Submitted:
12 March 2025
Posted:
13 March 2025
You are already at the latest version
Abstract
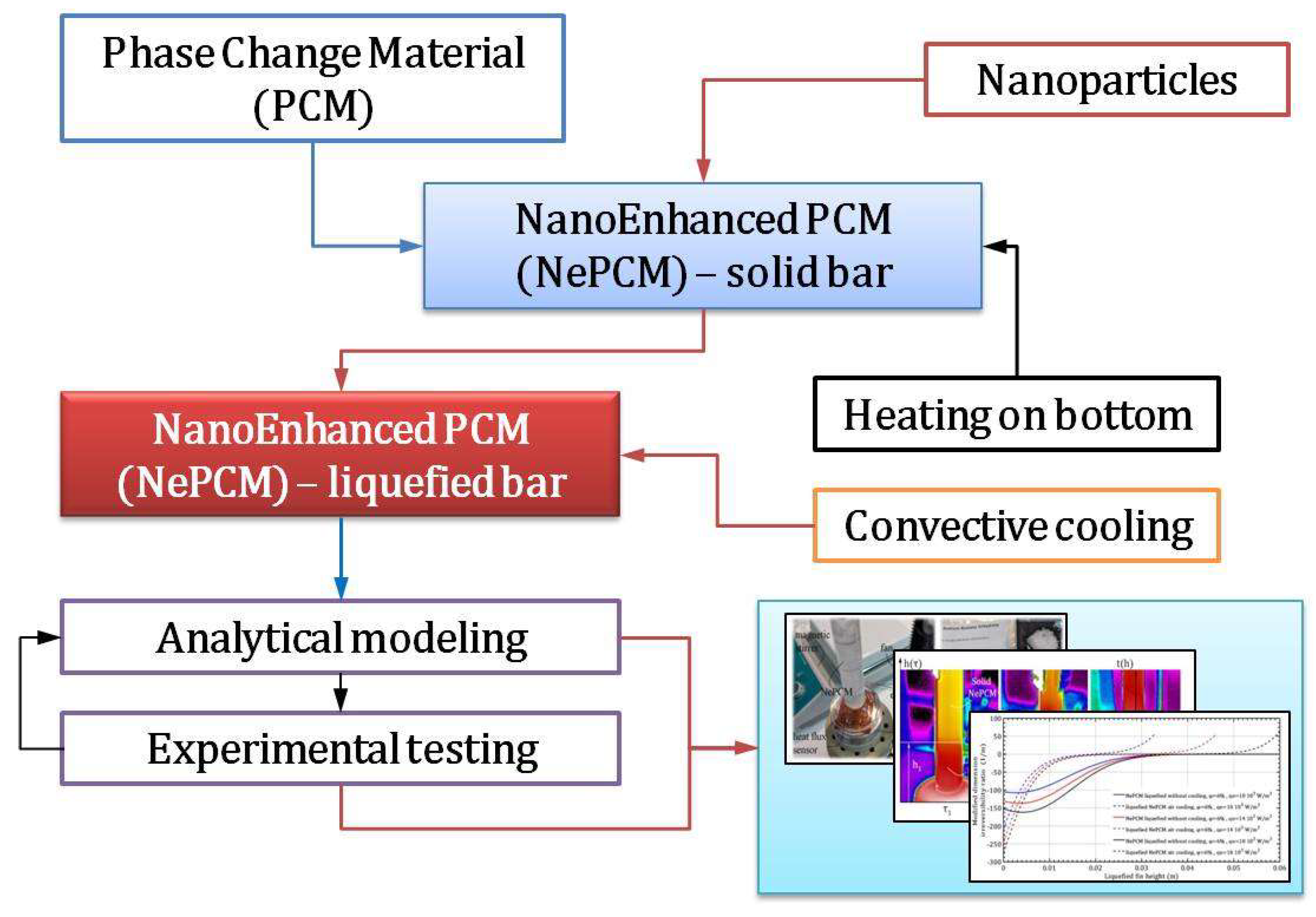
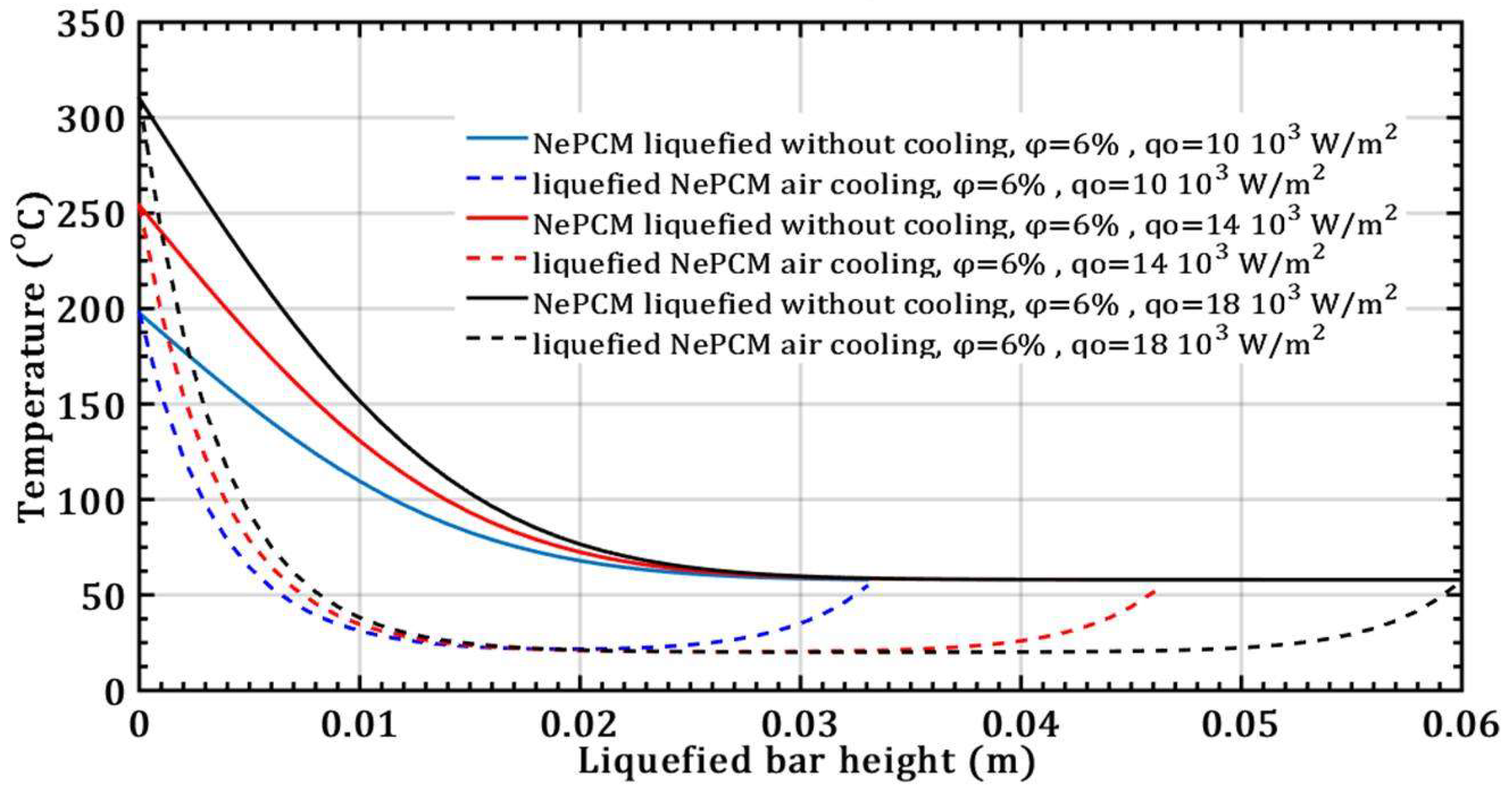
Inside the closed, thin-walled hollow cylinder, there is a solid state of phase change material (NePCM) that has been nano-enhanced. This NePCM is heated at its bottom. Nanoparticles (Al2O3) were inserted and homogenized within the PCM (sodium acetate trihydrate, C2H3O2Na), to create the NePCM. The hollow cylinder is thermally insulated from the outside ambient temperature, while the heat supplied is enough to cause a phase change. Once the entire NePCM has converted from a solid to a liquid due to heating, it is then cooled, and the thermal insulation is removed. The cylindrical liquefied NePCM bar is cooled in this manner. Thermal entropy, entransy dissipation rate, and bar efficiency during the heating and cooling of NePCM bar were analyzed by changing variables. The volume fraction ratio of nanoparticles, inlet heat flux, and liquefied bar height were the variables considered. The results indicate a significant impact on the NePCM bar during liquefaction and convective cooling when the values of these variables are altered. For instance, with an increase in the volume fraction ratio from 3% to 9%, at a constant heat flux of 104 Wm-2 and a liquefied bar height of 0.02m, the NePCM bar efficiency decreases to 99%. The thermal entropy from heat conduction through liquefied NePCM bar is significantly lower compared to thermal entropy from convective air cooling on its surface. The thermal entropy of the liquefied NePCM bar increases on average by 110% without any cooling. With a volume fraction ratio of 6%, there is an 80% increase in heat flux as the bar height increases to 0.02m.
Keywords:
1. Introduction
- -
- A well-defined methodology for analytically modeling transient thermal irreversibilities in a NePCM bar during combined conductive-convective heat transfer.
- -
- The abillity to optimize process and geometric parameters of the NePCM bar by on maximizing the modified irreversibility ratio.
- -
- the transient temperature field of a NePCM bar during its liquefaction an external heat source;
- -
- the transient temperature field of the liquefied NePCM bar during forced cooling of its outer surface;
- -
- thermal transient irreversibilities generated in both cases, which enable the establishment of an efficient optimization model based on minimizing them.
2. Methodology
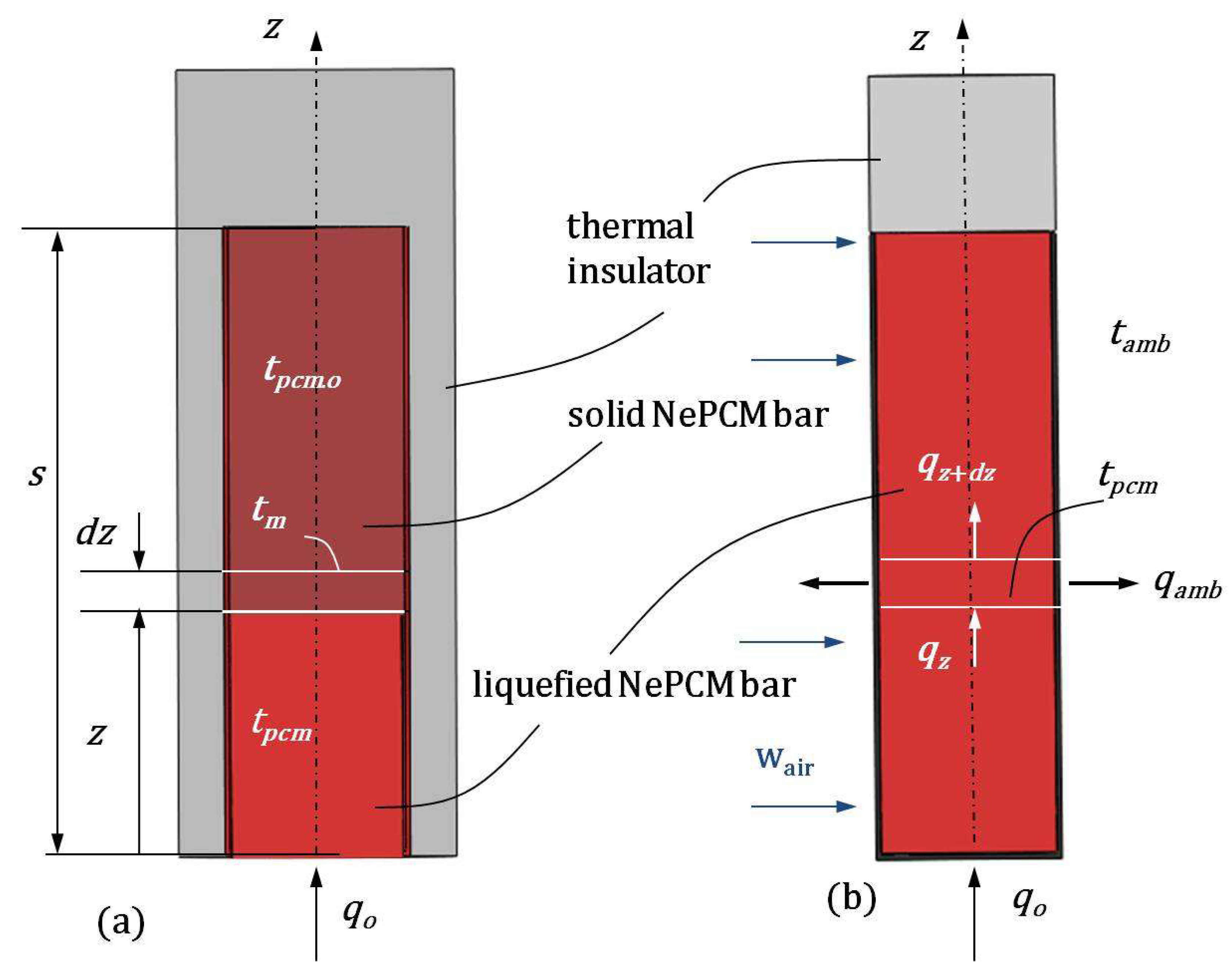
2.1. Temperature Distribution of a NePCM Cylindrical Bar
- -
- the temperature field of the cylindrical bar is one-dimensional and depends only on z coordinate;
- -
- incoming heat flux at the bottom of the bar is uniform across its circular cross-section;
- -
- the physical properties of NePCM are consistent throughout its volume;
- -
- volume concentration of nanoparticles does not alter the liquefaction temperature of the NePCM bar.
2.2. Temperature Distribution During the Cooling Process of a Cylindrical Bar Using Liquefied NePCM
2.3. Thermal Entropy of the Liquefied NePCM Bar Due to Heat Conduction
2.4. Entransy Dissipation Rate
2.5. Modified Dimension Irreversibility Ratio
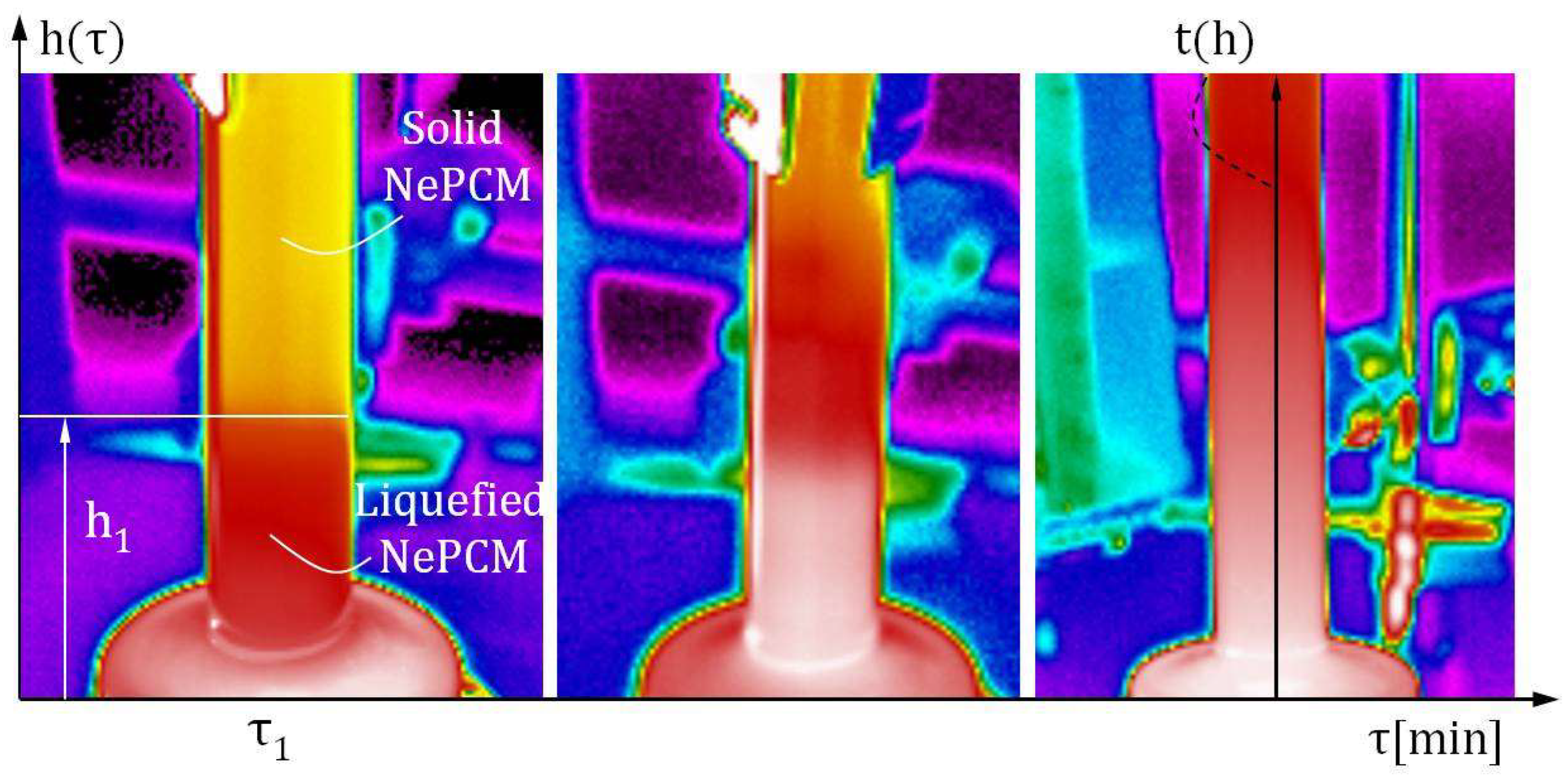
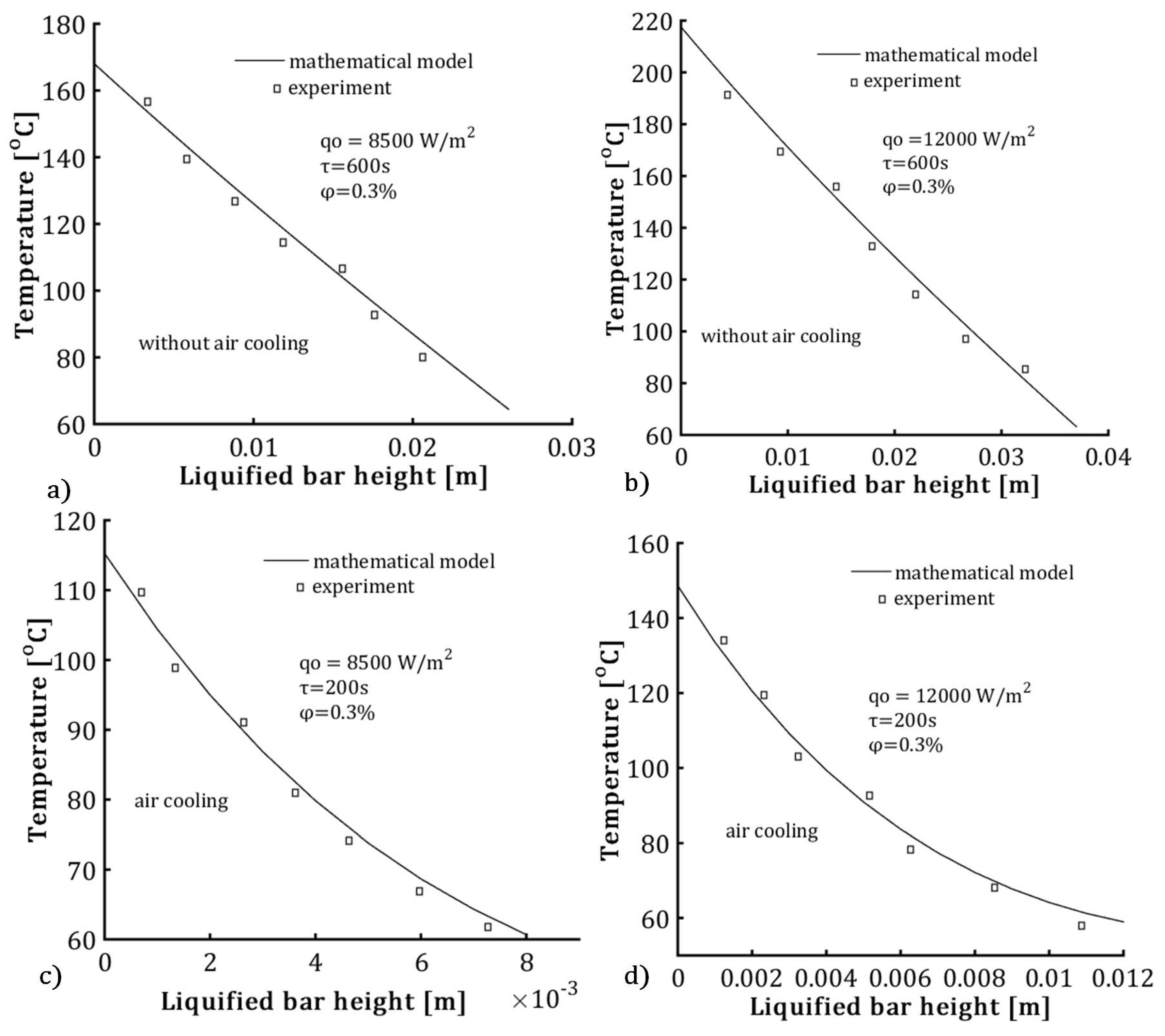
2.6. Experimental Testing
3. Results and Discussion
- -
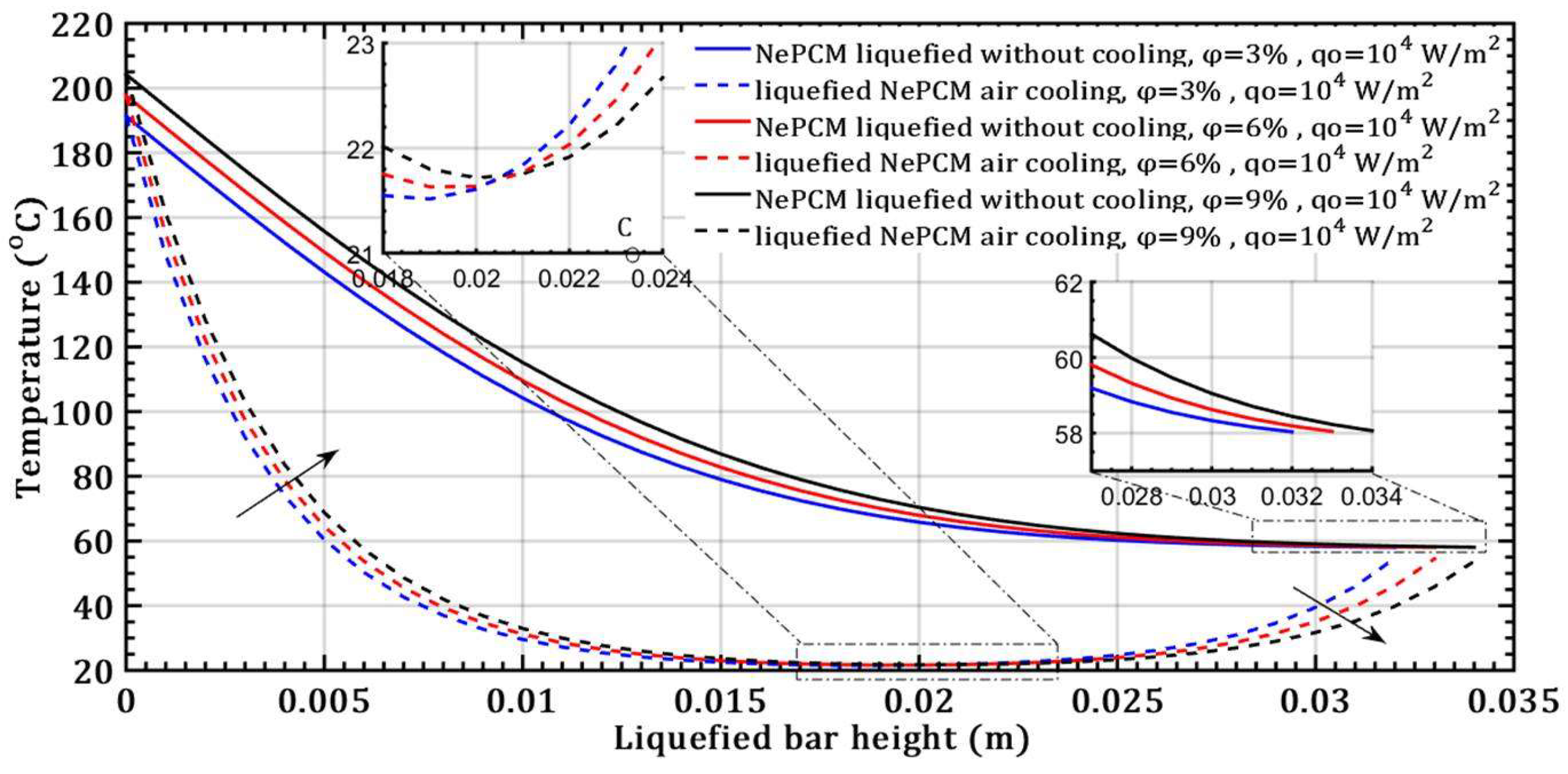
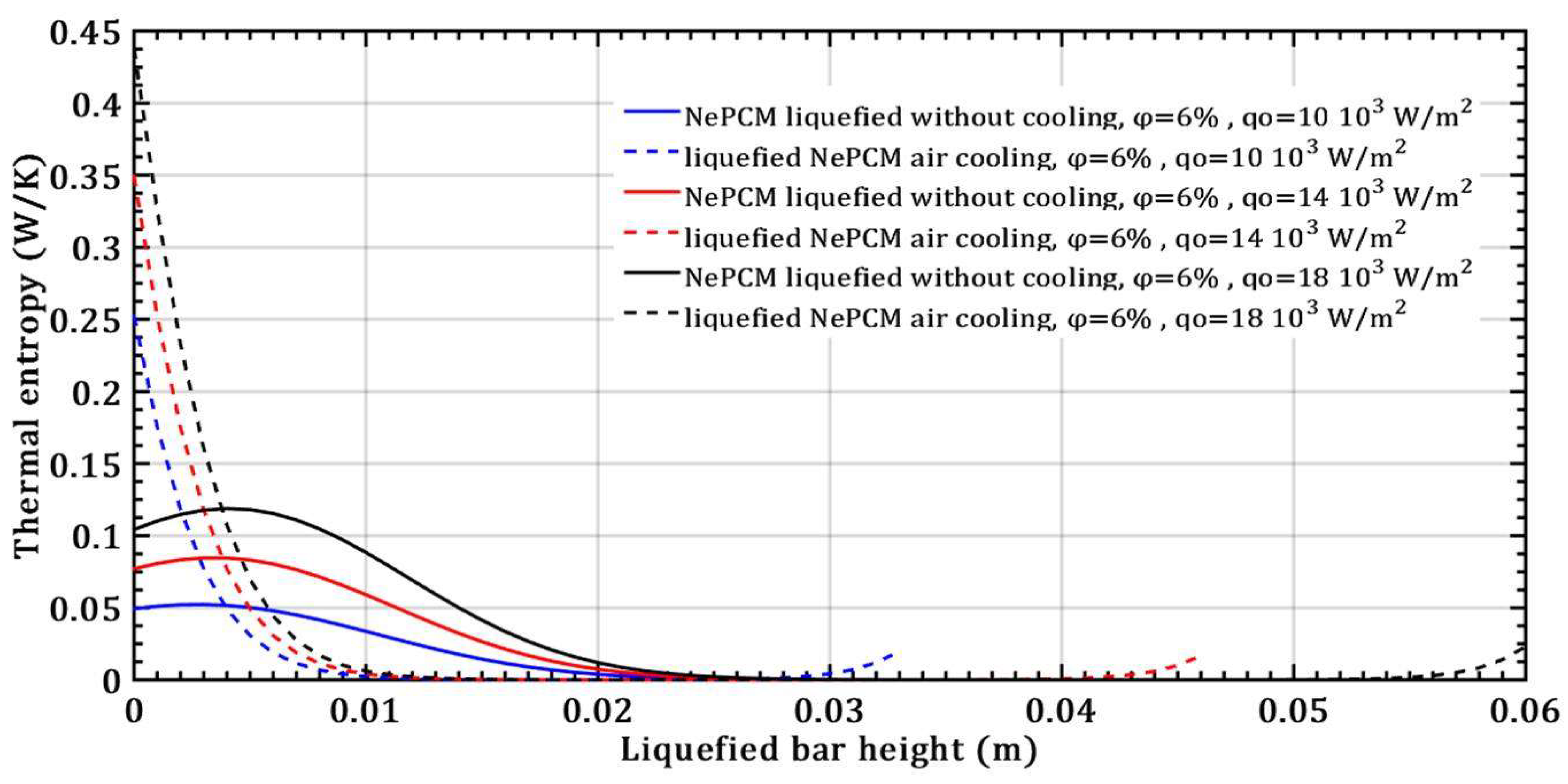
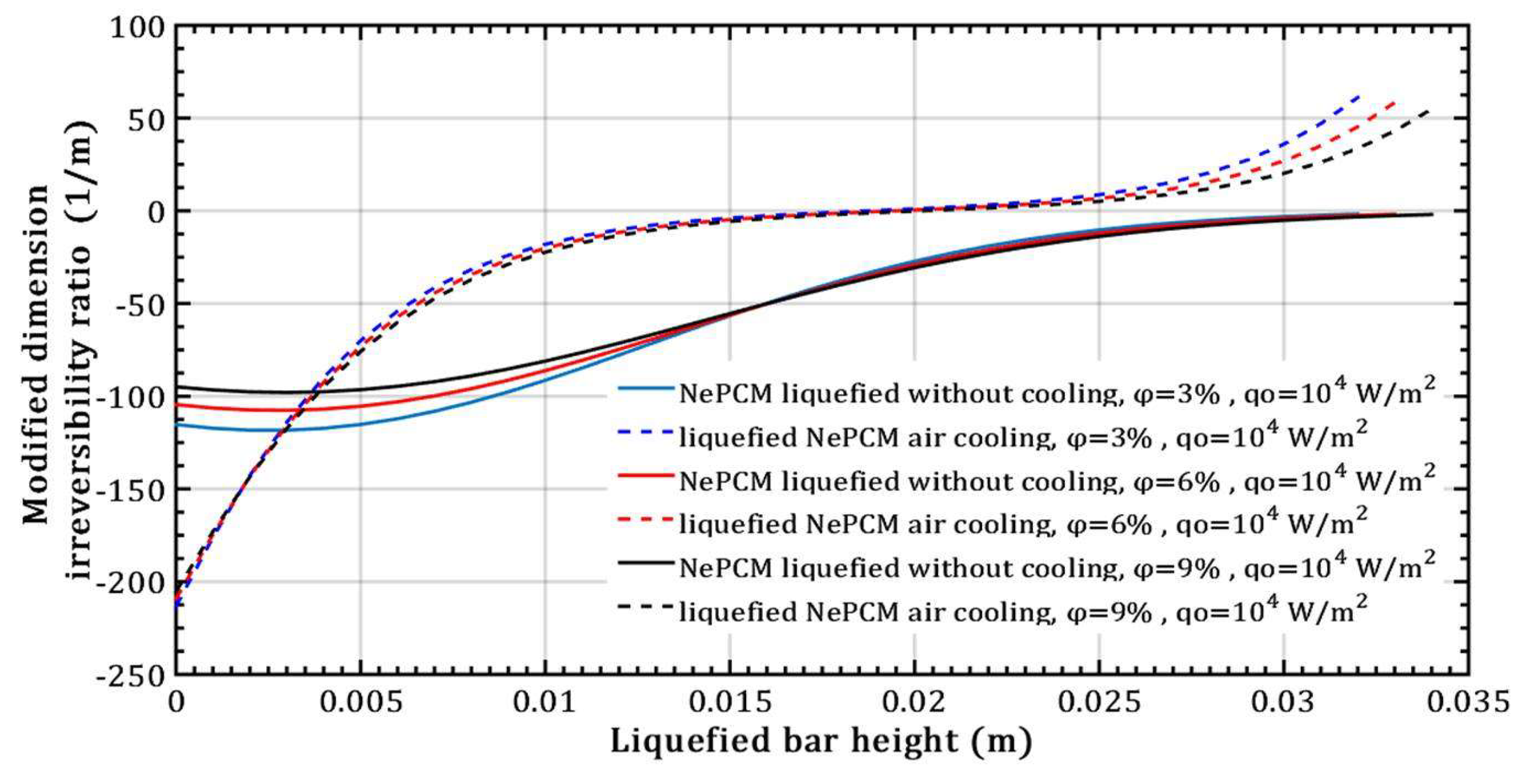
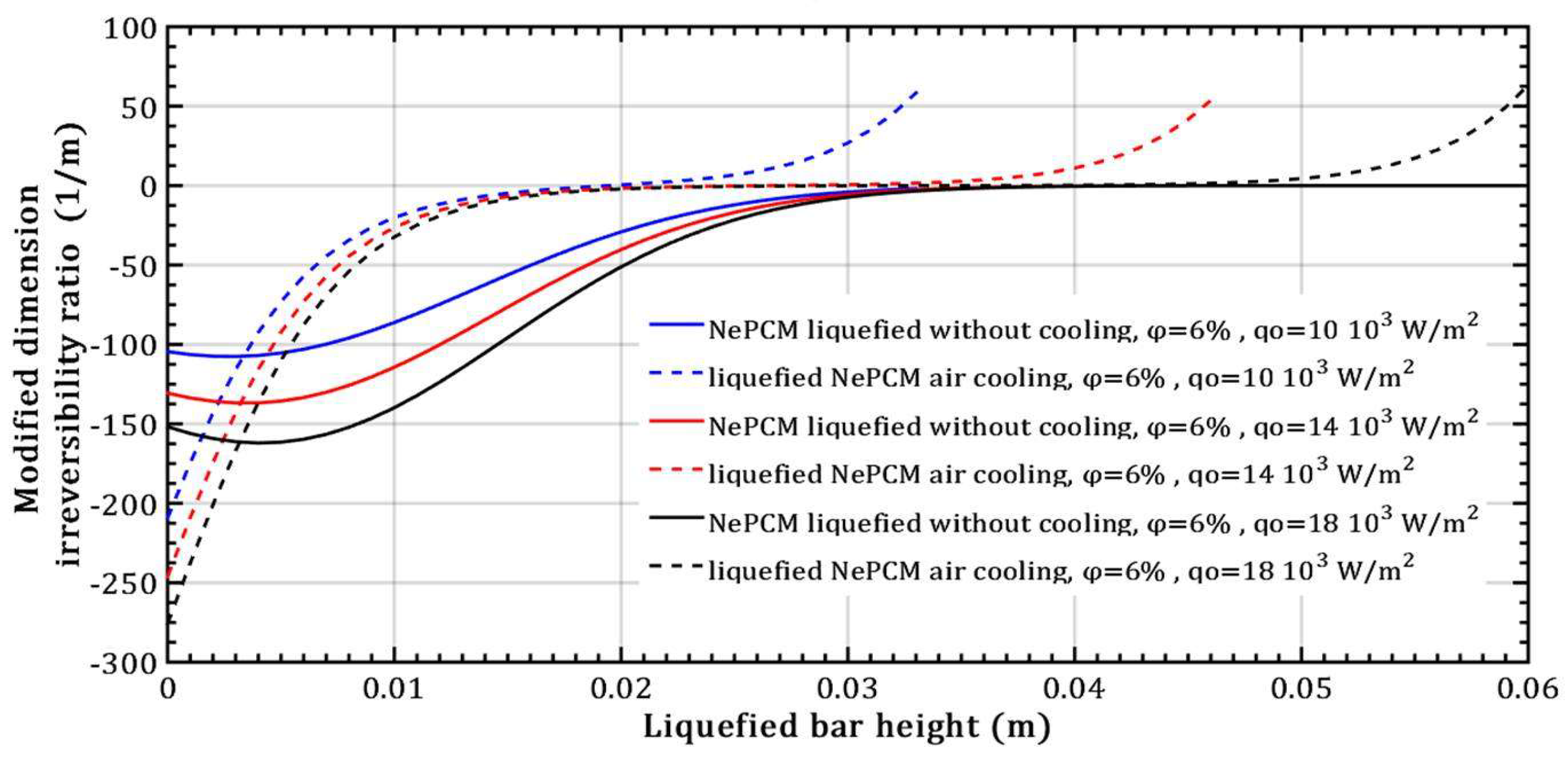
- There is no effect on the thermal entropy of the liquefied NePCM bar when increasing the volume fraction ratio from 3% to 9%. This is observed at a constant heat flux of 104 Wm-2 and a height of the liquefied bar of 0.012m.
- -
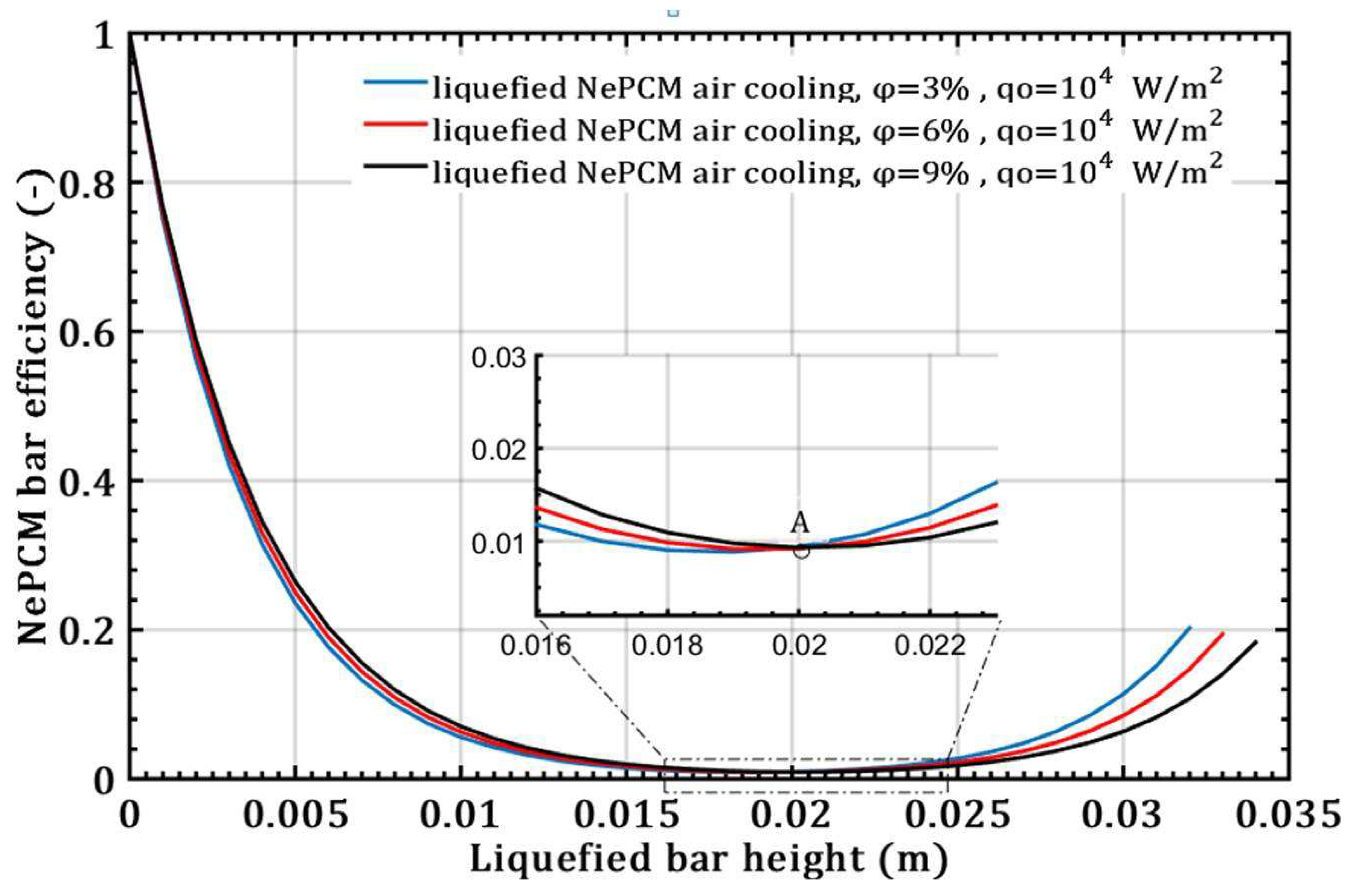
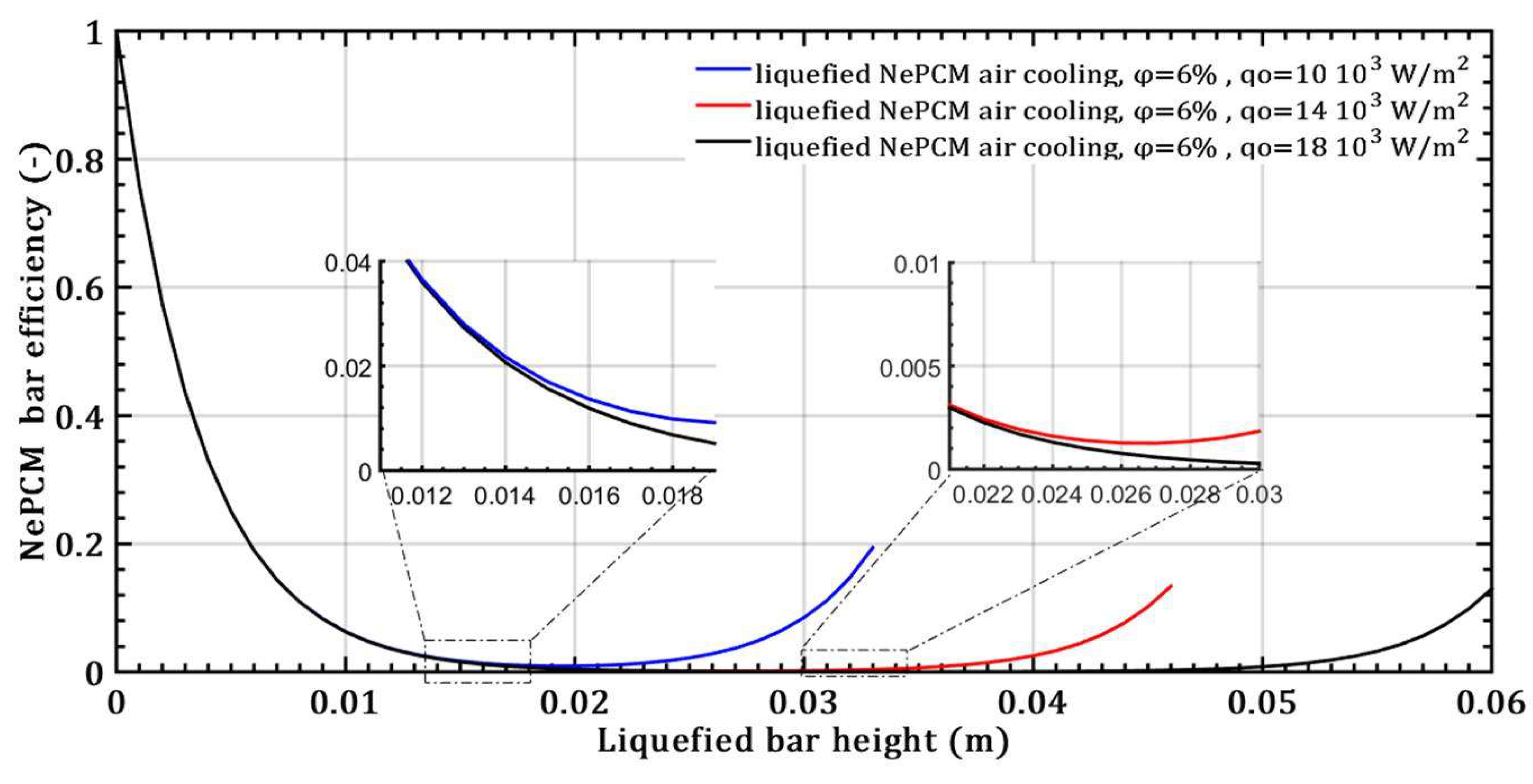
- However, with an increase in the volume fraction ratio from 3% to 9%, at a constant heat flux of 104 Wm-2 and a height of the liquefied bar of 0.02m, the NePCM bar efficiency decreases by 99%.
- -
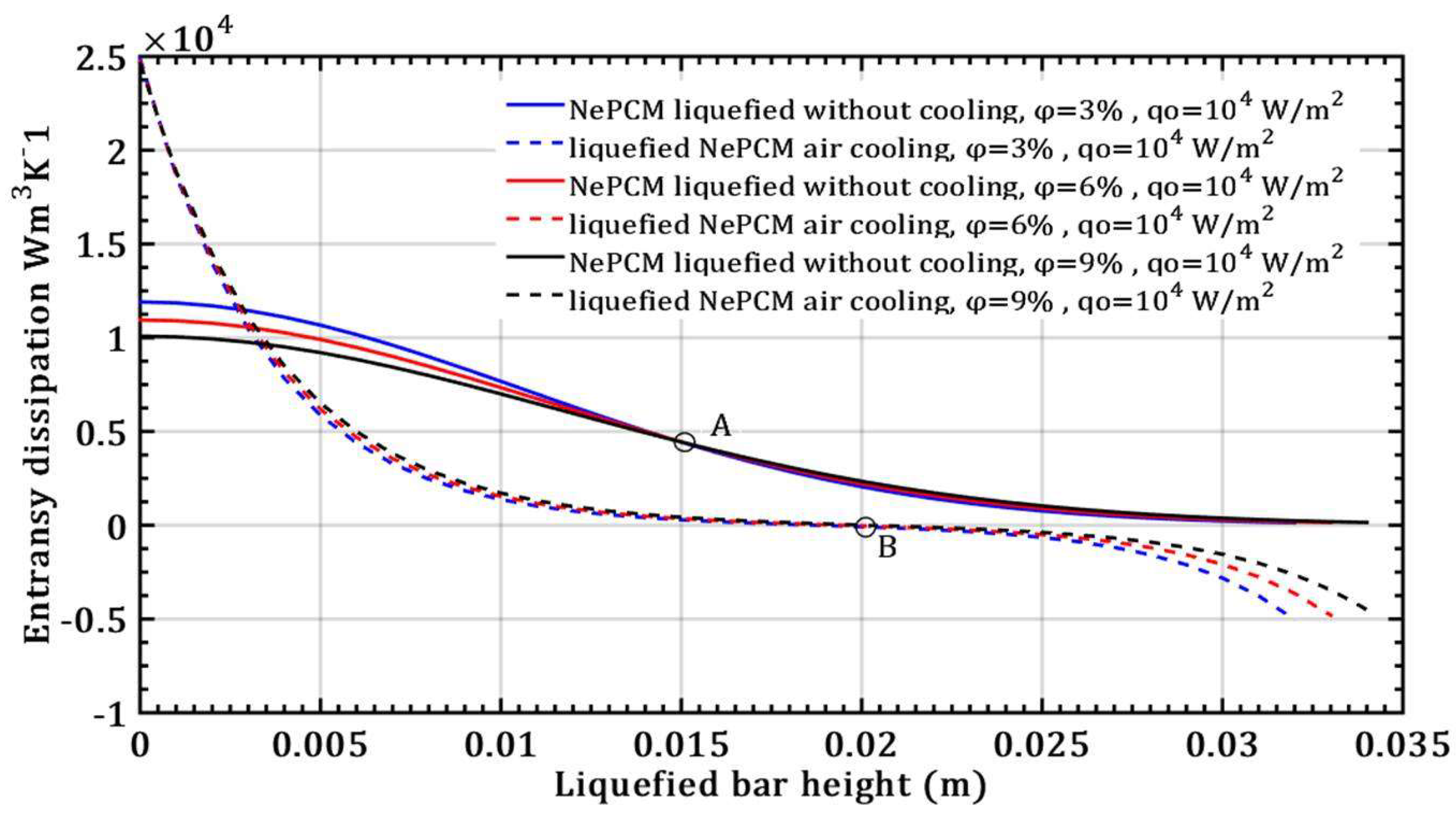
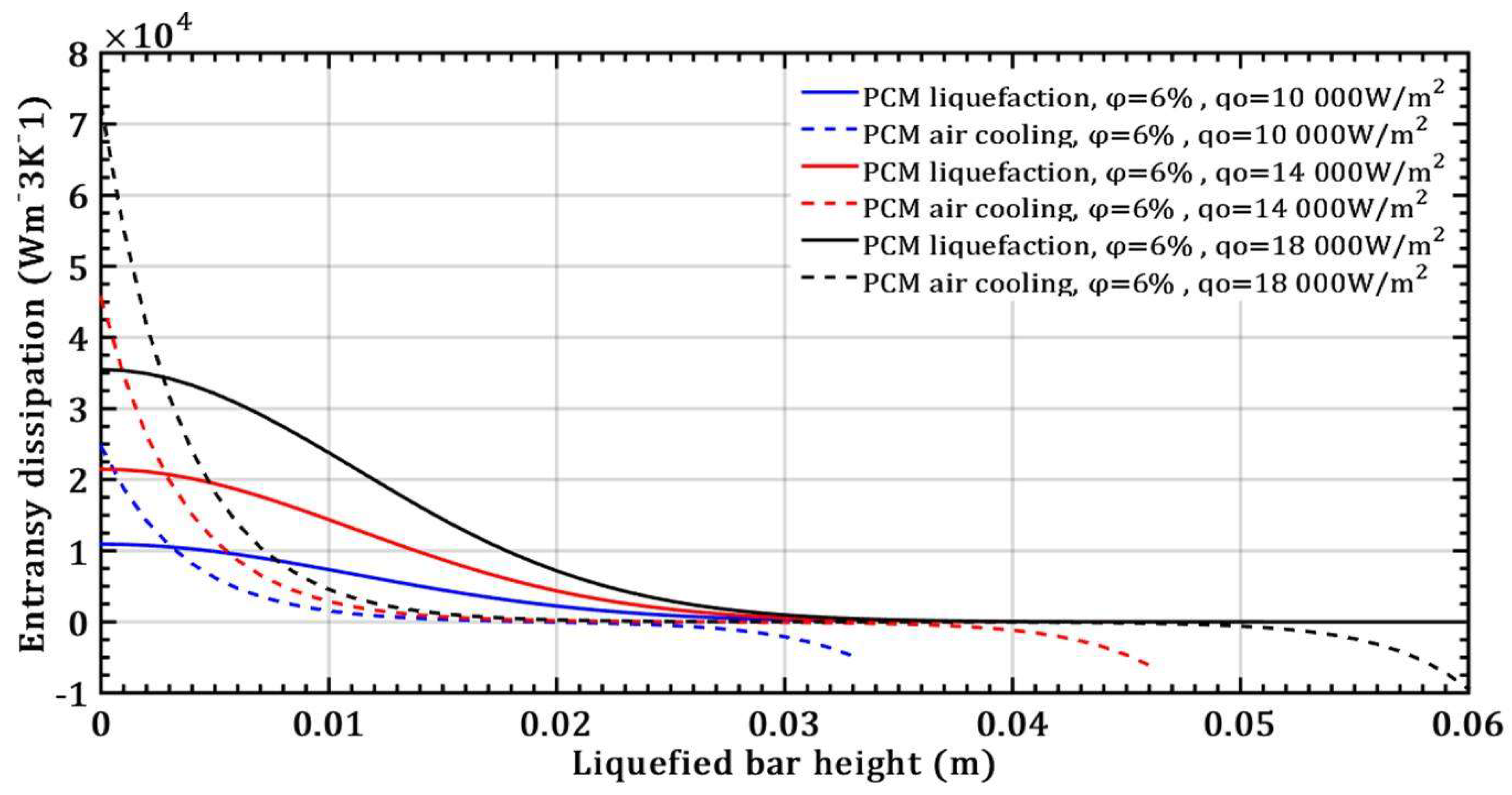
- The entransy dissipation value decreases by 50% and is not affected by the volume fraction ratio at a liquefied bar height of 0.015m and a heat flux of 104 Wm-2 for the case without bar cooling. However, with a liquefied bar height of 0.02m and a heat flux of 104 Wm-2, entransy dissipation decreases by 100% with bar cooling, becoming negative for bar heights over 0.02m.
- -
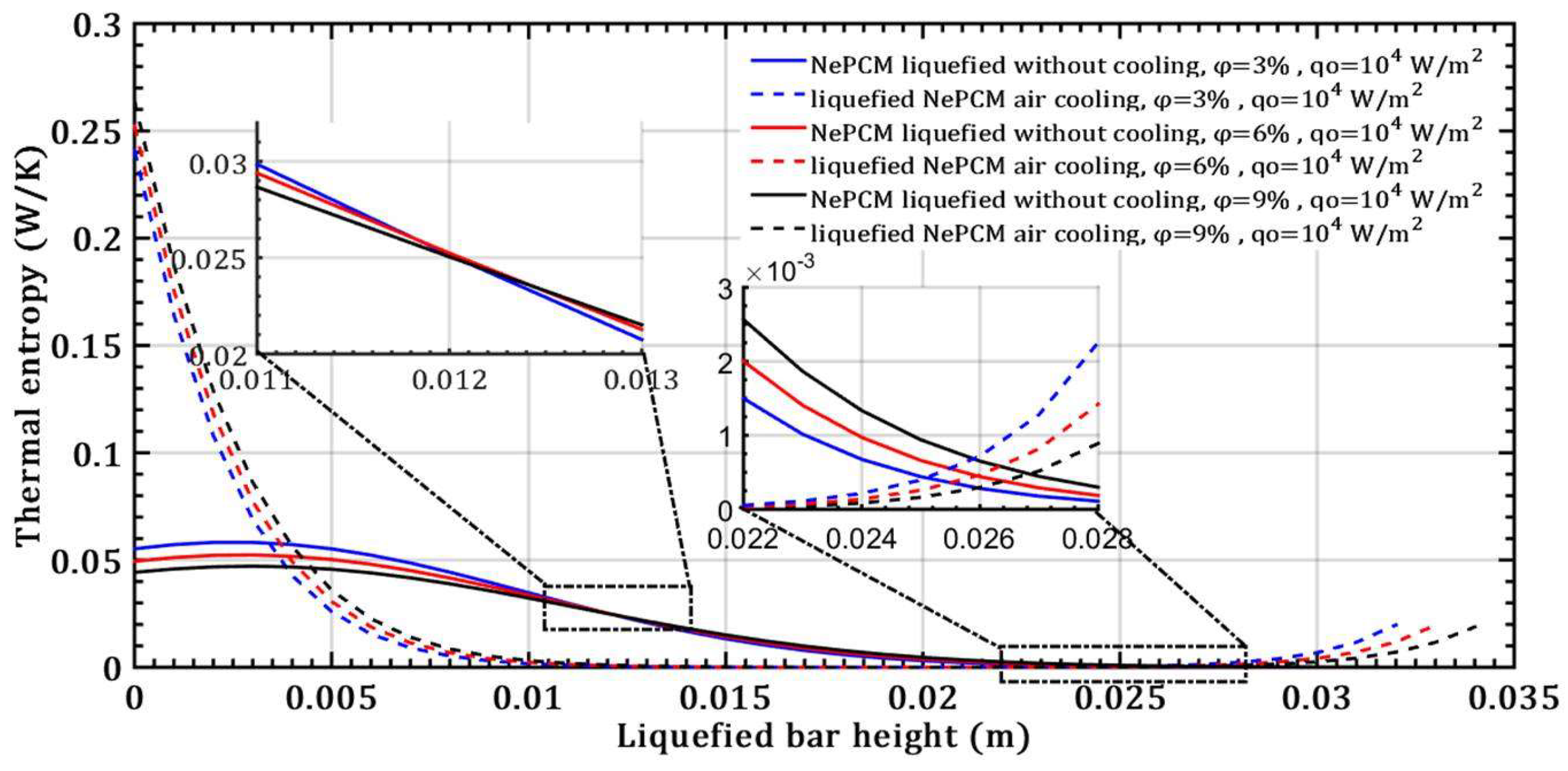
- The thermal entropy value of the liquefied NePCM bar increases by an average of 110% without cooling at a volume fraction ratio of 6% and an 80% increase in heat flux, with the bar height reaching up to 0.02m.
- -
- The entransy dissipation ratio of the liquefied NePCM bar at 0.035m without cooling is not affected by the increase in heat flux. However, with bar cooling the entransy dissipation decreases by about 98% when the heat flux increases by 80% and the bar height is up to 0.01m.
4. Conclusion
- -
- The effect of increasing the volume fraction ratio of Al2O3 nanoparticles in basic PCM results in an increase in the melting height and a decrease in the rate of convective cooling of NePCM bars.
- -
- The transient thermal entropy during the liquefaction of NePCM bar is significantly lower compared to the case of convective cooling of the liquefied NePCM bar.
- -
- Changing the value of the input heat flux has a significant impact on both the intensity and rate of change of the transient thermal entropy.
- -
- When it comes to the efficiency of the NePCM bar, varying the input heat flux has no significant effect at lower heights of the liquefaction NePCM bar.
- -
- The impact of the volume fraction ratio of Al2O3 nanoparticles on the entransy dissipation rate of NePCM bars is negligible compared to the variation in the input heat flux.
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| cpcm.o | specific heat capacity of PCM, kJkg-1K-1 |
| cp | specific heat capacity of nanoparticles, kJkg-1K-1 |
| cpcm | specific heat capacity of nano-enhanced phase change material, kJkg-1K-1 |
| ipcm | specific enthalpy of nano-enhanced phase change material, kJkg-1 |
| ip | specific enthalpy of nanoparticles, kJkg-1 |
| s | liquefied bar height, m |
| tpcm | temperature of liquefied bar in NePCM, °C |
| tpcm.o | temperature of solid bar in NePCM, °C |
| tm | temperature of phase change in PCM, °C |
| apcm | thermal diffusivity of nano-enhanced phase change material, m2 s-1 |
| tair | ambient air temperature, °C |
| Epcm.conv | entransy dissipation rate of NePCM in a liquefied bar is influenced by convective heat transfer, Wm-2K |
| Spcm.cond | thermal entropy of NePCM in a liquefied bar is influenced by conductive heat transfer, Wm-1K-1 |
| Spcm.conv | thermal entropy of NePCM in a liquefied bar is influenced by convective cooling, Wm-1K-1 |
| Epcm.cond | entransy dissipation rate of NePCM in a liquefied bar is influenced by conductive heat transfer, Wm-2K |
| Greek symbols | |
| φ | volume fraction ratio of nanoparticles,- |
| qo | inlet heat flux into the bottom of the bar, Wm-2 |
| τ | time, s |
| λpcm.o | thermal conductivity coefficient of PCM, Wm-1 K-1 |
| λpcm | thermal conductivity coefficient of NePCM, Wm-1 K-1 |
| λp | thermal conductivity coefficient of nanoparticles, Wm-1 K-1 |
| αpcm | convection heat transfer coefficient, Wm-2 K-1 |
| ρpcm.o | density of PCM, kgm-3 |
| ρpcm | density of NePCM, kgm-3 |
| ρp | density of nanoparticles, kgm-3 |
| ψ | dimension irreversibility ratio, K2 |
References
- Dhaidan, N.S.; Hassan, A.F.; Al-Gaheeshi, A.M.R.; Al-Mousawi, F.N.; Homod, R.Z. Experimental investigation of thermal characteristics of phase change material in finned heat exchangers. Journal of Energy Storage. 2023, 71, 108162. [Google Scholar] [CrossRef]
- Sarani, I.; Payan, S.; Nada, S.; Payan, A. Numerical investigation of an innovative discontinuous distribution of fins for solidification rate enhancement in PCM with and without nanoparticles. Applied Thermal Engineering. 2020, 176, 115017. [Google Scholar] [CrossRef]
- Al-Salami, H.A.; Dhaidan, N.S.; Abbas, H.H.; Al-Mousawi, F.N.; Homod, R.Z. Review of PCM charging in latent heat thermal energy storage systems with fins. Thermal Science and Engineering Progress. 2024, 51, 102640. [Google Scholar] [CrossRef]
- Shahsavar, A.; Shaham, A.; Yıldız, Ç.; Arıcı, M. Entropy generation characteristics of phase change material in a variable wavy walled triplex tube latent heat storage unit for battery thermal management system. Journal of Energy Storage. 2022, 51, 104374. [Google Scholar] [CrossRef]
- Sharma, S.; Micheli, L.; Chang, W.; Tahir, A.; Reddy, K.; Mallick, T. Nano-enhanced Phase Change Material for thermal management of BICPV. Applied Energy. 2017, 208, 719–733. [Google Scholar] [CrossRef]
- Colla, L.; Fedele, L.; Mancin, S.; Danza, L.; Manca, O. Nano-PCMs for enhanced energy storage and passive cooling applications. Appl Therm Eng. 2017, 110, 584–589. [Google Scholar] [CrossRef]
- Leong, K.Y.; Abdul Rahman, M.R.; Gurunathan, B.A. Nano-enhanced phase change mate-rials: a review of thermo-physical properties, applications and challenges. J Energy Storage. 2019, 21, 18–31. [Google Scholar] [CrossRef]
- He, M.; Yang, L.; Lin, W.; Chen, J.; Mao, X.; Ma, Z. Preparation, thermal characterization and examination of phase change materials (PCMs) enhanced by carbon-based nanoparticles for solar thermal energy storage. J Energy Storage 2019, 25, 100874. [Google Scholar] [CrossRef]
- Dhaidan, N.S.; Khodadadi, J.; Al-Hattab, T.A.; Al-Mashat, S.M. Experimental and numerical investigation of melting of phase change material/nanoparticle suspensions in a square container subjected to a constant heat flux. International Journal of Heat and Mass Transfer. 2013, 66, 672–683. [Google Scholar] [CrossRef]
- Al-Jethelah, M.S.M.; Tasnim, S.H.; Mahmud, S.; Dutta, A. Melting of nano-phase change material inside a porous enclosure. International Journal of Heat and Mass Transfer. 2016, 102, 773–787. [Google Scholar] [CrossRef]
- NematpourKeshteli, A.; Iasiello, M.; Langella, G.; Bianco, N. Using metal foam and nanoparticle additives with different fin shapes for PCM-based thermal storage in flat plate solar collectors. Thermal Science and Engineering Progress. 2024, 52, 102690. [Google Scholar] [CrossRef]
- Sharma, A.; Kothadia, H.B.; Singh, S.; Mondal, B. Solidification of nanoparticle-based PCM in a fin-aided triplex-tube energy storage system for cooling applications. Thermal Science and Engineering Progress. 2023, 42, 101872. [Google Scholar] [CrossRef]
- Zullo, F. Entropy Production in the Theory of Heat Conduction in Solids. Entropy. 2016, 18, 87. [Google Scholar] [CrossRef]
- Din, Z.U.; Ali, A.; De la Sen, M.; Zaman, G. Entropy generation from convective–radiative moving exponential porous bars with variable thermal conductivity and internal heat generations. Sci Rep. 2022, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, L. Heat conduction in cylinders: Entropy generation and mathematical inequalities. International Journal of Heat and Mass Transfer. 2018, 121, 1137–1145. [Google Scholar] [CrossRef]
- Alic, F. The modified dimension and dimensionless irreversibility analysis of low conductivity material of concentric hollow cylinders. J Therm Anal Calorim. 2022, 147, 14555–14569. [Google Scholar] [CrossRef]
- Alic, F. Transient Entropy Generation Analysis of Liquid vortex Isolated by Hollow Heated Cylinder. Appl. Math. Model. 2017, 44, 321–335. [Google Scholar] [CrossRef]
- Aziz, A.; Khan, W.A. Classical and minimum entropy generation analyses for steady state conduction with temperature dependent thermal conductivity and asymmetric thermal boundary conditions: Regular and functionally graded materials. Energy. 2011, 36, 6195–6207. [Google Scholar] [CrossRef]
- Alic, F. The non-dimensional analysis of nanofluid irreversibility within novel adaptive process electric heaters. Applied Thermal Engineering. 2019, 152, 13–23. [Google Scholar] [CrossRef]
- Guelpa, E.; Sciacovelli, A.; Verda, V. Entropy generation analysis for the design improvement of a latent heat storage system. Energy. 2013, 53, 128–138. [Google Scholar] [CrossRef]
- Zadeh, S.M.H.; Mehryan, S.A.M.; Sheremet, M.; Ghodrat, M.; Ghalambaz, M. Thermo-hydrodynamic and entropy generation analysis of a dilute aqueous suspension enhanced with nano-encapsulated phase change material. International Journal of Mechanical Sciences. 2020, 178, 105609. [Google Scholar]
- Guo, Z.Y.; Zhu, H.Y.; Liang, X.G. Entransy-A Physical Quantity describing Heat Transfer Ability. Int. J. Heat Mass Transf. 2007, 50, 2545–2556. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Q.; Liang, X. Analyses of entransy dissipation, entropy generation and entransy-dissipation-based thermal resistance on heat exchanger optimization. Applied Thermal Engineering. 2012, 38, 31–39. [Google Scholar] [CrossRef]
- Brinkman, H.C. The viscosity of concentrated suspensions and solution. J. Chem. Phys. 1952, 20, 571–581. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transfer. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Alic, F. Entransy Dissipation Analysis and New Irreversibility Dimension Ratio of Nanofluid Flow. Energies. 2020, 13, 114. [Google Scholar] [CrossRef]
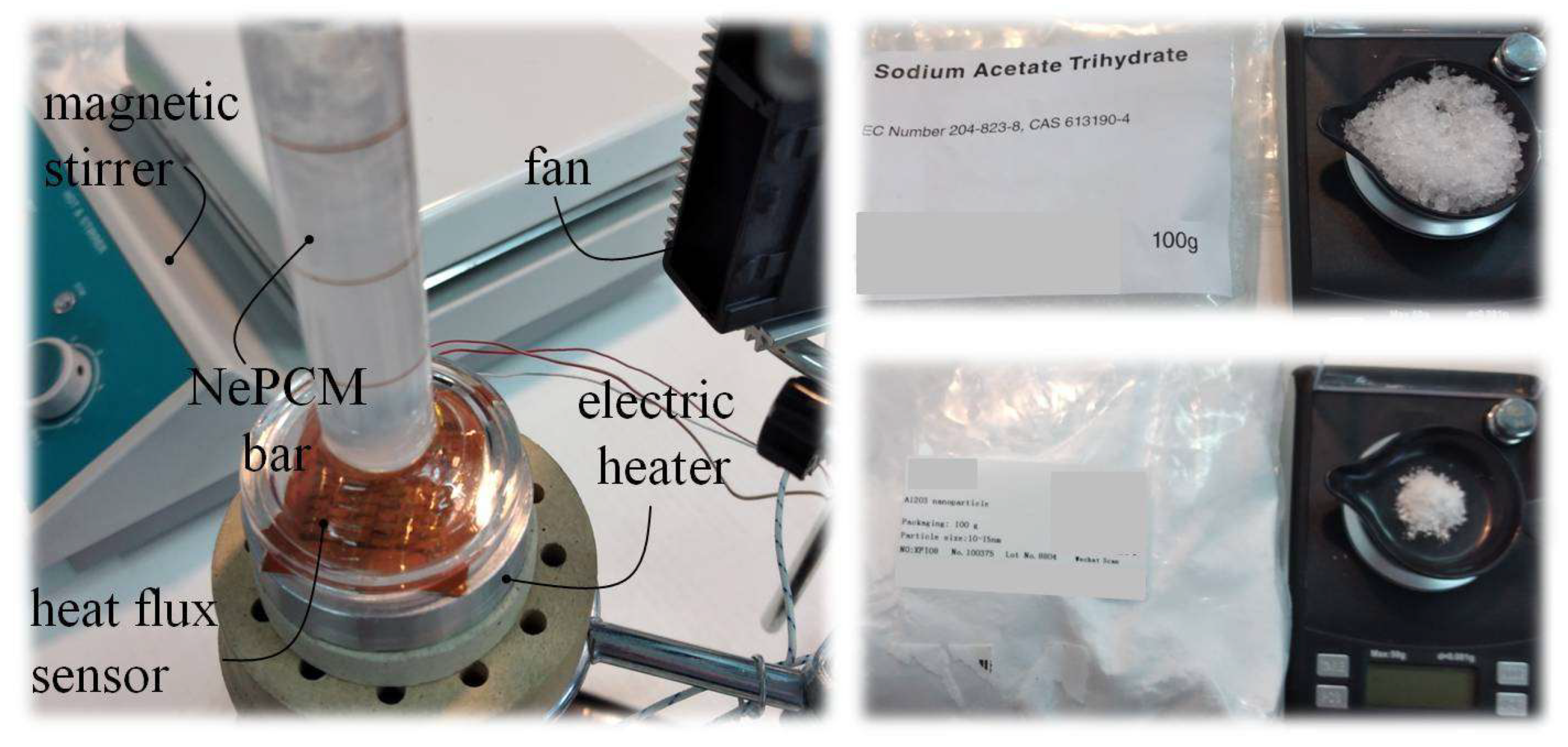
| Properties of nanoparticles (Al2O3) | Properties of the base PCM - sodium acetate trihydrate | |||||
|---|---|---|---|---|---|---|
|
λp [Wm-1K-1] |
ρp [kgm-3] |
cp [Jkg-1K-1] |
ibf [kJkg-1] |
λbf [Wm-1K-1] |
ρbf [kgm-3] |
cbf [Jkg-1K-1] |
| 40 | 3970 | 756 | 264 | 0.387 | 1450 | 3100 |
| Control parameter | Value |
|---|---|
| heat flux | 8500 Wm2, 10000 Wm2 , 12000 Wm2 , 14000 Wm2 ,18000 Wm2 |
| heating time | 200s , 500s, 600s |
| volume fraction ratio of Al2O3 nanoparticles | 3% , 6%, 9% |
| liquified bar height | to 0.035m |
| bottom bar temperature | from 115 °C to 350 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





