Submitted:
12 March 2025
Posted:
12 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
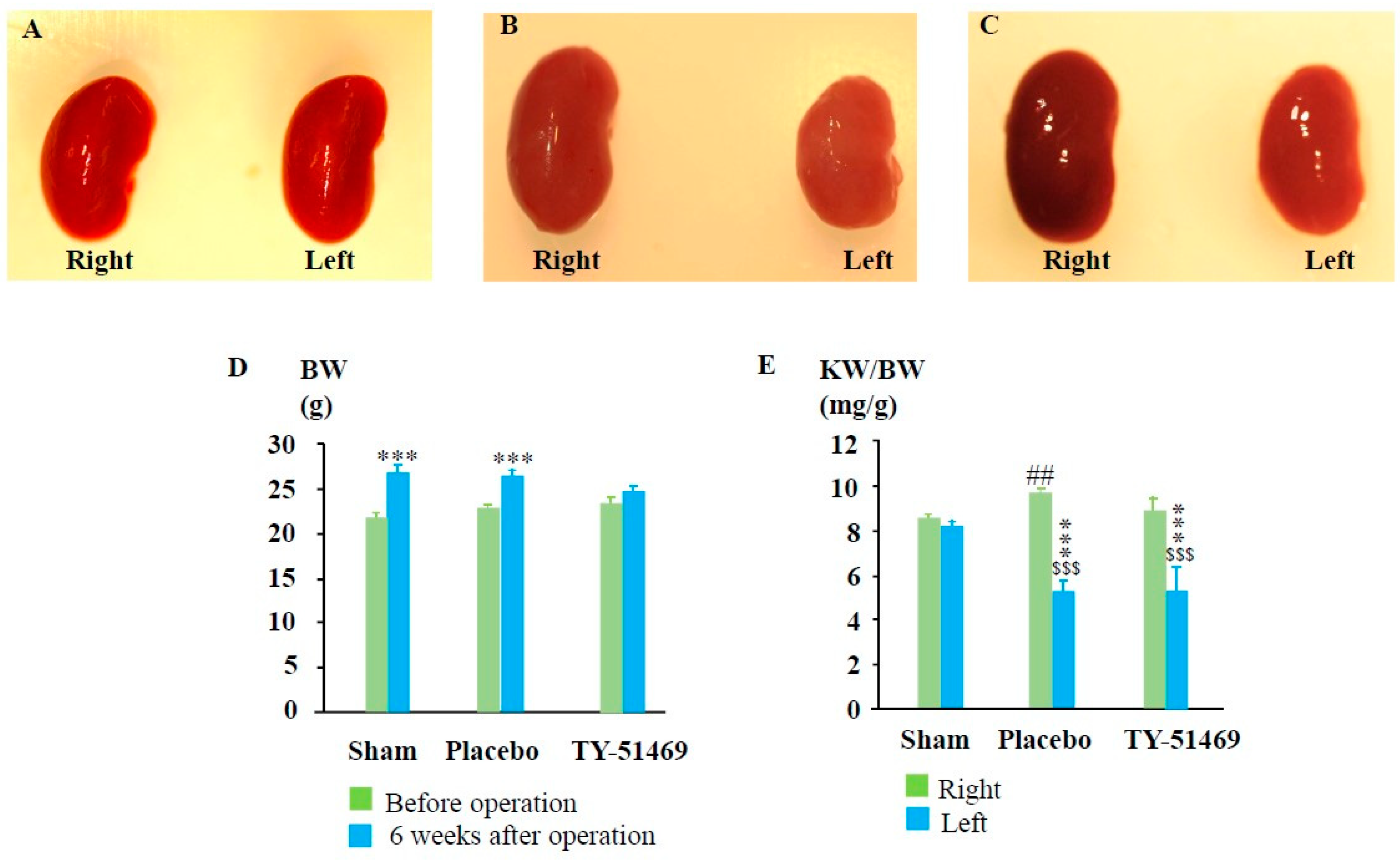
2.1. Procedure for creating the I/R model and subsequent changes in KW to BW ratio
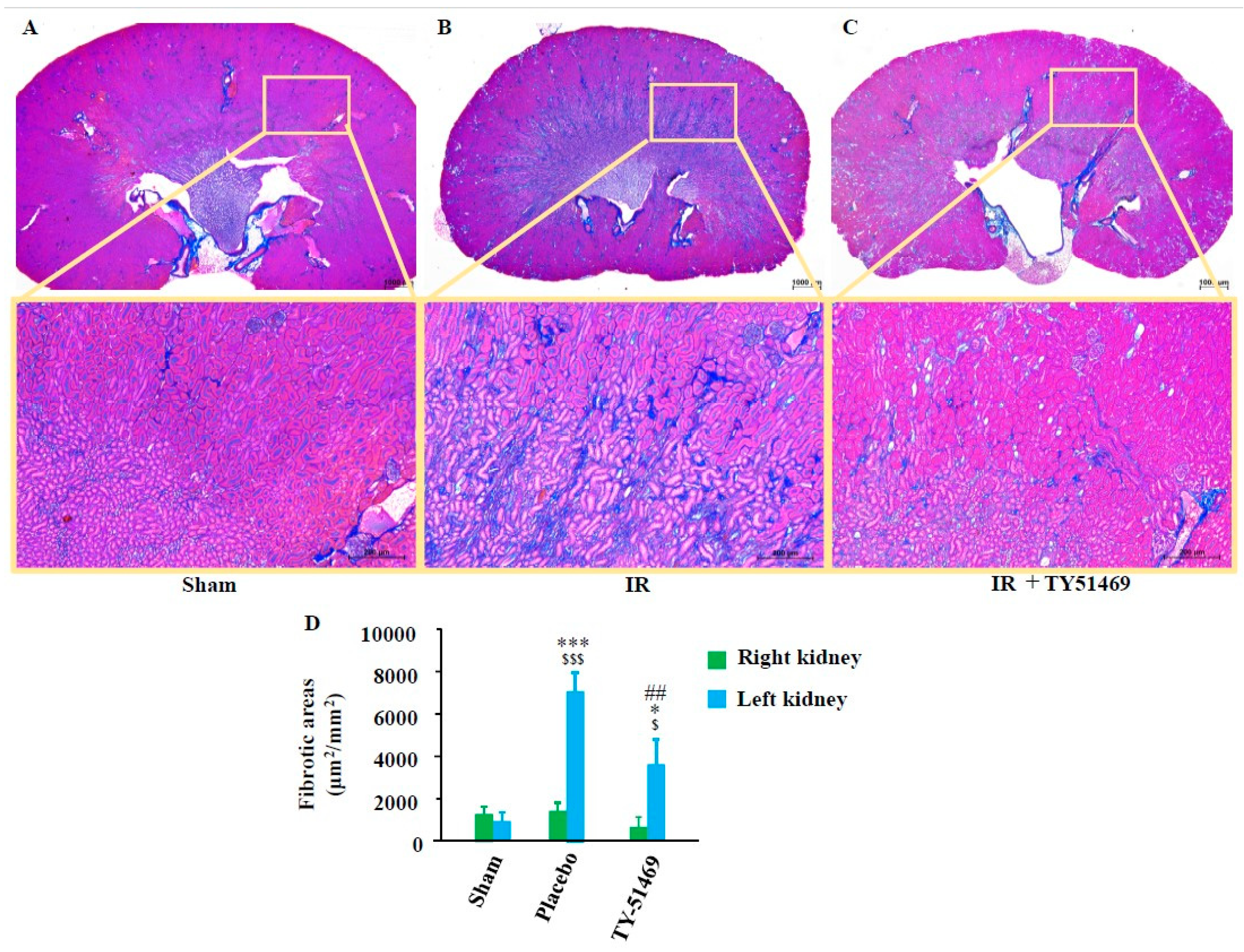
2.2. Characteristics of renal fibrosis after I/R
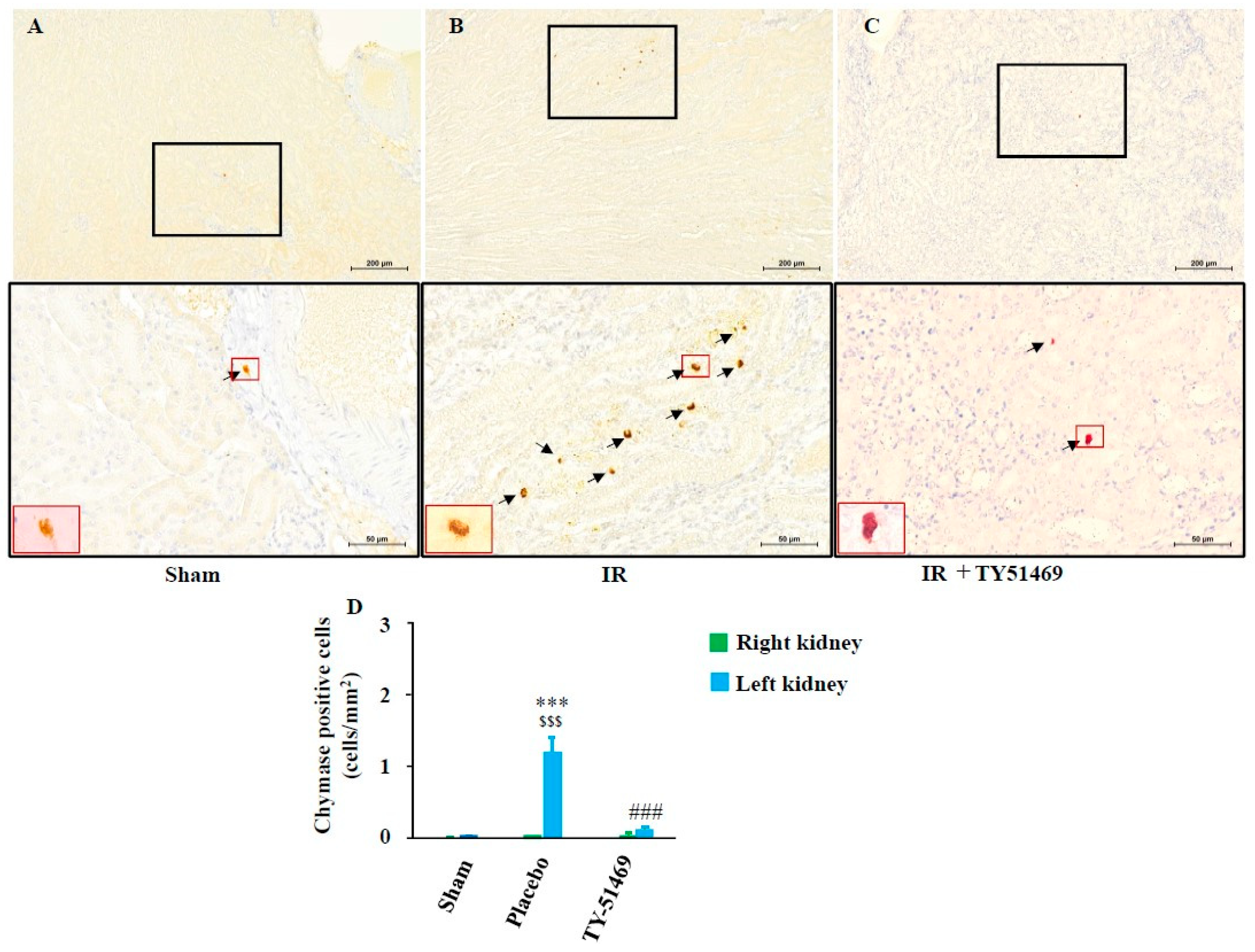
2.3. Changes in chymase-positive mast cells in renal fibrosis areas after I/R
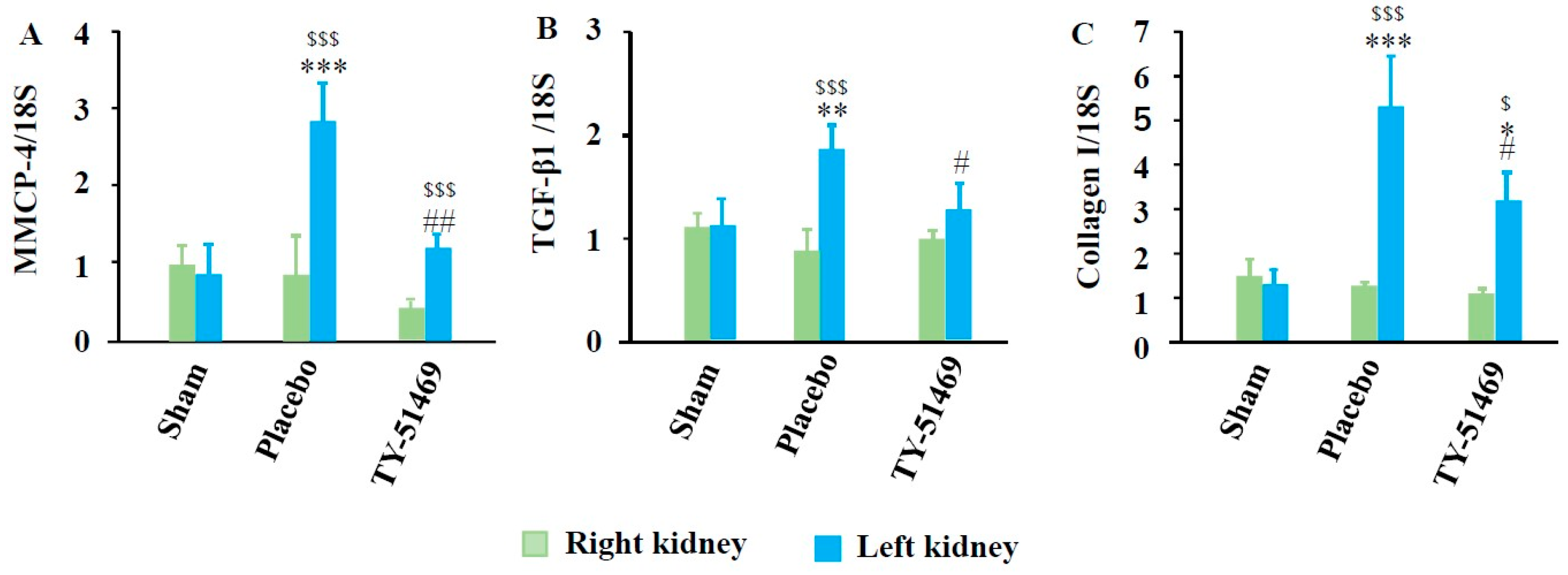
2.4. Changes in gene expression levels of MMCP-4, TGF-β1, and collagen I after I/R
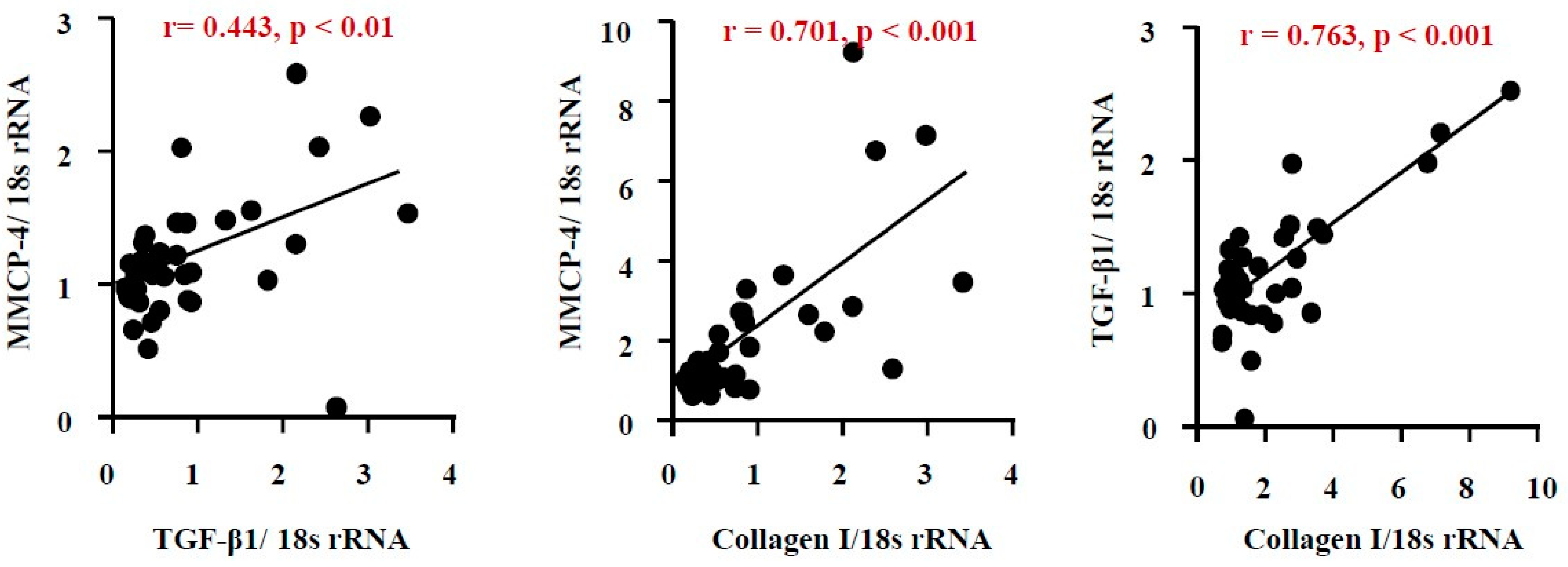
2.5. Correlation among renal MMCP-4, TGF-β1 and collagen I gene expression levels after I/R
3. Discussion
4. Materials and Methods
4.1. Animals
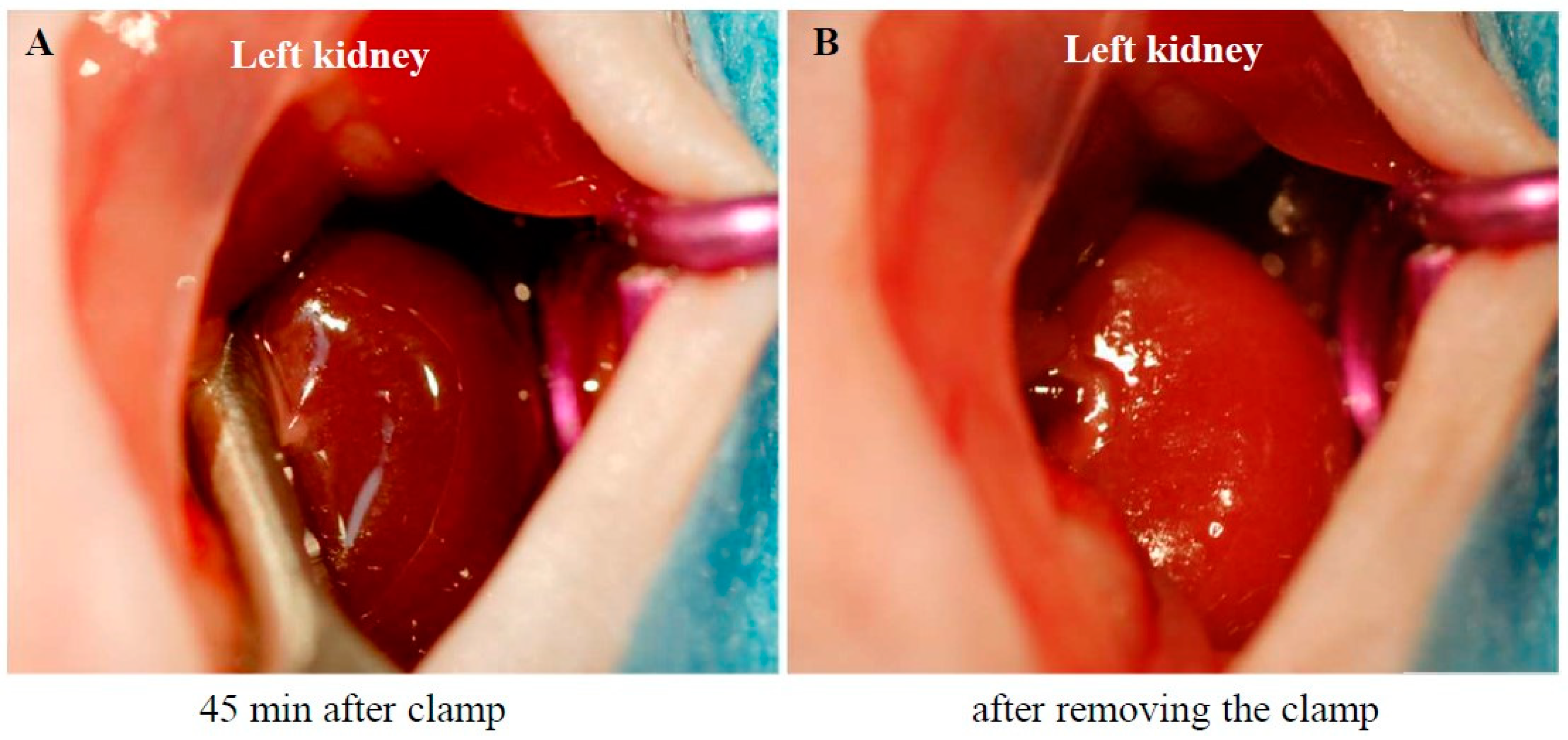
4.2. Creation of the unilateral renal I/R injury model
4.3. Grouping and Sampling
4.4. Reverse Transcriptio Polymerase Chain Reaction (RT-PCR)
4.5. Histological Studies
4.6. Statistical analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bonventre, J.V.; Weinberg, J.M. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 2003, 14, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E.; Perkins, R.M. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef]
- Li, Z.; Li, N. Epigenetic modification drives acute kidney injury-to-chronic kidney disease progression. Nephron 2021, 145, 737–747. [Google Scholar] [PubMed]
- Liu, J.; Kumar, S.; Dolzhenko, E.; Alvarado, G.F.; Guo, J.; Lu, C.; Chen, Y.; Li, M.; Dessing, M.C.; Parvez, R.K.; Cippà, P.E.; Krautzberger, A.M.; Saribekyan, G.; Smith, AD.; McMahon, A.P. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight. 2017, 2, e94716. [Google Scholar] [CrossRef]
- Liu, B.C.; Tang, T.T.; Lv, L.L.; Lan, H.Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Basile, D.P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef]
- Basile, D.P.; Collett, J.A.; Yoder, M.C. Endothelial colony-forming cells and pro-angiogenic cells: Clarifying definitions and their potential role in mitigating acute kidney injury. Acta. Physiol. Oxf. Engl. 2018, 222, e12914. [Google Scholar] [CrossRef]
- Anders, H.J.; Schaefer, L. Beyond Tissue Injury—Damage-Associated Molecular Patterns, Toll-Like Receptors, and Inflammasomes Also Drive Regeneration and Fibrosis. J. Am. Soc. Nephrol. 2014, 25, 1387–1400. [Google Scholar]
- Sato, Y.; Yanagita, M. Immune cells and inflammation in AKI to CKD progression. Am. J. Physiol. Renal Physiol. 2018, 315, 501–1512. [Google Scholar] [CrossRef]
- Mack, M.; Yanagita, M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015, 87, 297–307. [Google Scholar] [CrossRef]
- Nakamura, J.; Sato, Y.; Kitai, Y.; Wajima, S.; Yamamoto, S.; Oguchi, A.; Yamada, R.; Kaneko, K.; Kondo, M.; Uchino, E. Myofibroblasts acquire retinoic acid–producing ability during fibroblast-to-myofibroblast transition following kidney injury. Kidney Int. 2019, 95, 526–539. [Google Scholar] [CrossRef]
- Kaissling, B.; Lehir, M.; Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 2013, 1832, 931–939. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Wu, Y.; Zhao, C. Kruppel-like factor 4 modulates the miR-101/COL10A1 axis to inhibit renal fibrosis after AKI by regulating epithelial-mesenchymal transition. Ren Fail. 2024, 46, 2316259. [Google Scholar]
- Maryam, B.; Smith, M.E.; Miller, S.J.; Natarajan, H.; Zimmerman, K.A. Macrophage Ontogeny, Phenotype, and Function in Ischemia Reperfusion-Induced Injury and Repair. Kidney360. 2024, 5, 459–470. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati., M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Guzzi, F.; Cirillo, L.; Roperto, R.M.; Romagnani, P.; Lazzeri, E. Molecular Mechanisms of the Acute Kidney Injury to Chronic Kidney Disease Transition: An Updated View. Int J Mol Sci. 2019, 20, 4941. [Google Scholar] [CrossRef]
- Kim, D.J.; Kang, J.M.; Park, S.H.; Kwon, H.K.; Song, S.J.; Moon, H.; Kim, S.M.; Seo, J.W.; Lee, Y.H.; Kim, Y.G.; Moon, J.Y.; Lee, S.Y.; Son, Y.; Lee, S.H. Diabetes Aggravates Post-ischaemic Renal Fibrosis through Persistent Activation of TGF-beta(1) and Shh Signalling. Sci Rep. 2017, 7, 16782. [Google Scholar]
- Takai, S.; Shiota, N.; Yamamoto, D.; Okunishi, H.; Miyazaki, M. Purification and characterization of angiotensin II-generating chymase from hamster cheek pouch. Life Sci. 1996, 58, 591–597. [Google Scholar] [CrossRef]
- Lindstedt, K.A.; Wang, Y.; Shiota, N.; Saarinen, J.; Hyytiäinen, M.; Kokkonen, J.O.; Keski-Oja, J.; Kovanen, P.T. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001, 15, 1377–1388. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; Liu, J. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Fan, Y.; Yuan, Y.; Xiong, M.; Jin, M.; Zhang, D.; Yang, D.; Liu, C.; Petersen, R.B.; Huang, K.; Peng, A.; Zheng, L. Tet1 deficiency exacerbates oxidative stress in acute kidney injury by regulating superoxide dismutase. Theranostics 2023, 13, 5348–5364. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvnink, H.G.D. Ischemia and reperfusion injury in kidney transplantation: relevant mechanisms in injury and repair. J Clin Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemicacute kidney injury. J Clin Invest. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Burne-Taney, M.J.; Yokota, N.; Rabb, H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int. 2005, 67, 1002–1009. [Google Scholar] [CrossRef]
- Caughey, G.H.; Raymond, W.W.; Wolters, P.J. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta. 2000, 1480, 245–257. [Google Scholar]
- Andersson, M.K.; Karlson, U.; Hellman, L. The extended cleavage specificity of the rodent betachymases rMCP-1 and mMCP-4 reveal major functional similarities to the human mast cell chymase. Mol Immunol. 2008, 45, 766–775. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Garrido, A.M.; Griendling, K.K. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009, 302, 148–158. [Google Scholar] [CrossRef]
- Asehnoune, K.; Strassheim, D.; Mitra, S.; Kim, J.Y.; Abraham, E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004, 172, 2522–2529. [Google Scholar] [CrossRef]
- Terai, K.; Jin, D.; Watase, K.; Imagawa, A.; Takai, S. Mechanism of Albuminuria Reduction by Chymase Inhibition in Diabetic Mice. Int J Mol Sci. 2020, 21, 7495. [Google Scholar] [CrossRef]
- Nishimura, H.; Jin, D.; Kinoshita, I.; Taniuchi, M.; Higashino, M.; Terada, T.; Takai, S.; Kawata, R. Increased Chymase-Positive Mast Cells in High Grade Mucoepidermoid Carcinoma of the Parotid Gland. Int J Mol Sci. 2023, 24, 8267. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




