Submitted:
31 March 2025
Posted:
01 April 2025
You are already at the latest version
Abstract
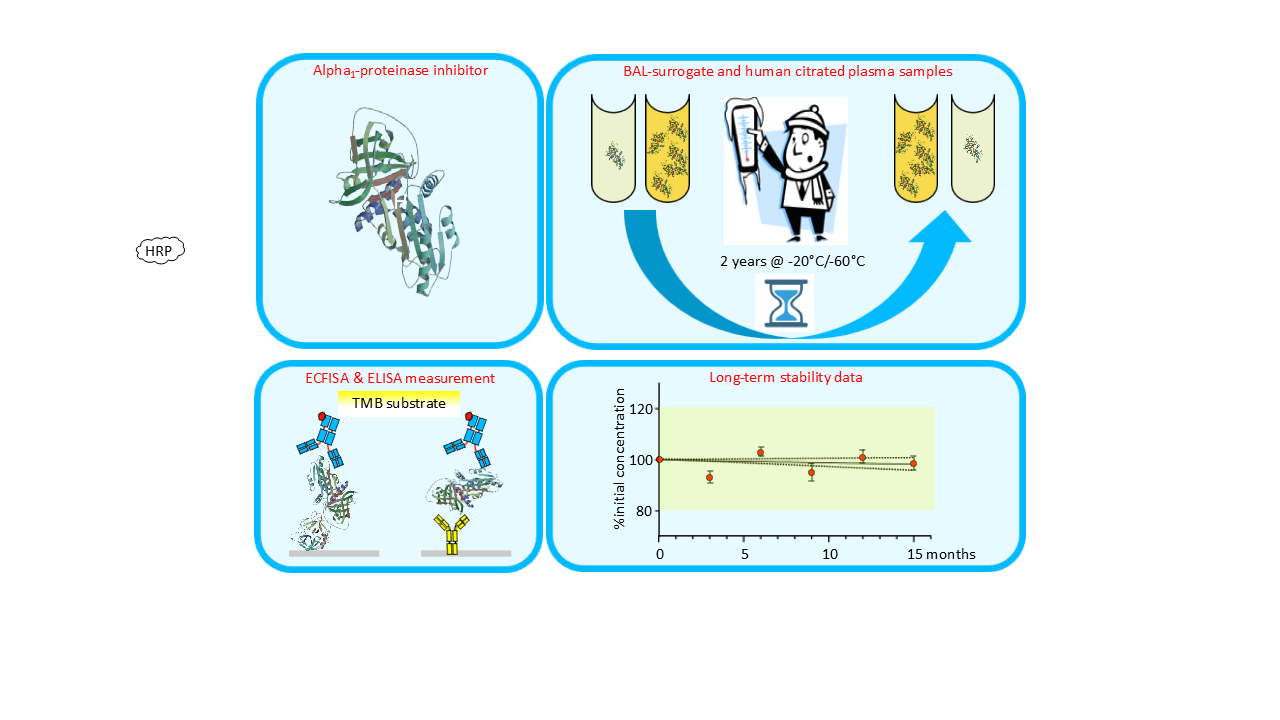
Keywords:
1. Introduction
2. Results
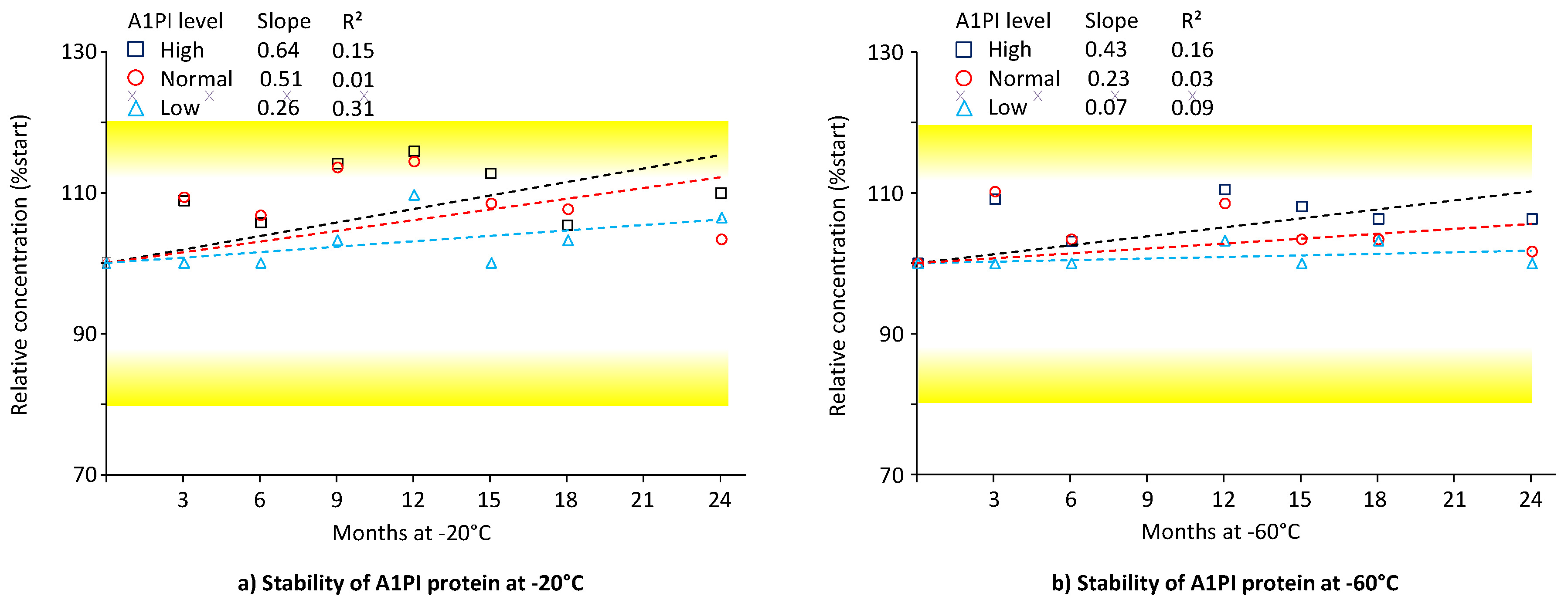
2.1. A1PI Protein Stability in Citrated Human Plasma as Measured with the Nephelometric Assay
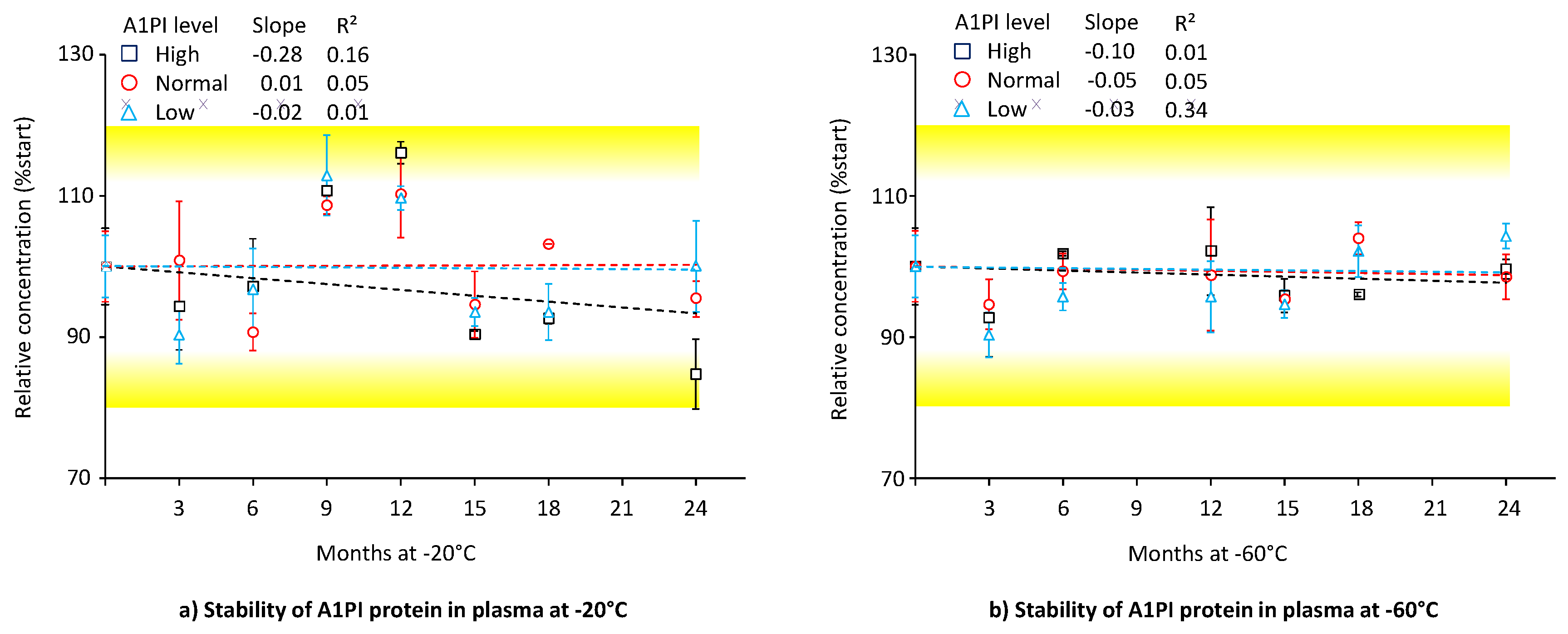
2.2. A1PI Protein Stability in Citrated Human Plasma as Measured with the ELISA
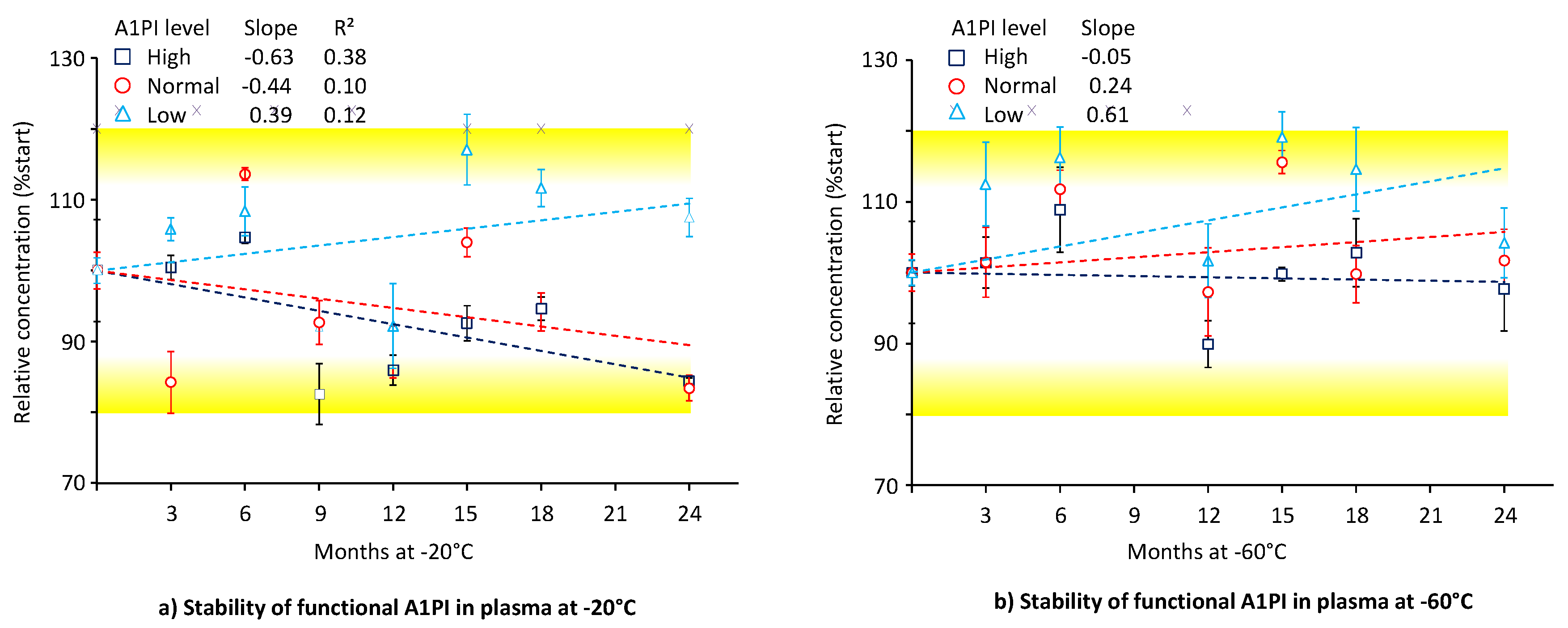
2.3. A1PI Functional Activity in Citrated Human Plasma as Measured with the ECFISA
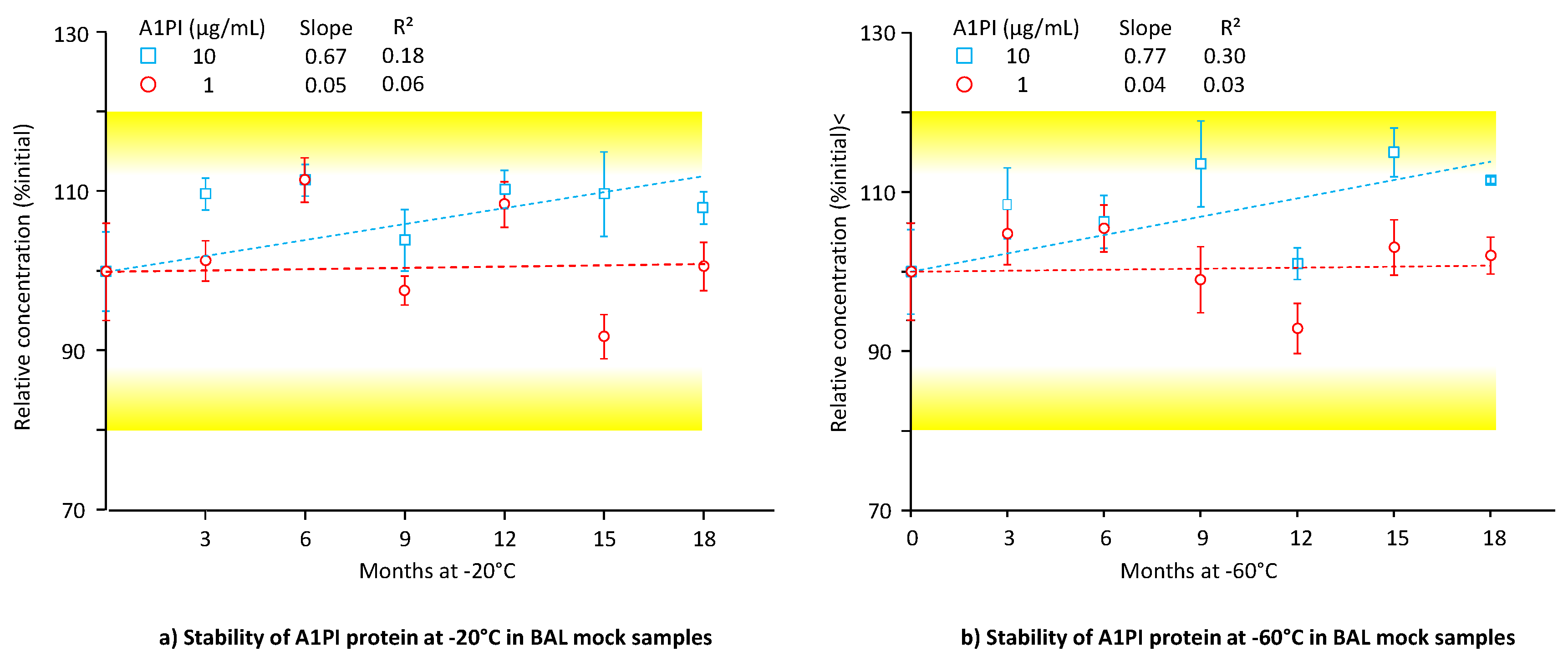
2.4. Stability of A1PI Protein in BAL Mock Samples as Measured with the ELISA
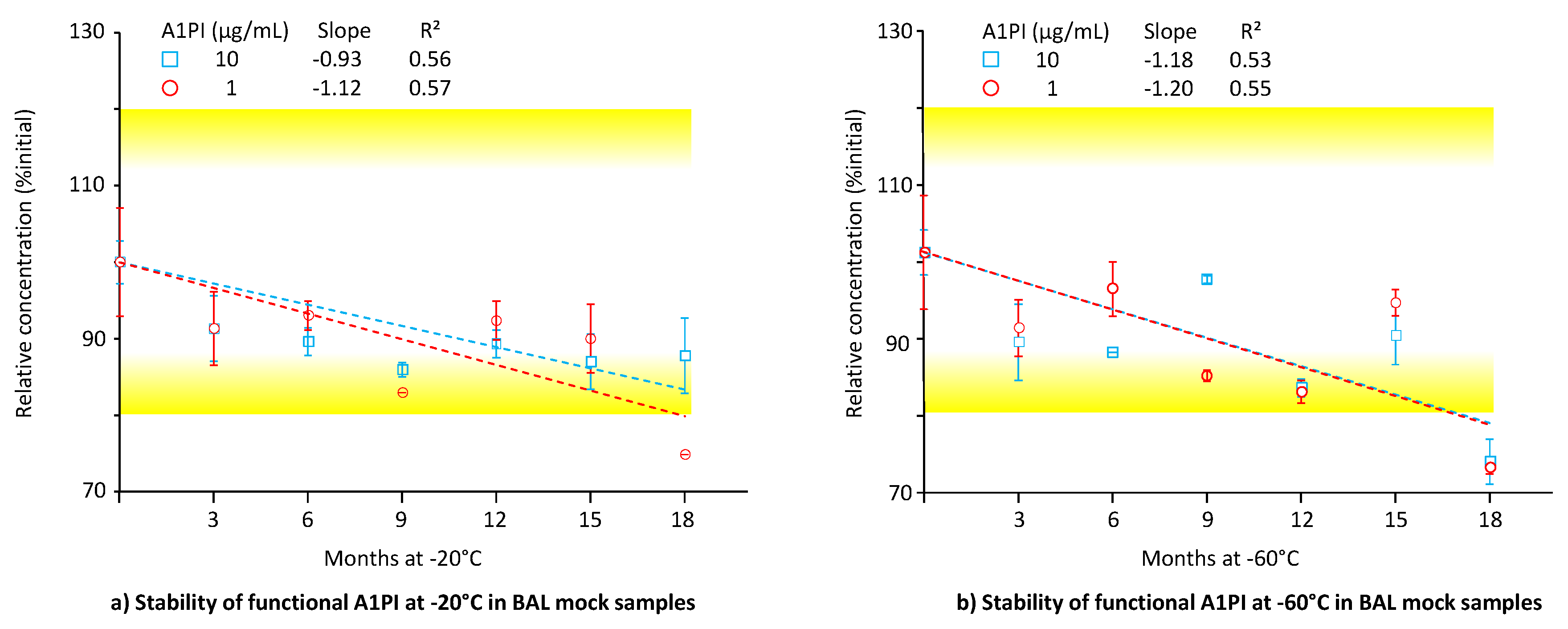
2.5. A1PI Functional Activity Measurement with the ECFISA in BAL Mock Samples
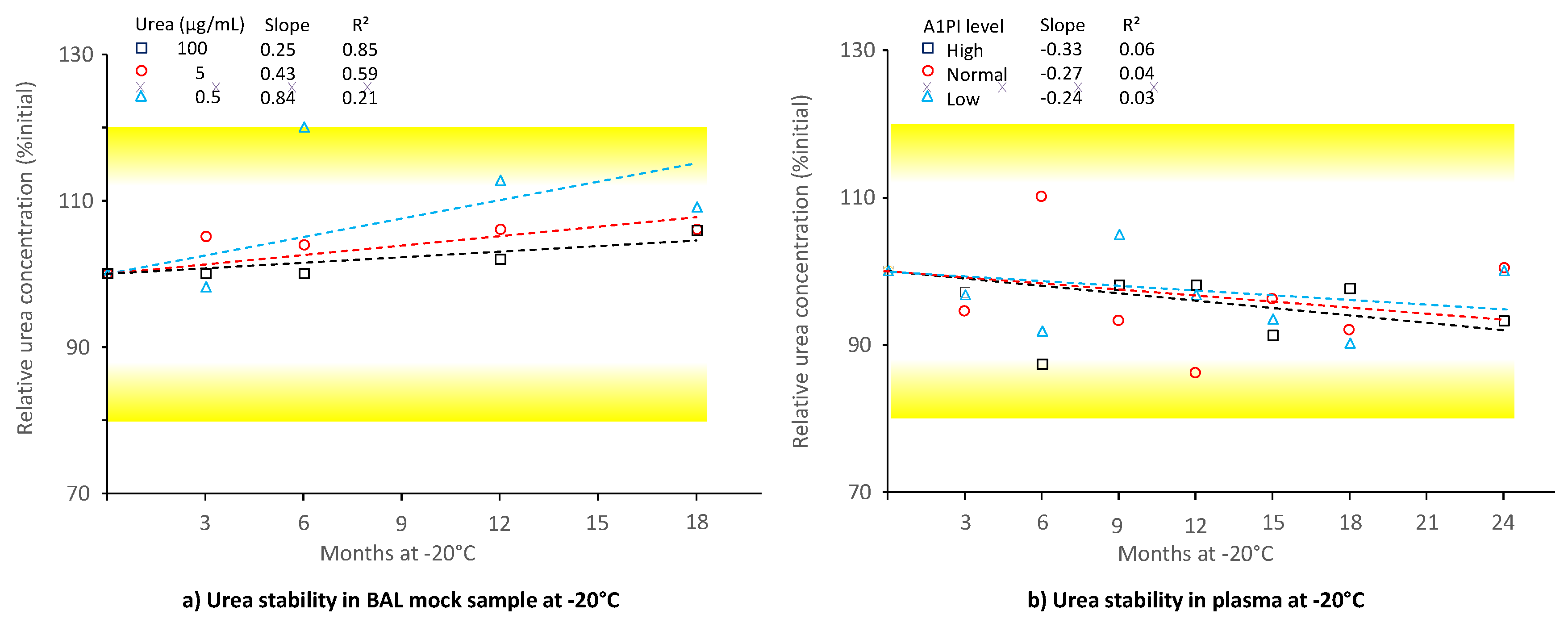
2.6. Stability of Urea in BAL and Human Citrated Plasma
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Stability Samples and Design of the Stability Study
4.3. Nephelometric A1PI Protein Measurement
4.4. A1PI Protein Measurement with the ELISA
4.5. Functional A1PI Activity Measurement
4.6. Urea Measurement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A1PI | α1-proteinase inhibitor |
| AAT | α1-antitrypsin |
| AATD | Alpha1-antitrypsin deficiency |
| BAL | Bronchoalveolar lavage |
| DB | Dilution buffer |
| ECFISA | Elastase complex formation immunosorbent assay |
| ELISA | Enzyme-linked immunosorbent assay |
| NADH | Reduced nicotinamide adenine dinucleotide |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PBST | Washing buffer |
| RCL | Reactive center loop |
| RSD | Relative standard deviation |
| RT | Room temperature |
| SD | Standard deviation |
| Serpin | Serine protease inhibitor |
Appendix A
Appendix A.1: Description of the Nephelometric A1PI Measurement
| Parameter |
A1PI level [mg/mL] |
Number of A1PI levels |
Mean [Min - Max] |
| Accuracy [%recovery] |
0.05 – 2.0 | 6 | 103.2% [100.0% - 107.5%] |
| Intra-run precision [RSD, n=6] |
0.05 – 2.0 | 6 | 0.8% [0.0% - 1.8%] |
| Inter-run precision [RSD, n=6] |
0.05 – 20 | 8 | 4.3% [3.4% - 6.5%] |
| Linearity | 0.05 – 2.0 | 6 | R² = 1.0000 |
| 3× freezing-thawing [%recovery of fresh sample] |
0.05 ; 0.5 ; 1.0 | 3 | 102.4% [98.8% - 105.7%] |
| 48 h RT stability [%recovery of fresh sample] |
0.05 ; 0.5 ; 1.0 | 3 | 96.6% [93.8% - 98.8%] |
Appendix A.2: Description of the A1PI Protein Measurement with the ELISA
- Coating buffer: 0.1 M NaHCO3, 0.1 M Na2CO3; dissolved in HPLC-grade water; pH 9.5 with HCl (25%)
- Washing buffer (PBST): phosphate-buffered saline (PBS), 0.8% NaCl, 0.02% KCl, 0.02% KH2PO4, 0.126% Na2HPO4 × 2 H2O with 0.05% Tween 20; pH 7.0-7.4
- Blocking/dilution buffer (DB): 0.1% non-fat dry milk, 2 mM benzamidine in PBST
- Stopping solution: 1.5 M sulfuric acid.
- Rabbit anti-human α1-antitrypsin IgG A0012 (DakoCytomation, Glostrup, Denmark) as the capturing antibody
- Sheep anti-human α1-antitrypsin IgG peroxidase PP034 (The Binding Site, Birmingham, UK) as the detection antibody
- Human serum calibrator ERM DA 470, 1.12 mg A1PI/mL, as the assay calibrator
- Human reference plasma 1A51 (Takeda, Vienna, Austria) as the control preparation
| Parameter |
A1PI level [µg/mL] |
Number of A1PI levels |
Mean [Min - Max] |
| Accuracy [%recovery] |
0.2 –10 | 6 | 94.7% [88.9% - 99.2%] |
| Intra-run precision [RSD, n=6] |
0.2 ; 10 | 2 | 4.3% [3.3% ; 5.2%] |
| Inter-run precision [RSD, n=6] |
0.2 - 10 | 6 | 7.0% [4.4% - 10.6%] |
| Linearity | 0.2 – 10 | 6 | R² = 0.9993 |
| 3× freezing-thawing [%recovery of unfrozen] |
0.2 ; 10 | 2 | 90.3% [85.7% ; 94.9%] |
| 48 h RT stability [%recovery of fresh] |
0.2 ; 10 | 2 | 98.6% [97.1% ; 100.0%] |
| Parameter |
A1PI level [mg/mL] |
Number of A1PI levels |
Mean [Min - Max] |
| Accuracy [%recovery] |
0.01 – 1.00 | 6 | 96.9% [94.0 – 103.7] |
| Inter-run precision [RSD, n=6] |
0.01– 2.00 | 7 | 8.1% [6.2% – 9.4%] |
| Linearity | 0.01 -2.00 | 7 | R² = 0.9990 |
Appendix A.3: Description of the A1PI ECFISA Measurement
- ECFISA coating buffer (PBS): 8.0 g/L NaCl, 0.2 g/L KCl, 0.2 g/L KH2PO4, 1.26 g/L Na2HPO4 × 2 H2O. Salts are dissolved in 1 L HPLC water; pH checked (target pH 7.2 ± 0.2) and 0.2 µm filtration with Nalgene filter unit (Sigma). The buffer can be stored at 4°C for two weeks.
- Washing buffer (PBST): PBS with 0.05% (v/v) Tween 20. This washing buffer can be stored at RT for one week.
- ECFISA dilution buffer (DB): 5 g BSA (Sigma A0281) are dissolved in 500 mL PBST. The DB used for the dilution of samples contains 0.025% (g/v) Patentblau V, added from a 2.5% aqueous stock solution. Dilution buffer has to be prepared freshly before use.
- Peroxidase substrate SureBlue – ready to use.
- Stopping solution – 3 N sulfuric acid
- Coating: Porcine elastase (Sigma, E7885) is dissolved in water (5 mg/mL) and kept frozen at -20°C in 50-µL aliquots for up to 12 months. Immediately for the plate coating, an aliquot is thawed and diluted 1/250 with coating buffer. Nunc Maxisorp F96 plates are incubated with 100 µL/well coating solution at 4°C overnight.
- Washing: Coating is terminated by a washing step with washing buffer done either manually or with a 96-well plate washer (Bio-Tek ELx-405). The washing is done three times, the emptied plate is then further processed.
- Blocking of wells: 200 µL/well ECFISA DB are added to the emptied wells using an 8-channel pipette or a dispenser (Multidrop 384 Dispenser). The plate is then incubated at 37°C for 60 min. Blocking is terminated by a single washing step. The emptied plate is then further processed.
- Standard and sample dilution, loading and incubation: Each well is filled with 100 µL/well DB ECFISA. Colored DB ECFISA is used for the dilution of standard and samples. The in-house assay standard with 19.2 mg A1PI/mL is diluted 1/50,000, samples are diluted to obtain AAT concentrations of about 400 ng/mL. Serial 1+1 dilution series comprising six dilutions are then prepared directly on the plate by mixing 100 µL of the colored sample dilution with the dilution buffer in the well. Two independent dilution series are prepared. The samples loaded to row B are measured in only five dilutions as the positions B11 and B12 serve as blank and contain DB only. Fading of the color with progressing dilution from the left to right side of the plate reflects the serial dilution series prepared. Each plate contains dilution series for the assay standard, the assay control and six samples. The dilutions (100 µL/well) are then incubated at RT for 60 min. Sample incubation is terminated by three washing steps, followed by a further three washing steps after the plate has been turned by 180°. The emptied plate is then further processed.
- Incubation with anti-AAT peroxidase: Anti-human α1-antitrypsin peroxidase (TBS PP034) is diluted 1/1,000 with DB. 100 µL/well are added and incubated at RT for 60 min. Incubation is terminated by a washing step, essentially carried out as described for the termination of the sample incubation.
- Color reaction: 100 µL/well SureBlue is added to the wells. The plate is incubated at RT for 15 min (protected for direct sun light), before 100 µL/well stopping solution (3 N sulfuric acid) is added. Both additions can be done either manually or using the dispenser.
- Plate measurement: The plate is measured within 60 min with an ELISA reader (Bio-Tek EL-808) at 450 nm using a reference wavelength of 620 nm.
- Data evaluation: The calibration curve, ranging from 6 to 192 ng active A1PI/mL, is obtained as a linear regression curve calculated for the blank-corrected mean ODs of the duplicates and the AAT concentrations of the six assay standards. For the sample evaluation, only ODs within the range defined by the calibration curve are considered. The concentrations obtained for the individual samples’ dilutions are multiplied with the dilution and averaged to yield the final results.
| Parameter |
A1PI level [µg/mL] |
Number of A1PI levels |
Mean [Min - Max] |
| Accuracy [%recovery] |
0.2 –10 | 6 | 97.6% [85.0% - 107.4%] |
| Intra-run precision [RSD, n=6] |
0.2 ; 10 | 2 | 1.9% [1.4% ; 2.4%] |
| Inter-run precision [RSD, n=6] |
0.2 - 10 | 6 | 4.5% [3.2% - 8.4%] |
| Linearity | 0.2 – 10 | 6 | R² = 0.9998 |
| 3× freezing-thawing [%recovery of unfrozen] |
0.2 ; 10 | 2 | 99.6% [99.1% ; 100.0%] |
| 4 h RT stability [%recovery of fresh] |
0.2 ; 10 | 2 | 99.1% [98.1% ; 100.0%] |
| Parameter |
A1PI level [mg/mL] |
Number of A1PI levels |
Mean [Min - Max] |
| Accuracy [%recovery] |
- 3 | 7 | 101.8% [94.0% - 106.0%] |
| Intra-run precision [RSD, n=6] |
0.01 - 3 | 7 | 3.0% [2.0% - 4.2%] |
| Inter-run precision [RSD, n=6] |
0.01 - 20 | 9 | 6.3% [4.5% - 8.2%] |
| Linearity | 0.01 - 3 | 7 | R² = 0.9993 |
| 3× freezing-thawing [%recovery of unfrozen] |
0.01 ; 1 | 2 | 103.7% [104.5% ; 102.9%] |
| 4 h RT stability [%recovery of fresh] |
0.01 ; 1 | 2 | 110.2% [106.6% ; 113.8%] |
Appendix A.4: Description of the Urea Measurement
| Parameter |
Urea level [µg/mL] |
Number of urea levels |
Mean [Min - Max] |
| Accuracy [%recovery] |
0.5 - 450 | 7 | 102.3% [97.5% – 110.7%] |
| Intra-run precision [RSD, n=6] |
0.5 ;100 ; 300 | 3 | 7.6% [0.7% – 11.5%] |
| Inter-run precision [RSD, n=6] |
0.5 - 450 | 8 | 4.8% [0.6% – 9.1%] |
| Linearity - low protein | 0.5 - 100 | 5 | R² = 0.9999 |
| Linearity - plasma | 150 - 450 | 3 | R² = 1.0000 |
| Linearity - all samples | 0.5 - 450 | 8 | R² = 1.0000 |
| 3× freezing-thawing [%recovery of unfrozen] |
0.5 ; 100 ; 300 | 3 | 98.7% [96.2% - 101.9%] |
| 4 h RT stability [%recovery of fresh] |
0.5 ; 100 ; 300 | 3 | 98.5% [95.8% - 101.8%] |
Appendix A.5: Stability of A1PI Protein in Citrated Plasma as Measured with the Nephelometric Method
| Months | Low | Normal | High | ||||
| mg/mL | %initial | mg/mL | %initial | mg/mL | %initial | ||
| Storage at -20°C | 0 | 0.31 | 100.0 | 1.18 | 100.0 | 2.86 | 100.0 |
| 3 | 0.31 | 100.0 | 1.29 | 109.3 | 3.11 | 108.7 | |
| 6 | 0.31 | 100.0 | 1.26 | 106.8 | 3.02 | 105.6 | |
| 9 | 0.32 | 103.2 | 1.34 | 113.6 | 3.26 | 114.0 | |
| 12 | 0.34 | 109.7 | 1.35 | 114.4 | 3.31 | 115.7 | |
| 15 | 0.31 | 100.0 | 1.28 | 108.5 | 3.22 | 112.6 | |
| 18 | 0.32 | 103.2 | 1.27 | 107.6 | 3.01 | 105.2 | |
| 24 | 0.33 | 106.5 | 1.22 | 103.4 | 3.14 | 109.8 | |
| Storage at -60°C | 0 | 0.31 | 100.0 | 1.18 | 100.0 | 2.86 | 100.0 |
| 3 | 0.31 | 100.0 | 1.30 | 110.2 | 3.12 | 109.1 | |
| 6 | 0.31 | 100.0 | 1.22 | 103.4 | 2.95 | 103.1 | |
| 12 | 0.32 | 103.2 | 1.28 | 108.5 | 3.16 | 110.5 | |
| 15 | 0.31 | 100.0 | 1.22 | 103.4 | 3.09 | 108.0 | |
| 18 | 0.32 | 103.2 | 1.22 | 103.4 | 3.04 | 106.3 | |
| 24 | 0.31 | 100.0 | 1.20 | 101.7 | 3.04 | 106.3 | |
Appendix A.6: Stability of A1PI Protein in Citrated Plasma as Measured with the ELISA
| Months | Low | Normal | High | ||||
| mg/mL | %initial | mg/mL | %initial | mg/mL | %initial | ||
| Storage at -20°C | 0 | 0.31 | 100.0 | 1.28 | 100.0 | 3.54 | 100.0 |
| 3 | 0.28 | 90.3 | 1.29 | 100.9 | 3.34 | 94.4 | |
| 6 | 0.30 | 96.8 | 1.16 | 90.7 | 3.44 | 97.2 | |
| 9 | 0.35 | 112.9 | 1.39 | 108.7 | 3.92 | 110.7 | |
| 12 | 0.34 | 109.7 | 1.41 | 110.2 | 4.11 | 116.1 | |
| 15 | 0.29 | 93.5 | 1.21 | 94.6 | 3.20 | 90.4 | |
| 18 | 0.29 | 93.5 | 1.32 | 103.2 | 3.28 | 92.7 | |
| 24 | 0.31 | 100.0 | 1.22 | 95.4 | 3.00 | 84.7 | |
| Storage at -60°C | 0 | 0.31 | 100.0 | 1.28 | 100.0 | 3.54 | 100.0 |
| 3 | 0.28 | 90.3 | 1.21 | 94.6 | 3.28 | 92.7 | |
| 6 | 0.30 | 95.7 | 1.27 | 99.3 | 3.60 | 101.8 | |
| 12 | 0.30 | 95.7 | 1.26 | 98.8 | 3.62 | 102.2 | |
| 15 | 0.29 | 94.6 | 1.22 | 95.4 | 3.39 | 95.9 | |
| 18 | 0.32 | 102.2 | 1.33 | 104.0 | 3.40 | 96.0 | |
| 24 | 0.32 | 104.3 | 1.26 | 98.5 | 3.53 | 99.6 | |
Appendix A.7: Stability of Functional A1PI Activity in Citrated Plasma
| Months | Low | Normal | High | ||||
| µg/mL | %initial | µg/mL | %initial | µg/mL | %initial | ||
| Storage at -20°C | 0 | 241 | 100.0 | 1027 | 100.0 | 2889 | 100.0 |
| 3 | 255 | 105.9 | 865 | 84.2 | 2901 | 100.4 | |
| 6 | 261 | 108.3 | 1167 | 113.6 | 3023 | 104.7 | |
| 9 | 222 | 92.2 | 952 | 92.7 | 2385 | 82.6 | |
| 12 | 222 | 92.2 | 909 | 88.5 | 2483 | 86.0 | |
| 15 | 282 | 117.1 | 1068 | 104.0 | 2674 | 92.6 | |
| 18 | 269 | 111.7 | 967 | 94.2 | 2733 | 94.6 | |
| 24 | 259 | 107.5 | 857 | 83.4 | 2439 | 84.4 | |
| Storage at -60°C | 0 | 241 | 100.0 | 1027 | 100.0 | 2889 | 100.0 |
| 3 | 271 | 112.5 | 1042 | 101.5 | 2929 | 101.4 | |
| 6 | 280 | 116.2 | 1148 | 111.8 | 3145 | 108.9 | |
| 12 | 245 | 101.7 | 999 | 97.3 | 2597 | 89.9 | |
| 15 | 287 | 119.1 | 1187 | 115.6 | 2882 | 99.8 | |
| 18 | 276 | 114.6 | 1025 | 99.8 | 2970 | 102.8 | |
| 24 | 251 | 104.2 | 1045 | 101.7 | 2824 | 97.8 | |
Appendix A.8: Stability of A1PI Protein in BAL Mock Samples
| Months | 1 µg/mL | 10 µg/mL | |||
| µg/mL | %initial | µg/mL | %initial | ||
| Storage at -20°C | 0 | 0.98 | 100.0 | 11.5 | 100.0 |
| 3 | 1.00 | 101.7 | 12.6 | 109.3 | |
| 6 | 1.10 | 111.9 | 12.8 | 111.0 | |
| 9 | 0.96 | 98.0 | 11.9 | 103.5 | |
| 12 | 1.07 | 108.8 | 12.6 | 109.9 | |
| 15 | 0.90 | 92.2 | 12.6 | 109.3 | |
| 18 | 0.99 | 101.0 | 12.4 | 107.5 | |
| Storage at -60°C | 0 | 0.98 | 100.0 | 11.5 | 100.0 |
| 3 | 1.03 | 105.1 | 12.4 | 108.1 | |
| 6 | 1.04 | 105.8 | 12.2 | 105.8 | |
| 9 | 0.97 | 99.3 | 13.0 | 113.0 | |
| 12 | 0.91 | 93.2 | 11.6 | 100.6 | |
| 15 | 1.01 | 103.4 | 13.2 | 114.5 | |
| 18 | 1.00 | 102.4 | 12.8 | 111.0 | |
Appendix A.9: Stability of functional A1PI activity in BAL mock samples
| Months | 1 µg/mL | 10 µg/mL | |||
| µg/mL | %initial | µg/mL | %initial | ||
| Storage at -20°C | 0 | 0.99 | 100.0 | 12.8 | 100.0 |
| 3 | 0.90 | 91.2 | 11.7 | 91.3 | |
| 6 | 0.92 | 92.9 | 11.5 | 89.6 | |
| 9 | 0.82 | 82.8 | 11.0 | 85.9 | |
| 12 | 0.91 | 92.3 | 11.4 | 89.3 | |
| 15 | 0.89 | 89.9 | 11.1 | 87.0 | |
| 18 | 0.74 | 74.7 | 11.2 | 87.8 | |
| Storage at -60°C | 0 | 0.99 | 100.0 | 12.8 | 100.0 |
| 3 | 0.90 | 90.5 | 11.4 | 88.8 | |
| 6 | 0.94 | 95.3 | 11.2 | 87.5 | |
| 9 | 0.84 | 84.5 | 12.4 | 96.6 | |
| 12 | 0.82 | 82.5 | 10.6 | 83.1 | |
| 15 | 0.93 | 93.6 | 11.5 | 89.6 | |
| 18 | 0.72 | 73.1 | 9.45 | 73.9 | |
Appendix A.10: Stability of urea in BAL mock samples and citrated human plasma
| Months | 0.5 µg/mL | 5 µg/mL | 100 µg/mL | |||
| µg/mL | %initial | µg/mL | %initial | µg/mL | %initial | |
| 0 | 0.55 | 100.0 | 5.12 | 100.0 | 102 | 100.0 |
| 3 | 0.54 | 98.2 | 5.38 | 105.1 | 102 | 100.0 |
| 6 | 0.66 | 120.0 | 5.32 | 103.9 | 102 | 100.0 |
| 12 | 0.62 | 112.7 | 5.43 | 106.1 | 104 | 102.0 |
| 18 | 0.60 | 109.1 | 5.43 | 106.1 | 108 | 105.9 |
| Months | Low A1PI | Normal A1PI | High A1PI | |||
| µg/mL | %initial | µg/mL | %initial | µg/mL | %initial | |
| 0 | 61 | 100.0 | 238 | 100.0 | 205 | 100.0 |
| 3 | 59 | 96.7 | 225 | 94.5 | 199 | 97.1 |
| 6 | 56 | 91.8 | 262 | 110.1 | 179 | 87.3 |
| 9 | 64 | 104.9 | 222 | 93.3 | 201 | 98.0 |
| 12 | 59 | 96.7 | 205 | 86.1 | 201 | 98.0 |
| 15 | 57 | 93.4 | 229 | 96.2 | 187 | 91.2 |
| 18 | 55 | 90.2 | 219 | 92.0 | 200 | 97.6 |
| 24 | 61 | 100.0 | 239 | 100.4 | 191 | 93.2 |
References
- Carrell, R.W.; Jeppsson, J.O.; Laurell, C.B.; Brennan, S.O.; Owen, M.C.; Vaughan, L.; Boswell, D.R. Structure and variation of human alpha1-antitrypsin. Nature 1982, 298, 329–334. [Google Scholar] [CrossRef]
- Lebing, W. Alpha1-Proteinase Inhibitor: The Disease, the Protein, and Commercial Production. In Production of plasma proteins for therapeutic use, 1st ed.; Bertolini, J.; Goss, N.; Curling, J. John Wiley & Sons, Inc., Hoboken, New Jersey, United States of America, 2013; pp.
- Kolarich, D.; Weber, A.; Turecek, P.; Schwarz, H.-P.; Altmann, F. Comprehensive glyco-proteomic analysis of human 1-antitrypsin and its charge isoforms. Proteomics 2006, 6, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Kolarich, D.; Turecek, P.L.; Weber, A.; Mitterer, A.; Graninger, M.; Matthiessen, P.; Nicolaes, G.A.F.; Altmann, F.; Schwarz, H.P. Biochemical, molecular characterization, and glycoproteomic analyses of 1-proteinase inhibitor products used for replacement therapy. Transfusion 2006, 46, 1959–1977. [Google Scholar] [CrossRef]
- Matthiessen, H.P.; Willemse, J.; Weber, A.; Turecek, P.L.; Deiteren, K.; Hendriks, D.; Ehrlich, H.J.; Schwarz, H.-P. Ethanol dependence of a1-antitrypsin C-terminal Lys truncation mediated by basic carboxypeptidases. Transfusion. [CrossRef]
- de Serres, F.; Blanco, I. Role of alpha-1 antitrypsin in human health and disease. J Intern Med. 2014, 276, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Dolmer, K.; Gettins, P.G.W. How the serpin a1-proteinase inhibitor folds. J Biol Chem 2012, 287, 12425–12432. [Google Scholar] [CrossRef]
- Taggart, C.; Cervantes-Laurean, D.; Kim, G.; McElvaney, N.G.; Wehr, N.; Moss, J.; Levine, R.L. Oxidation of either methionine 351 or methionine 358 in 1-antitrypsin causes loss of anti-neutrophil elastase activity. . J Biol Chem, /: 27258-27265; https, 2725. [Google Scholar] [CrossRef]
- Potempa, J.; Korzus, E.; Travis, J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem 1994, 269, 15957–15960. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.E.; Irving, J.A.; Lomas, D.A.; Luke, C.L.; Moyer, R.W.; Pemberton, P.A.; Remold-O'Donnell, E.; Salvesen, G.S.; Travis, J.; Whisstock, J.C. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 2001, 276, 33293–33296; [Google Scholar] [CrossRef]
- Lomas, D.A. Molecular mousetraps, α1-antitrypsin deficiency and the serpinopathies. Clin Med (Lond) 2005, 5, 249. [Google Scholar] [CrossRef]
- Bruch, M.; Weiss, V.; Engel, J. Plasma serine proteinase inhibitors (serpins) exhibit major conformational changes and a large increase in conformational stability upon cleavage at their reactive sites. J Biol Chem 1988, 263, 16626–16630. [Google Scholar] [CrossRef]
- Zhou, A.; Carrell, R.W.; Huntington, J.A. The serpin inhibitory mechanism is critically dependent on the length of the reactive center loop. J Biol Chem 2001, 276, 27541–27547. [Google Scholar] [CrossRef]
- Perlmutter, D.H.; Joslin, G.; Nelson, P.; Schasteen, C.; Adams, S.; Fallon, R. .Endocytosis and degradation of alpha1-antitrypsin-protease complex is mediated by the serpin-enzyme complex (SEC) receptor. J Biol Chem 1990, 265, 16713–16716. [Google Scholar] [CrossRef]
- Joslin, G; Fallon, R. J.; Bullock, J; Adams, S.P.; Perlmutter, D.H. The SEC receptor recognizes a pentapeptide neodomain of alpha 1- antitrypsin-protease complexes. J Biol Chem 1991, 266, 11282–11288.
- Mast, A.E.; Enghild, J.J.; Pizzo, S.V.; Salvesen, G. Analysis of the plasma elimination kinetics and conformational stabilities of native, proteinase-complexed and reactive site cleaved serpins: Comparison of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, antithrombin III, alpha 2-antiplasmin, angiotensinogen, and ovalbumin. Biochemistry 1991, 30, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.M.; Bals, R.; Koczulla, R.; Vogelmeier, C.; Köhnlein, T.; Welte, T. The discovery of a1-antitrypsin and its role in health and disease. Respir Med 2011, 105, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Laurell, C.-B.; Eriksson, S. The electrophoretic a1- globulin pattern of serum in a1-antitrypsin deficiency. Scand J Clin Lab Invest 1963, 15, 132–40. [Google Scholar] [CrossRef]
- Sharp, H.L.; Bridges, R.A.; Krivit, W.; Freier, E.F. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med 1969, 73, 934–939. [Google Scholar]
- Turino, G.M.; Senior, R.M.; Garg, B.D.; Keller, S.; Levi, M.M.; Mandl, I. Serum elastase inhibitor deficiency and alpha l-antitrypsin deficiency in patients with obstructive emphysema. Science 1969, 165, 709–710. [Google Scholar] [CrossRef]
- Owen, M.C.; Carrell, R.W.; Brennan, S.O. The abnormality of the S variant of human α-1-antitrypsin. BBA 1976, 453, 257–261. [Google Scholar] [CrossRef]
- Jeppsson, J.-O. Amino acid substitution Glu → Lys in alpha1-antitrypsin PiZ. FEBS Letters 1976, 65, 195–197. [Google Scholar] [CrossRef]
- de Serres, F.J.; Blanco, I. Prevalence of a1-antitrypsin deficiency alleles PI*S and PI*Z worldwide. /: Ther Adv Resp Dis 2012, 6, 277-295; https, 2012; 6. [Google Scholar] [CrossRef]
- Gadek, J.E.; Klein, H.G.; Holland, P.V.; Crystal, R.G. Replacement therapy of alpha-1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. 68, /: 1981, 68, 1158-1165; https, 1981; 68. [Google Scholar] [CrossRef]
- Janciauskiene, S.; Welte, T. Augmentation therapy with alpha1-antitrypsin: Novel perspectives. Cardiovasc Hematol Disord Drug Targets 2013, 13, 90–8. [Google Scholar] [CrossRef]
- Schmid, S.T.; Koepke, J.; Dresel, M.; Hattesohl, A.; Frenzel, E.; Perez, J.; Lomas, D.A.; Miranda, E.; Greulich, T.; Noeske, S.; Wencker, M.; Teschler, H.; Vogelmeier, C.; Janciauskiene, S.; Koczulla, A.R. The effects of weekly augmentation therapy in patients with PiZZ alpha1-antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis 2012, 7, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Teschler, H. Long-term experience in the treatment of a1-antitrypsin deficiency: 25 years of augmentation therapy. Eur Respir Rev 2015, 24, 46–51. [Google Scholar] [CrossRef]
- Schouten, I.G.M.; Kasteleyn, M.J.; Tsonaka, R.; Bals, R.; Turner, A.C.; Ferrarotti, I.; Corsico, A.G.; Lara, B.; Miravitlles, M.; Stockley, R.A.; Stolk, J.-. Long-term effect of α1-antitrypsin augmentation therapy on the decline of FEV1 in deficient patients: an analysis of the AIR database. ERJ Open Res 2021, 7, 00194–2021; https://. [Google Scholar] [CrossRef]
- Brantly, M.L.; Lascano, J.E.; Shahmohammadi, A. Intravenous alpha-1 antitrypsin therapy for alpha-1 antitrypsin deficiency: the current state of the evidence. Chronic Obstr Pulm Dis 2019, 6, 100–14. [Google Scholar] [CrossRef]
- Ellis, P.R.; Holm, K.E.; Choate, R.; Mannino, D.M.; Stockley, R.A.; Sandhaus, R.A.; Turner, A.M.L. Quality of life and mortality outcomes for augmentation naïve and augmented patients with severe alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis 2023, 10, 139–47. [Google Scholar] [CrossRef] [PubMed]
- Bianchera, A.; Alomari, E.; Bruno, S. Augmentation therapy with alpha 1-antitrypsin: present and future of production, formulation, and delivery. Curr Med Chem 2022, 29, 385–410. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, A.; Piitulainen, E.; Parr, D.G.; Deng, C.; Wencker, M.; Shaker, S.B.; Stockley, R.A. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J 2009, 33, 1345–53. [Google Scholar] [CrossRef]
- Stockley, R.A.; Parr, D.G.; Piitulainen, E.; Stolk, J.; Stoel, B.C.; Dirksen, A. Therapeutic efficacy of a-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res 2010, 11, 136–44. [Google Scholar] [CrossRef]
- Chapman, K.R.; Burdon, J.G.W.; Piitulainen, E.; Sandhaus, R.A.; Seersholm, N.; Stocks, J.M.; Stoel, B.C.; Huang, L.; Yao, Z.; Edelman, J.; McElvaney, N.G. ; on behalf of the RAPID Trial Study Group. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 360–8. [Google Scholar] [CrossRef]
- Brantly, M.L.; Kuhn, B.T.; Farah, H.W.; Mahadeva, R.; Cole, A.; Chang, C.L.; Brown, C.D.; Campos, M.A.; Lascano, J.E.; Babcock, E.K.; Bhagwat, S.P.; Boyea, T.F.; Veldstra, C.A.; Andrianov, V.; Kalabus, J.L.; Eckelman, B.P.; Veale, A.G. Recombinant alpha-1 antitrypsin–Fc fusion protein INBRX-101 in adults with alpha-1 antitrypsin deficiency: A phase 1 study. Chronic Obstr Pulm Dis 2014, 11, 282–92; [Google Scholar] [CrossRef]
- Bergin, D.A.; Hurley, K.; McElvaney, N.G.; Reeves, E.P. Alpha-1 antitrypsin: A potent anti-inflammatory and potential novel therapeutic agent. Arch Immunol Ther Exp (Warsz), 2012, 60, 81–97; https://. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Diciaccio, B.; Li, Y.; Elshikha, A.S.; Titov, D.; Brenner, B.; Seifer, L.; Pan, H.; Karic, N.; Akbar, M.A.; Lu, Y.; Song, S.; Zhou, L. Anti-inflammaging effects of human alpha-1 antitrypsin. Aging Cell 2018, 17, e12694; https://. [Google Scholar] [CrossRef]
- Schuster, R.; Motola-Kalay, N.; Baranovski, L.; Tov, N.; Stein, M.; Lewis, E.C.; Ayalon, M.; Sagiv, Y. Distinct anti-inflammatory properties of alpha1-antitrypsin and corticosteroids reveal unique underlying mechanisms of action. /: Cell Immunol 2020, 104177; https, 2020. [Google Scholar] [CrossRef]
- Janciauskiene, S.M.; Nita, I.M.; Stevens, T. Alpha1-antitrypsin, old dog, new tricks: Alpha1-antitypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem 2007, 282, 8573–8582. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.A.; Reeves, E.P.; Meleady, P.; Henry, M.; McElvaney, O.J.; Carroll, T.P.; Condron, C.; Chotirmall, S.H.; Clynes, M.; O'Neill, S.; McElvaney, N.G. a-1 antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest 2010, 120, 4236–4250. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, C.A.; O’Brien, M.E.; Wormald, M.R.; White, M.M.; Banville, N.; Hurley, K.; McCarthy, C.; McElvaney, N.G.; Reeves, E.P. The BLT1 inhibitory function of alpha-1 antitrypsin augmentation therapy disrupts leukotriene B4 neutrophil signaling. J Immunol 2015, 195, 3628–3641. [Google Scholar] [CrossRef]
- Lagarde, W.H.; Courtney, K.L.; Reiner, B.; Steinmann, K.; Tsalikian, E.; Willi, S.M. Human plasma-derived alpha1-proteinase inhibitor in patients with new-onset type 1 diabetes mellitus: a randomized, placebo-controlled proof-of-concept study. Pediatr Diabetes 2021, 22, 192–201. [Google Scholar] [CrossRef]
- Magenau, J.M.; Goldstein, S.C.; Peltier, D.; Soiffer, R.J.; Braun, T.; Pawarode, A.; Riwes, M.M.; Kennel, M. , Antin J. H.; Cutler, C.S.; Ho, V.T.; Alyea, E.P.; Parkin, B.L.; Yanik, G.A.; Choi, S.W.; Lewis, E.C.; Dinarello, C.A.; Koreth, J.; Reddy, P. a1-antitrypsin infusion for treatment of steroid resistant acute graft-versus-host disease. Blood 2018, 131, 1372–1379. [Google Scholar] [CrossRef]
- Toldo, S.; Seropian, I.M.; Mezzaroma, E.; Van Tassell, B.W.; Salloum, F.N.; Lewis, E.C.; Voelkel, N.; Dinarello, C.A.; Abbate, A. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol 2011, 51, 244–251. [Google Scholar] [CrossRef]
- EMA guideline Guideline on bioanalytical method validation. EMA/CHMP/EWP/192217/2009. Committee for Medicinal Products for Human Use (CHMP), , 2011 (effective: February 1, 2012). 21 July.
- Stoller, J.K.; Hupertz, V.; Aboussouan, L.S. Alpha-1 antitrypsin deficiency. In GeneReviews®, 2023. Adam, M.P.; Feldman, J.; Mirzaa, G.M. U: Seattle (WA), 1993. [Google Scholar]
- Long, G.L. , Chandra, T., Woo, S.L.C., Davie, E.W., Kurachi, K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry 1984, 23, 4828–4837. [Google Scholar] [CrossRef]
- Lomas, D.A.; Evans, D.L.; Finch, J.T.; Carrell, R.W. The mechanism of Z a1-antitrypsin accumulation in the liver. Nature, /: 605-607; https. [CrossRef]
- Lomas, D.A.; Elliott, P.R.; Chang, W.-S.W.; Wardell, M.R.; Carrell, R.W. Preparation and characterization of latent a1-antitrypsin. J Biol Chem 1995, 270. [Google Scholar] [CrossRef]
- Lomas, D.A.; Elliott, P.R.; Carrell, R.W. Commercial plasma α1-antitrypsin (Prolastin®) contains a conformationally inactive, latent component. Eur Respir J 1997, 10, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I. , Fijalkowska, I., Medler, T.R., Skirball, J., Cruz, P., Zhen, L., Petrache, H.I., Flotte, T.R., Tuder, R.M. a-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. American J of Pathology 2006, 169, 1155–1167. [Google Scholar] [CrossRef]
- Wang, Y. , He, Y., Abraham, B., Rouhani, F.N., Brantly, M.L., Scott, D.E., Reed, J.L. Cytosolic, autocrine alpha-1 proteinase inhibitor (A1PI) inhibits caspase-1 and blocks IL-1β dependent cytokine release in monocytes. PLoS One, /: e51078; https, 5107. [Google Scholar] [CrossRef]
- Lockett, A.D. , Kimani, S., Ddungu, G., Wrenger, S., Rubin, M., Tuder, R.M., Janciauskiene, S.M., Petrache, I. a1-antitrypsin modulates lung endothelial cell inflammatory responses to TNF-a. Am J Respir Cell Mol Biol 2013, 49, 143–150. [Google Scholar] [CrossRef]
- Lam, S. , LeRiche, J.C., Kijek, K. Effect of filtration and concentration on the composition of bronchoalveolar lavage fluid. /: Chest 1985, 87, 740-742; https, 1985; 87. [Google Scholar] [CrossRef]
- Noel-Georis, I.; Bernard, A.; Falmagne, P.; Wattiez, R. Database of bronchoalveolar lavage fluid proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2002, 771, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Wattiez, R.; Falmagne, P. Proteomics of bronchoalveolar lavage fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2005, 815, 169–178. [Google Scholar] [CrossRef]
- Capelli, A.; Cerutti, C.G.; Lusuardi, M.; Colombo, M.; Donner, C.F. 1993; 39.
- Olsen, G.N.; Harris, J.O.; Castle, J.R.; Waldman, R.H.; Karmgard, H.J. Alpha-1-antitrypsin content in the serum, alveolar macrophages and alveolar lavage fluid of smoking and nonsmoking normal subjects. J Clin Invest 1975, 55, 427–430. [Google Scholar] [CrossRef]
- Dati, F.; Schumann, G.; Thomas, L.; Aguzzi, F.; Baudner, S.; Bienvenu, J.; Blaabjerg, O.; Blirup-Jensen, S.; Carstöm, A.; Hytoft-Petersen, P.; Johnson, A.M.; Milford-Ward, A.; Ritchie, R.F.; Svendsen, P.J.; Whicher, J. Consensus of a group of professional and diagnostic companies on guidelines for interim ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM470). International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists Eur J Clin Chem Biochem 1996, 34, 517–520. [Google Scholar]
- Naser, W.L. Single incubation multilayer immune technique. J. Immunol. Method 1990, 129, 151–7. [Google Scholar] [CrossRef] [PubMed]
- Engelmaier, A. , Weber, A. Sensitive and specific measurement of alpha1-antitrypsin activity with an elastase complex formation immunosorbent assay (ECFISA). J Pharm Biomed Anal 2022, 209, 114476. [Google Scholar] [CrossRef]
- Thelwell, C. , Marszal, E. , Rigsby, P., Longstaff, C. An international collaborative study to establish the WHO 1st international standard for alpha-1-antitrypsin. Vox Sang 2011, 101, 83–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




