Submitted:
11 March 2025
Posted:
12 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Acquisition of S. malayensis Polyps and Ephyrae
2.2. Evaluation of Growth, Survival, and Feeding of S. malayensis Ephyrae According to the Effect of Salinity
3. Statistical Analysis
4. Results
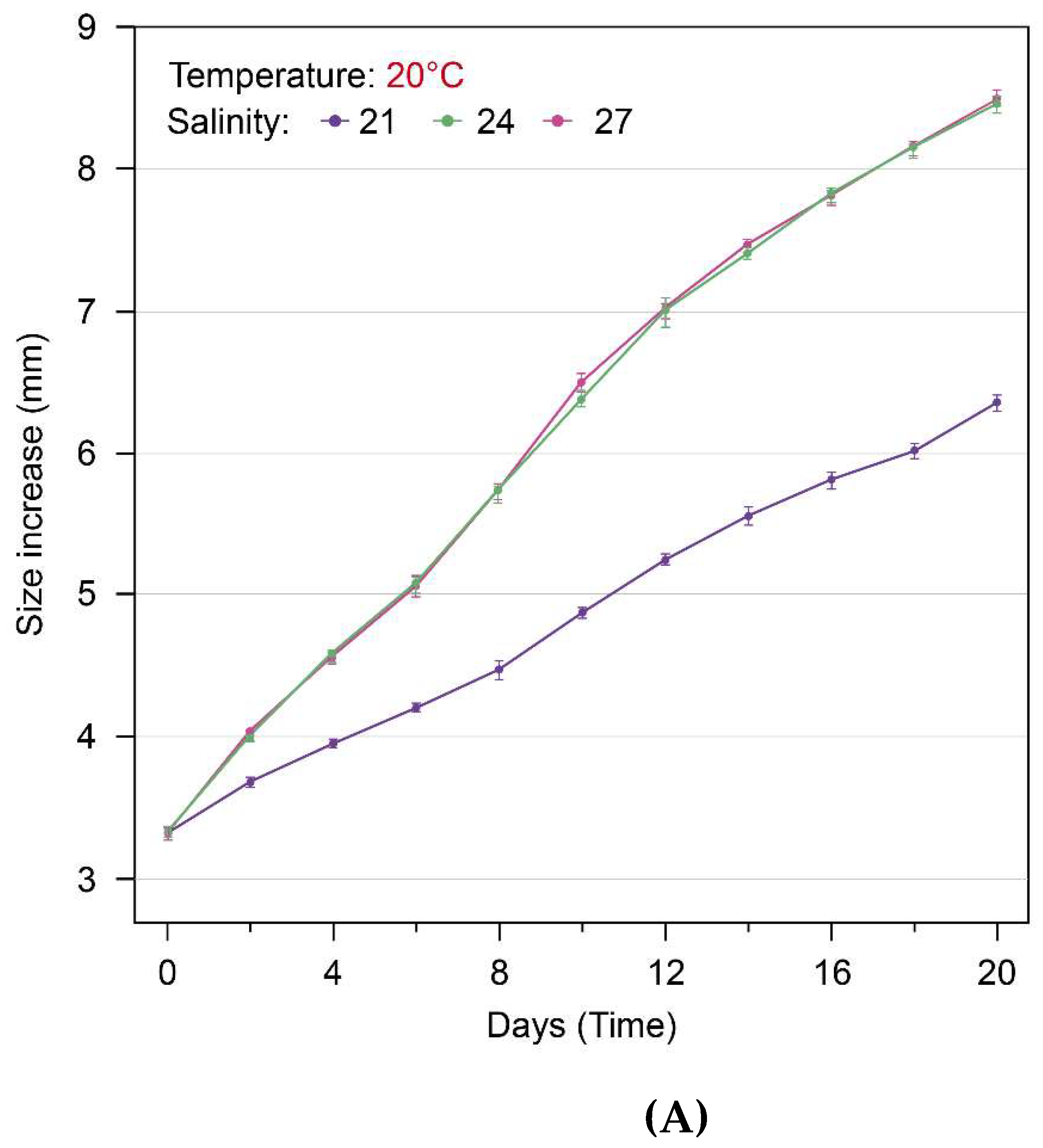
4.1. Effect of Salinity Environmental Factors on the Growth of S. malayensis Ephyrae
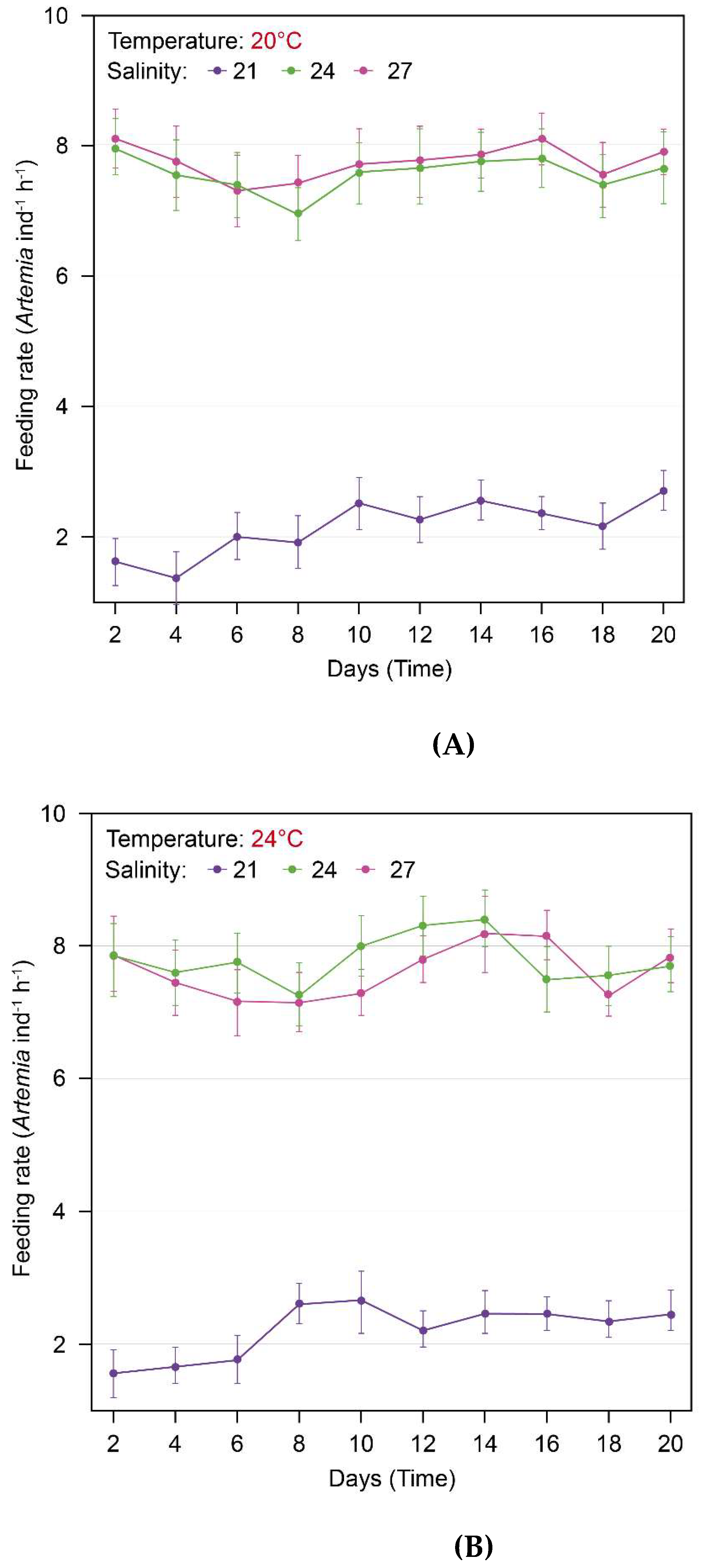
4.2. Effect of Salinity Environmental Factors on the Feeding of S. malayensis Ephyrae
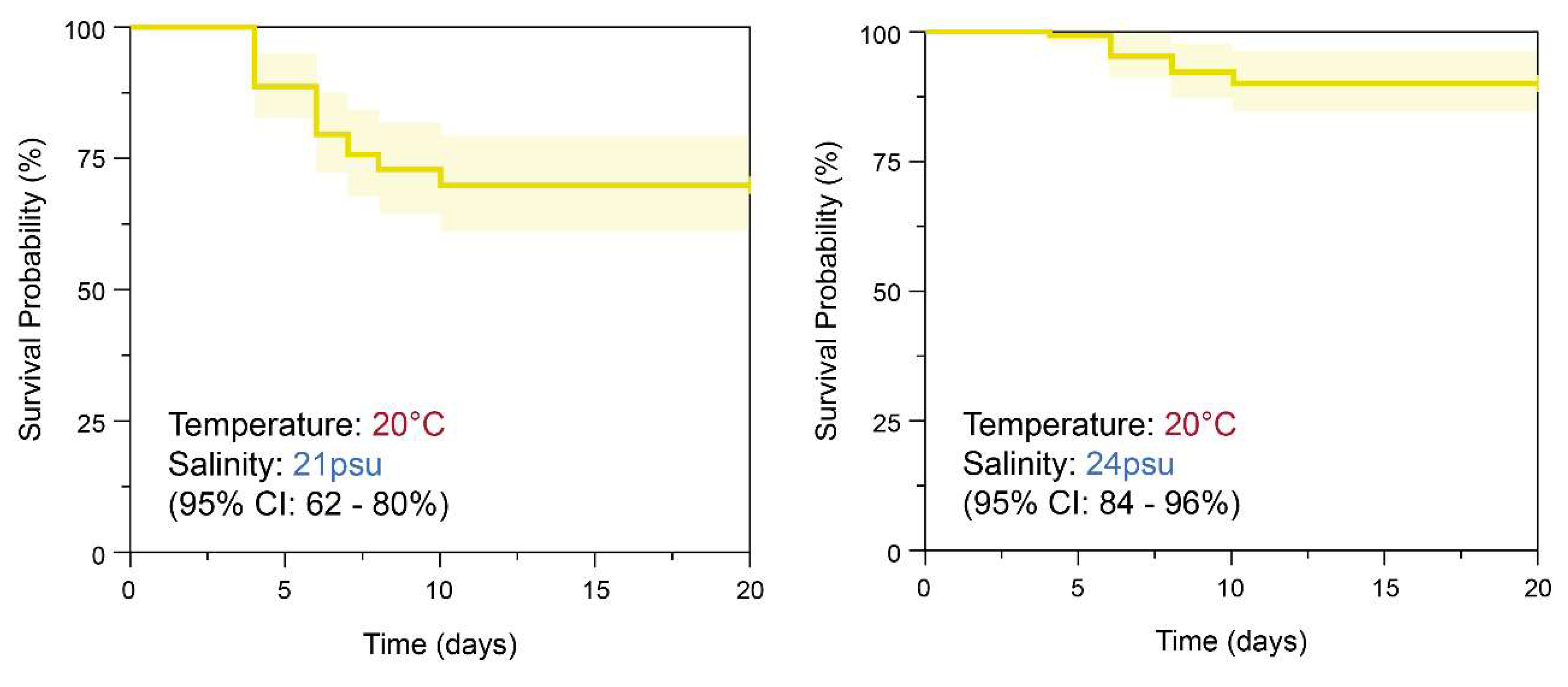
4.3. Effect of Salinity Environmental Factors on the Survival of S. malayensis Ephyrae
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Purcell, J.E.; Uye, S.; Lo, W.-T. Anthropogenic causes of jellyfish blooms their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153-174. https://doi.org/10.3354/meps07093. [CrossRef]
- Purcell, J.E.; Atienza, D.; Fuentes, V.; Olariaga, A.; Tilves, U.; Colahan, C.; Gili, J.M. Temperature effects on asexual reproduction rates of scyphozoan species from the northwest Mediterranean Sea. Hydrobiologia 2012, 690, 169-180. http://doi.org/10.1007s10750-012-1047-7. [CrossRef]
- Pitt, K.A.; Lucas, C.H.; Condon, R.H.; Duarte, C.M.; Stewart-Koster, B. Claims That Anthropogenic Stressors Facilitate Jellyfish Blooms Have Been Amplified Beyond the Available Evidence: A Systematic Review. Front. Mar. Sci. 2018, 5, 451. http://doi.org/10.3389/fmars.2018.00451. [CrossRef]
- Lee, S.H.; Scotti, M.; Jung, S.J.; Hwang, J.S. Jellyfish blooms challenge the provisioning of ecosystem services in the Korean coastal waters. Hydrobiologia 2023, 850, 2855-2870. http://doi.org/10.1007/s10750-022-05076-4. [CrossRef]
- Bosch-Belmar, M.; Milisenda, G.; Basso, L.; Doyle, T.K. Jellyfish Impacts on Marine Aquaculture and Fisheries. Rev. Fish. Sci. Aquac. 2023, 29, 242-259. http://doi.org/10.1080/23308249.2020.1806201. [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Ismael, J.; Sabah, S.; Pérez-Ruzafa, A. Population dynamics and growth in three scyphozoan jellyfishes, and their relationship with environmental conditions in a coastal lagoon. Estuar. Coast. Shelf. Sci. 2020, 243, 106901. https://doi.org/10.1016/j.ecss.2020.106901. [CrossRef]
- Quiñones, J.; Monroy, A.; Acha, E. M.; Mianzan, H. Jellyfish bycatch diminishes profit in an anchovy fishery off Peru. Fish. Res. 2013, 139, 47-50. http://doi.org/10.1016/j.fishres.2012.04.014. [CrossRef]
- Purcell, J.E.; Baxter, E.J.; Fuentes, V. 13-Jellyfish as products and problems of aquaculture. In Advances in Aquaculture Hatchery Technology, 1st ed.; Allan, G., Burnell, G., Eds.; Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2013; pp. 404-430. http://doi.org/10.1533/9780857097460.2.404. [CrossRef]
- De Donno, A.; Idolo, A.; Bagordo, F.; Grassi, T.; Leomanni, A.; Serio, F.; Guido, M.; Canitano, M.; Zampardi, S.; Boero, F.; Piraino, S. Impact of stinging jellyfish proliferations along south Italian coasts: Human health hazards, treatment and social costs. Int. J. Environ. Res. Public Health 2014, 1, 2488-2503. http://doi.org/10.3390/ijerph110302488. [CrossRef]
- Milisenda, G.; Martinez-Quintana, A.; Fuentes, V.L.; Bosch-Belmar, M.; Aglieri, G.; Boero, F.; Piraino, S. Reproductive and bloom patterns of Pelagia noctiluca in the Strait of Messina, Italy. Estuar. Coast. Shelf Sci. 2018, 201, 29-39. http://doi.org/10.1016/j.ecss.2016.01.002. [CrossRef]
- Tilves, U.; Fuentes, V.L.; Milisenda, G.; Parrish, C.C.; Vizzini, S.; Sabatés, A. Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: Evidence from stable isotope signatures and fatty acid composition. Mar. Ecol. Prog. Ser. 2018, 591, 101-116. http://doi.org/10.3354/meps12332. [CrossRef]
- Frolova, A.; Miglietta, M.P. Insights on Bloom Forming Jellyfish (Class: Scyphozoa) in the Gulf of Mexico: Environmental Tolerance Ranges and Limits Suggest Differences in Habitat Preference and Resistance to Climate Change Among Congeners. Front. Mar. Sci. 2020, 7, 93. http://doi.org/10.3389/fmars.2020.00093. [CrossRef]
- Tang, C.; Sun, S.; Zhang, F. Natural predators of polyps of three scyphozoans: Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum. J.Oceanol. Limnol. 2021, 39, 598-608. http://doi.org/10.1007/s00343-020-0284-2. [CrossRef]
- Ishii, H. The influence of environmental changes upon the coastal plankton ecosystems, with special reference to mass occurrence of jellyfish. Bull. Plankt. Soc. Jpn. 2001, 48, 55-61.
- Wang, S.; Zhang, G.; Sun, S.; Wang, Y.; Zhao, Z. Population dynamics of three Scyphozoan Jellyfish species during summer of 2011 in Jiaozhou Bay. Oceanol. Limnol. Sin. 2012, 43, 471-479.
- Sun, M.; Dong, J.; Purcell, J.E.; Li, Y.; Duan, Y.; Wang, A.; Wang, B. Testing the influence of previous–year temperature and food supply on development of Nemopilema nomurai blooms. Hydrobiologia 2015, 754, 85–86. http://doi.org/10.1007/s10750-014-2046-7. [CrossRef]
- Lee, S.H.; Hwang, J.S.; Kim, K.Y.; Molinero, J.C. Contrasting Effects of Regional and Local Climate on the Interannual Variability and Phenology of the Scyphozoan, Aurelia coerulea and Nemopilema nomurai in the Korean Peninsula. Diversity 2021, 13, 214. http://doi.org/10.3390/d13050214. [CrossRef]
- Helm, R.R. Evolution and development of scyphozoan jellyfish. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1228-1250. http://doi.org/10.1111/brv.12393. [CrossRef]
- Feng, S.; Wang, S.; Sun, S.; Zhang, F.; Zhang, G.; Mengtan, L.; Uye, S-I. Strobilation of three scyphozoans (Aurelia coelurea, Nemopilema nomurai and Rhopilema esculentum) in the field at Jiaozhou Bay, China. Mar. Ecol. Prog. Ser. 2018, 591, 141-153. http://doi.org/10.3354/meps12276. [CrossRef]
- Xing, Y.; Liu, Q.; Zhang, M.; Zhen, Y.; Mi, T.; Yu, Z. Effects of temperature and salinity on the asexual reproduction of Aurelia coerulea polyps. J. Oceanol. Limnol. 2019, 38, 133-142. http://doi.org/10.1007/s00343-019-8337-0. [CrossRef]
- Loveridge, A.; Lucas, C.H.; Pitt, K.A. Shorter, warmer winters may inhibit production of ephyrae in a population of the moon jellyfish Aurelia Aurita. Hydrobiologia 2021, 848, 739-749. https://doi.org/10.1007/s10750-020-04483-9. [CrossRef]
- Takauchi, S.; Miyake, H.; Hirata, N.; Nagai, M.; Suzuki, N.; Ogiso, S.; Ikeguchi, S. Morphological characteristics of ephyrae of Aurelia coerulea derived from planula strobilation. Fish. Sci. 2021, 87, 617- 679. http://doi.org/10.1007/s12562-021-01541-6. [CrossRef]
- Schäfer, S.; Gueroun, S.K.M.; Andrade, C.; Canning-Clode, J. Combined Effects of Temperature and Salinity on Polyps and Ephyrae of Aurelia solida (Cnidaria: Scyphozoa). Diversity 2021, 13, 573. http://doi.org/10.3390/d13110573. [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Perez-Ruzafa, A. Reconstructing the Biogeographic History of the Genus Aurelia Lamarck, 1816 (Cnidaria, Scyphozoa), and Reassessing the Nonindigenous Status of A. solida and A. coerulea in the Mediterranean Sea. Diversity 2023, 15, 1181. http://doi.org/10.3390/d15121181. [CrossRef]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2017, 180, 243-267. http://doi.org/10.1111/zoj.12494. [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Pérez-Ruzafa, A. The unpredictability of scyphozoan jellyfish blooms. Front, Mar. Sci 2024, 11. http://doi.org/10.3389/fmars.2024.1349956. [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Perez-Ruzafa, A. Larger scyphozoan species dwelling in temperate, shallow waters show higher blooming potential. Mar. pollut. Bull. 2021, 173, 113100. http://doi.org/10.1016/j.marpolbul.2021.113100. [CrossRef]
- Boero, F.; Brotz, L.; Gibbons, M.J.; Piraino, S.; Zampardi, S. 3.10 Impacts and Effects of Ocean Warming on Jellyfish. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; IUCN: Gland, Switzerland, 2016; pp. 213-237. Available online: http://www.researchgate.net/publication/309209466.
- Avian, M.; Motta, G.; Prodan, M.; Tordoni, E.; Macaluso, V.; Beran, A.; Goruppi, A.; Bacaro, G.; Tirelli, V. Asexual reproduction and strobilation of S. malayensis (Scyphozoa, Pelagiidae) in relation to temperature: Experimental evidence and implications. Diversity 2021, 13, 37. https://doi.org/10.3390/d13020037. [CrossRef]
- Adler, L.; Jarms, G. New insights into reproductive traits of scyphozoans: Special methods of propagation in S. malayensis Goette, 1886 (Pelagiidae, Semaeostomeae) enable establishing a new classification of asexual reproduction in the class Scyphozoa. Mar Biol 2009, 156, 1411-1420. https://doi.org/10.1007/s00227-009-1181-6. [CrossRef]
- Morandini, A.C.; Gul, S. Rediscovery of S. malayensis and remarks on Rhopilema nomadica record in Pakistan (Cnidaria: Scyphozoa) Pap. Avulsos Zool 2016, 56, 171-175. https://doi.org/10.1590/0031-1049.2016.56.15. [CrossRef]
- Kramp, P.L. Synopsis of the medusae of the world. J. Mar. Biol. Assoc. UK 1961, 40, 7-382.
- Gershwin, L.A.; Zeidler, W. Two new jellyfishes (Cnidaria: Scyphozoa) from tropical Australian waters. Zootaxa 2008, 1764, 41-52. https://doi.org/10.5281/zenodo.181987. [CrossRef]
- Uchida, T. Medusae in the vicinity of the Amakusa Marine Biological Station. Bull. Biogeogr. Soc. Japan 1938, 8, 143–149.
- Miyake, H.; Hashimoto, J.; Chikuchishin, M.; Miura, T. Scyphopolyps of Sanderia malayensis and Aurelia aurita attached to tubes of vestimentiferan tubeworm (Lamellibrachia satsuma) at submarine fumaroles in Kagoshima Bay. Mar. Biotechnol. 2004, 6, S174-S178. Straehler-Pohl, I.; Widmer, C.L.; Morandini, A.C. Characterizations of juvenile stages of some semaeostome Scyphozoa (Cnidaria), with recognition of a new family (Phacellophoridae). Zootaxa 2011, 2741, 1-37. https://doi.org/10.11646/zootaxa.2741.1.1. [CrossRef]
- Schiariti, A.; Morandini, A.C.; Jarms, G.; Paes, R.V.G.; Franke, S.; Mianzan, H. Asexual reproduction strategies and blooming potential in Scyphozoa. Mar. Ecol. Prog. Ser. 2014, 510, 241-253. http://doi.org/10.3354/meps10798. [CrossRef]
- Shin, K.H.; Choi, K.H. Effects of the seawater temperature and salinity on the survival and growth of the Sanderia malayensis (Cnidaria: Scyphozoa) ephyrae. Mar. Biol. Res. 2025, in press (accepted). https://doi.org/10.1080/17451000.2025.2462828. [CrossRef]
- Kubota, S. Appearance of Sanderia malayensis at Shirahama, Wakayama Prefecture, Japan after half a century absence. Nanki Biol. Soc. 2012, 54, 147-148.
- Minemizu, R.; Kobota, S.; Hirano, Y.; Lindsay, D.J. A photographic guide to the jellyfishes of Japan [Japanese]; Heibonsha: Tokyo, Japan, 2015; pp. 268.
- Toshino, S.; Yamashiro, H.; Tanimoto, M. New record of Sanderia malayensis from the Ryukyu Archipelago, southern Japan. Fauna Ryukyuana 2018, 43, 19-25.
- Båmstedt, U.; Lane, J.; Martinussen, M.B. Bioenergetics of ephyra larvae of the scyphozoan jellyfish Aurelia aurita in relation to temperature and salinity. Mar. Biol. 1999, 135, 89-98. http://doi.org/10.1007/s002270050605. [CrossRef]
- Widmer, C.L. Influences of temperature and salinity on asexual reproduction and development of scyphozoan jellyfish from the British Isles. Dissertation, University of St Andrews, 2014; pp. 72-85.
- Fuchs, B.; Wang, W.; Graspeuntner, S.; Li, Y.; Insua, S.; Herbst, E-M.; Dirksen, P.; Boehm, A-M.; Hemmrich, G.; Sommer, F.; et al. Regulation of polyp-to-jellyfish transition in Aurelia aurita. Curr. Biol. 2014, 24, 263-273. http://doi.org/10.1016/j.cub.2013.12.003. [CrossRef]
- Uchida, T.; Sugiura, Y. On the ephyra and postephyra of Semaeostome medusa, Sanderia malayensis Goette, 1886. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1975, 19, 879-881. Uchida, T.; Sugiura, Y. On the polyp of the Scyphomedusa, Sanderia malayensis and its reproduction. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1978, 21, 279-286.
- Riisgård, H.U. Superfluous Feeding and Growth of Jellyfish Aurelia aurita. J Mar Sci Eng 2022, 10, 1368. https://doi.org/10.3390/jmse10101368. [CrossRef]
- Straehler-Pohl, I.; Jarms, G. Identification key for young ephyrae: A first step for early detection of jellyfish blooms. Hydrobiologia 2010, 645, 3-21. http://doi.org/10.1007/s10750-010-0226-7. [CrossRef]
- Båmstedt, U.; Ishii, H.; Martinussen, M.B. Is the scyphomedusa Cyanea capillata (L.) dependent on gelatinous prey for its early development? Sarsia 1997, 82, 269-273.
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only Anova Procedures. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 143-146. http://doi.org/10.1145/1978942.1978963. [CrossRef]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. An Aligned Rank Transform procedure for multifactor contrast tests. In Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology, 10-14 October 2021. http://doi.org/10.1145/3472749.3474784. [CrossRef]
- Therneau, T.M.; Grambsch, P.M. The cox model. In Modeling Survival Data: Extending the Cox Model; Springer: Berlin/Heidelberg, Germany, 2000; pp. 39-77. http://doi.org/10.1007/978-1-4757-3294-8. [CrossRef]
- Heins, A.; Sötje, I.; Holst, S. Assessment of investigation techniques for scyphozoan statoliths, with focus on early development of the jellyfish Sanderia malayensis. Mar. Ecol. Prog. Ser. 2018, 591, 37-56.
- Ballesteros, A.; Siles, P.; Jourdan, E.; Gili, J-M. Versatile aquarium for jellyfish: A rearing system for the biomass production of early life stages in flow-through or closed systems. Front Mar Sci 2022, 9, 942094. https://doi.org/10.3389/fmars.2022.942094. [CrossRef]
- Crow, J.; Howard, M.; Lévesque, V.; Matsushige, L.; Spina, S.; Schaadt, M.; Sowinski, N, Widmer, C.L et al. AZA Aquatic Invertebrate TAG. In Jellyfish (Cnidaria/Ctenophora) Care Manual; Schaadt, M., Ed.; Association of Zoos and Aquariums (AZA): Silver Spring, MD, USA, 2013; pp. 5-79.
- Purcell, J.E.; White, J.R.; Nemazie, D.A.; Wright, D.A. Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Mar. Ecol. Prog. Ser. 1999, 180, 187-196. http://doi.org/10.3354/meps180187. [CrossRef]
- Fu, Z.; Li, J.; Wang, J.; Lai, J.; Liu, Y.; Sun, M. Combined effects of temperature and salinity on the growth and pulsation of moon jellyfish (Aurelia coerulea) ephyrae. Am. J. Life Sci. 2020, 8, 144-151. https://doi.org/10.11648/j.ajls.20200805.19. [CrossRef]
- Yu, S.; Song, D.; Fan, M.; Xie, C. Effects of temperature and salinity on growth of Aurelia aurita. Ecol. Model. 2023, 476, 110229. http://doi.org/10.1016/j.ecolmodel.2022.110229. [CrossRef]
- Duarte, I.M.; Marques, S.C.; Leandro, S.M.; Calado, R. An overview of jellyfish aquaculture: For food, feed, pharma and fun. Rev. Aquac. 2022, 14, 265–287. http://doi.org/10.1111/raq.12597. [CrossRef]
- Miranda, F.A.S.; Chambel, J.; Almeida C.; Pires, D.; Dauarte I.; Esteves, L.; Maranhão, P. Effect of different diets on growth and survival of the white-spotted jellyfish, Phyllorhiza punctata. Front. Mar. Sci. 2016, 3. http://doi.org/10.3389/conf.FMARS.2016.04.00042. [CrossRef]
- Widmer, C.L. Effects of temperature on growth of north-east Pacific moon jellyfish ephyrae, Aurelia labiata (Cnidaria: Scyphozoa). J. Mar. Biol. Assoc. United Kingd. 2005, 85, 569-573. https://doi.org/10.1017/S0025315405011495. [CrossRef]
- Experimental design: Breeding Air Kreisel tank setup for S.malayensis ephyrae.
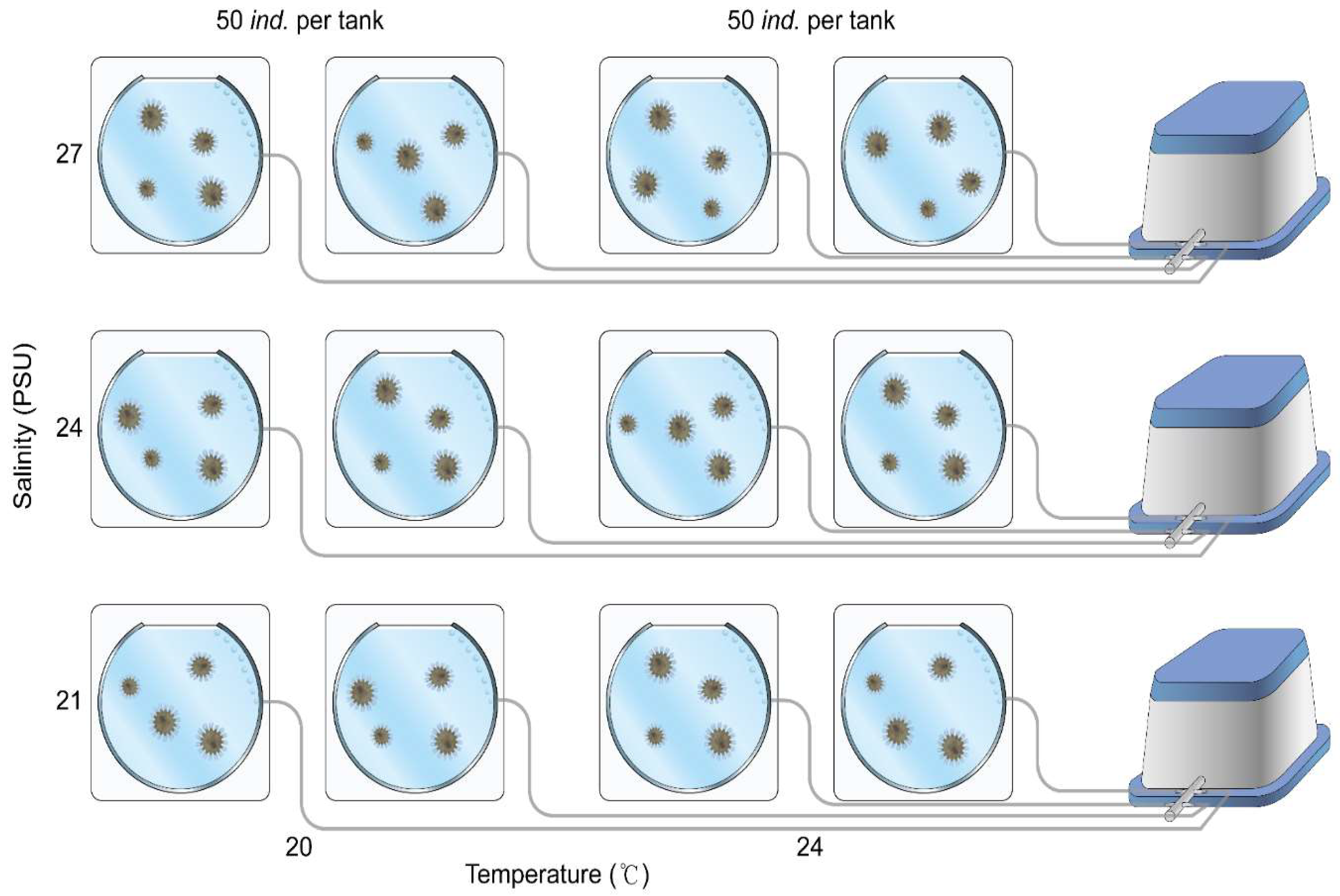
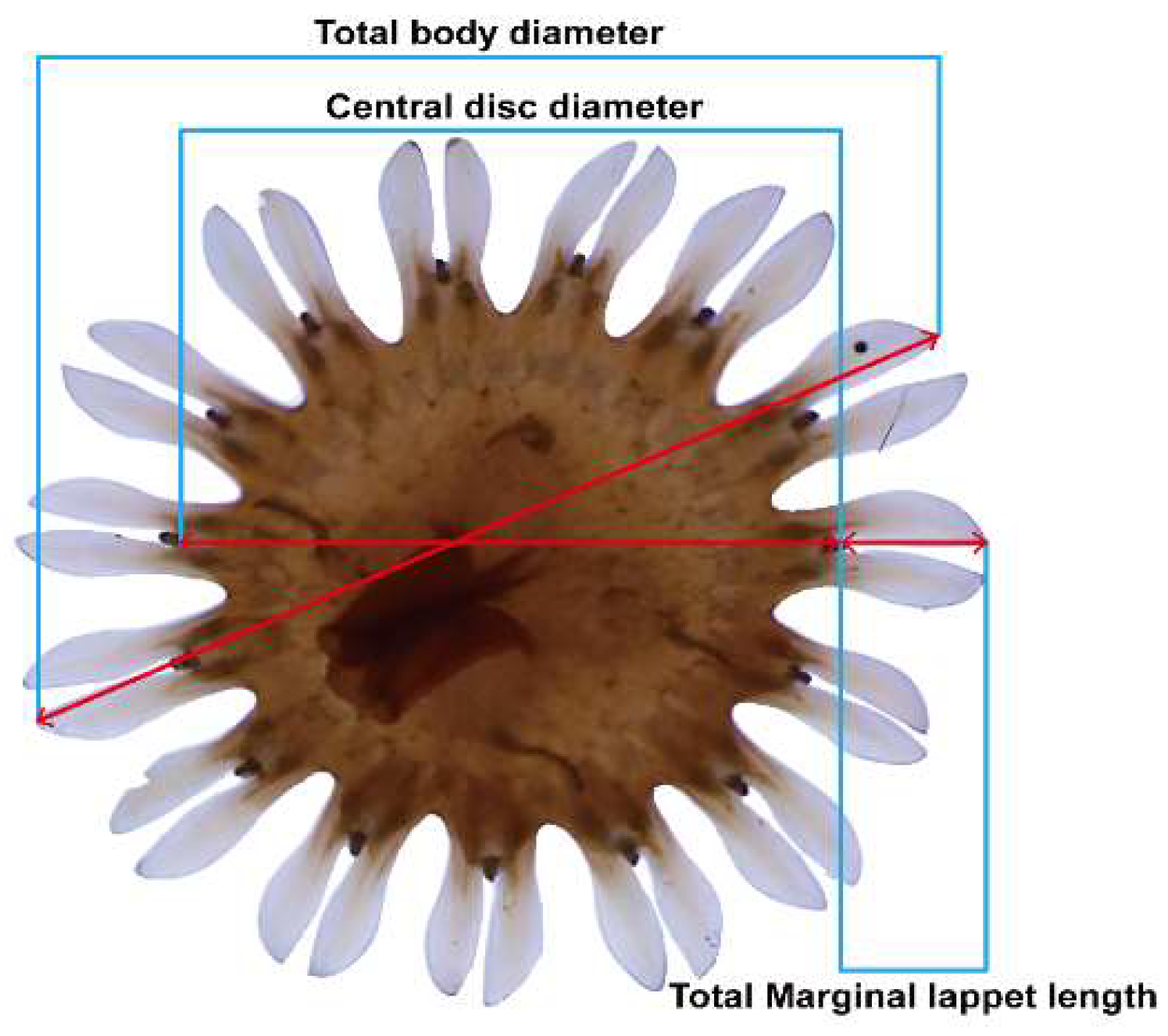
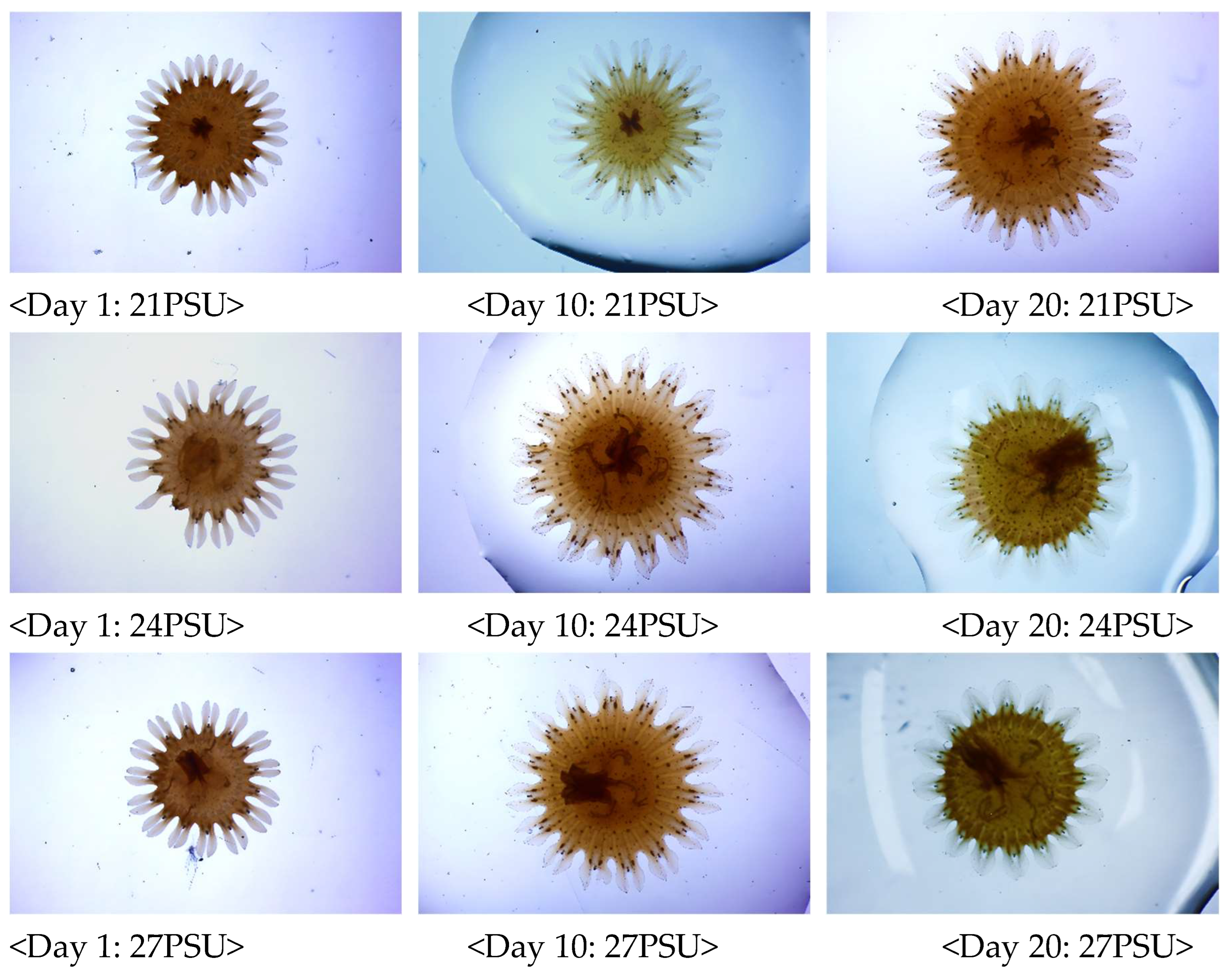
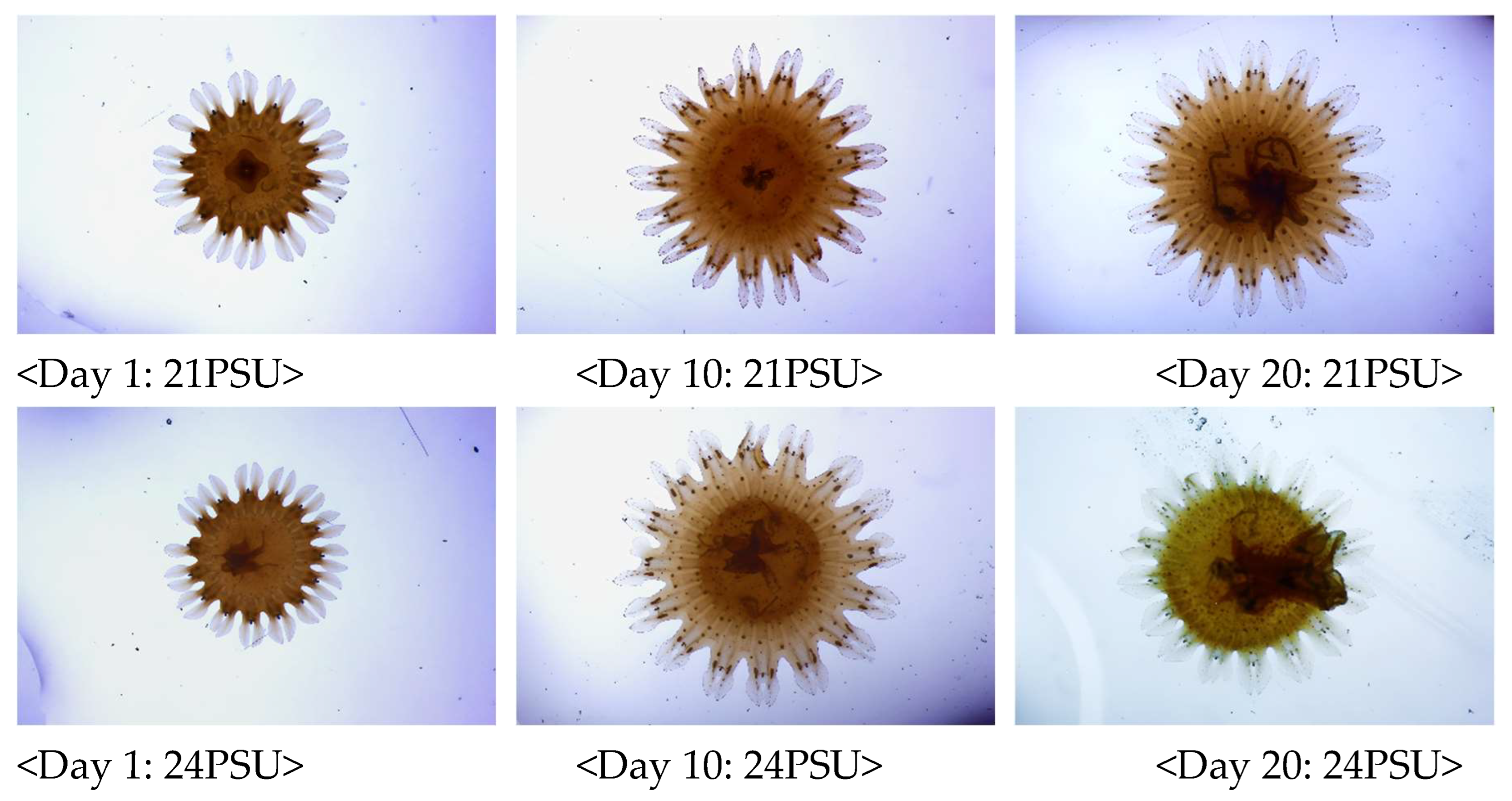



| Temperature (°C) | Salinity (PSU) |
Size Increase (mm, n = 10) | 95% Confidence interval | |||
|---|---|---|---|---|---|---|
| Mean SD | Lower Limit (CI) |
Upper Limit (CI) |
||||
| 20 | 21 | 6.34a | 0.14 | 6.10 | 6.70 | |
| 24 | 8.47b | 0.15 | 8.20 | 8.70 | ||
| 24 | 27 | 8.51b | 0.13 | 8.30 | 8.70 | |
| 21 24 27 |
6.35a 8.50b 8.50b |
0.14 0.13 0.13 |
6.108.30 8.30 |
6.608.70 8.70 |
||
| Temperature (°C) | Salinity (PSU) |
95% Confidence interval | ||||
|---|---|---|---|---|---|---|
| Growth rate (% d-1, n = 10) |
Lower Limit (CI) | Upper Limit (CI) | ||||
| 20 | 21 | 10.31 | 10.19 | 10.71 | ||
| 24 | 14.88 | 14.83 | 14.86 | |||
| 24 | 27 | 14.86 | 14.83 | 15.05 | ||
| 21 24 27 |
10.24 14.80 14.94 |
10.19 14.38 14.38 |
10.47 15.05 15.05 |
|||
| Temperature(°C) Source | Size Increase (mm, n = 10) | |||||
|---|---|---|---|---|---|---|
| Degrees of freedom |
Sum of Squares F-value | p-Value | ||||
| Time | 2 | 15988153.54 633.97 | <0.001 | |||
| 20 Salinity | 10 | 23703043.10 6023.03 | <0.001 | |||
| Salinity × Days | 20 | 20791713.35 234.03 | <0.001 | 9 | <0.001 | |
| Time | 2 | 15974650.91 633.98 | <0.001 | 2 | <0.001 | |
| 24 Salinity | 10 | 23715381.33 6284.18 | <0.001 | 18 | <0.001 | |
| Salinity × Days | 20 | 20437093.72 207.09 | <0.001 | |||
| Temperature (°C) | Salinity (PSU) |
Feeding rate (Artemia ind-1 h-1, n = 10) |
95% Confidence interval | |||
|---|---|---|---|---|---|---|
| Mean SD | Lower Limit (CI) |
Upper Limit (CI) |
||||
| 20 | 21 | 2.10a | 0.908 | 0.00 | 4.00 | |
| 24 | 7.58b | 1.138 | 5.00 | 10.00 | ||
| 24 | 27 | 7.69b | 1.165 | 4.00 | 10.00 | |
| 21 24 27 |
2.16a 7.75b 7.64b |
0.869 1.118 1.136 |
0.00 5.00 5.00 |
5.00 10.00 11.00 |
||
| Source | Feeding rate (Artemia ind-1 h-1, n = 10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of squares | Degrees offreedom | Mean ofsquares | F-value | p-Value | ||||||
| Time | 4489 | 2 | 2245 | 2029.04 | <0.001 | |||||
| Salinity | 35 | 10 | 3 | 3.16 | <0.001 | |||||
| Salinity × Days | 33 | 20 | 2 | 1.47 | 0.085 | 9 | <0.001 | |||
| Source | Feeding rate (Artemia ind-1 h-1, n = 10) | |||||||||
| Degress of freedom | Sum of squares |
F-value | p-Value | |||||||
| Time | 2 | 15983505.68 | 633.18 | <0.001 | ||||||
| Salinity | 10 | 1186144.58 | 3.27 | <0.001 | ||||||
| Salinity × Days | 20 | 1927731.32 | 2.75 | <0.001 | 9 | <0.001 | ||||
| Temperature (°C) | Salinity(PSU) | 95% Confidence interval | ||||
|---|---|---|---|---|---|---|
| Survival rate (%) | ObservedEvent | Lower CI | Upper CI | |||
| 20 | 21a | 70 30 | 0.62 | 0.80 | ||
| 24b | 90 10 | 0.84 | 0.96 | |||
| 24 | 27c | 87 13 | 0.81 | 0.94 | ||
| 21a 24b 27c |
55 45 79 21 86 14 |
0.46 0.69 0.79 |
0.66 0.86 0.93 |
|||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





