1. Introduction
White-tailed deer (
Odocoileus virginianus) (WTD) are the most abundant wild ungulate in North America. The transmission of SARS-CoV-2, the COVID virus, between humans and deer [
1], as well as the transmission of tuberculosis between deer and cattle [
2], demonstrate the central role white-tailed deer play at the human-livestock-wildlife interface. Furthermore, farmed WTD may play a dynamic role in zoonotic pathogen transmission as deer are susceptible to Q-fever, leptospirosis, salmonellosis, cryptosporidiosis and giardiasis [
3].
Chronic wasting disease (CWD), a spongiform encephalopathy, is present in both free-ranging and farmed deer, but has not been documented in humans or livestock. Since its first report in 1967, CWD has spread to 26 US states and 3 Canadian provinces [
4]. Additionally, captive wild ruminants in the United States including sambar deer (
Cervus unicolor), WTD, waterbuck (
Kobus ellipsiprymnus), and elk (
Cervus elaphus), harbor coronaviruses (CoVs) that are biologically, genetically, and antigenically closely related to bovine CoV (BCoV) [
5]. Lastly, WTD have been reported to be susceptible to bovine viral diarrhea virus (BVDV) [
6,
7] and can produce persistently infected (PI) fawns [
8], thus serving as a source for BVDV infection.
Given the susceptibility of WTD to a variety of diseases, methods for prevention and disease control in WTD are an important consideration. Currently in the United States, there are approximately 4,000 white-tailed deer agricultural operations with an approximate revenue of
$44 million annually [
9]. Knowledge of deer health is imperative to maintain such operations and routine vaccinations are part of any herd health program. Typically, ruminant vaccines used in WTD are used off-label, as no preventive label claims are specific for WTD. In general, knowledge of vaccine-induced immune responses or vaccine efficacy are lacking for most wildlife species. Given the use of commercial vaccines in farmed WTD, and their susceptibility and potential role in transmission for various diseases, understanding if and how WTD respond to these vaccines is critical for our ability to enhance animal health and implement disease management strategies. To bridge this knowledge gap, we use a modified live vaccine (MLV) and a killed viral vaccine (KV) against BVDV, an economically important pathogen, documented to be spread between WTD and cattle [
10], and assess humoral and cellular mediated immune (CMI) responses in WTD post-vaccination.
2. Materials and Methods
2.1. Animals, Vaccination, and Sample Collection
White-tailed deer were hand raised and housed at the National Animal Disease Center (NADC) in Ames, Iowa. All work involving animals was conducted in accordance with recommendations in the Care and Use of Laboratory Animals of the National Institutes of Health and the Guide for the Care and Use of Agricultural Animals in Research and Teaching. Additionally, all animal related procedures were approved by and carried out under the NADC Institutional Animal Care and Use Committee (IACUC) guidelines and oversight. Additionally, all procedures involving animals were approved by the NADC IACUC (Protocol number: ARS-2020-909). Eighteen, 7–9-month-old, female and castrated male white-tailed deer were divided into 3 experimental groups, non-vaccinated controls (n = 6), killed vaccine (KV) (n = 6) and modified live vaccine (MLV) (n = 6). Deer in the KV group were vaccinated with the commercially available multivalent vaccine Vira Shield™ 6 (Elanco Animal Health, Inc., Greenfield, IN) containing BVDV type 1 and type 2, bovine herpes virus-1 (BHV-1), bovine respiratory syncytial virus (BRSV) and parainfluenza-3 (PI-3). Deer in the MLV group were vaccinated with the commercially available pentavalent vaccine Bovi-Shield Gold 5 (Zoetis, Inc., Parsippany, NJ) containing BVDV type 1 and type 2, BHV-1, BRSV, and PI-3. The two vaccines were administered at the recommended volume and per recommended route, in the cervical area, according to manufacturers’ instructions. Approximately 4 weeks post-initial vaccination, deer in the KV group were re-vaccinated, again, per label dose and route of administration, according to the manufacturer’s instructions. Blood samples were collected from the jugular vein prior to initial vaccination and at approximately 4 and 8 weeks post-initial vaccination.
2.2. BVDV Strains
For
in vitro stimulation of peripheral blood mononuclear cells (PBMC), field strains of non-cytopathic BVDV-1a (PI34) and BVDV-2a (PI28) were utilized as previously described for cattle [
11]. For assessment of serological responses cytopathic reference strains BVDV-1a (Singer) and BVDV-2a (296c) were used. Propagation of strains was performed as previously described [
12]. Viral titers were determined through serial 10-fold dilutions, individually run in quintuplets on bovine turbinate (BTu) cells. Cytopathic effect (CPE) was evaluated as previously described [
13].
2.3. Serology
To assess responses to vaccination, titers to bovine viral diarrhea virus 1 and 2 (BVDV1 and BVDV2) were measured from serum samples. Blood was collected into serum separation tubes and centrifuged at 1,200x g at room temperature for 25 minutes. Serum was then aliquoted and stored at -80° Celsius (C) until analysis. Evaluation of titer responses to vaccination was performed as assessed by determination of a virus neutralization titer (VNT) using cytopathic isolates BVDV-1a-Singer and BVDV-2a-296c as described [
12]. Briefly, serum samples were serially diluted from 1:2 to 1:4096 in minimal essential media (MEM) using flat bottom 96 well plates, and 200 TCID
50 of virus was added to each well. Samples and virus were incubated for 1 hour at 37° C with 5% CO
2. Following incubation, 2 x 10
5 BTu cells were added to each well and incubated for 4 days at 37° C with 5% CO
2. Dilution endpoints were determined by observation of cytopathic effect (CPE) in the BTu cell monolayer. Results were expressed as the antilog base 10 of the highest serum dilution able to inhibit CPE in the monolayer. Sample dilutions were run in triplicates and VNT were calculated by the Spearman-Karber method.
2.4. Peripheral Blood Mononuclear Cell (PBMC) Isolation and Stimulation
Blood samples were collected into acid citrate dextrose (ACD) tubes and PBMC were isolated using SepMate tubes according to manufacturer’s recommendations (Stemcell Technologies, Cambridge, MA), as described previously [
11,
14]. Following isolation, PMBC were passed through a 40 μm filter to remove cellular debris. Viability and counts were then determined via the Muse™ Cell analyzer (Cytek, Fremont, CA) according to manufacturer’s recommendations. PMBC were adjusted to 1 x 10
7 cells per ml and approximately 1 x 10
6 live cells in 100 μl of cell suspension were added onto 96-well round bottom plates containing 100 μl of complete RPMI medium, supplemented as described previously [
14]. All samples were plated in duplicate for each stimulation condition and plates were incubated at 37° C with 5% CO
2.
2.5. In Vitro Stimulation Conditions
Mitogen and antigen stimulations were performed as previously described [
15]. Briefly, as a positive control for stimulation, 50 μl of media was removed from the respective wells and replaced with 50 μl of cell stimulation cocktail (Phorbol myristate acetate (PMA) and ionomycin; 8 μl diluted in 1 ml of complete RPMI media; (eBioscience, San Diego, CA). For antigen stimulations, 50 μl of media was removed from the respective wells and replaced with 50 μl of BVDV-1a (PI34) or 50 μl of BVDV-2a (PI28), at a multiplicity of infection (MOI) of approximately 1. Non-stimulated wells were left untouched. Following the addition of mitogen or antigen, plates were returned to the incubator and maintained at 37° C with 5% CO
2. Stimulation times were approximately 1.5 hours for mitogen and approximately 24 hours for antigen.
2.6. Staining for Flow Cytometry and PrimeFlow
Cells were processed for flow cytometry analysis as previously described [
15]. Briefly, plates were removed from the incubator and centrifuged at 300x g for 4 minutes at room temperature. The supernatant was discarded and all PMBC were resuspended in 100 μl of phosphate buffered saline (PBS). At this time duplicate wells were combined, and the pooled samples transferred to a new 96-well round bottom plate for further processing. PBMC were then washed twice at 300x g for 4 minutes and then incubated with 50 μl of a 1:100 dilution of a primary monoclonal antibody cocktail against CD4 (Clone 17D, IgG1), CD8 (Clone ST8, IgM), and γδ (Clone GB21A, IgG2b) for 15 minutes at room temperature (RT) (Washington State Monoclonal Antibody Center, Pullman, WA). After the incubation period, 50 μl of a cocktail including the following fluorescently-labeled secondary antibodies were added: anti-IgG1 BUV395 (BD Bioscience, San Diego, CA), FITC-labeled anti-IgM (Biolegend, San Diego, CA), and BV711-labeled anti-IgG2a (BD Bioscience). Secondary antibodies were incubated for 15 minutes at RT in the dark. PMBC were then washed twice in PBS as described above and further processed for the PrimeFlow assay for IFN-γ detection as previously described [
11,
14]. Proprietary specific oligonucleotide (RNA) probes designed specially to detect WTD IFN-γ (6007031-210V) labeled with AF750, were designed by Thermo Fisher Scientific. All data was collected using the BD FACS Symphony flow cytometer (BD Biosciences, San Diego, CA).
2.7. Data Analysis
The frequency of each T cell subset (CD4, CD8 or γδ), and frequencies of IFN-γ mRNA expressing T cell population were determined using FlowJo™ software (FlowJo, LLC, BD Bioscience, Ashland, OR). Average VNT were determined for each respective group using individual VNT values for each respective animal at each respective timepoint. As previously described in VNT methods, the neutralization results of the 3 wells at each respective dilution were evaluated for neutralization or lack of CPE. These results were calculated for the VNT and were used to convert to a log2 value according to Spearman-Karber method. All data were graphed and analyzed using GraphPad Prism 9 (GraphPad Software, Boston, MA). Statistical significance was evaluated using a 2-way ANOVA with multiple comparisons with a Tukey’s correction.
3. Results
3.1. Neutralizing Antibody Titers to BVDV-1 and BVDV-2a
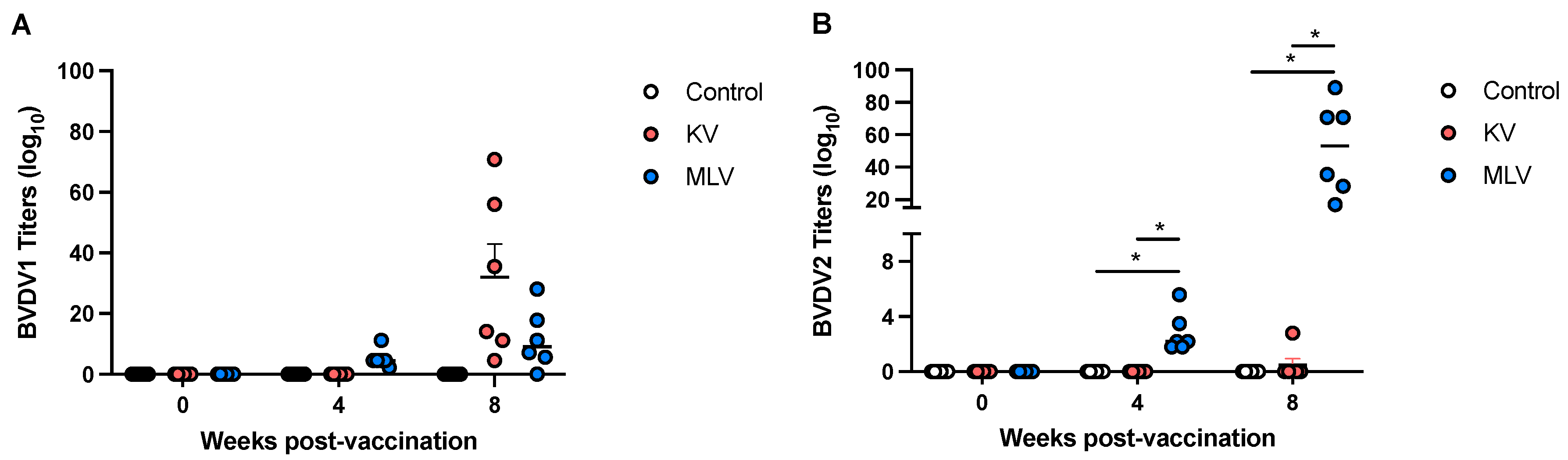
We assessed serological responses to BVDV-1a and BVDV-2a via VNT. Shown in
Figure 1 are individual and average VNT values for each experimental group. Prior to vaccination, none of the deer had any serological responses to either strain tested. At 4 weeks post vaccination, we observed measurable VNT to BVDV-1 following MLV vaccination, and by 8 weeks post-vaccination, titers to BVDV-1 were observed in both KV and MLV vaccination groups (
Figure 1A). Titers to BVDV-2a were only observed in the MLV vaccinate group, at both 4- and 8-weeks post-vaccination (
Figure 1B). These findings are consistent with the BVDV strains present in each vaccine used in this study, with the KV containing BVDV-1 and the MLV containing BVDV-2a. Overall, MLV vaccinated animals demonstrated a greater serological response at the last time point tested with mean VNT of 51.9 ±0.467 to BVDV-2a as compared to the KV vaccinated animals with mean VNT of 32 ±10.9 to BVDV-1. Additionally, statistically significant differences in BVDV-2a VNT were only observed for the MLV vaccinated animals as compared to both control and KV groups (
Figure 1B).
3.2. Frequency of Circulating T Cell Subsets and Antigen-Specific IFN-γ Responses
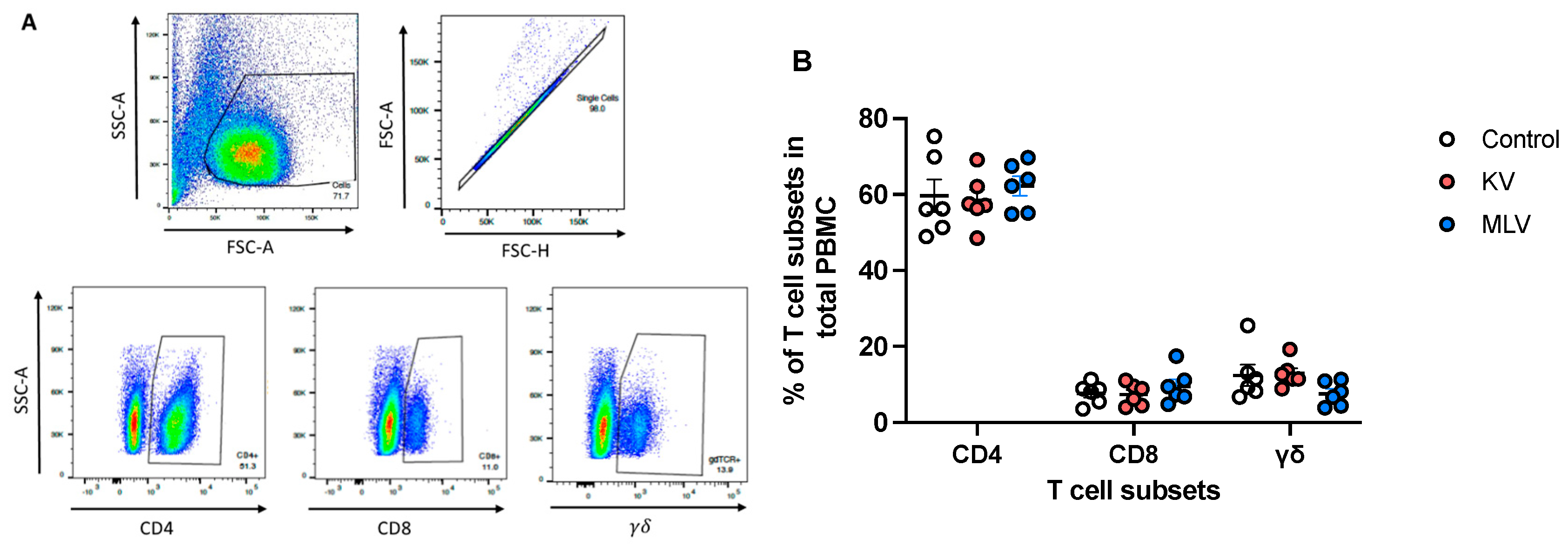
Peripheral blood mononuclear cells (PMBC) were isolated from whole blood samples collected at 8 weeks post-vaccination, stimulated
in vitro and analyzed via flow cytometry for frequencies of CD4, CD8, γδ T cell subsets (
Figure 2A, representative dot plots). Overall, CD4
+ T cells constituted the majority of circulating PBMC (~60%), followed by CD8
+ T cells (~8%), and γδ T cells (~10%) (
Figure 2B). Frequencies of these different T cell subsets were similar across experimental groups (Control, KV and MLV), suggesting that vaccination did not significantly affect the distribution of these cells, at least at the time point analyzed.
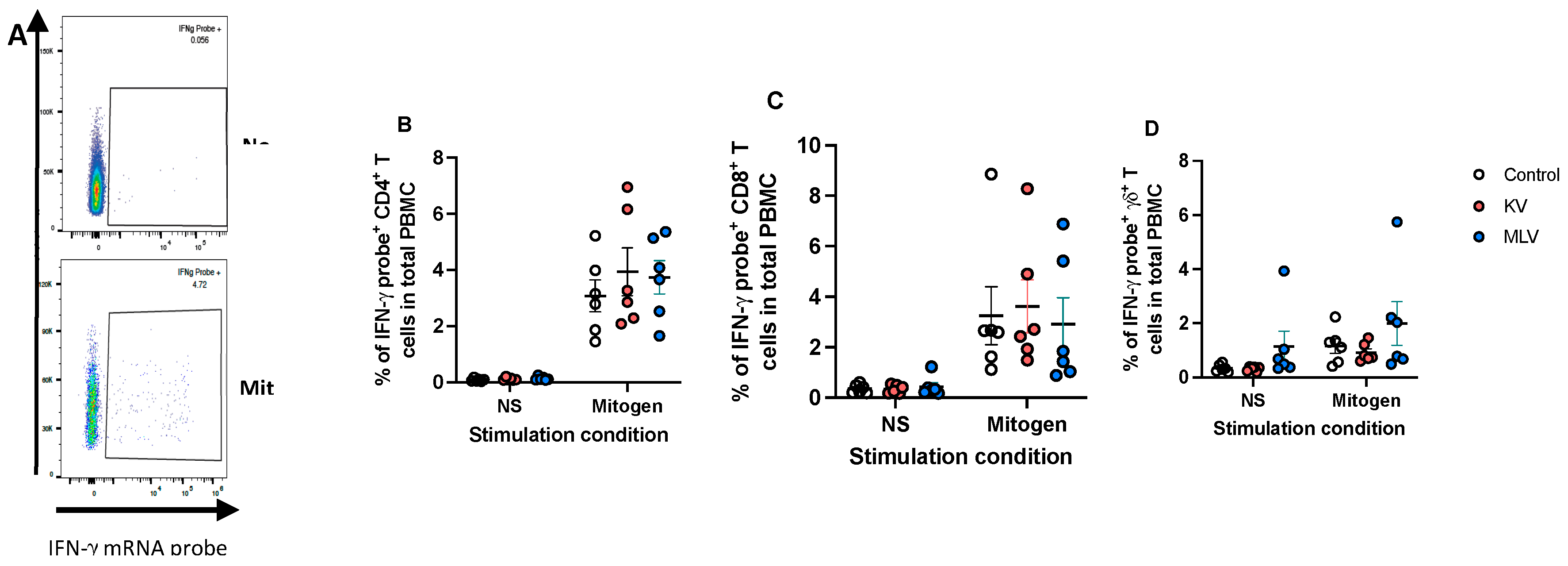
To assess cell-mediated responses, particularly IFN-γ responses, we utilized PrimeFlow followed by flow cytometry to measure intracellular IFN-γ mRNA. As previously described [
14], PrimeFlow utilizes probes to detect target mRNA, which can be designed to be species specific. Therefore, we designed probes to detect WTD IFN-γ mRNA. Following PMA/ionomycin stimulation as a positive control, we observed an increase in the frequency of IFN-γ mRNA expressing PMBC as compared to non-stimulated PBMC (
Figure 3A, representative dot plots). Additionally, when combined with surface staining for CD4 (
Figure 3B), CD8 (
Figure 3C) and γδ (
Figure 3D), we observe that all three T cell subsets are capable of expressing IFN-γ mRNA in response to stimulation. Altogether, these data demonstrate that this approach can successfully detect IFN-γ mRNA in white-tailed deer PMBC.
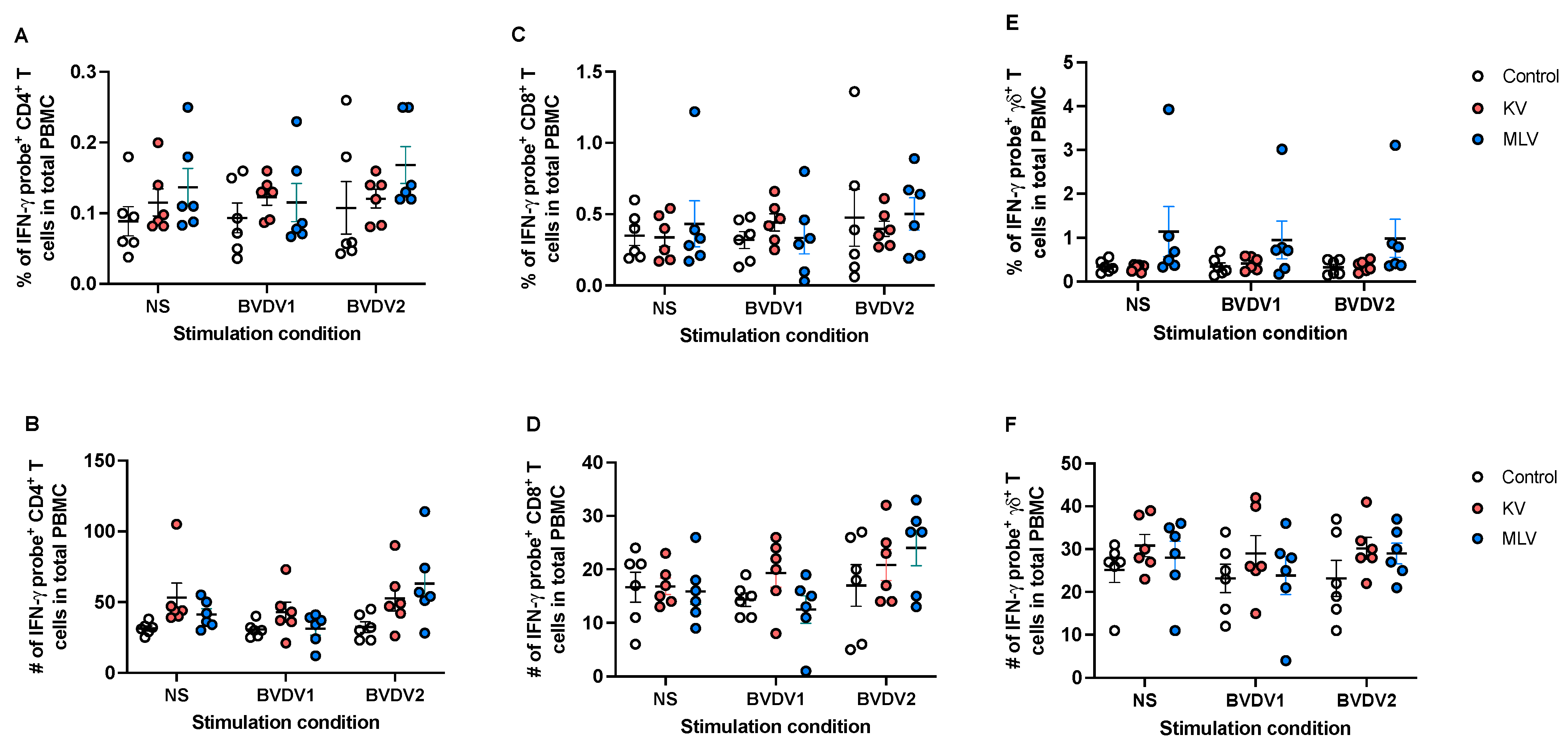
We then applied this approach to assess antigen-specific IFN-γ responses following vaccination. Following
in vitro stimulation with BVDV-1 or BVDV-2a, we did not observe any significant changes in the frequency (
Figure 4A) or number (
Figure 4B) of CD4
+ T cell expressing IFN-γ mRNA in vaccinated animals as compared to control animals. Similarly, we did not observe any significant changes in the frequency or number of CD8
+ (
Figure 4C and 4D) or γδ (
Figure 4E and 4F) T cells expressing IFN-γ mRNA in response to BVDV antigen stimulation. Altogether, these data would suggest that while this assay can detect IFN-γ mRNA, neither KV nor MLV vaccinations induced an increased IFN-γ response, at the time point analyzed.
4. Discussion
In this study we set out to assess the serological and cellular immune responses induced by vaccination with commercial BVDV vaccines for domestic livestock in WTD. The data presented demonstrates that KV and MLV can induce measurable antibody titers against BVDV1 and BVDV2, respectively. Similar to cattle, antibody responses to BVDV in cattle following vaccination, antibody titers to the MLV appear earlier, at approximately 4 weeks post-vaccination. In contrast, measurable antibody titers following inoculation with the KV do not appear until 8 weeks post-vaccination, 4 weeks following the manufacturer’s recommended boost. These findings are consistent with previously reported data from cattle vaccinated with these same vaccines [
15], suggesting similar immunogenicity in inducing humoral responses in cattle and WTD.
Assessment of CMI responses, can sometimes be hindered by the lack of reagents available for use in non-traditional laboratory species. The assay used in this study to measure cellular responses following vaccination, utilizes oligonucleotide probes to detect target gene products. While this requires knowledge of the sequence for the gene of interest, generating probes is faster and easier than generating antibodies against a specific protein target. We combined traditional cellular staining with commercially available antibodies against T cell surface markers with intracellular detection of host IFN-γ mRNA using oligonucleotide probes, PrimeFlow. When we stimulated WTD PBMC with a mitogen to induce IFN-γ production, our assay detected IFN-γ mRNA from various T cell subsets, including CD4 and CD8 T cells. To our knowledge, this is the first time that PrimeFlow was utilized to detect intracellular IFN-γ mRNA using WTD PBMC. Characterization of IFN-γ production in the context of CMI is important as this cytokine is a key player in the orchestration of anti-viral, anti-bacterial and anti-parasitic infections [
16]. Since multiple cell types can produce IFN-γ, ELISAs do not provide information as to the cellular source of this cytokine. Therefore, development of an assay, such as the one described here, that can determine the source of IFN-γ, provides additional information regarding the nature of the cellular immune response.
Unlike the response observed with mitogen stimulation, we were not able to detect BVDV-specific IFN-γ mRNA in any of the T cell populations analyzed following vaccination. The time point chosen for analysis was based on previous experience with analysis of T cell responses in cattle. It is possible, that this was not an appropriate timepoint for measuring T cell responses in WTD, and that a later timepoint (i.e. 12- or 16-weeks post-vaccination) would have allowed detection. One of the major limitations of this study, was the limited time points used to assess CMI. Therefore, we cannot conclusively state that CMI responses to the vaccines were not elicited. However, this limitation can be overcome in subsequent studies analyzing different timepoints and/or by optimizing antigen stimulation conditions.
5. Conclusions
Altogether, the data presented here demonstrates that vaccination with commercially available livestock vaccines can be effective at inducing measurable humoral responses in WTD. As the farmed deer industry continues to grow, along with the use of livestock vaccines in non-approved species, this information is valuable to producers and veterinarians alike as husbandry and veterinary care practices are developed and modified. Here we established PrimeFlow as a method to detect IFN-γ responses in specific T cell populations, adding another level of resolution to our ability to understand WTD immune responses.
Author Contributions
P.B.: Funding acquisition, conceptualization, investigation, project administration, formal analysis, writing – original draft. M. P.: Funding acquisition, conceptualization, investigation, project administration, supervision, writing – review and editing. S.O.: Funding acquisition, supervision, writing – review and editing. S.M.: Conceptualization, investigation, methodology, validation, formal analysis, writing – review and editing.
Funding
This research was funded by The work presented here was supported by intramural funding from the United States Department of Agriculture, Agricultural Research Service Project CRIS #3625-32000-222.
Institutional Review Board Statement
The study was conducted in accordance with recommendations in the Care and Use of Laboratory Animals of the National Institutes of Health and the Guide for the Care and Use of Agricultural Animals in Research and Teaching. Additionally, all animal related procedures were approved by and carried out under that National Animal Disease Center (NADC) Institutional Animal Care and Use Committee (IACUC) guidelines and oversight (Protocol #: ARS-2020-909).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated is presented in the manuscript.
Acknowledgments
The authors would like to thank the NADC Animal Resources Unit, specifically Dr. Rebecca Cox and animal care staff Jonathan Gardner, Jacob Fritz, Kolby Stallman, Derek Vermeer, and Tiffany Williams for their excellent work in animal care and husbandry. We would also like to thank the NADC Flow Cytometry core and Sam Humphrey for his expertise in flow cytometry and Kathryn Bickel for her assistance with virus neutralization assays.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BVDV |
Bovine viral diarrhea virus |
| WTD |
White-tailed deer |
| MLV |
Modified live vaccine |
| KV |
Killed vaccine |
| CMI |
Cell mediated immunity |
| PBMC |
Peripheral blood mononuclear cells |
References
- Willgert, K., et al., Transmission history of SARS-CoV-2 in humans and white-tailed deer. Sci Rep, 2022. 12(1): p. 12094. [CrossRef]
- VerCauteren, K.C., M.J. Lavelle, and H. Campa, 3rd, Persistent Spillback of Bovine Tuberculosis From White-Tailed Deer to Cattle in Michigan, USA: Status, Strategies, and Needs. Front Vet Sci, 2018. 5: p. 301. [CrossRef]
- Campbell, T.A. and K.C. Vercauteren, Diseases and Parasites in Biology and Management of White-Tailed Deer, D.G. Hewitt, Editor. 2011, CRC Press: Boca Raton, FL. p. 219-249.
- Rivera, N.A., et al., Chronic Wasting Disease In Cervids: Prevalence, Impact And Management Strategies. Vet Med (Auckl), 2019. 10: p. 123-139. [CrossRef]
- Saif, L.J. and K. Jung, Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans. J Clin Microbiol, 2020. 58(8). [CrossRef]
- Ridpath, J.F., et al., Reproductive tract disease associated with inoculation of pregnant white-tailed deer with bovine viral diarrhea virus. Am J Vet Res, 2008. 69(12): p. 1630-6. [CrossRef]
- Ridpath, J.F., et al., Febrile response and decrease in circulating lymphocytes following acute infection of white-tailed deer fawns with either a BVDV1 or a BVDV2 strain. J Wildl Dis, 2007. 43(4): p. 653-9. [CrossRef]
- Passler, T., et al., Experimental persistent infection with bovine viral diarrhea virus in white-tailed deer. Vet Microbiol, 2007. 122(3-4): p. 350-6. [CrossRef]
- Deer, W. Deer Farming. 2024; Available from: https://worlddeer.org/deer-farming/.
- Passler, T., et al., Transmission of bovine viral diarrhea virus among white-tailed deer (Odocoileus virginianus). Vet Res, 2010. 41(2): p. 20. [CrossRef]
- Falkenberg, S.M., et al., Evaluation of Antigenic Comparisons Among BVDV Isolates as it Relates to Humoral and Cell Mediated Responses. Front Vet Sci, 2021. 8: p. 685114. [CrossRef]
- Bauermann, F.V., et al., Lack of evidence for the presence of emerging HoBi-like viruses in North American fetal bovine serum lots. J Vet Diagn Invest, 2014. 26(1): p. 10-7. [CrossRef]
- Bolin, S.R., P.J. Matthews, and J.F. Ridpath, Methods for detection and frequency of contamination of fetal calf serum with bovine viral diarrhea virus and antibodies against bovine viral diarrhea virus. J Vet Diagn Invest, 1991. 3(3): p. 199-203. [CrossRef]
- Falkenberg, S.M., et al., Measuring CMI responses using the PrimeFlow RNA assay: A new method of evaluating BVDV vaccination response in cattle. Vet Immunol Immunopathol, 2020. 221: p. 110024. [CrossRef]
- Falkenberg, S.M., et al., Response to Bovine Viral Diarrhea Virus in Heifers Vaccinated with a Combination of Multivalent Modified Live and Inactivated Viral Vaccines. Viruses, 2023. 15(3). [CrossRef]
- Kak, G., M. Raza, and B.K. Tiwari, Interferon-gamma (IFN-gamma): Exploring its implications in infectious diseases. Biomol Concepts, 2018. 9(1): p. 64-79. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).