Submitted:
08 March 2025
Posted:
10 March 2025
You are already at the latest version
Abstract
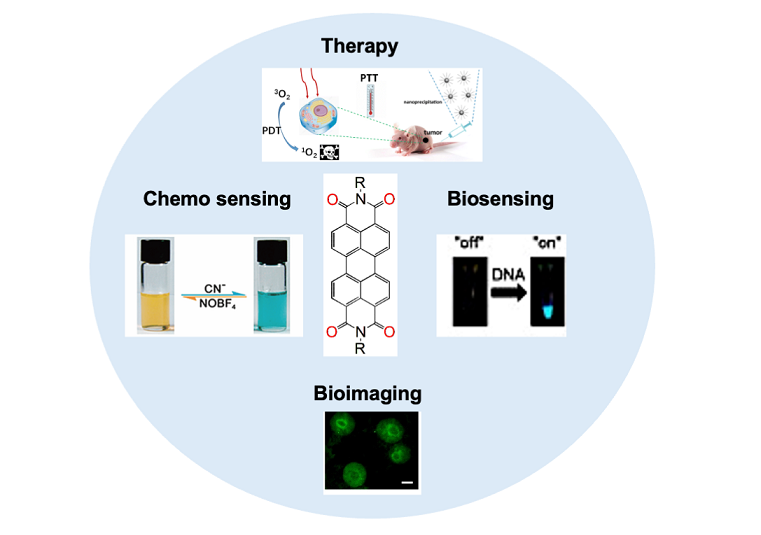
Keywords:
1. Introduction
2. General Synthetic Routes
2.1. Traditional Synthesis Methods
2.2. Room Temperature Synthesis
2.3. Green Synthesis Approaches
2.4. Transition Metal-Catalyzed Methods
3. Photophysics of PDIs
4. Synthesize of Water Soluble PDI Derivatives
4.1. Substituting with Anionic Groups
4.2. Substituting with Cationic Groups
4.3. PDI Derivatives with Non-Ionic Substituents
5. Biomedical Application of PDI
5.1. Biosensing Applications
5.2. Bioimaging Applications
5.2.1. PDI Molecules
5.2.2. PDI Based Nanoparticles
5.2.3. Photoacoustic Based Imaging (PAI)
5.3. PDI NPs for Cellular pH Measurements
5.4. PDI for Drug Delivery and Cancer Therapy
6. Conclusions
7. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Xue, Z.; Gao, N.; Yang, X.; Zang, L. Perylene Diimide-Based Fluorescent and Colorimetric Sensors for Environmental Detection. Sensors 2020, 20, 917. [Google Scholar] [CrossRef] [PubMed]
- Krupka, O.; Hudhomme, P. Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 6308. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, M.; Zhu, L.; Yang, X.; Zang, L. Architectures and Mechanisms of Perylene Diimide-Based Optical Chemosensors for pH Probing. Chemosensors 2023, 11, 293. [Google Scholar] [CrossRef]
- Soh, N.; Ueda, T. Perylene Bisimide as a Versatile Fluorescent Tool for Environmental and Biological Analysis: A Review. Talanta 2011, 85, 1233–1237. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Z.; Yin, M. Perylenediimide-Cored Dendrimers and Their Bioimaging and Gene Delivery Applications. Prog. Polym. Sci. 2015, 46, 25–54. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, N.; Wang, Y.; Ling, G.; Zhang, P. Perylene Diimide-Based Treatment and Diagnosis of Diseases. J. Mater. Chem. B 2021, 9, 8937–8950. [Google Scholar] [CrossRef]
- Sun, M.; Müllen, K.; Yin, M. Water-Soluble Perylenediimides: Design Concepts and Biological Applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Li, D.; Liu, D.; Jiang, W.; Han, C.; Shi, Z. Water-soluble 3,4:9,10-perylene Tetracarboxylic Ammonium as a High-performance Fluorochrome for Living Cells Staining. Luminescence 2009, 24, 140–143. [Google Scholar] [CrossRef]
- Zhou, W.; He, D.D.; Zhang, K.; Liu, N.; Li, Y.; Han, W.; Zhou, W.; Li, M.; Zhang, S.; Huang, H.; et al. A Perylene Diimide Probe for NIR-II Fluorescence Imaging Guided Photothermal and Type I/Type II Photodynamic Synergistic Therapy. Biosens. Bioelectron. 2024, 259, 116424. [Google Scholar] [CrossRef]
- Özçil, F.; Yükrük, F. Evaluation of Singlet Oxygen Generators of Novel Water-Soluble Perylene Diimide Photosensitizers. RSC Adv. 2023, 13, 15416–15420. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Q. Recent Advances in Perylene Diimides (PDI)-based Small Molecules Used for Emission and Photothermal Conversion. Chem.Photo.Chem. 2024, 8, e202300213. [Google Scholar] [CrossRef]
- Cheng, H.; Qu, B.; Qian, C.; Xu, M.; Zhang, R. Synthesis, Fluorescence Property and Cell Imaging of a Perylene Diimide-Based NIR Fluorescent Probe for Hypochlorite with Dual-Emission Fluorescence Responses. Mater. Adv. 2020, 1, 814–819. [Google Scholar] [CrossRef]
- Görl, D.; Zhang, X.; Würthner, F. Molecular Assemblies of Perylene Bisimide Dyes in Water. Angew. Chem. Int. Ed. 2012, 51, 6328–6348. [Google Scholar] [CrossRef]
- Laine, R.F.; Sinnige, T.; Ma, K.Y.; Haack, A.J.; Poudel, C.; Gaida, P.; Curry, N.; Perni, M.; Nollen, E.A.A.; Dobson, C.M.; et al. Fast Fluorescence Lifetime Imaging Reveals the Aggregation Processes of α-Synuclein and Polyglutamine in Aging Caenorhabditis Elegans. ACS Chem. Biol. 2019, 14, 1628–1636. [Google Scholar] [CrossRef]
- Su, P.; Ran, G.; Wang, H.; Yue, J.; Kong, Q.; Bo, Z.; Zhang, W. Intramolecular and Intermolecular Interaction Switching in the Aggregates of Perylene Diimide Trimer: Effect of Hydrophobicity. Molecules 2023, 28, 3003. [Google Scholar] [CrossRef]
- Cho, J.; Keum, C.; Lee, S.-G.; Lee, S.-Y. Aggregation-Driven Fluorescence Quenching of Imidazole-Functionalized Perylene Diimide for Urea Sensing. Analyst 2020, 145, 7312–7319. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, K.; Wang, Y.; Ma, L. Perylene Imide Derivatives: Structural Modification of Imide Position, Aggregation Caused Quenching Mechanism, Light-Conversion Quality and Photostability. Dyes and Pigments 2023, 210, 110948. [Google Scholar] [CrossRef]
- Wu, J.; Peng, M.; Mu, M.; Li, J.; Yin, M. Perylene Diimide Supramolecular Aggregates: Constructions and Sensing Applications. Supramol. Mater. 2023, 2, 100031. [Google Scholar] [CrossRef]
- Rostami-Tapeh-Esmail, E.; Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Perylene-3,4,9,10-Tetracarboxylic Diimide and Its Derivatives: Synthesis, Properties and Bioapplications. Dyes and Pigments 2020, 180, 108488. [Google Scholar] [CrossRef]
- Nowak-Król, A.; Würthner, F. Progress in the Synthesis of Perylene Bisimide Dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Kardos, M. Über Einige Aceanthrenchinon- Und 1.9-Anthracen-Derivate. Ber. Dtsch. Chem. Ges. 1913, 46, 2086–2091. [Google Scholar] [CrossRef]
- Langhals, H. Cyclic Carboxylic Imide Structures as Structure Elements of High Stability. Novel Developments in Perylene Dye Chemistry. HETEROCYCLES 1995, 40, 477. [Google Scholar] [CrossRef]
- Langhals, H. Synthese von Hochreinen Perylen-Fluoreszenzfarbstoffen in Großen Mengen – Gezielte Darstellung von Atrop-Isomeren. Chem. Ber. 1985, 118, 4641–4645. [Google Scholar] [CrossRef]
- Würthner, F. Perylene Bisimide Dyes as Versatile Building Blocks for Functional Supramolecular Architectures. Chem. Commun. 2004, 1564–1579. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K.; Ortiz, J.; Gutiérrez, A.M.; Fernández-Lázaro, F.; Sastre-Santos, Á. Formation of a Long-Lived Charge-Separated State of a Zinc Phthalocyanine-Perylenediimide Dyad by Complexation with Magnesium Ion. Chem. Commun. 2005, 3814. [Google Scholar] [CrossRef]
- Nagao, Y. Synthesis and Properties of Perylene Pigments. Prog. Org. Coat. 1997, 31, 43–49. [Google Scholar] [CrossRef]
- Wicklein, A.; Kohn, P.; Ghazaryan, L.; Thurn-Albrecht, T.; Thelakkat, M. Synthesis and Structure Elucidation of Discotic Liquid Crystalline Perylene Imide Benzimidazole. Chem. Commun. 2010, 46, 2328. [Google Scholar] [CrossRef]
- Iverson, I.K.; Tam-Chang, S.-W. Cascade of Molecular Order by Sequential Self-Organization, Induced Orientation, and Order Transfer Processes. J. Am. Chem. Soc. 1999, 121, 5801–5802. [Google Scholar] [CrossRef]
- Sommer, M.; Lindner, S.M.; Thelakkat, M. Microphase-Separated Donor–Acceptor Diblock Copolymers: Influence of HOMO Energy Levels and Morphology on Polymer Solar Cells. Adv. Funct. Mater. 2007, 17, 1493–1500. [Google Scholar] [CrossRef]
- Sommer, M.; Lang, A.S.; Thelakkat, M. Crystalline–Crystalline Donor–Acceptor Block Copolymers. Angew. Chem. Int. Ed. 2008, 47, 7901–7904. [Google Scholar] [CrossRef]
- Lindner, S.M.; Hüttner, S.; Chiche, A.; Thelakkat, M.; Krausch, G. Charge Separation at Self-Assembled Nanostructured Bulk Interface in Block Copolymers. Angew. Chem. Int. Ed. 2006, 45, 3364–3368. [Google Scholar] [CrossRef]
- Kwakernaak, M.C.; Koel, M.; Van Den Berg, P.J.L.; Kelder, E.M.; Jager, W.F. Room Temperature Synthesis of Perylene Diimides Facilitated by High Amic Acid Solubility. Org. Chem. Front. 2022, 9, 1090–1108. [Google Scholar] [CrossRef] [PubMed]
- Kelber, J.; Bock, H.; Thiebaut, O.; Grelet, E.; Langhals, H. Room-Temperature Columnar Liquid-Crystalline Perylene Imido-Diesters by a Homogeneous One-Pot Imidification–Esterification of Perylene-3,4,9,10-tetracarboxylic Dianhydride. Eur. J. Org. Chem. 2011, 2011, 707–712. [Google Scholar] [CrossRef]
- Dubey, R.K.; Westerveld, N.; Grozema, F.C.; Sudhölter, E.J.R.; Jager, W.F. Facile Synthesis of Pure 1,6,7,12-Tetrachloroperylene-3,4,9,10-Tetracarboxy Bisanhydride and Bisimide. Org. Lett. 2015, 17, 1882–1885. [Google Scholar] [CrossRef]
- Baumgartner, B.; Svirkova, A.; Bintinger, J.; Hametner, C.; Marchetti-Deschmann, M.; Unterlass, M.M. Green and Highly Efficient Synthesis of Perylene and Naphthalene Bisimides in Nothing but Water. Chem. Commun. 2017, 53, 1229–1232. [Google Scholar] [CrossRef]
- Cao, Q.; Crawford, D.E.; Shi, C.; James, S.L. Greener Dye Synthesis: Continuous, Solvent-Free Synthesis of Commodity Perylene Diimides by Twin-Screw Extrusion. Angew. Chem. Int. Ed. 2020, 59, 4478–4483. [Google Scholar] [CrossRef]
- Moura, H.M.; Peterlik, H.; Unterlass, M.M. Green Hydrothermal Synthesis Yields Perylenebisimide–SiO2 Hybrid Materials with Solution-like Fluorescence and Photoredox Activity. J. Mater. Chem. A 2022, 10, 12817–12831. [Google Scholar] [CrossRef]
- Wang, H.Z.; Ning, L.G.; Lv, W.Y.; Xiao, L.; Li, C.M.; Lu, Z.S.; Wang, B.; Xu, L.Q. Green Synthesis of Perylene Diimide-Based Nanodots for Carbon Dioxide Sensing, Antibacterial Activity Prediction and Bacterial Discrimination. Dyes and Pigments 2020, 176, 108245. [Google Scholar] [CrossRef]
- Aivali, S.; Tsimpouki, L.; Anastasopoulos, C.; Kallitsis, J.K. Synthesis and Optoelectronic Characterization of Perylene Diimide-Quinoline Based Small Molecules. Molecules 2019, 24, 4406. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, S.; Hwang, D.K.; Barlow, S.; Kippelen, B.; Marder, S.R. 2-Bromo Perylene Diimide: Synthesis Using C–H Activation and Use in the Synthesis of Bis(Perylene Diimide)–Donor Electron-Transport Materials. J. Mater. Chem. C 2013, 1, 5093. [Google Scholar] [CrossRef]
- Ganesamoorthy, R.; Vijayaraghavan, R.; Ramki, K.; Sakthivel, P. Synthesis, Characterization of Bay-Substituted Perylene Diimide Based D-A-D Type Small Molecules and Their Applications as a Non-Fullerene Electron Acceptor in Polymer Solar Cells. J. Sci.: Adv. Mater. Devices. 2018, 3, 99–106. [Google Scholar] [CrossRef]
- Siddiqui, A.; Thawarkar, S.; Singh, S.P. A Novel Perylenediimide Molecule: Synthesis, Structural Property Relationship and Nanoarchitectonics. J. Solid State Chem. 2022, 306, 122687. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, S.; Sun, X.; Liu, Y.; Zhu, D. Suzuki Coupling Reaction of 1,6,7,12-Tetrabromoperylene Bisimide. Org. Lett. 2006, 8, 867–870. [Google Scholar] [CrossRef] [PubMed]
- El-Berjawi, R.; Hudhomme, P. Synthesis of a Perylenediimide-Fullerene C60 Dyad: A Simple Use of a Nitro Leaving Group for a Suzuki-Miyaura Coupling Reaction. Dyes and Pigments 2018, 159, 551–556. [Google Scholar] [CrossRef]
- Li, A.; Zhang, X.; Wang, S.; Wei, K.; Du, P. Synthesis and Physical Properties of a Perylene Diimide-Embedded Chiral Conjugated Macrocycle. Org. Lett. 2023, 25, 1183–1187. [Google Scholar] [CrossRef]
- Kuklin, S.A.; Safronov, S.V.; Khakina, E.A.; Buyanovskaya, A.G.; Frolova, L.A.; Troshin, P.A. New Perylene Diimide Electron Acceptors for Organic Electronics: Synthesis, Optoelectronic Properties and Performance in Perovskite Solar Cells. Mendeleev Communications 2023, 33, 314–317. [Google Scholar] [CrossRef]
- Huo, L.; Zhou, Y.; Li, Y. Synthesis and Absorption Spectra of n-Type Conjugated Polymers Based on Perylene Diimide. Macromol. Rapid Commun. 2008, 29, 1444–1448. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, W.; Wang, Z. Synthesis and Application of Rylene Imide Dyes as Organic Semiconducting Materials. Chem. Asian J. 2018, 13, 20–30. [Google Scholar] [CrossRef]
- Wang, L.-H.; Liu, L.-L.; Liu, H.; Chen, Y.; Ye, D.-N.; Fu, W.; Liu, S.-Y. Diketopyrrolopyrrole and Perylene Diimine-Based Large π-Molecules Constructed via C–H Direct Arylation. Dyes and Pigments 2022, 204, 110468. [Google Scholar] [CrossRef]
- Hoffman, E.; Kozakiewicz, K.; Rybczyńska, M.; Mońka, M.; Grzywacz, D.; Liberek, B.; Bojarski, P.; Serdiuk, I.E. Photochemical Transformation of a Perylene Diimide Derivative Beneficial for the in Situ Formation of a Molecular Photocatalyst of the Hydrogen Evolution Reaction. J. Mater. Chem. A 2024, 12, 5233–5243. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Chi, Y.; Yu, H.; Li, Y.; Jiang, T.; Wei, X.; Shi, J. Self-Assembly, Optical and Electrical Properties of Perylene Diimide Dyes Bearing Unsymmetrical Substituents at Bay Position. Sci. Rep. 2018, 8, 8208. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, C.; Schuck, P.J.; Kaufman, L.J. Aggregation Pathway Complexity in a Simple Perylene Diimide. Sci Rep 2024, 14, 31989. [Google Scholar] [CrossRef] [PubMed]
- Almuhana, A.R.Y.; Langer, P.; Griffin, S.L.; Lodge, R.W.; Rance, G.A.; Champness, N.R. Retention of Perylene Diimide Optical Properties in Solid-State Materials through Tethering to Nanodiamonds. J. Mater. Chem. C 2021, 9, 10317–10323. [Google Scholar] [CrossRef]
- He, J.; Li, S.; Zeng, H. The Photostability of Two Optical Materials Based on Perylene Diimide Substituted by Different Aromatic Groups at the Bay Area. J. Heterocyclic Chem. 2017, 54, 2800–2807. [Google Scholar] [CrossRef]
- Asir, S.; Zanardi, C.; Seeber, R.; Icil, H. A Novel Unsymmetrically Substituted Chiral Amphiphilic Perylene Diimide: Synthesis, Photophysical and Electrochemical Properties Both in Solution and Solid State. J. Photochem. Photobiol. C Photochem. Rev. 2016, 318, 104–113. [Google Scholar] [CrossRef]
- Chao, C.-C.; Leung, M.; Su, Y.O.; Chiu, K.-Y.; Lin, T.-H.; Shieh, S.-J.; Lin, S.-C. Photophysical and Electrochemical Properties of 1,7-Diaryl-Substituted Perylene Diimides. J. Org. Chem. 2005, 70, 4323–4331. [Google Scholar] [CrossRef]
- Tang, N.; Zhou, J.; Wang, L.; Stolte, M.; Xie, G.; Wen, X.; Liu, L.; Würthner, F.; Gierschner, J.; Xie, Z. Anomalous Deep-Red Luminescence of Perylene Black Analogues with Strong π-π Interactions. Nat. Commun. 2023, 14, 1922. [Google Scholar] [CrossRef]
- Al-Khateeb, B.; Dinleyici, M.; Abourajab, A.; Kök, C.; Bodapati, J.B.; Uzun, D.; Koyuncu, S.; Icil, H. Swallow Tail Bay-Substituted Novel Perylene Bisimides: Synthesis, Characterization, Photophysical and Electrochemical Properties and DFT Studies. J. Photochem. Photobiol. A. Chem. 2020, 393, 112432. [Google Scholar] [CrossRef]
- Das, L.; Das, P.; Ahamed, S.M.; Datta, A.; Pal, A.K.; Datta, A.; Malik, S. Bay-Substituted Perylene Diimide-Based Donor–Acceptor Type Copolymers: Design, Synthesis, Optical and Energy Storage Behaviours. J. Mater. Chem. A 2025, 13, 1842–1852. [Google Scholar] [CrossRef]
- Vajiravelu, S.; Ramunas, L.; Juozas Vidas, G.; Valentas, G.; Vygintas, J.; Valiyaveettil, S. Effect of Substituents on the Electron Transport Properties of Bay Substituted Perylene Diimide Derivatives. J. Mater. Chem. 2009, 19, 4268. [Google Scholar] [CrossRef]
- Yang, S.K.; Zimmerman, S.C. Polyglycerol-Dendronized Perylenediimides as Stable, Water-Soluble Fluorophores. Adv. Funct. Mater. 2012, 22, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Battagliarin, G.; Davies, M.; Mackowiak, S.; Li, C.; Müllen, K. Ortho -Functionalized Perylenediimides for Highly Fluorescent Water-Soluble Dyes. Chem. Phys. Chem. 2012, 13, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Kohl, C.; Pottek, M.; Müllen, K. Ionic Perylenetetracarboxdiimides: Highly Fluorescent and Water-Soluble Dyes for Biolabeling. Angew. Chem. Int. Ed. 2004, 43, 1528–1531. [Google Scholar] [CrossRef]
- Kohl, C.; Weil, T.; Qu, J.; Müllen, K. Towards Highly Fluorescent and Water-Soluble Perylene Dyes. Chem. Eur. J. 2004, 10, 5297–5310. [Google Scholar] [CrossRef]
- Yukruk, F.; Dogan, A.L.; Canpinar, H.; Guc, D.; Akkaya, E.U. Water-Soluble Green Perylenediimide (PDI) Dyes as Potential Sensitizers for Photodynamic Therapy. Org. Lett. 2005, 7, 2885–2887. [Google Scholar] [CrossRef]
- Draper, E.R.; Walsh, J.J.; McDonald, T.O.; Zwijnenburg, M.A.; Cameron, P.J.; Cowan, A.J.; Adams, D.J. Air-Stable Photoconductive Films Formed from Perylene Bisimide Gelators. J. Mater. Chem. C 2014, 2, 5570–5575. [Google Scholar] [CrossRef]
- Kozma, E.; Grisci, G.; Mróz, W.; Catellani, M.; Eckstein-Andicsovà, A.; Pagano, K.; Galeotti, F. Water-Soluble Aminoacid Functionalized Perylene Diimides: The Effect of Aggregation on the Optical Properties in Organic and Aqueous Media. Dyes and Pigments 2016, 125, 201–209. [Google Scholar] [CrossRef]
- Heek, T.; Fasting, C.; Rest, C.; Zhang, X.; Würthner, F.; Haag, R. Highly Fluorescent Water-Soluble Polyglycerol-Dendronized Perylene Bisimide Dyes. Chem. Commun. 2010, 46, 1884–1886. [Google Scholar] [CrossRef]
- You, S.; Cai, Q.; Müllen, K.; Yang, W.; Yin, M. pH-Sensitive Unimolecular Fluorescent Polymeric Micelles: From Volume Phase Transition to Optical Response. Chem. Commun. 2014, 50, 823–825. [Google Scholar] [CrossRef]
- Yin, M.; Feng, C.; Shen, J.; Yu, Y.; Xu, Z.; Yang, W.; Knoll, W.; Müllen, K. Dual-Responsive Interaction to Detect DNA on Template-Based Fluorescent Nanotubes. Small 2011, 7, 1629–1634. [Google Scholar] [CrossRef]
- Yin, M.; Shen, J.; Gropeanu, R.; Pflugfelder, G.O.; Weil, T.; Müllen, K. Fluorescent Core/Shell Nanoparticles for Specific Cell-Nucleus Staining. Small 2008, 4, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, C. Fluorescence Turn-On Detection of a Protein through the Reduced Aggregation of a Perylene Probe. Angew. Chem. Int. Ed. 2010, 49, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cheng, W.; Guo, K.; Yu, J.; Shen, J.; Tang, J.; Yang, W.; Yin, M. Molecular Size, Shape, and Electric Charges: Essential for Perylene Bisimide-Based DNA Intercalator to Localize in Cell Nuclei and Inhibit Cancer Cell Growth. ACS Appl. Mater. Interfaces 2015, 7, 9784–9791. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Cai, Q.; Zheng, Y.; He, B.; Shen, J.; Yang, W.; Yin, M. Perylene-Cored Star-Shaped Polycations for Fluorescent Gene Vectors and Bioimaging. ACS Appl. Mater. Interfaces 2014, 6, 16327–16334. [Google Scholar] [CrossRef]
- Gryszel, M.; Schlossarek, T.; Würthner, F.; Natali, M.; Głowacki, E.D. Water-Soluble Cationic Perylene Diimide Dyes as Stable Photocatalysts for H2 O2 Evolution. Chem.Photo.Chem. 2023, 7, e202300070. [Google Scholar] [CrossRef]
- Bag, K.; Halder, R.; Jana, B.; Malik, S. Solvent-Assisted Enhanced Emission of Cationic Perylene Diimide Supramolecular Assembly in Water: A Perspective from Experiment and Simulation. J. Phys. Chem. C 2019, 123, 6241–6249. [Google Scholar] [CrossRef]
- Xu, Z.; He, B.; Wei, W.; Liu, K.; Yin, M.; Yang, W.; Shen, J. Highly Water-Soluble Perylenediimide-Cored Poly(Amido Amine) Vector for Efficient Gene Transfection. J. Mater. Chem. B 2014, 2, 3079–3086. [Google Scholar] [CrossRef]
- Gao, B.; Li, H.; Liu, H.; Zhang, L.; Bai, Q.; Ba, X. Water-Soluble and Fluorescent Dendritic Perylene Bisimides for Live-Cell Imaging. Chem. Commun. 2011, 47, 3894. [Google Scholar] [CrossRef]
- Bo, F.; Gao, B.; Duan, W.; Li, H.; Liu, H.; Bai, Q. Assembly–Disassembly Driven “off–on” Fluorescent Perylene Bisimide Probes for Detecting and Tracking of Proteins in Living Cells. RSC Adv. 2013, 3, 17007. [Google Scholar] [CrossRef]
- Yang, S.K.; Shi, X.; Park, S.; Doganay, S.; Ha, T.; Zimmerman, S.C. Monovalent, Clickable, Uncharged, Water-Soluble Perylenediimide-Cored Dendrimers for Target-Specific Fluorescent Biolabeling. J. Am. Chem. Soc. 2011, 133, 9964–9967. [Google Scholar] [CrossRef]
- Singh, P.; Hirsch, A.; Kumar, S. Perylene Diimide-Based Chemosensors Emerging in Recent Years: From Design to Sensing. Trends Anal. Chem. 2021, 138, 116237. [Google Scholar] [CrossRef]
- Kaur, N.; Kour, R.; Kaur, S.; Singh, P. Perylene Diimide-Based Sensors for Multiple Analyte Sensing (Fe2+ /H2 S/ Dopamine and Hg2+ /Fe2+ ): Cell Imaging and INH, XOR, and Encoder Logic. Anal. Methods 2023, 15, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, R.; Gao, Y.; Xu, J.; Sun, Y.; Bao, J.; Fang, L.; Gou, S. Realizing Near-Infrared (NIR)-Triggered Type-I PDT and PTT by Maximizing the Electronic Exchange Energy of Perylene Diimide-Based Photosensitizers. ACS Materials Lett. 2023, 5, 1752–1759. [Google Scholar] [CrossRef]
- Yan, L.; Ye, Z.; Peng, C.; Zhang, S. A New Perylene Diimide-Based Fluorescent Chemosensor for Selective Detection of ATP in Aqueous Solution. Tetrahedron 2012, 68, 2725–2727. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, J.; Bai, W.; Yang, S.; Chen, A. A Simple and Sensitive Label-Free Fluorescent Approach for Protein Detection Based on a Perylene Probe and Aptamer. Biosens. Bioelectron. 2015, 64, 530–534. [Google Scholar] [CrossRef]
- Zong, L.; Zhang, H.; Li, Y.; Gong, Y.; Li, D.; Wang, J.; Wang, Z.; Xie, Y.; Han, M.; Peng, Q.; et al. Tunable Aggregation-Induced Emission Nanoparticles by Varying Isolation Groups in Perylene Diimide Derivatives and Application in Three-Photon Fluorescence Bioimaging. ACS Nano 2018, 12, 9532–9540. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, F.; Zhang, J.; Jiang, T.; Li, X.; Wu, J.; Ren, H. A Water-soluble Fluorescent pH Probe Based on Perylene Dyes and Its Application to Cell Imaging. Luminescence 2016, 31, 102–107. [Google Scholar] [CrossRef]
- Jana, A.; Nguyen, K.T.; Li, X.; Zhu, P.; Tan, N.S.; Ågren, H.; Zhao, Y. Perylene-Derived Single-Component Organic Nanoparticles with Tunable Emission: Efficient Anticancer Drug Carriers with Real-Time Monitoring of Drug Release. ACS Nano 2014, 8, 5939–5952. [Google Scholar] [CrossRef]
- Jana, A.; Devi, K.S.P.; Maiti, T.K.; Singh, N.D.P. Perylene-3-Ylmethanol: Fluorescent Organic Nanoparticles as a Single-Component Photoresponsive Nanocarrier with Real-Time Monitoring of Anticancer Drug Release. J. Am. Chem. Soc. 2012, 134, 7656–7659. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, H.; Ji, C.; Yang, R.; Yin, M. A Perylenediimide-Based Nanocarrier Monitors Curcumin Release with an “off–on” Fluorescence Switch. Polym. Chem. 2019, 10, 2551–2558. [Google Scholar] [CrossRef]
- Zhou, P.; Aschauer, U.; Decurtins, S.; Feurer, T.; Häner, R.; Liu, S.-X. Merging of Azulene and Perylene Diimide for Optical pH Sensors. Molecules 2023, 28, 6694. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Garnier, U.; Ghasemi, R.; Pierre Lefevre, J.; Mongin, C.; Brosseau, A.; Frédéric Audibert, J.; Pansu, Robert. ; Dauzères, A.; Leray, I. Perylene Based PET Fluorescent Molecular Probes for pH Monitoring. J. Photochem. Photobiol. A. Chem. 2022, 432, 114035. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A Novel Water-Soluble Perylenetetracarboxylic Diimide as a Fluorescent pH Probe: Chemosensing, Biocompatibility and Cell Imaging. Dyes and Pigments 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Zhang, B.; Suo, Z.; Xing, F.; Feng, L. Sensitive and Reversible Perylene Derivative-Based Fluorescent Probe for Acetylcholinesterase Activity Monitoring and Its Inhibitor. Anal. Biochem. 2020, 607, 113835. [Google Scholar] [CrossRef]
- Muthuraj, B.; Mukherjee, S.; Chowdhury, S.R.; Patra, C.R.; Iyer, P.K. An Efficient Strategy to Assemble Water Soluble Histidine-Perylene Diimide and Graphene Oxide for the Detection of PPi in Physiological Conditions and in Vitro. Biosens. Bioelectron. 2017, 89, 636–644. [Google Scholar] [CrossRef]
- Dey, S.; Sukul, P.Kr. Selective Detection of Pyrophosphate Anions in Aqueous Medium Using Aggregation of Perylene Diimide as a Fluorescent Probe. ACS Omega 2019, 4, 16191–16200. [Google Scholar] [CrossRef]
- Feng, C.L.; Yin, M.; Zhang, D.; Zhu, S.; Caminade, A.M.; Majoral, J.P.; Müllen, K. Fluorescent Core-Shell Star Polymers Based Bioassays for Ultrasensitive DNA Detection by Surface Plasmon Fluorescence Spectroscopy. Macromol. Rapid Commun. 2011, 32, 679–683. [Google Scholar] [CrossRef]
- Feng, X.; An, Y.; Yao, Z.; Li, C.; Shi, G. A Turn-on Fluorescent Sensor for Pyrophosphate Based on the Disassembly of Cu2+ -Mediated Perylene Diimide Aggregates. ACS Appl. Mater. Interfaces 2012, 4, 614–618. [Google Scholar] [CrossRef]
- Wang, K.-R.; Wang, Y.-Q.; Li, J.; An, H.-W.; Zhang, L.-P.; Zhang, J.-C.; Li, X.-L. Synthesis of Perylene Bisimide-Centered Glycodendrimer and Its Interactions with Concanavalin A. Bioorg. Med. Chem. Lett. 2013, 23, 480–483. [Google Scholar] [CrossRef]
- Wang, K.-R.; An, H.-W.; Qian, F.; Wang, Y.-Q.; Zhang, J.-C.; Li, X.-L. Synthesis, Optical Properties and Binding Interactions of a Multivalent Glycocluster Based on a Fluorescent Perylene Bisimide Derivative. RSC Adv. 2013, 3, 23190. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, X.; Dong, Y.; Zhang, N.; Wang, H.; Li, X.; Ren, X.; Ma, H.; Wei, Q. Self-Assembled Perylene Diimide (PDI) Nanowire Sensitized In2 O3 @MgIn2 S4 S-Scheme Heterojunction as Photoelectrochemical Biosensing Platform for the Detection of CA15–3. Anal. Chem. 2024, 96, 13197–13206. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Choudhary, N.; Vasave, A.T.; Sonpal, V.; Paital, A.R. Perylene Diimide Functionalized Nano-Silica: Green Emissive Material for Selective Probing and Remediation of 4-Nitrocatechol, Ru3+, and Cu2+ with Biosensing Applications. Mater. Adv. 2024, 5, 8937–8952. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Lai, Y.; Zhou, Z.; Zhao, Y.; Wang, H.; Wang, Z. Guanidinium-Dendronized Perylene Bisimides as Stable, Water-Soluble Fluorophores for Live-Cell Imaging. New J. Chem. 2013, 37, 2983. [Google Scholar] [CrossRef]
- Yin, M.; Kuhlmann, C.R.W.; Sorokina, K.; Li, C.; Mihov, G.; Pietrowski, E.; Koynov, K.; Klapper, M.; Luhmann, H.J.; Weil, T. Novel Fluorescent Core–Shell Nanocontainers for Cell Membrane Transport. Biomacromolecules 2008, 9, 1381–1389. [Google Scholar] [CrossRef]
- Heek, T.; Nikolaus, J.; Schwarzer, R.; Fasting, C.; Welker, P.; Licha, K.; Herrmann, A.; Haag, R. An Amphiphilic Perylene Imido Diester for Selective Cellular Imaging. Bioconjugate Chem. 2013, 24, 153–158. [Google Scholar] [CrossRef]
- Cui, X.; Shi, B.; Qiu, Z.; Yang, F.; Wang, X.; Xu, Y.; Wei, W. Highly Fluorescent, Water-Soluble Tetrapodal Perylene Diimides Insulated by Cationic Pendants for Live-Cell Imaging. Dyes and Pigments 2025, 232, 112460. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Sun, T.; Bian, J.; Bu, X.; Ge, X.; Sun, J.; Liu, Z.; Xie, Z.; Xi, P.; et al. Multicolor Tuning of Perylene Diimides Dyes for Targeted Organelle Imaging In Vivo. Anal. Chem. 0160; c1. [Google Scholar] [CrossRef]
- Yang, F.; Li, R.; Wei, W.; Ding, X.; Xu, Z.; Wang, P.; Wang, G.; Xu, Y.; Fu, H.; Zhao, Y. Water-Soluble Doubly-Strapped Isolated Perylene Diimide Chromophore. Angew. Chem. Int. Ed. 2022, 61, e202202491. [Google Scholar] [CrossRef]
- Casagrande, V.; Salvati, E.; Alvino, A.; Bianco, A.; Ciammaichella, A.; D’Angelo, C.; Ginnari-Satriani, L.; Serrilli, A.M.; Iachettini, S.; Leonetti, C.; et al. N -Cyclic Bay-Substituted Perylene G-Quadruplex Ligands Have Selective Antiproliferative Effects on Cancer Cells and Induce Telomere Damage. J. Med. Chem. 2011, 54, 1140–1156. [Google Scholar] [CrossRef]
- Kulkarni, B.; Malhotra, M.; Jayakannan, M. Perylene-Tagged Polycaprolactone Block Copolymers and Their Enzyme-Biodegradable Fluorescent Nanoassemblies for Intracellular Bio-Imaging in Cancer Cells. ACS Appl. Polym. Mater. 2019, 1, 3375–3388. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y.; Jiang, R.; Fu, N.; Lu, X.; Tian, C.; Hu, W.; Fan, Q.; Huang, W. Homogeneous Near-Infrared Emissive Polymeric Nanoparticles Based on Amphiphilic Diblock Copolymers with Perylene Diimide and PEG Pendants: Self-Assembly Behavior and Cellular Imaging Application. Polym. Chem. 2014, 5, 1372–1380. [Google Scholar] [CrossRef]
- Ye, Y.; Zheng, Y.; Ji, C.; Shen, J.; Yin, M. Self-Assembly and Disassembly of Amphiphilic Zwitterionic Perylenediimide Vesicles for Cell Membrane Imaging. ACS Appl. Mater. Interfaces 2017, 9, 4534–4539. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, M.; Aly, S.; Lant, J.T.; Zhang, X.; Charpentier, P. Energy/Electron Transfer Switch for Controlling Optical Properties of Silicon Quantum Dots. Sci. Rep. 2018, 8, 17068. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Raja, S.; Rodrigues, A.S.; Fernandes, F.; Baleizão, C.; Farinha, J.P.S. NIR and Visible Perylenediimide-Silica Nanoparticles for Laser Scanning Bioimaging. Dyes and Pigments 2014, 110, 227–234. [Google Scholar] [CrossRef]
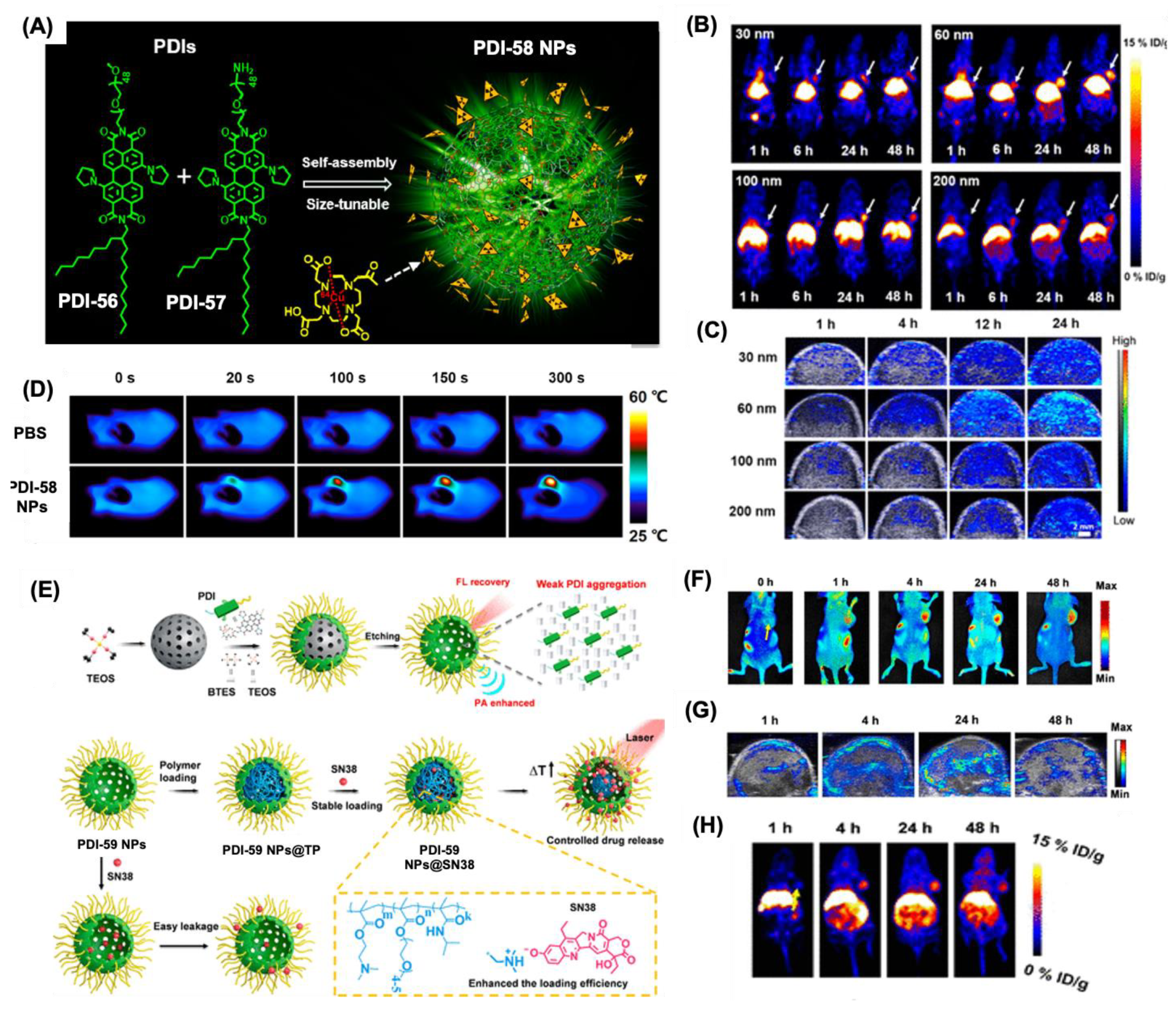
- Fan, Q.; Cheng, K.; Yang, Z.; Zhang, R.; Yang, M.; Hu, X.; Ma, X.; Bu, L.; Lu, X.; Xiong, X.; et al. Perylene-Diimide-Based Nanoparticles as Highly Efficient Photoacoustic Agents for Deep Brain Tumor Imaging in Living Mice. Adv. Mater. 2015, 27, 843–847. [Google Scholar] [CrossRef]
- Yang, Y.; Fryer, C.; Sharkey, J.; Thomas, A.; Wais, U.; Jackson, A.W.; Wilm, B.; Murray, P.; Zhang, H. Perylene Diimide Nanoprobes for In Vivo Tracking of Mesenchymal Stromal Cells Using Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2020, 12, 27930–27939. [Google Scholar] [CrossRef]
- Cui, C.; Yang, Z.; Hu, X.; Wu, J.; Shou, K.; Ma, H.; Jian, C.; Zhao, Y.; Qi, B.; Hu, X.; et al. Organic Semiconducting Nanoparticles as Efficient Photoacoustic Agents for Lightening Early Thrombus and Monitoring Thrombolysis in Living Mice. ACS Nano 2017, 11, 3298–3310. [Google Scholar] [CrossRef]
- Li, S.-A.; Meng, X.-Y.; Zhang, Y.-J.; Chen, C.-L.; Jiao, Y.-X.; Zhu, Y.-Q.; Liu, P.-P.; Sun, W. Progress in pH-Sensitive Sensors: Essential Tools for Organelle pH Detection, Spotlighting Mitochondrion and Diverse Applications. Front. Pharmacol. 2024, 14, 1339518. [Google Scholar] [CrossRef]
- Ghosh, S.; Lai, J.-Y. Recent Advances in the Design of Intracellular pH Sensing Nanoprobes Based on Organic and Inorganic Materials. Environmental Research 2023, 237, 117089. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Hou, S.; Zhang, J.; Shi, Z.; Jiang, T.; Wei, X. pH-Sensitive Perylene Tetra-(Alkoxycarbonyl) Probes for Live Cell Imaging. New J. Chem. 2016, 40, 6615–6622. [Google Scholar] [CrossRef]
- Ye, F.; Liang, X.-M.; Wu, N.; Li, P.; Chai, Q.; Fu, Y. A New Perylene-Based Fluorescent pH Chemosensor for Strongly Acidic Condition. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2019, 216, 359–364. [Google Scholar] [CrossRef]
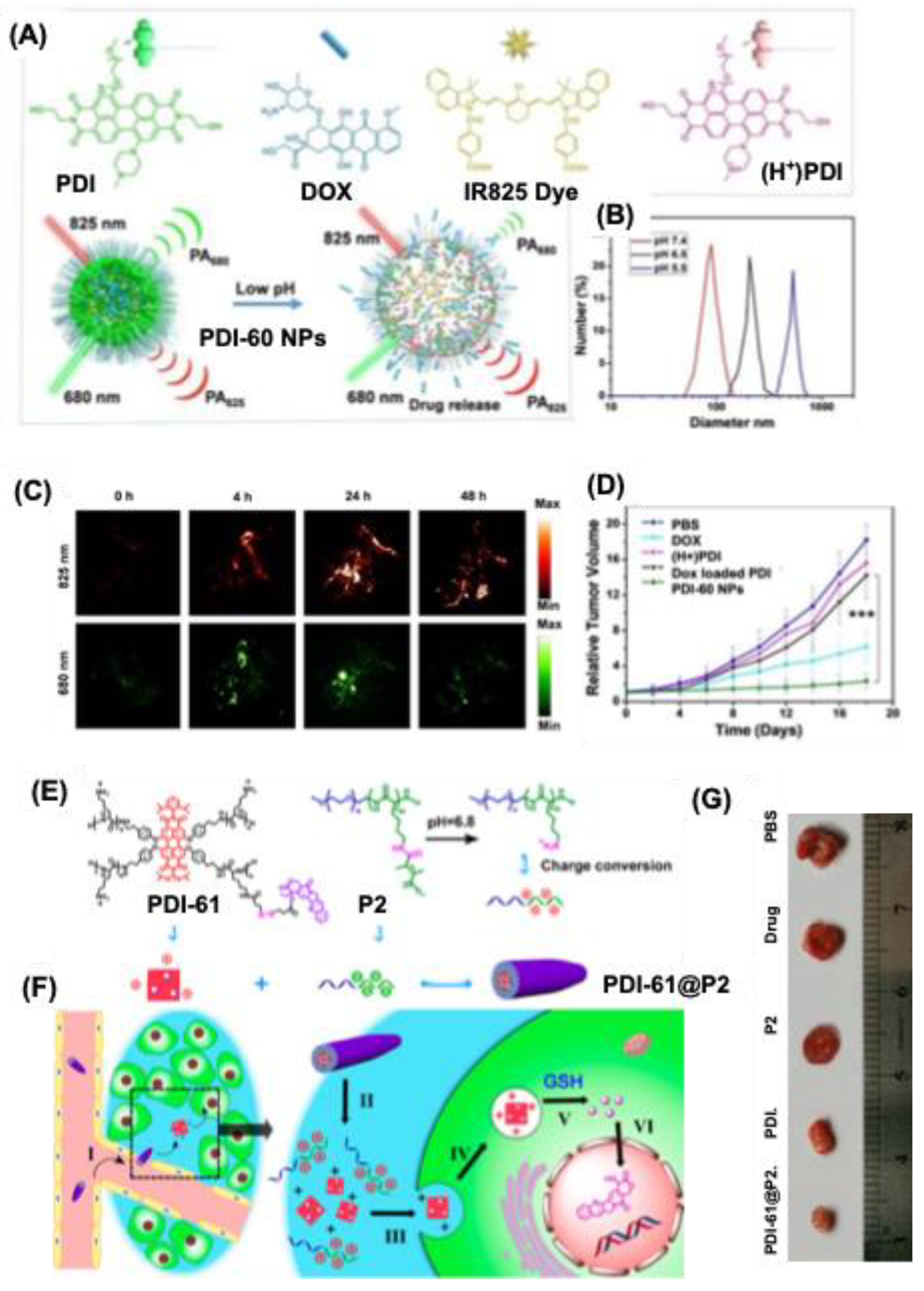
- Sun, M.; Yin, W.; Dong, X.; Yang, W.; Zhao, Y.; Yin, M. Fluorescent Supramolecular Micelles for Imaging-Guided Cancer Therapy. Nanoscale 2016, 8, 5302–5312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Song, J.; Dai, Y.; Chen, J.; Wang, F.; Lin, L.; Liu, Y.; Zhang, F.; Yu, G.; Zhou, Z.; et al. Self-Assembly of Semiconducting-Plasmonic Gold Nanoparticles with Enhanced Optical Property for Photoacoustic Imaging and Photothermal Therapy. Theranostics 2017, 7, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yuan, P.; Wang, G.; Deng, W.; Tian, S.; Wang, C.; Lu, X.; Huang, W.; Fan, Q. High Density Glycopolymers Functionalized Perylene Diimide Nanoparticles for Tumor-Targeted Photoacoustic Imaging and Enhanced Photothermal Therapy. Biomacromolecules 2017, 18, 3375–3386. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tian, R.; Wu, J.; Fan, Q.; Yung, B.C.; Niu, G.; Jacobson, O.; Wang, Z.; Liu, G.; Yu, G.; et al. Impact of Semiconducting Perylene Diimide Nanoparticle Size on Lymph Node Mapping and Cancer Imaging. ACS Nano 2017, 11, 4247–4255. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, W.; Zou, J.; Tang, W.; Li, L.; He, L.; Shen, Z.; Wang, Z.; Jacobson, O.; Aronova, M.A.; et al. Precision Cancer Theranostic Platform by In Situ Polymerization in Perylene Diimide-Hybridized Hollow Mesoporous Organosilica Nanoparticles. J. Am. Chem. Soc. 2019, 141, 14687–14698. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current Update on Nanoplatforms as Therapeutic and Diagnostic Tools: A Review for the Materials Used as Nanotheranostics and Imaging Modalities. Asian J. Pharm. Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Tang, W.; Fan, W.; Dai, Y.; Shen, Z.; Lin, L.; Cheng, S.; Liu, Y.; Niu, G.; et al. Stimuli-Responsive Nanotheranostics for Real-Time Monitoring Drug Release by Photoacoustic Imaging. Theranostics 2019, 9, 526–536. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Y.; Yin, C.; Fan, Q.; Zhang, W.; Song, J.; Yu, G.; Tang, W.; Fan, W.; Yung, B.C.; et al. Activatable Semiconducting Theranostics: Simultaneous Generation and Ratiometric Photoacoustic Imaging of Reactive Oxygen Species In Vivo. Adv. Mater. 2018, 30, 1707509. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; Wang, S.; Wang, Z.; Song, J.; Yu, G.; Fan, W.; Dai, Y.; Wang, J.; Shan, L.; et al. Organic Semiconducting Photoacoustic Nanodroplets for Laser-Activatable Ultrasound Imaging and Combinational Cancer Therapy. ACS Nano 2018, 12, 2610–2622. [Google Scholar] [CrossRef]
- Cheng, W.; Cheng, H.; Wan, S.; Zhang, X.; Yin, M. Dual-Stimulus-Responsive Fluorescent Supramolecular Prodrug for Antitumor Drug Delivery. Chem. Mater. 2017, 29, 4218–4226. [Google Scholar] [CrossRef]
- Li, H.; Yue, L.; Li, L.; Liu, G.; Zhang, J.; Luo, X.; Wu, F. Triphenylamine-Perylene Diimide Conjugate-Based Organic Nanoparticles for Photoacoustic Imaging and Cancer Phototherapy. Colloids and Surfaces B: Biointerfaces 2021, 205, 111841. [Google Scholar] [CrossRef] [PubMed]
- Yip, A.M.; Shum, J.; Liu, H.; Zhou, H.; Jia, M.; Niu, N.; Li, Y.; Yu, C.; Lo, K.K. Luminescent Rhenium(I)–Polypyridine Complexes Appended with a Perylene Diimide or Benzoperylene Monoimide Moiety: Photophysics, Intracellular Sensing, and Photocytotoxic Activity. Chem. Eur. J. 2019, 25, 8970–8974. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liang, C.; Gong, H.; Chen, Q.; Wang, C.; Liu, Z. Photosensitizer-Conjugated Albumin−Polypyrrole Nanoparticles for Imaging-Guided In Vivo Photodynamic/Photothermal Therapy. Small 2015, 11, 3932–3941. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, J.-Y.; Tung, C.-H.; Kim, I.-H.; Choi, Y. Gold Nanorod−Photosensitizer Complex for Near-Infrared Fluorescence Imaging and Photodynamic/Photothermal Therapy In Vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef]
- Sun, P.; Wang, X.; Wang, G.; Deng, W.; Shen, Q.; Jiang, R.; Wang, W.; Fan, Q.; Huang, W. A Perylene Diimide Zwitterionic Polymer for Photoacoustic Imaging Guided Photothermal/Photodynamic Synergistic Therapy with Single near-Infrared Irradiation. J. Mater. Chem. B 2018, 6, 3395–3403. [Google Scholar] [CrossRef]
- Sun, P.; Huang, T.; Wang, X.; Wang, G.; Liu, Z.; Chen, G.; Fan, Q. Dynamic-Covalent Hydrogel with NIR-Triggered Drug Delivery for Localized Chemo-Photothermal Combination Therapy. Biomacromolecules 2020, 21, 556–565. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.; Wang, C.-H.; Chang, K.-H.; Ho, J.A.; Chou, P.-T. Comprehensive Thione-Derived Perylene Diimides and Their Bio-Conjugation for Simultaneous Imaging, Tracking, and Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Jin, X.; Deng, Q.; Yin, Z.; Tong, S.; Qing, W.; Huang, Y. Regioisomer-Manipulating Thio-Perylenediimide Nanoagents for Photothermal/Photodynamic Theranostics. J. Mater. Chem. B 2020, 8, 5535–5544. [Google Scholar] [CrossRef]
- Llewellyn, B.A.; Davies, E.S.; Pfeiffer, C.R.; Cooper, M.; Lewis, W.; Champness, N.R. Thionated Perylene Diimides with Intense Absorbance in the Near-IR. Chem. Commun. 2016, 52, 2099–2102. [Google Scholar] [CrossRef]
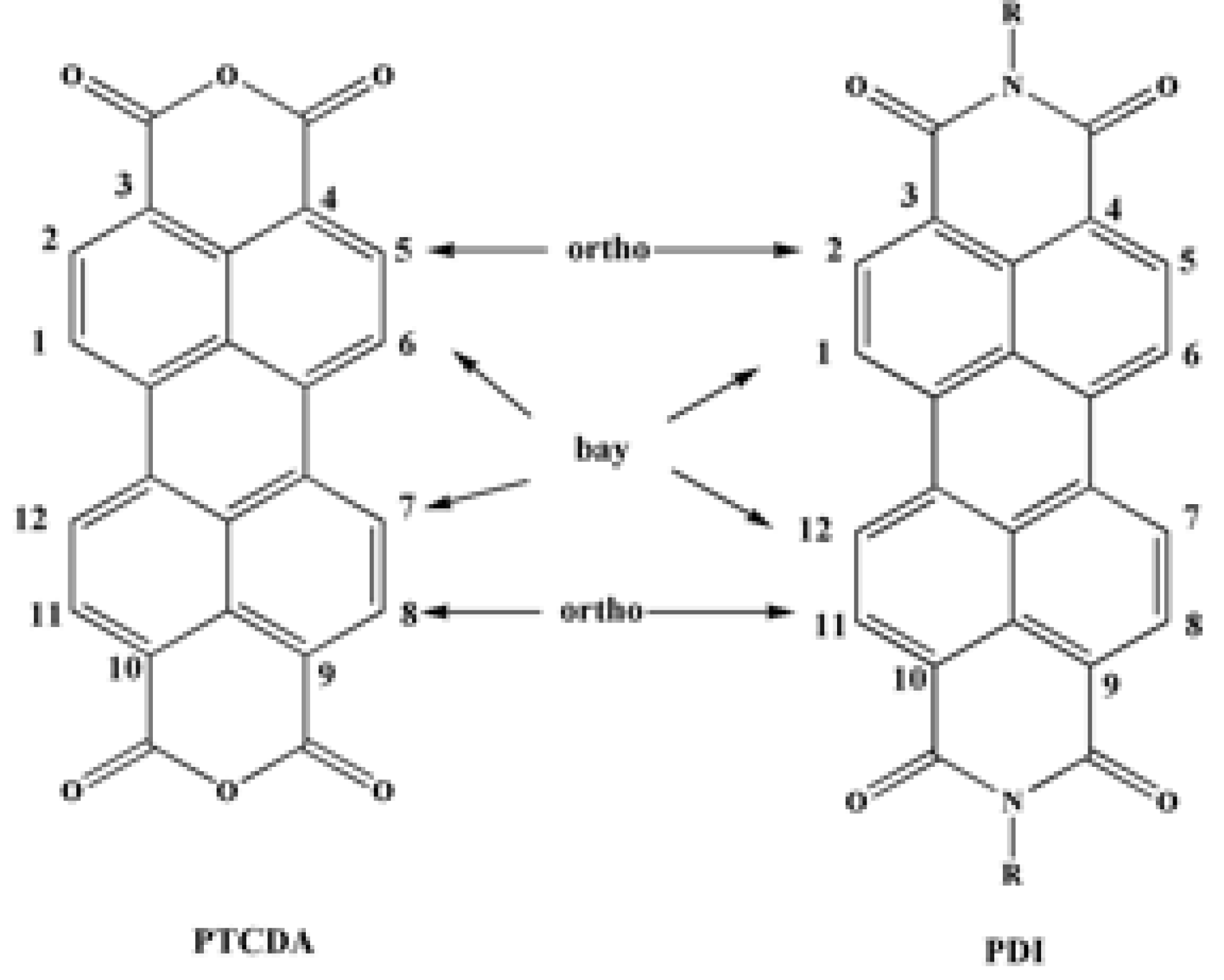
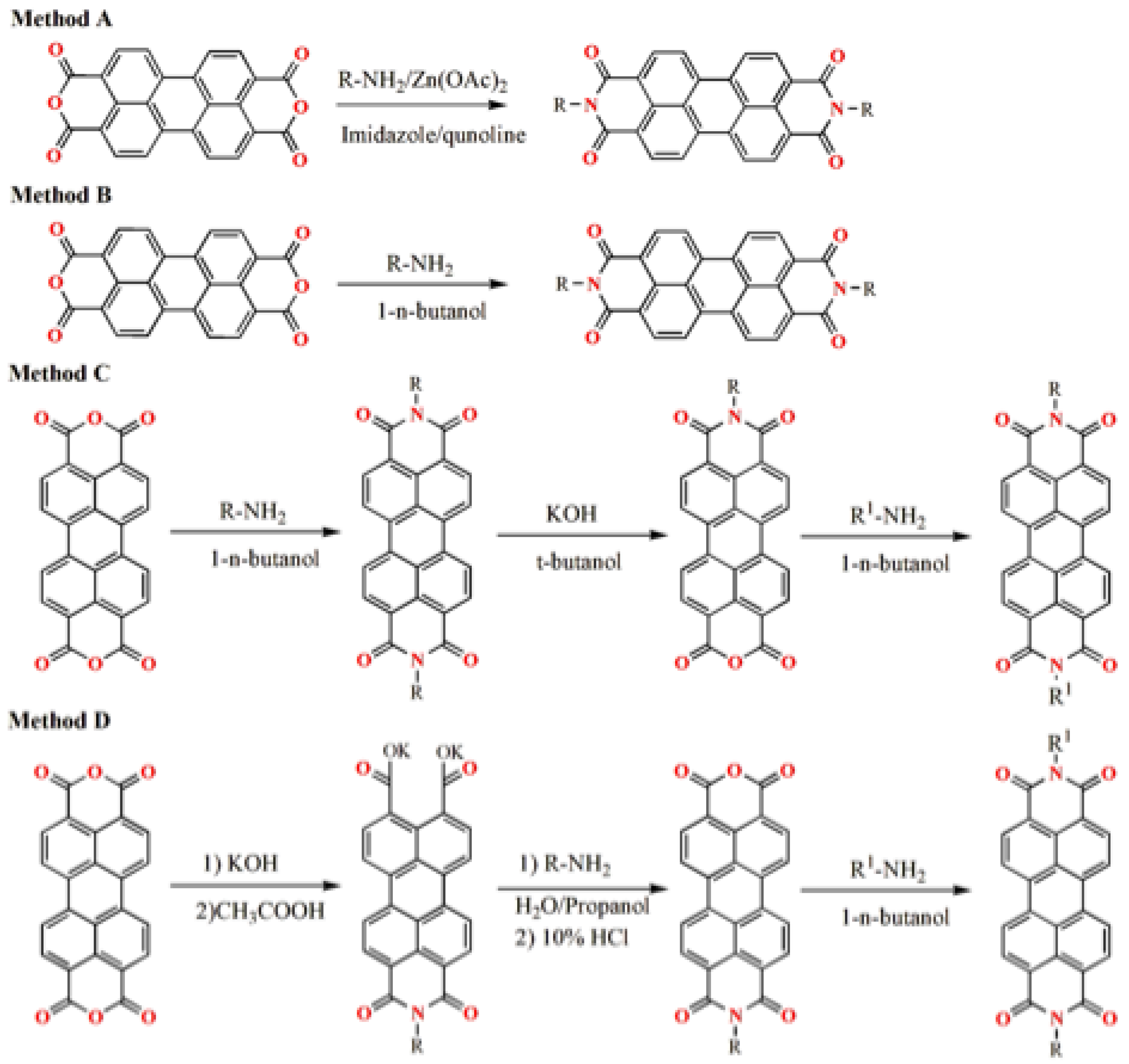
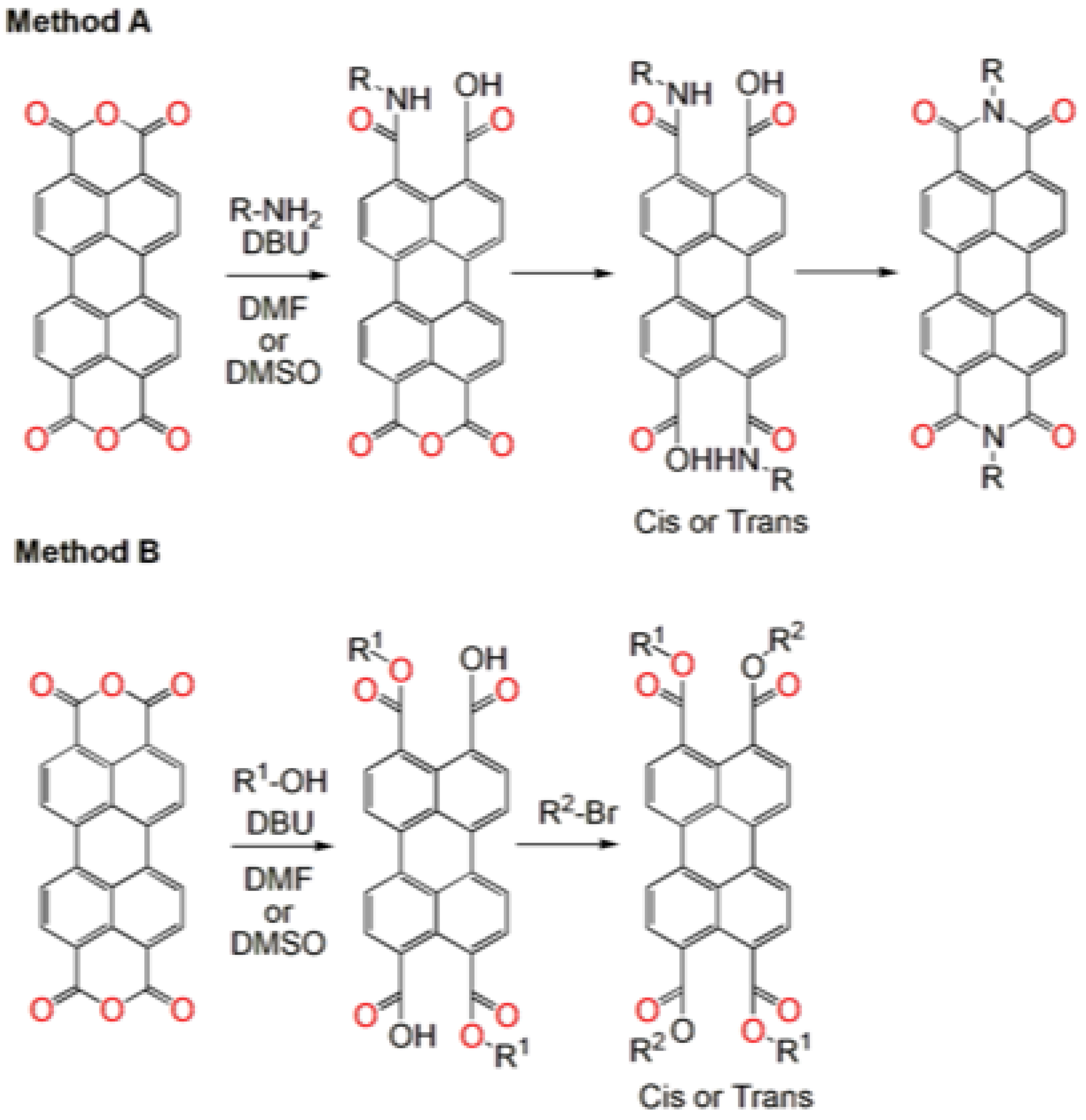
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




