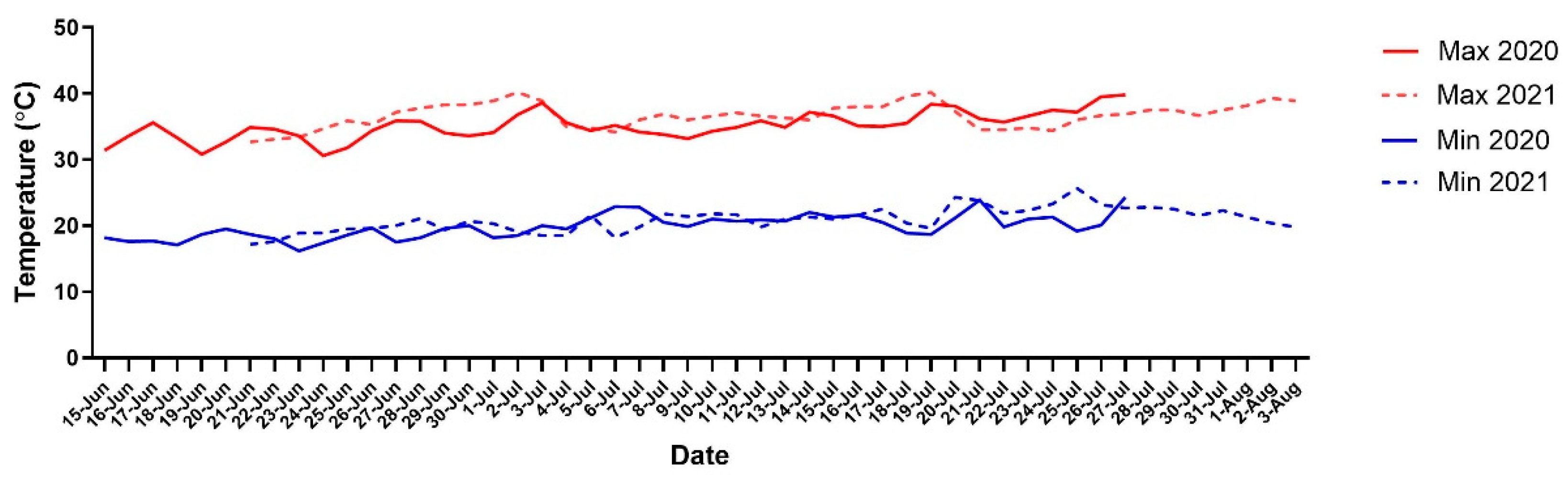
2.1. Experimental Site
2.2. Plant Material and Experimental Design
2.3. Plant Density at Harvest and Yield Measurements
2.4. Plant PC, Protein Yield, and Amino Acid Composition
2.5. Statistical Analysis
3. Results
3.1. Effect of Row Spacing on YVQ
3.1.1. Plant Density and DM
3.1.2. Fresh and DM Yield
3.1.3. PC and Protein Yield
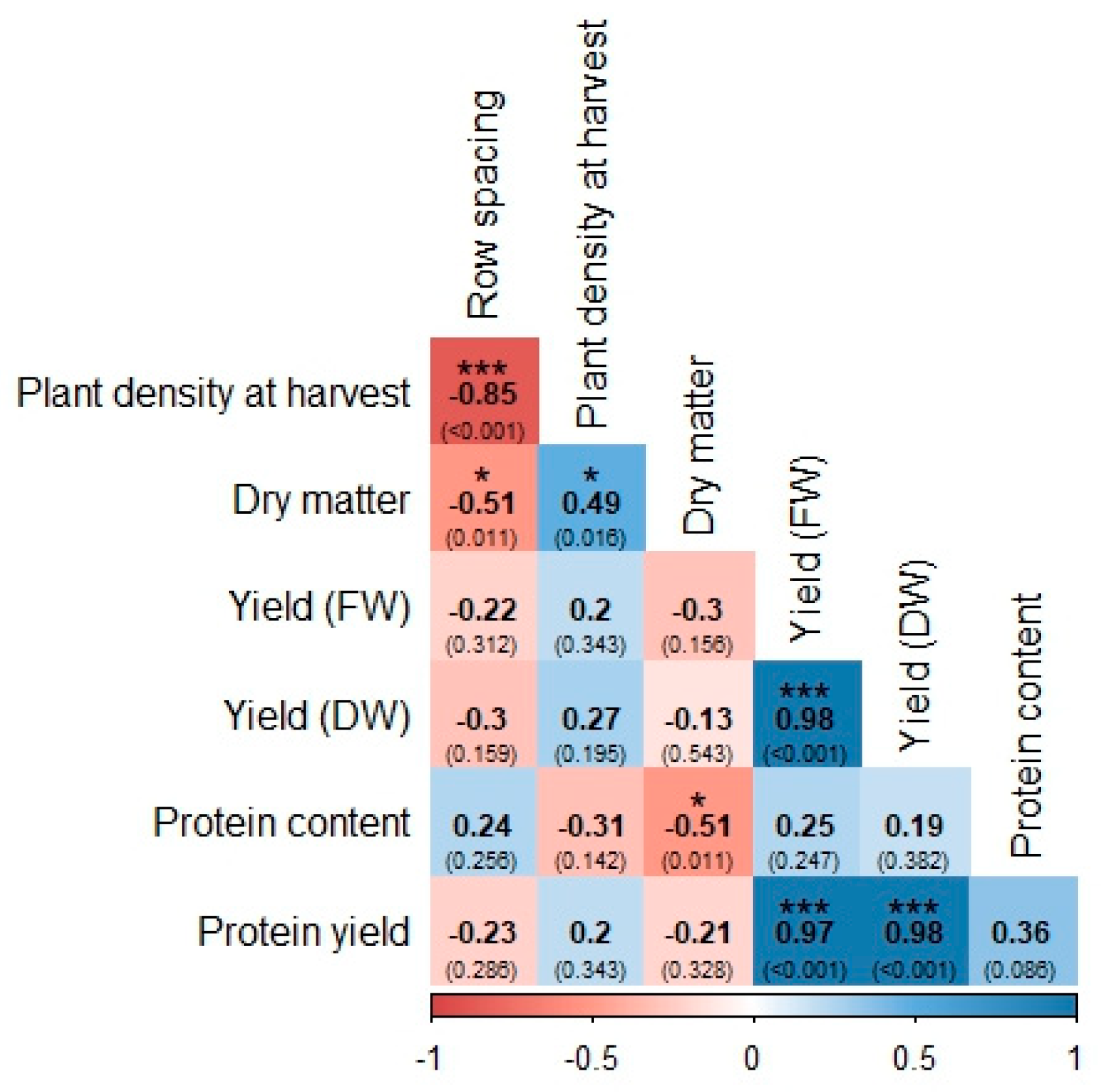
3.1.4. Correlations Between Traits
3.2. Effect of Genotypes on YVQ
3.2.1. Plant Density and DM
3.2.2. Fresh and DM Yield
3.2.3. PC and Protein Yield
3.3. Amino Acid Composition of YVQ
4. Discussion
4.1. Effect of Row Spacing on Yield Parameters
4.2. Effect of Genotype on Yield Parameters
4.3. Amino Acid Composition of YVQ
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAS | days after sowing |
| DM | dry matter |
| FW | fresh weight |
| EAA | essential amino acids |
| PC | protein content |
| YVQ | young vegetative quinoa |
References
- Grandview Research Global Protein Ingredients Market Size Report, 2021-2028. Available online: https://www.grandviewresearch.com/industry-analysis/protein-ingredients-market (accessed on 29 January 2023).
- De Boer, J.; Aiking, H. On the Merits of Plant-Based Proteins for Global Food Security: Marrying Macro and Micro Perspectives. Ecol. Econ. 2011, 70, 1259–1265. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-Based Food and Protein Trend from a Business Perspective: Markets, Consumers, and the Challenges and Opportunities in the Future. Crit Rev Food Sci Nutr 2020, 1–10. [Google Scholar] [CrossRef]
- Pam Ismail, B.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein Demand: Review of Plant and Animal Proteins Used in Alternative Protein Product Development and Production. Anim Front 2020, 10, 53–63. [Google Scholar] [CrossRef]
- Andreotti, F.; Bazile, D.; Biaggi, C.; Callo-concha, D.; Jacquet, J.; Jemal, O.M.; King, O.I.; Mbosso, C.; Padulosi, S.; Speelman, E.N.; et al. When Neglected Species Gain Global Interest: Lessons Learned from Quinoa’s Boom and Bust for Teff and Minor Millet. Glob Food Sec 2022, 32, 100613. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front Plant Sci 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front Plant Sci 2016, 7. [Google Scholar] [CrossRef]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Gill, S.; Butt, K.U.R. Quinoa for Marginal Environments: Toward Future Food and Nutritional Security in MENA and Central Asia Regions. Front Plant Sci 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Pulvento, C.; Bazile, D. Worldwide Evaluations of Quinoa—Biodiversity and Food Security under Climate Change Pressures: Advances and Perspectives. Plants 2023, 12, 868. [Google Scholar] [CrossRef]
- Scanlin, L.; Lewis, K.A. Quinoa as a Sustainable Protein Source: Production, Nutrition, and Processing. In Sustainable Protein Sources; Elsevier Inc., 2017; pp. 223–238 ISBN 9780128027769.
- García-Parra, M.; Zurita-Silva, A.; Stechauner-Rohringer, R.; Roa-Acosta, D.; Jacobsen, S.E. Quinoa (Chenopodium Quinoa Willd.) and Its Relationship with Agroclimatic Characteristics: A Colombian Perspective. Chil J Agric Res 2020, 80, 290–302. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; C.F.R. Ferreira, I. Chemical and Nutritional Characterization of Chenopodium Quinoa Willd (Quinoa) Grains: A Good Alternative to Nutritious Food. Food Chem 2019, 280, 110–114. [CrossRef]
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium Quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9. [Google Scholar] [CrossRef]
- Ceyhun Sezgin, A.; Sanlier, N. A New Generation Plant for the Conventional Cuisine: Quinoa (Chenopodium Quinoa Willd.). Trends Food Sci Technol 2019, 86, 51–58. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa Protein: Composition, Structure and Functional Properties. Food Chem 2019, 299. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Vlachostergios, D.; Baxevanos, D.; Karyotis, T.; Iliadis, C. Adaptation, Agronomic Potential, and Current Perspectives of Quinoa Under Mediterranean Conditions: Case Studies from the Lowlands of Central Greece. Commun Soil Sci Plant Anal 2017, 48, 2612–2629. [Google Scholar]
- Jacobsen, S.E. The Scope for Adaptation of Quinoa in Northern Latitudes of Europe. J Agron Crop Sci 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global Expansion of Quinoa and Challenges for the Andean Region. Glob Food Sec 2020, 26. [Google Scholar] [CrossRef]
- Sellami, M.H.; Pulvento, C.; Lavini, A. Agronomic Practices and Performances of Quinoa under Field Conditions: A Systematic Review. Plants 2021, 10, 1–20. [Google Scholar] [CrossRef]
- Asher, A.; Dagan, R.; Galili, S.; Rubinovich, L. Effect of Row Spacing on Quinoa (Chenopodium Quinoa) Growth, Yield, and Grain Quality under a Mediterranean Climate. Agriculture 2022, 12, 1298. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.; Shock, C.C.; Meng, C.; Qiao, L. Effects of Management Practices on Quinoa Growth, Seed Yield, and Quality. Agronomy 2020, 10. [Google Scholar] [CrossRef]
- Walters, H.; Carpenter-Boggs, L.; Desta, K.; Yan, L.; Matanguihan, J.; Murphy, K. Effect of Irrigation, Intercrop, and Cultivar on Agronomic and Nutritional Characteristics of Quinoa. Agroecol. Sustain. Food Syst. 2016, 40, 783–803. [Google Scholar] [CrossRef]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The Potential of Quinoa (Chenopodium Quinoa) Cultivation in Israel as a Dual-Purpose Crop for Grain Production and Livestock Feed. Sci Hortic 2020, 272, 109534. [Google Scholar] [CrossRef]
- Filik, G. Biodegradability of Quinoa Stalks: The Potential of Quinoa Stalks as a Forage Source or as Biomass for Energy Production. Fuel 2020, 266, 117064. [Google Scholar] [CrossRef]
- Matías, J.; Cruz, V.; Reguera, M. Heat Stress Impact on Yield and Composition of Quinoa Straw under Mediterranean Field Conditions. Plants 2021, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Ramos, N.; Cruz, A.M. Evaluation of Seven Seasonal Crops for Forage Production during the Dry Season in Cuba. Cuban J. Agric. Sci. 2002, 36, 271–276. [Google Scholar]
- Ebeid, H.M.; Kholif, A.E.; El-Bordeny, N.; Chrenkova, M.; Mlynekova, Z.; Hansen, H.H. Nutritive Value of Quinoa (Chenopodium Quinoa) as a Feed for Ruminants: In Sacco Degradability and in Vitro Gas Production. Environ Sci Pollut Res Int 2022, 29, 35241–35252. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Tan, J.; Zeng, C.; Wang, J.; Huang, J.; Hu, Y.; Wu, Q.; Wu, X.; Liu, C.; et al. Quinoa Greens as a Novel Plant Food: A Review of Its Nutritional Composition, Functional Activities, and Food Applications. Crit Rev Food Sci Nutr 2024. [Google Scholar] [CrossRef]
- Rubinovich, L.; Dagan, R.; Lugasi, Y.; Galili, S.; Asher, A. The Potential of Young Vegetative Quinoa (Chenopodium Quinoa) as a New Sustainable Protein-Rich Winter Leafy Crop under Mediterranean Climate. PLoS One 2023, 18, e0290000. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium Quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 1–12. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Sowiński, J.; Jama-Rodzeńska, A. The Effect of Sowing Date and Harvest Time on Leafy Greens of Quinoa (Chenopodium Quinoa Willd.) Yield and Selected Nutritional Parameters. Agriculture (Switzerland) 2021, 11. [Google Scholar] [CrossRef]
- Pathan, S.; Ndunguru, G.; Islam, M.R.; Jhumur, S.T.; Ayele, A.G. Production of Quinoa Leafy Greens in High Tunnel for Season Extension in Missouri. Horticulturae 2023, 9. [Google Scholar] [CrossRef]
- Vazquez-Luna, A.; Cortés, V.P.; Carmona, F.F.; Díaz-Sobac, R. Quinoa Leaf as a Nutritional Alternative. Cienc Investig Agrar 2019, 46, 137–143. [Google Scholar] [CrossRef]
- Pathan, S.; Eivazi, F.; Valliyodan, B.; Paul, K.; Ndunguru, G.; Clark, K. Nutritional Composition of the Green Leaves of Quinoa (Chenopodium Quinoa Willd.). J Food Res 2019, 8, 55. [Google Scholar] [CrossRef]
- Gómez, M.J.R.; Magro, P.C.; Blázquez, M.R.; Maestro-Gaitán, I.; Iñiguez, F.M.S.; Sobrado, V.C.; Prieto, J.M. Nutritional Composition of Quinoa Leafy Greens: An Underutilized Plant-Based Food with the Potential of Contributing to Current Dietary Trends. Food Res Int 2024, 178. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Sułkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and Anticancer Activities of Chenopodium Quinoa Leaves Extracts - in Vitro Study. Food Chem Toxicol 2013, 57, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, V.; Jacobsen, S.E.; Vitanescu, M.; Jitareanu, G.; Butnariu, M.; Munteanu, N.; Stan, T.; Teliban, G.C.; Cojocaru, A.; Mihalache, G. Nutritional and Antinutritional Compounds in Leaves of Quinoa. Food Biosci 2022, 45. [Google Scholar] [CrossRef]
- Lim, J.G.; Park, H.M.; Yoon, K.S. Analysis of Saponin Composition and Comparison of the Antioxidant Activity of Various Parts of the Quinoa Plant (Chenopodium Quinoa Willd.). Food Sci Nutr 2020, 8, 694–702. [Google Scholar] [CrossRef]
- Dick Mastebroek, H.; Limburg, H.; Gilles, T.; Marvin, H.J. Occurrence of Sapogenins in Leaves and Seeds of Quinoa (Chenopodium Quinoa Willd). J Sci Food Agric 2000, 80, 152–156. [Google Scholar] [CrossRef]
- Bernardo Solíz-Guerrero, J.; Jasso De Rodriguez, D.; Rodríguez-García, R.; Luis Angulo-Sánchez, J.; Méndez-Padilla, G. Quinoa Saponins: Concentration and Composition Analysis. In Trends in New Crops and New Uses; J. Janick, A. Whipkey, Eds.; ASHS Press: Alexandria, VA, 2002.
- Abd El-Samad, E.H.; Hussin, S.A.; El-Naggar, A.M.; El-Bordeny, N.E.; Eisa, S.S. The Potential Use of Quinoa as a New Non-Traditional Leafy Vegetable Crop. Biosci. Res. 2018, 15, 3387–3403. [Google Scholar]
- Sief, A.S.; El-Deepah, H.R.A.; Kamel, A.S.M.; Ibrahim, J.F. Effect of Various Inter and Intra Spaces on the Yield and Quality of Quinoa (Chenopodium Quinoa Willd.). J. Plant Production, Mansoura Univ 2015, 6, 371–383. [Google Scholar] [CrossRef]
- Bellalou, A.; Daklo-Keren, M.; Abu Aklin, W.; Sokolskaya, R.; Rubinovich, L.; Asher, A.; Galili, S. Germination of Chenopodium Quinoa Cv. ‘Mint Vanilla’ Seeds under Different Abiotic Stress Conditions. Seed Sci. Technol. 2022, 50, 41–45. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis, 21st Edition (2019) - AOAC International; 2019;
- WHO Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation.; 2007; ISBN 9789241209359.
- Alvar-Beltrán, J.; Verdi, L.; Marta, A.D.; Dao, A.; Vivoli, R.; Sanou, J.; Orlandini, S. The Effect of Heat Stress on Quinoa (Cv. Titicaca) under Controlled Climatic Conditions. J. Agric. Sci. 2020, 158, 255–261. [Google Scholar] [CrossRef]
- Hinojosa, L.; Sanad, M.N.M.E.; Jarvis, D.E.; Steel, P.; Murphy, K.; Smertenko, A. Impact of Heat and Drought Stress on Peroxisome Proliferation in Quinoa. Plant J. 2019, 99, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Areej, F.; Asad, S.A.; Saqib, M.; Anwar-ul-Haq, M.; Afzal, S.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Akram, M.; et al. Differential Effect of Heat Stress on Drought and Salt Tolerance Potential of Quinoa Genotypes: A Physiological and Biochemical Investigation. Plants 2023, 12. [Google Scholar] [CrossRef]
- Matías, J.; Rodríguez, M.J.; Cruz, V.; Calvo, P.; Reguera, M. Heat Stress Lowers Yields, Alters Nutrient Uptake and Changes Seed Quality in Quinoa Grown under Mediterranean Field Conditions. J Agron Crop Sci 2021, 207, 481–491. [Google Scholar] [CrossRef]
- Alon, E.; Shapira, O.; Azoulay-Shemer, T.; Rubinovich, L. Shading Nets Reduce Canopy Temperature and Improve Photosynthetic Performance in ‘Pinkerton’ Avocado Trees during Extreme Heat Events. Agronomy 2022, 12, 1360. [Google Scholar] [CrossRef]
- Spehar, C.R.; Rocha, J.E. da S. Effect of Sowing Density on Plant Growth and Development of Quinoa, Genotype 4.5, in the Brazilian Savannah Highlands. Biosci. J. 2009, 25, 53–58.
- Risi, J.; Galwey, N.W. Effects of Sowing Date and Sowing Rate on Plant Development and Grain Yield of Quinoa (Chenopodium Quinoa) in a Temperate Environment. J Agric Sci 1991, 117, 325–332. [Google Scholar] [CrossRef]
- Bhargava, A.; Sudhir, S.; Deepak, O. Effect of Sowing Dates and Row Spacings on Yield and Quality Components of Quinoa (Chenopodium Quinoa) Leaves. Indian Journal of Agricultural Sciences 2007, 77, 748–751. [Google Scholar]
- Prommarak, S. Response of Quinoa to Emergence Test and Row Spacing in Chiang Mai-Lumphun Valley Lowland Area. Khon Kaen Agric. J. 2014, 42, 8–14. [Google Scholar]
- Casini, P.; Biancofiore, G. Influence of Row Spacing on Canopy and Seed Production in Grain Amaranth (Amaranthus Cruentus L.). Agron. Res 2020, 18, 53–62. [Google Scholar] [CrossRef]
- Phillipus Du Plooy, C.; Beletse, Y.; Mulandana, N.; Mamadi, N.; Plooy, C. DU Effect of Spacing and Transplanting Time on Amaranths Yield. In Proceedings of the African Crop Science Conference Proceedings; 2015; Vol. 9, pp. 243–246.
- Nascimento, C.S.; Filho, A.B.C.; Mendoza-Cortez, J.W.; Nascimento, C.S.; Neto, F.B.; Grangeiro, L.C. Effect of Population Density of Lettuce Intercropped with Rocket on Productivity and Land-Use Efficiency. PLoS One 2018, 13. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Stølen, O.; Jørgensen, I. Cultivation of Quinoa (Chenopodium Quinoa) under Temperate Climatic Conditions in Denmark. J Agric Sci 1994, 122, 47–52. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO The State of Food Security and Nutrition in the World 2022: Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable.; FAO, Rome, 2022.
- Delgado-Iniesta, M.J.; Girona-Ruíz, A.; Sánchez-Navarro, A. Agro-Ecological Impact of Irrigation and Nutrient Management on Spinach (Spinacia Oleracea L.) Grown in Semi-Arid Conditions. Land (Basel) 2023, 12. [Google Scholar] [CrossRef]
| Year | Row spacing (cm) | Plant density at harvest (plant m–2) | Dry matter (%) | Fresh yield (kg ha–1) | Dry yield (kg DMha–1) | Protein content (%) | Protein yield (kg ha–1) |
|---|---|---|---|---|---|---|---|
| 16 | 336 ± 6 a | 13.1 ± 0.6 | 28469 ± 639 a | 3737 ± 243 a | 23.9 ± 1.4 | 884 ± 24 a | |
| 2020 | 26 | 193 ± 27 b | 13.4 ± 0.7 | 27262 ± 251 a | 3662 ± 176 a | 23.5 ± 1.1 | 865 ± 76 a |
| 80 | 55 ± 4 c | 11.4 ± 0.2 | 20986 ± 555 b | 2396 ± 54 b | 26.6 ± 2.2 | 633 ± 39 b | |
| 16 | 345 ± 37 a | 14.4 ± 0.1 a | 7423 ± 209 | 1072 ± 41 | 20.5 ± 0.4 | 220 ± 13 | |
| 2021 | 26 | 166 ± 157 b | 13.4 ± 0.1 b | 6747 ± 1609 | 902 ± 212 | 23.8 ± 1.3 | 209 ± 43 |
| 80 | 65 ± 3 c | 13.3 ± 0.4 b | 4957 ± 775 | 661 ± 105 | 22.3 ± 2 | 147 ± 28 |
| Year | Accession | Plant density at harvest (plant m–2) | Dry matter (%) | Fresh yield (kg ha–1) | Dry yield (kg DM ha–1) |
Protein content (%) | Protein yield (kg ha–1) |
|---|---|---|---|---|---|---|---|
| Red Head | 176 ± 6 a | 12.2 ± 0.6 | 17776 ± 794 | 2175 ± 196 | 24.4 ± 0.8 | 533 ± 56 | |
| Mint Vanilla | 183 ± 16 a | 13.1 ± 0.3 | 16852 ± 389 | 2199 ± 54 | 26.3 ± 1.1 | 579 ± 25 | |
| 2020 | Ivory | 207 ± 14 a | 13.6 ± 0.6 | 15969 ± 752 | 2182 ± 173 | 24.6 ± 2.1 | 539 ± 69 |
| Oro de Valle | 111 ± 4 b | 12.3 ± 0.4 | 14923 ± 1056 | 1852 ± 182 | 26.5 ± 1.7 | 486 ± 40 | |
| Peppermint | 186 ± 6 a | 12.7 ± 0.7 | 16531 ± 1609 | 2136 ± 298 | 25.9 ± 1.4 | 556 ± 85 | |
| Red Head | 279 ± 7 a | 14 ± 0.4 | 9342 ± 1620 | 1292 ± 209 | 22.7 ± 1 | 290 ± 44 | |
| Mint Vanilla | 168 ± 13 b | 14.5 ± 0.3 | 8337 ± 686 | 1209 ± 98 | 21.9 ± 0.5 | 265 ± 19 | |
| 2021 | Ivory | 181 ± 14 b | 14.5 ± 0.4 | 9821 ± 1241 | 1417 ± 177 | 21.5 ± 1 | 300 ± 29 |
| Oro de Valle | 163 ± 6 b | 15.1 ± 0.2 | 7477 ± 1387 | 1122 ± 198 | 23.4 ± 0.7 | 260 ± 42 | |
| Peppermint | 213 ± 20 b | 15.1 ± 0.5 | 9847 ± 793 | 1494 ± 153 | 21.2 ± 1.3 | 315 ± 33 |
| Amino acids (g 100 g DM–1) |
Recommended daily intake (g per 70 kg body weight) a | |
|---|---|---|
| Essential | ||
| Histidine | 0.23 ± 0.01 | 0.7 |
| Isoleucine | 0.55 ± 0.04 | 1.4 |
| Leucine | 1.01 ± 0.05 | 2.73 |
| Lysine | 0.51 ± 0.04 | 2.1 |
| Methionine | 0.22 ± 0.01 | 0.7 |
| Phenylalanine | 0.65 ± 0.04 | 1.75b |
| Threonine | 0.63 ± 0.03 | 1.05 |
| Tryptophan | 0.0588 ± 0.0069 | 0.28 |
| Valine | 0.64 ± 0.05 | 1.82 |
| Non-essential | ||
| Alanine | 0.74 ± 0.05 | |
| Arginine | 0.64 ± 0.04 | |
| Aspartic acid | 1.24 ± 0.06 | |
| Cystine + Cysteine | 0.20 ± 0.01 | |
| Glutamic acid | 1.54 ± 0.5 | |
| Glycine | 0.75 ± 0.03 | |
| Proline | 0.63 ± 0.03 | |
| Serine | 0.65 ± 0.03 | |
| Tyrosine | 0.36 ± 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





