Submitted:
22 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
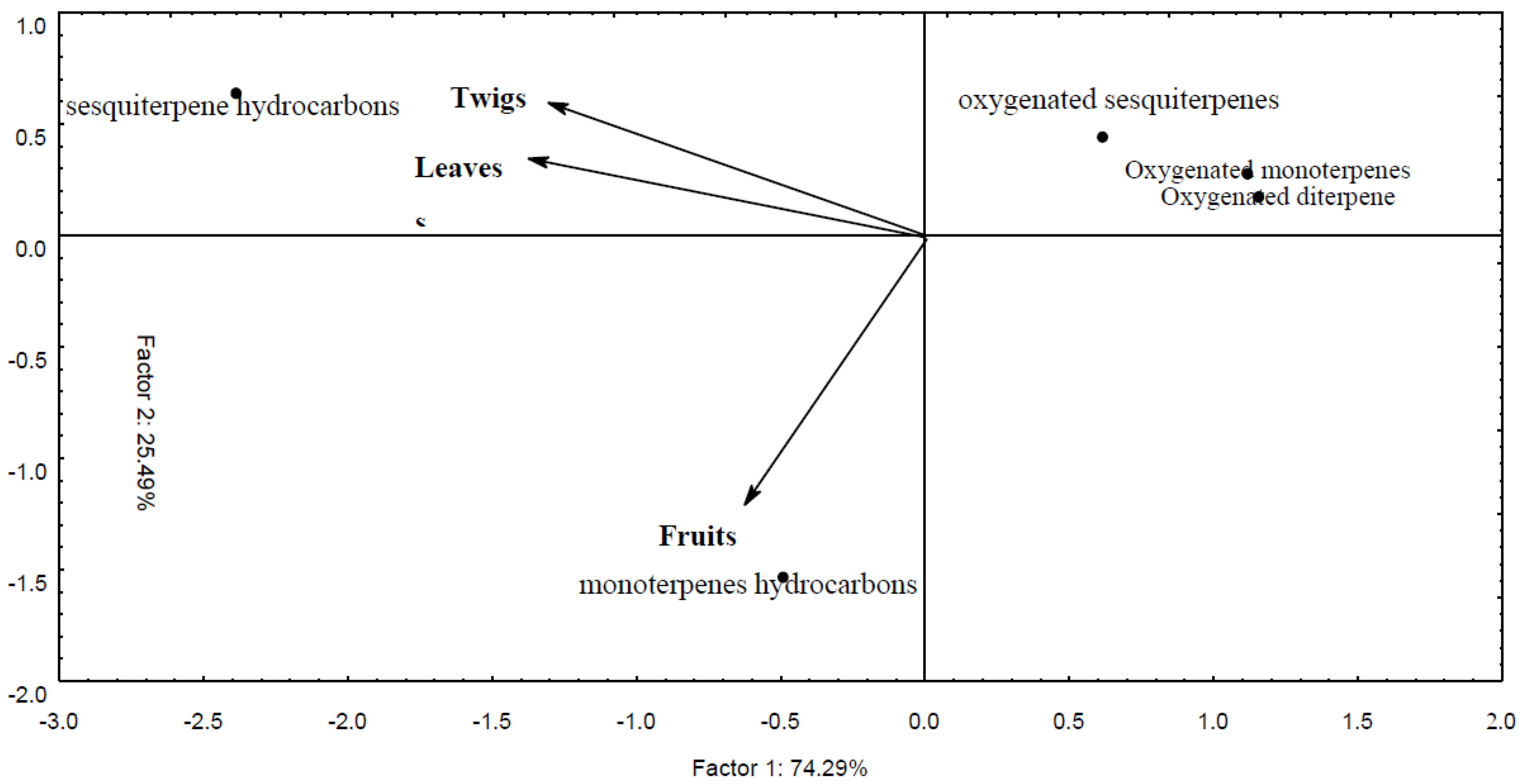
2.1. Yield and Chemical Composition of Essential Oil
2.2. Antioxidant Activity
| Samples | DPPH | FRAP | Total Phenolics |
|---|---|---|---|
| IC50 (mg mL−1) | (µM ferrous sulphate mg−1) | (µg AGE mg−1) | |
| Leaves | 5.368 ± 0.132b | 0.434 ± 0.005b | 23.66 ± 1.60b |
| Fruits | 14.760 ± 0.108d | 0.438 ± 0.002b | 24.79 ± 0.37a |
| Twigs | 12.690 ± 0.483c | 0.437 ± 0.004b | 19.17 ± 0.72c |
| Quercetin | 0.01 ± 0.01a | - | - |
| Trolox | - | 9.175 ± 0.01a | - |
2.3. Antibacterial Activity
| Bacteria | Leaves | Fruits | Twigs | Sodium Nitrite |
|---|---|---|---|---|
| (mg mL−1) | (mg mL−1) | (mg mL−1) | (mg mL−1) | |
| MIC | MIC | MIC | MIC | |
| MBC | MBC | MBC | MBC | |
| Staphylococcus aureus | 1.25 ± 0.00b | 10.00 ± 0.01d | 2.50 ± 0.00c | 5.00 ± 0.00c |
| 5.00 ± 0.01b | 20.00 ± 0.01d | 10.00 ± 0.00c | >20.00 ±0.00d | |
| Escherichia coli | 0.62 ± 0.00b | 10.00 ± 0.00d | 20.00 ±0.002e | 5.00 ± 0.00c |
| 20.00 ± 0.00b | >20.00 ± 0.00b | >20.00 ±0.00b | >20.00 ± 0.00b | |
| Bacillus cereus | 0.62 ± 0.00b | 10.00 ± 0.00d | 10.00 ± 0.00d | 5.00 ± 0.00c |
| 10.00 ± 0.01b | 10.00 ± 0.00b | 20.00 ± 0.00c | >20.00 ± 0.00c | |
| Salmonella Typhi | 2.50 ± 0.00b | 10.00 ± 0.01d | >20.00 ± 0.02e | 5.00 ± 0.00c |
| 20.00 ±.0.00b | >20.00 ± 0.00b | >20.00 ± 0.00b | >20.00 ± 0.01b | |
| Pseudomonas aeruginosa | 2.50 ± 0.00b | 5.00 ± 0.00c | 20.00 ±0.00d | 5.00 ± 0.00c |
| 2.50 ± 0.00b | 10.00 ± 0.00c | 20.00 ±0.00d | >20.00 ± 0.00d |
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius
4.3. Analysis of the Chemical Composition of Essential Oil
4.4. Analysis of the Main Components of Essential Oils
4.5. Antioxidant Activity
4.5.1. Determination of Total Phenol Content (FT)
4.5.2. Free Radical Scavenging Method 2,2 Diphenyl-1-picrylhydrazyl (DPPH)
4.5.3. β-Carotene/Linoleic Acid Co-Oxidation System
4.5.4. Ferrous Reduction Method (FRAP)
4.6. Antibacterial Activity
4.6.1. Microorganisms and Inoculum Preparation
4.6.2. Antibacterial Activity by Broth Microdilution Method
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Žarković, N. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [CrossRef]
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods a review. Int. J. Food Microbiol. 2004, 94, 223- 253. [CrossRef]
- Zantar, S.; Yedri, F.; Mrabet, R.; Laglaoui, A.; Bakkali, M.; Zerrouk, M. H. Effect of Thymus vulgaris and Origanum compactum essential oils on the shelf life of fresh goat cheese. J. Essent. Oil Res. 2014, 26, 76 - 84. [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/facts-in-pictures/detail/food-safety (accessed on 02 December 2023).
- Almeida Filho, E. S.; Nader Filho, A. Occurrence of Staphylococcus aureus in cheese made in Brazil. Rev. Saúde Pública 2000, 34, 578-580. [CrossRef]
- Bulhões, C. C. C.; Rossi Júnior, O. D. Occurrence of the genus Aeromonas in minas frescal cheese. Arq. Bras. Med. Vet. Zootec. 2002 54, 320-324. [CrossRef]
- Komatsu, R. S.; Rodrigues, M. A. M.; Loreno, W. B. N.; Santos, K. A. Ocorrência de Staphylococcus coagulase positiva em queijos Minas frescal produzidos em Uberlândia-MG. Biosci. J. 2010, 26, 316-321.
- Lourenço, S. C.; Moldão-Martins, M.; Alves, V. D. Antioxidants of natural plant origins: from sources to food industry applications. Molecules 2019, 24, 4132. [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K. M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [CrossRef]
- Phaniendra, A.; Jestadi, D. B.; Periyasamy, L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11-26. [CrossRef]
- Andrade, M. A.; Cardoso, M. G.; Batista, L. R.; Mallet, A. C. T.; Machado, S. M. F. Essential oils of Cinnamomum zeylanicum, Cymbopogon nardus and Zingiber officinale: composition, antioxidant and antibacterial activities. Rev. Ciênc. Agron. 2012, 43, 399-408. [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M. A. Synthetic phenolic antioxidants: Metabolism, hazards, and mechanism of action. Food Chem. 2021, 353, 129488. [CrossRef]
- Lenzi, M.; Orth, A. I. Fenologia reprodutiva, morfologia e biologia floral de Schinus terebinthifolius Raddi (Anacardiaceae), em restinga da ilha de Santa Catarina, Brasil. Biotemas 2004, 17, 67-89.
- Cesário, L. F.; Gaglianone, M. C. Pollinators of Schinus terebinthifolius Raddi (Anacardiaceae) in vegetational formations of restinga in northern Rio de Janeiro state. Biosci. J. 2013, 29, 458- 467.
- Oliveira, L. F. M.; Oliveira Junior, L. F. G.; Santos, M. C.; Narain, N.; Leite Neto, M. T. S. Distillation time and volatile profile of the essential oil of Brazilian pepper (Schinus terebinthifolius) in Sergipe, Brazil. Rev. Bras. Plantas Med. 2014, 16, 243-249. [CrossRef]
- Ferreira-Filho, P. J.; Pinã-Rodrigues, F. C. M.; Silva, J. M. S.; Guerreiro, J. C.; Ghiotto, T. C.; Piotrowski, I.; Dias, L. P.; Wilcken, C. F.; Zanuncio, J. The exotic wasp Megastigmus transvaalensis (Hymenoptera: Torymidae): first record and damage on the Brazilian peppertree, Schinus terebinthifolius drupes, in São Paulo, Brazil. An. Acad. Bras. Cienc. 2015, 87, 2091-2095. [CrossRef]
- Carvalho, M. G.; Melo, A. G. N.; Aragão, C. F. S.; Raffin, F. N.; Moura, T. F. A. L. Schinus terebinthifolius Raddi: chemical composition, biological properties and toxicity. Rev. Bras. Plantas Med. 2013, 15, 158-169. [CrossRef]
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Monografia da espécie Schinus terebinthifolius Raddi (Aroeira-da-praia). Anvisa: Brasília, 2014.
- Estevão, L. R. M.; Simões, R. S.; Cassini-Vieira, P.; Canesso, M. C. C.; Barcelos, L. D. S.; Rachid, M. A.; Câmara, C. A. G. D.; Evêncio-Neto, J. Schinus terebinthifolius raddi (aroeira) leaves oil attenuates inflammatory responses in cutaneous wound healing in mice. Acta Cir. Bras. 2017, 32, 726-735. [CrossRef]
- Rosas, E.C.; Correa, L. B.; M.; Henriques, M. G. Antiinflammatory properties of Schinus terebinthifolius and its use in arthritic condition, In Book Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Editor(s): Ronald Ross Watson, R. R.; Victor R. Preedy, V. R., Eds.; Academic Press: London, United Kingdom, 2019; pp. 489-505. [CrossRef]
- Food and Agriculture Organization of the United Nations World Health Organization. Discussion paper on the grouping of spices and culinary herbs. Codex committee on spices and culinary herbs. FAO/WHO, 2015.
- Ruas, E. A.; Ruas, C. F.; Medri, P. S.; Medi, C.; Medri, M. E.; Bianchini, E.; Pimenta, J. A.; Rodrigues, L. A.; Ruas, P. M. Anatomy and genetic diversity of two populations of Schinus terebinthifolius (Anacardiaceae) from the Tibagi River basin in Paraná, Brazil. Genet. Mol. Res. 2011, 10, 526-536. [CrossRef]
- Dannenberg, G. S.; Funck, G. D.; Silva, W. P.; Fiorentini, A. M. Essential oil from pink pepper (Schinus terebinthifolius Raddi): chemical composition, antibacterial activity and mechanism of action. Food Control 2019, 95, 115–120. [CrossRef]
- Bortolucci, W. C.; Oliveira, H. L. M.; Silva, E. S.; Campo, C. F. A. A.; Gonçalves, J. E.; Piau Junior, R.; Colauto, N. B.; Linde, G. A.; Gazim, Z. C. Schinus terebinthifolius essential oil and fractions in the control of Aedes aegypti. Biosci. J. 2019, 35, 391-404. [CrossRef]
- Mohamed, A. A.; Behiry, S. I.; Ali, H. M.; El-Hefny, M.; Salem, M. Z. M.; Ashmawy, N. A. Phytochemicals from branches of the oily liquid extract of P. halepensis and essential oil of S. terebinthifolius and their potential antifungal activity. Processes 2020, 8, 330. [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157-160. [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M. S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49-55. [CrossRef]
- Ennigrou, A.; Casabianca, H.; Vulliet, E.; Hanchi, B.; Hosni, K. Assessing the fatty acid, essential oil composition, their radical scavenging and antibacterial activities of Schinus terebinthifolius Raddi leaves and twigs. J. Food Sci. Technol. 2018, 55, 1582-1590. [CrossRef]
- Belhoussaine, O.; El Kourchi, C.; Harhar, H.; Bouyahya, A.; El Yadini, A.; Fozia, F.; Alotaibi, A.; Ullah, R.; Tabyaoui, M. Chemical composition, antioxidant, insecticidal activity, and comparative analysis of essential oils of leaves and fruits of Schinus molle and Schinus terebinthifolius. Evid. Based Complement. Alternat. Med. 2022, 30, 4288890. [CrossRef]
- Baser, K. H. C.; Buchbauer, G. Handbook of essential oils: science, technology and applications, 1st ed.; CRC Press: Boca Raton/London/New York, USA, 2010; 991 p.
- Nemeth, E.; Bernath J. Biological activities of yarrow species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151-3167. [CrossRef]
- European Pharmacopoeia. Council of Europe, Strasbourg, 2013, ed. VIII, pp. 1-43.
- Bendaoud, H.; Romdhane, M.; Souchard, J. P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466-C472. [CrossRef]
- Becker, E. M.; Nissen, L. R.; Skibsted, L. H. Antioxidant evaluation protocols: food quality or health effects. Eur. Food Res. Technol. 2004, 219, 561-571. [CrossRef]
- Sahin, F.; Gulluce, M.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M.; Agar, G.; Ozer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549-557. [CrossRef]
- Dannenberg, G. D.; Funck, G. D., Mattei, F. J.; Silva, W. P., Fiorentini, Â. M. Antimicrobial and antioxidant activity of essential oil from pink pepper tree (Schinus terebinthifolius Raddi) in vitro and in cheese experimentally contaminated with Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2016, 36, 120-127. [CrossRef]
- Sokmen, A.; Abdel-Baki, A. A. S.; Al-Malki, E. S.; Al-Quraishy, S.; Abdel-Hallem, H. B. Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities. J. King Saud. Univ. Sci. 2020, 32, 2377-2382. [CrossRef]
- Neha, K.; Haider, M. R.; Pathak, A.; Yar, M. S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687-704. [CrossRef]
- Yunfeng, Z.; Lin, L.; Lan, S.; Zhou Lidong, Z.; Yang, X. In comparison with vitamin C and butylated hydroxytoluene, the antioxidant capacity of aqueous extracts from buds and flowers of Lonicera japonica Thunb. J. Tradit. Chin. Med. 2018, 38, 373-379. [CrossRef]
- Kulisic, T.; Radanic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633-640. [CrossRef]
- Hassimotto, N. M. A.; Genovese, M. I.; Lajolo, F. M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928-2935. [CrossRef]
- Yunes, R. A; Cechinel Filho, V. Química de Produtos Naturais, Novos fármacos e a Moderna Farmacognosia, 2nd ed.; Univale: Itajaí, Brasil, 2009; pp. 219-256.
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451-1474. [CrossRef]
- Bajpai, V. K.; Baek, K. H.; Kang, S. C. Control of Salmonella in foods by using essential oils: a review. Food Res. Int. 2012, 45, 722-734. [CrossRef]
- Falleh, H.; Jemaa, M. B.; Saada, M.; Ksouri, R. Essential oils: a promising eco-friendly food preservative. Food Chem. 2020, 330, 1-40. [CrossRef]
- Sousa, E. L. F.; Arias, T. C.; Ferreira, S. B.; Ferreira, P. B.; Lima, Z. N.; Ferreira, S. B. Antibacterial activity and Time-kill kinetics of positive enantiomer of α-pinene against strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917-924. [CrossRef]
- Tundis, R.; Iacopetta, D.; Sinicropi, M. S.; Bonesi, M.; Leporini, M.; Passalacqua, N. G.; Ceramella, J.; Menichini, F.; Loizzo, M. R. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [CrossRef]
- Ceramella, J.; Loizzo, M. R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicano, T. M.; Saturnino, C.; Malzert-Freon, A.; Tundis, R.; Sinicropi, M. S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. J. 2019, 10, 4280–4290. [CrossRef]
- Machado, K. D. C.; Islam, M. T.; Ali, E. S.; Rouf, R.; Uddin, S. J.; Dev, S.; Shilpi, J. A.; Shill, M. C.; Reza, H. M.; Das, A. K. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [CrossRef]
- Novais, C.; Molina, A. K.; Abreu, R. M. V.; Santo-Buelga, C.; Ferreira, I. C. F. R.; Pereira, C.; Barros, L. Natural food colorants and preservatives: a review, a demand, and a challenge. J. Agric. Food. Chem. 2022, 70, 2789-2805. [CrossRef]
- Gundimeda U.; Naidu, A. N.; Krishnaswamy, K. Dietary intake of nitrate in India. J. Food Compos. Anal. 1993, 6, 242–249. [CrossRef]
- Ezeagu, I. E. Nitrate and nitrite contents in ogi and the changes occurring during storage. Food Chem. 1996, 56, 77-79. [CrossRef]
- Zhang, D. S. T.; Lin, X. Accumulation of nitrate in vegetables and its possible implications to human health. Agr. Sci. China, 2007, 6, 1246-1255. [CrossRef]
- Chan, T. Y. K. Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol. Lett. 2011, 200, 107-108, 2011. [CrossRef]
- Santos, M. R. A.; Lima, R. A.; Silva, A. G.; Lima, D. K. S.; Sallet, L. A. P.; Teixeira, C. A. D.; Facundo, V. A. Chemical composition and insecticidal activity of the essential oil of Schinus terebinthifolius Raddi (Anacardiaceae) on coffee berry borer (Hypothenemus hampei) Ferrari. Rev. Bras. Plantas Med. 2013, 15, 757-762. [CrossRef]
- Adams, R. P. Identification of essential oils components by gas chromatography/quadrupole mass spectrometry, 5th ed.; Texensis Publishing: Gruver, United States of America 2017; 698p.
- Moita Neto, J. M.; Moita, G. C. An introduction analysis exploratory multivariate date. Quim. Nova 1998, 21, 467-469. [CrossRef]
- Hair, J. F.; Anderson, R. E.; Tatham, R. L.; Black W, Hair J. Análise multivariada de dados, 1st ed.; Bookman: Porto Alegre, Brasil, 2005; 688 p.
- Ferré, L. Selection of components in principal component analysis: a comparison of methods. Comput. Stat. Data Anal. 1995, 19, 669-682. [CrossRef]
- Camacho, J.; Picó, J.; Ferrer, A. Data understanding with PCA: structural and variance information plots. Chemometr. Intell. Lab. Syst. 2010, 100, 48-56. [CrossRef]
- Statsoft Inc. Statistica for Windows (Computer Program Manual). Available online: http://www.statsoft.com/ (accessed on 20 July 2018).
- Swain, T.; Hillis, W.E. The Phenolic Constituents of Prunus domestica. I.—The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959, 10, 63-68. [CrossRef]
- Sousa De Sá, P.G., Guimarães, A.L., Oliveira, A.P., Filho, J.A.S., Fontana, A.P., Damasceno, P.K.F., Branco, C.R.C., Branco, A., Almeida, J.R.G.S. Total phenols, total flavonoids and antioxidant activity of Selaginella convoluta (Arn.) Spring (Selaginellaceae). Rev. Ciênc. Farm. Básica Apl. 2012, 33, 561-566.
- Rufino, M. S. M.; Alves, R. E., Brito, E. S.; Morais, S. M.; Sampaio, C. G.; Jiménez, J. P.; Calixto, F. D. S. Metodologia científica: determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Embrapa 2007, 127, p. 1–4.
- Rufino, M. S. M.; Alves, R. E.; Brito, E. S.; Mancini Filho, J.; Moreira, A. V. B. Metodologia Científica: determinação da atividade antioxidante total em frutas no sistema β-caroteno/ácido linoleico. Embrapa 2006a, 126, 1-4.
- Benzie, I. F.; Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70-76. [CrossRef]
- Rufino, M. S. M.; Alves, R. E.; Brito, E. S.; Morais, S. M.; Sampaio, C. G.; Jiménez, J. P.; Calixto, F. D. S. Metodologia científica: determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Embrapa 2006b, 125, 1-4.
- Clinical Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard M7-A10. CLSI 2015, 7th.
| Peak | RT1 | Compound | RI2 | RI | RA %3 | ||
|---|---|---|---|---|---|---|---|
| Leaves | Fruits | Twigs | |||||
| 1 | 5.833 | α- pinene | 934 | 932 | 11.6 | 17.16 | 2.99 |
| 2 | 7.13 | β-pinene | 976 | 974 | 5.68 | 43.34 | 5.60 |
| 3 | 7.13 | α- phellandrene | 1003 | 1002 | 0.61 | 0.85 | 0.44 |
| 4 | 8.523 | 3-carene | 1009 | 1008 | 0.79 | - | 0.54 |
| 5 | 8.803 | α-terpinene | 1014 | 1014 | - | - | 0.17 |
| 6 | 9.144 | p-cymene | 1019 | 1020 | - | - | 0.43 |
| 7 | 9.32 | D-limonene | 1018 | 1024 | - | 1.92 | 1.50 |
| 8 | 9.321 | β- phellandrene | 1023 | 1025 | 0.43 | - | 0.66 |
| 9 | 9.324 | eucalyptol | 1025 | 1026 | 1.49 | - | - |
| 10 | 10.274 | β-cis-ocimene | 1039 | 1032 | - | - | 0.26 |
| 11 | 10.717 | γ-terpinene | 1048 | 1054 | - | - | 0.40 |
| 12 | 17.051 | terpinen-4- ol | 1178 | 1174 | - | - | 1.18 |
| 13 | 17.882 | α- terpineol | 1185 | 1186 | - | - | 0.42 |
| 14 | 26.657 | δ-eIemene | 1330 | 1335 | 0.71 | - | - |
| 15 | 27.442 | α- cubebene | 1343 | 1345 | - | - | 1.31 |
| 16 | 28.963 | α-copaene | 1373 | 1374 | 5.25 | - | 5.73 |
| 17 | 29.88 | β-cubebene | 1383 | 1387 | 1.55 | - | 1.94 |
| 18 | 30.03 | β-elemene | 1390 | 1389 | 4.57 | - | 1.44 |
| 19 | 31.549 | α- gurjunene | 1409 | 1409 | 2.15 | 2.12 | 0.82 |
| 20 | 31.549 | caryophyllene | 1418 | 1417 | 15.97 | 3.12 | 11.73 |
| 21 | 32.621 | (-) -aristolene | 1427 | 1428 | - | - | 0.40 |
| 22 | 33.155 | α-himachalene | 1446 | 1449 | - | - | 1.42 |
| 23 | 33.493 | α-humulene | 1452 | 1452 | 1.91 | - | 1.99 |
| 24 | 34.913 | E-β-farnesene | 1454 | 1454 | - | - | 1.31 |
| 25 | 34.212 | aromadendrene-allo | 1459 | 1458 | 2.25 | - | 1.49 |
| 26 | 34.928 | γ-gurjunene | 1475 | 1475 | 16.85 | 3.15 | - |
| 27 | 35.241 | γ-muurolene | 1476 | 1478 | - | - | 1.27 |
| 28 | 35.45 | germacrene D | 1481 | 1484 | 12.04 | 15.78 | 20.41 |
| 29 | 36.435 | valencene | 1495 | 1496 | - | - | 6.38 |
| 30 | 36.569 | α-selinene | 1497 | 1498 | 1.33 | - | - |
| 31 | 37.148 | α-muurolene | 1499 | 1500 | - | - | 0.63 |
| 32 | 37.262 | δ-amorphene | 1509 | 1511 | 4.94 | 3.21 | 0.77 |
| 33 | 37.811 | δ-cadinene | 1503 | 1522 | - | - | 5.59 |
| 34 | 38.212 | cadina-1,4-diene | 1510 | 1524 | - | - | 0.33 |
| 35 | 39.29 | elemol | 1530 | 1548 | - | 2.7 | - |
| 36 | 40.628 | spathulenol | 1573 | 1577 | 3.75 | 1.21 | 5.47 |
| 37 | 40.92 | caryophyllene oxide | 1578 | 1582 | 1.99 | 0.77 | 4.83 |
| 38 | 41.041 | globulol | 1589 | 1590 | 1.11 | - | - |
| 39 | 42.167 | viridiflorol | 1590 | 1592 | - | - | 0.61 |
| 40 | 43.577 | epicubenol | 1603 | 1617 | - | - | 0.66 |
| 41 | 44.316 | t-cadinol | 1618 | 1625 | 1.02 | - | 2.17 |
| 42 | 44.632 | torreyol | 1624 | 1632 | - | - | 0.39 |
| 43 | 45.046 | t-muurulol | 1638 | 1640 | - | - | 1.08 |
| 44 | 69.658 | mandenol | 2151 | 2159 | - | 2.02 | - |
| Total Identified | 97.99 | 97.35 | 94.76 | ||||
| monoterpenes hydrocarbons | 19.11 | 63.27 | 12.99 | ||||
| monoterpenes oxygenated | 1.49 | 0 | 1.6 | ||||
| sesquiterpenes hydrocarbons | 69.52 | 30.08 | 64.96 | ||||
| Sesquiterpenes oxygenated | 7.87 | 1.98 | 15.21 | ||||
| Diterpene oxygenated | 0 | 2.02 | 0 | ||||
| Unidentified | 0.99 | 2.17 | 5.27 | ||||
| Samples | Concentrations (mg mL−1) | |||
|---|---|---|---|---|
| 1 | 0.75 | 0.5 | 0.25 | |
| Leave | 63.65±1.38dC | 55.23±2.25cB | 50.63±2.75bB | 44.93±2.85aA |
| Fruits | 61.52±1.13dB | 54.60±1.27cA | 49.69±2.45aA | 54.09±2.94bC |
| Twigs | 40.75±2.10aA | 58.76±0.71dC | 51.62±2.53cC | 47.95±1.24bB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





