Submitted:
05 March 2025
Posted:
05 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
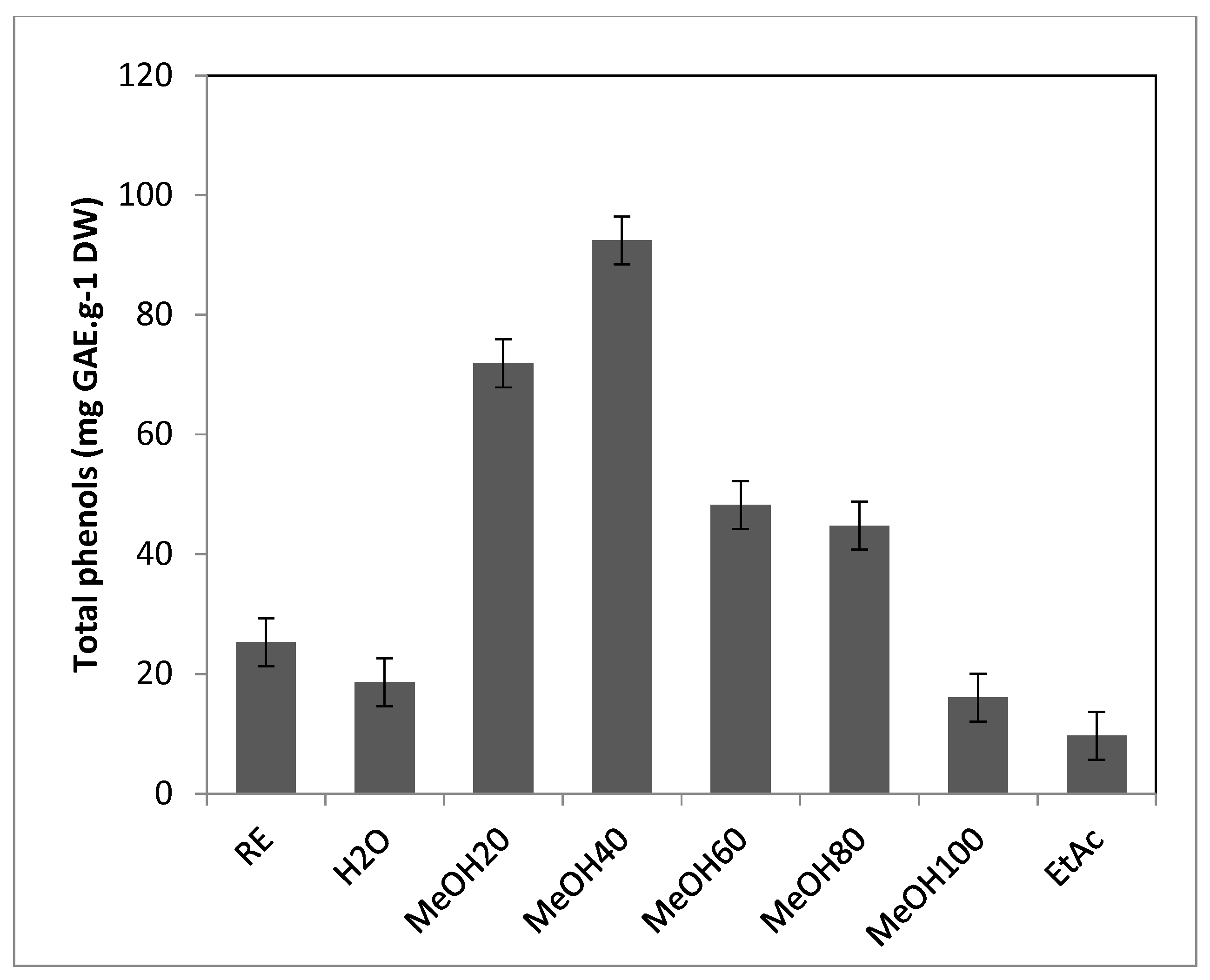
2.1. Total Phenolic Content
2.2. Antioxidant Activities
2.3. Other Biological Activities
2.3.1. Anti-Ageing Activity
2.3.2. Anti-Inflammatory Activity
2.3.3. Neuroprotective Activity
2.3.4. Antidiabetic and Anti-Obesity Activities
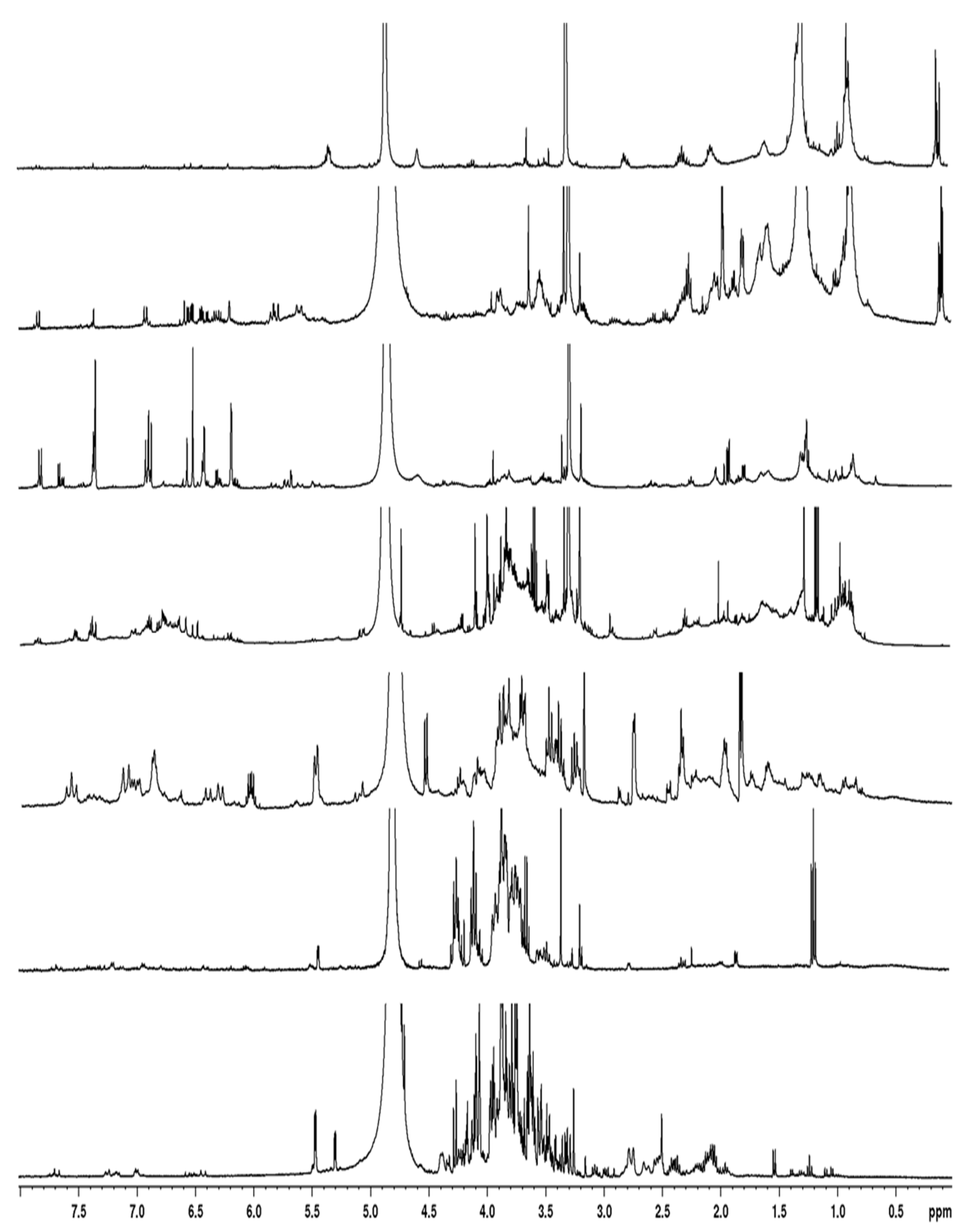
2.4. Solute Identification
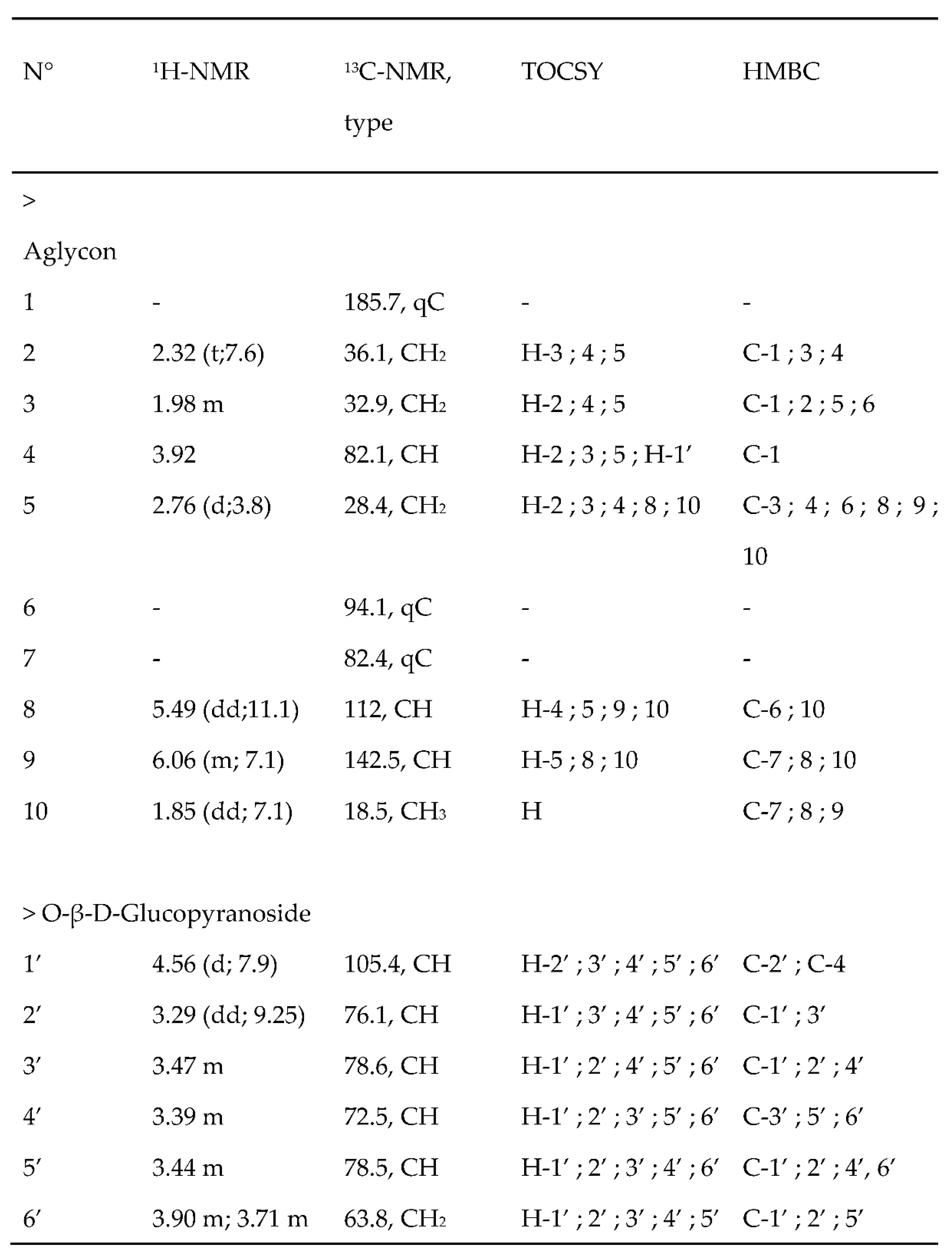
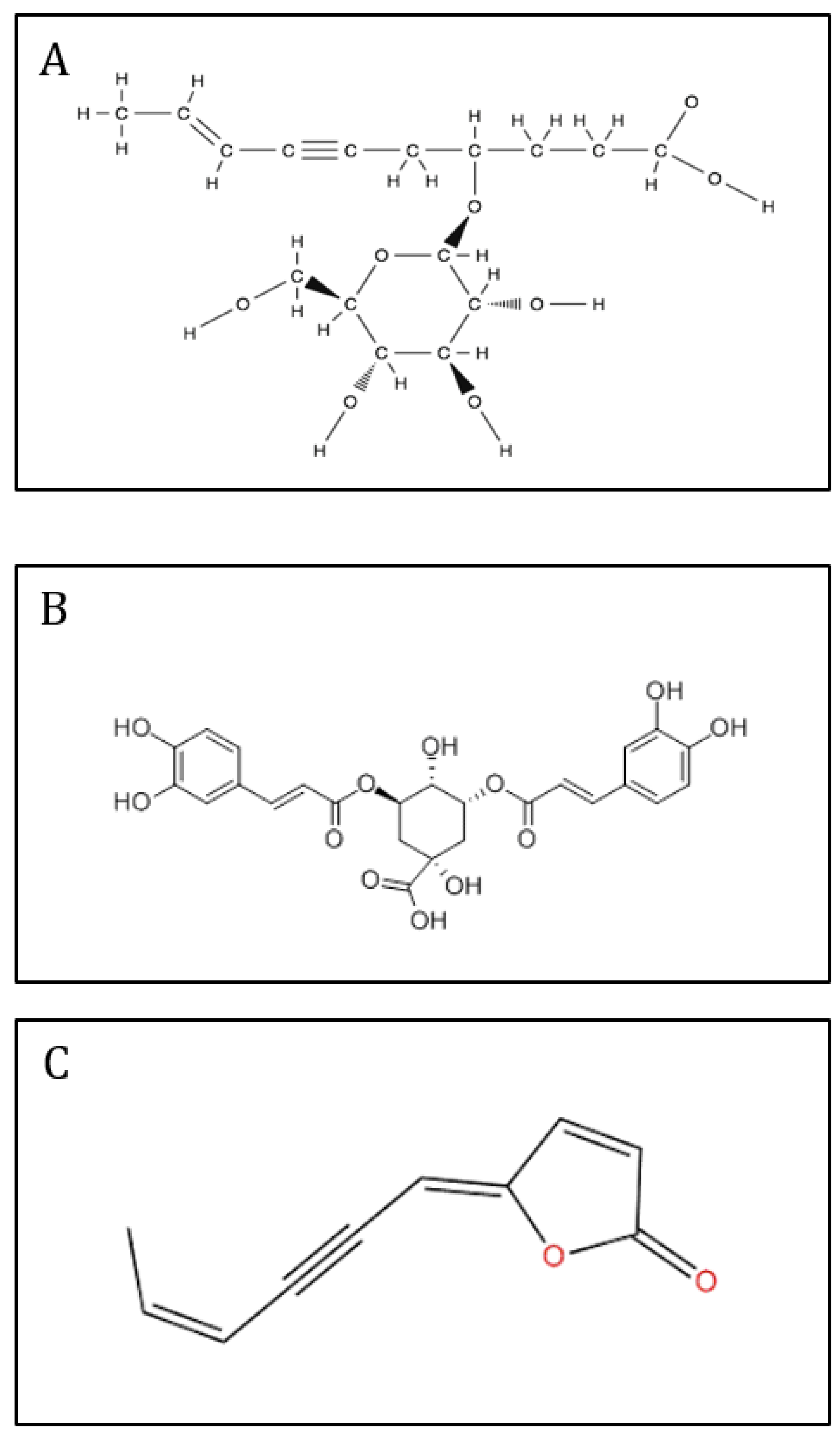
3. Discussion
4. Materials and Methods
4.1. Chemicals, Culture Media and Supplements
4.2. Plant Material
4.3. Extraction and Fractionation
4.4. Antioxidant Activities
4.4.1. DPPH Scavenging Activity
4.4.2. Ferric Reducing Activity (FRAP)
4.4.3. ABTS Scavenging Activity
4.5. Anti-Ageing Activity
4.6. Neuroprotective Activity
4.7. Anti-Diabetic Activity
4.8. Anti-Inflammatory Activity
4.9. NMR Analyses
4.10. Mass Spectrometry Analysis
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Conflict of Interest
Abbreviations
References
- Applequist, W.L. A Reassessment of the Nomenclature of Matricaria L. and Tripleurospermum Sch. Bip. (Asteraceae). Taxon 2002, 51(4), 757. [CrossRef]
- Oberprieler, C.; Himmelreich, S.; Vogt, R. A new subtribal classification of the tribe Anthemideae (Compositae). Willdenowia 2007, 37, 89–114. [CrossRef]
- Kim, S.; Jung, E.; Kim, J.H.; Park, Y.H.; Lee, J.; Park, D. Inhibitory effects of (-)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580-2585.
- Bulgari, M.; Sangiovanni, E.; Colombo, E.; Maschi, O.; Caruso, D.; Bosisio, E.; Dell’Agli, M. Inhibition of Neutrophil Elastase and Metalloprotease-9 of Human Adenocarcinoma Gastric Cells by Chamomile (Matricaria recutita L.) Infusion. Phytother. Res. 2012, 26, 1817-1822. [CrossRef]
- Ranpariya, V.L.; Parmar, S.K.; Sheth, N.R.; Chandrashekhar, V.M. Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharm. Biol. 2011, 49(7), 696-701. [CrossRef]
- Chandrashekhar, V.M.; Halagali, K.S.; Nidavani, R.B.; Shalavadi, M.H.; Biradar, B.S.; Biswas, D.; Muchchandi, I.S. Anti-allergic activity of German chamomile (Matricaria recutita L.) in mast cell mediated allergy model. J. Ethnopharmacol. 2011, 137, 336-340. [CrossRef]
- Silva, N.; Barbosa, L.; Seito, L.; Fernandes Junior, A. Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat. Prod. Res. 2012, 26, 1510-1514. [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Bezerra Morais-Braga, M.F.; Rocha, J.E.; Melo Coutinho, H.D.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; Yousaf, Z.,; Setzer, W.N.; Verma, D.R.; Martorell, M.; Sureda, A.; Sharifi-Rad, J. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76-88. [CrossRef]
- Matić, I.Z.; Juranić, Z.; Savikin, K.; Zdunić, G.; Nađvinski, N.; Gođevac, D. Chamomile and marigold tea: chemical characterization and evaluation of anticancer activity. Phytother. Res. 2013, 27(6), 852-858. [CrossRef]
- Sebai, H.; Jabri, M.A.; Souli, A.; Rtibi, K.; Selmi, S.; Tebourbi, O.; El-Benna, J.; Sakly, M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J. Ethnopharmacol. 2014, 152(2), 327-32. [CrossRef]
- Zekovic, Z.; Pekic, B.; Lepojevic Z.; Petrovic L. Study of the extraction of chamomile flowers with supercritical carbon dioxide. Chromatographia 1994, 39, 587-590.
- Öztürk, E.; Ozer, H.; Cakir, A.; Mete, E.; Kandemir, A.; Polat, T. Chemical Composition of the Essential Oil of Tripleurospermum corymbosum E. Hossain, an Endemic Species from Turkey. J. Essent. Oil Bear. Pl. 2013, 13, 148-153. [CrossRef]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vincieri, F.; Prucher, D. Characterization of Matricaria recutita L-flower extracts by HPLC-MS and HPLC-DAD analysis. Chromatographia 2000, 51(5), 301-307. [CrossRef]
- Raal, A.; Püssa, T.; Sepp, J.; Malmiste, B.; Arak, E. Content of phenolic compounds in aerial parts of Chamomilla suaveolens from Estonia. Nat. Prod. Commun. 2011, 6(8), 1107-1110. [CrossRef]
- Xie, X.Y.; Wang, R.; Shi, Y.P. Flavonoids from the Flowers of Matricaria chamomilla. Chem. Nat. Compd., 50, 910-911. [CrossRef]
- Kovacik, J.; Repcak, M. Accumulation of coumarin-related compounds in leaves of Matricaria chamomilla related to sample processing. Food Chem. 2008, 111 (3), 755-757. [CrossRef]
- Kovalikova Ducaiova, Z.; Sajko, M.; Mihaličová, S.; Repčák, M. Dynamics of accumulation of coumarin-related compounds in leaves of Matricaria chamomilla after methyl jasmonate elicitation. Plant Growth Regul. 2015, 79, 81-94. [CrossRef]
- Petronilho, S.; Maraschin, M.; Coimbra, M.A.; Rocha, S.M. In vitro and in vivo studies of natural products: A challenge for their valuation. The case study of chamomile (Matricaria recutita L.). Indus. Crops Prod. 2012, 40, 1–12. [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5(9), 82-95.
- Parvini, S.; Hosseini, M.J.; Bakhtiarian, A. The Study of Analgesic Effects and Acute Toxicity of Tripleurospermum disciforme in Rats by Formalin Test. Toxicol. Mech. Method. 2007, 17(9), 575-580. [CrossRef]
- Tofighi, Z.; Molazem, M.; Doostdar, B.; Taban, P.; Shahverdi, A.R.; Samadi, N.; Yassa, N. Antimicrobial Activities of Three Medicinal Plants and Investigation of Flavonoids of Tripleurospermum disciforme. Iran J. Pharm. Res. 2015, 14(1), 225-31.
- Al-Saleem, M.S.; Awaad, A.S.; Alothman, M.R.; Alqasoumi, S.I. Phytochemical standardization and biological activities of certain desert plants growing in Saudi Arabia. Saudi Pharm. J. 2018, 26(2), 198-204. [CrossRef]
- Cérantola, S.; Kervarec, N.; Pichon, R.; Magné, C.; Bessières, M.A.; Deslandes, E. NMR characterisation of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima L. Carbohydr. Res. 2004, 339, 2445-2449. [CrossRef]
- Ksouri, R.; Megdiche, W.; Jallali, I.; Debez, H.; Magné, C.; Isoda, H.; Abdelly, C. Medicinal halophytes: potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32 (4), 289-326.
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 2(3), 181-192. [CrossRef]
- Ćavar Zeljković, S.; Ayaz, F.A.; Inceer, H.; Hayirlioglu-Ayaz, S.; Colak, N. Evaluation of chemical profile and antioxidant activity of Tripleurospermum insularum, a new species from Turkey. Nat. Prod. Res. 2015, 29(3), 293-296. [CrossRef]
- Davies, P.; Bourmaud, A.; Pajot, A.; Baley, C.A preliminary evaluation of Matricaria maritimum fibres for polymer reinforcement. Indus. Crops Prod. 2011, 34, 1652-1654. [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933-956. [CrossRef]
- Méot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum (L.) leaves. Plant Physiol. Bioch. 2009, 47(1), 37-41. [CrossRef]
- Falleh, H.; Oueslati, S.; Guyot, S.; Ben Dali, A.; Magné, C.; Abdelly, C.; Ksouri, R. LC/ESI-MS/MS characterisation of procyanidins and propelargonidins responsible for the strong antioxidant activity of the edible halophyte Mesembryanthemum edule L. Food Chem. 2011, 127(4), 1732-1738. [CrossRef]
- Jdey, A.; Falleh, H.; Ben Jannet, S.; Mkadmini Hammi, K.; Dauvergne, X.; Ksouri, R.; Magné, C. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. S. Afr. J. Bot. 2017a, 112, 508-514. [CrossRef]
- Jdey, A.; Falleh, H.; Ben Jannet, S.; Hammi, K.M.; Dauvergne, X.; Magné, C.; Ksouri, R. Anti-aging activities of extracts from Tunisian medicinal halophytes and their aromatic constituents. EXCLI J. 2017b, 16, 755-769. [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [CrossRef]
- Choi, H.K.; Lim, Y.S.; Kim, Y.S.; Park, S.Y.; Lee, C.H.; Hwang, K.W. Free-radical-scavenging and tyrosinase-inhibition activities of Cheonggukjang samples fermented for various times. Food Chem. 2008, 106, 564–568. [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [CrossRef]
- Song, J.L.; Song, R.K.; Gao, Y.Y. Anti-inflammatory effect of methanolic extract of Conyza canadensis in lipopolysaccharide (LPS)-stimulated RAW264.7 murine macrophage cells. Pak. J. Pharm. Sci. 2016, 29(3), 935-940.
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29-35. [CrossRef]
- Idres, A.Y.; Tousch, D.; Dhuyque-Mayer, C.; Hammad, I.; Lambert, K.; Cazals, G.; Portet, K.; Ferrare, K.; Bidel, L.P.R.; Poucheret, P. An Original Asteraceae Based Infused Drink Prevents Metabolic Syndrome in Fructose-Rat Model. Antioxidants (Basel) 2023, 12(2), 340. [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Plants and natural products for the treatment of skin pigmentation – A review. Planta Med. 2018, 84(14), 988-1006. [CrossRef]
- Shahidi, F.; Yeo, J.D. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19(6), 1573-1589. [CrossRef]
- Vidari, G.; Abdo, S.; Gilardoni, G.; Ciapessoni, A.; Gusmeroli, M.; Zanoni, G. Fungitoxic metabolites from Erigeron apiculatus. Fitoterapia 2006, 77(4), 318-320. [CrossRef]
- Queiroz, S.C.; Cantrell, C.L.; Duke, S.O.; Wedge, D.E.; Nandula, V.K.; Moraes, R.M.; Cerdeira, A.L. Bioassay-directed isolation and identification of phytotoxic and fungitoxic acetylenes from Conyza canadensis. J. Agric. Food Chem. 2012, 60(23), 5893-5898. [CrossRef]
- Ekundayo, O.; Laasko, I.; Hiltunen, R. Essential oil of Ageratum conyzoides. Planta Med. 1988, 54, 55-57.
- Wiedenfeld, H.; Roder, E. Pyrrolizidine Alkaloids from Ageratum conyzoides. Planta Med. 1991, 57, 578-579. [CrossRef]
- Okunade, A.L. Ageratum conyzoides L. (Asteraceae). Fitoterapia 2002, 73, 1-16.
- Magid, A.A.; Voutquenne-Nazabadioko, L.; Bontemps, G.; Litaudon, M.; Lavaud, C. Tyrosinase inhibitors and sesquiterpene diglycosides from Guioa villosa. Planta Med. 2008, 74, 55-60. [CrossRef]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1-13. [CrossRef]
- Stefanis, I.; Hadjipavlou-Litina, D.; Bilia, A.R.; Karioti, A. LC-MS- and NMR-guided isolation of monoterpene dimers from cultivated Thymus vulgaris Varico 3 hybrid and their antityrosinase activity. Planta Med. 2019, 85, 941-946. [CrossRef]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2(4), 320-330. [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition. Technical Communication No. 22. Commonwealth Bureau, London. 2nd ed., 1966.
- Marwah, R.G.; Fatope, M.O.; Mahrooqi, R.A.; Varma, G.B.; Abadi, H.A.; Al-Burtamani, S.K.S. Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem. 2007, 101, 465–470.
- Jimenez-Alvarez, D.; Giuffrida, F.; Vanrobaeys, F.; Golay, P.A.; Cotting, C.; Lardeau, A.; Keely, B.J. High-throughput methods to assess lipophilic and hydrophilic antioxidant capacity of food extracts in vitro. J. Agric. Food Chem. 2008, 56, 3470–3477. [CrossRef]
- Bolanos de la Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197-201. [CrossRef]
- Kim, Y.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechinaldehyd polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256-261. [CrossRef]
- Bouzaiene, N.N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Rodrigues, M.J.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Botryococcus braunii and Nanochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J. Appl. Phycol. 2015, 27, 839–848. [CrossRef]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: new sources of natural inhibitors for public health problems. Ind. Crops Prod. 2016, 83, 39-43. [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [CrossRef]


| Tyrosinase inhibition (mgKAE.g-1DW) |
Melanogenesis inhibition (% at 125 μg.mL-1) |
NO inhibition (IC50, μg.mL-1) |
AchE inhibition (IC50, mg.mL-1) |
Anti-α glucosidase (IC50, mg.mL-1) |
|
|
Raw extract MeOH20 MeOH40 MeOH60 MeOH80 MeOH100 EtAc Arbutin L-NAME Galantamine Acarbose |
294.29 ± 49.56c 178.42 ± 26.98d 452.59 ± 72.73b 527.89 ± 15.65b 472.92 ± 42.70b 707.52 ± 59.97a 719.67 ± 53.45a |
ND ND ND ND ND ND 23.77 ± 1.17b 15.95 ± 0.50a |
ND ND ND 5.69 ± 0.30a 8.63 ± 0.55a ND 103.17 ± 2.08c 27.81 ± 1.93b |
ND ND ND 0.06 ± 0.00b ND ND ND 0.01 ± 0.00a |
ND ND 0.20 ± 0.01 c 0.02 ± 0.00 a 0.57 ± 0.04 d ND 0.06 ± 0.02 b 3.14 ± 0.09 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





