Submitted:
13 March 2025
Posted:
14 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
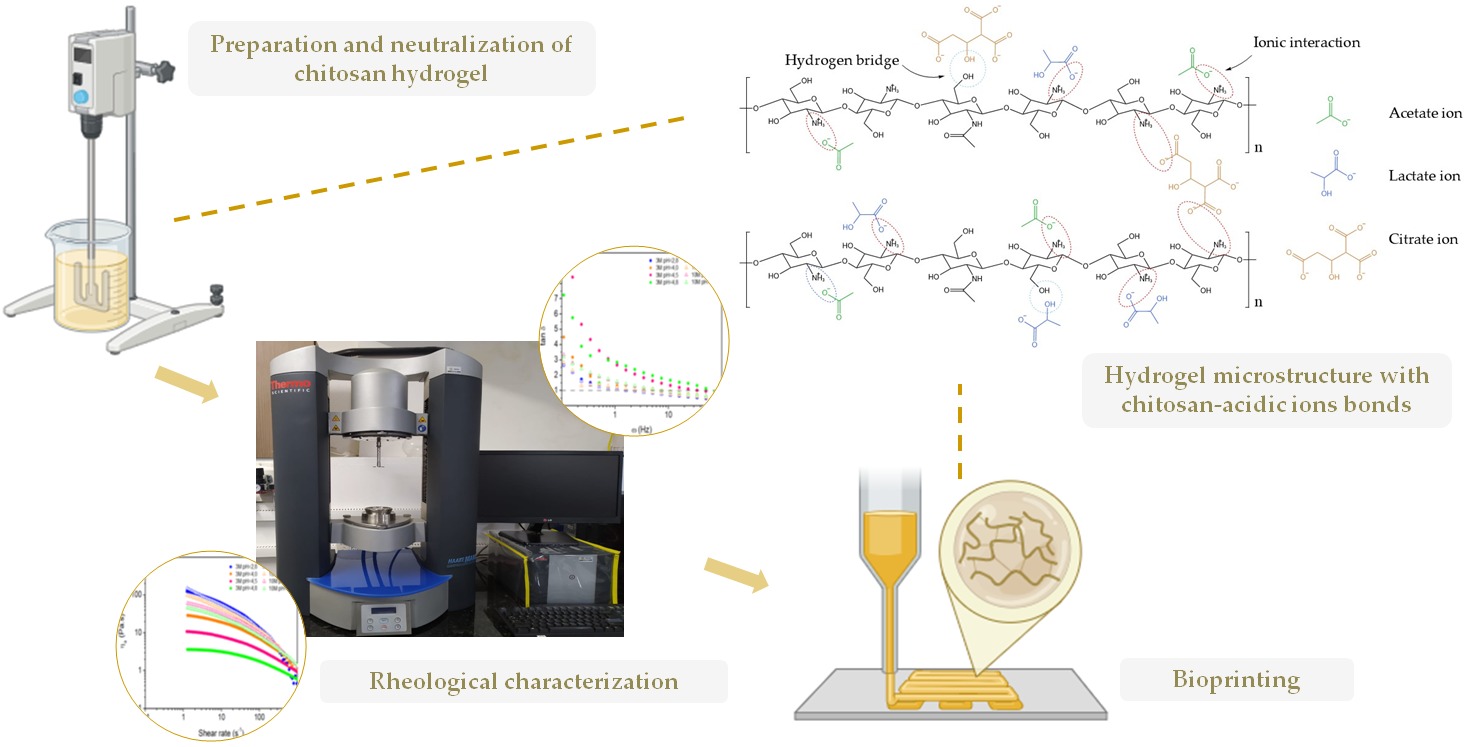
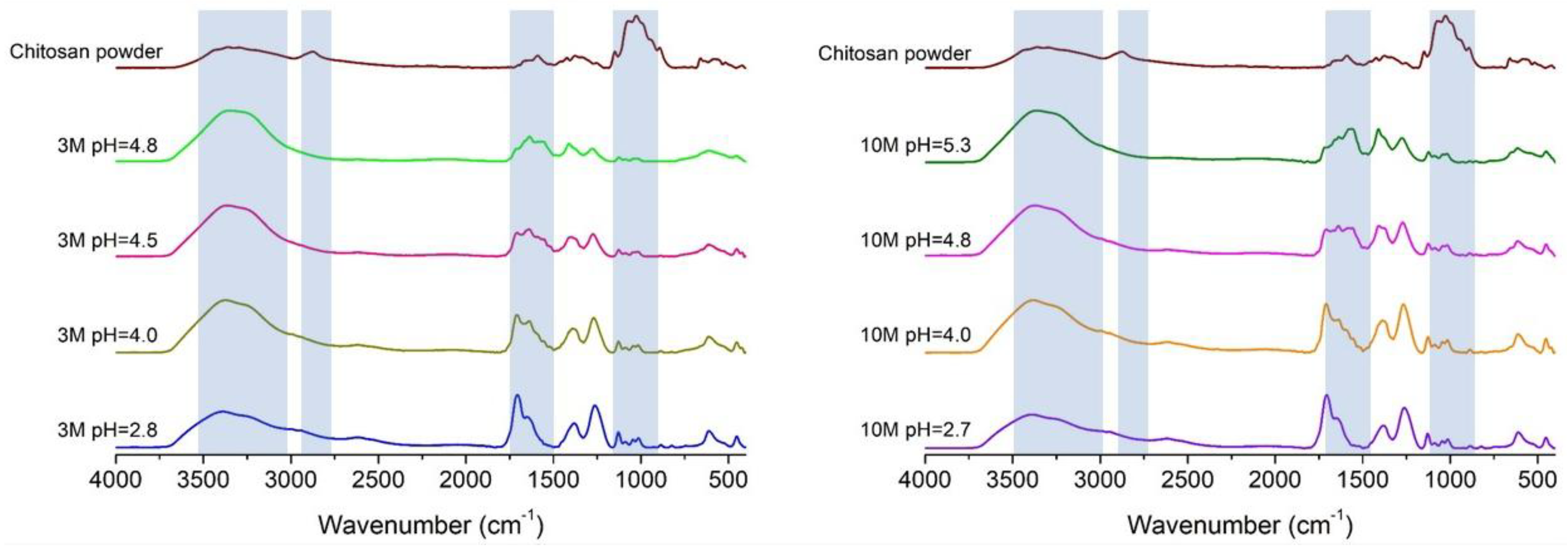
2.1. Organoleptic Properties and FTIR of Chitosan Hydrogels
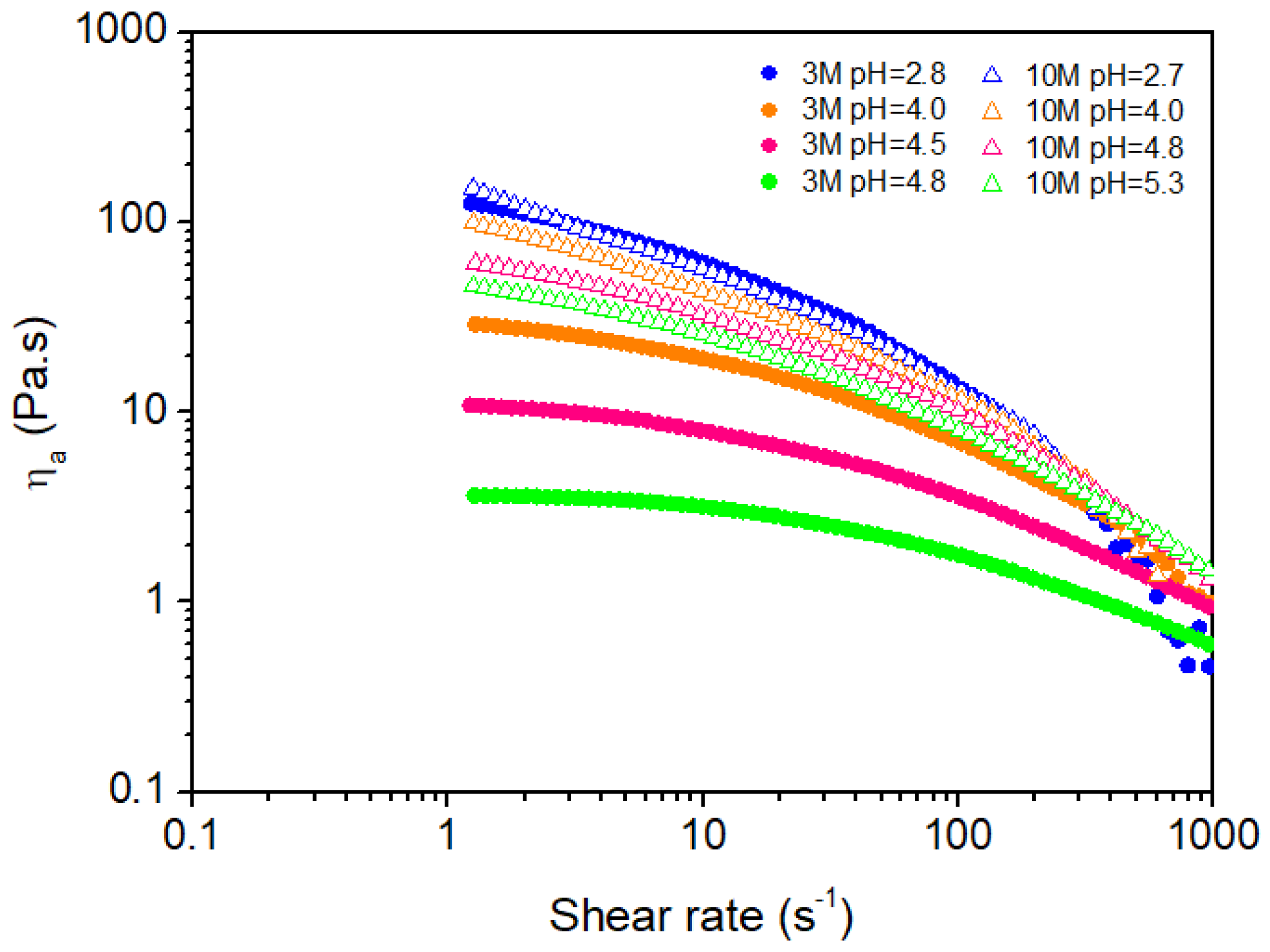
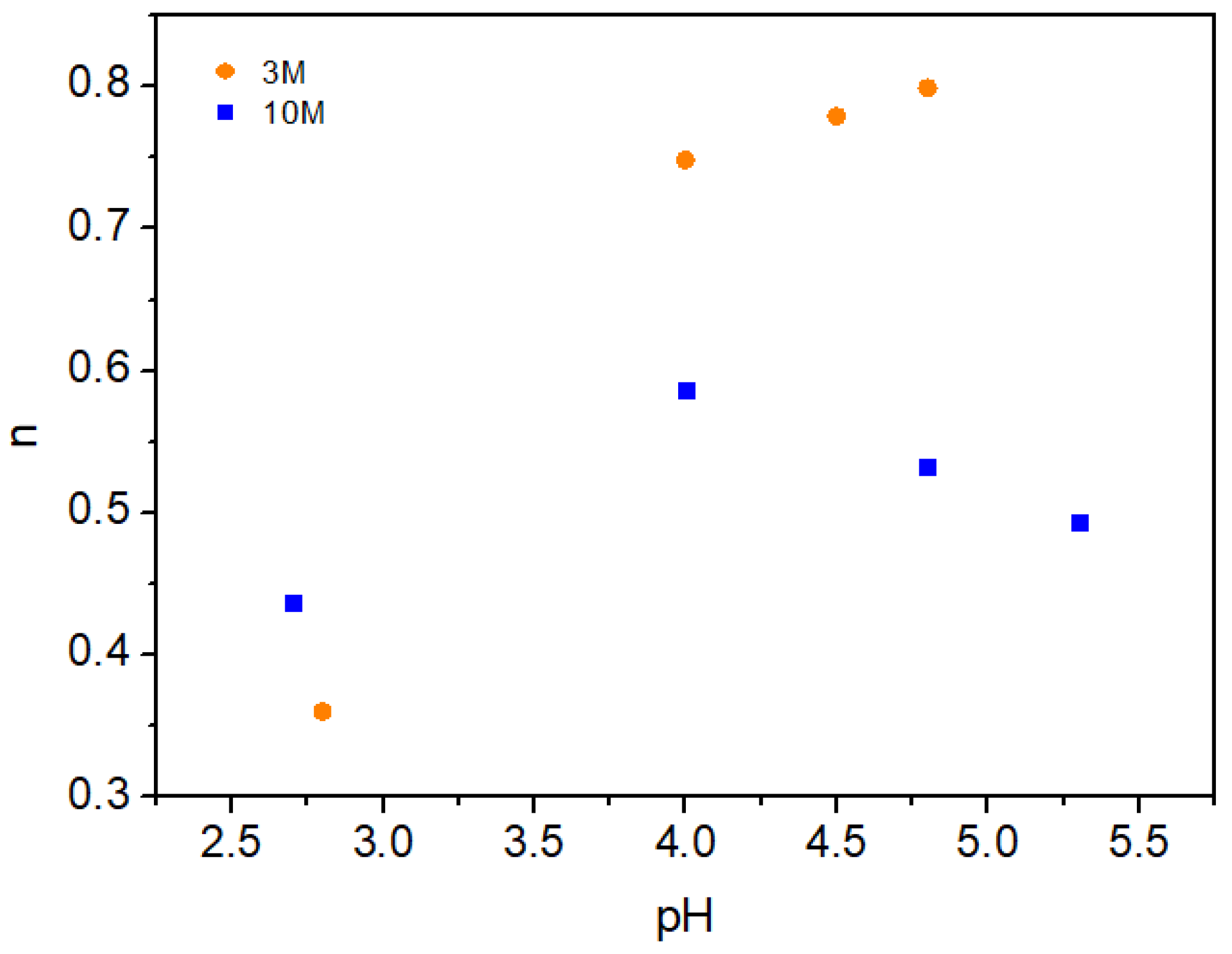
2.2. Rheological Properties of Chitosan Hydrogels
2.2.1. Shear Behavior
2.2.2. Oscillatory Behavior
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Chitosan Hydrogels
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Advanced drug delivery reviews 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Shan, B.H.; Wu, F.G. Hydrogel-based growth factor delivery platforms: strategies and recent advances. Advanced Materials 2024, 36, 2210707. [Google Scholar] [CrossRef]
- Gosecka, M.; Gosecki, M.; Jaworska-Krych, D. Hydrophobized hydrogels: construction strategies, properties, and biomedical applications. Advanced Functional Materials 2023, 33, 2212302. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Advanced drug delivery reviews 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Gierszewska-Drużyńska, M. Effect of ionic crosslinking on the water state in hydrogel chitosan membranes. Carbohydrate polymers 2009, 77, 590–598. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. European journal of pharmaceutics and biopharmaceutics 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: characteristics and pharmaceutical applications. Research in pharmaceutical sciences 2015, 10, 1–16. [Google Scholar]
- Clark, A.H. Structural and mechanical properties of biopolymer gels. In Food polymers, gels and colloids; Elsevier: 1991; 322-338. [CrossRef]
- Palacio, D.A.; Urbano, B.F.; Rivas, B.L. Hydrogels based on alkylated chitosan and polyelectrolyte copolymers. Journal of Applied Polymer Science 2018, 135, 46556. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: an update on potential biomedical and pharmaceutical applications. Marine drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Hisham, F.; Akmal, M.H.M.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer chitosan: Potential sources, extraction methods, and emerging applications. Ain Shams Engineering Journal 2024, 15, 102424. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. Reactive and Functional Polymers 2023, 191, 105634. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress in polymer science 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Rizwanullah, M.; Ahmad, J.; Alasmary, M.Y.; Akhter, M.H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. International Journal of Polymeric Materials and Polymeric Biomaterials 2022, 71, 602–623. [Google Scholar] [CrossRef]
- Franzén, H.M.; Draget, K.I.; Langebäck, J.; Nilsen-Nygaard, J. Characterization and properties of hydrogels made from neutral soluble chitosans. Polymers 2015, 7, 373–389. [Google Scholar] [CrossRef]
- Afzal, S.; Maswal, M.; Dar, A.A. Rheological behavior of pH responsive composite hydrogels of chitosan and alginate: Characterization and its use in encapsulation of citral. Colloids and Surfaces B: Biointerfaces 2018, 169, 99–106. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Alonso-González, M.; Romero, A.; Perez-Puyana, V. Applied rheology as tool for the assessment of chitosan hydrogels for regenerative medicine. Polymers 2021, 13, 2189. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vibrational Spectroscopy 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films: Effect of the type of solvent acid. Lwt 2021, 135, 109984. [Google Scholar] [CrossRef]
- Branca, C.; D'Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Takara, E.A.; Marchese, J.; Ochoa, N.A. NaOH treatment of chitosan films: Impact on macromolecular structure and film properties. Carbohydrate polymers 2015, 132, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sampath, U.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.-C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Kowalonek, J. Studies of chitosan/pectin complexes exposed to UV radiation. International journal of biological macromolecules 2017, 103, 515–524. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. Composite chitosan hydrogels for extended release of hydrophobic drugs. Carbohydrate polymers 2016, 136, 570–580. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H. Chitosan nanoparticles conjugate with trypsin and trypsin inhibitor. Carbohydrate Polymers 2016, 144, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and antimicrobial activity of some novel cross-linked chitosan hydrogels. International journal of molecular sciences 2012, 13, 11194–11209. [Google Scholar] [CrossRef] [PubMed]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Christou, C.; Philippou, K.; Krasia-Christoforou, T.; Pashalidis, I. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers. Carbohydrate polymers 2019, 219, 298–305. [Google Scholar] [CrossRef]
- Eddya, M.; Tbib, B.; Khalil, E.-H. A comparison of chitosan properties after extraction from shrimp shells by diluted and concentrated acids. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Yan, E.; Hao, X.; Cao, M.; Fan, Y.; Zhang, D.; Xie, W.; Sun, J.; Hou, S. Preparation and characterization of carboxymethyl chitosan hydrogel. Pigment & Resin Technology 2016, 45, 246–251. [Google Scholar] [CrossRef]
- Kang, Y.; Zhao, X.; Han, X.; Ji, X.; Chen, Q.; Pasch, H.; Lederer, A.; Liu, Y. Conformation and persistence length of chitosan in aqueous solutions of different ionic strengths via asymmetric flow field-flow fractionation. Carbohydrate Polymers 2021, 271, 118402. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, I.; Montembault, A.; David, L.; Perrier, A.; Vibert, R.; Duclaux, L.; Reinert, L. Prilling and characterization of hydrogels and derived porous spheres from chitosan solutions with various organic acids. International journal of biological macromolecules 2019, 129, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Visan, R.M.; Angelescu, D.G. Coarse-Grained Model of Phytic Acid for Predicting the Supramolecular Architecture of Ionically Cross-Linked Chitosan Hydrogels. The Journal of Physical Chemistry B 2023, 127, 5718–5729. [Google Scholar] [CrossRef]
- Beserra Junior, I.M.; de Sousa Lopes, D.; da Silva Barbosa, M.C.; da Silva Neto, J.E.; da Silva, H.N.; Fook, M.V.L.; Navarro, R.F.; Silva, S.M.d.L. Rheological Characterization of Genipin-Based Crosslinking Pigment and O-Carboxymethyl Chitosan–Oxidized Hyaluronic Acid In Situ Formulable Hydrogels. Polymers 2024, 16, 2615. [Google Scholar] [CrossRef]
- Navarro, R.F. The Use of Strain Density Energy to Identify Self-Healing Behavior in Chitosan Hydrogels. Biomed. J. Sci & Tech. Res. 2024, 59, 52052–52058. [Google Scholar] [CrossRef]
- Navarro, R.F., Silva, S.M.L., Fook, M.V.L. Shear Thickening - Shear Thinning Transitions in Chitosan Hydrogel. Polymers 2025. https://www.preprints.org/manuscript/202503.0475/v1.
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.C. 3D printing of microstructured and stretchable chitosan hydrogel for guided cell growth. Advanced Biosystems 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Richa, A.R.C. pH mediated rheological modulation of chitosan hydrogels. International journal of biological macromolecules 2020, 156, 591–597. [Google Scholar] [CrossRef]

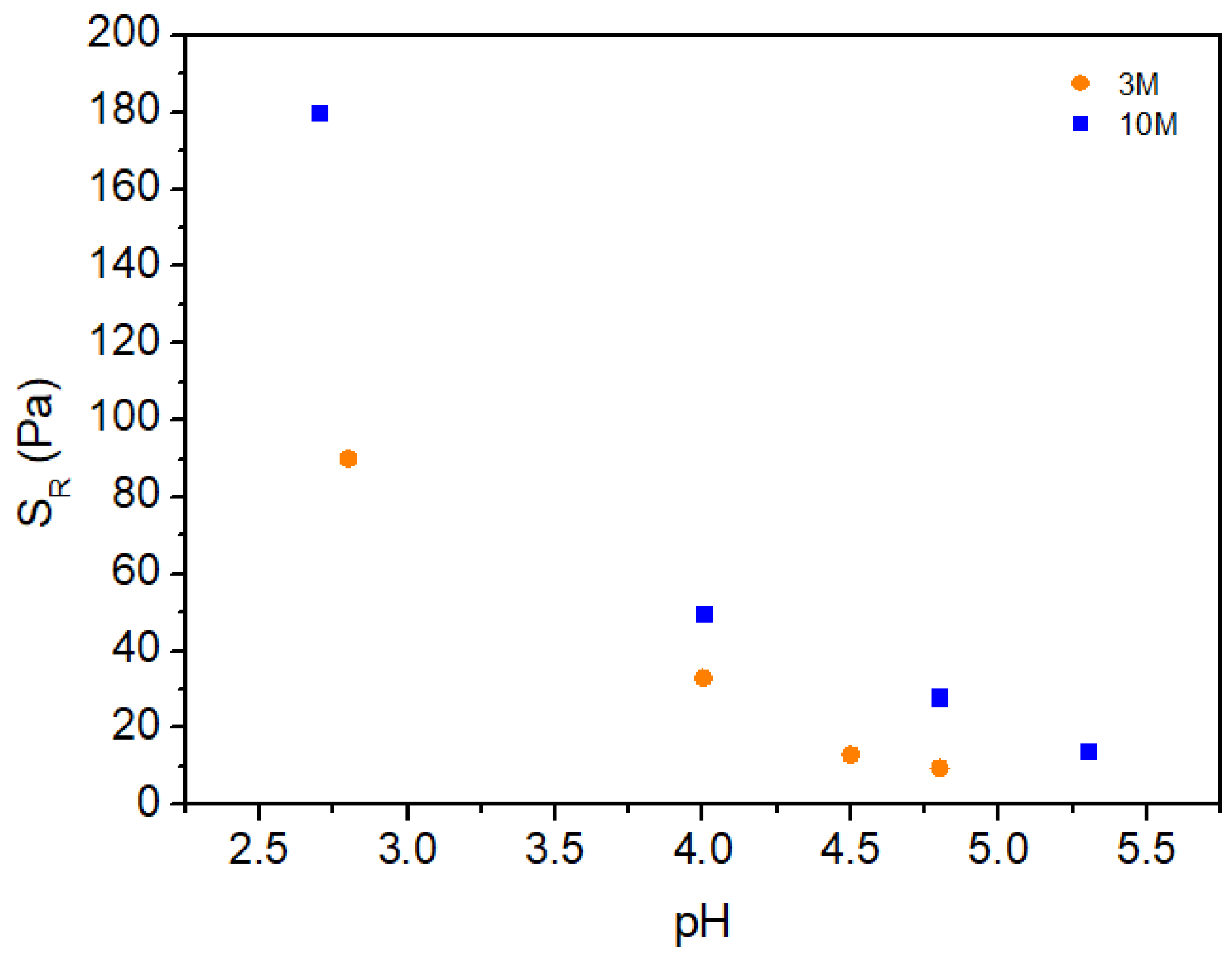
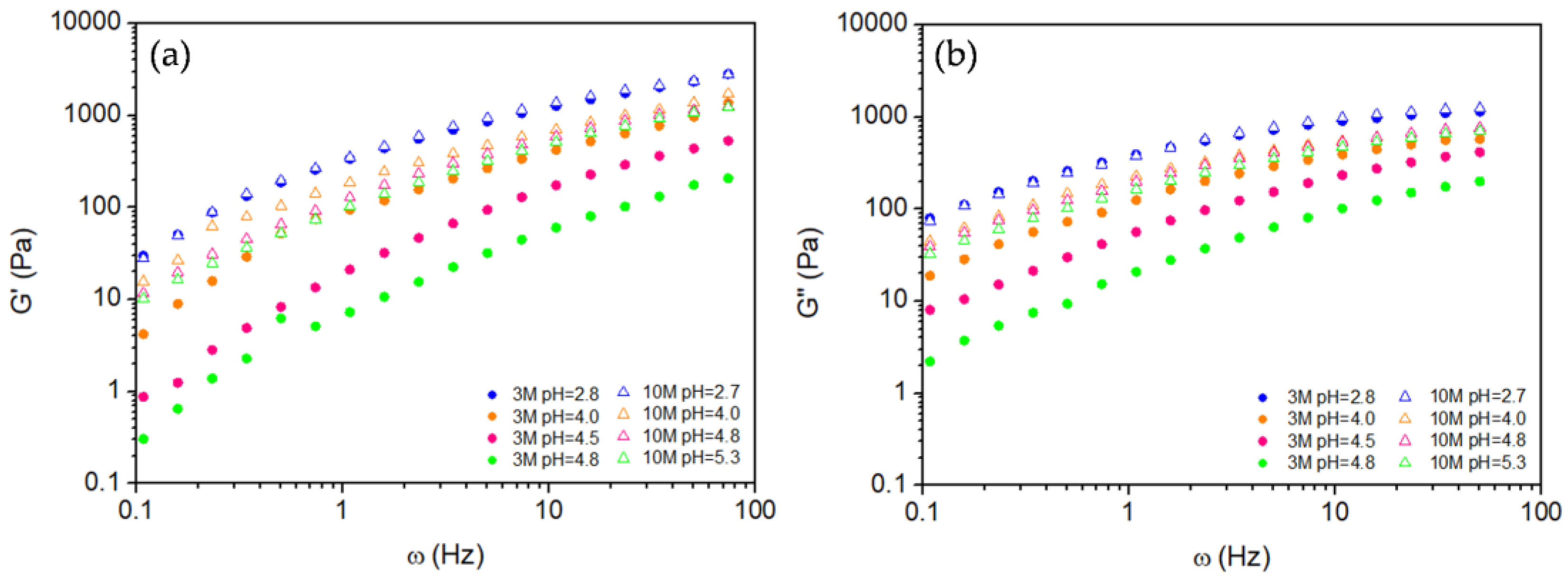
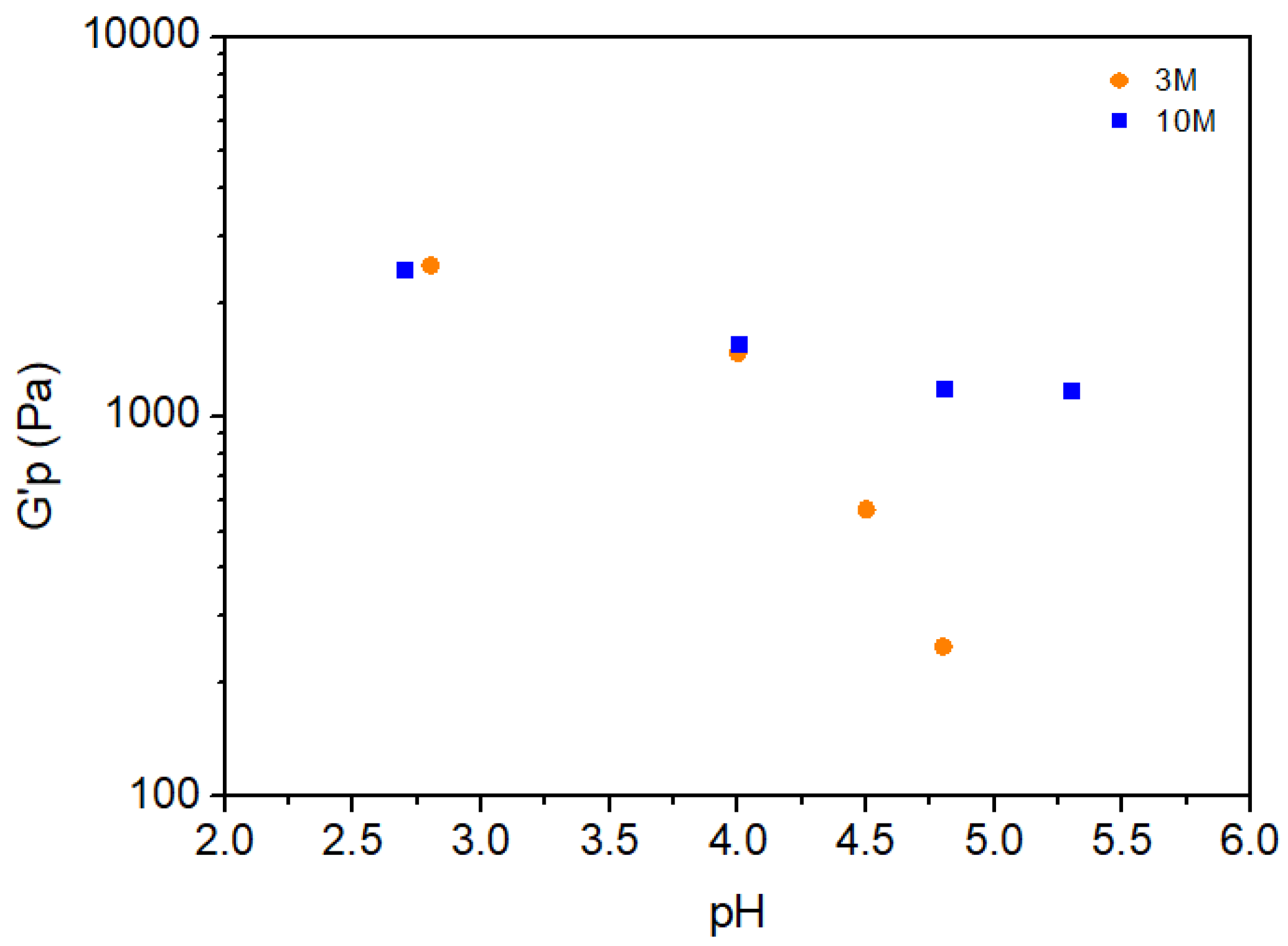
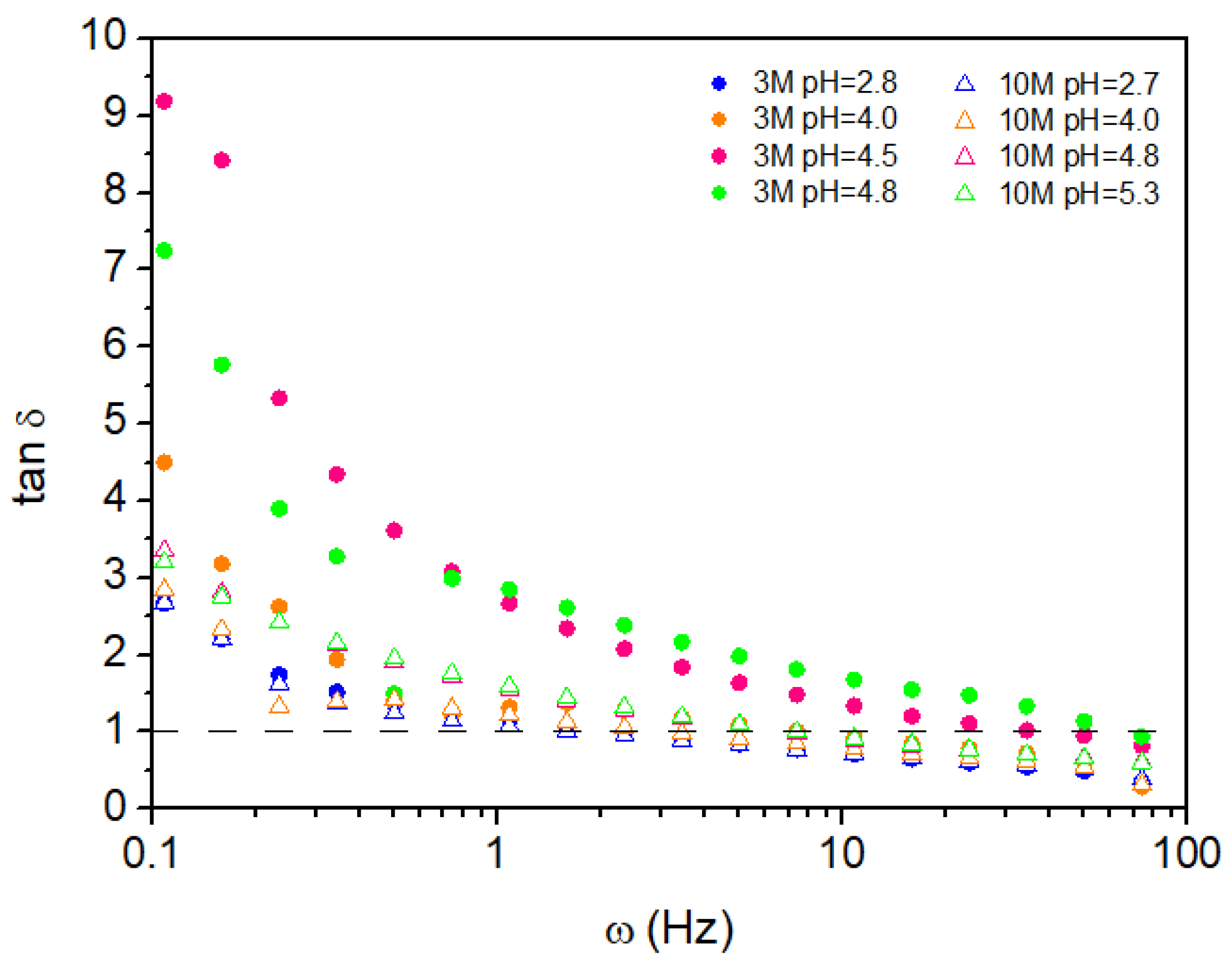
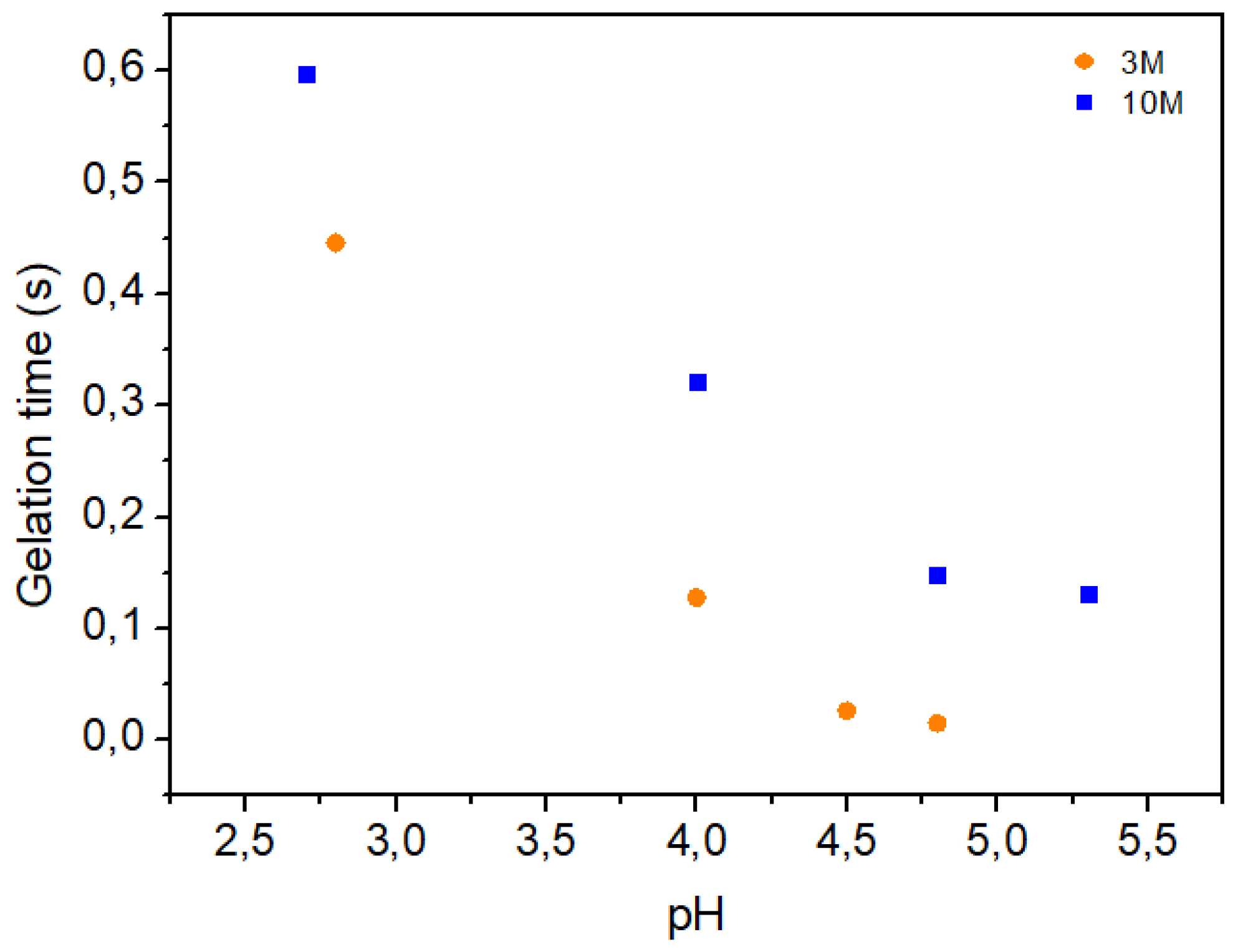
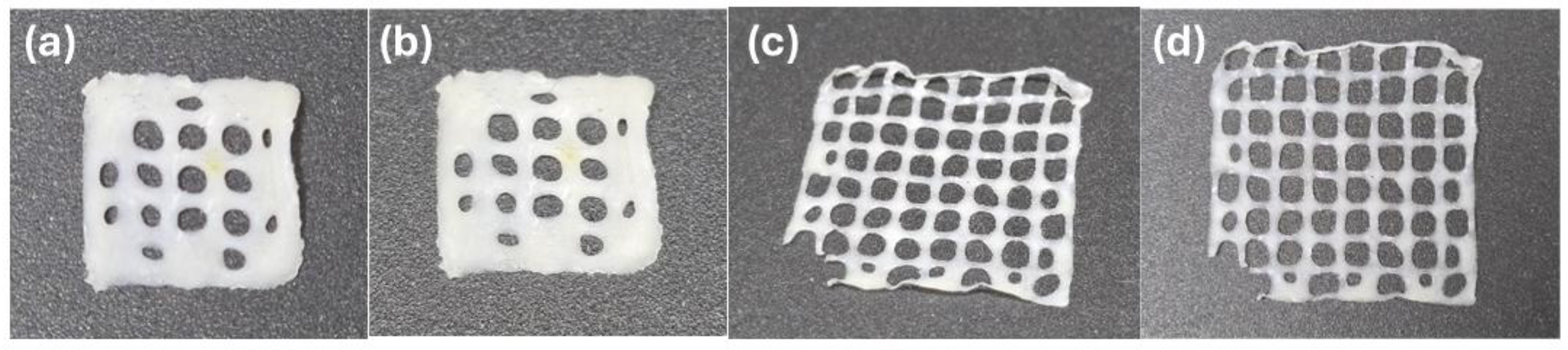
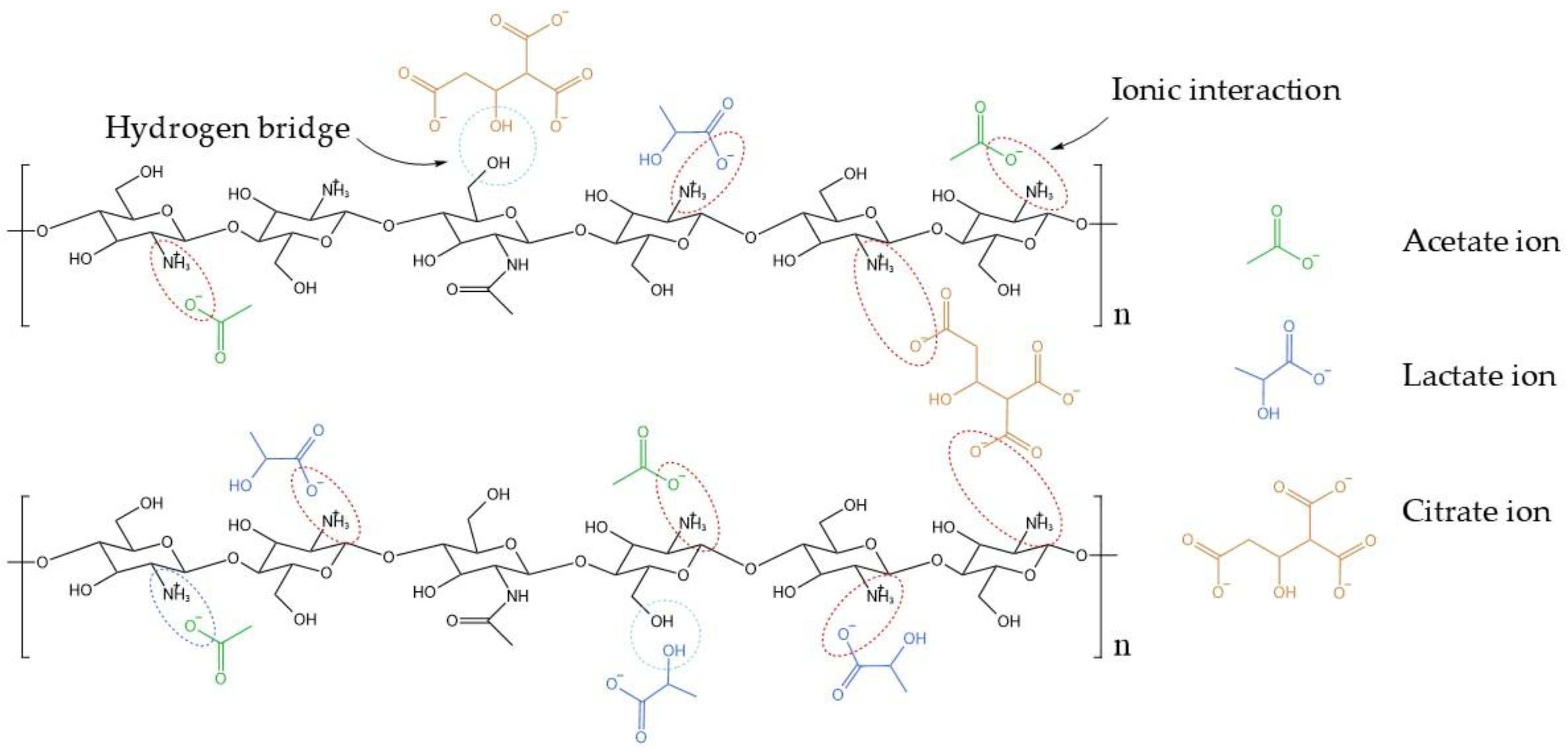
| NaOH solution concetration | pH | Volume (mL) of NaOH solution per g of chitosan |
|---|---|---|
| 3M | 2.8 | 0 |
| 3M | 4.0 | 5.38 |
| 3M | 4.5 | 4.69 |
| 3M | 4.8 | 6.92 |
| 10M | 2.7 | 0 |
| 10M | 4.0 | 2.00 |
| 10M | 4.8 | 2.80 |
| 10M | 5.3 | 2.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





