1. Introduction
Urological cancer leads the ranking of the most common neoplasms in Western countries and represent one of the most active test bench for basic and clinical cancer research nowadays. The estimation of new cases in the USA in 2024 include prostate, urinary bladder, and kidney cancer in the top-ten list of the most frequent tumors amounting for 40% of malignancies in men and the expected deaths of prostate and bladder cancer in the male US population in the same period ranks up to 15% [
1]. Prostate cancer (PCa) leads the list of new cases and is the second most common tumor of estimated deaths. GLOBOCAN estimates show similar figures in Europe [
2].
Renal cell carcinoma (RCC), with almost 200,000 deaths per year, is the eighth most common cancer in the US. Tobacco, obesity, and hypertension are well recognized risk factors. The urinary bladder carcinoma (BC) is the most common tumor in the urinary tract, and represents the eleventh most frequent malignancy in the general population (males and females). Occupational exposure to diverse compounds, particularly chlorate hydrocarbons, aromatic amines, polycyclic hydrocarbons, is a well-known risk factor. PCa is the most common malignancy in men with almost 300,000 new cases and the second cause of deaths by cancer with more than 35,000 deaths in the USA. Aside from genetic predisposition and race, there are no evident risk factors detected so far. Penile cancer (PeCa) is quite common in less developed regions of the world but it is a rare neoplasm in USA accounting for less than 1% of cancers in men. Well-known risk factors are human papillomavirus (HPV) infection, tobacco, phimosis, and immunosuppression, i.e., HIV infection. Although testicular cancer (TC) shows also figures under 1%, it is the most common malignancy in young men.
2. Methods
Our aim in this narrative has been to identify the main current avenues in urological cancer research and clinics serving as a start point for more detailed analysis by the interested reader. However, our goal is not to perform the standard literature review, since the enormous amount of literature covering clinical and research topics of urological cancer makes this objective unrealistic within the limits of a standard narrative.
We have tested the urological cancer trends since 2021 by reviewing the original articles published in a selection of widespread outstanding journals in Anatomic Pathology, i.e.,
Modern Pathology,
American Journal of Surgical Pathology,
American Journal of Clinical Pathology,
Journal of Pathology,
Histopathology,
Virchow’s Archives, Kidney Cancer, Archives of Pathology and Laboratory Medicine, Human Pathology,
American Journal of Pathology, and
Annals of Diagnostic Pathology, Urology, i.e.,
Urology, Journal of Urology, European Urology European Urology Oncology, European Urology Focus, and basic science, i.e.,
Cell,
Cell Reports Medicine,
Cancer Cell,
Evolutionary Applications,
Clinical Therapy, Nature Cancer,
Nature Communications, Nature Medicine,
Nature, Ecology & Evolution,
PNAS,
JAMA,
Bulletin of Mathematical Biology, Genome Medicine,
Cancer Research,
Clinical Cancer Research,
Lancet Oncology, and
Cancer Letters. The abstract of 278 articles, including 98 of kidney, 93 of prostate, 55 of urinary tract, 16 of testicle, and 6 of penile cancers have been analyzed to detect the current tendencies in urological basic cancer research and clinics. These hot spots are summarized in
Table 1.
3. 2022 WHO Update in the Pathologic Classification of Urinary and Male Genital Tumors
3.1. Prostate Cancer
2022 WHO classification of PCa [
3] recognizes PIN-like prostate adenocarcinoma as a new form of low-grade acinar adenocarcinoma (Gleason index = 6). Cribriform and/or papillary structures are not a feature in this tumor subtype, which typically presents mutations in
RAF/
RAS. PIN-like adenocarcinoma must be distinguished from ductal adenocarcinoma. The term ductal adenocarcinoma is maintained only in radical prostatectomy specimens with cancers containing more than 50% of ductal morphology. Then, the term “adenocarcinoma with ductal changes” is recommended for cancers detected in core biopsy specimens. Ductal adenocarcinoma may show a wide spectrum of mutations (
ERG,
SPOP,
FOXA1,
CTNBB1, APC, etc.).
The 2022 WHO update [
3] recognizes the neuroendocrine carcinoma secondary to antiandrogenic treatment. To note, this tumor variant develops in up to 17% of the patients treated with androgenic blockade (abiraterone, enzalutamide…). Synaptophysin and/or chromogranin immunohistochemistry is not recommended for its identification in the clinical practice, and most of them are positive with TTF-1, p53, PSA, and prostatic acid phosphatase.
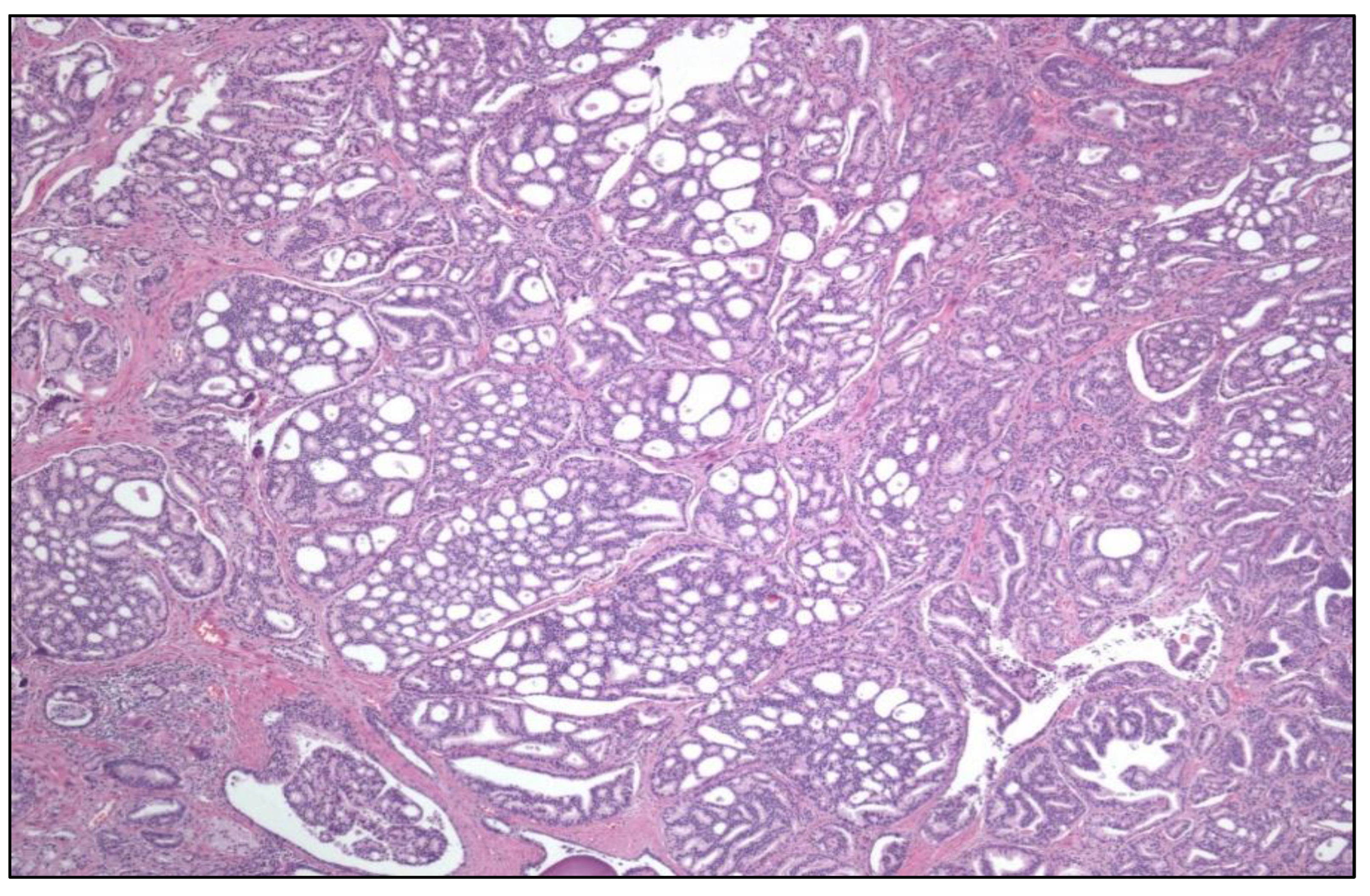
The presence of a cribriform pattern worsens the prognosis of Gleason indexes 3+4, 4+3, and 4+4. Also, large (>12 lumina) cribriform structures (
Figure 1) impact negatively the prognosis of PCa.
Finally, the term “basal cell carcinoma” is no longer used and is changed by “adenoid-cystic carcinoma” based on specific molecular findings.
3.2. Renal Cancer
New renal tumor entities have been included in the long list of RCC [
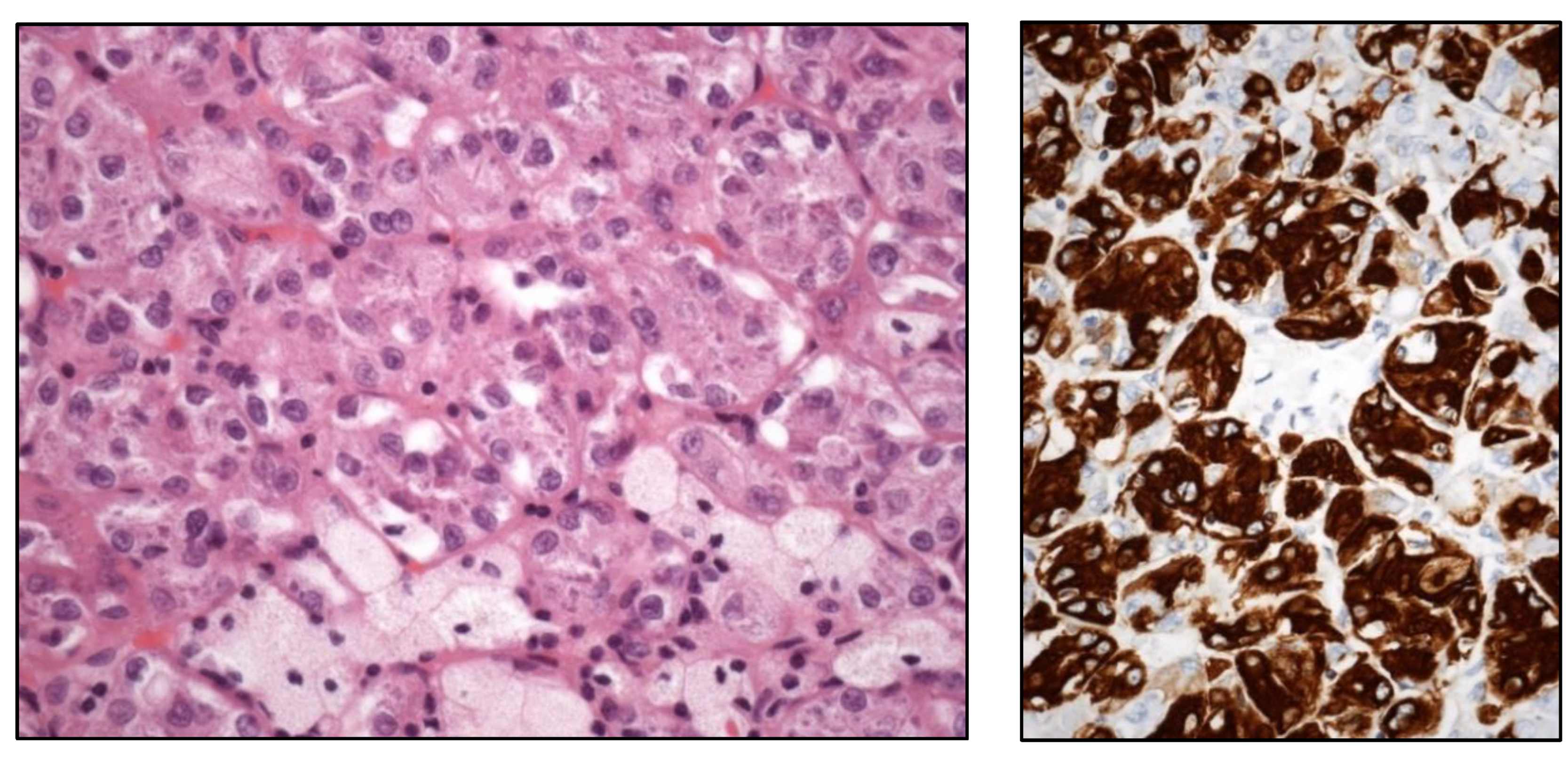
4], i.e., eosinophilic solid and cystic RCC (ESC RCC) (
Figure 2),
ELOC-mutated RCC,
ALK-rearranged RCC,
SMARCB1-deficient medullary RCC,
TFEB-altered RCC, and
FH-deficient RCC.
Papillary RCC (PRCC) classification has been fully rearranged. The formerly called PRCC type 1 is not a unique entity and includes several new variants, i.e., renal neoplasm with inverted polarity (mutated in KRAS), psammomatous hyalinizing RCC (mutated in NF2), biphasic alveolo-squamoid renal RCC, and thyroid-like follicular RCC (EWSR1-PATZ1 fusion). On the other hand, the “old” PRCC type 2 is no longer a specific entity and has been broken down into several new ones, i.e., FH-deficient RCC, tubulo-cystic RCC, eosinophilic solid and cystic RCC (ESC RCC), SMARCB1-deficient medullary RCC, and RCC of the MiTF group.
Several neoplasms are emerging within the spectrum between renal oncocytoma and chromophobe RCC, i.e., SDH-deficient RCC, eosinophilic vacuolated tumor, and low-grade oncocytic tumor.
Finally, the molecular classification of RCC in the 2022 WHO classification of renal tumors is still under construction.
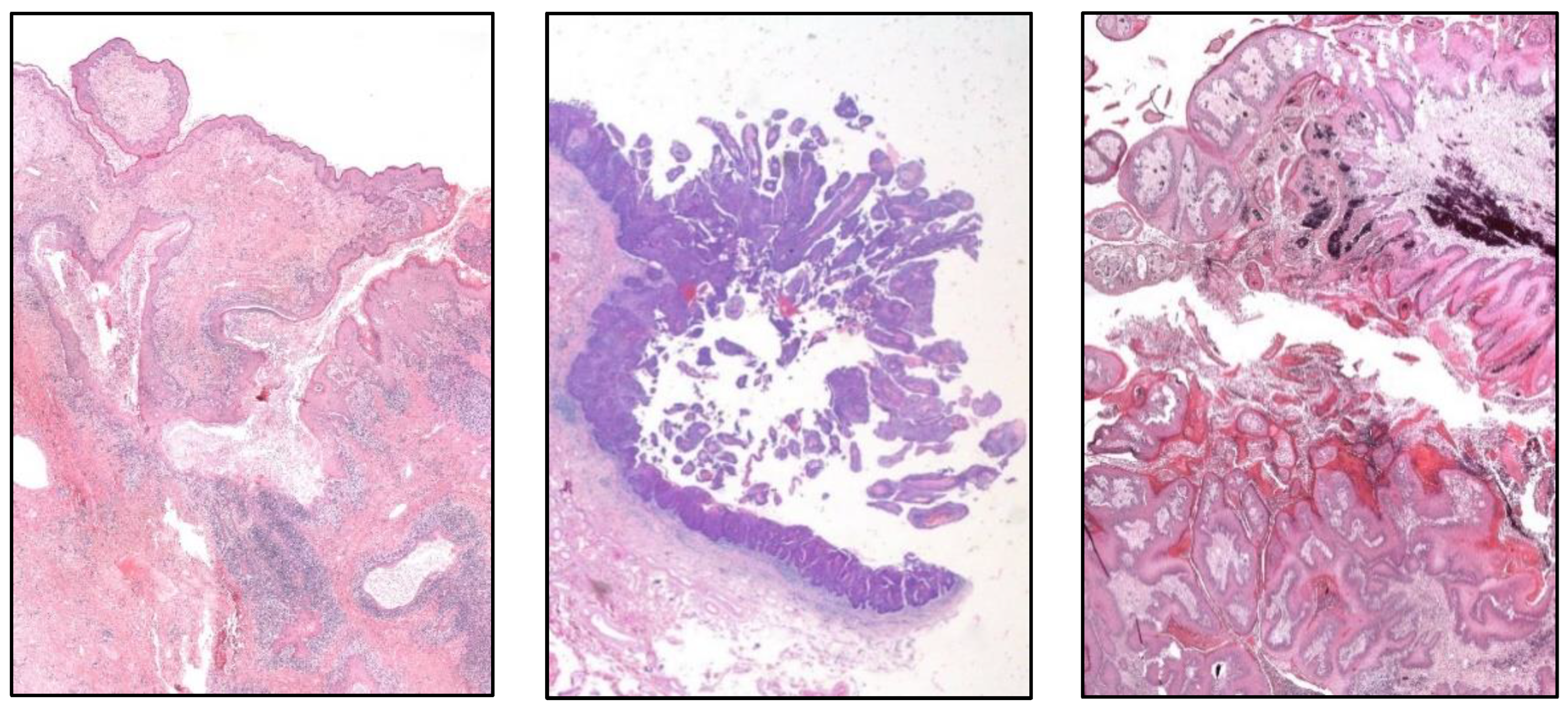
3.3. Urinary Tract Cancer
The 2022 WHO classification of urinary tract carcinoma [
3] maintains the division between high and low grades thus correlating with the cytologic approach of the Paris system [
5].
It is not mandatory to define the level of invasion (pTa vs. pT1) in the low-grade non-invasive papillary urothelial carcinoma (UC). It is important to try to distinguish between inverted growth and true invasion.
The term “papillary urothelial neoplasia of low malignant potential” is maintained and the term urothelial dysplasia is retained, but dysplasia is not a synonym of in situ carcinoma.
The following histological subtypes of UC are specifically recognized:
Conceptual or terminological modifications in pre-existing tumor subtypes are the following:
- -
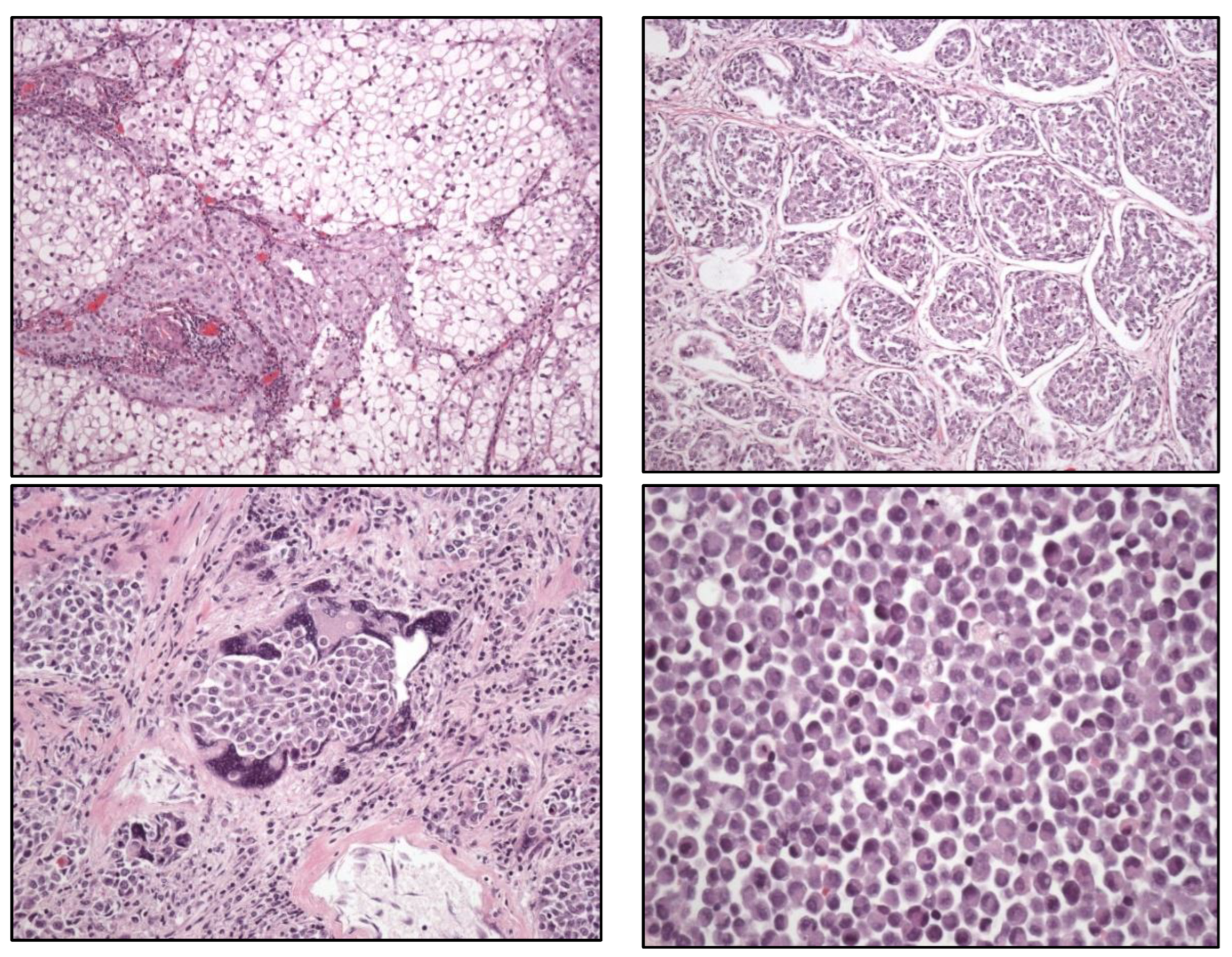
Clear cell UC is renamed as “glycogen-rich” clear cell UC (
Figure 3) to distinguish it from the adenocarcinoma of Müllerian-type.
- -
Plasmacytoid UC should no longer be termed as “signet-ring cell” / “diffuse”.
- -
Glandular, squamous, trophoblastic (
Figure 3), Müllerian, and neuroendocrine morphologies must be specifically mentioned in the pathological report of UC, including its approximate percentage.
- -
Micropapillary, plasmacytoid (
Figure 3), and other “wolves in lamb clothes” must be recognized and specifically reported, either pure or mixed, in the pathological report of UC.
Still non solved issues in the UC diagnosis are:
- -
The sub-staging of pT1 carcinomas (pT1a/pT1b).
- -
The applicability of the molecular classification (luminal, basal, basal-squamous…) in muscle-invasive UC.
- -
The clinical usefulness of FGFR3, TP53, ERCC2 mutations.
3.4. Testicular Cancer
The testis is a territory conditioned by the extreme histological variability. The 2022 WHO classification of TC [
4] recommends not to speak of “variants” but of types and subtypes because “variant” usually refers to genetic mutations. In general terms, the subclassification is maintained with respect to previous editions, although sex-cord tumors and stromal tumors non-gonadoblastoma are no longer accepted in this edition.
Seminoma is included in the group of germinomas. Indeed, seminoma with syncytiotrophoblastic cells deserves to be individualized as a specific subtype, while the rest of seminomas do not. The group of non-seminomatous germ cell tumors does not change (yolk-sac tumor, embryonal carcinoma, trophoblastic tumors).
Consider using mm2 as the surface unit for mitotic counting, and not high-power fields to avoid possible variations in the magnification from one microscope to another.
The term PNET must not be used in the group of teratomas with somatic transformation, neither in the testis nor in the ovary, to avoid the misunderstanding with the PNETs in central nervous system; instead, the name ENET (embryonal neuroectodermal tumor) has been coined for these cases.
Within the group of germ cell tumors not related with
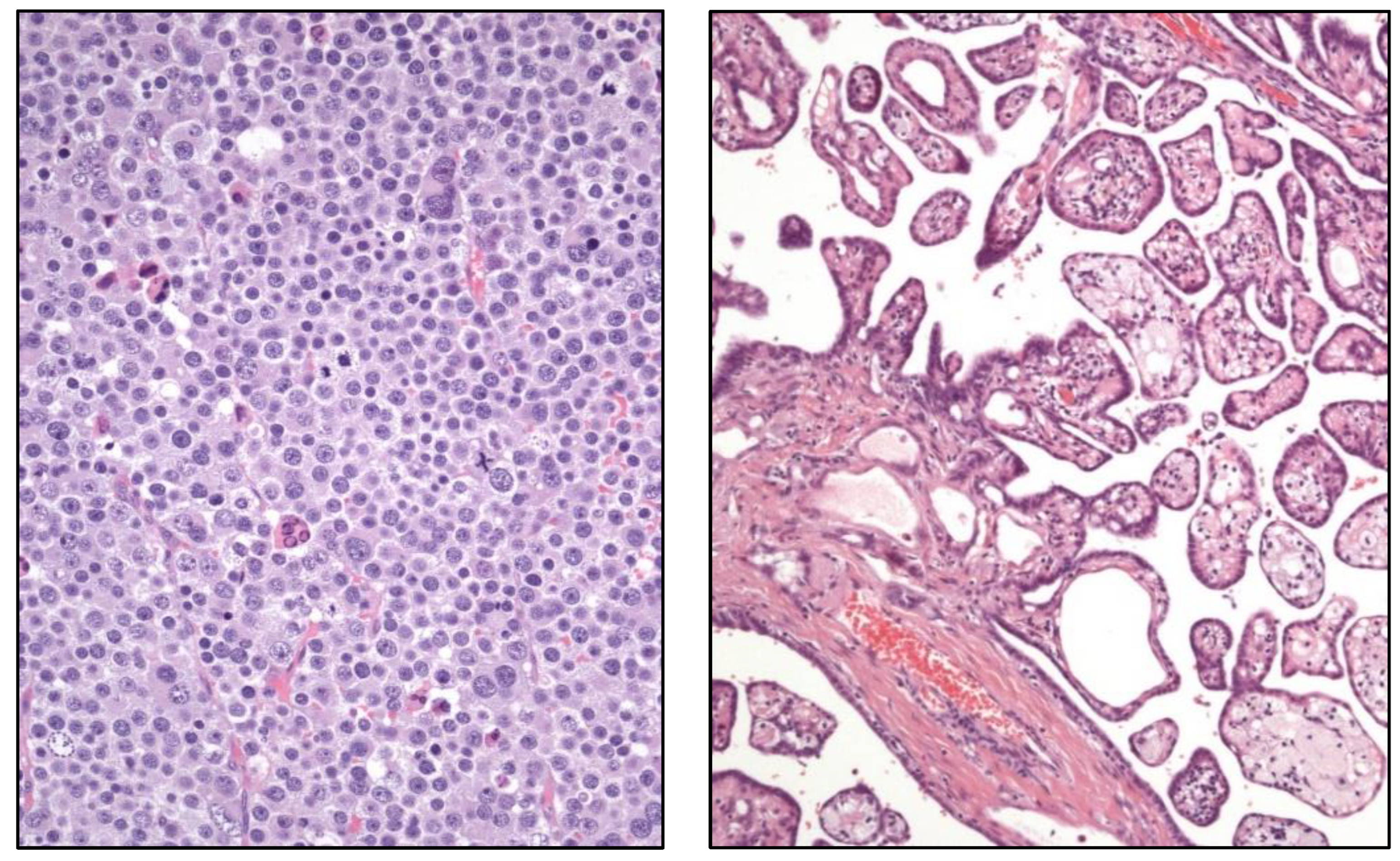
in situ germ cell neoplasia, the term spermatocytic tumor (
Figure 4) is maintained. To note, the name “carcinoid tumor” is no longer used. The testicular neuroendocrine tumor is recalled as “prepuberal testicular neuroendocrine tumor” since most of them arise in prepuberal teratomas. The sertoliform cystadenoma moves from the adnexal tumors group to the cluster of Sertoli cell tumors.
Newly recognized entities in this edition are:
- -
Stromal tumor with signet-ring cells.
- -
“Myoid” gonadal stromal tumor.
- -
Well-differentiated papillary mesothelial tumor (
Figure 4).
3.5. Penile Cancer
The 2022 WHO classification of PeCa [
4] distinguishes HPV-related and HPV-unrelated carcinomas.
- -
-
HPV-related squamous cell carcinomas are:
- ▪
- ▪
Basaloid PeCa.
- ▪
Clear cell squamous PeCa.
- ▪
Lymphoepithelioma-like PeCa.
- -
-
HPV-unrelated squamous cell PeCa are:
- ▪
Conventional squamous PeCa, either pseudo-hyperplastic or acantholytic/pseudo-glandular.
- ▪
Verrucous PeCa, including carcinoma “cuniculatum” (
Figure 5).
- ▪
- ▪
Sarcomatoid squamous PeCa.
- -
Squamous cell PeCa, not otherwise specified.
- -
Mixed squamous cell PeCa.
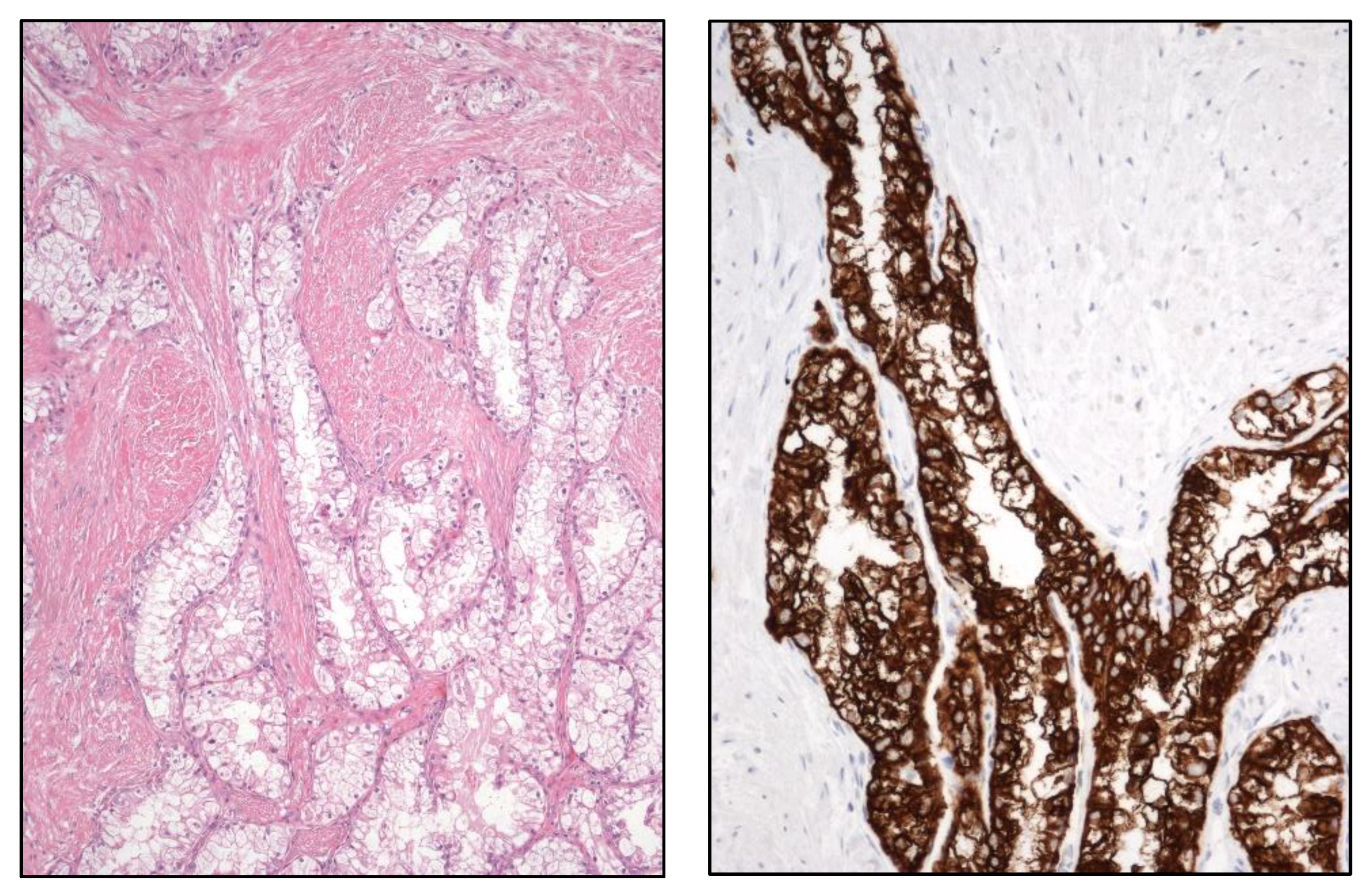
4. New Entities in Kidney Cancer
RCC is becoming in the last decade a neoplasm with an increasingly complex constellation of clinical, histological, and molecular features conforming one of the hottest and exciting topics in Pathology. In this particular context, the Genitourinary Pathology Society (GUPS) has brought order to this puzzle updating in 2021 the last findings in existing WHO RCC [
6] as well as an update of the list of renal tumor novelties [
7]. New tumors includes novel [eosinophilic solid and cystic RCC, ALK rearrangement-associated RCC, and RCC with fibromyomatous stroma (
Figure 6)], emerging (eosinophilic vacuolated tumor and thyroid-like follicular RCC), and provisional (low-grade oncocytic tumor, atrophic kidney-like lesion, and biphasic hyalinizing psammomatous RCC) tumor entities. A detailed compiled description with a literature review of all these tumors is provided in the aforementioned GUPS article [
7].
5. Urinary Cancer -omics
Thousands of studies are being implemented in the last decade analyzing genomics, proteomics, transcriptomics, and other -omics in urological cancer providing interesting and promising data for patients’ management. Here, we overview some hot spots in renal, urinary tract, prostate and penile cancers.
5.1. Renal Cancer
Aside from the well-known
VHL inactivation responsible of clear cell renal cell carcinoma (CCRCC) genesis, deletions of chromosome 3p also involve other suppressor genes like
PBRM1,
SETD2, and
BAP-1. Interestingly,
PBRM1 and
BAP-1 mutations in CCRCC are associated to specific clinical courses, attenuated and aggressive respectively [
8]. Other implicated genes include histone modifying genes like
KDM5C and
KDM6A, and mTOR pathway genes like
TSC1,
TSC2,
MTOR,
PIK3CA, and
PTEN, and
TP53. One of the most important limitations in defining the molecular profile of CCRCC is its inherent high levels of intratumor heterogeneity (ITH) by which different regions of the same tumor display different molecular alterations [
9] associated to specific prognosis and clinical aggressiveness. This characteristic has made CCRCC a perfect test bench for analyzing tumor complexity [
10].
Genomics of PRCC includes MET oncogene upregulation in up to 80% of the cases, a gene which has raised different clinical attempts for targeted therapies. Mutations on TERT, a gene malfunction also involved in many other cancers including UC, have been also detected in PRCC, where it has been associated to high metastatic capability and bad prognosis. Other less frequently mutated genes in PRCC are CDKN2A and CDKN2B.
Chromophobe renal cell carcinoma (ChRCC) displays a lower mutation frequency compared with the precedent RCC, being TP53, TERT, mTOR, and PTEN genes the most frequently involved.
NF2, FBXW7 tumor suppressor, and CDKN2A are genes whose malfunctioning has been detected in collecting-duct RCC, a rare and aggressive form of RCC.
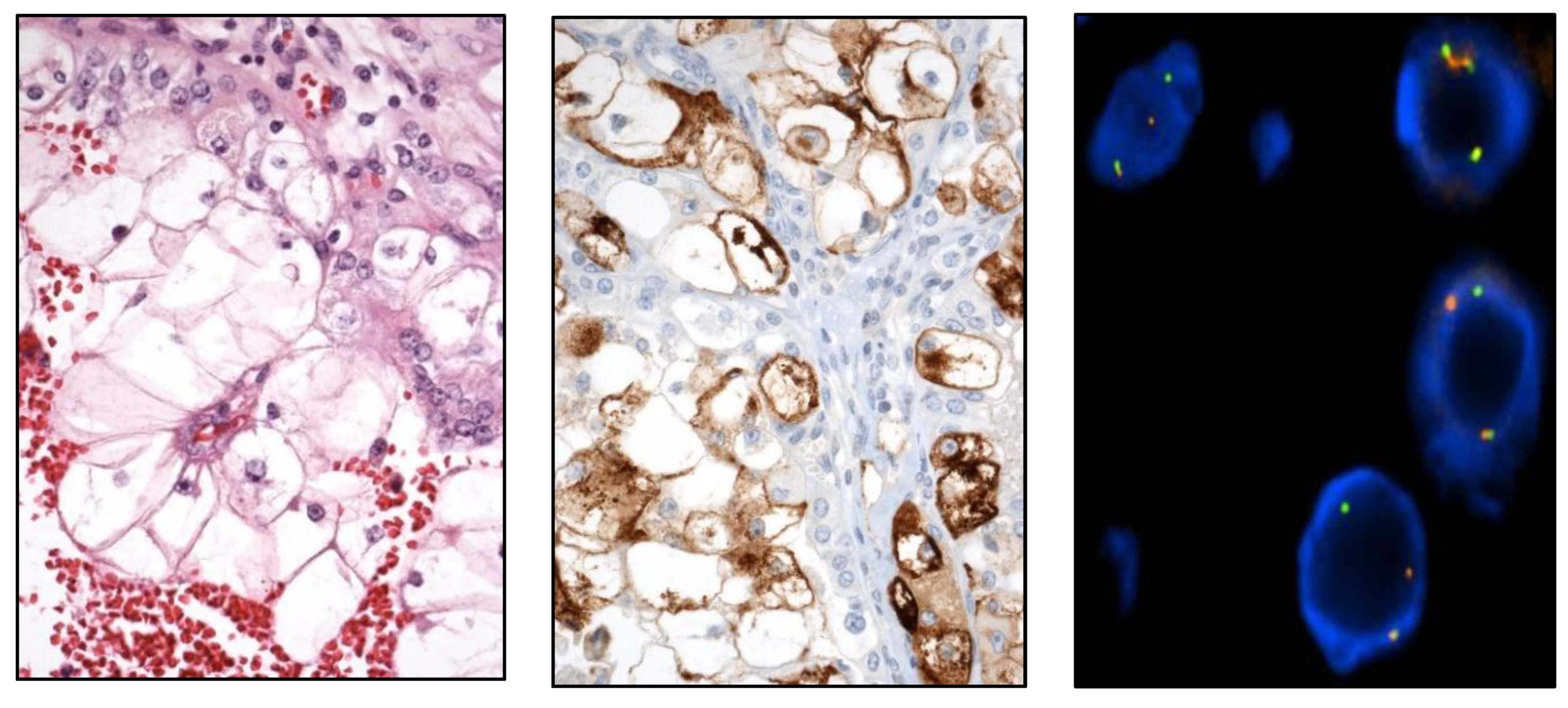
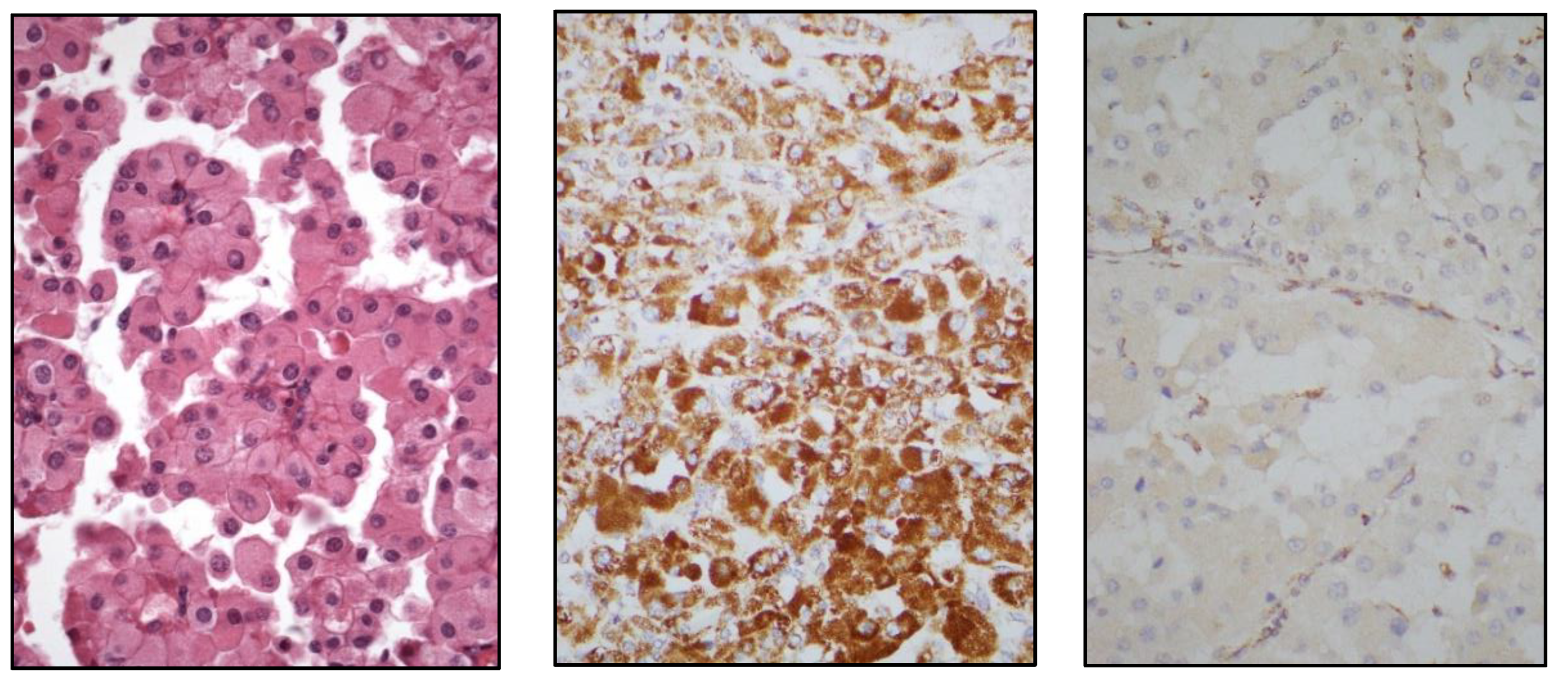
Aside from that, an important practical issue is to know the molecular alterations with clinical significance in RCC, a promising perspective conducting to a future molecular classification of renal neoplasia [
6,
7]. Among them,
TFE3-rearranged RCC, t(6;11) translocated (
TFEB) RCC (
Figure 7),
FH-deficient RCC,
SDH-deficient RCC (
Figure 8),
SMARCB1-deficient medullary RCC, ALK-rearranged RCC, and ELOC-mutated RCC are included.
5.2. Prostate Cancer
Localized Pca shows multiple genomic and proteomic alterations. A single-cell proteomic study has revealed that some cases at early-stage already contained subclones typically detected in castration-resistant and metastatic cases [
11].
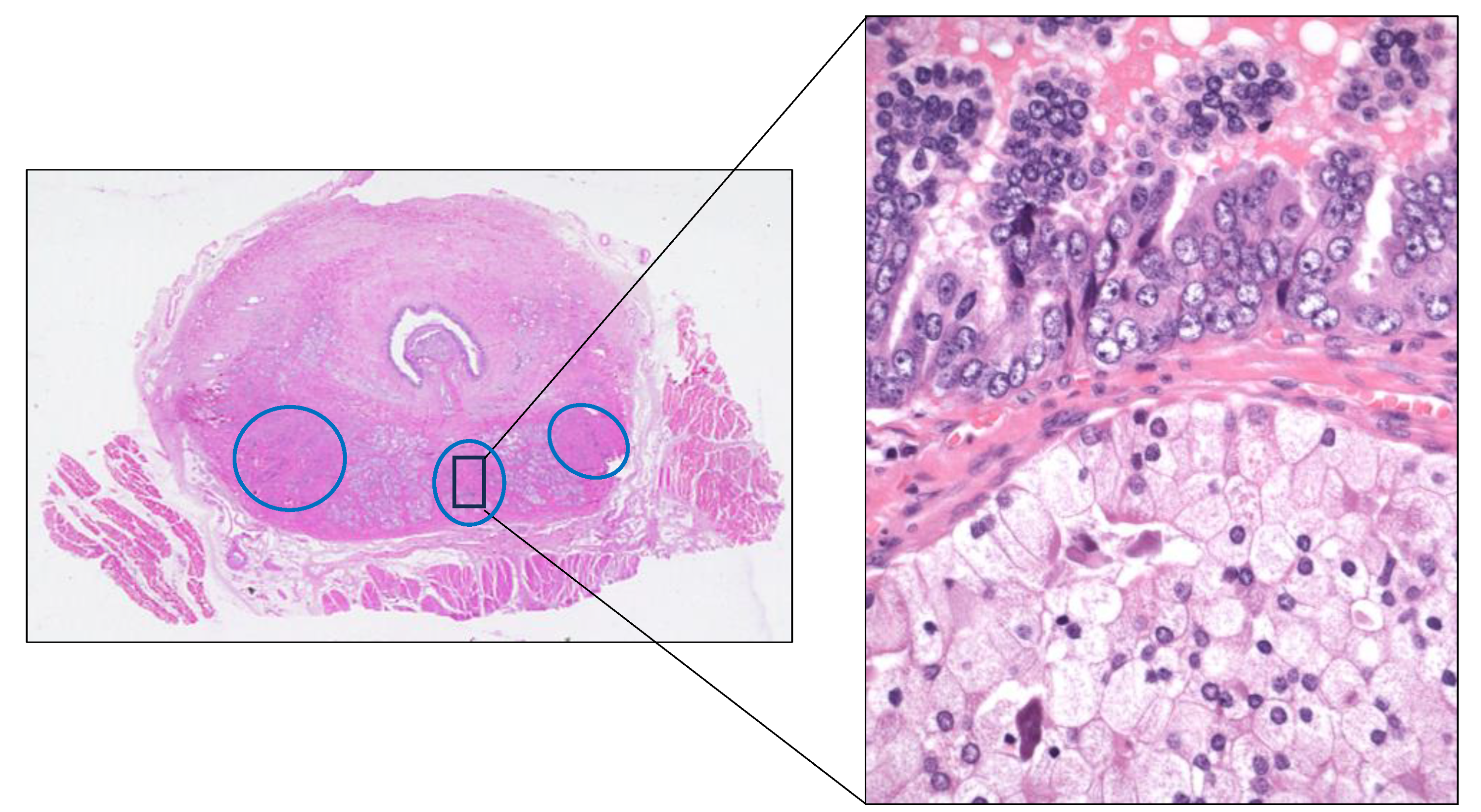
Focusing on the advanced Pca, many studies have confirmed the marked multifocality and ITH, both histologically (
Figure 9) and genetically, of the primary Pca itself. This condition has been evidenced by analyzing multi-region sampling in radical prostatectomy specimens and in their paired metastases, this way identifying significant differences in gene expression between different tumor areas. For example, whole-exome sequencing analyses of primary and metastatic Pca samples have demonstrated that androgen receptor (
AR) gene activating alterations were the most frequent genomic event found in the metastases followed by
TP53 mutations and
MYC amplifications. Interestingly,
AR alterations were not present in their paired primary tumors. The
AR activations found in the metastases of many Pca may be a sign of an over-imposed castration resistance status.
FOXA1,
KMT2C,
KMT2D are other genes whose alterations are more frequent in the metastases than in the primary tumors.
BRCA2 germline alterations has been detected in a minority of cases. Since
PTEN loss has been previously associated to progression, and metastases, some authors propose to consider the immunohistochemical PTEN loss of expression as a reliable marker of clinical aggressiveness in Pca [
12].
5.3. Urinary Tract Cancer
Molecular subtyping of UC [
13] distinguishes several tumor types with different clinical significances, luminal (including luminal papillary and luminal infiltrating) and basal (including squamous) subtypes being the most common ones (
Table 2).
Papillary UC -derived from intermediate cells in the urothelium- and UC in situ -derived from basal cell in the urothelium- follow two different pathways on urothelial carcinogenesis with specific clinic-pathological and molecular features.
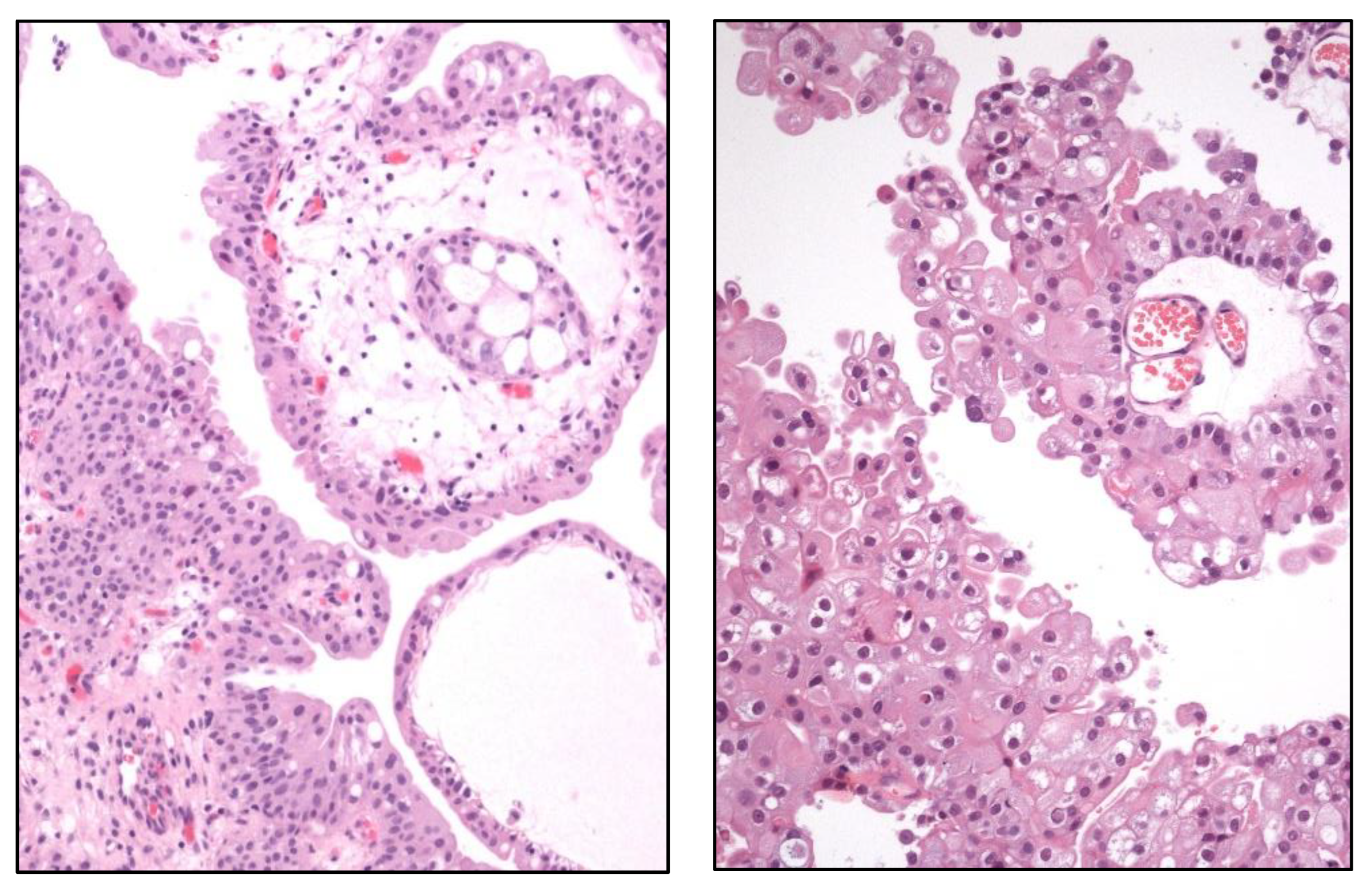
Proteogenomic analyses have clarified the distinction between urothelial papilloma and papillary UC (
Figure 10), a classic differential diagnosis under the light microscope. So, urothelial papilloma shows a higher mutation rate in
MPRIP,
HRAS, and
MAP3K1 genes whereas papillary UC carries mutations in
FGFR3 and
PPFIBP1. Of them,
HRAS mutations have been reported as predominant in the inverted type of urothelial papilloma. HRAS, ALDH7A1, CBLB, and MPRIP proteins were differently expressed in urothelial papilloma and papillary UC.
DNA damage related to APOBEC signature is a key pathway in the progression of in situ to invasive forms of UC, a fact that occurs in roughly 60% of the cases. Approximately 15% of UC patients develop distant metastases, a fact probably associated to RBPMS losses through the activation of AP-1 transcription factors.
5.4. Penile Carcinoma
Squamous PeCa displays a wide spectrum of mutations [
14], being
TP53,
TERT promoter, and
PIK3CA among the most frequently identified. Besides,
CDKN2A losses and CCND1 amplifications have also been detected in a small number of cases.
6. Update on Gleason Grading System
Gleason system for PCa grading was proposed by Donald Gleason in 1966. It has demonstrated high levels of inter- and intra-observer reproducibility along decades among pathologists and an optimal correlation with prognosis of PCa patients. It has been updated several times on the light of new histological findings and clinical evidences in large cohort studies. Changes included in the last 2022 WHO update [
3] have been previously mentioned in this article.
Prognostic groups in PCa were proposed in the last decade [
15]. The goal was to eliminate some misunderstandings, for example, if the lowest possible Gleason index in clinical practice is 3+3=6 (
Figure 11) and the highest is 5+5=10 in a range from 2 to 10, that means that a sum of 6 might represent an erroneous perception of an intermediate grade with unnecessary stress for patients. Another imprecision concerns to Gleason index 7, because the clinical evidence had showed that 3+4 is not the same that 4+3. For interested readers, precise definitions and additional reasons for a generalized adoption of these prognostic groups have been recently reviewed elsewhere [
16,
17].
In recent years the question has arisen as to whether prognostic group 1 PCa (Gleason index 3+3) (
Figure 11) should be considered cancer or not [
18]. From a clinical viewpoint there are some reasons for reconsidering this diagnostic category, for example, the well-known indolence of these cases, the risk of overtreatment, the possibility to adopt an active surveillance on these patients, and the unnecessary stress generated on patients when hearing the word “cancer”. However, the general consensus of pathologists is to continue considering these cases as low-grade cancers in core biopsies [
19] for several reasons. First, core biopsy may not reflect the reality of the tumor because high-grade prostate cancer may not be sampled even using appropriate protocols. Second, although infrequent, the possibility of tumor progression in the form of extraprostatic extension and metastatic spread does persist in these cases. Third, Gleason index 3+3=6 prostatic adenocarcinoma maintains the immunohistochemical staining and molecular changes of high-grade PCa.
7. Targeted Therapies and Other Novel Treatments in Urologic Cancer
This is a huge extremely dynamic area in permanent updating by numerous trials. The classical pillars of cancer care include surgery, radiotherapy, cytotoxic chemotherapy, and precision (molecular targeted) therapy. However, immunotherapy has emerged as a new (re)volution in the standard of care in certain patients with genitourinary malignancies. While the benefit of classical immunotherapy obtained in the past for metastatic CCRCC based on interferon-α and IL-2, and for early-stage bladder UC using Bacillus Calmette-Guerin therapy has been modest, therapies with immune checkpoint inhibitors demonstrate meaningful survival benefit and durable clinical response in RCC, UC, and some patients with PCa [
20]. The combination or sequencing of immunomodulators, either with chemotherapy or other targeted therapies, is the current hot topic in urologic cancer therapy, not only for metastatic cancer but also in the early disease, either as neoadjuvant or adjuvant setting. Interested readers are advised to address the contemporary information in every tumor type. Here, only general outlines for non-specialists are mentioned.
Partial or radical surgery remains the first elective treatment in RCC. In the metastatic stage there is a great option of therapies, including tyrosine kinase inhibitors such as sunitinib, axitinib, or cabozantinib, to block VEGF pathways and mTOR receptors, alone or in combination with immune checkpoint inhibitors such as nivolumab, ipilimumab, or pembrolizumab [
21]. Although this wealth of options has established RCC at the forefront of solid tumor immunology, many questions still remain unanswered [
22]. An open-label, randomized, phase 3 study has revealed in 2022 a higher overall survival of nivolumab plus ipilimumab compared with sunitinib alone in advanced RCC [
23], but the issue of which is the best therapy is still unsolved as synergistic toxicity needs to be minimized. Besides, many patients treated with immune chechpoint inhibitors will experience disease progression and a standardized second-line approach in these cases remains unknown as rechallenge with combined immunotherapy after early progression is not recommended [
24]. Although precision immunotherapy is a tremendous promise in advanced RCC there has unfortunately been limited success thus far. A deeper understanding of tumor immune microenvironment in RCC could help to target newer immune checkpoints to generate a more vigorous anti-tumor response in a near future. Also, the incorporation of mass spectrometry and single cell sequencing technologies to develop immunogenomic tools will enable the discovery of newer tumor antigens serving as targets for chimeric antigen receptor (CAR) T cell therapies, same as in hematologic malignancies [
25]. Several other estrategies have potential interest to improve immunotherapy and could be used in a not so close future. They include targeted lactate-lactylation and the modulation of the microbiome to potentially improve responses to immunotherapy [
26,
27,
28].
Surgery plus cisplatin-based chemotherapy remains the elective treatment in muscle invasive bladder UC and intravesical Bacillus Calmette-Guerin (BCG) instillations is still considered the best option in non-muscle invasive (pTa/pT1) high-grade bladder UC [
29]. However, BCG fails in nearly 40% of patients, thus requiring alternative treatments. Traditionally, radical cystectomy (that severely impacts the quality of life) has been the standard treatment for BCG-unresponsive disease, although recent advances have focused on bladder-preserving therapies that leverage immune checkpoint inhibitors , viral gene therapies, novel drug delivery systems, and other targeted molecular agents. Very recent immune checkpoint inhibitors, such as pembrolizumab and durvalumab, and other immunomodulators have demonstrated potential for systemic treatments in BCG-unresponsive non-muscle invasive disease [
30,
31,
32]. Also, the role of platinum-based chemotherapy in the evolving treatment landscape of advanced UC must be reviewed. Immune checkpoint inhibitors and antibody-drug conjugates have proven survival benefits in the metastatic contexts, and are expanding their therapeutic applications to the perioperative setting for non-metastatic muscle-invasive disease, either as substitutes or in combination with cytotoxic chemotherapy [
33,
34]. Other therapeutic models like the oncolytic adenovirus XVir-N-31 are being investigated in bladder UC [
35].
The therapeutic approach to PCa is complex, spanning from active surveillance to radical surgery or radiotherapy and hormone blockade. Several factors influence therapeutic decisions like age and patient comorbidities, stage, Gleason index, and histological risk group, among others. Immunotherapy is not an option so far and newly targeted therapies are emerging and require further investigations. The heterogeneity of the genomic landscape of metastatic castration-resistant tumors makes the advances in this issue very difficult. This heterogeneity and the complexity of tumor immune microenvironment in late forms of advanced and metastatic PCa have brought a delay in establishing immunotherapy as a standard option in this serious disease. Ipilimumab and olaparib have proved to prolong survival compared to placebo, but are still very far to be incorporated in clinical practice. More recent options such as PSMA-targeted treatments and other PARP inhibitors are being currently evaluated [
36]. Integrated machine learning could facilitate extensive analysis to identify epithelial cell marker genes that could be used to enhance immunotherapy in PCa [
37].
Targeted therapies are still under investigation in penile cancer. Specifically, the analysis of PD-L1 expression seems to be higher in the pT2 stage and correlates with regional lymph node metastasis [
38]. Currently, several trials are analyzing the potential use of avelumab [
39] and retifanlimab [
40] in this disease.
8. News on Non-Muscle-Invasive Urothelial Carcinoma
Non-muscle-invasive neoplasm is the most frequent form of UC in Western countries [
1]. Main issues of discussion in the literature are the histological grading, the precise histological definition of basal membrane disruption, that is, the distinction between pTa/pTis (intraepithelial) and pT1 (lamina propria/submucosa invasion), and the clinical approach to the disease, in other words, how to manage these patients using the armamentarium currently available.
From the pathologist viewpoint, the identification of stromal microscopic invasion (disruption of the basal membrane) is still a sliding issue in non-muscle-invasive UC -as well as in squamous cell carcinomas of other topographies- because many of these tumors grow into the lamina propria with a “pushing” border where no isolated cells and/or small tumor cell infiltrating nests are identifiable. This problem is old [
41,
42] and still persists nowadays. Once defined a tumor as superficially (non-muscle) invasive, its level of invasion, either into the lamina propria or submucosa, matters in terms of prognosis [
43,
44]. Several methods based on microscopic analysis were designed but the misorientation of tissue fragments and the inherent artifacts related to transurethral resection procedures make such methods poorly reproducible. Although this issue has been recently revisited in a single-institution study [
45], it remains not resolved so far.
UC grading has received a lot of attention since 1973, when a unified WHO grading system was adopted [
46]. This WHO system has been updated several times and comparisons between different versions of this system have been published [
47,
48].
Molecular analyses of UC have recently received full attention showing significant different routes between in situ UC and papillary intraepithelial UC [
49]. More specifically,
CCDC 138 is the most frequently mutated gene in situ UC (pTis) [
50]. The topic is too broad to be condensed here, so the interested reader is invited to address the specific literature.
The therapeutic spectrum of non-muscle-invasive UC has been very recently reviewed considering low-risk, intermediate, and high-risk, BCG-naïve, and BCG-unresponsive non-muscle-invasive variants [
51].
9. Artificial Intelligence in Urologic Cancer
Diverse tools of artificial intelligence have been applied in urological cancer studies to help in the diagnosis, especially in PCa [
52,
53,
54], to predict treatment response, to select patients for specific therapies and, finally, to predict clinical evolution [
55].
The identification of targetable molecular alterations [
56] and histological data with prognostic implications like renal capsule invasion in RCC [
57] are being unveiled by using radiomics models. Indeed, several recent studies show that machine learning models seem to be useful in predicting the risk of recurrence in non-metastatic localized RCC, a fact that might help in the selection of patients for personalized therapies [
58,
59].
These tools are being also applied, for example, to predict lymph node metastases [
60] or HER2 status [
61] in UC of the urinary bladder, and to predict the histological composition of testicular tumors [
62] and even to discriminate between the benign or malignant nature of testicular masses [
63].
Artificial intelligence tools are being implemented also in PCa, mainly for diagnostic purposes of core biopsies using morphological and immunohistochemical parameters [
52,
53,
54]. An innovative approach combining diagnostic immunohistochemical markers (cytokeratins, p63, and racemase) and a customized segmentation network [
64] has been developed to discriminate between PCa, prostate high-grade intraepithelial neoplasia (HG-PIN), and benign tissue in prostate core biopsies. This methodology would be potentially helpful to assist pathologists in the routine diagnosis of a quickly growing diagnostic area which highly impacts pathology lab burdens.
10. Intratumor Heterogeneity Influences Therapeutic Failures in Urologic Neoplasms
Massive sequencing studies [
65,
66] have demonstrated that ITH is an inherent condition in the evolution of cancer. Multi-region studies [
8,
67] are discovering the intricacies and particularities of every tumor type, so we already know that temporo-spatial ITH may develop in a diverse manner depending on the tumor and on the patient; in brief, ITH is a tumor- and patient-specific event that makes every neoplasm a unique and unrepeatable process. This acknowledgement is at the basis of the so-called personalized therapy and, at the same time, justifies therapeutic failures if the tumor is not deeply analyzed and ITH correctly unveiled.
CCRCC is a good example to analyze ITH (
Figure 12) from histological, immunohistochemical, and molecular point of views. Interestingly, specific clonal and sub-clonal mutations are linked with different tumor evolutions, metastatic potential, clinical course, and prognosis [
8,
68].
Multi-regional studies have also detected immunohistochemical ITH in muscle-invasive [
69] and non-muscle-invasive [
70] UC, as well as in PCa [
71], and in non-seminomatous germ cell tumors of the testis [
72].
Recent studies have demonstrated that resistances to therapy are closely linked to the presence of ITH [
73] which, in this context, can be considered as a survival response of tumor cells following ecological principles. The ecological perspective of ITH involves not only tumor cells but also the tumor microenvironment [
74]. This fact makes the understanding of tumor biology and the implementation of successful targeted treatments even more complex.
Advances in single-cell RNA sequencing (scRNA-seq) have been crucial in unveiling the heterogeneity of both tumor cells and tumor microenvironment [
75]. Complementary techniques like spatial transcriptomic [
76], multiplex immunohistochemistry [
77], and lipid imaging mass spectrometry [
78] provide critical insights into the localization, density, spatial interactions, and metabolic-functional states of the diverse cells involved within the tumor ecosystem. Furthermore, computational tools are being developed to integrate these datasets [
79]; for instance, Tumoroscope, a recently described method tested in PCa, enables comprehensive analysis of the genetic, functional, and spatial architecture of tumors, facilitating a deeper understanding of ITH and tumor evolution [
80].
Understanding the interactions between tumor cells and tumor microenvironment provides valuable opportunities to refine the classification of urological tumors, evaluate progression risk, and guide more precise therapeutic decisions [
75]. In the era of immunotherapy, one of the most intensively studied biological phenomena is the generation of immunosuppressive microenvironments as a consequence of these interactions [
81]. Immunosuppressive players such as M2 macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs) orchestrate these environments, which also harbor immunologically exhausted cytotoxic T lymphocytes [
82]. In turn, cancer-associated fibroblasts (CAFs) can hamper the infiltration of cytotoxic cells into the tumor through the extracellular matrix remodeling, induce M2 macrophage polarization, and drive the differentiation of naïve T cells into Tregs, collectively fostering a microenvironment conducive to tumor immune evasion [
83].
These microenvironmental interactions often occur in different ways in distinct tumor regions [
75]. In CCRCC, a paradigmatic example of ITH [
68], synergies between specific Treg populations and M2 macrophage polarization have been observed predominantly at the tumor periphery, correlating with poor prognosis [
76]. In these same peripheral zones, aggressive mesenchymal-like tumor cells interact with specific CAF subpopulations, particularly myofibroblastic CAFs, influencing immunotherapy response and patient survival [
77]. The abundance of Tregs (FOXP3+) and CAFs expressing markers such as transgelin and fibroblast activation protein (FAP) in CCRCC has been associated with the presence of exhausted cytotoxic T lymphocytes (CD8+/PD-1+) in the tumor microenvironment, poor prognosis, and unfavorable responses to immune checkpoint inhibitors (ICIs) [
77,
84,
85].
At this point, unveiling the precise profile of ITH seems mandatory in clinical settings [
86]. Total tumor sampling is the gold standard procedure, but many tumors are too large, for instance some RCC, so a total sampling of these cases is not a realistic option in routine practice. A multisite tumor sampling methodology based in the divide-and-conquer algorithm [
87] has been successfully developed in the routine practice to overcome this hurdle. This methodology couples clinical affordability and diagnostic efficiency in CCRCC [
88] with applicability to other large neoplasms [
89].
11. Intratumor Microbiome and Its Influence in Urologic Tumor Aggressiveness
The symbiotic dynamic interactions between bacteria and other microorganisms with human cells are known since decades [
90]. In this vein, many studies analyzing their influence in cancer evolution have demonstrated the presence of complex interconnections between the microbiome, cancer cells, and tumor microenvironment [
91]. In this chapter we will focus strictly on urological cancer.
Some studies using 16S DNA sequencing show that RCC and normal renal tissue differ significantly in their bacterial composition [
92]. Importantly, the microbiome alters important biological functions in tumor cells such as membrane transport, transcription, and cell growth, while in non-tumor cells, among other functions, modifies cell motility, signal transduction, and energy metabolism. The influence of microbiome in the expression of PD-L1 in normal tissue, tumor, and tumor thrombus has also been analyzed [
93].
UC has been a test bench for analyzing the effect of the local microbiome on tumor genesis and development [
94]. Several studies have found that UC displays a decreased microbial diversity compared with non-tumor tissue and tobacco smoking seems to be involved in this change [
95]. A conclusion could be that specific microbial signatures might be used to detect and/or monitor UC. On the other hand, a classically well-known precursor of bladder squamous cell carcinoma is the Schistosoma
sp. infection through inflammatory/immunological reactions.
Changes in the gut bacterial [
96] and circulating fungal [
97] microbiome has been extensively investigated in PCa patients. Since specific changes in urine microbiome is associated with high-grade PCa and PCa biochemical recurrence [
98], urine analyses could potentially serve as tumor biomarkers with diagnostic and prognostic implications.
Seminal plasma analysis is an accessible non-invasive method to obtain specimens to investigate the microbiome of TC. However, very few studies have been performed in germ cell tumors of the testis so far [
99], so the topic is open for promising future studies.
12. Ecological Principles and Mathematics Applied to Urological Cancer Study
Considering neoplasia as a huge community of individuals (cells) permanently interacting one each other, either cooperating or competing, has represented one of the most important steps ahead in the study of cancer in the last times. From this ecological perspective many tumor behaviors can be better understood and predicted, allowing the development of new therapeutic approaches. The number of studies on different cancers analyzed under this viewpoint is rapidly growing, ending even to coin the term “eco-oncology” [
100]. In this context, the interaction among tumor cells themselves are only a part of the general picture since the role of the microenvironment and tumor/non-tumor cells interactions seem also crucial. Tumor-infiltrating lymphocytes [
101], tumor-associated macrophages [
102], and tumor-associated fibroblasts [
103] have demonstrated to play a preeminent role in tumor evolution and treatment.
From this prospect, up to four different tumor evolution types have been identified [
104], i.e., linear, neutral, branching, and punctuated. In the linear evolution, driver mutations are so selective that outcompete all previous clones giving rise to an intratumor quasi-homogeneity following a Darwinian model of evolution. Instead, in neutral evolution new clones coexist without inter-clonal competition giving rise to extreme levels of ITH following a non-Darwinian model. Branching tumors is a model where clones developed from a common ancestry evolve in parallel and expand simultaneously giving rise to a tumor with high levels of ITH following a Darwinian model of evolution. Finally, the punctuated type of evolution is characterized by the development of a very aggressive clone in early phases of tumor development generating a tumor with low levels of ITH, following also a Darwinian model. The interesting point of these conceptual types of tumors is that they correlate quite well with tumor progression and prognosis in the clinical practice [
105].
Most CCRCC display either branching or punctuated evolutions, as reflected in a multiregional genomic analysis with specific clinical behavior [
8]. Branching-type tumors showed high ITH and an attenuated clinical course, with late and single metastasis, while punctuated-type ones behaved aggressively, with multiple and early metastases, and low-levels of ITH. These molecular data correlate well with histological findings [
106].
To note, ITH is not randomly distributed throughout the tumor. The tumor interior areas, where hypoxia and nutrient scarcity is high, develop aggressive clones with metastatic competencies, whereas tumor periphery, where oxygen and nutrient supply is maximum, develops invasive capabilities at a local level [
107,
108]. As ecology rules predicted, local/regional environmental pressures influence changes in cellular genomics of cells in a fight for survival [
109].
Coupled with Ecology, applied mathematics have significantly improved our knowledge on cancer intricacies [
110]. The use of Game Theory, for example, has explained why tumor cells behave as they do thus envisaging hidden reasons for ITH development thus predicting future behaviors which may eventually guide therapy [
111,
112]. For example, recent data suggest that histological and genomic data converge with game theory modeling using the hawk-dove game in analyzing the implications and significance of ITH in CCRCC [
113].
Mathematics and ecology have also provided some plausible explanations and possible solutions to the problem of tumor resistances to therapy. As an example, some authors have proposed that a strategy of tumor containment using drugs under the maximum tolerable dose could benefit patients [
114]. The ecological explanation of this anti-intuitive approach sustains that tumor cells developing under normal conditions spend all their energy to grow; so, a treatment following the maximum tolerable dose regime will force tumor cells to deviate all their energy expenditure to generate resistances in order to survive. Latter or sooner, a subset of tumor cells will attempt to develop resistances, and this fact will give rise to a uniformly resistant tumor. Since the amount of energy available in the cell is limited, a dose under the maximum tolerable threshold will make tumor cells to divide their energy expenditure in two mutually exclusive tasks, to grow and to cope with the drug. The final result in this scenario is that both tasks, tumor growth and development of resistances to therapy, will be necessarily slow down thus allowing a retardation in both of them. This argument is at he basis to understand the Parrondo’s paradox applied to cancer [
115], which reads that the combination of two loosing strategies working together may win.
13. Conclusions
Urological cancer is a health problem in Western countries and represents an excellent test bench for basic and clinical research, as reflected in the literature. The spectrum of neoplasms in this field is broad and shows different pathogenic mechanisms with morphological, clinical, and therapeutic repercussions. This narrative focuses on ten current hot topics trying to update interested readers in this area. These topics are 2022 WHO update in the classification of urinary and male genital tumors, new entities in kidney cancer, urinary cancer -omics, update on Gleason grading system, targeted therapies and other novel therapies, news on non-muscle invasive urothelial carcinoma, artificial intelligence in urologic cancer, intratumor heterogeneity influence in therapeutic failures in urologic neoplasms, intratumor microbiome and its influence in urologic tumor aggressiveness, and ecological principles and mathematics applied to urological cancer study.
Author Contributions
Conceptualization, C.M., J.I.L.; methodology, J.I.L.; data curation, C.M., G.L., J.C.A., J.I.L.; writing—original draft preparation, C.M., G.L., J.C.A.; writing—review and editing, J.I.L.; supervision, C.M., J.I.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Pca |
Prostate carcinoma |
| RCC |
Renal cell carcinoma |
| CCRCC |
Clear cell renal cell carcinoma |
| ITH |
Intratumor heterogeneity |
| PRCC |
Papillary renal cell carcinoma |
| HPV |
Human papilloma virus |
| PeCa |
Penile carcinoma |
| UC |
Urothelial carcinoma |
| TC |
Testicular carcinoma |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 26 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Netto, G.J.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization classification of tumors of the urinary system and male genital organs-Part B: Prostate and urinary tract tumors. Eur. Urol. 2022, 82, 469–482. [Google Scholar] [CrossRef]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization classification of tumours of the urinary system and male genital organs-Part A: Renal, penile, and testicular tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.P.; Seide, S.; Proctor, T.; Kleinaki, Z.; Kleinaki, M.; Reynolds, J.P. The Paris System for reporting urinary cytology: A meta-analysis. J. Pers. Med. 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Hes, O.; Williamson, S.R.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. New developments in existing WHO entities and evolving molecular concepts: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1392–1424. [Google Scholar] [CrossRef]
- Trpkov, K.; Williamson, S.R.; Gill, A.J.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1167–1184. [Google Scholar] [CrossRef]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Horswell, S.; Chambers, T.; O’Brien, T.; López, J.I.; Watkins, T.B.; Nicol, D.; et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell 2018, 173, 595–610. [Google Scholar] [CrossRef]
- Manini, C.; López-Fernández, E.; Larrinaga, G.; López, J.I. Clear cell renal cell carcinoma: A test bench for investigating tumor complexity. Cancers 2024, 16, 829. [Google Scholar] [CrossRef]
- López, J.I.; Hogan, M.F.; Sutton, B.; Church, S.E.; Angulo, J.C.; Nunes-Xavier, C.E. Distinct spatial landscapes in clear-cell renal cell carcinoma as revealed by whole transcriptome analysis. Immunooncol. Technol. 2023, 21, 100690. [Google Scholar] [CrossRef]
- De Vargas Roditi, L.; Jacobs, A.; Rueschoff, J.H.; Bankhead, P.; Chevrier, S.; Jackson, H.W.; Hermanns, T.; Fankhauser, C.D.; Poyet, C.; Chun, F.; et al. Single-cell proteomics defines the cellular heterogeneity of localized prostate cancer. Cell Rep. Med. 2022, 3, 100604. [Google Scholar] [CrossRef] [PubMed]
- Cyrta, J.; Prandi, D.; Arora, A.; Hovelson, D.H.; Sboner, A.; Rodriguez, A.; Fedrizzi, T.; Beltran, H.; Robinson, D.R.; Gopalan, A.; et al. Comparative genomics of primary prostate cancer and paired metastases: insights from 12 molecular case studies. J. Pathol. 2022, 257, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Xu, N.; Shang, G.; Wang, H.; Tao, H.; Wang, Y.; Qin, Z.; Tan, S.; Feng, J.; Zhu, J.; et al. Proteogenomics of different urothelial bladder cancer stages reveals distinct molecular features for papillary cancer and carcinoma in situ. Nat. Commun. 2023, 14, 5670. [Google Scholar] [CrossRef]
- Necchi, A.; Spiess, P.E.; Bandini, M.; Basile, G.; Grivas, P.; Bratslavsky, G.; Jacob, J.; Danzinger, N.; Lin, D.; Decker, B.; Sokol, E.S.; et al. Advanced squamous cell carcinomas of the pelvic and perineal region: A comprehensive genomic profiling study. Oncologist 2022, 27, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Montironi, R.; Cheng, L.; Cimadamore, A.; Mazzucchelli, R.; Scarpelli, M.; Santoni, M.; Massari, F.; Lopez-Beltran, A. Narrative review of prostate cancer grading systems: will the Gleason scores be replaced by the Grade Groups. Transl. Androl. Urol. 2021, 10, 1530–1540. [Google Scholar]
- Epstein, J.I.; Amin, M.B.; Fine, S.W.; Algaba, F.; Aron, M.; Baydar, D.E.; Lopez-Beltran, A.; Brimo, F.; Cheville, J.C.; Colecchia, M.; et al. The 2019 Genitourinary Pathology Society (GUPS) white paper on contemporary grading of prostate cancer. Arch. Pathol. Lab. Med. 2021, 145, 461–493; [Google Scholar] [CrossRef]
- Epstein, J.I. Is Grage Group 1 (Gleason score 3+3=6) adenocarcinoma of the prostate really cancer? Curr. Opin. Urol. 2022, 32, 91–95. [Google Scholar] [CrossRef]
- Saramatunga, H.; Egevad, L.; Yaxley, J.; Perry-Keene, J.; Le Fevre, I.; Kench, J.; Matsika, A.; Bostwick, D.; Iczkowski, K.; Delahunt, B. Gleason score 3+3=6 prostatic adenocarcinoma is not benign and the current debate is unhelpful to clinicians and patients. Pathology 2024, 56, 33–38. [Google Scholar] [CrossRef]
- Lu, K.; Chiu, K.-. .; Cheng, C.-L. Immunotherapy in genitourinary malignancy: Evolution in revolution or revolution in evolution. Cancer Treat. Res. 2022, 183, 201–223. [Google Scholar] [CrossRef]
- Schindler, N.R.; Braun, D.A. Antigenic targets in clear cell renal cell carcinoma. Kidney Cancer 2023, 7, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Bakouny, Z.; Hirsch, L.; Flippot, R.; Van Allen, E.M.; Wu, C.J.; Choueiri, T.K. Beyond conventional immune-checkpoint inhibition - novel immunotherapies for renal cell carcinoma. Nat. Rev. Clin. Oncol. 2021, 18, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Shah, A.Y.; Suarez, C.; Hamzaj, A.; Porta, C.; Hocking, C.M.; et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomized, phase 3 trial. Lancet Oncol. 2022, 23, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Carrillo, R.; Saad, E.; Saliby, R.M.; Sun, M.; Albiges, L.; Bex, A.; Heng, D.; Mejean, A.; Motzer, R.J.; Plimack, E.R.; et al. First and second-line treatments in metastatic renal cell carcinoma. Eur. Urol. 2025, 87, 143–154. [Google Scholar] [CrossRef]
- D'Agostino, M.; Raje, N. Anti-BCMA CAR T-cell therapy in multiple myeloma: can we do better? Leukemia 2020, 34, 21–34. [Google Scholar] [CrossRef]
- Zha, J.; Zhang, J.; Lu, J.; Zhang, G.; Hua, M.; Guo, W.; Yang, J.; Fan, G. A review of lactate-lactylation in malignancy: its potential in immunotherapy. Front. Immunol. 2024, 15, 1384948. [Google Scholar] [CrossRef]
- Mjaess, G.; Karam, A.; Aoun, F.; Albisinni, S.; Roumeguère, T. Fecal microbiota transplantation for immunotherapy-resistant urological tumors: Is it time? An update of the recent literature. Cancer 2022, 128, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Quan, C.; He, Y.; Matsika, J.; Huang, J.; Liu, B.; Chen, J. Targeting gut and intratumoral microbiota: a novel strategy to improve therapy resistance in cancer with a focus on urologic tumors. Expert Opin. Biol. Ther. 2024, 24, 747–759. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- Hannouneh, Z.A.; Hijazi, A.; Alsaleem, A.A.; Hami, S.; Kheyrbek, N.; Tanous, F.; Khaddour, K.; Abbas, A.; Alshehabi, Z. Novel immunotherapeutic options for BCG-unresponsive high-risk non-muscle-invasive bladder cancer. Cancer Med. 2023, 12, 21944–21968. [Google Scholar] [CrossRef]
- Oh, E.L.; Redfern, A.; Hayne, D. An evaluation of durvalumab across the spectrum of urothelial carcinoma. Expert Rev. Anticancer Ther. 2024, 24, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Filon, M.; Schmidt, B. New treatment options for non-muscle-invasive bladder cancer. Am. Soc. Clin. Oncol. Educ. Book. 2025, 45, e471942. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, H.W.; Ha, H.K.; Seo, H.K. Perioperative systemic therapy in muscle invasive bladder cancer: Current standard method, biomarkers and emerging strategies. Investig. Clin. Urol. 2023, 64, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tan, A.; Shah, A.Y.; Iyer, G.; Morris, V.; Michaud, S.; Sridhar, S.S. Reevaluating the role of platinum-based chemotherapy in the evolving treatment landscape for patients with advanced urothelial carcinoma. Oncologist. 2024, 29, 1003–1013. [Google Scholar] [CrossRef]
- Lichtenegger, E.; Koll, F.; Haas, H.; Mantwill, K.; Janssen, K.-P.; Laschinger, M.; Gschwend, J.; Steiger, K.; Black, P.C.; Moskalev, I.; et al. The oncolytic adenovirus XVir-N-31 as a novel therapy in muscle-invasive bladder cancer. Hum. Gene Ther. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Mitsogiannis, I.; Tzelves, L.; Dellis, A.; Issa, H.; Papatsoris, A.; Moussa, M. Prostate cancer immunotherapy. Expert Opin. Biol. Ther. 2022, 22, 577–590. [Google Scholar] [CrossRef]
- Zhu, W.; Zeng, H.; Huang, J.; Wu, J.; Wang, Y.; Wang, Z.; Wang, H.; Luo, Y.; Lai, W. Integrated machine learning identifies epithelial cell marker genes for improving outcomes and immunotherapy in prostate cancer. J. Transl. Med. 2023, 21, 782. [Google Scholar] [CrossRef]
- Udager, A.M.; Liu, T.Y.; Skala, S.L.; Magers, M.J.; McDaniel, A.S.; Spratt, D.E.; Feng, F.Y.; Siddiqui, J.; Cao, X.; Fields, K.L.; et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: Potential opportunities for immunotherapeutic approaches. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1706–1712. [Google Scholar] [CrossRef]
- Gassian, N.; Frontczak, A.; Mouillet, G.; Vernerey, D.; Manseur, O.; Goujon, M.; Meurisse, A.; Berthod, D.; Robert, E.; Calcagno, F.; et al. Activity and tolerability of maintenance avelumab immunotherapy after first line polychemotherapy including platinum in patients with locally advanced or metastatic squamous cell penile carcinoma: PULSE. Bull. Cancer 2020, 107, eS16–eS21. [Google Scholar] [CrossRef]
- Garcia Del Muro, X.; Paez Lopez-Bravo, D.; Cuellar-Rivas, M.A.; Maroto, P.; Giannatempo, P.; Castellano, D.; Climent, M.A.; Valderrama, B.P.; Gomez de Liano, A.; Lopez-Montero, L.; et al. Retifanlimab in advanced penile squamous cell carcinoma: The phase 2 ORPHEUS study. Eur. Urol. Oncol. 2024. [Google Scholar] [CrossRef]
- Angulo, J.C.; Lopez, J.I.; Flores, N.; Toledo, J.D. The value of tumor spread, grading and growth pattern as morphological predictive parameters in bladder carcinoma. A critical revision of the 1987 TNM classification. J. Cancer Res. Clin. Oncol. 1993, 119, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.I.; Angulo, J.C. Growth pattern in superficial urothelial bladder carcinomas. Histological review and clinical relevance. Int. Urol. Nephrol. 2009, 41, 847–854. [Google Scholar] [CrossRef]
- Chang, W.C.; Chang, Y.H.; Pan, C.C. Prognostic significance in substaging of T1 urinary bladder urothelial carcinoma on transurethral resection. Am. J. Surg. Pathol. 2012, 36, 454–461. [Google Scholar] [CrossRef]
- Angulo, J.C.; Lopez, J.I.; Grignon, D.J.; Sanchez-Chapado, M. Muscularis mucosa differentiates two populations with different prognosis in stage T1 bladder cancer. Urology 1995, 45, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kläger, J.; Koeller, M.C.; Oszwald, A.; Wasinger, G.; D’Andrea, D.; Compérat, E. A single-center retrospective comparison of pT1 substaging methods in bladder cancer. Virchows Arch. 2024. [Google Scholar] [CrossRef]
- Mostofi, F.K.; Sobin, L.H.; Torloni, H. Histological Typing of Urinary Bladder Tumors: International Classification of Tumors. Geneva: World Health Organization, 1973: no. 10: 17.
- May, M.; Brookman-Amissah, S.; Roigas, J.; Hartmann, A.; Störkel, S.; Kristiansen, G.; Gilfrich, C.; Borchardt, R.; Hoschke, B.; Kaufmann, O.; et al. Prognostic accuracy of individual uropathologists in noninvasive urinary bladder carcinoma: a multicentre study comparing the 1973 and 2004 WHO World Health Organisation classifications. Eur. Urol. 2010, 57, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Otto, W.; Denzinger, S.; Fritsche, H.M.; Burger, M.; Wieland, W.F.; Hofstädter, F.; Hartmann, A.; Bertz, S. The WHO classification of 1973 is more suitable than the WHO classification of 2004 for predicting survival in pT1 urothelial bladder cancer. BJU Int. 2011, 107, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Xu, N.; Shang, G.; Wang, H.; Tao, H.; Wang, Y.; Qin, Z.; Tan, S.; Feng, J.; Zhu, J.; et al. Proteogenomics of different urothelial bladder cancer stages reveals distinct molecular features of papillary cancer and carcinoma in situ. Nat. Commun. 2023, 14, 5670. [Google Scholar] [CrossRef]
- Anurag, M.; Strandgaard, T.; Kim, S.H.; Dou, Y.; Comperat, E.; Al-Ahmadie, H.; Inman, B.A.; Taber, A.; Nordentoft, I.; Jensen, J.B.; et al. Multiomics profiling of urothelial carcinoma in situ reveals CIS-specific gene signature and immune characteristics. iScience 2024, 27, 109179. [Google Scholar] [CrossRef]
- McNall, S.; Hooper, K.; Sullivan, T.; Rieger-Christ, K.; Clements, M. Treatment modalities for non-muscle invasive bladder cancer: An updated review. Cancers 2024, 16, 1843. [Google Scholar] [CrossRef]
- Chatrian, A.; Colling, R.T.; Browning, L.; Alham, N.K.; Sirinukunwattana, R.; Malacrino, S.; Haghighat, M.; Aberdeen, A.; Monks, A.; Moxley-Wyles, B.; et al. Artificial intelligence for advance requesting of immunohistochemistry in diagnostically uncertain prostate biopsies. Mod. Pathol. 2021, 34, 1780–1794. [Google Scholar] [CrossRef] [PubMed]
- Perincheri, S.; Levi, A.W.; Celli, R.; Gershkovich, P.; Rimm, D.; Morrow, J.S.; Rothrock, B.; Raciti, P.; Klimstra, D.; Sinargd, J.; et al. An independent assessment of an artificial intelligence system for prostate cancer detection shows strong diagnostic accuracy. Mod. Pathol. 2021, 34, 1588–1595. [Google Scholar] [CrossRef]
- Bulten, W.; Kartasalo, K.; Cameron Chen, P.-H.; Ström, P.; Pinckaers, H.; Nagpal, K.; Cai, Y.; Steiner, D.F.; van Boven, H.; Vink, R.; et al. Artificial intelligence for diagnosis and Gleason grading of prostate cancer: the PANDA challenge. Nat. Med. 2022, 28, 154–163. [Google Scholar] [CrossRef]
- Wang, Y.; Xuan, Y.; Su, B.; Gao, Y.; Fan, Y.; Huang, Q.; Zhang, P.; Gu, L.; Niu, S.; Shen, D.; et al. Predicting recurrence and survival in patients with non-metastatic renal-cell carcinoma after nephrectomy: a prospective population-based study with multicenter validation. Int. J. Surg. 2024, 110, 820–831. [Google Scholar] [CrossRef]
- Orton, M.R.; Hann, E.; Doran, S.J.; Shepherd, S.T.C.; Dafydd, D.A.; Spencer, C.E.; Lopez, J.I.; Albarrán-Artahona, V.; Comito, F.; Warren, H.; et al. Interpretability of radiomics models is improved when using feature group selection strategies for predicting molecular and clinical targets in clear-cell renal cell carcinoma: insights from the TRACERx Renal study. Cancer Imaging 2023, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Li, Z.; Yang, M.; Ye, Z. Preoperative prediction of renal fibrous capsule invasion in clear cell renal cell carcinoma using CT-based radiomics model. Br. J. Radiol. 2024, 97, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Khene, Z.E.; Bigot, P.; Doumerc, N.; Ouzaid, I.; Boissier, R.; Nouhaud, F.X.; Albiges, L.; Bernhard, J.C.; Ingels, A.; Borchiellini, D.; et al. Application of machine learning models to predict recurrence after surgical resection of nonmetastatic renal cell carcinoma. Eur. Urol. Oncol. 2023, 6, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Golkaram, M.; Kuo, F.; Gupta, S.; Carlo, M.I.; Salmans, M.L.; Vijayaraghavan, R.; Tang, C.; Makarov, V.; Rappold, P.; Blum, K.A.; et al. Spatiotemporal evolution of clear cell renal cell carcinoma microenvironment links intra-tumoral heterogeneity to immune escape. Genome Med. 2022, 14, 143. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, T.; Zhu, L.; Yao, Y.; Mei, J.; Sun, L.; Zhang, G. Using machine learning to develop preoperative model for lymph node metastasis in patients with bladder urothelial carcinoma. BMC Cancer 2024, 24, 725. [Google Scholar] [CrossRef]
- Peng, J.; Tang, Z.; Li, T.; Pan, X.; Feng, L.; Long, L. Contrast-enhanced computed tomography-based radiomics nomogram for predicting HER2 status in urothelial bladder carcinoma. Front. Oncol. 2024, 14, 1427122. [Google Scholar] [CrossRef]
- Fang, F.; Wu, L.; Luo, X.; Bu, H.; Huang, Y.; Xian Wu, Y.; Lu, Z.; Li, T.; Yang, G.; et al. Differentiation of testicular seminomas from nonseminomas based on multiphase CT radiomics combined with machine learning: A multicenter study. Eur. J. Radiol. 2024, 175, 111416. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Z.; Wang, L.; Lv, W.; Liu, Z.; Min, X.; Li, J.; Zhang, J. Comparison and analysis of multiple machine learning models for discriminating benign and malignant testicular lesions based on magnetic resonance imaging radiomics. Front. Med. 2023, 10, 1279622. [Google Scholar] [CrossRef]
- Salvi, M.; Manini, C.; López, J.I.; Fenoglio, D.; Molinari, F. Deep learning approach for accurate prostate cancer identification and stratification using combined immunostaining of cytokeratin, p63, and racemase. Comput. Med. Imaging Graph. 2023, 109, 102288. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Jolly, C.; Leshchiner, I.; Dentro, S.C.; Gonzalez, S.; Rosebrock, D.; Mitchell, T.J.; Rubanova, Y.; Anur, P.; Yu, K.; et al. The evolutionary history of 2,658 cancers. Nature 2020, 578, 122–128. [Google Scholar] [CrossRef]
- Bailey, C.; Black, J.R.M.; Reading, J.L.; Litchfield, K.; Turajlic, S.; McGranahan, N.; Jamal-Hanjani, M.; Swanton, C. Tracking cancer evolution through the disease course. Cancer Discov. 2021, 11, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Chambers, T.; Lopez, J.I.; Nicol, D.; O'Brien, T.; Larkin, J.; Horswell, S.; et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 2018, 173, 581–594. [Google Scholar] [CrossRef]
- Schallenberg, S.; Dragomir, M.P.; Anders, P.; Ebner, B.; Volz, Y.; Eismann, L.; Rodler, S.; Casuscelli, J.; Buchner, A.; Klauschen, F.; et al. Intratumoral heterogeneity of molecular subtypes in muscle-invasive bladder cancer. An extensive multiregional immunohistochemical analysis. Eur. Urol. Focus 2023, 9, 788–798. [Google Scholar] [CrossRef]
- Garczyk, S.; Bischoff, F.; Schneider, U.; Golz, R.; von Rundstedt, F.C.; Knüchel, R.; Degener, S. Intratumoral heterogeneity of surrogate molecular subtypes in urothelial carcinoma in situ of the urinary bladder: implications for prognostic stratification of high-risk non-muscle-invasive bladder cancer. Virchows Arch. 2021, 479, 325–335. [Google Scholar] [CrossRef]
- Singhal, U.; Nallandhighal, S.; Tosoian, J.J.; Hu, K.; Pham, T.M.; Stangl-Kremser, J.; Liu, C.J.; Karim, R.; Plouffe, K.R.; Morgan, T.M.; et al. Integrative multi-region molecular profiling of primary prostate cancer in men with synchronous lymph node metastasis. Nat. Commun. 2024, 15, 4341; [Google Scholar] [CrossRef]
- Singh, Y.; Barua, S.K.; Singh, V.K.; Trivedi, S.; Rajeev, T.P.; Koti, S.R.; Garg, N. Intratumoral heterogeneity, chemoresistance and lymph node landing zone prognosis in testicular tumors based on histopathological characteristics. Ann. Surg. Oncol. 2024, 31, 3544–3553; [Google Scholar] [CrossRef]
- Rhinehart, D.P.; Lai, J.; Sanin, D.E.; Vakkala, V.; Mendes, A.; Bailey, C.; Antonarakis, E.S.; Paller, C.J.; Wu, X.; Lotan, T.L.; et al. Intratumoral heterogeneity drives acquired therapy resistance in a patient with metastatic prostate cancer. NPJ Precis. Oncol. 2024, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- van Wilpe, S.; Gorris, M.A.J.; van der Woude, L.L.; Sultan, S.; Koornstra, R.T.H.; van der Heijden, A.G.; Gerritsen, W.R.; Simons, M.; de Vries, I.J.M.; Mehra, N. Spatial and temporal heterogeneity of tumor-infiltration lymphocytes in advanced urothelial cancer. Front. Immunol. 2022, 12, 802877. [Google Scholar] [CrossRef]
- Grausenburger, R.; Herek, P.; Shariat, S.F.; Englinger, B. Recent contributions of single-cell and spatial profiling to the understanding of bladder cancer. Curr. Opin. Urol. 2024, 34, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, Y.; Geng, W.; Jiao, J.; Liu, H.; Chen, R.; He, Q.; Wang, L.; Sun, X.; Qin, W.; et al. Spatial and single-cell transcriptomics reveal cellular heterogeneity and a novel cancer-promoting Treg cell subset in human clear-cell renal cell carcinoma. J. Immunother. Cancer 2025, 13, e010183. [Google Scholar] [CrossRef]
- Davidson, G.; Helleux, A.; Vano, Y.A.; Lindner, V.; Fattori, A.; Cerciat, M.; Elaidi, R.T.; Verkarre, V.; Sun, C.M.; Chevreau, C.; et al. Mesenchymal-like Tumor Cells and Myofibroblastic Cancer-Associated Fibroblasts Are Associated with Progression and Immunotherapy Response of Clear Cell Renal Cell Carcinoma. Cancer Res. 2023, 83, 2952–2969. [Google Scholar] [CrossRef]
- Calvo, I.; Fresnedo, O.; Mosteiro, L.; López, J.I.; Larrinaga, G.; Fernández, J.A. Lipid imaging mass spectrometry: Towards a new molecular histology. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159568. [Google Scholar] [CrossRef]
- Mi, H.; Sivagnanam, S.; Ho, W.J.; Zhang, S.; Bergman, D.; Deshpande, A.; Baras, A.S.; Jaffee, E.M.; Coussens, L.M.; Fertig, E.J.; et al. Computational methods and biomarker discovery strategies for spatial proteomics: a review in immuno-oncology. Brief Bioinform. 2024, 25, bbae421. [Google Scholar] [CrossRef]
- Shafighi, S.; Geras, A.; Jurzysta, B.; Sahaf Naeini, A.; Filipiuk, I.; Ra Czkowska, A.; Toosi, H.; Koperski, Ł.; Thrane, K.; Engblom, C.; et al. Integrative spatial and genomic analysis of tumor heterogeneity with Tumoroscope. Nat. Commun. 2024, 15, 9343. [Google Scholar] [CrossRef]
- Bi, K.; He, M.X.; Bakouny, Z.; Kanodia, A.; Napolitano, S.; Wu, J.; Grimaldi, G.; Braun, D.A.; Cuoco, M.S.; Mayorga, A.; et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell 2021, 39, 649–661.e5. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, W.; Lin, S.; Liu, B.; Wu, P.; Li, L. Fibroblast diversity and plasticity in the tumor microenvironment: roles in immunity and relevant therapies. Cell Commun. Signal. 2023, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Tanaka, N.; Takamatsu, K.; Hakozaki, K.; Fukumoto, K.; Masuda, T.; Mikami, S.; Shinojima, T.; Kakimi, K.; Tsunoda, T.; et al. Multiplexed single-cell pathology reveals the association of CD8 T-cell heterogeneity with prognostic outcomes in renal cell carcinoma. Cancer Immunol. Immunother. 2021, 70, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Larrinaga, G.; Redrado, M.; Loizaga-Iriarte, A.; Pérez-Fernández, A.; Santos-Martín, A.; Angulo, J.C.; Fernández, J.A.; Calvo, A.; López, J.I. Spatial expression of fibroblast activation protein-α in clear cell renal cell carcinomas revealed by multiplex immunoprofiling analysis of the tumor microenvironment. Cancer Immunol. Immunother. 2025, 74, 53. [Google Scholar] [CrossRef]
- Soultati, A.; Stares, M.; Swanton, C.; Larkin, J.; Turajlic, S. How should clinicians address intratumour heterogeneity in clear cell renal cell carcinoma? Curr. Opin. Urol. 2015, 25, 358–366. [Google Scholar] [CrossRef]
- Cormen, T.H.; Leiserson, C.E.; Rivest, R.L.; Stein, C. Introduction to Algorithms. 3rd Edition, MIT Press, 2009. ISBN: 9780262533058.
- López, J.I.; Cortés, J.M. Multisite tumor sampling : a new tumor selection method to enhance intratumor heterogeneity detection. Hum. Pathol. 2017, 64, 1–6. [Google Scholar] [CrossRef]
- Cortés, J.M.; de Petris, G.; López, J.I. Detection of intratumor heterogeneity in modern pathology : A multisite tumor sampling perspective. Front. Med. 2017, 4, 25. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–81. [Google Scholar] [CrossRef]
- Jain, T.; Sharma, P.; Are, A.C.; Vickers, S.M.; Dudeja, V. New insights into the cancer–microbiome–immune axis: decrypting a decade of discoveries. Front. Immunol. 2021, 12, 622064. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, X.; Wang, Z.; Cao, G.; Liu, K.; Yan, T. Uncovering the microbiota in renal cell carcinoma tissue using 16S rRNA gene sequencing. J. Cancer Res. Clin. Oncol. 2021, 147, 481–491. [Google Scholar] [CrossRef]
- Liss, M.A.; Chen, Y.; Rodriguez, R.; Pruthi, D.; Johnson-Pais, T.; Wang, H.; Mansour, A.; White, J.R.; Kaushik, D. Microbiome within primary tumor tissue from renal cell carcinoma may be associated with PD-L1 expression of the venous tumor thrombus. Adv. Urol. 2020, 2020, 9068068. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.E.; Memon, A.; Ahmed, S. Bladder Cancer and the Urinary Microbiome-New Insights and Future Directions: A Review. Clin. Genitourin. Cancer 2024, 22, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, W.; Shen, L.; Liu, J.; Yang, F.; Maskey, N.; Wang, H.; Zhang, J.; Yan, Y.; Yao, X. Can smoking cause differences in urine microbiome in male patients with bladder cancer? A retrospective study. Front. Oncol. 2021, 11, 677605. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Motooka, D.; Hatano, K.; Fukae, S.; Kawamura, N.; Tomiyama, E.; Hayashi, Y.; Banno, E.; Takao, T.; et al. The gut microbiota associated with high-Gleason prostate cancer. Cancer Sci. 2021, 112, 3125–3135. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Turner, D.; Lilly, M.; Ou, T.; Jiang, W. Differential circulating fungal microbiome in prostate cancer patients compared to healthy control individuals. J. Immunol. Res. 2022, 2022, 2574964. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Banerjee, A.; Datta, C.; Pal, D.K.; Ghosh, A.; Saha, A. Differential microbial signature associated with benign prostatic hyperplasia and prostate cancer. Front. Cell. Infect. Microbiol. 2022, 12, 894777. [Google Scholar] [CrossRef]
- Mørup, N.; Main, A.M.; Jørgensen, N.; Daugaard, G.; Juul, A.; Almstrup, K. The seminal plasma microbiome of men with testicular ger cell tumours described by small RNA sequencing. Andrology 2023, 11, 756–769. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Oli, M.W.; Oli, M.K. Eco-oncology: Applying ecological principles to understand and manage cancer. Ecol. Evol. 2020, 10, 8538–8553. [Google Scholar] [CrossRef]
- Badalamenti, G.; Fanale, D.; Incorvaia, L.; Barraco, N.; Listì, A.; Maragliano, R.; Vincenzi, B.; Calò, V.; Iovanna, J.L.; Bazan, V.; et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2019, 343, 103753. [Google Scholar] [CrossRef]
- Hochstadt, J.; Martínez Pacheco, S.; Casanova-Acebes, M. Embracing diversity: macrophage complexity in cancer. Trends Cancer 2025. [Google Scholar] [CrossRef]
- Errarte, P.; Larrinaga, G.; López, J.I. The role of cancer-associated fibroblasts in renal cell carcinoma. An example of tumor modulation through tumor/non-tumor cell interactions. J. Adv. Res. [CrossRef]
- Davis, A.; Gao, R.; Navin, N. Tumor evolution: linear, branching, neutral, or punctuated? Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 151–161. [Google Scholar] [CrossRef]
- Vendramin, R.; Litchfield, K.; Swanton, C. Cancer evolution: Darwin and beyond. EMBO J. 2021, 40, e108389. [Google Scholar] [CrossRef] [PubMed]
- Manini, C.; López-Fernández, E.; Lawrie, C.H.; Laruelle, A.; Angulo, J.C.; López, J.I. Clear cell renal cell carcinomas with aggressive behavior display low intratumor heterogeneity at the histological level. Curr. Urol. Rep. 2022, 23, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, X.; López, J.I.; Rowan, A.; Au, L.; Fendler, A.; Xu, H.; Horswell, S.; Shepherd, S.T.C.; Spain, L.; et al. Selection of metastasis competent subclones in the tumour interior. Nat. Ecol. Evol. 2021, 5, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- López, J.I.; Hogan, M.F.; Sutton, B.; Church, S.E.; Angulo, J.C.; Nunes-Xavier, C.E. Distinct spatial landscapes in clear-cell renal cell carcinoma as revealed by whole transcriptome analysis. Immunooncol. Technol. 2023, 21, 100690. [Google Scholar] [CrossRef]
- Dujon, A.M.; Aktipis, A.; Alix-Panabières, C.; Amend, S.R.; Boddy, A.M.; Brown, J.S.; Capp, J.P.; DeGregori, J.; Ewald, P.; Gatenby, R.; et al. Identifying key questions in the ecology and evolution of cancer. Evol. Appl. 2021, 14, 877–892. [Google Scholar] [CrossRef]
- Manini, C.; López, J.I. Ecology and games in cancer: new insights into the disease. Pathologica 2022, 114, 347–351. [Google Scholar] [CrossRef]
- Wölfl, B.; te Rietmole, H.; Salvioli, M.; Kaznatcheev, A.; Thuisjman, F.; Brown, J.S.; Burgering, B.; Stanková, K. The contribution of evolutionary game theory to understanding and treating cancer. Dyn. Games Appl. 2022, 12, 313–342. [Google Scholar] [CrossRef]
- Laruelle, A.; Rocha, A.; Manini, C.; López, J.I.; Inarra, E. Effects of heterogeneity on cancer: a game theory perspective. Bull. Math. Biol. 2023, 85, 72. [Google Scholar] [CrossRef]
- Manini, C.; Laruelle, A.; Rocha, A.; López, J.I. Convergent insights into intratumor heterogeneity. Trends Cancer 2024, 10, 12–14. [Google Scholar] [CrossRef]
- Viossat, Y.; Noble, R. A theoretical analysis of tumour containment. Nat. Ecol. Evol. 2021, 5, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Capp, J.P.; Nedelcu, A.M.; Dujon, A.M.; Roche, B.; Catania, F.; Ujvari, B.; Alix-Panabières, C.; Thomas, F. Does cancer biology rely on Parrondo’s principles? Cancers 2021, 13, 2197. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Big cribriform structures indicative or poorer prognosis in a prostate adenocarcinoma.
Figure 1.
Big cribriform structures indicative or poorer prognosis in a prostate adenocarcinoma.
Figure 2.
Eosinophilic solid and cystic renal cell carcinoma with its characteristic CK20 positivity (right).
Figure 2.
Eosinophilic solid and cystic renal cell carcinoma with its characteristic CK20 positivity (right).
Figure 3.
Histologic images of a clear cell (upper left), large nested (upper right), trophoblastic differentiation (lower left), and plasmacytoid (lower right) urothelial carcinoma of the urinary bladder.
Figure 3.
Histologic images of a clear cell (upper left), large nested (upper right), trophoblastic differentiation (lower left), and plasmacytoid (lower right) urothelial carcinoma of the urinary bladder.
Figure 4.
Typical histology of spermatocytic tumor (left) (original magnification, x250) and well differentiated papillary mesothelial tumor (right).
Figure 4.
Typical histology of spermatocytic tumor (left) (original magnification, x250) and well differentiated papillary mesothelial tumor (right).
Figure 5.
Histological features of “cuniculatum” (left), papillary (center), and verrucous (right) squamous cell carcinomas.
Figure 5.
Histological features of “cuniculatum” (left), papillary (center), and verrucous (right) squamous cell carcinomas.
Figure 6.
Histological and immunohistochemical (CK7+) (right) views of RCC with fibromyomatous stroma.
Figure 6.
Histological and immunohistochemical (CK7+) (right) views of RCC with fibromyomatous stroma.
Figure 7.
Histological view of a TFEB [t(6;11)] translocated renal cell carcinoma (left) with HMB-45 positivity (center), and FISH image (right).
Figure 7.
Histological view of a TFEB [t(6;11)] translocated renal cell carcinoma (left) with HMB-45 positivity (center), and FISH image (right).
Figure 8.
Histological view of a SDHB-deficient renal cell carcinoma (left) with retained SDHA (center) and lost SDHB (right) proteins.
Figure 8.
Histological view of a SDHB-deficient renal cell carcinoma (left) with retained SDHA (center) and lost SDHB (right) proteins.
Figure 9.
Example of prostate adenocarcinoma multifocality (blue circles) and intratumor heterogeneity (black rectangle) in an autopsy case.
Figure 9.
Example of prostate adenocarcinoma multifocality (blue circles) and intratumor heterogeneity (black rectangle) in an autopsy case.
Figure 10.
Histological image of a urothelial papilloma (left) and a papillary urothelial carcinoma (right).
Figure 10.
Histological image of a urothelial papilloma (left) and a papillary urothelial carcinoma (right).
Figure 11.
Gleason index 3+3=6 (Group 1) prostatic adenocarcinoma in a core biopsy sample.
Figure 11.
Gleason index 3+3=6 (Group 1) prostatic adenocarcinoma in a core biopsy sample.
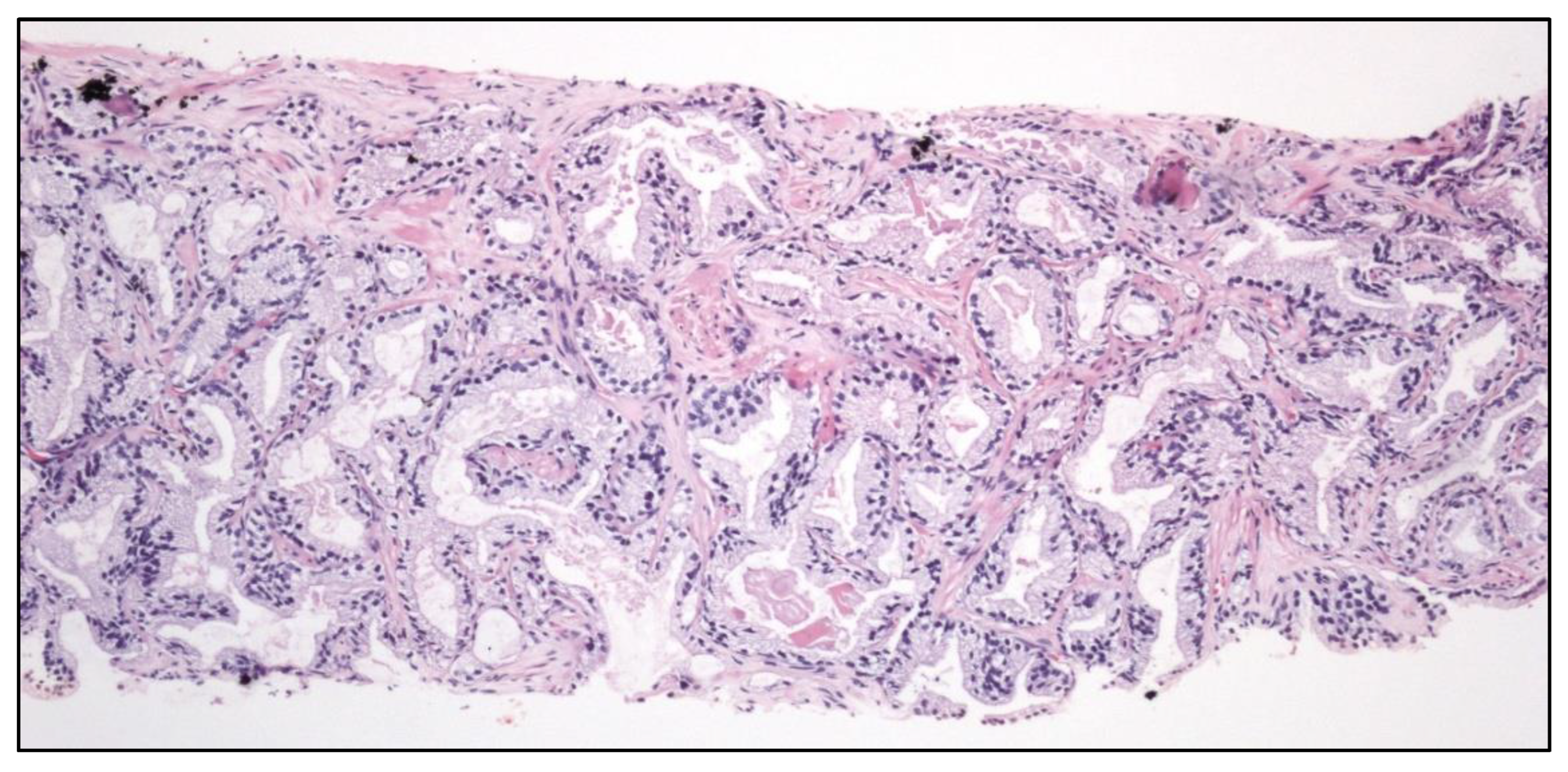
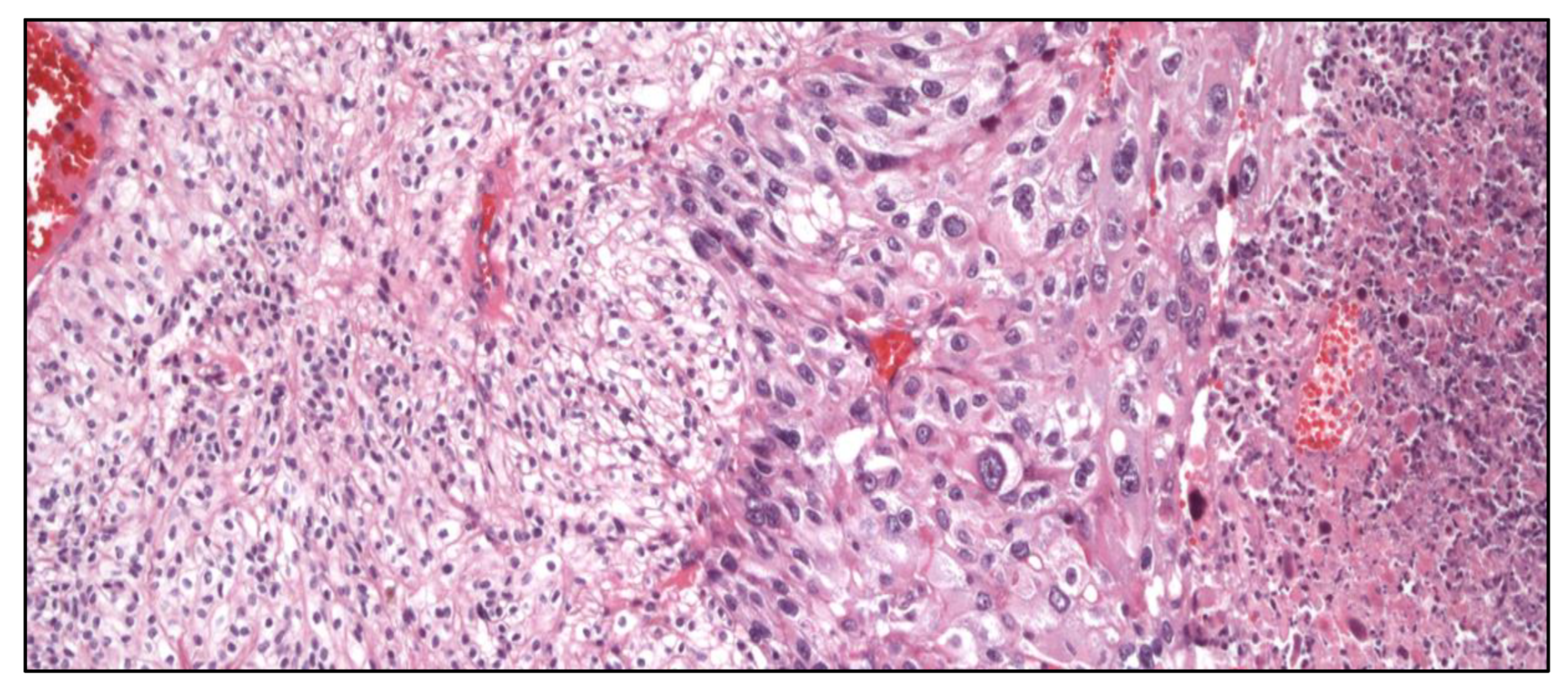
Figure 12.
Histologic example of intratumor heterogeneity in clear cell renal cell carcinoma showing low (left) and high (right) grades.
Figure 12.
Histologic example of intratumor heterogeneity in clear cell renal cell carcinoma showing low (left) and high (right) grades.
Table 1.
Hot spots in urological cancer research and clinics.
Table 1.
Hot spots in urological cancer research and clinics.
| 2022 WHO update in the classification of urinary and male genital tumors |
| New entities in kidney cancer |
| Urinary cancer -omics |
| Gleason grading system (update) |
| Targeted therapies and other novel treatments in urologic cancer |
| News on non-muscle invasive urothelial carcinoma |
| Artificial intelligence in urologic cancer |
| Intratumor heterogeneity influence in therapeutic failures in urologic neoplasms |
| Intratumor microbiome and its influence in urologic tumor aggressiveness |
| Ecological principles and mathematics applied to urological cancer study |
Table 2.
Molecular, histological, immunohistochemical, and gene expression in urothelial carcinoma.
Table 2.
Molecular, histological, immunohistochemical, and gene expression in urothelial carcinoma.
Luminal (positive KRT20, GATA3, and FOXA1 markers)
Luminal-papillary
Papillary Histology
(FGFR3 mutation/fusion/amplification)
Luminal-infiltrated
positive EMT markers (TWIST1, ZEB1, etc.)
Myofibroblast markers
Wild-type p53 |
Basal-squamous (positive KRT5, KRT6, and KRT14 and negative GATA3 and FOXA1)
Carcinoma in situ
Squamous differentiation
Basal keratin markers (CK 5.6, etc.)
Immune inflammatory infiltrates (PD-1, PD-L1, etc) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).