1. Introduction to Liposomal and Nanocarrier Drug Delivery
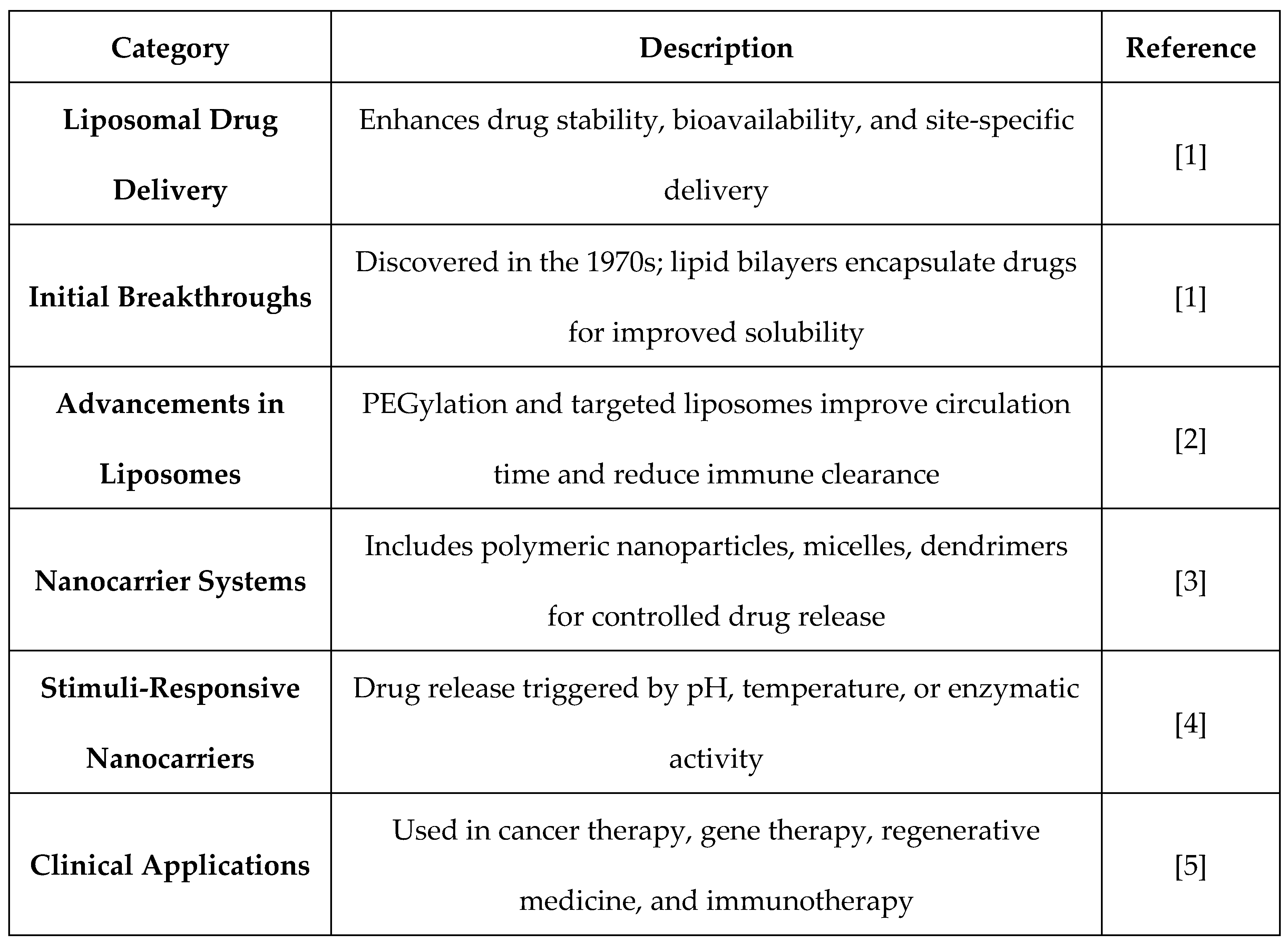
Liposomal and nanocarrier drug delivery systems have revolutionized modern therapeutics to a large degree by increasing the stability of drugs, bioavailability, and site-specific delivery. Drug release can be controlled better with these systems, eliminating side effects and increasing therapeutic effectiveness. The strategies of formulation are more sophisticated over time, and newer and improved drug carriers have appeared to treat several diseases.
1.1. Evolution of Liposomal Drug Delivery and Initial Breakthroughs
The idea of liposomes as drug carriers was initially envisioned in the 1970s, a milestone in pharmaceutical sciences. Vesicular systems formed from lipid bilayers were discovered to be capable of encapsulating hydrophilic and hydrophobic drugs, improving solubility and drug stability [
1]. Early research showed that they could improve the pharmacokinetics of therapeutic drugs, opening the door to clinical applications.
Subsequent developments in liposomal technology resulted in increasingly advanced formulations, including long-circulating and targetable liposomes. Lipid structure improvements and surface modifications, including PEGylation, prolonged circulation half-life and suppressed immune clearance, thus maximizing drug performance [
2]. These developments have resulted in successful clinical use of liposomal drug delivery, with a number of FDA-approved products now being marketed for cancer, infection, and other disease treatment.
1.2. Emergence of Nanocarrier Systems and Their Impact on Therapeutics
The evolution of nanocarriers like polymeric nanoparticles, micelles, and dendrimers extended the frontier of targeted drug delivery beyond liposomes. The nanoscale carriers have some benefits like controlled release of the drug, site-specific targeting, and increased permeation across biological barriers [
3]. Their capacity to alter pharmacokinetic profiles has rendered them of immense utility for the treatment of life-threatening and chronic diseases.
Nanomedicine has also evolved with the introduction of stimuli-responsive carriers that enable drug delivery based on some of the physiological conditions like pH, temperature, or enzymatic activity. The technology has enabled enhanced drug targeting and reduced systemic toxicity [
4]. Nanocarriers have also proven efficient in gene therapy, regenerative medicine, and immunotherapy and thus have become revolutionary in today's healthcare [
5].
Table 1.
Liposomal and Nanocarrier Drug Delivery Systems.
Table 1.
Liposomal and Nanocarrier Drug Delivery Systems.
2. Liposomal Drug Delivery Mechanisms and Structural Composition
Liposomal drug delivery systems aim to improve therapeutic effect through encapsulation of lipids within drug bilayers, shielding them from degradation and delivering them to a specific site. The vesicles have varying structural features and may be constructed with the most suitable pharmacokinetics and biodistribution for site-specific therapeutic use.
2.1. Liposome Formation, Classification, and Functionalization
Liposomes are natural spherical bilayer phospholipid vesicles with an aqueous interior. The form depends on hydrophobic as well as hydrophilic interaction and is capable of being flexible to encapsulate lipid-soluble as well as water-soluble drugs [
6]. Depending on size, composition, and lamellarity, liposomes are classified as unilamellar, multilamellar, and multivesicular vesicles with different drug capacity and release profile [
7].
Surface modifications have led to complex formulations with increased circulation and targeting. Enhancing their stability and receptor-mediated targeting for site-specific drug delivery involves some of the methods that include PEGylation, conjugation of ligands, and modification of charges [
8]. These modifications have done a lot in improving the efficiency of liposomal carriers for clinical therapy.
2.2. Mechanisms of Drug Encapsulation and Release Kinetics
The drugs are retained within the liposomes through passive or active drug entrapance processes. Hydrophobic and electrostatic interaction loading happens in a passive mode, while pH gradients or ion exchange is responsible for active loading for drug entrapance in concentrated drugs [
9]. The two processes have an important role to play in the stability and bioavailability of the drug entrapped.
Drug release from liposomes is influenced by multifarious parameters like lipid composition, bilayer stability, and extrinsic stimuli including temperature, pH, and enzymatic activity. Liposomes have been developed as stimulus-sensitive and made possible the controlled release of the target site, while minimizing off-target drug side effects and drug activity amplifications [
10].
3. Advancements in Liposomal Formulations for Targeted Therapy
Liposomal drugs have been significantly advanced to improve the stability, half-life circulation, and drug targeting of the drugs. Stealth liposomes and FDA-approved drugs have transformed drug delivery into the form of enhanced bioavailability and minimized systemic toxicity.
3.1. Stealth Liposomes and Their Role in Prolonged Circulation
Stealth liposomes are obtained through PEG conjugation to liposome surfaces to prevent opsonization and removal by mononuclear phagocytes. This modification greatly extends half-life in circulation, enabling the drug to penetrate easily to reach its site of action [
11]. High stability and low immunogenicity of stealth liposomes render them suitable for the treatment of long-duration diseases and targeted therapeutics [
12].
3.2. Liposome-Based Cancer Therapy and FDA-Approved Formulations
Liposomal drugs have also been thoroughly investigated in oncology with several FDA-approved liposomal drugs displaying clinical utility. The initial among these to gain FDA approval, Doxil®, utilizes pegylated liposomes to enhance doxorubicin delivery with sparing of cardiotoxicity and maintained anti-tumoral effects [
13]. Liposomal Amphotericin B (AmBisome®) has also revolutionized antifungal treatment with enhanced drug solubility with reduced nephrotoxicity [
14]. More research on liposomal formulation has opened the gate for safer and more effective therapeutic agents [
15].
4. Comparative Analysis of Liposomes with Other Nanocarriers
Liposomal drug delivery has been employed on a wide scale in relation to other nanocarrier systems in an attempt to analyze their relative advantages and disadvantages in pharmaceutical treatment. Although liposomes provide enhanced biocompatibility along with controlled drug release, micellar systems, SLNs, polymeric nanoparticles, and hybrid vesicular systems provide some advantage in drug solubility, stability, and target delivery.
4.1. Micellar Nanocarriers and Solid Lipid Nanoparticles (SLNs)
Micellar nanocarriers, which consist of amphiphilic molecules, increase the hydrophobic drug solubility to improve bioavailability and targeted delivery at the targeted locations. Micellar carriers are also extremely convenient in drug formulation for intravenous administration since they can spontaneously self-assemble to create stable core-shell nanoparticles under water conditions [
16].
Solid lipid nanoparticles (SLNs) are a compromise between polymeric nanoparticles and liposomes in that they possess the advantage of solid-state lipid with controlled delivery. They are stable, simple to make in large quantities, and less toxic, and thus highly promising as drug-delivery systems, especially for sustained release [
17].
4.2. Polymeric Nanoparticles and Hybrid Vesicular Systems
Polymeric nanoparticles have also been termed as a great drug delivery system because of their adjustable properties, ability to encapsulate hydrophobic and hydrophilic drugs, and for controlled release of drugs. The nanoparticles tend to utilize biodegradable polymers like poly(lactic-co-glycolic acid) (PLGA) to increase the bioavailability and stability of the drug [
18].
Hybrid vesicular drug delivery systems like effervescent-based carriers and proniosomes have the additional benefit of controlling drug release, drug solubility, and permeability. The systems inherit the advantages of polymeric nanoparticles and liposomes and provide maximum stability with high drug encapsulation efficiency [
19]. Proniosomal system advancements with cutting-edge technology also improve their use in target therapy with an improved pharmacokinetic profile and minimizing systemic toxicity [
20].
5. Challenges, Limitations, and Future Prospects in Liposomal Drug Delivery
Liposomal drug delivery has been demonstrated to be vast potential in contemporary therapeutics, yet a number of limitations exist that make its application on a large scale a nonstarter. Stability and scalability are two of these limitations, as well as targeting and cost-effectiveness efficiency. Transcending all these limitations is required for the next generation of liposomes to have a greater therapeutic impact.
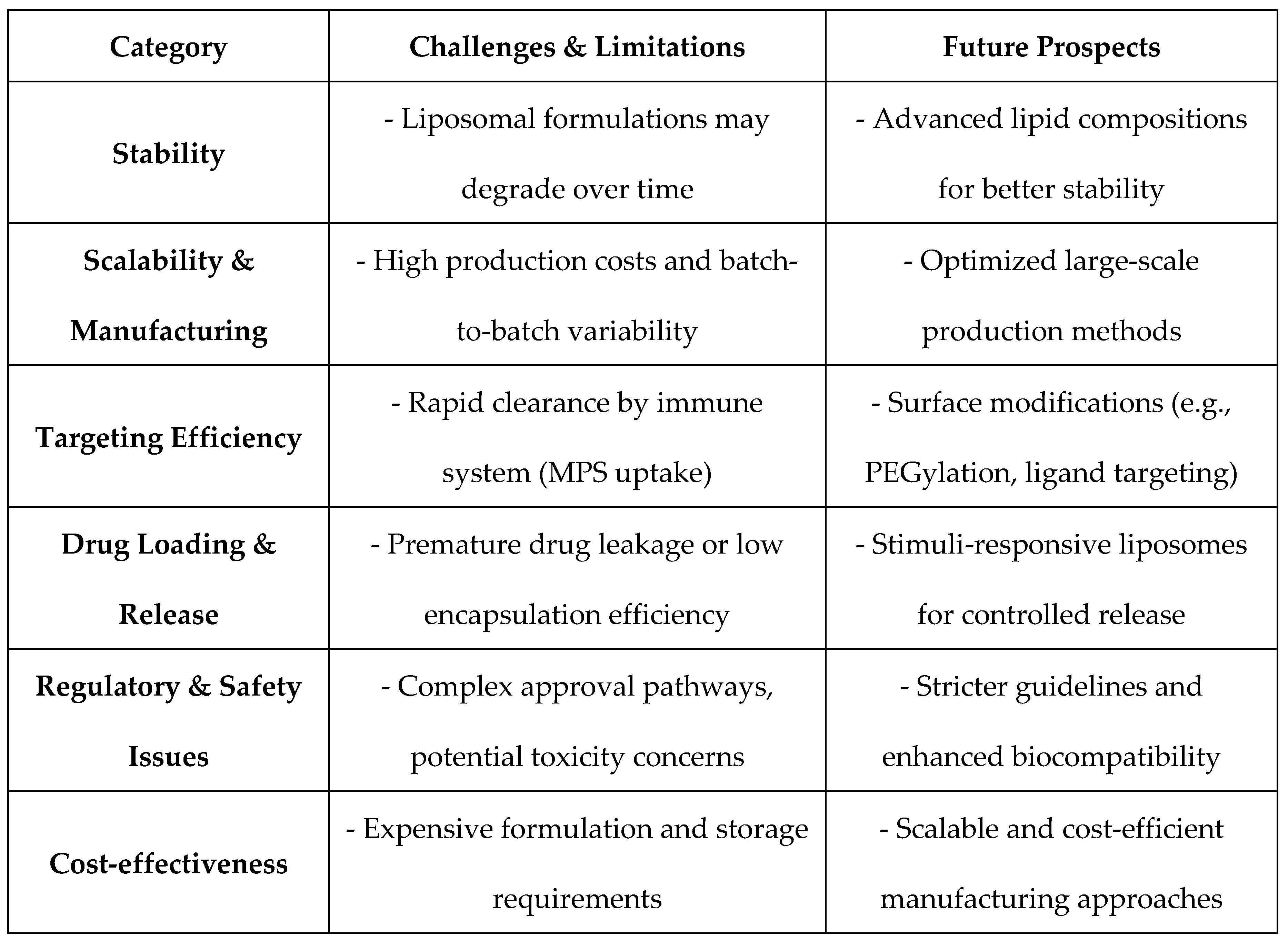
Table 2.
Challenges, Limitations, and Future Prospects of Liposomal Drug Delivery.
Table 2.
Challenges, Limitations, and Future Prospects of Liposomal Drug Delivery.
5.1. Current Limitations in Stability, Scalability, and Targeting Efficiency
One of the major problems with liposomal drug delivery is liposome stability at storage conditions and physiological conditions. Liposomal dispersions are susceptible to degradation, leakage, and agglutination and resultant loss of drug potency. Besides, structural stability of liposomes without compromising effective drug loading and drug release is still a problem in drug design [
21].
A further principal challenge is scalability since lab-to-manufacture translation for most is unresponsive and hence batch-to-batch variation comes along with it. Specialty hardware requirements and high-cost manufacturability, as well as government approval issues, make scaling-up manufacture difficult, along with commercial readying of liposomal drugs [
22]. Furthermore, while high precision targeting has been challenging due to non-selective RES uptake that is bound to decrease bioavailability, in addition to off-site targeting, shortening the overall drug efficacy of the liposomal formulation [
22].
5.2. Emerging Trends in Nanomedicine for Next-Gen Therapeutics
Newer development in nanotechnology and biomedical engineering has brought into the picture newer approaches to challenge the existing frontiers of drug delivery using liposomes. Releasing drugs by using pH-, temperature-, or enzyme-sensitive liposomes has been seen to amplify site-specific release of drugs at the expense of systemic toxicity [
23].
Additionally, the innovation of multifunctional nanocarriers that combine liposomes with polymeric or inorganic ingredients is broadening the scope of hybrid delivery systems. Such strategies target the enhancement of circulation time, targeting specificity, and drug release kinetics for maximizing therapeutic gain [
24]. Besides, ongoing research into lipid modification and surface engineering like PEGylation and ligand conjugation is facilitating the advancement of liposomal drug delivery for enhanced therapeutic efficacy and target specificity against numerous diseases like cancer and infections [
25].
Conclusions
Drug delivery by liposomes and nanocarrier systems have transformed contemporary therapeutics by improving drug bioavailability, prolonging release, and targeted release.
Dominating uninterrupted decades of R&D, breakthrough research gave rise to liposomal structural design, strategy design, and clinico-helical applications of liposomes. Stealth liposomes transformed circulation half-lives with FDA-approved products such as Doxil® marking a validation of clinico-designs of therapeutically used liposomes. Relative views comparing with other nanocarriers illustrate strengths, benefits of the liposomal system over the equivalent micellar systems, SLN, polymeric particles, and hybrid vesicles. In spite of such innovations, stability issues, scalability limitations, and ineffective targeting persist as a problem, requiring further research.
The application of nanotechnology-based systems such as stimuli-responsive liposomes and hybrid carrier systems has significant solutions in overcoming the limitations. The future of nanomedicine is focused on the development of liposomal systems for more efficient, targeted, and personalized therapeutic uses. With continued research, liposomal drug delivery should be included in the future of targeted therapeutic modalities.
References
- Gregoriadis, G. (1976). The carrier potential of liposomes in biology and medicine. New England Journal of Medicine, 295(14), 765-770. [CrossRef]
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. [CrossRef]
- Peer, D., Karp, J. M., Hong, S., Farokhzad, O. C., Margalit, R., & Langer, R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology, 2(12), 751-760. [CrossRef]
- Moghimi, S. M., Hunter, A. C., & Murray, J. C. (2005). Nanomedicine: Current status and future prospects. The FASEB Journal, 19(3), 311-330.
- Farokhzad, O. C., & Langer, R. (2009). Impact of nanotechnology on drug delivery. ACS Nano, 3(1), 16-20. [CrossRef]
- Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S. W., Zarghami, N., Hanifehpour, Y., Samiei, M., Kouhi, M., & Nejati-Koshki, K. (2013). Liposome: Classification, preparation, and applications. Nanoscale Research Letters, 8(1), 102.
- Sharma, A., & Sharma, U. S. (1997). Liposomes in drug delivery: Progress and limitations. International Journal of Pharmaceutics, 154(2), 123-140.
- Garg, T., & Goyal, A. K. (2014). Liposomes: Targeted and controlled delivery system. Drug Delivery Letters, 4(1), 62-71. [CrossRef]
- Bozzuto, G., & Molinari, A. (2015). Liposomes as nanomedical devices. International Journal of Nanomedicine, 10, 975-999.
- Paliwal, R., Paliwal, S. R., Mishra, N., & Mehta, A. (2011). Engineered polymeric nanoparticles: Current status and future directions. Expert Opinion on Drug Delivery, 8(4), 469-482.
- Immordino, M. L., Dosio, F., & Cattel, L. (2006). Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. International Journal of Nanomedicine, 1(3), 297-315.
- Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145-160. [CrossRef]
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release, 160(2), 117-134.
- Bulbake, U., Doppalapudi, S., Kommineni, N., & Khan, W. (2017). Liposomal formulations in clinical use: An updated review. Pharmaceutics, 9(2), 12. [CrossRef]
- Silverman, J. A., & Elmer, G. W. (1990). Liposomal amphotericin B (AmBisome) for the treatment of systemic fungal infections. Antimicrobial Agents and Chemotherapy, 34(7), 1281-1283.
- Torchilin, V. P. (2006). Micellar nanocarriers: Pharmaceutical perspectives. Pharmacology & Therapeutics, 120(2), 119-127. [CrossRef]
- Müller, R. H., Mäder, K., & Gohla, S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery – A review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics, 50(1), 161-177.
- Sercombe, L., Veerati, T., Moheimani, F., Wu, S. Y., Sood, A. K., & Hua, S. (2015). Advances and challenges of liposome assisted drug delivery. Frontiers in Pharmacology, 6, 286. [CrossRef]
- Sengar, A., Tile, S. A., Sen, A., Malunjkar, S. P., Bhagat, D. T., & Thete, A. K. (2024). Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research, 13(18), 1424-1435.
- Sengar, A., Saha, S., Sharma, L., Hemlata, Saindane, P. S., & Sagar, S. D. (2024). Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research, 13(21), 1063-1071.
- Sengar, A. (2024). Liposomes and beyond: Pioneering vesicular systems for drug delivery. Preprints. [CrossRef]
- Sengar, A. (2024). Precision in practice: Nanotechnology and targeted therapies for personalized care. International Journal of Advanced Nano Computing and Analytics, 3(2), 56-67. [CrossRef]
- Sengar, A. (2025). Liposomal drug delivery systems: An intro as a primer for advanced therapeutics. Preprints. [CrossRef]
- Bozzuto, G., & Molinari, A. (2015). Liposomes as nanomedical devices. International Journal of Nanomedicine, 10, 975-999.
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release, 160(2), 117-134.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).