Submitted:
03 March 2025
Posted:
05 March 2025
Read the latest preprint version here
Abstract
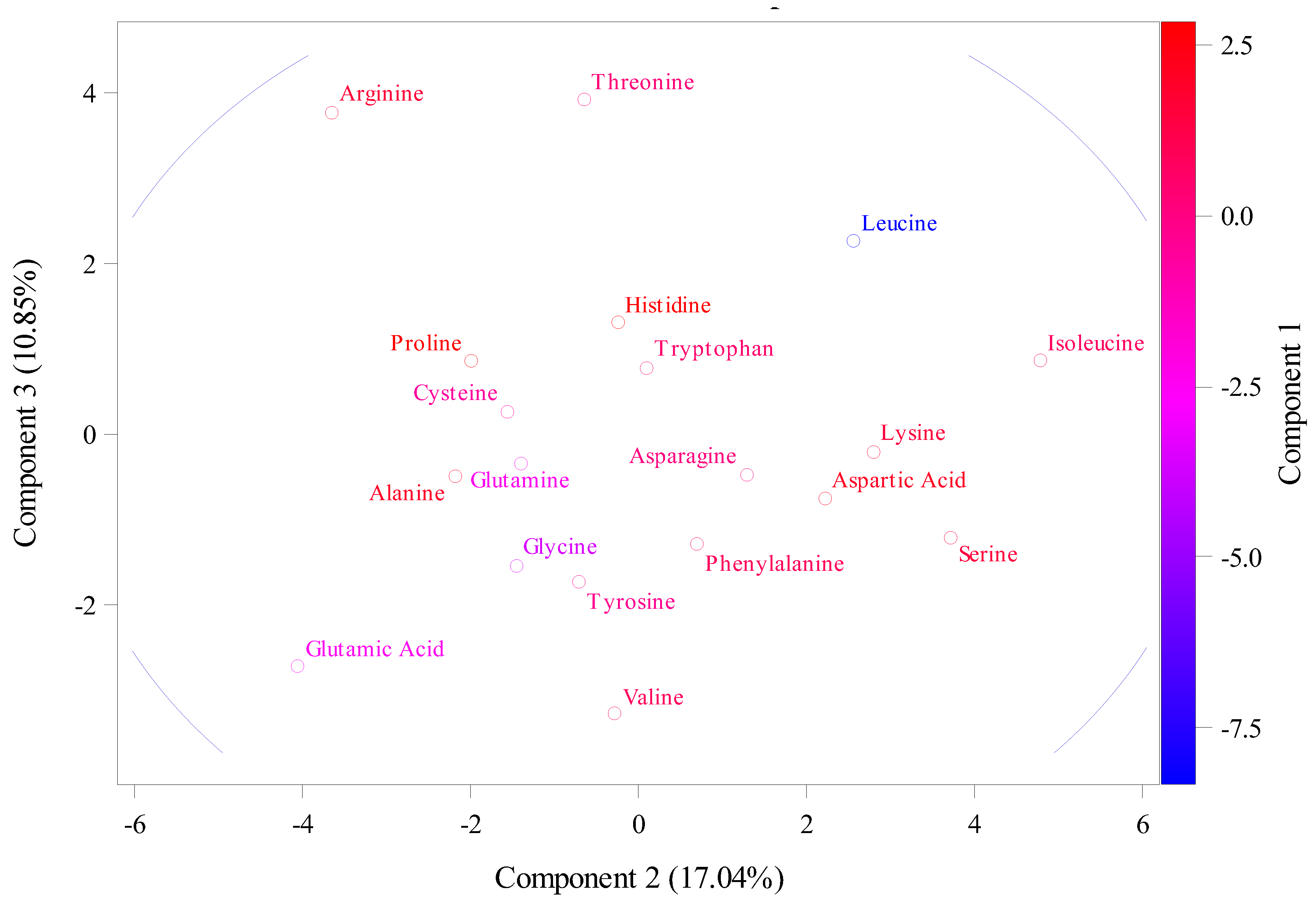
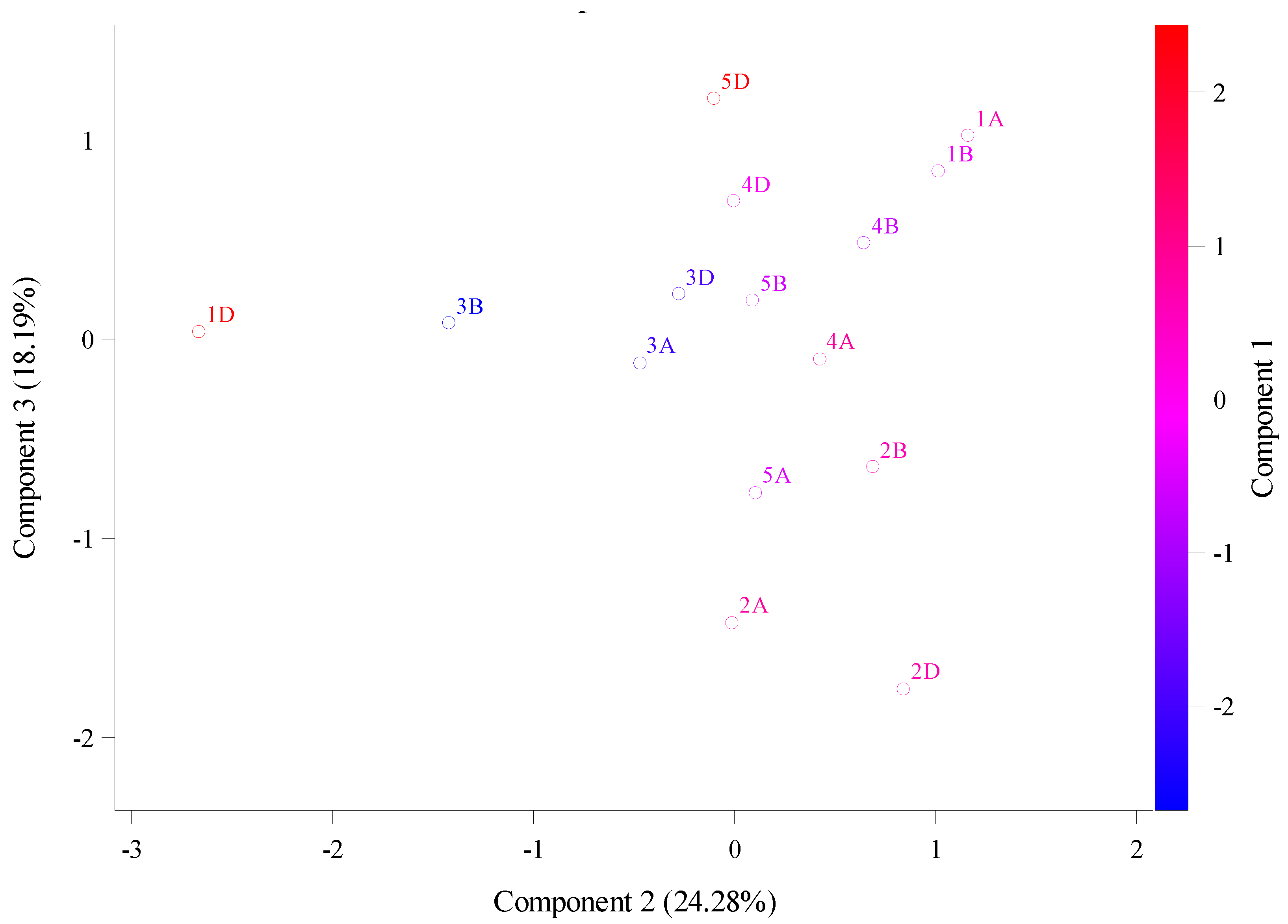
Cytokinin is play strongly role and implicated in wheat breeding in terms of flowering and yield. The aims here was to explain the wheat cytokinin riboside 5'-monophosphate phosphoribohydrolase sequences from two different databases through the using relative synonymous codon usage (RSCU), molecular weight (g/mol), theoretical isoelectric point, instability index, aliphatic index, and hydrophobicity. The RSCU divided values into two crops. First crop, significant overrepresentation were values above 1.6 that as in phenylalanine (TTC) located in 5B chromosomes, Leucine (TTA) located in 5A, and 1D chromosomes, leucine (TTG) located in 5B chromosome, leucine (CTC) located in 5D, 7D, and 7B. Leucine (CTA) located in 5D and second crop, underrepresentation were values below 0.6 as in leucine (CTA) located in 3A, 5D, 1B, 5B, and 7B. Valine (GTT) located in 4D, 5B, 4A, and 5B. In addition, theoretical isoelectric point (PI) ranged from 4.81 to 6.6 in chromosomes 3A and 4D respectively that were instability index was 34.3 and 38.16 respectively. The high instability was found at 1D and 5D with 54.16 and 50.36 respectively with decreasing in their stability as shown in aliphatic index at 92.98 and 88.91. Principal component analysis (PCA) of RSCU were explained main of variation is assigned to PCA1 with total variation about 72.11% and these amino acids are Isoleucine, Leucine, Lysine, Aspartic Acid, and Serine while the PCA2 and PCA3 had total variation, 17.04% and 10.85. While PCA of the theoretical isoelectric point results explained main of variation was assigned to PCA1 with total variation about 58.88 % and these chromosomes are 5D, 4D, 1A, 4B, 3D. While the PCA2 and PCA3 had total variation, 27.52% and 13.6% respectively assigned with 1D, 3B, 3A, 2D, 2A and 2B. The future objective from these results would be benefit for in nutrition and industrial and aid in breeding programs.
Keywords:
1. Introduction
2. Results
2.2. The Number of Chromosomes for Cytokinin Riboside 5'-Monophosphate Phosphoribohydrolase Genes Through NCBI and Ensemble Databases
2.3. The Relative Synonymous Codon Usage (RSCU)
3. Discussion
4. Materials and Methods
Funding
Data Availability Statement
Conflicts of Interest
References
- Jameson, P.E. and Song, J. (2016) Cytokinin: a key driver of seed yield. J. Exp. Bot. 67, 593–606. [CrossRef]
- Yamburenko, M.V., Kieber, J.J. and Schaller, G.E. (2017) Dynamic patterns of expression for genes regulating cytokinin metabolism and signaling during rice inflorescence development. PLoS ONE, 12, e0176060. [CrossRef]
- Cortleven, A., Leuendorf, J.E., Frank, M., Pezzetta, D., Bolt, S. and Schmülling, T. (2019) Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 42, 998–1018. [CrossRef]
- Pavlů, J., Novák, J., Koukalová, V., Luklová, M., Brzobohatý, B. and Černý, M. (2018) Cytokinin at the crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 19, 2450. [CrossRef]
- Prasad, R. 2022. Cytokinin and Its Key Role to Enrich the Plant Nutrients and Growth Under Adverse Conditions-An Update. Frontiers. An Update. Front. Genet. 13:883924. [CrossRef]
- Wang, W., Hao, Q., Tian, F., Li, Q., Wang, W. 2016. Cytokinin-Regulated Sucrose Metabolism in Stay-Green Wheat Phenotype. PLoS One. 11(8):e0161351. [CrossRef] [PubMed] [PubMed Central]
- Grewal, S., Yang, Cy., Scholefield, D. et al. Chromosome-scale genome assembly of bread wheat’s wild relative Triticum timopheevii. Sci Data 11, 420 (2024). [CrossRef]
- Nagar, S. (2011). Physiological basis of cytokinin induced drought tolerance in wheat (triticum aestivum l.). Journal of AgriSearch. 1(3): 139-144.
- Criado, M., Caputo, C., Roberts, I., Castro, M., Barneix, A. 2009. Cytokinin-induced changes of nitrogen remobilization and chloroplast ultrastructure in wheat (Triticum aestivum). Journal of Plant Physiology. 166. 1775-1785. [CrossRef]
- Mohanta, T.K., Khan, A., Hashem, A. et al. The molecular mass and isoelectric point of plant proteomes. BMC Genomics 20, 631 (2019). [CrossRef]
- Jablonski, B, Ogonowska H, Szala K, Bajguz A, Orczyk W, Nadolska-Orczyk A. Silencing of TaCKX1 Mediates Expression of Other TaCKX Genes to Increase Yield Parameters in Wheat. Int J Mol Sci. 2020;21(13):1–21. [CrossRef]
- Mameaux, S, Cockram J, Thiel T, Steuernagel B, Stein N, Taudien S, Jack P, Werner P, Gray JC, Greenland AJ, et al. Molecular, phylogenetic and comparative genomic analysis of the cytokinin oxidase/dehydrogenase gene family in the Poaceae. Plant Biotechnol J. 2012;10(1):67–82. [CrossRef]
- Zhang, J, Vankova R, Malbeck J, Dobrev P, Xu Y, Chong, K and Neff, M. (2009) AtSOFL1 and AtSOFL2 Act Redundantly as Positive Modulators of the Endogenous Content of Specific Cytokinins in Arabidopsis. PLoS ONE 4(12): e8236. [CrossRef]
- Gun L, Yumiao R, Haixian P, Liang Z. Comprehensive Analysis and Comparison on the Codon Usage Pattern of Whole Mycobacterium tuberculosis Coding Genome from Different Area. Biomed Res Int. 2018 May 8;2018:3574976. [CrossRef] [PubMed] [PubMed Central]
- Debnath S, Sarkar A, Perveen K, Bukhari NA, Kesari KK, Verma A, Chakraborty NR, Tesema M. Principal Component and Path Analysis for Trait Selection Based on the Assessment of Diverse Lentil Populations Developed by Gamma-Irradiated Physical Mutation. Biomed Res Int. 2022 Jul 18;2022:9679181. Retraction in: Biomed Res Int. 2024 Jan 9;2024:9835247. doi: 10.1155/2024/9835247. [CrossRef] [PubMed] [PubMed Central]
- Yang, Q., Xin, C., Xiao, Q., Lin, Y., Li, L., Zhao, J. 2023. Codon usage bias in chloroplast genes implicate adaptive evolution of four ginger species. Front. Plant Sci. 14. [CrossRef]
- Khalid, A, Hameed A, Tahir MF. Wheat quality. 2023. A review on chemical composition, nutritional attributes, grain anatomy, types, classification, and function of seed storage proteins in bread making quality. Front Nutr. ;10:1053196. [CrossRef]
- Wong, E. H., Smith, D. K., Rabadan, R., Peiris, M., and Poon, L. L. (2010). Codon usage bias and the evolution of influenza a viruses. Codon usage biases of influenza virus. BMC Evolutionary Biology, 10, 253. [CrossRef]
- Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wilkins, M., Appel, R., Bairoch A. 2005. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker, J.M. (eds) The Proteomics Protocols Handbook. Springer Protocols Handbooks. Humana Press. pp. 571-607. [CrossRef]
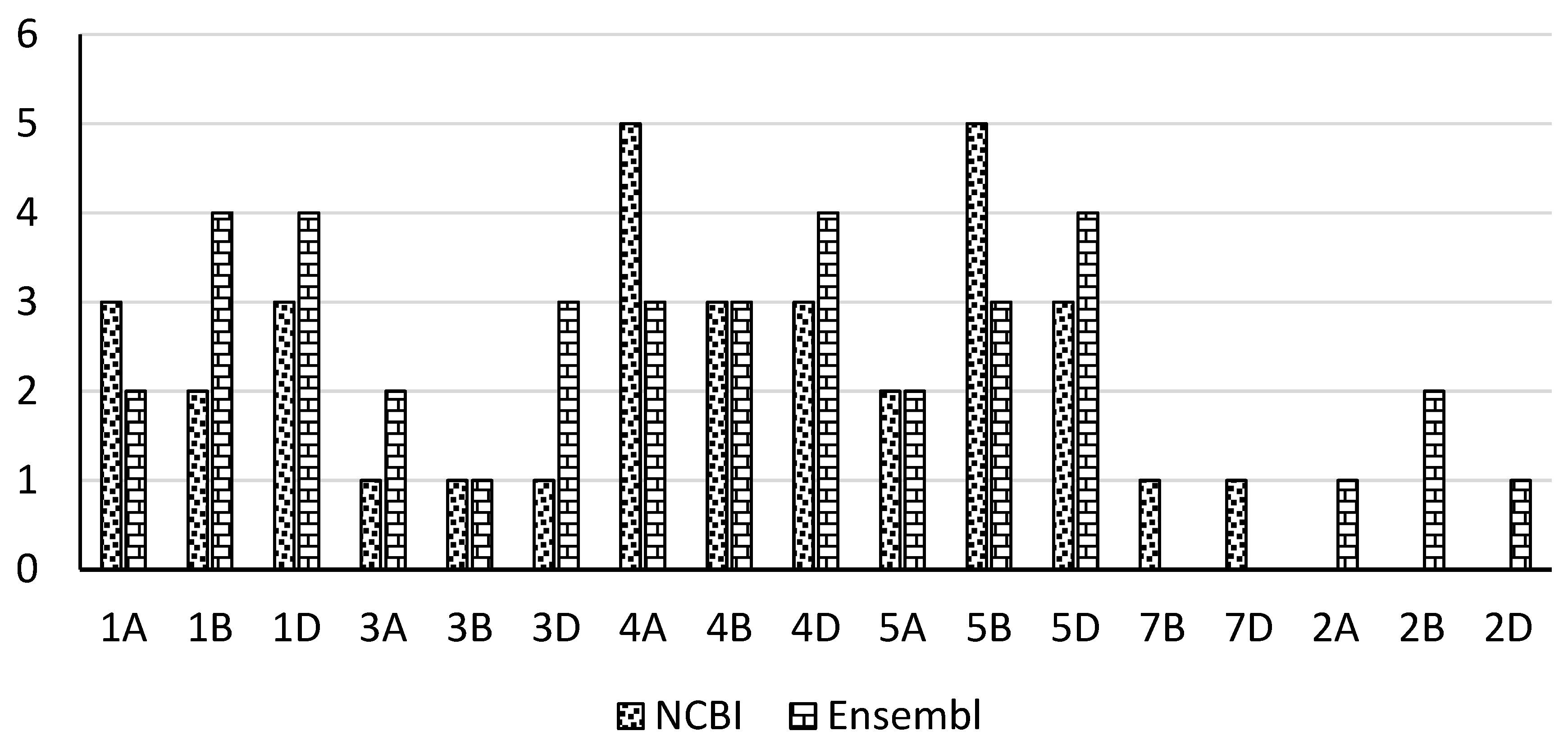
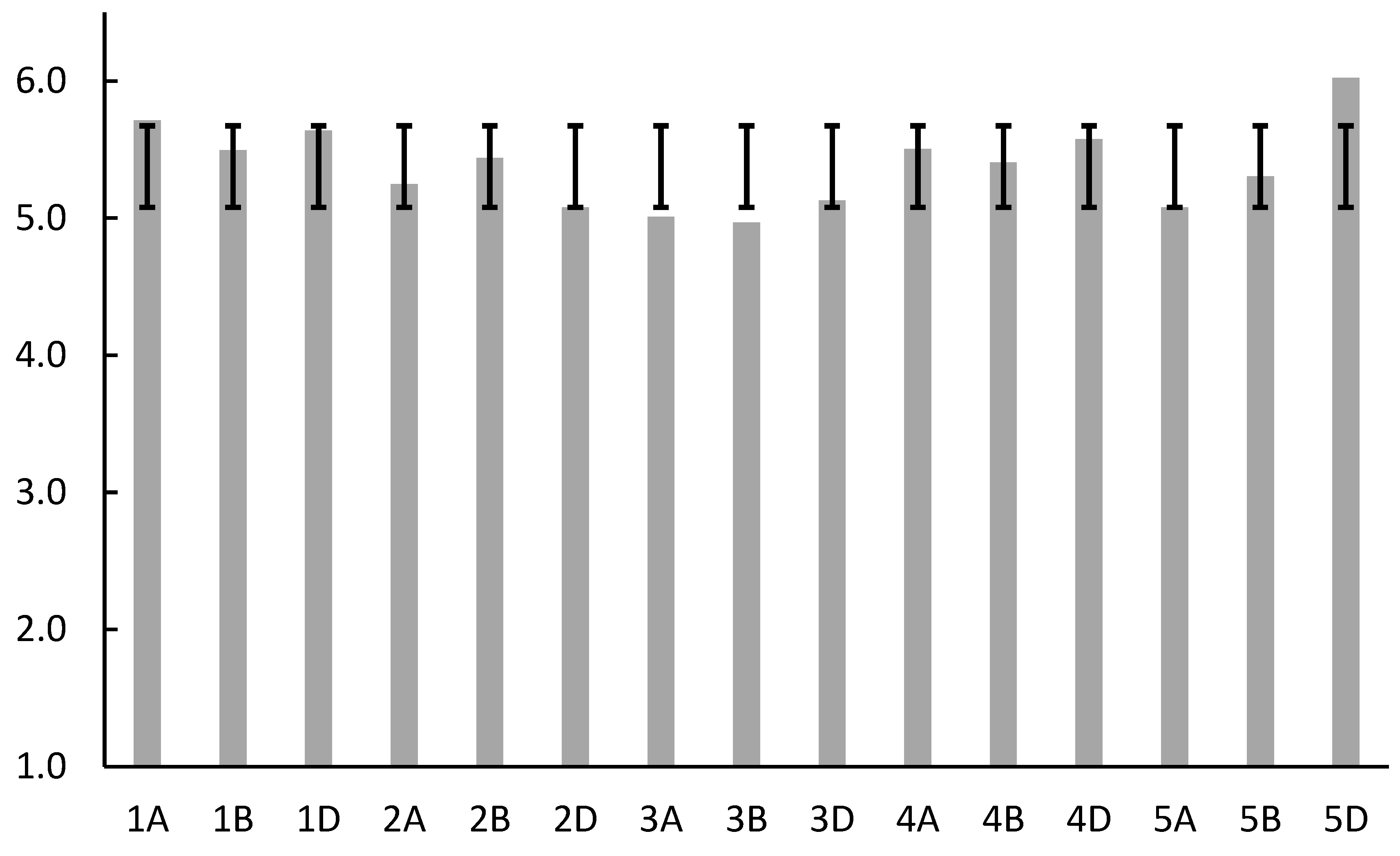
| Location | bp | molecular weight (g/mol) | theoretical isoelectric point (pI) | instability index (II), | aliphatic index (AI) | hydrophobicity (HY) |
| 4A | 218 | 22991.1 | 5.38 | 38.33 | 93.53 | -0.105 |
| 2A | 243 | 26618.74 | 5.25 | 40.31 | 91.44 | -0.096 |
| 4B | 205 | 22513.91 | 5.78 | 41.69 | 94.63 | 0.008 |
| 3A | 234 | 25265.85 | 5.21 | 34.3 | 95 | -0.017 |
| 1D | 215 | 23152.61 | 5.77 | 54.16 | 92.98 | 0.035 |
| 3D | 241 | 25737.38 | 5.21 | 33.91 | 93.49 | 0.01 |
| 1B | 268 | 28401.58 | 5.06 | 38.27 | 96.12 | 0.071 |
| 1D | 239 | 26037.71 | 5.66 | 43.3 | 84.1 | -0.38 |
| 1A | 208 | 22818.19 | 6.13 | 35.3 | 97.5 | -0.045 |
| 2B | 280 | 30712.62 | 5.71 | 39.74 | 95.71 | -0.03 |
| 2B | 246 | 26897.94 | 5.17 | 38.94 | 93.09 | -0.118 |
| 5D | 235 | 24482.96 | 6.22 | 42.68 | 88.04 | 0.008 |
| 3A | 229 | 24220.69 | 4.81 | 29.84 | 88.69 | 0.04 |
| 4D | 245 | 25850.59 | 6.6 | 38.16 | 89.59 | -0.049 |
| 5D | 248 | 26288.03 | 6.23 | 50.36 | 88.91 | -0.108 |
| 5D | 247 | 26070.71 | 6.17 | 41.8 | 86.52 | -0.082 |
| 5A | 246 | 26030.62 | 5.23 | 38.94 | 88.82 | -0.063 |
| 1B | 208 | 22819.17 | 5.98 | 36.23 | 97.5 | -0.045 |
| 5B | 241 | 25631.11 | 5.15 | 35.92 | 92.24 | -0.012 |
| 1D | 273 | 28817.09 | 5.15 | 36.73 | 39.99 | 0.067 |
| 2D | 246 | 26898.99 | 5.08 | 43.64 | 94.31 | -0.085 |
| 1A | 215 | 23217.63 | 5.3 | 50 | 92.05 | 0.02 |
| 5B | 241 | 25631.11 | 5.15 | 35.92 | 92.24 | -0.012 |
| 4A | 206 | 22487.78 | 5.15 | 37.95 | 95.58 | 0.052 |
| 4D | 225 | 23599.69 | 5.26 | 36.46 | 93.24 | -0.106 |
| 1B | 215 | 23221.66 | 5.45 | 48 | 91.12 | -0.003 |
| 5B | 246 | 26101.79 | 5.62 | 41.79 | 91.99 | -0.002 |
| 4D | 250 | 26176.7 | 5.28 | 36.82 | 87.8 | -0.004 |
| 3D | 226 | 24032.55 | 4.97 | 28.66 | 88.98 | 0.023 |
| 5A | 245 | 26310.95 | 4.93 | 39.99 | 95.51 | -0.001 |
| 1D | 208 | 22819.17 | 5.98 | 36.23 | 97.5 | -0.045 |
| 3B | 226 | 24032.55 | 4.97 | 27.99 | 88.98 | 0.023 |
| 4A | 248 | 26111.76 | 5.99 | 48.45 | 88.06 | -0.105 |
| 5D | 249 | 26428.14 | 5.48 | 43.07 | 90.48 | -0.032 |
| 3D | 239 | 25637.3 | 5.21 | 34.82 | 94.64 | 0.014 |
| 4B | 205 | 22282.61 | 5.29 | 34.03 | 97.51 | 0.107 |
| 4D | 205 | 22315.99 | 5.17 | 36.73 | 96.05 | 0.08 |
| 4B | 226 | 23572.58 | 5.15 | 37.49 | 91.15 | -0.125 |
| Mean | 233.947 | 25058.883 | 5.455 | 39.130 | 90.923 | -0.029 |
| StD | 19.385 | 1982.593 | 0.439 | 5.748 | 9.140 | 0.084 |
| NCBI | Ensembl | |||
| Gene symbol ID | Chro. | Gene symbol ID | Chro. | |
| 1 | LOC123045576 | 1A | TraesCS4A02G277200 | 4A |
| 2 | LOC123045860 | 1A | TraesCS2A02G380600 | 2A |
| 3 | LOC123061754 | 3A | TraesCS4B02G250400 | 4B |
| 4 | LOC123065219 | 1A | TraesCS3A02G211100 | 3A |
| 5 | LOC123070388 | 3B | TraesCS1D02G367400 | 1D |
| 6 | LOC123078846 | 3D | TraesCS3D02G213900 | 3D |
| 7 | LOC123080662 | 1B | TraesCS1B02G471300 | 1B |
| 8 | LOC123082392 | 4A | TraesCS1D02G003500 | 1D |
| 9 | LOC123083722 | 4A | TraesCS1A02G156100 | 1A |
| 10 | LOC123087480 | 4A | TraesCS2B02G397600 | 2B |
| 11 | LOC123087481 | 4A | TraesCS5D02G568400 | 5D |
| 12 | LOC123088143 | 4A | TraesCS3A02G251500 | 3A |
| 13 | LOC123093512 | 4B | TraesCS4A02G317900 | 4A |
| 14 | LOC123094293 | 4B | TraesCS5D02G568500 | 5D |
| 15 | LOC123095076 | 4B | TraesCS5B02G561400 | 5B |
| 16 | LOC123097019 | 4D | TraesCS5A02G347400 | 5A |
| 17 | LOC123098787 | 4D | TraesCS1B02G173200 | 1B |
| 18 | LOC123099424 | 4D | TraesCS5B02G348600 | 5B |
| 19 | LOC123107636 | 5A | TraesCS1D02G444500 | 1D |
| 21 | LOC123107637 | 5A | TraesCS2D02G376900 | 2D |
| 22 | LOC123112988 | 5B | TraesCS1A02G362500 | 1A |
| 23 | LOC123112989 | 5B | TraesCS5B02G561300 | 5B |
| 24 | LOC123114455 | 5B | TraesCS4A02G413200 | 4A |
| 25 | LOC123116784 | 5B | TraesCS4D02G033800 | 4D |
| 26 | LOC123117873 | 5B | TraesCS1B02G379700 | 1B |
| 27 | LOC123121452 | 1B | TraesCS5B02G348400 | 5B |
| 28 | LOC123123922 | 5D | TraesCS5B02G348300 | 5B |
| 29 | LOC123125354 | 5D | TraesCS3D02G251900 | 3D |
| 30 | LOC123126254 | 5D | TraesCS5A02G347500 | 5A |
| 31 | LOC123157422 | 7B | TraesCS1D02G154700 | 1D |
| 32 | LOC123160435 | 1D | TraesCS3B02G281000 | 3B |
| 33 | LOC123161849 | 1D | TraesCS4A02G318100 | 4A |
| 34 | LOC123165646 | 7D | TraesCS5D02G353600 | 5D |
| 35 | LOC123181086 | 1D | TraesCS3B02G241600 | 3B |
| 36 | TraesCS4B02G313800 | 4B | ||
| 37 | TraesCS4D02G310800 | 4D | ||
| 38 | TraesCS4B02G035700 | 4B | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




