Bambusoideae is one of the largest subfamilies among the 12 subfamilies of the family Poaceae, which is distinguished from other grasses by the wood fibers of the bamboo culms. Sinocalamus is a genus of the Bambusoideae under the family Poaceae, and is mainly distributed in Asia, especially in China, where it has a rich diversity. It is mainly distributed in Asia, especially in China. Bamboo species in the genus
Chimonobamboo are usually hardy and can grow at lower temperatures, hence the name "
Chimonobambusa marmorea".
Chimonobambusa sichuanensis is a typical plant of the genus
Chimonobambusa, with strong individual proliferation, often cultivated as a hedge. Because of its beautiful appearance and strong adaptability, it is considered a representative of ornamental bamboo species[
1].
In our laboratory, blue light was found to be the main environmental factor inducing the color change of
Chimonobambusa sichuanensis, which significantly increased the carotenoid and anthocyanin contents of
Chimonobambusa sichuanensis, and caused its sunny-side culms to turn purple in autumn and winter. The transcriptome analysis of
Chimonobambusa sichuanensis culms and non-colored culms showed that blue light promoted the synthesis of flavonoids such as anthocyanins and lignin through the enhancement of phenylpropane metabolic pathway[
2]. Sequencing results showed that a total of 10872 genes were differentially expressed, of which 5854 genes were up-regulated and 5018 genes were down-regulated. Analysis of the results showed that among the different light qualities, blue light was the main influencing factor, and blue light receptors CRY and ZTL were significantly up-regulated in the
Chimonobambusa sichuanensis transcriptome[
3]. Thus, the Cryptochrome
CRY gene was selected for investigation in this study to provide a theoretical basis for further study of
Chimonobambusa sichuanensis gene sequences.
Cryptochrome (CRY) is a very important class of blue light receptors in plants, which is not only crucial for plant growth and development, but also extremely important for photoperiod regulation. Studies have shown that Cryptochrome is a class of flavoprotein that receives blue light (400-500 nm) and near-ultraviolet light (320-400 nm), with a molecular weight of 70-80 kD[
4]. The N-terminus of Cryptochrome consists of about 500 amino acids, which are highly homologous to photolytic enzymes, and the chromophore consists of the photocatalytic factor, flavin adenine dinucleotide (FAD), and the light capture factor, folate (MTHF,). 5,10-methyltetrahydrofolate). The length of the sequence at the C-terminus and its composition varies significantly between species, and includes a conserved but not contiguous DAS (DQXVP-acidic-STAESSS) motif , thus playing a role in the construction of plant photomorphology, modulation of floral changes , regulation of the biological clock , and stomatal opening and closing and are widely found in eukaryotes and prokaryotes. Previous studies have found that plants contain three main classes of Cryptochrome proteins: CRY1, CRY2, and CRY3[
5]. It is generally believed that CRY1 plays a dominant role in blue-light-induced photomorphogenesis, whereas CRY2 mainly regulates photoperiodicity to affect flowering time, and CRY3 plays an important role in the regulation of photoperiods and biological clocks. There is an overlap in the functions of the two proteins, CRY1 and CRY2, in regulating flowering. Although CRY1, CRY2, and CRY3 are the same blue-light receptor, their structural domains are very different and they have different light-responsive functions[
6,
7].
The Cryptochrome gene sequences of
Chimonobambusa sichuanensis, an excellent bamboo species, are still unknown. In this study, four Cryptochrome gene sequences of
Chimonobambusa sichuanensis were comparatively identified using published sequences of
PheCRYs of
Phyllostachys edulis[
8]. The sequences of these four Cryptochrome genes, named
CsCRYs:
CsCRY1a,
CsCRY1b,
CsCRY2,
CsCRY3 were identified and named as
CsCRYs. The results showed that in the
Chimonobambusa sichuanensis CsCRYs gene family,
CsCRY1a,
CsCRY1b, and
CsCRY2 have the PRK 10674 structural domain, which is involved in signaling and metabolic processes, and the same structural domains as
Phyllostachys edulis PheCRY1a-d,
PheCRY2, and
PheCRY2s identified by the previous authors. In
Arabidopsis,
AtCRY1 and
AtCRY2 were identified by the previous authors as differing in the amino acid sequences of their photocleavage enzyme-like structural domains (PHRs), and therefore in the rates of their photochemical reactions. This is reflected in the genes of
Phyllostachys edulis
PheCRY1 and
PheCRY2 and
Chimonobambusa sichuanensis CsCRY1 and
CsCRY2. Based on the previously published sequence analysis of
Phyllostachys edulis, it was found that
PheCRY2 has more PLN structural domains than
PheCRY1, which is involved in intracellular homeostatic regulation, which is not reflected in
Chimonobambusa sichuanensis, and in
Chimonobambusa sichuanensis CsCRY1 has more Cryptochrome-C structural domains than
CsCRY2, which is involved in environmental stress, and this may affect the ability of
CsCRY1 and
CsCRY2 in response to different environmental stresses. In
Chimonobambusa sichuanensis, the structural domain of
CsCRY3 possesses the Cryptochrome_DASH structural domain, which is not found in
Phyllostachys edulis PheCRY3, and is the hallmark structural domain for identification as a subfamily of
CRY3. The structure can sense light signals in the environment and is involved in the process of synchronization and regulation of the biological clock, which is closely related to the physiological function of
CRY3. The different structural domains between
CRY1,
CRY2, and
CRY3 lead to their different physiological functions.
CRY1 is involved in many aspects of the de-etiolation response and photomorphogenesis, as well as the provision of nutrients for plant growth. In
Arabidopsis thaliana AtCRY1 is involved in the inhibition of hypocotyl elongation in blue light, the accumulation of floral pigmentation glycosides, etc., and the
CRY mutants all exhibit delayed flowering under different light conditions[
9]. It was found that
OsCRY1s in rice functioned when exposed to blue light to inhibit the growth of embryonic sheaths and leaves, as well as to regulate the de-yellowing response in rice. When two
CRY genes,
SbCRY1a, and
SbCRY1b, were studied in sweet sorghum, it was found that transgenic
Arabidopsis showed high sensitivity to abscisic acid after overexpression of the two genes in
Arabidopsis and that overexpression of
SbCRY1b promoted flowering in
Arabidopsis.
SbCRY1 was also involved in a novel mechanism in which blue light and ambient temperature synergistically regulate plant hypocotyl elongation[
10].
CRY2 also plays a role in seedling photomorphogenesis, including suppressing embryonic axis growth inhibition and promoting cotyledon expansion.
CRY2 also plays a role in seedling photomorphogenesis, including suppressing embryonic axis growth inhibition and promoting cotyledon expansion. However,
CRY2 has a pronounced de-yellowing function mainly at low blue light intensity, but not at high light. In
Arabidopsis, the expression of
AtCRY2 was found to be rapidly down-regulated by blue light in a light-intensity-dependent manner compared with that of
AtCRY1[
11]. It has been reported that
CRY1 in different plants exhibit light-dependent inhibition of hypocotyl elongation and show inhibitory effects on plant growth.
CRY3 is mainly involved in the regulation of the biological clock, for example,
AtCRY3 can regulate the different expression patterns of certain genes in chloroplasts during the daytime and nighttime, so that processes, such as photosynthesis, are carried out efficiently during the daytime, while the level of activity is appropriately reduced at night[
12]. Although
CRYs genes have been studied in a variety of plants by previous researchers, there is still great room for development of genetic studies on numerous bamboo species.
In this study, the CsCRYs gene family of Chimonobambusa sichuanensis was identified and analyzed for its basic physicochemical properties, phylogeny, conserved motifs, secondary structure and expression pattern, which will provide theoretical references for the subsequent studies on gene cloning and functional analysis of the CsCRYs gene family of Chimonobambusa sichuanensis.
1. Materials and Methods
1.1. Screening and Cloning of Chimonobambusa sichuanensis CsCRYs Gene Family
Using the published
Phyllostachys edulis CRY sequences, the
Chimonobambusa sichuanensis genome assembled in our laboratory was compared with the protein annotation database and the transcriptome database obtained after blue light stress using blast from TBtools, with the parameters of E-value<1x10
-5, per.ident>90%, query cover>90%, and at the same time, the sequences were screened for redundancy and named using the SnapGene and NCBI database (
https://www.ncbi.nlm.nih.gov/cdd/) (E-value set to 0.001, other parameters set to default) were compared and screened to remove redundancy and named, and a total of four gene sequences were obtained[
13].
1.2. Selection of Structural Domains and Conserved Motifs Analyzed for the Chimonobambusa Sichuanensis CsCRYs Gene Family
The conserved motifs of
Chimonobambusa sichuanensis CRY protein sequences were identified using the online analysis tool MEME (
http://meme-suite.org/). Parameters were set to a maximum of 10 motifs, motif lengths were set to 20-50 amino acid residues, and site distributions were set to either zero or one occurrence per sequence (contributing motif sites)[
14]. The protein sequences of 55 CRY genes from 11 other species will also be analyzed by motif analysis to compare the
Chimonobambusa sichuanensis CsCRY protein motifs with those of other species NCBI Batch CD-search (
https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) was used to obtain the conserved structural domains of
Chimonobambusa sichuanensis, and TBtools software was used to input the CDD Batch results and visualize the conserved structural domains of the
Chimonobambusa sichuanensis CsCRYs family. The conserved domains were visualized and analyzed using TBtools software.
1.3. Phylogenetic Analysis of the CsCRYs Gene Family in Chimonobambusa Sichuanensis
Sequences were obtained from the TAIR10 database (
http://www.
Arabidopsis.org/), Ensembe Plant database (http: plants.ensembl.org/index.html), and PFAM database (
http://pfam,sanger.ac.uk), respectively.
Arabidopsis thaliana (
A. thaliana), Rice (
O. sativa), Sorghum (
Sorghum bicolor (L.) Moench), Barley (
Hordeum vulgare L.), Wheat (
Triticum aestivum L.), Soybean (
Glycine max (L.) Merr.), Gossypium hirsutum (
Gossypium hirsutum L.), Populus trichocarpa (
Populus trichocarpa (Torr. & Gray)), Maize (
Zea mays L.), Physcomitrium sphaericum (
Physcomitrium sphaericum (C. Ludw.) Brid.), and
Phyllostachys edulis (Carrière) J. Houzeau) with
CRY gene sequences. Mega X software was used to perform multiple alignment analysis of
Arabidopsis thaliana AtCRY,
O. sativa OsCRY,
Sorghum bicolor SbCRY,
Triticum aestivum TaCRY,
Hordeum vulgare HvCRY,
Populus trichocarpa PtCRY,
Zea mays
ZmCRY,
Glycine max GmCRY,
Gossypium hirsutum GhCRY,
Physcomitrium sphaericum PsCRY,
Phyllostachys edulis PheCRY, and the conserved domain sequences of
CRY in
Chimonobambusa sichuanensis; set Bootstrap=1000。And use the online software Evolview(
https://www.evolgenius.info/ )Beautify the evolutionary tree. The parameters Neighbor-Join; Bootstrap=1000 were set. and the evolutionary tree was beautified using the online software Evolview (
https://www.evolgenius.info/).
1.4. Secondary Structure, Subcellular Localization, Protein Characterization and Signal Peptide Analysis of Chimonobambusa sichuanensis CsCRYs Gene Family Proteins
Subcellular localization of four CsCRYs proteins was predicted using the online tool site WoLF PSORT: Protein Subcellular Localization Prediction (
https://wolfpsort.hgc.jp/).
1.5. Expression Pattern of Cryptochrome CsCRYs Gene in Chimonobambusa sichuanensis Under Different Environmental Conditions
Firstly, light quality was set as the environmental variable, and Chimonobambusa sichuanensis hydroponic seedlings cultivated in the greenhouse of Chimonobambusa Institute of Bamboo Research, Nanjing Forestry University, were used at a constant temperature of 20 ℃, with a single light intensity of 100 µmol·m-²·s-1 of blue and red light, and a total light intensity of 100 µmol·m-²·s-1 of mixed light, which consisted of a 1:1 ratio of red and blue light, and were exposed to a constant temperature of 20 ℃. The hydroponic seedlings of Chimonobambusa sichuanensis were continuously irradiated with these three treatments.
Next, light intensity was set as the environmental variable, and the bamboo seedlings were irradiated uninterruptedly using blue light with total light intensities of 50, 75, and 100 µmol·m-²·s-1. Finally, temperature was set as the environmental variable, and bamboo seedlings were irradiated using constant natural light with a light intensity of 100 µmol·m-²·s-1, and treated with different temperature gradients: -5 ℃, 0 ℃, 5 ℃, 10 ℃, and 20 ℃, and three pots of bamboo seedlings with similar growth were set as the replicate control, and leaves were collected every 4 h. The sampling times were: 0 h, 4 h, 8 h, 12 h, 16 h, 20 h and 24 h. The expression of Cryptochrome CsCRYs in Chimonobambusa sichuanensis leaves under different light conditions was detected by qRT-PCR quantitative comparison using the reagent SYBR GreenPro Taq HS Plant qRT-PCR Kit.
2. Results and Analysis
2.1. Screening and Cloning of Gene Family Members of Cryptochrome CsCRYs in Chimonobambusa sichuanensis
A total of four
Chimonobambusa sichuanensis CRYs gene sequences were obtained by homology search comparison. Blast comparison was performed on the NR database on the website NCBI (URL:
https://www.ncbi.nlm.nih.gov/), and the four sequences were identified as
CRYs gene families by blast comparison of genes of possible classes. Based on
Chimonobambusa sichuanensis scientific names, structural domains, and affinities, the four
Chimonobambusa sichuanensis CRYs gene family members obtained were named
CsCRY, namely:
CsCRY1a,
CsCRY1b,
CsCRY2, and
CsCRY3.
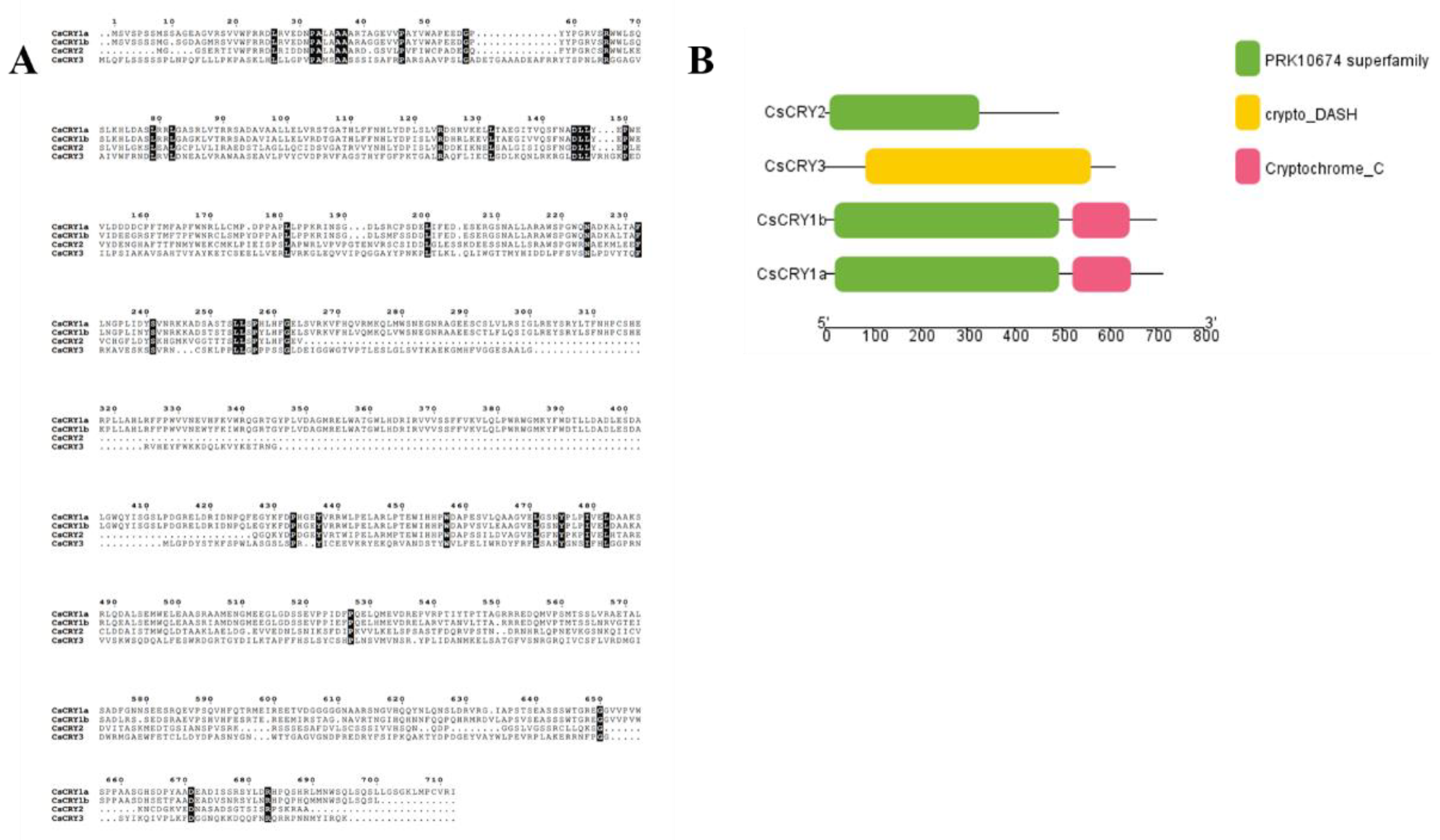
The portion of the
Chimonobambusa sichuanensis Cryptochrome gene family protein comparison sequences with high sequence similarity was presented as a visualization plot using the program ESPript 3 (URL:
https://espript.ibcp.fr/ESPript/ESPript/index.php). The Sequence similarities depiction parameter was set to PAM250 and the results are shown in
Figure 1.
Of the four sequences shown in
Figure 1A CsCRY1a is 2139 bp in length and encodes 712 proteins; CsCRY1b is 2094 bp in length and encodes 697 proteins; CsCRY2 is 1476 bp in length and encodes 491 proteins; CsCRY3 is 1833 bp in length; and encodes 610 proteins.
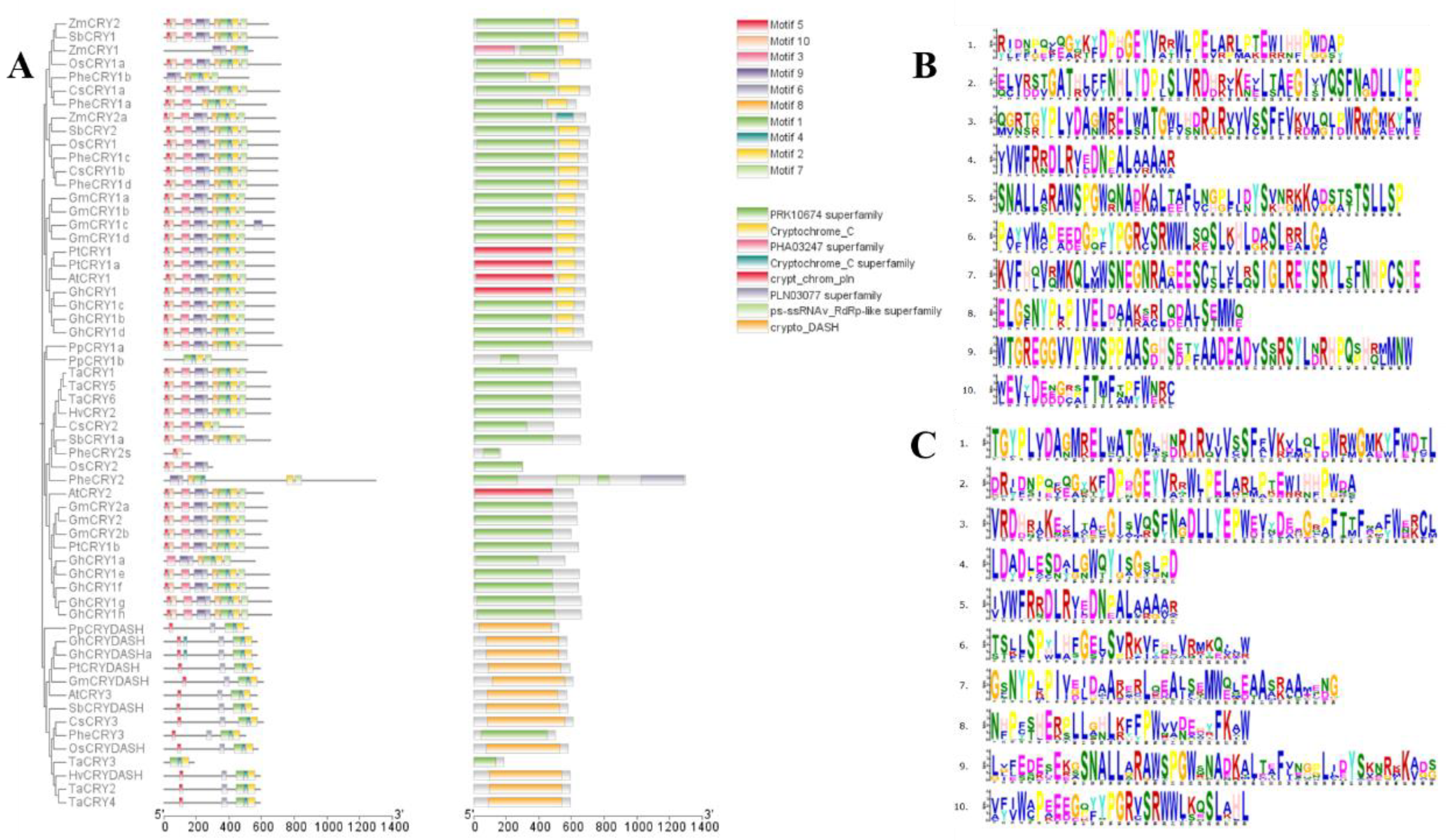
The four protein sequences were compared, as shown in the figure, it was found that they had multiple identical sites, indicating that the four sequences were extremely close in affinity, and there were similar parts of the sequences, which might be linked to their physiological functions and their common physiological roles. According to the motif analysis, the protein conserved structural domains of the four Chimonobambusa sichuanensis CsCRYs families CsCRY1a~CsCRY3 were screened, and among the four gene family sequences, two Cryptochrome_C family, three PRK10674 family and one Cryptochrome_DASH family were screened, respectively. By analyzing the structural distribution of Motif, it can be seen that the distribution of Motif within each sequence is more consistent.
2.2. Structural Characterization of Chimonobambusa sichuanensis CsCRYs Gene Family
From
Figure 2A, Motif analysis showed that Motifs 1, 4, 6, and 7 were present in the four CsCRY proteins
CsCRY1a,
CsCRY1b,
CsCRY2, and
CsCRY3, and the conserved structural domains analysis (Fig. 2C) showed that Motifs 1, 8, and 9 together constituted homologous structural domains in the
CsCRY1 subfamily, and that the conserved motifs in
CsCRY1a and
CsCRY1b were the same and were merged into the same subfamily. The
CsCRY1 subfamily is identified by the presence of Motif 9. The
CsCRY2 subfamily differs from the other subfamilies in the absence of Motif 3. Similarly, the
CsCRY3 subfamily does not contain Motifs 2, 5, 8, and 10 in comparison to the other subfamilies.
Motif 1, 2, 4, 5, 6, 8, and Motif 10 constitute the PRK10674 conserved domain, which is common to photoreceptors, and Motif 9 constitutes the Cryptochrome_C conserved domain, which is unique to the CsCRYs family. Conserved motif analysis revealed that Motif 2, Motif 4, and Motif 6 are highly conserved among all family members, indicating that these motifs play a fundamental and important role in CRY protein function, Motif 6 are highly conserved among all family members, suggesting that these motifs play fundamental and important roles in CRY protein function. The motif differences between subclasses are as follows: CsCRY1 has Motif 3 and Motif 7 compared to CsCRY2, and CsCRY3 was identified as Cryptochrome_DASH due to the lack of Motif 2 and Motif 10, whereas CsCRY1 and CsCRY2 are from different subclasses but contain Motif 5, and there is no difference between different subclasses. There was no difference between the subclasses. These phenomena reflect the potential diversity of these genes in light transmission and regulatory functions.
To further explore the similarities and differences between the
CsCRYs gene family and other species, the conserved motifs of the
CRY gene family in the four
CsCRYs genes and several species were compared and analyzed. A total of 59 gene sequences, distinguished by 10 conserved motifs (Motif 1~Motif 10), were examined using the online software MEME for the
CRY gene families in the four
CsCRYs genes and other species. The results showed that each
CRY gene contains 1 to 10 motifs.
Figure 2C shows that
CsCRY1a and
CsCRY1b have the same motif and both consist of two structural domains, motifs 3, 5, 6, 9, and 10 form the PRK10674-Superfamily structural domain, which is related to signaling, and the rest of motifs 1, 2, 7, and 8 form the Cryptochrome-C The remaining motifs 1, 2, 7, and 8 form the Cryptochrome-C structural domain, which is related to photosynthetic signaling and interaction with downstream signals, and is similar to other species such as wheat, sorghum, and maize, which also consist of these two structural domains in their CRY1 sequences and have the same motif composition, which can be regarded as a strong evidence for the identification of
CRY1. Interestingly,
Phyllostachys edulis PheCRY1a, which is very close to
Chimonobambusa sichuanensis, lacks motifs 3, 5, and 10, which suggests that there are differences in their sequence composition despite the same structural domains, and
CsCRY2 is similar to
CRY2 genes of other species in that it possesses a PRK10674-Superfamily structural domain, which is not of the same length in different species.
CsCRY2 consists of motifs 2, 3, 5, 7, 9, and 10, with motif 8 missing compared to wheat, sorghum, and maize, and this fragment is not found in
Phyllostachys edulis.
CRY3 is more consistent in all species and consists of the Crypto-DASH structural domain, which is a marker for identifying CRY3 and consists of motifs 1, 2, 4, 5, and 6, which is present in both
CsCRY3 and
AtCRY3 and
ZmCRY3.
2.3. Chimonobambusa sichuanensis CsCRYs Gene Affinities
The protein sequences of the four Chimonobambusa sichuanensis CsCRYs were aligned with the CRY protein sequences of 10 species for tree construction, as shown in Fig. 3. From the analysis of the evolutionary tree, the four Chimonobambusa sichuanensis CsCRY genes were divided into three subfamilies (subclades I-III). Each subfamily has members distributed, among which, there are two members in subfamily I (CsCRY1a, CsCRY1b), one member in subfamily П (CsCRY2), and one member in subfamily III (CsCRY3). The members belonging to the same subclade have the same protein structure, and the branches (CsCRY1a~3) are consistent with the results of the conserved motif analysis. And the evolutionary relationship can be seen that CsCRY1a and CsCRY1b are clustered into a subfamily with PheCRY1a and PheCRY1b of Phyllostachys edulis, and CsCRY1a and PheCRY1a are clustered into a branch in the evolutionary tree before polymerizing with CsCRY1b, which proves that in this subfamily, CsCRY1a is more closely related to PheCRY1a. CsCRY1a, CsCRY1b and PheCRY1a, PheCRY1b all have the PRK10674-Superfamily as well as Cryptochrome-C structural domains, which are shared by members of this subfamily in the evolutionary tree, which is a common feature of subfamily I. The CsCRY1a, CsCRY1b, and PheCRY1a are all related to PheCRY1a. Similarly, CsCRY2 is located in subfamily II and is closer to PheCRY2s, which proves that in this subfamily CsCRY2 is more closely related to PheCRY2s. Both CsCRY2 and PheCRY2 have the PRK10674-Superfamily structural domain, which is shared by members of subfamily П in the evolutionary tree. CsCRY3, in turn, constitutes a small branch with PheCRY3, which proves that CsCRY3 is more closely related to Phyllostachys edulis PheCRY3 in subfamily III. Both CsCRY3 and PheCRY3 have the Crypto-DASH structural domain, which is shared by members of this subfamily in the evolutionary tree, and is a common characteristic of subfamily III as well as a criterion for its identification. All four CsCRYs family sequences of Chimonobambusa sichuanensis showed very close affinity with the PheCRYs family sequences of Phyllostachys edulis and were highly similar in classification, which proved the correctness of the screening. The branching of the phylogenetic tree can effectively reflect the evolutionary relationship of CRY genes in each species.
Figure 2.
Evolutionary tree and motif comparison of CRYs gene families in multiple species. Note: Plant species include Chimonobambusa sichuanensis (Cs), Arabidopsis thaliana (At), O. sativa (Os), Triticum aestivum (Ta), Zea mays (Zm), Glycine max (Gm), Hordeum vulgare (Hv), Sorghum bicolor (Sb), Populus trichocarpa (Pt), Gossypium hirsutum (Gh), Physcomitrella patens (Pp) and Phyllostachys edulis (Phe). A: Evolutionary tree and conserved structural domain analysis of CRY gene families in multiple species; B: Chimonobambusa sichuanensis CsCRYs gene family motifs; C: CRY gene family motifs in multiple species.
Figure 2.
Evolutionary tree and motif comparison of CRYs gene families in multiple species. Note: Plant species include Chimonobambusa sichuanensis (Cs), Arabidopsis thaliana (At), O. sativa (Os), Triticum aestivum (Ta), Zea mays (Zm), Glycine max (Gm), Hordeum vulgare (Hv), Sorghum bicolor (Sb), Populus trichocarpa (Pt), Gossypium hirsutum (Gh), Physcomitrella patens (Pp) and Phyllostachys edulis (Phe). A: Evolutionary tree and conserved structural domain analysis of CRY gene families in multiple species; B: Chimonobambusa sichuanensis CsCRYs gene family motifs; C: CRY gene family motifs in multiple species.
Among them, some motifs are common to all members, such as Motif 5 (except TaCRY3, PpCRY1b, and ZmCRY1), and CRY gene family members of most species contain motifs Motif 1, Motif 2, Motif 3, Motif 4, Motif 5, and Motif 10, and the results indicate that they are CRY gene family members play the Motifs 3, 5, 6, 9, and 10 constitute the PRK10674 conserved domain, Motifs 1, 2, 4, 7, and 8 constitute the Cryptochrome_C conserved domain, and Motifs 1, 2, 4, and 6 constitute the Cryptochrome_DASH domain. The above results indicate that there are both similarities and differences in the functions of CRY genes among different subfamilies.
A total of four members of the Chimonobambusa sichuanensis CsCRYs gene family were accurately identified through the mining of Chimonobambusa sichuanensis transcriptome data, and this number was determined from seven Phyllostachys edulis sequences screened by previous authors, of which Phyllostachys edulis sequences among which PheCRY3 and PheCRY4 were demonstrated to have repetitive sites and could be spliced into a single sequence in the present study. Consistent with the classification of CRY families in other plant species such as Arabidopsis, rice, companion mineral Sedum, alfalfa, and soybean. All four screened sequences of Chimonobambusa sichuanensis corresponded to Phyllostachys edulis sequences, which can be classified into three subfamilies (subfamilies I-III) based on their structural features. Compared with the classification results of other species, which are similar to that of Arabidopsis, the Arabidopsis cryptochrome gene family consists of members of three subfamilies: AtCRY1, AtCRY2, and AtCRY3. Among them, CRY3 belongs to the CRY-DASH branch, and evolutionarily CRY3 is an intermediate between Cryptochrome and photolyase. In contrast, although the PheCRY gene family of Phyllostachys edulis is divided into three subfamilies, the number in each subfamily is not equally distributed, which is different from Arabidopsis and Chimonobambusa sichuanensis. By analyzing other species classifications, all of these results showed differences, implying that there are structural and functional differences in CRY proteins from different plant species.
2.5. Protein physicochemistry of Four CsCRYs Families of Chimonobambusa sichuanensis
Subcellular localization of Chimonobambusa sichuanensis CsCRYs family member proteins was performed by Plant-mPloc, and the subcellular localization prediction showed that Chimonobambusa sichuanensis CsCRYs family members were mainly distributed in the cytoplasm. The results indicate that none of the four proteins has a transmembrane structure and thus belongs to the extracellular proteins. CsCRY1a, CsCRY1b, and CsCRY3 are distributed in the cytoplasm, and only CsCRY2 is distributed not only in the cytoplasm but also partially in the nucleus.
Physicochemical properties such as protein relative molecular mass and isoelectric point of CsCRYs were predicted by using Expasy website (
Table 1). Their relative molecular masses ranged from 54.15 kD to 80.10 kD (CsCRY1a to CsCRY3). The relative molecular mass of CsCRY2 was the smallest, and the molecular masses of all four genes in the CsCRYs family differed by a small range. The isoelectric points of the four protein sequences ranged from 5.08 to 9.30 (CsCRY1~CsCRY3), and only CsCRY3 had an isoelectric point greater than 7, which was basic, while the isoelectric points of the other members were about 5, which was acidic. The instability coefficient of the CsCRYs The instability coefficients of the CsCRYs proteins ranged from 41.41 to 54.36, which were all greater than 40, and all members were unstable proteins. The average hydrophilicity coefficients of the CsCRYs proteins ranged from -0.433 to -0.317, which were all less than 0. The average hydrophilicity coefficients were negative, and the smaller the coefficient, the more hydrophilic they were, and so it was hypothesized that all CsCRYs were hydrophilic proteins. The protein lengths of
Chimonobambusa sichuanensis CsCRYs ranged from 491 to 712 bp, and most of the genes and proteins of
Chimonobambusa sichuanensis CsCRYs did not have a large span of lengths, except for the smallest protein length of CsCRY2.
The four protein sequences of the
CsCRYs gene family were analyzed for their coding physicochemical properties using the online website Expasy. The results are shown in
Table 1 of the Annex. Of these four protein sequences, in CsCRY1a Leu (76, 10.7%), Ser (71, 10.0%), Ala (59, 8.3%), Arg (59, 8.3%), these 4 amino acids were relatively more abundant; in CsCRY1b Leu (73, 10.5%), Ser (68, 9.8%), Ala (57, 8.2%), Arg (55, 7.9%), these 4 amino acids were relatively more abundant, and the higher content of Leu was more hydrophobic, which was involved in inter-protein interactions, and it was hypothesized that the CsCRY1 protein might be involved in signaling; in CsCRY1, the protein was more hydrophobic and involved in inter-protein interaction. involved in signaling; in CsCRY2 Ser (56, 11.4%), Leu (48, 9.8%), Asp (35, 7.1%), Glu (33, 6.7%), the content of these 4 amino acids is relatively more, Ser is one of the common sites of protein phosphorylation modification and glycosylation modification, Ser content is more, then CsCRY2 protein may play a role in the recognition and binding of signaling molecules; in CsCRY3 Leu (63, 10.3%), Ser (52, 8.5%), Gly (47, 7.7%), and Ala (45, 7.4%), which are the four amino acids with relatively high content, then the CsCRY3 protein may also play a role in signaling. As can be seen from the table,
CRY genes of the same subfamily also have extremely similar protein amino acid compositions, suggesting that they are extremely closely related and have more similar compositions.
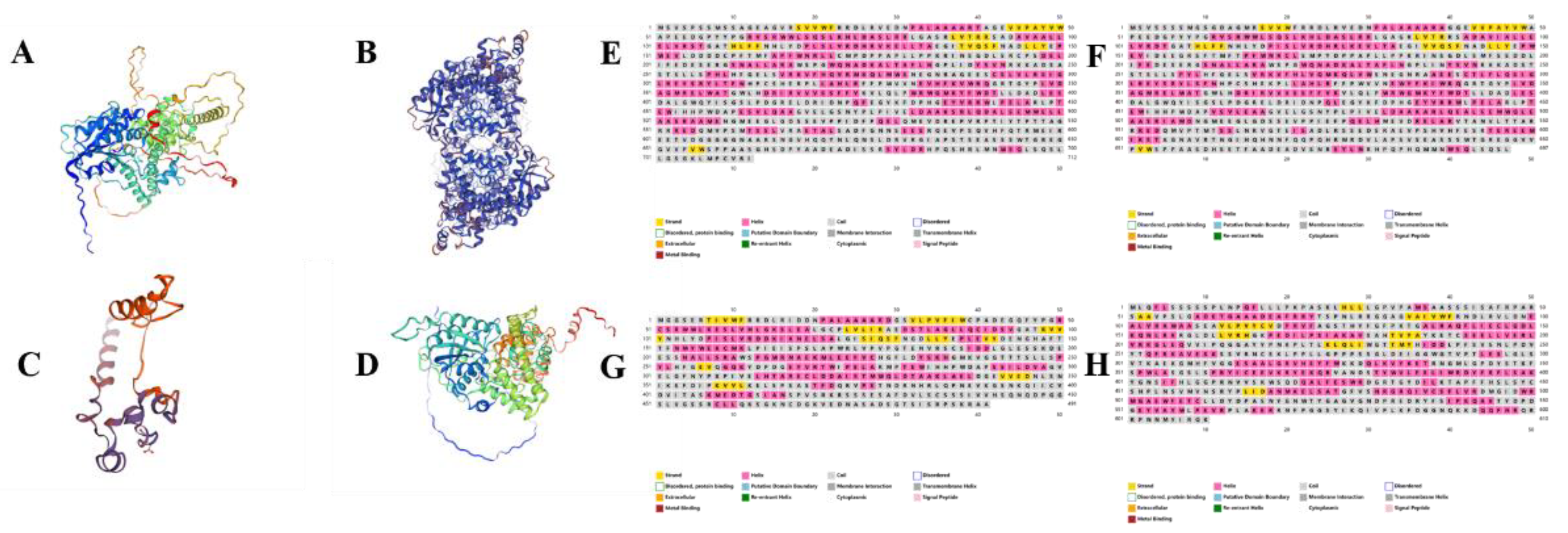
The Swiss-MODEL online website (URL:
https://swissmodel.expasy.org/) was used to predict the secondary levels of the proteins of
Chimonobambusa sichuanensis CsCRYs family members and to construct protein structure models. The results are shown in
Figure 3 below, which shows that the protein mainly consists of the structures of α-helix, extended chain, β-folding and random curl. According to the analysis of the data in
Table 2, the random curl is the component with the largest proportion, followed by a larger proportion of α-helices, and the other parts also contain β-folding and extended chain (
Table 2,
Figure 4A-D). The protein secondary structures of the
CsCRYs family members of
Chimonobambusa sichuanensis were also analyzed and constructed using the database PSIPRED (URL:
http://bioinf.cs.ucl.ac.uk/psipred/)[
17], the secondary structure usually includes the following parts: α-helix, β-strand, Intrinsically Disordered Regions (IDRs), and other structures, and the results are shown in Fig. 4E-H.
The alpha helix (α-helix) is a right-handed helical structure stabilized by hydrogen bonding, which usually consists of 3.6 amino acid residues forming a complete helical loop. the hydrogen bonding network and hydrophobicity of the α-helix make it a very stable structural element in many proteins. From the results presented in Fig. 3, it can be seen that the proteins of
Chimonobambusa sichuanensis CsCRYs family members all contain 34.62 %-39.17 % of α-helices, and they are all unstable proteins. β-fold is a relatively stretched section of peptide chain in a protein, usually consisting of 4-16 amino acids. Analysis of the results of protein secondary structure prediction showed that the proteins of
Chimonobambusa sichuanensis CsCRYs family members all contain 4.16%-8.35% β-folding, forming a relatively stretched sheet-like structure. Intrinsically Disordered Regions (IDRs) are regions of a protein that lack a fixed three-dimensional structure. These regions are highly flexible in their natural state and do not form well-defined secondary structures such as α-helices or β-folds, but have the ability to bind proteins. These regions or structural elements play an important role in protein function, especially in signaling, cellular localization and metabolic regulation. As can be seen from
Table 2, CsCRY3 had the largest proportion of extended chain at 12.9%, while the proportion of other CsCRY proteins with extended chain ranged from 8.8% to 10.7%.
Signal peptide analysis was performed through the Signalp 6.0 online website (URL:
https://services.healthtech.dtu.dk/services/SignalP-6.0/). Secondary structure, phosphorylation sites, and signal peptide sites play important roles in protein structure and protein folding[
18]. The four
Chimonobambusa sichuanensis protein sequences were analyzed by TMHMM online website (URL:
https://services.healthtech.dtu.dk/services/TMHMM-2.0/)[
19].
Table 3 shows the predicted signal peptide results for the CsCRYs proteins, indicating that the signal peptide ratios Sec/SPI for all four proteins are 0. The signal peptide indices of the family members are all less than 0.5 and most of them are 0, which indicates that none of the
Chimonobambusa sichuanensis CsCRYs family members contain signal peptides.
The closer the values of C-Score, S-Core, Y-Score, are to 1 the more likely they are to have a signal peptide, Mean-S is the average value of the S-Score of each amino acid at the amino acid from the N-terminus to the shear site, and Mean-D is the weighted average of Mean-S and Y-Max. Analysis of the results in
Table 3 and Fig. 2 in the Appendix shows that none of the four proteins has a signal peptide, and their absence of transmembrane structural domains is consistent with the subcellular prediction results described above.
Chimonobambusa sichuanensis CsCRYs proteins are monomeric proteins, mainly composed of α-helices and irregular coiled-coils, with β-turns and extended strands distributed throughout the protein sequences, which act as auxiliary modifications. The secondary and tertiary structures of Chimonobambusa sichuanensis CsCRYs proteins exhibit high conservation, a feature that confers Chimonobambusa sichuanensis CsCRYs proteins with excellent structural stability and functional diversity. In Chimonobambusa sichuanensis, CsCRY1a, CsCRY1b, CsCRY2, and CsCRY3 proteins are devoid of signaling peptide structures, and at the same time, their functional domains are mainly responsible for protein binding, which provides assistance for light signaling. This precise regulatory mechanism not only ensures the ionic balance of the intra- and extracellular environments, but also is the key for plant photosynthesis to show its function.
2.6. Expression Patterns of Cryptochrome CsCRYs Genes in Chimonobambusa sichuanensis Under Different Environmental Conditions
In order to screen the expression pattern of
CsCRYs genes involved in the regulation of light response under light stress, different intensities of blue light as well as different light qualities of
Chimonobambusa sichuanensis irradiated with different light qualities were used as the experimental materials, and the light conditions that could lead to the highest expression of
CsCRYs genes in
Chimonobambusa sichuanensis were screened. In this study, bamboo seedlings were irradiated with blue light at light intensities of 50, 75, and 100 µmol·m
-²·s
-1 for 24 h. The expression of
CsCRY2 gene was also examined under blue light at light intensity of 100 µmol·m
-²·s
-1, red light, and red-blue mixed light at light intensity of 1:1 by qRT-PCR, and leaves of the same treatment height were collected every 2-4 h. In this study, the expression response of
CsCRYs was analyzed in the light conditions that resulted in the highest expression of
CsCRYs in the bamboo seedlings, which were irradiated with the same treatment height. leaves as samples. The sampling times were: 2 h, 4 h, 6 h, 8 h, 12 h, 16 h, 20 h, 24 h. The quantitative primers are shown in the attached Table 4, and the spectra of treatment conditions are shown in the attached
Figure 2.
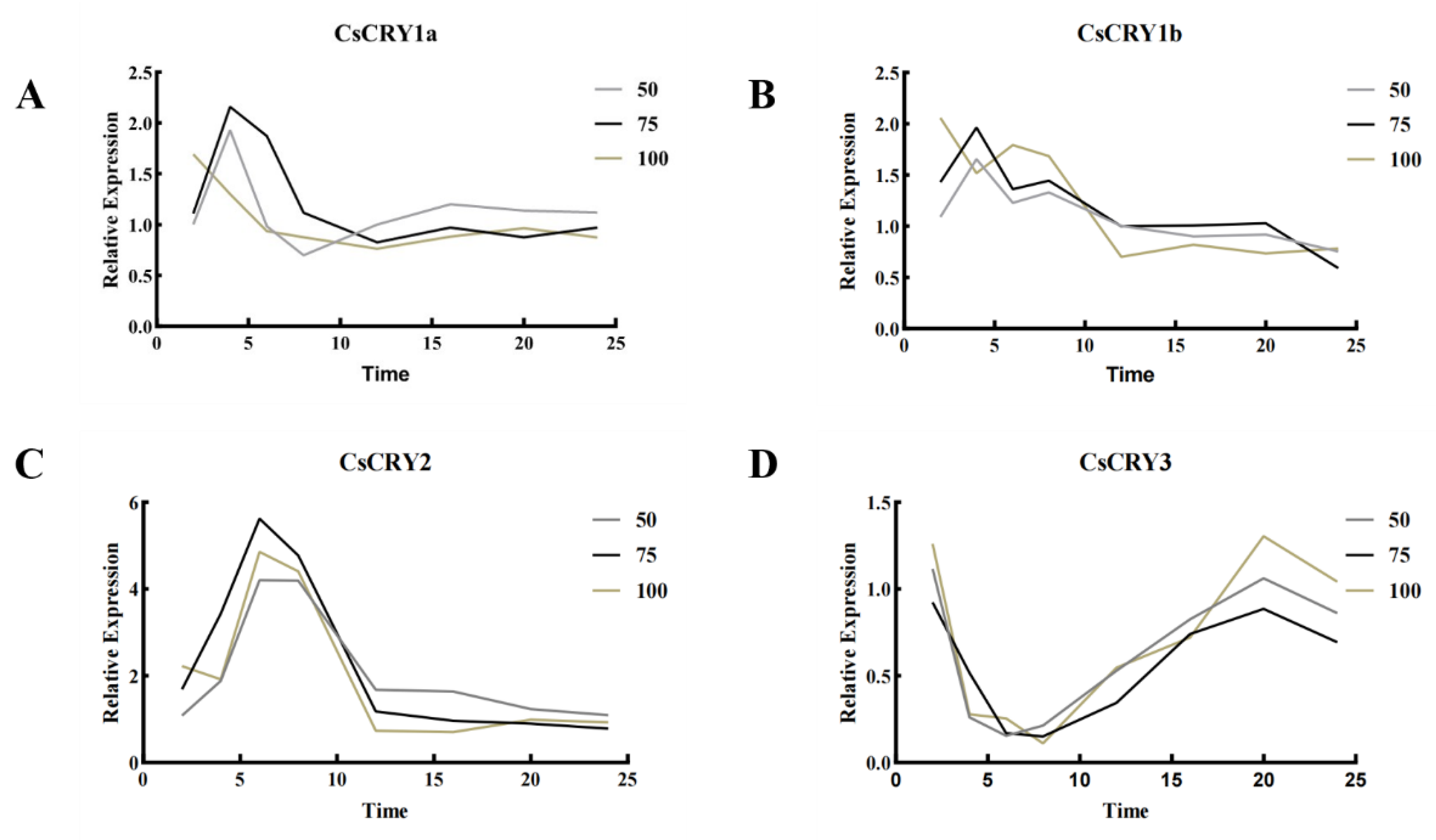
Figure 4.
Gene expression of Chimonobambusa sichuanensis CsCRYs family members under different blue light treatments. Note: A: CsCRY1a gene expression; B: CsCRY1b gene expression; C: CsCRY2 gene expression; D: CsCRY3 gene expression.
Figure 4.
Gene expression of Chimonobambusa sichuanensis CsCRYs family members under different blue light treatments. Note: A: CsCRY1a gene expression; B: CsCRY1b gene expression; C: CsCRY2 gene expression; D: CsCRY3 gene expression.
The results showed that the expression patterns of CsCRY1a, CsCRY1b and CsCRY2 were more consistent, all of them increased under blue light stress for a short period of time, and with the increase of the stress time, the expression decreased and stabilized under the three kinds of stress light intensities, and the light intensity around 75 µmol·m-²·s-1 was the category of the highest expression of them. The expression of CsCRY3 showed a trend of decreasing, then increasing and then decreasing with the increase of the stress time, and it was guessed that it might be related to the environmental adaptation with the long time. CsCRY3 showed a tendency of decreasing, then increasing and then decreasing with the increase of stress treatment time, which was guessed to be related to the environmental adaptation, and gradually adapted with the length of stress, and the gene expression of CsCRY3 was higher than that of the other two treatments in most of the time under the blue light stress of 100 µmol·m-²·s-1. It is worth mentioning that CsCRY2 is the gene with the highest expression under blue light stress among the Cryptochrome gene family in Chimonobambusa sichuanensis, and the reason for this may be similar to the fact that AtCRY2 of Arabidopsis is a specific receptor for blue light, where AtCRY2 is activated by the blue light response and undergoes a conformational change that activates downstream signaling. The structures of these four genes were explored in this study, and the structural domains of CsCRY1a, CsCRY1b, and CsCRY2 are relatively similar, whereas CsCRY3 has a distinctive Crypto-DASH structural domain, despite the differences in their sequences and lengths of structural domains, and it was hypothesized that the similarity of the domains may be associated with their tendency to show similar expression levels under physiological stress. It is hypothesized that the similar structural domains may be associated with their tendency to show similar expression under physiological stress.
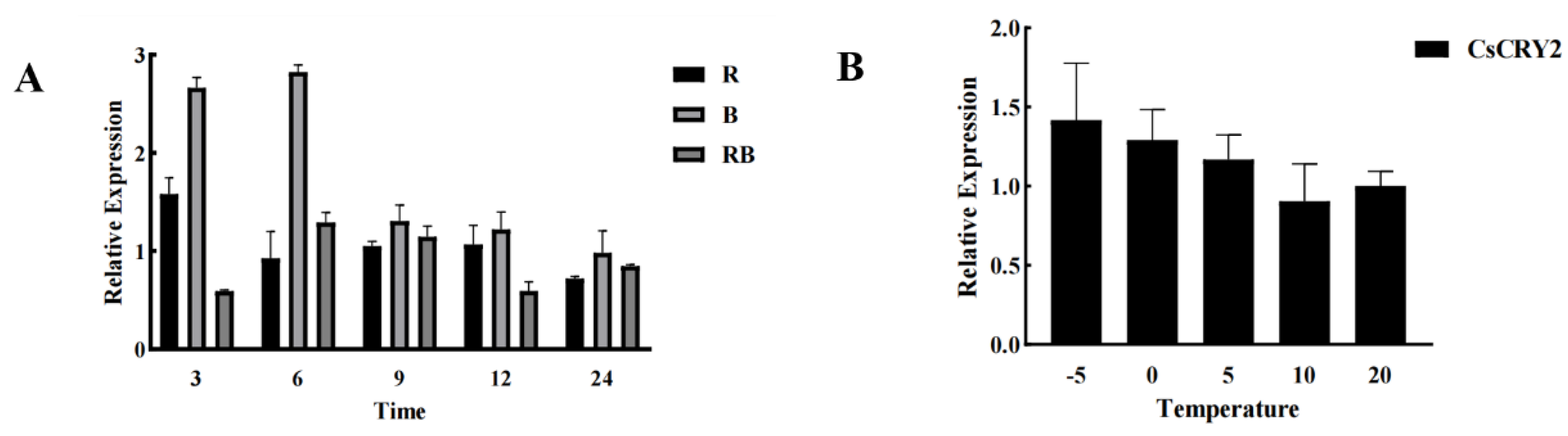
In
Chimonobambusa sichuanensis,
CsCRY2 was selected from the
CsCRYs gene family as the focus of the study, and bamboo seedlings were treated with different light qualities, and the gene expression of
CsCRY2 was shown in
Figure 5A. The results showed that the qRT-PCR results were highly consistent with the results of the previous transcriptome analysis, and the blue light irradiation group showed a significant increase in
CsCRY2 gene expression compared with the red light as well as the mixed light treatment groups. The expression of
Chimonobambusa sichuanensis CsCRY2 gene under different light treatments gradually stabilized with the increase of treatment time. All of them showed a rise in gene expression under blue light stress for a short period of time, and all of them showed that their expression would be higher under a single blue light irradiation than that of a single red light irradiation group and a 1:1 red and blue light co-irradiation treatment group, and that their expression was higher than that of the natural light control group under blue light stress during the 24 h treatment time. Therefore, it is initially hypothesized that
CsCRY2 is a key gene involved in responding to blue light stress in
Chimonobambusa sichuanensis.
In addition, previous studies have found that blue light affects the early process of cold domestication in plants, and
CRYs, as plant blue light receptors, are also involved in cold resistance in plants. In order to investigate the relationship between
CsCRYs and temperature in
Chimonobambusa sichuanensis, a key gene in the family,
CsCRY2, was selected as a research target. Different temperature gradients were set to explore whether cold stress affects
CsCRY2 gene expression, and the results are shown in
Figure 5B.
As can be seen in
Figure 5, the expression of
CsCRY2 gradually increased with decreasing temperature using room temperature treatment as a standard control, which is in line with the results of previous studies that
CRY is not only a blue light receiver, but also tightly linked to temperature. In terms of structural domains, structural domain mapping defines gene function and thus helps to elucidate the molecular mechanism of
CRY activity. Among the members of the
CsCRYs family in
Chimonobambusa sichuanensis, the gene expression of
CsCRY2 was significantly higher than that of the other members under stress, and previous studies found that
CRY1 and
CRY2 in
Arabidopsis interacted with COP1/SPA in seedlings grown under blue light, which represses genes related to the construction of plant photomorphology, and is therefore a negative regulator, but not with COP1/SPA in seedlings grown under dark light. COP1/SPA complex in dark-light-grown seedlings, whereas the COP1/SPA complex preferentially interacts with the
CRY1 and
CRY2 phosphorylated isoforms that are present only after blue-light irradiation.
CRY2 does not dissociate from the COP1-SPA1 complex, and blue light enhances the CRY2-COP1 interaction. In contrast, in this study,
CsCRY1a and
CsCRY1b were found to have Cryptochrome-C structural domains, which was not found in
CsCRY2, and this structural domain could bind to the COP1/SPA complex and thus inhibit its action, which was also consistent with the qRT-PCR results. Taxonomically,
Chimonobambusa sichuanensis belongs to the genus Cold Bamboo, which can survive under low-temperature stress, and the expression of
CsCRY2 peaked when the bamboo seedlings were subjected to subzero low-temperature stress, so it was hypothesized that
Chimonobambusa sichuanensis resisted to the low-temperature in a way that might increase the expression of the
CRY genes, and thus participated in the process of stress response.
3. Discussion
Chimonobambusa sichuanensis has become a preferred plant for urban greening due to its multiple ecological advantages such as evergreenness and cold tolerance, coupled with its beautiful system. In this study, the enrichment and expression characteristics of Cryptochrome in Chimonobambusa sichuanensis under different light conditions were further investigated on the basis of previous studies.
Plant light receptors are components necessary to establish signaling networks to regulate plant growth and stress-related responses. And blue light affects key biological processes in plants, including the promotion of flowering, seedling de-yellowing[
20], stomatal development[
21], and circadian rhythm regulation[
22]. In this study, the following findings were obtained by analyzing and identifying the
Chimonobambusa sichuanensis CsCRYs gene family by bioinformatics methods:
The composition of conserved motifs showed that among the four
Chimonobambusa sichuanensis sequences,
CsCRY1a,
CsCRY1b,
CsCRY2,
CsCRY3, 10 conserved sequences were predicted, and all the
CsCRYs had PRK 10674 super family conserved structural domains, which were highly conserved in the family and there were differences in conserved motifs among
CsCRYs of different subfamilies,
CsCRY3 had Crypto-DASH structural domains, which could participate in signaling processes and affect downstream gene expression and cellular activities after combining FAD and FMN.
CsCRY3 has a Crypto-DASH domain, which can be involved in signaling process and affect the expression of downstream genes and cellular activities after binding to FAD and FMN, unlike
Phyllostachys edulis sequences screened out by the previous authors, we did not find any
Phyllostachys edulis proteins that are very similar to each other to have the domain, and we analyzed the domains of
Phyllostachys edulis proteins published by the previous authors and found out that the domains of
Phyllostachys edulis proteins were very similar[
23]. The structural domains of
PheCRY3 and
PheCRY4 were analyzed in this paper and found that the
PheCRY3 and
PheCRY4 sequences published by the previous authors had very high duplications and might be the same sequence.
CsCRY1a and
CsCRY1b in all three subfamilies have PRK10674-Superfamily as well as Cryptochrome-C structural domains,
CsCRY2 in subfamily II has PRK10674-Superfamily structural domains, and
CsCRY3 has Crypto-DASH structural domains in the evolutionary tree. In the evolutionary tree, all members of this subfamily have this structural domain, which is involved in the biological clock, which is the identification criterion for subfamily III. This sequence was not found in
Phyllostachys edulis PheCRY3 published by the previous authors, and the reason why
PheCRY3 is the closest related sequence to
CsCRY3 in the evolutionary tree is not yet known. Since members of subfamilies I and П have similar structural domains,
CsCRY1a,
CsCRY1b, and
CsCRY2 showed a similar trend of expression under blue-light stress, confirming once again that the structure of the genes is closely related to their function[
24]. These results reflect their structural unity and variability, which provide diversity in the functions exercised by
Chimonobambusa sichuanensis CsCRY proteins.
Based on the analysis of the expression pattern of Chimonobambusa sichuanensis CsCRYs genes under different light qualities, it was shown that the expression of CsCRY1a, CsCRY1b, CsCRY2, and CsCRY3 had different degrees of changes. Since the CsCRYs gene its function as a blue light receptor, based on the gene expression results of the leaves of the stressed plants, it can be seen that the expression of CsCRY1a, CsCRY1b, CsCRY2, CsCRY3 were all increased under blue light stress. By comparing the trends of CsCRY2 gene expression in leaves subjected to single red and blue light stress and in the control group under combined red and blue light irradiation, combined with the expression of these genes under different treatments, it was concluded that CsCRY2 genes might be involved in the short-term response to resist blue light stress. The expression of CsCRY2 gene was significantly increased in the first 6 h under blue light stress, and was much higher than that in the red light stress group and the control group under red and blue light co-irradiation, whereas the expression of CsCRY2 gene tended to be stabilized in the different treatment groups after 6 h, which suggests that CsCRY2 may be involved in the resistance to the response to short-term blue light stress. It has been reported that Cryptochromes are involved in multiple aspects of plant responses to adversity stress and play different positive and negative regulatory roles, but most of the CRY genes reported so far negatively regulate abiotic stress processes, and in Chimonobambusa sichuanensis CsCRY2 was positively regulated by abiotic stress, and its expression increased with stress. The results showed that members of subfamily III in Chimonobambusa sichuanensis were significantly less responsive to stress than the other subfamilies, which supports the conclusion that members of subfamilies CRYI and П are mainly involved in plant stress tolerance. The results also showed that the expression increment of CsCRY2 was significantly higher than that of other members under high-throughput blue light stress treatment, and since the structural domain of this gene is different from that of other CsCRYs genes, it is hypothesized that this gene is a key member of the Chimonobambusa sichuanensis family of CsCRYs in responding to light.
In this study,
Chimonobambusa sichuanensis seedlings were irradiated with different light intensities of blue light stress to determine the expression of the
CsCRYs gene family. In this study, the expression trends of different
CsCRYs genes in the face of stress varied, suggesting that different
CsCRYs genes in
Chimonobambusa sichuanensis play different roles in adversity stress[
25]. The expression levels of
CsCRY1a,
CsCRY1b, and
CsCRY2 showed high levels of
CsCRY1a,
CsCRY1b, and
CsCRY2 in short-term
CsCRY1a,
CsCRY1b, and
CsCRY2 showed high expression in short-term irradiation of medium-amplitude blue light, suggesting that they may be involved in the short-term response to blue light stress, whereas the expression of
CsCRY3 showed an opposite trend to that of other members of the gene family, which indicated that
CsCRY3 may be involved in the long-term response to blue light stress.
Current studies have only found CRY genes linked to temperature in a few plants, for example, in Arabidopsis thaliana, AtCRY2 was found to regulate the participation of Arabidopsis thaliana in cold domestication under blue light in conjunction with COP1, HY5, etc. The number of CRY genes varies from plant to plant, and so do the functions they play, so there are still many prospects for exploration of CRY gene excavation. In order to fully search for the functions of the gene members of the CsCRYs family of Chimonobambusa sichuanensis, Chimonobambusa sichuanensis was subjected to low-temperature stress, and from the results, it can be seen that the Chimonobambusa sichuanensis CsCRY2 gene is also involved in Chimonobambusa sichuanensis's defense against cold stress, and with the decrease of the temperature, the expression of CsCRY2 gradually increased and reached a peak, which indicates that CsCRY2 is not only a blue-light receptor, but also responds to the low-temperature stress, and that it is the This indicates that CsCRY2 is not only a blue light receptor, but also a response to low temperature stress, which is one of the reasons why Chimonobambusa sichuanensis can safely survive the winter, and is also in line with the results of transcriptome sequencing of Chimonobambusa sichuanensis culms in winter.
The above results indicate that Chimonobambusa sichuanensis CsCRYs, as a blue light receptor gene, is actively involved in the growth and development of Chimonobambusa sichuanensis and in the defense of Chimonobambusa sichuanensis against cold stress. The present results provide a valuable reference for the comprehensive investigation of the functions of Chimonobambusa sichuanensis CsCRYs family of genes, and lays a foundation for the further analysis of the molecular mechanisms.
Author Contributions
Conceptualization, Kong, Y. N. and Liu, C. L; resources, Liu, G. H and Ji, Fang; writing—original draft preparation, Kong, Y. N; writing—review and editing Kong, Y. N. and Liu, C. L; Software, Li, T. S.; project administration, Liu, G. H; funding acquisition, Liu, G. H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Key Research and Development Program 2023YFD2201901-5, 2023YFD220120302.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dlamini, L.C.; Fakudze, S.; Makombe, G. G.; Muse, S.; Zhu, J.G. Bamboo as a Valuable Resource and its Utilization in Historical and Modern-day China. Bioresources 2022, 17, 1926–1938. [Google Scholar]
- Liu, C. L.; Xiong, X.; Li, T. S.; Duan, Y. H.; Yang, F.; Liu, G.H. The discoloration mechanism of Chimonobambusa sichuanensis clumunder colored film covering based on transcriptome analysis. Plant Physiology Journal 2025, 61, 83–96. [Google Scholar]
- Shao, X. Y.; Xiong, X.; Yang, F.; Zhao, Y. F.; Liu, C. L.; Liu, G.H. Full-length transcriptome sequencing analysis of Chimonobambusa sichuanensis after discoloration of bamboo culm. Jiangsu Journal of Agricultural Sciences 2024, 40, 538–531. [Google Scholar]
- Batschauer, A. New insights into the regulation of Arabidopsis cryptochrome 1. New Phytologist. 2022, 234, 1109–1111. [Google Scholar] [PubMed]
- Li, Q. H.; Yang, H.Q. Cryptochrome signaling in plants. Photochemistry and Photobiology 2007, 83, 94–101. [Google Scholar] [PubMed]
- Emery, P.; So, W. V.; Kaneko, M.; Hall, J. C.; Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 1998, 95, 669–79. [Google Scholar]
- Lin, C.; Yang, H.; Guo, H.; Mockler, T.; Chen, J.; Cashmore, A.R. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proceedings of the National Academy of Sciences of the United States of America 1998, 95, 2686–2690. [Google Scholar]
- Chen, Z.Y. Functional study of PhemiR156-PheSPLand PheCRY in moso. Dissertation, Fujian Agriculture and Forestry Universit 2022.
- Gao, L.; Liu, Q.; Zhong, M.; Zeng, N. N.; Deng, W. X.; Li, Y. X.; Wang, D.; Liu, S. Y.; Wang, Q. Blue light-induced phosphorylation of Arabidopsis cryptochrome 1 is essential for its photosensitivity. Journal of Integrative Plant Biology 2022, 64, 1724–1738. [Google Scholar]
- Hirose, F.; Inagaki, N.; Hanada, A.; Yamaguchi, S.; Kamiya, Y.; Miyao, A.; Hirochika, H.; Takano, M. Cryptochrome and Phytochrome Cooperatively but Independently Reduce Active Gibberellin Content in Rice Seedlings under Light Irradiation. Plant and Cell Physiology 2012, 53, 1570–1582. [Google Scholar] [CrossRef]
- Banerjee, R.; Schleicher, E.; Meier, S.; Viana, R. M.; Pokorny, R.; Ahmad, M.; Bittl, R.; Batschauer, A. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. Journal of Biological Chemistry 2007, 282, 14916–14922. [Google Scholar]
- Klar, T.; Pokorny, R.; Moldt, J.; Batschauer, A.; Essen, L.O. Cryptochrome 3 from Arabidopsis thaliana: Structural and functional analysis of its complex with a folate light antenna. Journal of Molecular Biology 2007, 366, 954–964. [Google Scholar] [CrossRef]
- Chen, C. J.; Wu, Y.; Xia, R. A painless way to customize Circos plot: From data preparation to visualization using TBtools. Imeta 2022, 3, 11. [Google Scholar] [CrossRef]
- Brown, P.; Baxter, L.; Hickman, R.; Beynon, J.; Moore, J. D.; Ott, S. MEME-LaB: motif analysis in clusters. Bioinformatics 2013, 29, 1696–1697. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deleage, G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Computer applications in the biosciences : CABIOS 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Bienert, S. ; Waterhouse, A; de Beer, T. A. P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Research 2017, 45, 313–319. [Google Scholar]
- Buchan, D.W. A.; Moffat, L.; Lau, A.; Kandathil, S. M.; Jones, D.T. Deep learning for the PSIPRED Protein Analysis Workbench. Nucleic Acids Research 2024, 52, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T. N. ; Brunak, S; von Heijne, G. ; Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods 2011, 8, 785–786. [Google Scholar]
- Krogh, A. ; Larsson, B; von Heijne, G. ; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. Journal of Molecular Biology 2011, 305, 567–580. [Google Scholar]
- Banas, A. K.; Leja, K.; Zglobicki, P.; Jedynak, P.; Kowalska, E.; Strzalka, W.; Grzyb, J.; Mysliwa-Kurdziel, B. De-etiolation is Almost Color Blind: The Study of Photosynthesis Awakening under Blue and Red Light. Plant and Cell Physiology 2024, 25. [Google Scholar]
- Pfeifer, A.; Mathes, T.; Lu, Y. H.; Hegemann, P.; Kottke, T. Blue Light Induces Global and Localized Conformational Changes in the Kinase Domain of Full-Length Phototropin. Biochemistry 2010, 49, 1024–1032. [Google Scholar] [CrossRef]
- Emery, P.; So, W. V.; Kaneko, M.; Hall, J. C.; Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 1998, 95, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, R.; Klar, T.; Hennecke, U.; Carell, T.; Batschauer, A.; Essen, L.O. Recognition and repair of UV lesions in loop structures of duplex DNA by DASH-type cryptochrome. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 21023–21027. [Google Scholar] [PubMed]
- Zuo, Z. C.; Liu, H. T.; Liu, B.; Liu, X. M.; Lin, C.T. Blue Light-Dependent Interaction of CRY2 with SPA1 Regulates COP1 activity and Floral Initiation in Arabidopsis. Current Biology 2011, 21, 841–847. [Google Scholar] [PubMed]
- Li, Y. P.; Shi, Y. T.; Li, M. Z.; Fu, D. Y.; Wu, S. F.; Li, J. G.; Gong, Z. Z.; Liu, H. T.; Yang, S.H. The CRY2-COP1-HY5-BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).