Introduction
Colorectal cancer (CRC) is the second most common cancer in Malaysia [
1]. Globally, it ranks second in terms of cancer-related mortality. Colonoscopy is the gold standard diagnostic modality for CRC screening and diagnosis of other colorectal pathologies. It also possesses a therapeutic role by detecting and removing colorectal adenomas, the precursor lesions to CRC. However, However, missed lesions remain a key limitation of colonoscopy, contributing to interval colorectal cancer development. [
2,
3,
4,
5].
Adenoma detection rate (ADR) is defined as the number of adenomas that are successfully detected by colonoscopies, thus serving as the most critical, reproducible and quantifiable intraprocedural quality indicator [
3,
4,
5]. ADR reflects the ability of an endoscopist to detect and subsequently remove precancerous adenomas, the primary precursor for CRC. It is hence regarded as a benchmark of endoscopists' performance, enabling audits and inter-endoscopists or inter-institutional comparisons. If an endoscopist has low ADR, this indicates the endoscopist's failure to detect polyps. Corley and co-workers showed that a 1% increase in the ADR reduced CRC risk and CRC-related mortality by 3% and 5%, respectively, thus establishing the importance of ADR as an indicator of successful colonoscopies [
4].
ADR, however, can be affected by various factors such as poor colonoscopy technique, suboptimal bowel preparation, failed caecal intubation, surreptitious adenoma located posterior to the proximal haustral folds, subtle flat lesions, colonic flexure adenomas, and shorter withdrawal time [
6]. Hence, the use of enhanced imaging technologies (EITs) such as high-resolution monitors, high-definition colonoscopes or distal attachment device endoscopy are essential modalities for improving ADR. Nevertheless, the widespread adoption of EITs is limited by its exorbitant costs [
7,
8]. Therefore, the relatively cheaper add-on endoscopy devices are attractive alternatives for enhancing the ADR.
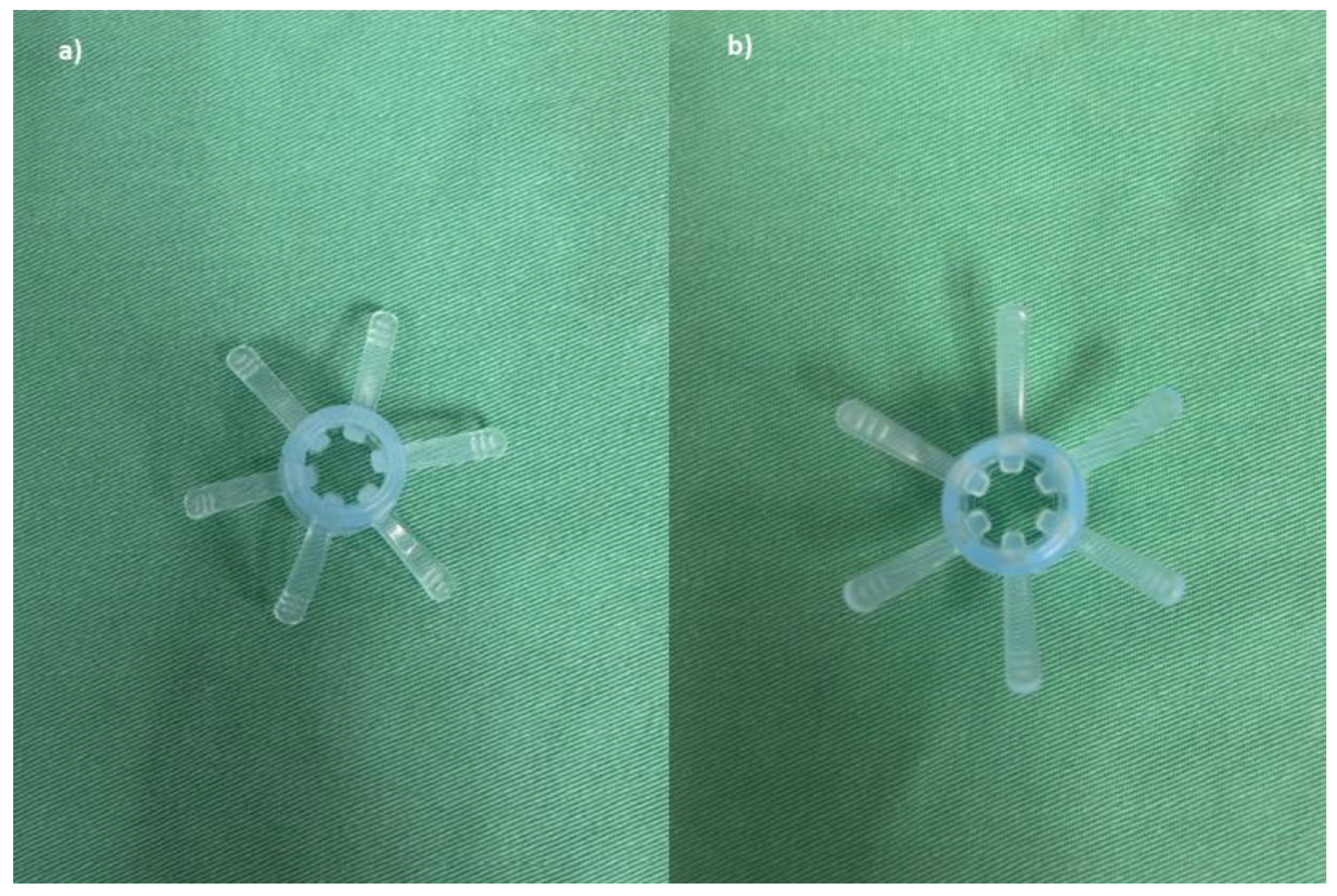
Endo-Wing™ (Shangxian) is a new, single-used distal attachment device attached at the distal end of standard colonoscopes which facilitates mucosal inspection by flattening out the mucosal folds and stabilizing the colonoscopes for better mucosal view. It cost only Ringgit Malaysia (RM) 5.00 or USD 1.10. It has the unique shape of a soft cap with six wing-like projections and is made of bendable and pliable soft silicone medical-grade material. It was design to have a single row of rounded wing-like projections, preventing mucosal abrasions observed with other distal attachment devices like the Endo-Cuff. During withdrawal, it flattens and widens the mucosal folds and field of view, enhancing lesion detection and removal. (
Figure 1,
Figure 2 and
Figure 3). Nevertheless, no study has yet compared the ADR between Endo-Wing™-assisted colonoscopy and standard colonoscopy.
Hence, we aimed to compare the ADRs between Endo-Wing™-assisted colonoscopy and standard colonoscopy. Additionally, we also assessed the caecal intubation rate, differences in polyp distribution between the proximal and distal colon, variations in the number and size of adenomas detected by both modalities, as well as differences in total sedation use and adverse event rates.
Methods
Study Design & Patient Recruitment
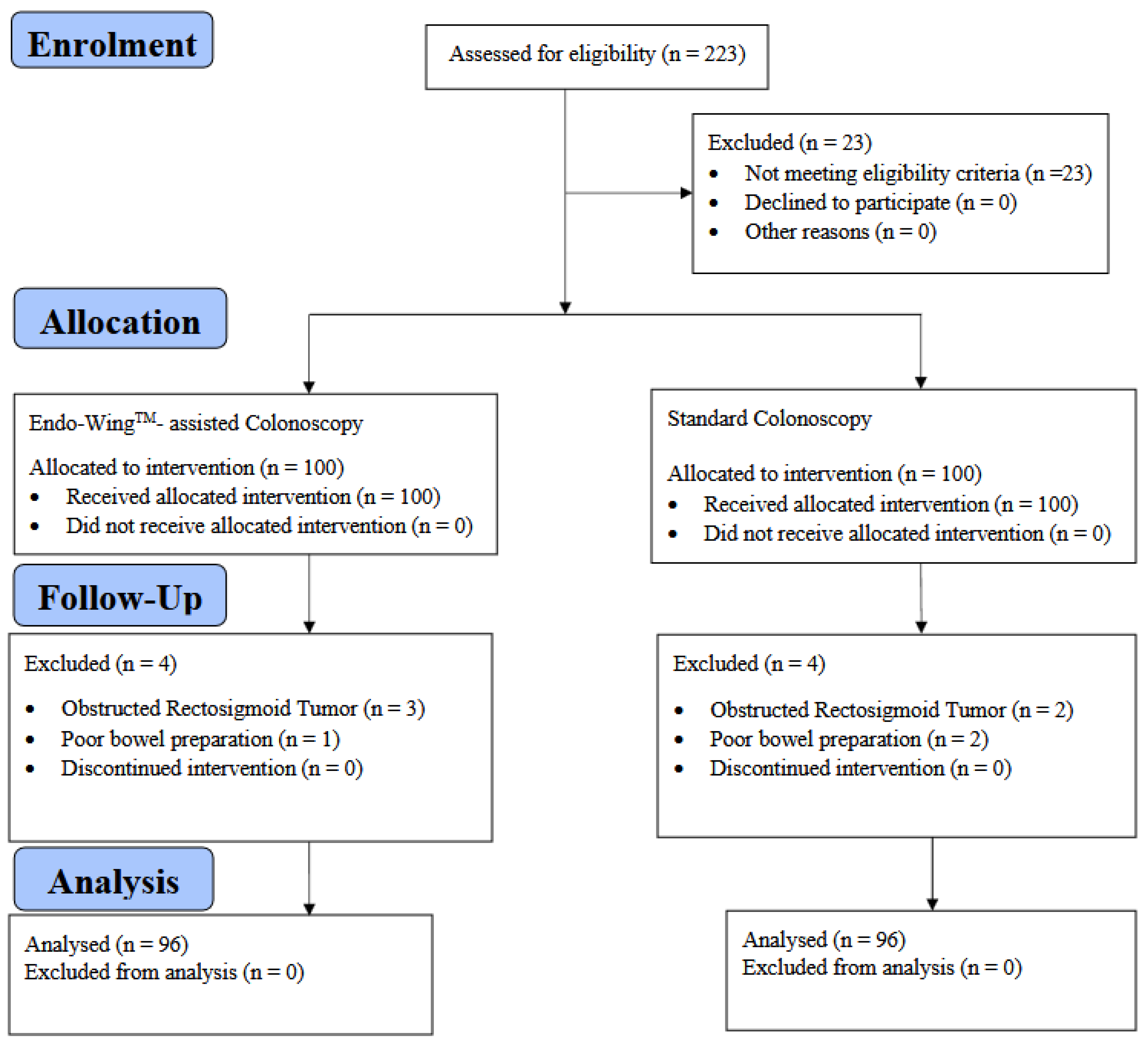
This is a Phase II, single-center, open-label, parallel-group, active-controlled, randomized, exploratory clinical trial conducted at Hospital Canselor Tuanku Muhriz, Faculty of Medicine, the National University of Malaysia (UKM), from July 2019 until April 2020. This study was approved by the National University of Malaysia Ethics Board Committee (Ethics ID: FF-2019-355; Date:30th August 2019) and adhered to the Declaration of Helsinki and Good Clinical Practice (GCP). The reporting of this article adhered to the CONSORT guideline to ensure reporting transparency. This study was funded by the National University of Malaysia from the Medical Faculty Fundamental Research Grant (Grant number: FF-2019-355) and registered in the ClinicalTrials.gov registry (Registration ID: NCT06859125).
Eligibility Criteria
Participants with symptoms indicative of colorectal cancer, such as chronic constipation, per rectal bleeding, and altered bowel habits and those with a prior history of adenoma and under repeated surveillance were included in this study whilst those with obstructed colonic tumors, colonic strictures, active colitis, underwent previous colonic surgery for benign and malignant conditions, had known polyposis syndrome, had colostomy/ileostomy, and poor bowel preparation defined as Boston Bowel Preparation Scale (BBPS) score of 0 were excluded.
Colonoscopy was performed by accredited surgeons and postgraduate surgical trainees who have completed their compulsory endoscopic accreditation training. All patients voluntarily consented to study participation and have signed informed consent. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP).
Sample Size Calculation
The sample size was calculated using PS Software Version 3.1.4.2 (Dupont and Plummer, 2018) based on the chi-square procedure for independent design. The power (1-β) and type I error (α) were fixed at 80% and 0.05 (two-sided), respectively. The dropout rate was chosen as 5% and the control-to-case ratio was fixed at 1:1.
For the values of other parameters required for sample size calculation, a recent study employing colonoscopy adjuncts for visualization enhancement was found to have an adenoma detection rate (ADR) of 45.1% [
9]. We hypothesized that the standard colonoscopy has an ADR of 28.8%, with a hypothesized difference of 20. Using the Pearson chi-square procedure, the calculated sample size was found to be 89 patients per colonoscopy arm (n
total = 178). After considering a 5% drop-out rate, the final sample size is 94 patients per group (n
total = 188 participants).
Randomization, Masking and Colonoscopy Procedures
On the day of colonoscopy, patients arrived in the endoscopy suite and were evaluated by medical officers for indication and suitability for eligibility. Patients who were eligible and consented were randomly allocated through central block randomization of varying block sizes using randomizer package version 4.1 into either of 2 groups: a standard colonoscopy or Endo-WingTM colonoscopy. The participants were blinded to treatment assignment, whilst the endoscopists were only masked to the procedural assignment until the commencement of colonoscopy. Other healthcare givers and statistician analyzing the data were not masked to the procedural allotment.
Three liters of polyethylene glycol electrolyte solution (Fortrans
TM, Ipsen Pharma) were prescribed as bowel preparation prior to colonoscopy to ensure adequate bowel preparation. Colonoscopy was then performed in left lateral position and under monitored sedation according to standard protocol (IV Midazolam 3 mg and IV Pethidine 25 mg) and if the endoscopists deemed the patient was in discomfort or pain, then an additional doses of IV Midazolam 2 mg and IV Pethidine 25 mg were administered. Blood pressure, heart rate and oxygen saturation were monitored throughout the procedure. Bowel preparation quality was assessed using the BBPS system during colonoscopy [
10,
11] by the performing endoscopists.
All colonoscopies were performed using Olympus Evis Exera III 90 series (Evis Exera III 90 series, Olympus) or Olympus Evis Lucera 260 series (Evis Lucera, Olympus) colonoscopes. If a participant was allotted to the Endo-Wing™ group, Endo-Wing™ would be attached to the distal tip of the scope, as per the manufacturer's recommendations. In both groups, the advancement of colonoscopy was done according to the standard insertion technique. Once the caecum was intubated, the minimum time for withdrawal and examination of the colonic mucosa was set at 6 minutes.
Trial Endpoints
The primary outcome of this study is the ADR, which was defined as the proportion of colonoscopies with at least one adenoma detected to the total colonoscopies in that group. Secondary outcomes were the distribution of adenomas by location, number of adenomas detected, size of adenomas, caecal intubation rate, the total amount of sedation used, and adverse effects. Any identified adenomas/polyps were subsequently noted for their size (classified into three groups: 1-5 mm, 6-9 mm and more than 10 mm), location proximal (caecum until transverse colon) or distal (descending colon until rectum)) and number (group into three categories: 1-2, 3-5 and more than 5 adenomas).
Post-Colonoscopy Procedure Follow-Up and Monitoring for Adverse Events
Patients were reviewed in the endoscopy suite post-colonoscopy for any immediate adverse events (bleeding, perforation) and monitored for at least 1 hour at a dedicated recovery bay in the endoscopy suite. Vital signs and oxygen saturation were monitored every 15 minutes. Prior to discharge, the medical officer in-charge examined the abdomen for signs of tenderness. Once fully conscious, patients were discharged home accompanied by their respective family members or caretakers. All patients were followed up with a telephone call 24 hours and one week after for further information on late adverse events.
Statistical Analysis
All analyses were performed using IBM SPSS Version 25 (Armonk, NY, IBM Corps, 2017). All variables were summarized with mean and standard deviation for continuous variables and frequency and percentage for categorical variables. The normality of continuous variables was evaluated using the Shapiro-Wilks test, Fisher’s coefficient of skewness (normality threshold: ±1.96 for small-to-moderate sized sample) and Q-Q plot. No multiple imputation was carried out since all variables were completely observed.
Comparison of categorical variables, adenoma detection rate and adenoma characteristics were analyzed using the exact version of the Pearson chi-squared test (Patel-Mehta algorithm), whilst for continuous variables, the non-parametric Mann-Whitney test was employed. A P-value of 0.05 (two-sided) was used as the significance threshold and the estimates of ADR in both groups were reported with their 95% confidence intervals.
Discussion
ADR is an important key indicator for quality colonoscopy and efforts must be geared towards maintaining and enhancing ADR. The development of new technologies, including distal attachment devices, is a solution to the challenges of improving ADR during colonoscopy [
12]. Experienced endoscopists achieve an ADR as high as 25% [
3,
4,
13]. In this study, we noted that there is a significantly higher ADR in the Endo-Wing
TM group compared to standard colonoscopy. With ADR at 49%, it surpassed our expectation and making Endo-Wing
TM an enticing distal attachment device to enhance ADR.
Our result also demonstrated that more small sized adenomas were detected using this distal attachment device. We postulate that Endo-Wing
TM enhances visualization of the colonic folds by flattening them during the withdrawal phase of colonoscopy. The effect of six wing-like projections provided by Endo-Wing
TM, which fans out during withdrawal, can provide the endoscopist with a superior view. Moreover, Endo-Wing
TM keeps the view centered, focusing on the colonic lumen whilst maintaining the colonoscope tip distance to the colon mucosa. These wing-like projections have the ability to augment the ADR, as observed by Manti and co-workers [
8]. This distal attachment device did not interfere with the colonoscope's usual functions, such as water suctioning, washing and performing diagnostic and therapeutic interventions such as polypectomy and biopsy of colorectal tissues.
Regarding safety, no adverse events, such as iatrogenic colonic perforation, tear or bleeding, were observed during the study. We have followed up with all our patients 24 hours as well as one week after colonoscopy through telephone calls, asking about their bowel function, pain, and discomfort. The results were encouraging since the patients did not report any additional adverse events. Thus, our study suggests that Endo-WingTM is a safe and reliable distal attachment device that does not cause any short-term complications.
It is also worth noting that several endoscopists performing the colonoscopic procedures were postgraduate surgical trainees. However, all of them have been accredited by the hospital to perform colonoscopies after undergoing a structured accredited training program and shadowing. Nonetheless, they were still considered less experienced than the specialist surgeons who have years of practice. Therefore, Endo-WingTM is an enticing distal attachment device that seems to be an invaluable adjunct for improving ADR among the less experienced surgical trainees performing colonoscopies.
Endo-Wing
TM provides an attractive option for increasing the ADR as it is a cheaper alternative compared to a more expensive apparatus such as a narrow-band imaging modality or an artificial-intelligence-assisted colonoscopy device. Manti et al, in their systematic review and meta-analysis highlighted that ADRs were comparable with lower cost measures such as water-aided colonoscopy and other distal attachment devices [
8]. This would certainly benefit health centers that have limited budgetary resources and cannot afford to procure a state-of-the-art colonoscopy system with a narrow-band imaging function. As mentioned earlier, there are other distal attachment devices in the market with structural variations, such as EndoCuff and EndoRings. Other technologies using retrograde flexion, such as Third Eye Retroscope colonoscopy, FUSE Endoscopy and Extra-Wide-angle Colonoscopy, are also available, but these are significantly costlier [
14].
In contrast, other studies using distal attachment devices to increase the ADR have shown varying results [
15,
16,
17]. Biecker et al. observed that the ADR was significantly increased with Endocuff compared to standard colonoscopy [
18]. On the contrary, another RCT from the Netherlands showed mixed results, and ADR did not differ significantly between Endocuff and standard colonoscopy [
19]. EndoRing, a structurally similar device to Endo-Wing™, showed a significant improvement in ADR compared to standard colonoscopy [
20]. Our results are thus consistent with previous studies in terms of ADR improvement facilitated by the use of distal attachment colonoscopy devices.
In this study, we detected more patients with adenomas of more than 1cm in size as well as smaller adenomas that are smaller than 5 mm in the Endo-Wing
TM than in the standard colonoscopy group. The Endo-Wing™ group also showed a significantly higher percentage of detected single or double adenomas than the standard colonoscopy group. We hypothesize that Endo-Wing™'s superior visualization is the reason for its enhanced ability to detect the readily missed smaller and single adenomas. In the National Polyp Study, increasing adenoma size in those with multiple adenomas was associated with an increased percentage of at least one adenoma with high-grade dysplasia [
21]. The adenoma size, especially more than 1 cm, is associated with higher grades of dysplasia and a greater risk of adenocarcinoma progress. The number of adenomas detected is also important, as each adenoma has the potential to become dysplastic and progress to malignant transformation. Besides, interval colorectal cancer incidence is inversely proportional to the number of adenomas detected at colonoscopy [
22]. Kaminski et al. have shown that endoscopists with an ADR of less than 11% have a higher chance of missing high-grade adenomas and, therefore, contributing to interval colorectal cancer [
5]. Therefore, we believe that Endo-Wing
TM can provide the solution to this challenge.
In addition, we also observed that more adenomas were detected on the right side of the colon in the Endo-Wing
TM group, with almost more than half of adenomas detected in the caecum, ascending colon and proximal transverse colon. Based on the literature, right-sided colon adenomas with a size of more than 5mm tend to be high-risk adenomas compared to left-sided colon adenomas of similar sizes. A right-sided colonic adenoma is frequently missed, 70% histologically more aggressive and has higher propensity to become cancerous [
22,
23]. With increasing adenoma size, the malignant transformation rate showed a right-sided shift with a significant interaction between adenoma size and right-sided location [
24]. Moreover, they also tend to have poorer histological grades, thus further predisposing them to adenocarcinomatous progression [
25]. Again, Endo-Wing
TM proves to be an effective tool to enhance ADR, especially for adenoma detection in the right colon.
We also found that the caecal intubation rate was slightly, albeit insignificantly, better in the Endo-Wing™ group (99.0% vs 95.8%). Four patients in the standard colonoscopy group experienced failed caecal intubation due to excessive looping; in three cases, the colonoscope could not advance beyond the hepatic flexure, while in the remaining case, it reached only the proximal transverse colon. We thus assume that the use of Endo-Wing™ facilitated caecal intubation by helping to centre the scope within the colonic lumen, thereby improving visibility, particularly in darker areas, and enhancing the passage and maneuverability of the colonoscope.
In contrast, there is a slightly higher percentage of patients requiring added sedation in the Endo-Wing™ group. The result, however, is not statistically significant. Those affected patients experienced more discomfort rather than pain, which necessitates an additional dose of intravenous Midazolam and intravenous Pethidine at the discretion of the endoscopists. This discomfort was temporary and resolved completely after the completion of the colonoscopy.
Considering that this device is safe, low-cost and easy to use, we can conclude that the Endo-WingTM distal attachment device is a suitable adjunct for colonoscopic screening of CRC and suitable for use in follow-up colonoscopy in patients with previously diagnosed adenomas, especially the right-sided adenomas. Moreover, Endo-WingTM is also suitable for use in less experienced endoscopists and in a resource-limited setting.
Study Limitations
Our findings must be interpreted within the context of several limitations. First, this is a single-center, randomized control trial that recruits participants around the Klang Valley area, thus limiting the generalizability of our findings. In addition, despite the inclusion of sufficient participants, our sample size may still be inadequate to achieve the minimum statistical power to draw up definitive conclusions by statistically controlling the confounders (e.g. the endoscopists' years of experience, the level of expertise- surgical trainee vs surgeon specialists, elective versus emergency colonoscopy etc. that have significant influence on the trial outcomes. Hence, to establish more robust evidence, future research should be conducted in a multi-center fashion and recruit a larger number of participants, which will improve the study power and enable the adjustments of confounders using multivariable statistical methods.
Second, our study was not designed to analyze missed adenoma rates since it did not employ a tandem colonoscopy design. This design would have provided a more accurate assessment of Endo-Wing™'s effectiveness by enabling per-lesion analysis. Third, our study has included patients who have undergone colonic resections, resulting in the reduction of bowel length that might lead to diminished chances for adenomas being detected. Moreover, performing colonoscopies in these patients was more challenging due to bowel adhesions, contributing to the looping problem, which would potentially lessen the caecal intubation rate and increase sedation requirements. Nevertheless, our study showed low rates of failed caecal intubation and added sedation requirements in both colonoscopic groups.
Finally, no histological analysis of the identified adenomas was performed. A better clinical correlation between the identified adenoma's size, numbers, gross morphological features and histological grade could have made our results more impactful in terms of the effectiveness of different colonoscopic modalities in detecting high-grade adenomas. Therefore, a future trial that adequately addresses our study limitations will further verify our findings.