Submitted:
09 April 2025
Posted:
10 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Hospitalization Costs
2.3. Statistics
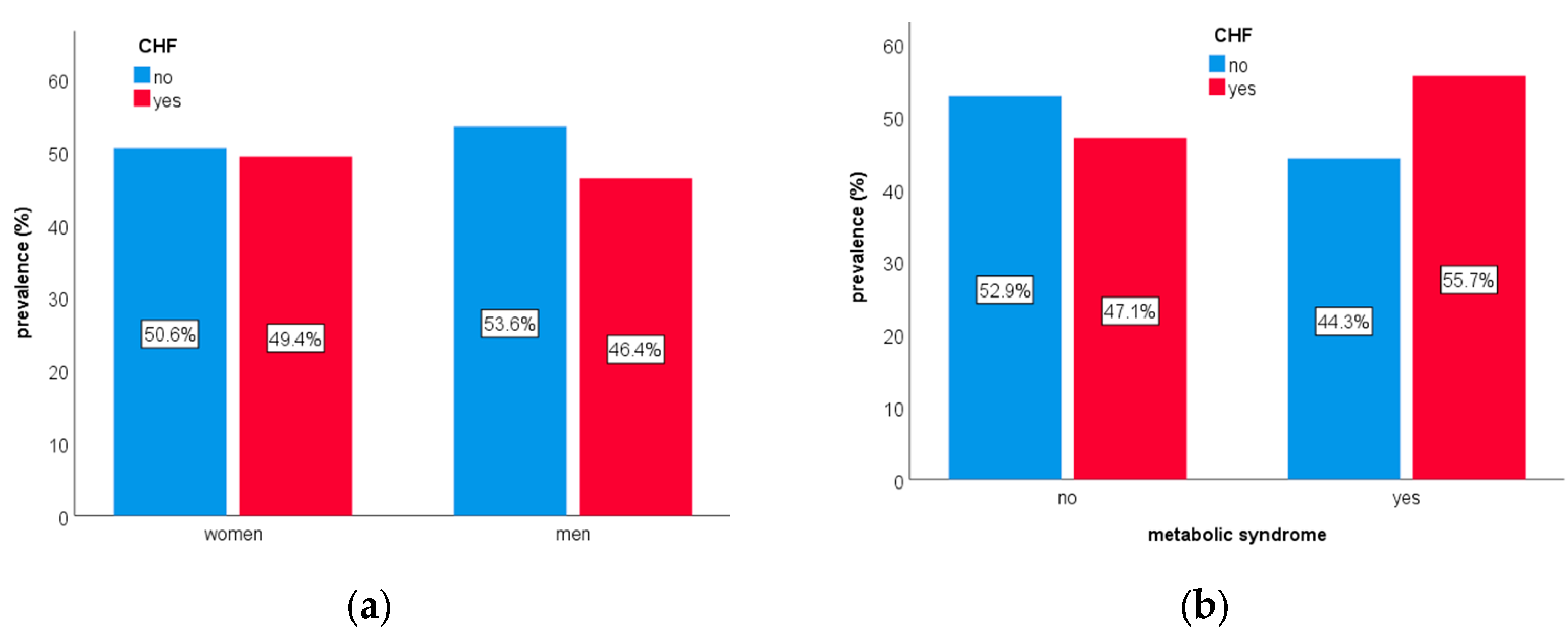
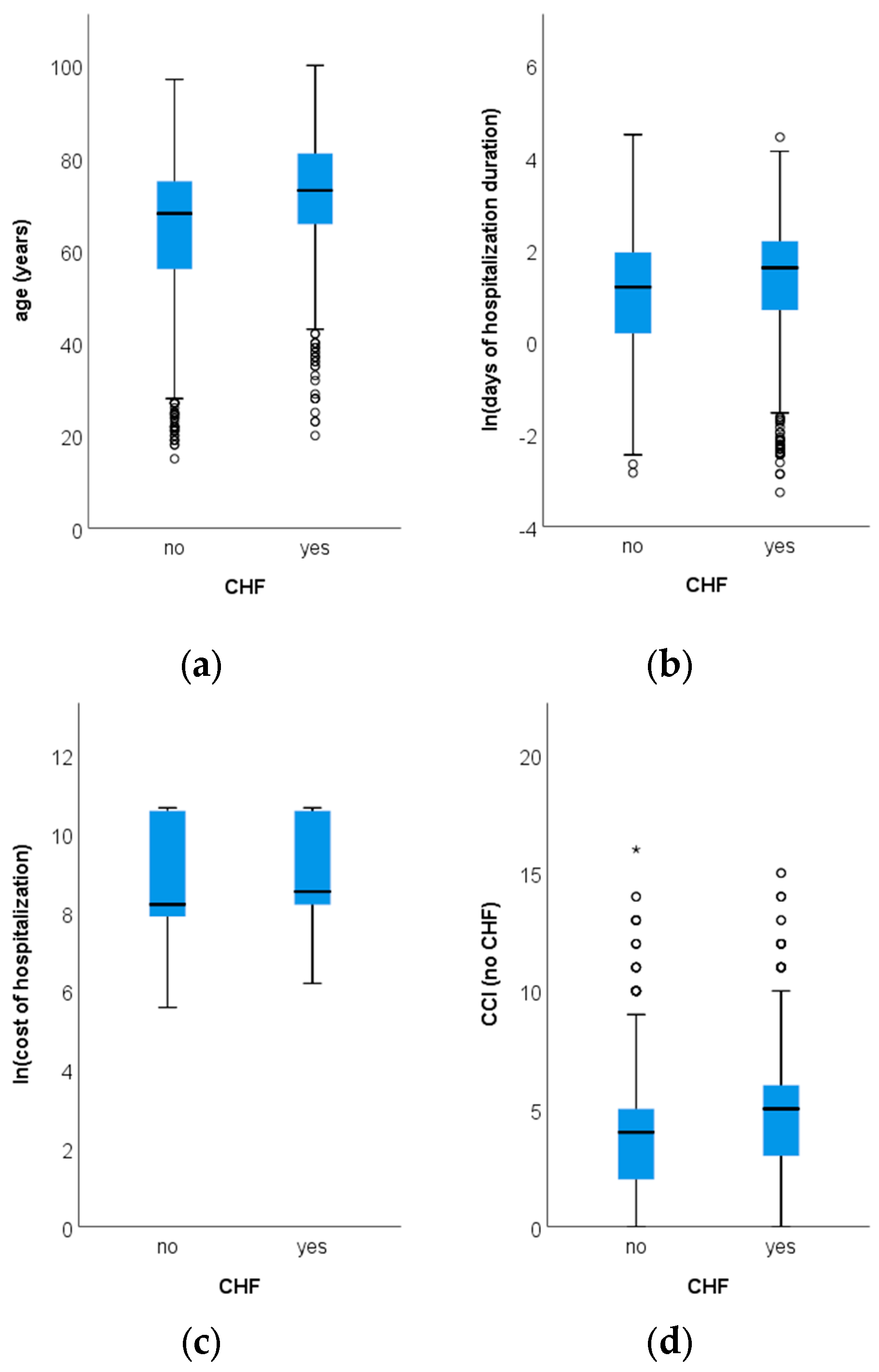
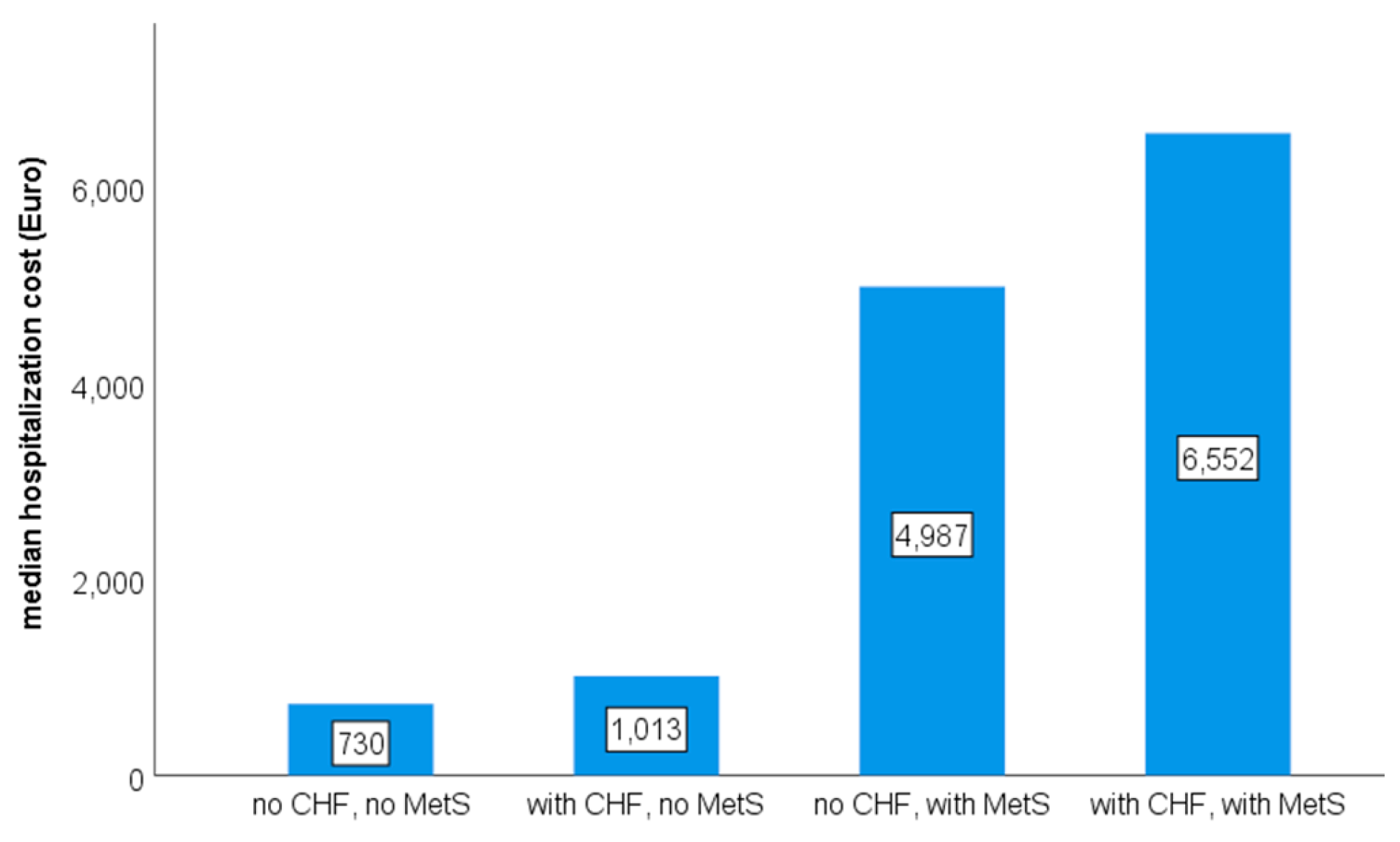
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | acquired immune-deficiency syndrome |
| CCI | Charleston comorbidity index |
| CHF | chronic heart failure |
| CRP | C-reactive protein |
| DRG | Diagnosis-Related Groups |
| HDL | high density lipoproteins |
| ICD | International Classification of Diseases |
| IDF | International Diabetes Foundation |
| IQR | interquartile range |
| MetS | metabolic syndrome |
| SD | standard deviation |
References
- Brake R, Jones ID. Chronic heart failure part 1: pathophysiology, signs and symptoms. Nurs Stand. 2017;31(19):54-63. [CrossRef]
- Elendu C, Amaechi DC, Elendu TC, Fiemotonghan BE, Okoye OK, Agu-Ben CM, et al. A comprehensive review of heart failure: Unraveling the etiology, decoding pathophysiological mechanisms, navigating diagnostic modalities, exploring pharmacological interventions, advocating lifestyle modifications, and charting the horizon of emerging therapies in the complex landscape of chronic cardiac dysfunction. Medicine (Baltimore). 2024;103(3):e36895. [CrossRef]
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-726. [CrossRef]
- Tomasoni D, Fonarow GC, Adamo M, Anker SD, Butler J, Coats AJS, et al. Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2022;24(3):431-41. [CrossRef]
- Cunningham JW, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, et al. Dapagliflozin in Patients Recently Hospitalized With Heart Failure and Mildly Reduced or Preserved Ejection Fraction. J Am Coll Cardiol. 2022;80(14):1302-10. [CrossRef]
- Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021;384(2):117-28. [CrossRef]
- Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272-87. [CrossRef]
- Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404-13. [CrossRef]
- Heidenreich PA, Fonarow GC, Opsha Y, Sandhu AT, Sweitzer NK, Warraich HJ, Chair HSSCM. Economic Issues in Heart Failure in the United States. J Card Fail. 2022;28(3):453-66. [CrossRef]
- Tran DT, Ohinmaa A, Thanh NX, Howlett JG, Ezekowitz JA, McAlister FA, Kaul P. The current and future financial burden of hospital admissions for heart failure in Canada: a cost analysis. CMAJ Open. 2016;4(3):E365-E70. [CrossRef]
- Khan SU, Khan MZ, Alkhouli M. Trends of Clinical Outcomes and Health Care Resource Use in Heart Failure in the United States. J Am Heart Assoc. 2020;9(14):e016782. [CrossRef]
- Rosano GMC, Seferovic P, Savarese G, Spoletini I, Lopatin Y, Gustafsson F, et al. Impact analysis of heart failure across European countries: an ESC-HFA position paper. ESC Heart Fail. 2022;9(5):2767-78. [CrossRef]
- Norhammar A, Bodegard J, Vanderheyden M, Tangri N, Karasik A, Maggioni AP, et al. Prevalence, outcomes and costs of a contemporary, multinational population with heart failure. Heart. 2023;109(7):548-56. [CrossRef]
- Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-Effectiveness of Left Ventricular Assist Devices in Ambulatory Patients With Advanced Heart Failure. JACC Heart Fail. 2017;5(2):110-9. [CrossRef]
- Urbich M, Globe G, Pantiri K, Heisen M, Bennison C, Wirtz HS, Di Tanna GL. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014-2020). Pharmacoeconomics. 2020;38(11):1219-36. [CrossRef]
- Berry C, Murdoch DR, McMurray JJ. Economics of chronic heart failure. Eur J Heart Fail. 2001;3(3):283-91. [CrossRef]
- Khan MS, Shahid I, Bennis A, Rakisheva A, Metra M, Butler J. Global epidemiology of heart failure. Nat Rev Cardiol. 2024;21(10):717-34. [CrossRef]
- Koutlas A, Jenkins P. Reducing Hospital Admissions for Patients with Heart Failure by Implementing the Chronic Care Management Framework: A Cost, Quality and Satisfaction Improvement Project. J Dr Nurs Pract. 2022. [CrossRef]
- Leon-Justel A, Morgado Garcia-Polavieja JI, Alvarez-Rios AI, Caro Fernandez FJ, Merino PAP, Galvez Rios E, et al. Biomarkers-based personalized follow-up in chronic heart failure improves patient’s outcomes and reduces care associate cost. Health Qual Life Outcomes. 2021;19(1):142. [CrossRef]
- Maru S, Byrnes J, Carrington MJ, Chan YK, Thompson DR, Stewart S, et al. Cost-effectiveness of home versus clinic-based management of chronic heart failure: Extended follow-up of a pragmatic, multicentre randomized trial cohort - The WHICH? study (Which Heart Failure Intervention Is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care). Int J Cardiol. 2015;201:368-75. [CrossRef]
- Miura Y, Fukumoto Y, Shiba N, Miura T, Shimada K, Iwama Y, et al. Prevalence and clinical implication of metabolic syndrome in chronic heart failure. Circ J. 2010;74(12):2612-21. [CrossRef]
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469-80. [CrossRef]
- Purwowiyoto SL, Prawara AS. Metabolic syndrome and heart failure: mechanism and management. Med Pharm Rep. 2021;94(1):15-21. [CrossRef]
- van der Hoef CCS, Boorsma EM, Emmens JE, van Essen BJ, Metra M, Ng LL, et al. Biomarker signature and pathophysiological pathways in patients with chronic heart failure and metabolic syndrome. Eur J Heart Fail. 2023;25(2):163-73. [CrossRef]
- Roytman AP, Sedova NA, Godkov MA. Laboratory indicators of pathological changes in patients with chronic heart failure with metabolic syndrome. Klin Lab Diagn. 2021;66(2):75-9. [CrossRef]
- Bossone E, Arcopinto M, Iacoviello M, Triggiani V, Cacciatore F, Maiello C, et al. Multiple hormonal and metabolic deficiency syndrome in chronic heart failure: rationale, design, and demographic characteristics of the T.O.S.CA. Registry. Intern Emerg Med. 2018;13(5):661-71. [CrossRef]
- Kearney MT. Chronic heart failure: a missing component of the metabolic syndrome? Diab Vasc Dis Res. 2009;6(3):145. [CrossRef]
- Nichols GA, Amitay EL, Chatterjee S, Steubl D. The Bidirectional Association of Chronic Kidney Disease, Type 2 Diabetes, Atherosclerotic Cardiovascular Disease, and Heart Failure: The Cardio-Renal-Metabolic Syndrome. Metab Syndr Relat Disord. 2023;21(5):261-6. [CrossRef]
- Leon-Roman J, Azancot MA, Marouco C, Patricio-Liebana M, Zamora JI, Ramos Terrades N, et al. A New Era in the Management of Cardiorenal Syndrome: The Importance of Cardiorenal Units. Cardiorenal Med. 2025;15(1):174-83. [CrossRef]
- Yaqoob N, Khalid F, Khan MF, Anwar W, Khan MF, Iqbal MH. Prevalence Of Cardiorenal Syndrome In Patients Admitted For Acute Decompensated Heart Failure And Its Correlation With In-Hospital Outcomes. J Ayub Med Coll Abbottabad. 2024;36(4):773-7. [CrossRef]
- Perrone-Filardi P, Savarese G, Scarano M, Cavazzina R, Trimarco B, Minneci S, et al. Prognostic impact of metabolic syndrome in patients with chronic heart failure: data from GISSI-HF trial. Int J Cardiol. 2015;178:85-90. [CrossRef]
- Tadaki S, Sakata Y, Miura Y, Miyata S, Asakura M, Shimada K, et al. Prognostic Impacts of Metabolic Syndrome in Patients With Chronic Heart Failure - A Multicenter Prospective Cohort Study. Circ J. 2016;80(3):677-88. [CrossRef]
- Ricardo SJ, Araujo MYC, Santos LLD, Romanzini M, Fernandes RA, Turi-Lynch BC, Codogno JS. Burden of metabolic syndrome on primary healthcare costs among older adults: A cross-sectional study. Sao Paulo Med J. 2024;142(6):e2023215. [CrossRef]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83. [CrossRef]
- Radu CP, Chiriac DN, Vladescu C. Changing patient classification system for hospital reimbursement in Romania. Croat Med J. 2010;51(3):250-8. [CrossRef]
- Bonapace S, Mantovani A. Do Sex and Gender-Related Differences Account to Different Risk of Developing Heart Failure in Middle-Aged People with Metabolic Syndrome? Metabolites. 2024;14(10). [CrossRef]
- Chandra A, Skali H, Claggett B, Solomon SD, Rossi JS, Russell SD, et al. Race- and Gender-Based Differences in Cardiac Structure and Function and Risk of Heart Failure. J Am Coll Cardiol. 2022;79(4):355-68. [CrossRef]
- Ciutac AM, Pana T, Dawson D, Myint PK. Sex-related differences in heart failure patients: physiological mechanisms of cardiovascular ageing and evidence-based sex-specific medical therapies. Ther Adv Cardiovasc Dis. 2025;19:17539447241309673. [CrossRef]
- Kim TE, Kim DY, Kim H, Kim SH. Sex and Age Differences in the Impact of Metabolic Syndrome on Heart Failure Development. Metabolites. 2024;14(12). [CrossRef]
- Yang X, Wen Y, Peng H, Zhu H, Wang WE, Zhou J. Gender Differences in Anxiety, Depression, Insomnia, and Quality of Life in Heart Failure With Preserved Ejection Fraction: A Multicenter, Cross-sectional Study. J Cardiovasc Nurs. 2023;38(5):425-32. [CrossRef]
- Tapia J, Basalo M, Enjuanes C, Calero E, Jose N, Ruiz M, et al. Psychosocial factors partially explain gender differences in health-related quality of life in heart failure patients. ESC Heart Fail. 2023;10(2):1090-102. [CrossRef]
- Lim A, Benjasirisan C, Tebay J, Liu X, Badawi S, Himmelfarb CD, et al. Gender Differences in Disease Burden, Symptom Burden, and Quality of Life Among People Living With Heart Failure and Multimorbidity: Cross-Sectional Study. J Adv Nurs. 2025. [CrossRef]
- Shi K, Zhang G, Fu H, Li XM, Jiang L, Gao Y, et al. Sex differences in clinical profile, left ventricular remodeling and cardiovascular outcomes among diabetic patients with heart failure and reduced ejection fraction: a cardiac-MRI-based study. Cardiovasc Diabetol. 2024;23(1):266. [CrossRef]
- Tang WHW. Targeting Inflammation in Heart Failure Prevention: Are There Sex Differences? JACC Heart Fail. 2025;13(3):450-2. [CrossRef]
- Fluschnik N, Strangl F, Kondziella C, Gossling A, Becher PM, Schrage B, et al. Gender differences in characteristics and outcomes in heart failure patients referred for end-stage treatment. ESC Heart Fail. 2021;8(6):5031-9. [CrossRef]
- Saldarriaga C, Garcia-Arango M, Valentina Lopez L, Contreras J. Sex Differences in Worsening Heart Failure: Learning From Real-world Evidence. J Card Fail. 2024;30(8):991-3. [CrossRef]
- Kocabas U, Kivrak T, Yilmaz Oztekin GM, Tanik VO, Ozdemir I, Kaya E, et al. Gender-related clinical and management differences in patients with chronic heart failure with reduced ejection fraction. Int J Clin Pract. 2021;75(3):e13765. [CrossRef]
- Cediel G, Codina P, Spitaleri G, Domingo M, Santiago-Vacas E, Lupon J, Bayes-Genis A. Gender-Related Differences in Heart Failure Biomarkers. Front Cardiovasc Med. 2020;7:617705. [CrossRef]
- Lee SY, Park SM. Sex differences in diagnosis and treatment of heart failure: toward precision medicine. Korean J Intern Med. 2025;40(2):196-207. [CrossRef]
- Qiu W, Cai A, Wu S, Zhu Y, Zheng H, Feng Y. Sex- and age-specific differences in the associations between comorbidity and incident heart failure. QJM. 2025. [CrossRef]
- Qiu W, Wang W, Wu S, Zhu Y, Zheng H, Feng Y. Sex differences in long-term heart failure prognosis: a comprehensive meta-analysis. Eur J Prev Cardiol. 2024;31(17):2013-23. [CrossRef]
- Chen CC, Chiu CC, Hao WR, Hsu MH, Liu JC, Lin JL. Sex differences in clinical characteristics and long-term clinical outcomes in Asian hospitalized heart failure patients. ESC Heart Fail. 2024;11(5):3095-104. [CrossRef]
- Salem K, ElKhateeb O. Gender-adjusted and age-adjusted economic inpatient burden of congestive heart failure: cost and disability-adjusted life-year analysis. ESC Heart Fail. 2017;4(3):259-65. [CrossRef]
- Cremers HP, Theunissen LJHJ, Essers PPM, van de Ven ART, Spee R, Verbunt R, et al. Gender differences in Heart Failure; Data on Outcomes and Costs. European Society of Cardiology (Virtual Journal). 2020. DOI:.
- Janwanishstaporn S, Karaketklang K, Krittayaphong R. National trend in heart failure hospitalization and outcome under public health insurance system in Thailand 2008-2013. BMC Cardiovasc Disord. 2022;22(1):203. [CrossRef]
- Aizpuru F, Millan E, Garmendia I, Mateos M, Librero J. Hospitalizations for heart failure: Epidemiology and health system burden based on data gathered in routine practice. Medicina Clínica Práctica. 2020;3(4-5):100140. [CrossRef]
- Alosaimi FD, Abalhassan M, Alhaddad B, Alzain N, Fallata E, Alhabbad A, Alassiry MZ. Prevalence of metabolic syndrome and its components among patients with various psychiatric diagnoses and treatments: A cross-sectional study. Gen Hosp Psychiatry. 2017;45:62-9. [CrossRef]
- Nguyen NT, Nguyen TN, Nguyen KM, Tran HPN, Huynh KLA, Hoang SV. Prevalence and impact of metabolic syndrome on in-hospital outcomes in patients with acute myocardial infarction: A perspective from a developing country. Medicine (Baltimore). 2023;102(45):e35924. [CrossRef]
- Park HJ, Jung JH, Han K, Shin J, Lee Y, Chang Y, et al. Association between metabolic syndrome and mortality in patients with COVID-19: A nationwide cohort study. Obes Res Clin Pract. 2022;16(6):484-90. [CrossRef]
- Checa C, Canelo-Aybar C, Suclupe S, Ginesta-Lopez D, Berenguera A, Castells X, et al. Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19(21). [CrossRef]
- Wang C, Ba Y, Ni J, Huang R, Du X. Role of Telemedicine Intervention in the Treatment of Patients with Chronic Heart Failure: A Systematic Review and Meta-analysis. Anatol J Cardiol. 2024;28(4):177-86. [CrossRef]
| diagnosis | ICD10 code |
|---|---|
| chronic heart failure | I50 |
| myocardial infarction | I21, I25 |
| peripheral vascular disease | I73 |
| cerebrovascular disease | I60, I61, I63, I64, I67, G45.8, G45.9 |
| dementia | F00, F01, F03 |
| chronic pulmonary disease | J44 |
| rheumatic disease | M05, M06, M45, L40.5, M07, M32, M33, M34, M35 |
| peptic ulcer disease | K27 |
| cirrhosis | K70, K71, K74, K76 |
| variceal bleeding | I85.0, I98.3 |
| diabetes mellitus | E10, E11, E12, E13, E14 |
| hemiplegia | G81 |
| chronic kidney disease | N18 |
| solid tumor | C |
| metastatic cancer | C78, C79, C80 |
| leukemia | C90, C91, C92, C93, C94, C95 |
| lymphoma | C81, C82, C83, C84, C85, C86, C88 |
| AIDS | B20, B21, B22, B23, B24 |
| obesity | E66 |
| arterial hypertension | I10 |
| dyslipidemia | E78 |
| variable | observed |
|---|---|
| women | 53.9% |
| men | 46.1% |
| age (years, average ± SD) | 68.7 ± 13.4 |
| hospitalization duration (days, median (IQR) | 4.0 (5.9) |
| total cost of hospitalization (€, median (IQR)* | 1002 (7338) |
| cost per day of hospitalization (€, median (IQR)* | 322 (1477) |
| Charlson Comorbidity Index (average ± SD) | 4.8 ± 2.5 |
| diagnosis | prevalence | diagnosis | prevalence |
| chronic heart failure | 48.0% | hemiplegia | 0.4% |
| myocardial infarction | 18.8% | chronic kidney disease | 19.8% |
| peripheral vascular disease | 0.9% | solid tumor | 8.7% |
| cerebrovascular disease | 6.5% | metastatic cancer | 2.8% |
| dementia | 4.8% | leukemia | 0.5% |
| chronic pulmonary disease | 9.6% | lymphoma | 0.3% |
| rheumatic disease | 2.9% | AIDS | 0.1% |
| peptic ulcer disease | 0.0% | obesity | 19.8% |
| cirrhosis | 17.3% | arterial hypertension | 50.0% |
| variceal bleeding | 0.0% | dyslipidemia | 38.5% |
| diabetes mellitus | 30.5% | metabolic syndrome | 11.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





