1. Introduction
In present, a great number of solid wastes generated especially from industrial activities constitutes major problems for the terrestrial environment. Their disposal in landfills is no longer acceptable because they pollute the soil, groundwater and surface water, as well as atmospheric air.
Recently (in the last decades of the previous millennium), it has been found that among the many available wastes, silica and alumina-rich wastes have cementitious and pozzolanic properties almost similar to those of Portland cement, the traditional binder for concrete manufacturing.
Under the current conditions, in which greenhouse gas emissions into the atmosphere (mainly CO2) are responsible for the danger of overheating the planet, causing a real global ecological crisis, the exceptional scientific discovery of the French researcher J. Davidovits allowed the use of alkaline-activated alumina-silicate wastes for their transformation into so-called ”geopolymers”.
Geopolymers are materials with remarkable newly-created value, whose mechanical and physical properties are recommended for use in various applications: construction materials, repair and consolidation materials, heat-resistant structural components, fire-resistant fiber composites, sealants, ceramics, etc. (Majidi, 2009).
Davidovits characterizes this new material type as an ”inorganic alumina-silicate polymer” (Davidovits, 2008), the precursors being natural materials (clay, kaolinite, volcanic ash) or industrial by-products (coal fly ash and granulated blast furnace slag). The alkaline activators recommended by the inventor are aqueous solutions containing sodium/potassium hydroxide and sodium/potassium silicate (Chithambar Ganesh and Muthukannan, 2018).
The current work focuses on the category of porous geopolymers, i.e. lightweight materials with high porosity, low heat conductivity, rapid hardening at low temperature, good thermal and chemical stability and adsorption properties.
Porous geopolymers do not require sintering process, the energy consumption during their manufacturing and CO2 emissions are very low and the required raw materials can come from a wide range of sources including not only natural minerals, but also alumina-silicate industrial wastes such as: fly ash, bottom ash, slag, red mud, rice husk ash (Li et al., 2022; Zhang et al., 2021).
According to the literature (Wang and Sun, 2023), the preparation of porous geopolymers can be achieved by one of the following methods: (i) stacking method of particles, (ii) direct foaming method, (iii) solvent evaporation method, and (iiii) adding porous filler method.
The porous geopolymer made by the first method usually has good permeability and satisfactory mechanical properties, being suitable for use in low-permeability concretes and porous pavements. The second method is the most commonly used and is based on the addition of an expanding agent and/or a surfactant, which facilitates the formation of the porous structure.
The pore size is generally quite large. In the third method, the volatilization of the solvent allows the formation of pores. The resulting porous structure has a very fine porosity. This geopolymer type with low pore size is often used as a membrane separation material and adsorbent. The last method is characterized by the addition of porous fillers or fillers that can generate a porous structure. The addition of porous polystyrene, porous siliceous material, cork, lightweight aggregate, shells, etc., quality porous products can be obtained.
In this work, the direct foaming method was chosen aiming at producing porous geopolymers with insulating properties intended for the construction sector.
According to the paper (Movais et al., 2016), a porous geopolymer was manufactured utilizing a mixture including natural alumina-silicate material (metakaolin) in a ratio of 67 % and industrial waste (fly ash) originated from porous biomass in a ratio of 33 %, hydrogen peroxide (H2O2) being additionally used as an expanding agent.
The H2O2 amount influenced porosity, heat conductivity, and mechanical strength of the geopolymer. The low heat conductivity (up to 0.107 W·m-1·K-1) ensures adequate properties for thermal insulation applications.
The aqueous solution composed of sodium silicate and sodium hydroxide was applied for the alkaline activation of alumina-silicate materials and initiation of the geopolymerization reaction.
The effect of alkaline activator concentration, alkaline activator ratio, and metakaolin/alkaline activator ratio on the thermal conductivity of metakaolin-based geopolymer was analyzed in (Jaya et al., 2020). Also, the effect of the foaming agent (H2O2) and surfactant on physical properties, compressive strength, and pore appearance was investigated in this work.
The results showed that the addition of H2O2 and surfactant contributed to the production of a porous geopolymer with compression strength within the limits of 0.4-6 MPa, bulk density between 471-1212 kg·m-3, porosity in the range of 36-86 %, and heat conductivity between 0.11-0.30 W·m-1·K-1.
The paper (Pedziwiatr et al., 2018) provides a critical review of hydrogen peroxide (H2O2), used as a foaming agent in the production of porous geopolymers, one of the most versatile chemical substances.
Numerous fields of its application are shown in the paper: cosmetics, medicine, pulp and paper industry, textile industry, etc. However, the use of H2O2 remains limited due to its high manufacturing cost, chemical instability (in the case of lower concentrations) requiring the addition of foam stabilizers, which modify the kinetics of the decomposition reaction.
According to (Bai et al., 2018), vegetable oils (sunflower oil, olive oil, canola oil) have proven their ability to stabilize the geopolymer foam, facilitating obtaining porous materials with excellent insulating characteristics: density between 370-740 kg·m-3, porosity in the range of 66-83 %, heat conductivity in the limits of 0.11-0.17 W·m-1·K-1, while compression strength had values in the range of 0.3-11.6 MPa.
Another type of foaming agent used as an alternative to H2O2 for making the porous geopolymer was sodium perborate (NaH2BO4) (Wattanarach et al., 2022). Mixed with metakaolin in ratios in the range of 0.5-2 wt. % and then with the aqueous solution of the alkaline activator (NaOH and Na2SiO3) a paste was obtained, which, poured into a silicone mould, was subjected to the hardening process at 60 ℃ for 24 hours.
The geopolymer was then cured at room temperature for 28 days before determining the physical, thermal, and mechanical features.
Increasing the sodium perborate ratio within the limits mentioned above led to an increase of the geopolymer porosity from 54.7 to 67.6 %, along with the decrease of the material density from 1077 to 750 kg·m-3, heat conductivity from 0.325 to 0.218 W·m-1·K-1, and compression strength from 6.7 to 5.2 MPa.
In another work published in the literature (Phavongkham et al., 2020), fly ash-based porous geopolymer was made using sodium perborate as a pore-providing agent and washing liquid as a surfactant.
The work was mainly focused on investigating the effects of surfactant on the thermal and mechanical characteristics of the geopolymer. Weight proportions of surfactant within the limits of 0.1-0.5 % showed that this has an important role in creating finer pores.
The compression strength, under the conditions of applying a 28-day curing process, had values between 4.2-4.8 MPa, with additional increases through the addition of surfactant.
Heat conductivity of the porous geopolymer was between 0.27-0.32 W·m-1·K-1. The work especially highlighted the excellent effect of using surfactant between 0.3-0.5 % on the fire resistance of the porous geopolymer, showing that a 40 mm thick plate heated on one of its surfaces to 1000 ℃ indicates a temperature of only 300 ℃ on the opposite surface of the plate, the fire resistance being superior compared to a plate of similar dimensions, made without or with 0.1 % surfactant.
The fabrication of fly ash-based porous geopolymer by alkaline activation choosing sodium perborate monohydrate (NaH2BO4) as a pore-providing agent and sodium dodecyl sulfate (NaC12 H25SO4) as a foam stabilizing agent, both agents in weight ratios in the range of 0.9-2.8 % was presented in the paper (Korat and Ducman, 2020).
The parameters of the curing process into the mould were the temperature of 70 ℃ for 24 hours, followed by maintaining the specimens removed from the mould at room temperature for 3 days. The hardened geopolymer specimens had the density between 330-670 kg·m-3, heat conductivity within the limits of 0.143-0.205 W·m-1·K-1, and compression strength between 1.02-6.33 MPa.
A porous geopolymer based on coal fly ash and metakaolin was designed and tried by authors of the current paper (Paunescu et al., 2023). The geopolymer formation was favourized by the development of the geopolymerization reaction of these alumina-silicate wastes into the alkaline medium based on NaOH and Na2SiO3 solution.
The usual expanding agent (H2O2) was replaced with sodium perborate, more stable and easier to handle. The work originality was the use of a nanomaterial (bentonite clay) able to increase the strength. The density and heat conductivity of the new product had low values (470 kgꞏm-3 and 0.104 Wꞏm-1ꞏK-1, respectively), and the compression strength reached 7.5 MPa.
The quality of the precursors used for producing geopolymers plays a major role (Furtos et al., 2024). The precursors nominated by the French inventor J. Davidovits are alumina-silicate materials with low calcium content.
Zhuang et al. (Zhuang et al., 2016) observed that the Si/Al molar ratio and the composition of the alkaline solution are factors that strongly influence the properties of the geopolymer. Higher density of materials can negatively influence the polymerization degree of the geopolymer, while a lower density increases the polymerization degree.
According to various experimental results, it was found that the composition of the alkaline activator plays a much more important role than previously thought in ensuring the required quality of the geopolymer.
The most commonly used alkaline activators in the manufacture of geopolymers are mixtures of sodium/potassium hydroxide with sodium/potassium silicate. The physical and structural characteristics of alumina and silica-based precursors are clearly influenced by the concentration of the activator.
Some authors have experimentally found that the cation size of the activating solution allows the formation of a stronger network after the polycondensation process, the potassium-based solution being more suitable compared to the sodium-based one (Khale and Chaudhary, 2007).
On the other hand, it was found that the use of NaOH favours porous network in fly ash precursors with Si/Al ratio of 1.5 and Na/Al ratio of 0.48 during the hydration process. The mixture including sodium hydroxide and sodium silicate leads to a microporous network in fly ash with Si/Al molar ratios of 2.8 and Na/Al of 0.46.
The research presented further in this paper aimed at creating a porous geopolymer with better thermal insulation properties (lower values of density and heat conductivity as well as higher porosity), while maintaining the mechanical resistance of the product at a high level.
2. Materials and Methods
The precursor chosen for this experiment was type F-coal fly ash (with low calcium content) resulting from burning the anthracite in power plant boilers, the proportion of calcium being around 3.5 % (ASTM C618).
The precursor was procured from the Romanian Paroseni-thermal power plant about 10 years ago with a grain size below 200 µm. Further mechanical processing by grinding was necessary to reduce the maximum grain size below 80 µm.
The chemical composition of fly ash included the following components: 54.4 % SiO2, 26.5 % Al2O3, 4.3 % Fe2O3, 3.5 % CaO, 2.5 % MgO, 1.5 % TiO2, 0.4 % Na2O, 0.6 % K2O, and 1.7 % SO3.
Silica fume, as one of the most well-known nanomaterials, was added due to its major potential contribution in increasing the mechanical strength of the geopolymer. Also, this nanomaterial has remarkable pozzolanic properties. In principle, silica fume cannot be activated in alkaline medium alone as a binder, requiring a calcium source (Khale and Chaudhary, 2007).
Available on the market as a crystalline powder, free flowing, and water-soluble, sodium perborate (NaH2BO4) was the expanding agent chosen by the authors as an alternative to hydrogen peroxide (H2O2).
This material constitutes a stable source of active oxygen in the field of detergents, cleaning products, bleaches for various textiles as well as disinfectants. Sodium perborate easily releases oxygen at low temperatures (above 60 ℃).
Also, readily available commercially, olive oil was adopted as a surfactant acting as a stabilizer for the geopolymer foam. Used at a maximum ratio of 15 %, the surfactant was added to the mixture influencing the formation of geopolymer pores. The density and mechanical strength of the geopolymer showed a decreasing trend with increasing the oil addition, while porosity and water uptake increased. The compression strength was directly influenced by the total porosity (Lertcumfu et al., 2019).
Regarding the composition of the alkaline activator, it was chosen to use potassium hydroxide (KOH) mixed with sodium silicate (Na2SiO3). KOH is available on the market in the form of solid water-soluble pellets. The molarity of the KOH solution obtained by dissolving the pellets in distilled water was adopted at M12. Also, sodium silicate is commercially available as an aqueous solution with the concentration of almost 40 %.
The method of producing porous geopolymer is based on the hydrolysis of the pore-forming agent (sodium perborate) in contact with water, releasing hydrogen peroxide (H2O2) and the tetrahydroxyborate anion [B(OH)4]- (Brotherton, 1994). Further, through the decomposition process of H2O2, molecular hydrogen and molecular oxygen are released, that form bubbles and contribute to the geopolymer foaming.
Also, the activation of alumina-silicate materials in a liquid alkaline medium creates the conditions for the initiation and development of the geopolymerization reaction. This reaction leads to obtaining the final material in the geopolymer state, i.e. a three-dimensional polymer chain and ring structure, containing bonds of the Si-O-Al-O type (Provis and Rees, 2009).
Preparation of the starting mixture for making porous geopolymer consisted in separate processing into individual containers of the liquid alkaline activator and the solid mixture, respectively. The preparation of the liquid activator was carried out by mechanical stirring at 300 rpm for 3 min in a mixer and the preparation of the solid mixture (previously ground to grain sizes below 80 µm, except for the nanomaterial, which has a grain size below 1 µm) by mixing at 700 rpm for 10 min in an electrically driven mixer.
After completion of these processes, the liquid mixture was poured over the solid mixture and the mixing process was restarted at 700 rpm for about 6 min, until a paste was formed. This was poured into a metal mould and introduced into a laboratory electric oven.
The temperature adopted for hardening the geopolymer paste was 70 ℃ for 24 hours. Then, the hardened specimen was removed from the mould and subjected to the curing process at room temperature under low humidity conditions for 7 and 28 days, respectively, before identifying the physical, thermal, mechanical, and microstructural features of the cured geopolymer specimen.
Methods used for determining the porous geopolymer features are usual methods. Apparent density was measured by weighing the mass with an electronic balance relating the mass value to that of the sample volume (Methodology, 2015).
Apparent porosity was identified using a vacuum saturation procedure (Kearsley and Wainwright, 2002). TA.XTplus Texture Analyzer was the equipment used for measuring the compression strength and the flexural strength was determined according to SR EN ISO 1412:2000 (Curtu and Stanciu, 2011). The heat conductivity was measured through the guarded-comparative-longitudinal heat flow method (ASTM E1225-04). The water-absorbing was determined using the immersion method under water of specimens for 24 hours (ASTM D570). The microstructural aspect of porous geopolymer samples was investigated with ASONA 100X Zoom Smartphone Digital Microscope.
3. Results and Discussion
The experiment was conducted by trying four experimental versions, in which the weight proportions of the mixture components were varied.
Table 1 shows the particularities of these versions regarding their composition.
Components whose values varied in the four experimental versions were sodium perborate within the limits of 1.8-5.0 g (representing 0.5-1.4 %) and olive oil in the range of 1.5-4.2 g (representing 0.42-1.18 %). The other components of the mixture had constant values according to the data in
Table 1.
Determining the main characteristics of porous geopolymer samples (physical, heat, mechanical and microstructural) was carried out after 28 curing days. Mechanical features (compression and flexural strength) were also measured at early age (after only 7 days). Results of these determinations are shown in
Table 2.
Examining experimental results in
Table 2, the excellent correlation between the values of thermal insulation properties and their mechanical resistances (compression and flexural strength) of the porous geopolymer making in this experiment is observed.
Thus, values of apparent density and heat conductivity are relatively low (between 475-518 kg·m-3 in the case of density and in the range of 0.107-0.129 W·m-1·K-1 in the case of the thermal conductivity).
Instead, the compression and flexural strength values are high enough to meet the requirements of a material usable in building construction. The compression strength has values included in the limits of 6.89-7.42 MPa after the completion of the 28 days curing process. Also, flexural strength values (after 28 curing days) are within a fairly high range of 3.65-5.30 MPa.
The geopolymer's ability to reach high mechanical strength values (both compression and flexural) at early age in all experimental versions is remarkable. Thus, the values of resistances at early age (after 7 days) are included in the range of 4.71-5.18 MPa corresponding to the compression strength and between 2.85-3.82 MPa in the case of flexural strength.
Water-absorbing determination indicated a normal level of this parameter of porous geopolymer specimens (between 2.98-3.16 vol. %).
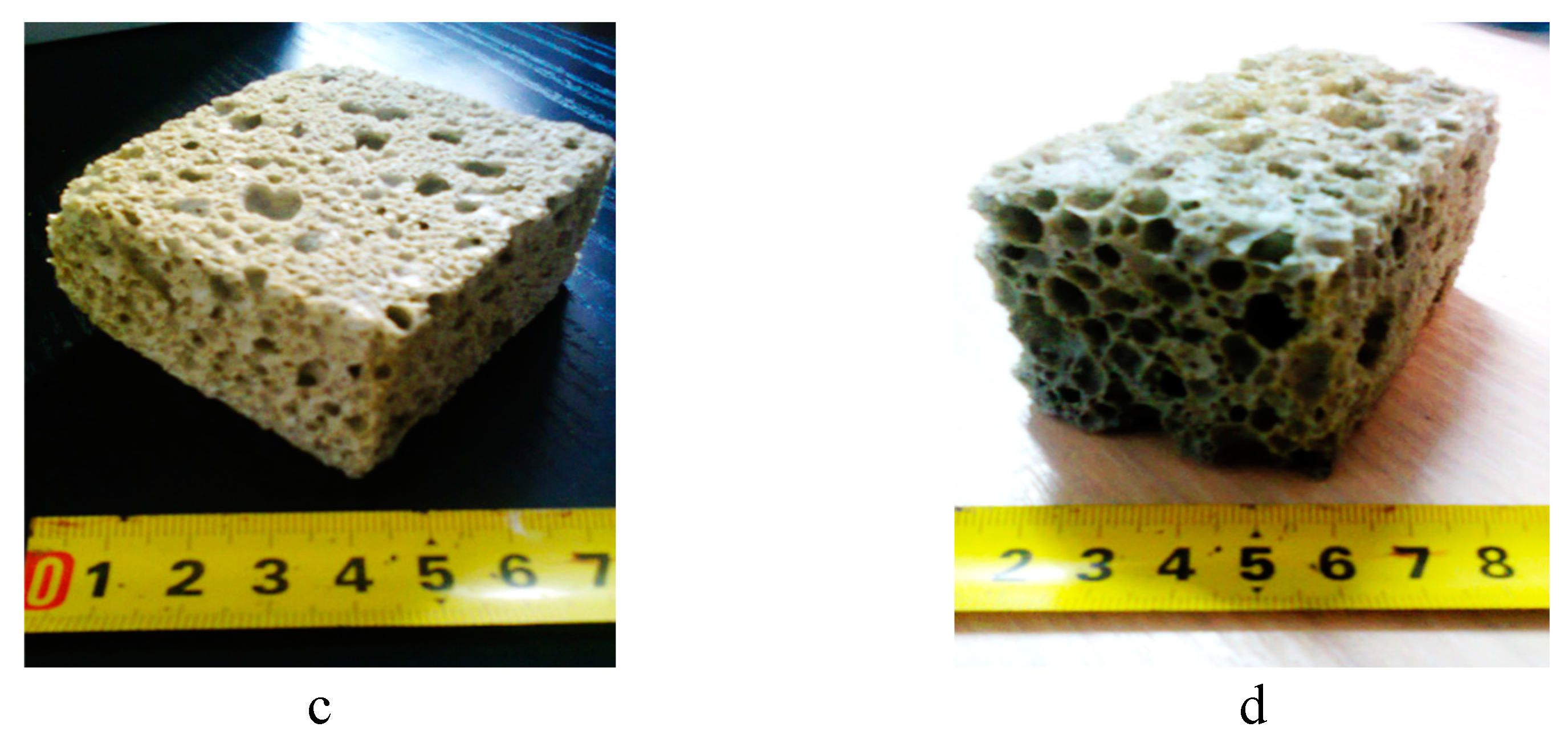
Images of porous geopolymer specimens in the form of parallelepiped pieces are presented in
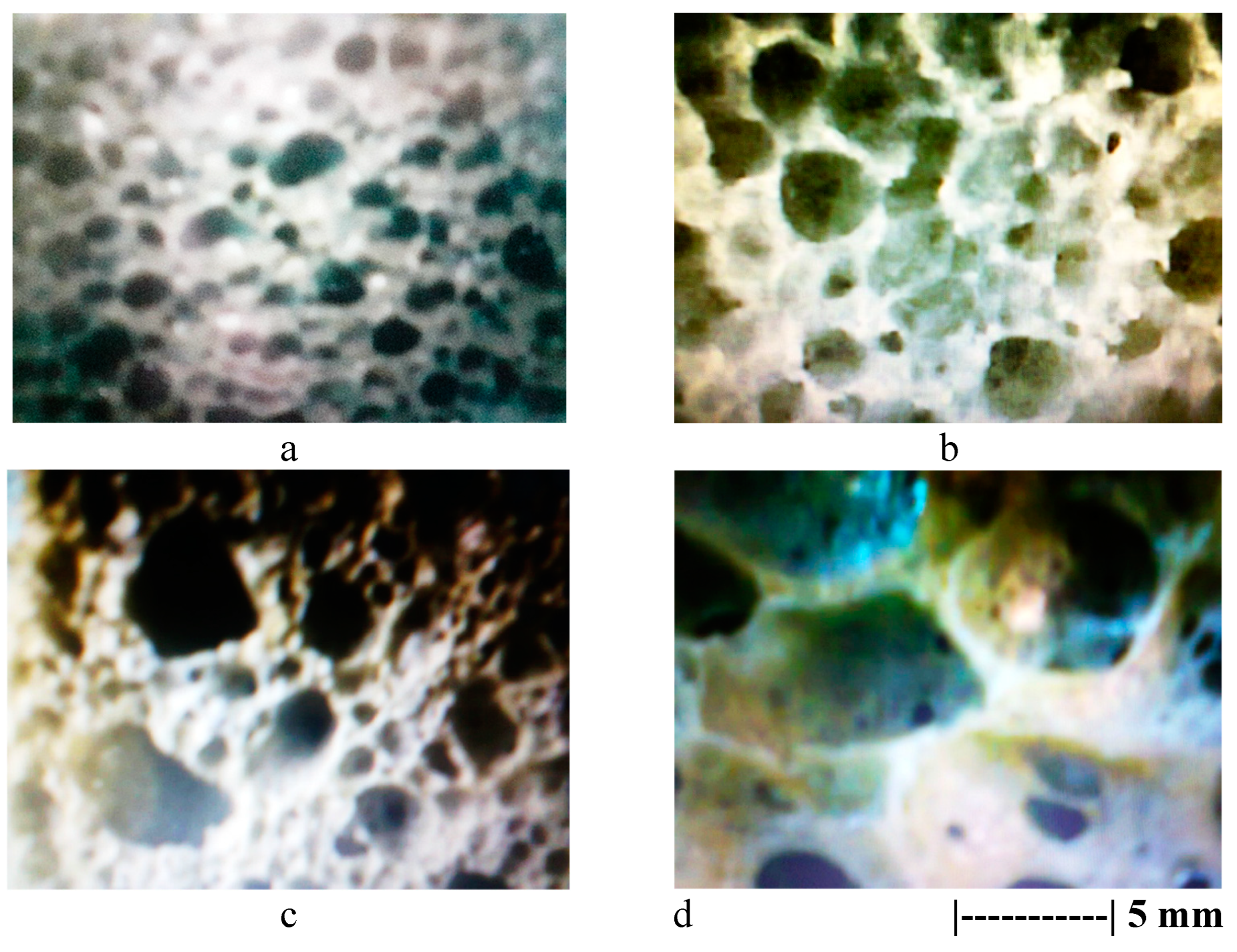
Figure 1 and the microstructural appearance of these specimens under microscope is shown in
Figure 2.
According to both the pictures in
Figure 1 and those in
Figure 2, increasing the content of expanding agent (sodium perborate) as well as of surfactant (olive oil), the porous structure of specimens expanded leading to improving the porosity and decreasing the heat transfer through the material pores. The pore size of specimens increased from the range of 0.4-1.1 mm (in version 1) to the range of 3.8-5.5 mm (in version 4).
The work aimed to create a porous fly ash-based geopolymer, in which the necessary thermal insulation properties would be in an optimal correlation with the mechanical strength features, so as to correspond to the qualitative requirements for applying in building construction.
According to the experimental results presented above, this objective was reached. Comparing the characteristics of fly ash-geopolymer specimens made with sodium perborate in this experiment with the similar features of a porous metakaolin-based geopolymer (Wattanarach et al., 2022) showed that the heat conductivity values were considerably lower than those of the metakaolin-based geopolymer, whose values were between 0.218-0.325 W·m-1·K-1 for sodium perborate proportions between 0.5-2 %.
On the other hand, the mechanical performance was also superior in the case of the porous geopolymer based on fly ash compared to that made on the basis of metakaolin. The compression strength after 28 days of the fly ash-based geopolymer varied between 6.89-7.42 MPa, being higher than compression strength of metakaolin-based porous geopolymer (5.23-6.73 MPa) in similar material curing conditions.
A similar observation resulted from determining the flexural strength after 28 days. The corresponding strength of the fly ash-based geopolymer varied in the range of 3.65-5.30 MPa compared to the range of values valid for the metakaolin-based geopolymer (3.03-5.15 MPa).
The work has used the reference (Wattanarach et al., 2022) for the comparison between results obtained in the current experiment and in the experimental production of metakaolin-based porous geopolymer.
Also, results of the experiment presented in the current paper were compared with results previously obtained (Paunescu et al., 2023) by some authors of this paper. According to this reference, a porous geopolymer was produced using as alumina-silicate precursors coal fly ash and metakaolin, the sodium perborate being the expanding agent, while bentonite clay was used as a nanomaterial due to its effect on increasing the geopolymer strength.
Results showed the heat conductivity in the range of 0.104-0.122 W·m-1·K-1, compression strength at early age between 3.9-5.2 MPa and after 28 days within the limits of 6.8-7.5 MPa. Flexural strength had values at early age in the range of 2.9-3.3 MPa and after 28 days between 3.1-3.5 MPa. The comparison between the characteristics of the two types of porous geopolymer showed many similarities, especially in the case of heat conductivity and compression strength. Flexural strength achieved in the current experiment reached higher values both after 7 days (2.85-3.82 MPa) and after 28 days of curing treatment (3.65-5.30 MPa).
4. Conclusions
The paper aimed at producing a porous geopolymer based on fly ash, obtaining better correlation between thermal insulation peculiarities and those of mechanical strength of this product usable in construction.
The manufacturing method was based on the activation in alkaline medium (KOH and Na2SiO3 solution) of type F-coal fly ash as an alumina-silicate industrial by-product, mixed with silica fume as a nanomaterial, olive oil as a surfactant and sodium perborate as an expanding agent that replaces the traditional hydrogen peroxide.
The originality of the adopted recipe consisted in the combination of less frequently used materials (such as KOH solution, olive oil, and sodium perborate), but mainly in the dosages chosen for the materials during the experiment. The geopolymer paste obtained by this mixing was first subjected to oven hardening at 70 ℃ for 24 hours and then to the curing process at ambient temperature for 7 and 28 days, respectively, before determining the characteristics of the porous geopolymer.
Results showed that the main objective of research was reached, being obtained excellent thermal insulation properties (density between 475-518 kg·m-3 and heat conductivity between 0.107-0.129 W·m-1·K-1), in an adequate correlation with the mechanical strength values (compressive and flexural) of the final product.
Also, the relatively high values of these resistances obtained at early age (after only 7 days) were remarkable.
Author Contributions
Conceptualization, A.I. and L.P.; methodology, A.S.; software, IL.C.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Majidi B., Geopolymers Technology, from Fundamentals to Advanced Applications: A Review, Mater. Technol., 24, 2, 79-87 (2009). [CrossRef]
- Chithambar Ganesh A., Muthukannan M., A Review of Recent Developments of Geopolymer Concrete, Int. J. Eng. Technol., 774, 4, 696-699 (2018). [CrossRef]
- Li X., Bai C., Qiao Y., Wang X., Yang K., Colombo P., Preparation, Properties and Applications of Fly Ash-Based Porous Geopolymers: A Review, J. Clean. Prod., Elsevier, 359, (2022). [CrossRef]
- Zhang X., Bai C., Qiao Y., Wang X., Jia D., Li H., Colombo P., Porous Geopolymer Composites: A Review, Compos. Part A: Appl. Sci. Manufact., Elsevier, 150, (2021).
- Wang L., Sun W., Research Progress of Geopolymer Porous Materials, J. Education Educ. Res., 6, 2, 136-137 (2023). [CrossRef]
- Movais R.M., Burubberi L.H., Ascensão G., Scabra M.P., Labrincha J.A., Porous Biomass Fly Ash-Based Geopolymer with Tailored Thermal Conductivity, J. Clean. Prod., Elsevier, 119, 99-107 (2016). [CrossRef]
- Jaya N.A., Liew Y.M., Heah C.Y., Mohd Mustafa Al Bakri A., Kamarudin H., Correlation between Pore Structure, Compressive Strength and Thermal Conductivity of Porous Metakaolin Geopolymer, Constr. Build. Mater., Elsevier, 247, 1-12 (2020). [CrossRef]
- Pedziwiatr P., Micotajczyk F., Zawadski D., Micotajczyk K., Bedka A., Decomposition of Hydrogen Peroxide-Kinetics and Review of Chosen Catalyst, Acta Innovations, 26, 45-62 (2018). [CrossRef]
- Bai C., Ni T., Wang Q., Li H., Colombo P., Porosity, Mechanical and Insulating Properties of Geopolymer Foam Using Vegetable Oil as the Stabilizing Agent, J. Eur. Ceram. Soc., 38, 2, 799-805 (2018). [CrossRef]
- Wattanarach S., Supothina S., Thavorniti P., Preparation and Properties of Metakaolin-Based Porous Geopolymer Formed with Sodium Perborate, J. Asian Ceram. Soc., 10, 3, 567-574 (2022). [CrossRef]
- Phavongkham V., Wattanasiriwech S., Cheng T., Wattanasiriwech D., Effects of Surfactant on Thermo-Mechanical Behavior of Geopolymer Foam Paste Made with Sodium Perborate Foaming Agent, Constr. Build. Mater., Elsevier, 243, 1-8 (2020). [CrossRef]
- Korat L., Ducman V., Characterization of Fly Ash Alkali Activated Foams Obtained Using Sodium Perborate Monohydrate as a Foaming Agent at Room and Elevated Temperatures, Front. Mater., Struct. Mater. Sect., 7, (2020). [CrossRef]
- Paunescu L., Paunescu B.V., Volceanov E., Geopolymer Foam Based on Coal Fly Ash and Metakaolin as an Economic and Environment Friendly Porous Construction Material, Rom. J. Civil Eng., Matrix Rom Publishing House, Bucharest, 14, 4, 317-329 (2023). [CrossRef]
- Furtos G., Prodan D., Sarosi C., Popa D., Moldovan M., Korniejenko K., The Precursor Used for Developing Geopolymer Composites for Circular Economy-A Review, Materials (Basel), MDPI, Koenders E. (ed.), 17, 7, (2024). [CrossRef]
- Zhuang X.Y., Chen L., Komarneni S., Zhou D.C.H., Tong S., Yang H.M., Yu W.H., Wang H., Fly Ash-Based Geopolymer: Clean Production, Properties, and Applications, J. Clean. Prod., Elsevier, 125, 253-267 (2016). [CrossRef]
- Khale D., Chaudhary R., Mechanism of Geopolymerization and Factors Influencing its Development: A Review, J. Mater. Sci., Springer Nature Link, 42, 729-746 (2007). https://link.springer.com/article/10.1007/s10853-006-0401-4.
- Lertcumfu N., Kaewapai K., Jaita P., Tunkasiri T., Sirisoonthorn S., Rujijanagul G., Effects of Olive Oil on Physical and Mechanical Properties of Ceramic Waste-Based Geopolymer Foam, J. Reinfor. Plast. Compos., 39, 3-4, (2019). [CrossRef]
- Brotherton B.J., Boron: Inorganic Chemistry, in: Encyclopedia of Inorganic Chemistry, 2nd edition, Bruce King R. (ed.), John Wiley & Sons, New Jersey, the United States, ISBN: 0-470-86078-2, (1994).
- Provis J.L., Rees C.A., Geopolymers Synthesis Kinetics, in: Geopolymers-Structures, Processing, Properties and Industrial Applications, Woodhead Publishing Series in: Civil and Structural Engineering, Provis J.L. and van Deventer J.S.J. (eds.), Sawston, Cambridge, UK, 118-136 (2009).
-
*** Metrology in Laboratory-Measurement of Mass and Derived Values, in: Radwag Balances and Scales, 2nd edition, Randon, Poland, 72-73 (2015).
- Kearsley E.P., Wainwright P.J., The Effect of Porosity on the Strength of Foamed Concrete, Cem. Concr. Res., Elsevier, 32, 2, 233-239 (2002). [CrossRef]
- Curtu I., Stanciu A.E., Determinarea Caracteristicilor Mecanice ale Epruvetelor din Material Compozit de Tip Mat&Roving (Determining Mechanical Characteristics of Mat&Roving Type Composite Material Specimens), Buletinul AGIR, Bucharest, Romania, 1, 78-81 (2011).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).