1. Introduction
Lead and zinc are critical metals extensively utilized in the battery industry, construction, metallurgy, and anti-corrosion applications [
1]. Their primary minerals, galena (PbS) and sphalerite (ZnS), are typically processed via flotation [
2]. However, the depletion of high-grade sulfide ores has shifted attention toward the beneficiation of oxidized ores, which present complex mineralogy, fine dissemination, and high levels of clays and soluble salts [
3,
4].
One of the most effective methods for the preliminary enrichment of oxidized lead-zinc ores is froth flotation with prior sulfidization of mineral surfaces. Sulfidization aims to alter the hydrophilicity of minerals by forming sulfide films, thereby enhancing their flotation activity [
5]. The main sulfidization techniques include surface sulfidization, sulfidizing roasting, mechanochemical processing, and hydrothermal treatment [
6,
7]. However, the high cost and complexity of most of these methods limit their industrial application. Currently, the use of sulfur-containing compounds is widespread in production, but the resulting films are often porous and prone to desorption during flotation, reducing separation efficiency [
8].
Advancing sulfidization methods and investigating the relationship between mineral crystal structures, sulfidization processes, and the flotation ability of oxidized ores are key research directions. Further development of sulfide reconstruction technologies could improve the recovery of lead and zinc from refractory oxidized ores, enabling their more efficient integration into industrial processing.
Oxidized lead and zinc ores exhibit high hydrophilicity, complicating direct flotation. The use of long-chain collectors (e.g., amines) results in high reagent consumption and low selectivity. Sulfide reconstruction is an effective technique for enhancing the flotation ability of such ores by improving surface hydrophobicity, increasing electronegativity, and promoting the adsorption of cationic collectors. Current sulfidization methods include surface sulfidization, sulfidizing roasting, mechanochemical sulfidization, and hydrothermal sulfidization.
Surface Sulfidization. This method involves treating oxidized minerals with solutions of sulfidizing agents (Na2S, NaHS, (NH4)2S) to form a sulfide film. The sulfidization reactions for the main lead and zinc oxide minerals are as follows:
ZnCO3/ZnCO3 + HS⁻ → ZnCO3/ZnS + HCO3⁻
PbCO3/PbCO3 + HS⁻ → PbCO3/PbS + HCO3⁻
Zn4Si2O7(OH)2·H2O/Zn4Si2O7(OH)2·H2O + HS⁻ → Zn4Si2O7(OH)2·H2O/ZnS + +H2(Si2O7)4⁻ + H2O
Studies using ToF-SIMS have shown that S²⁻ ions penetrate tens of nanometers into the mineral, facilitating the formation of stable sulfide phases. The optimal pH for this process is 9–11, where the HS⁻ ion exhibits the highest sulfidizing activity. Elevated temperatures increase the diffusion rate of sulfide ions, leading to denser sulfide films [
9,
10].
Sulfidizing Roasting. This process occurs at high temperatures in a reducing environment with the addition of sulfur or pyrite [
11]. Decarbonization of cerussite and smithsonite occurs at 300–500°C, followed by the formation of metallic sulfides at 700–1000°C [
12,
13]. Optimizing the temperature regime enables selective sulfidization and reduces energy costs. Studies have demonstrated that gradient heating achieves sulfidization rates of 96.50% for Pb and 97.29% for Zn [
14].
Mechanochemical Sulfidization. This method relies on mechanical action (impact, friction, shear) on oxidized minerals and a sulfidizing agent, creating active sites and lattice defects [
15]. Adding reducing agents (Fe, S) enhances efficiency [
16]. However, the presence of iron particles can complicate subsequent flotation, necessitating additional separation stages.
Hydrothermal Sulfidization. This process mimics geothermal conditions, converting metal oxides into sulfides via sulfur disproportionation in an aqueous solution [
17]. At 220°C with 180 minutes of stirring, the sulfidization rate of hemimorphite reaches 73% [
18]. Microwave heating improves efficiency and reduces reaction time [
19]. While promising for heavy metal recovery, industrial application is limited by heat transfer challenges.
Enhanced Sulfidization. To improve sulfide film stability and increase target mineral yields, ammonium salts [
20], Cu²⁺ and Pb²⁺ ions, and chloride ions [
21] are used. These catalyze sulfidization reactions, lower the energy barrier, and enhance flotation reagent adsorption. Experimental data confirm that pretreating oxidized minerals with Cu²⁺ or Pb²⁺ ions increases surface sulfide content, improving flotation performance [
22,
23].
Sulfidizing the surface of oxidized lead and zinc minerals is a critical step in preparing raw materials for flotation. The application of various sulfidization methods allows the technology to be tailored to specific processing conditions.
The objective of this study is to investigate the oxidized ores of the Koskuduk deposit using calcium polysulfide (CaSₙ) as a sulfidizing agent to enhance enrichment efficiency and replace sodium sulfide to reduce environmental impact.
2. Materials and Methods
The material composition of the analyzed ores and enrichment products was determined at the accredited laboratories of the KazHydroMed Testing Center LLP using various analytical chemistry methods. Studies were conducted in specialized groups: atomic absorption and emission analysis, classical chemical and photometric analysis, and assay gravimetric analysis. The analysis was performed on representative samples prepared according to established methodological requirements to ensure high accuracy and reliability of the results.
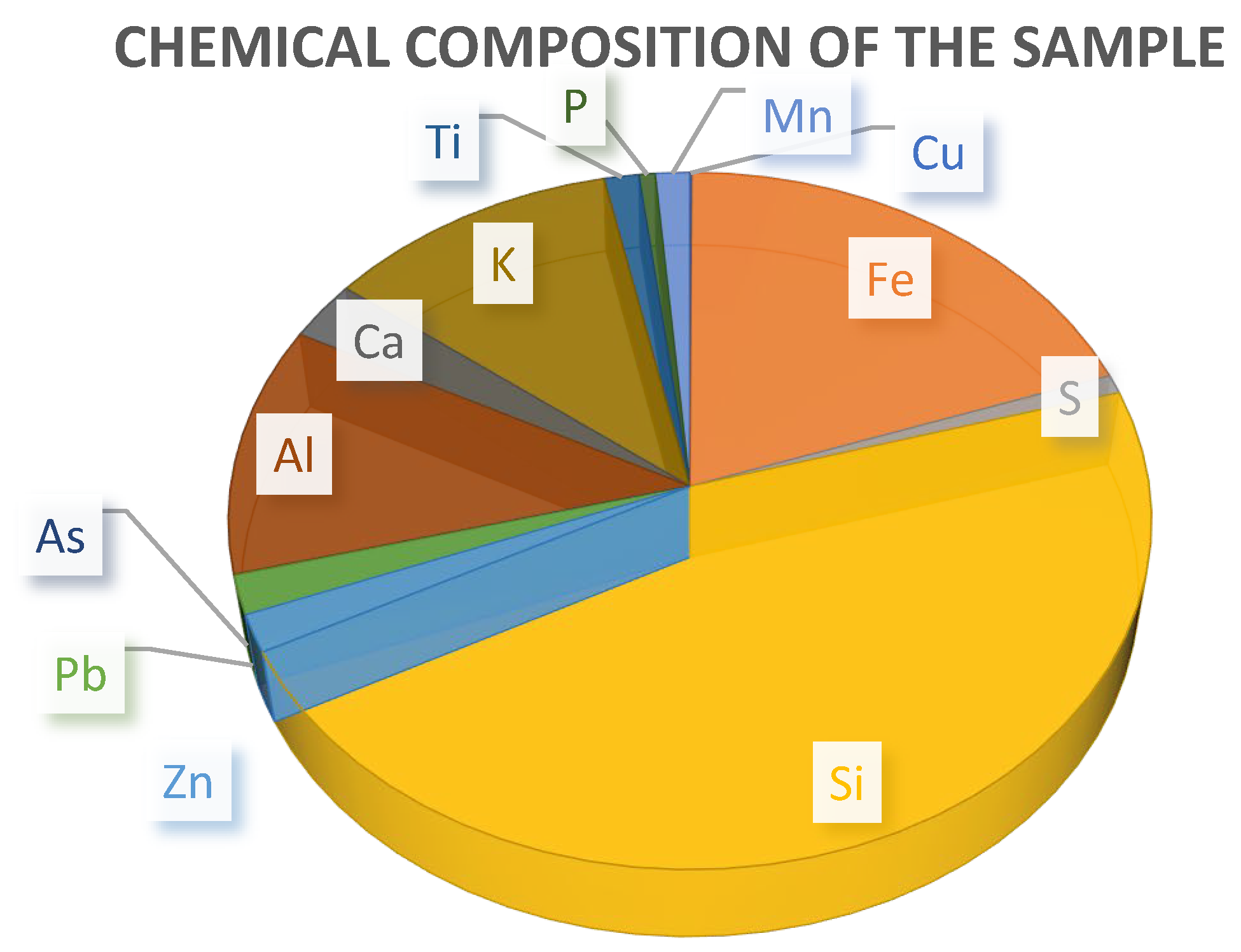
From
Figure 1, the sample contains 0.58% lead and 0.56% zinc, with a low copper content (0.02%) of no industrial significance. Notable amounts of precious metals are present: gold at 0.6 g/t and silver at 5.5 g/t. When studying the material composition, particular attention was paid to the forms of zinc, lead, and iron to assess their processability and potential enrichment methods.
The phase analysis results (
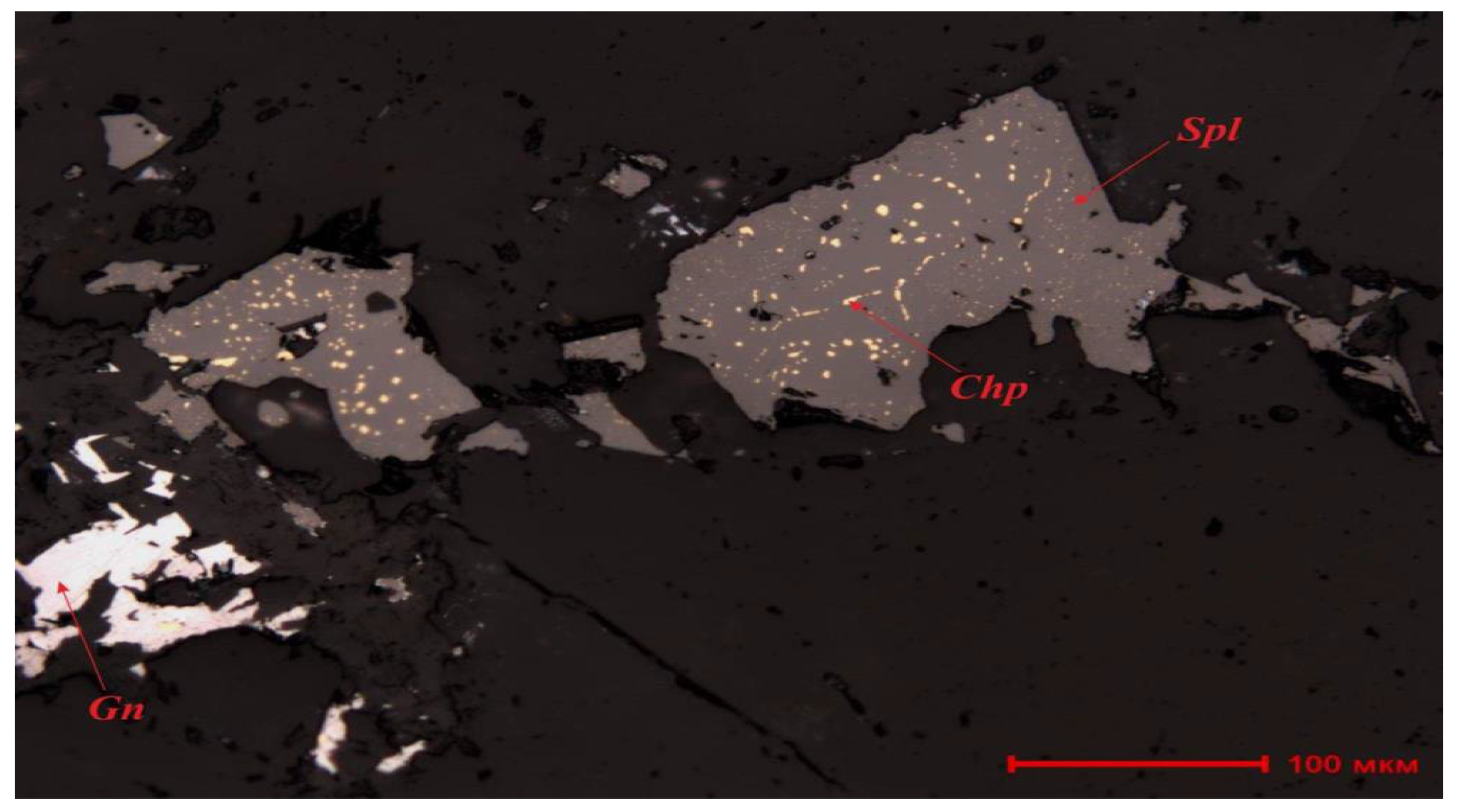
Table 1) indicate that the sample is of the oxidized type, with lead present in oxidized and silicate forms at 77.59% relative and zinc at 87.5% relative. Iron is almost entirely in oxidized form, with sulfide forms below 0.05% absolute. Ore minerals primarily consist of dull ores, chalcocite, covellite, goethite, and hematite, with sulfide minerals such as chalcopyrite, galena, pyrite, and sphalerite also identified.
Mineral aggregates form disseminated, vein-disseminated, and replacement structures. Chalcopyrite and dull ore occur as fine grains (0.005–0.015 mm) unevenly distributed in sphalerite and associated with dull ore. Sphalerite contains inclusions of chalcopyrite (0.003–0.01 mm) and galena (0.005–0.05 mm). Replacement structures of pyrite by goethite with needle-like hematite in disseminated forms were observed. Goethite also forms replacement zones along pyrite grain peripheries. Within sphalerite grains (0.05–0.3 mm), galena inclusions (0.005–0.05 mm) are present (
Figure 2).
The mineralogical characteristics of the ore and its enrichment products are complicated by the presence of fine-grained ore minerals, clay suspensions, and significant replacement of sulfide minerals by oxidized forms such as goethite and hematite. During these processes, sulfide mineral particles suitable for liberation and recovery decrease to sizes of 5 μm or less.
The results of sieve analysis, reflecting the distribution of chemical elements across size fractions, are presented in
Table 2. These data include mass fractions of individual classes and the content of target components in each fraction, enabling the evaluation of mineral liberation, distribution by size, and determination of optimal technological parameters for further enrichment.
The sieve analysis shows that the largest mass is concentrated in the -0.2 + 0.1 mm fraction (32.89%), while fine fractions (-0.045 + 0 mm) account for 7.96%. Zinc and lead predominantly accumulate in finer fractions, particularly -0.045 + 0 mm, with contents of 0.93% and 1.17%, respectively. The main distribution of lead and silver is observed in the -0.2 + 0.1 mm class.
Based on the chemical, mineralogical, and granulometric analyses, the oxidized ores of the Koskuduk deposit are classified as finely disseminated and difficult-to-enrich due to their degree of oxidation, structural-textural characteristics, and inclusion sizes.
3. Results
Flotation studies of oxidized ores began with determining the sulfidization regime using sodium sulfide. According to [
24], increasing sodium sulfide dosage from 200 g/t to 700 g/t improves lead enrichment efficiency by 10.8% (from 34.3% to 45.1%), with silver and gold recoveries of 60.2% and 59.6%, respectively. The optimal dosage for precious metals was 500 g/t, yielding 65.5% silver and 65.8% gold recovery. Notably, sulfidization has little effect on zinc enrichment [
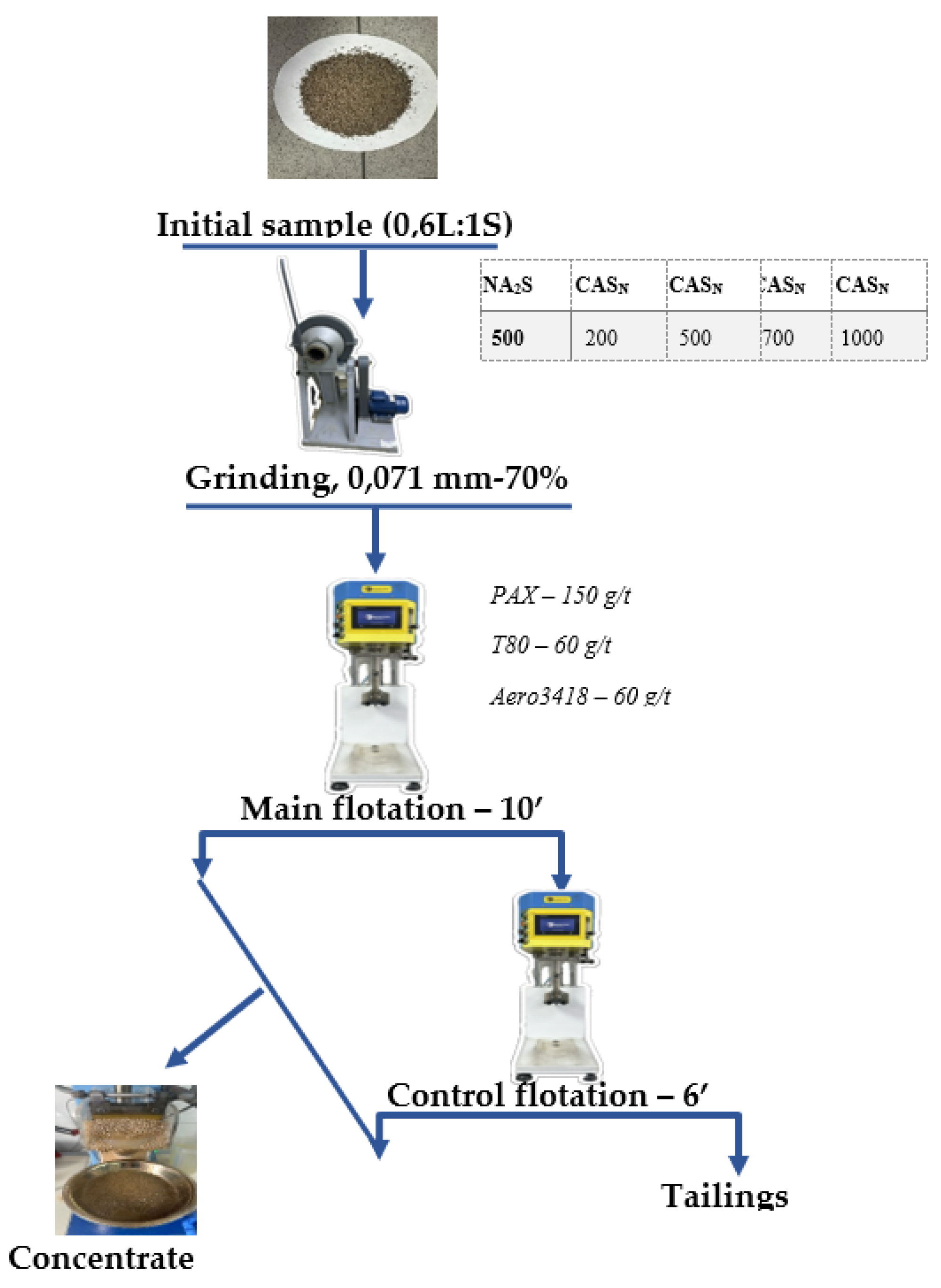
25]. Studies on the flotation of oxidized lead-bearing ores from the Zarechnoye deposit explored the effectiveness of calcium polysulfide as an alternative reagent. At a dosage of 1000 g/t, it increased lead concentration in the final product by 3.6% (from 64.4% to 68%) and recovery from 86.6% to 88.76%. These results confirm the potential of replacing sodium sulfide with calcium polysulfide to enhance flotation efficiency and lead recovery in the concentrate. The technological scheme for laboratory test experiments is shown in
Figure 3.
Calcium polysulfide was prepared by reacting hydrated lime (CaO) with sulfur (S) in an aqueous medium. The optimal component ratio was S : CaO : H2O = 10 : 5 : 85 (by weight), ensuring maximum polysulfide ion content in the solution for effective mineral sulfidization. Calcium polysulfide was introduced during the ore grinding stage, allowing uniform distribution and contact with oxidized minerals.
Table 3.
Results of Open Flotation Tests on the Initial Oxidized Ore Sample from the Koskuduk Deposit Using CaSₙ as a Sulfidizing Agent.
Table 3.
Results of Open Flotation Tests on the Initial Oxidized Ore Sample from the Koskuduk Deposit Using CaSₙ as a Sulfidizing Agent.
| Dosage, g/t |
Product |
Yield, % |
Content, %/g/t* |
Recovery, % |
| Pb |
Zn |
Ag* |
Fe |
Pb |
Zn |
Ag |
Fe |
| Base Test |
| Na2S - 500 |
Concentrate |
3.10 |
8.77 |
1.34 |
116.21 |
6.31 |
46.90 |
7.40 |
65.50 |
3.10 |
| Tailings |
96.90 |
0.32 |
0.54 |
1.96 |
6.31 |
53.10 |
92.60 |
34.50 |
96.60 |
| Initial Sample |
100.0 |
0.58 |
0.56 |
5.50 |
6.31 |
100.0 |
100.0 |
100.0 |
100.0 |
| Test 1 |
| CaSₙ - 200 |
Concentrate |
3.50 |
7.24 |
1.30 |
92.90 |
8.11 |
43.70 |
8.10 |
59.10 |
4.50 |
| Tailings |
96.50 |
0.34 |
0.53 |
2.33 |
6.24 |
56.30 |
91.90 |
40.90 |
95.50 |
| Initial Sample |
100.0 |
0.58 |
056 |
5.50 |
6.31 |
100.0 |
100.0 |
100.0 |
100.0 |
| Test 2 |
| CaSₙ - 500 |
Concentrate |
3.71 |
7.58 |
1.19 |
92.36 |
6.97 |
48.51 |
7.90 |
62.30 |
4.10 |
| Tailings |
96.29 |
0.31 |
0.54 |
2.15 |
6.28 |
51.49 |
92.10 |
37.70 |
95.90 |
| Initial Sample |
100.0 |
0.58 |
0.56 |
5.50 |
6.31 |
100.0 |
100.0 |
100.0 |
100.0 |
| Test 3 |
| CaSₙ - 700 |
Concentrate |
3.82 |
8.13 |
1.06 |
95.20 |
6.61 |
53.56 |
7.2 |
66.10 |
4.00 |
| Tailings |
96.18 |
0.28 |
0.54 |
1.94 |
6.30 |
46.44 |
92.80 |
33.90 |
96.00 |
| Initial Sample |
100.0 |
0.58 |
0.56 |
5.50 |
6.31 |
100.0 |
100.0 |
100.0 |
100.0 |
| Test 4 |
| CaSₙ - 1000 |
Concentrate |
4.02 |
7.39 |
0.99 |
87.84 |
5.65 |
51.23 |
7.10 |
64.20 |
3.60 |
| Tailings |
95.98 |
0.29 |
0.54 |
2.05 |
6.34 |
48.77 |
92.90 |
35.80 |
96.40 |
| Initial Sample |
100.0 |
0.58 |
0.56 |
5.50 |
6.31 |
100.0 |
100.0 |
100.0 |
100.0 |
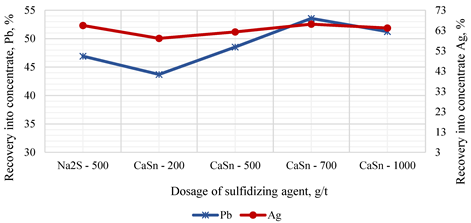
Analysis of flotation tests with varying calcium polysulfide dosages showed that at 200 g/t, lead content in the concentrate was 7.24% with a recovery of 43.7%. Silver content reached 92.90 g/t with a recovery of 59.1%, lower than with sodium sulfide, indicating insufficient sulfidization. At 500 g/t, lead content increased to 7.58% with a recovery of 48.51%, and silver content was 92.36 g/t with a recovery of 62.3%, showing improved sulfidization but still below base test silver values. The highest recovery was achieved at 700 g/t, with lead content at 8.13% and recovery at 53.56%, and silver content at 95.20 g/t with a recovery of 66.1%, surpassing sodium sulfide results. This dosage offers the best balance of content and recovery. At 1000 g/t, lead content dropped to 7.39% with a recovery of 51.23%, and silver content decreased to 87.84 g/t with a recovery of 64.2%, possibly due to excessive sulfide film formation impairing flotation properties.
Thus, the optimal results for lead and silver content and recovery were obtained at a calcium polysulfide dosage of 700 g/t, outperforming sodium sulfide in sulfidization efficiency and flotation characteristics.
4. Conclusions
This study demonstrated that using calcium polysulfide (CaSₙ) as a sulfidizing agent for enriching oxidized lead-zinc ores from the Koskuduk deposit enhances flotation efficiency. Comparative analysis showed that a dosage of 700 g/t increased lead content in the concentrate from 7.24% (at 200 g/t) to 8.13%, with recovery rising from 43.7% to 53.56%. Silver content increased from 92.90 g/t to 95.20 g/t, with recovery improving from 59.1% to 66.1%. These results exceed those obtained with sodium sulfide, where lead content was 8.77% with a recovery of 46.9%, and silver recovery reached 65.5%.
Chemical and mineralogical analyses confirmed the complex composition and high oxidation degree of the ores, necessitating sulfidization as a critical preparation step for flotation. Calcium polysulfide formed more stable sulfide films on mineral surfaces, enhancing interaction with flotation reagents and improving the hydrophobicity of ore minerals. Compared to sodium sulfide, it provided greater sulfide coating stability, improving recovery and content of key components.
The best results were achieved at a calcium polysulfide dosage of 700 g/t, increasing lead recovery by 6.66% and silver recovery by 0.6% compared to sodium sulfide. This not only enhanced flotation efficiency but also ensured more stable outcomes, making calcium polysulfide a promising alternative to traditional sulfidizing agents. Future research should focus on optimizing process parameters, exploring combinations of sulfidization methods, and studying the impact of flotation conditions on concentrate quality. Implementing this technology could enable more comprehensive processing of difficult-to-enrich oxidized ores, improving economic efficiency and reducing environmental impact.
Author Contributions
Conceptualization, G. M. and A.M.; methodology, T. T.; formal analysis L.S.; investigation, A.M., data curation, A.M. and L.S.; writing—original draft preparation, T.T.; writing—review and ed-iting, G. M.; project administration, A.M., M.B., and K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grant AR 23489765, funded by the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| РАХ |
potassium xanthogenate amyl |
References
- Nayak, M.S. Jena, N.R. Mandre. Beneficiation of lead–zinc ores—a review/ Miner. Process. Extr. Metall. Rev., 43 (5), 2022, pp.564-583. [CrossRef]
- Weiping Liu, Zuoxing Wang, Xuming Wang, Jan D. Miller. Smithsonite flotation with lauryl phosphate // Minerals Engineering, Volume 147, 1 March 2020, № 106155. [CrossRef]
- Wengang Liu, Liang Zhao, Wenbao Liu, Yongxing Zheng, Lingyun Huang, Yong Mao, Shengyuan Ding. Enhanced flotation of smithsonite from calcite based on the synergistic action of carboxylated chitosan and sodium carbonate // Advanced Powder Technology, Volume 34, Issue 12, December 2023, № 104261. [CrossRef]
- Sajjad Maghfouri, Mohammad Reza Hosseinzadeh, Abdorrahman Rajabi, Flavien Choulet. A review of major non-sulfide zinc deposits in Iran // Geoscience Frontiers, Volume 9, Issue 1, January 2018, рр. 249-272 . [CrossRef]
- 5. Yahui Yi, Peixuan Li, Ga Zhang, Qicheng Feng, Guang Han, Stepwise activation of hemimorphite surfaces with lead ions and its contribution to sulfidization flotation // Separation and Purification Technology, Volume 299, 15 October 2022, №121679 . [CrossRef]
- Martha Araceli Elizondo-Álvarez, Alejandro Uribe-Salas, Fabiola Nava-Alonso, Flotation studies of galena (PbS), cerussite (PbCO3) and anglesite (PbSO4) with hydroxamic acids as collectors // Minerals Engineering, Volume 155, 15 August 2020, № 106456 . [CrossRef]
- Jiwei Xue, Yaoben Qu, Yao Chen, Chonghui Zhang, Xianzhong Bu, Effective sulfide flotation of cerussite by using trithiocyanuric acid as a novel sulfurizing reagent // Minerals Engineering, Volume 198, July 2023, №108087 . [CrossRef]
- Song Zhang, Yongjun Xian, Shuming Wen, Guanyu Liang, Enhancement of xanthate adsorption on lead-modified and sulfurized smithsonite surface in the presence of ammonia // Minerals Engineering, Volume 189, November 2022, №107872 . [CrossRef]
- Qi Zuo, Jing Yang, Yunfeng Shi, Dandan Wu, Activating hemimorphite using a sulfidation-flotation process with sodium sulfosalicylate as the complexing agent // Journal of Materials Research and Technology, Volume 9, Issue 5, September–October 2020, рр. 10110-10120 . [CrossRef]
- Shaojun Bai, Pan Yu, Zhan Ding, Chunlong Li, Yongjun Xian, Shuming Wen, Ammonium chloride catalyze sulfidation mechanism of smithsonite surface: Visual MINTEQ models, ToF-SIMS and DFT studies // Minerals Engineering, Volume 146, 15 January 2020, № 106115 . [CrossRef]
- Y.X. Zheng, J.F. Lv, H. Wang, S.M. Wen, J. Pang, Formation of zinc sulfide species during roasting of ZnO with pyrite and its contribution on flotation // Scientific Reports., Volume 8, Issue 1, 1 December 2018, № 7839. [CrossRef]
- B.L. Ge, J. Pang, Y.X. Zheng, J.L. Ning, J.F. Lü, Sulfidation mechanism of cerussite in the presence of sulphur at high temperatures // J. Cent. South Univ., 27 (11) (2020), pp. 3259-3268 . [CrossRef]
- Y. Ke, N. Peng, K. Xue, X.B. Min, L.Y. Chai, Q.L. Pan, Y.J. Liang, R.Y. Xiao, Y.Y. Wang, C.J. Tang, H. Liu, Sulfidation behavior and mechanism of zinc silicate roasted with pyrite // Applied Surface Science., Volume 435, 30 March 2018, рр. 1011-1019. [CrossRef]
- X.Y. Wei, J.W. Han, Y.W. Wang, R. Huang, X.S. Gao, W.Q. Qin, Sulfidation behaviors and phase transformation mechanism of zinc silicate // Chinese Journal of Nonferrous Metals, Volume 32, Issue 12, 28 December 2022, рр. 3811-3822. [CrossRef]
- Hui Liu, Kai Pan, Chenglang Xiang, Dong Ye, Haining Wang, Xiaoqing Gou, Mechanochemical effect of spontaneous combustion of sulfide ore // Fuel, Volume 329, 1 December 2022, №125391. [CrossRef]
- Zhao Li, Min Chen, Peng-wu Huang, Qi-wu Zhang, Shao-xian Song, Effect of grinding with sulfur on surface properties and floatability of three nonferrous metal oxides // Transactions of Nonferrous Metals Society of China, Volume 27, Issue 11, November 2017, рр. 2474-2480. [CrossRef]
- Eric A. Runge, Muammar Mansor, Jeremiah Shuster, Stefan Fischer, Yali Liu, Dominique J. Lunter, Andreas Kappler, Jan-Peter Duda, Sulfidation of nano-magnetite to pyrite: Implications for interpreting paleoenvironmental proxies and biosignature records in hydrothermal sulfide deposits // Earth and Planetary Science Letters, Volume 617, 1 September 2023, №118261. [CrossRef]
- C.X. Li, C. Wei, Z.G. Deng, X.B. Li, M.T. Li, H.S. Xu, Hydrothermal sulfidation and flotation of oxidized zinc–lead ore // Metallurgical and Materials Transactions B, Volume 45, pр. 833–838, (2014). [CrossRef]
- H. Tan, J.X. Jin, Y.C. Shao, D. Zhou, Y. Zhou, Z.X. Wu, M.J. Wu, D.S. Shen, Y.Y. Long, Microwave hydrothermal sulfidation process for zinc-containing plating sludge // Separation Science and Technology, 56(17), рр. 3001–3010. [CrossRef]
- Yuanjia Luo, Leming Ou, Jianhua Chen, Guofan Zhang, Understanding the impact of ammonium salt on the sulfidization of smithsonite from coordination chemistry // Applied Surface Science, Volume 635, 30 October 2023, №157723. [CrossRef]
- X. Zhang, J.S. Deng, Y. Wang, G.Y. Wang, H.X. Xu, Novel insight into the lead sulfide species formed on hemimorphite surface during lead ions improved sulfidation // Colloids and Surfaces A: Physicochemical and Engineering Aspects, 653 (2022), № 129959. [CrossRef]
- Qicheng Feng, Meili Wang, Ga Zhang, Wenjuan Zhao, Guang Han, Enhanced adsorption of sulfide and xanthate on smithsonite surfaces by lead activation and implications for flotation intensification // Separation and Purification Technology, Volume 307, 15 February 2023, №122772. [CrossRef]
- Wenjuan Zhao, Bin Yang, Dianwen Liu, Qicheng Feng, Effect of copper ions on sulfidization flotation of smithsonite: Surface properties and adsorption mechanism // Colloids and Surfaces A: Physicochemical and Engineering Aspects, Volume 650, 5 October 2022, 1№ 29515. [CrossRef]
- A.R. Mambetalieva, A. Mukhtarkyzy, M.R. Shautenov. Study of the effect of sulfidization using sodium sulfide on oxidized lead-zinc ores of the Koskuduk deposit. Vestnik KazUTB, 2025. ISSN: 2708-4132. рр.
- N. K. Tussupbayev, A.A. Mukhanova, S.M. Narbekova, L.V. Syemushkina, D.K. Turysbekov. Application of calcium polysulphide as sulfidizing agent at flotation of oxidized lead – bearing ores // Complex Use of Mineral Resources № 4. 2016, рр 12 - 16.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).