Submitted:
04 March 2025
Posted:
04 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theoretical and Computational Methods
2.1. Electronic Structure Methods
2.2. Reaction Kinetics
3. Result and Discussion
3.1. Potential Energy Surfaces
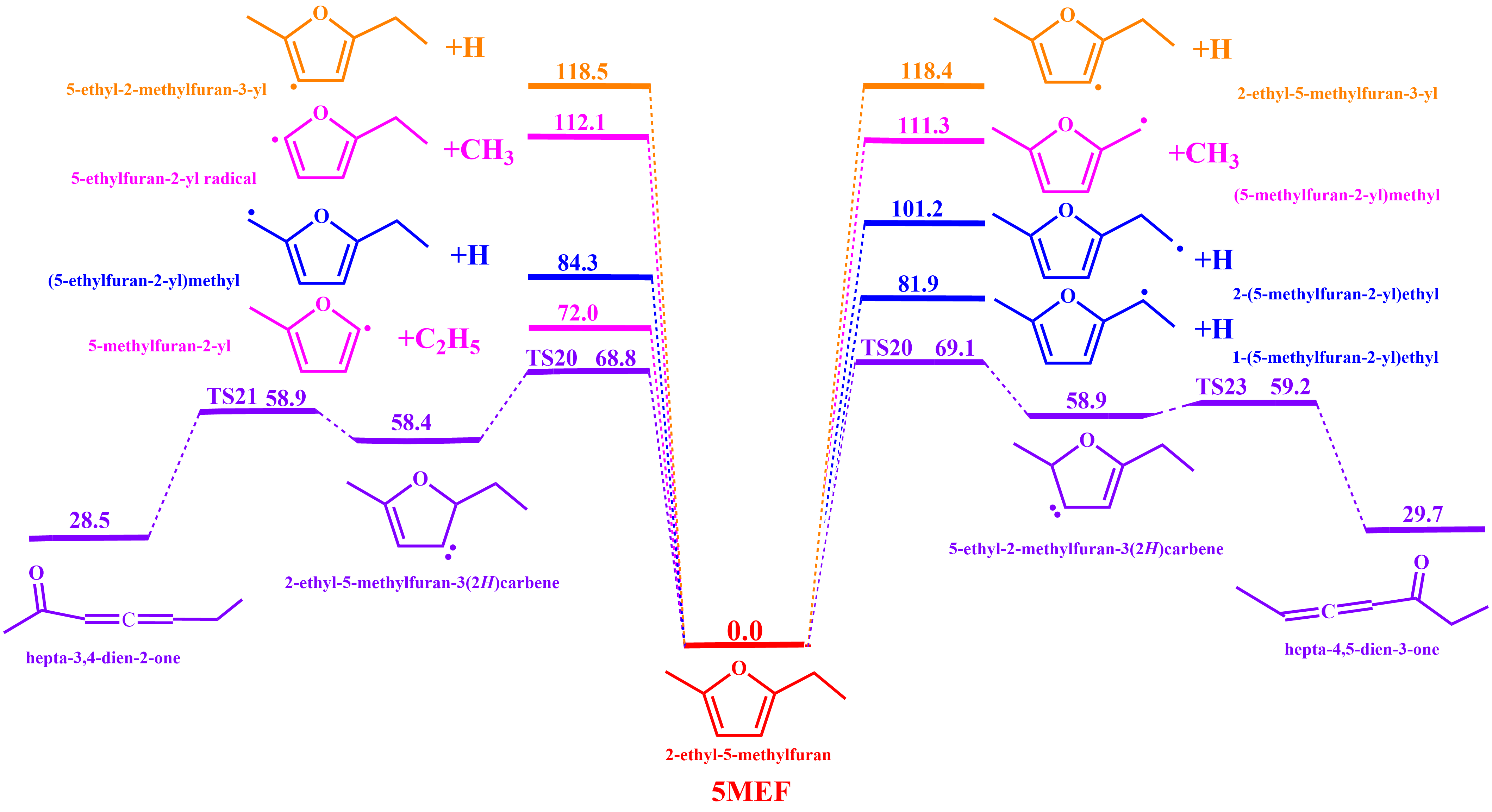
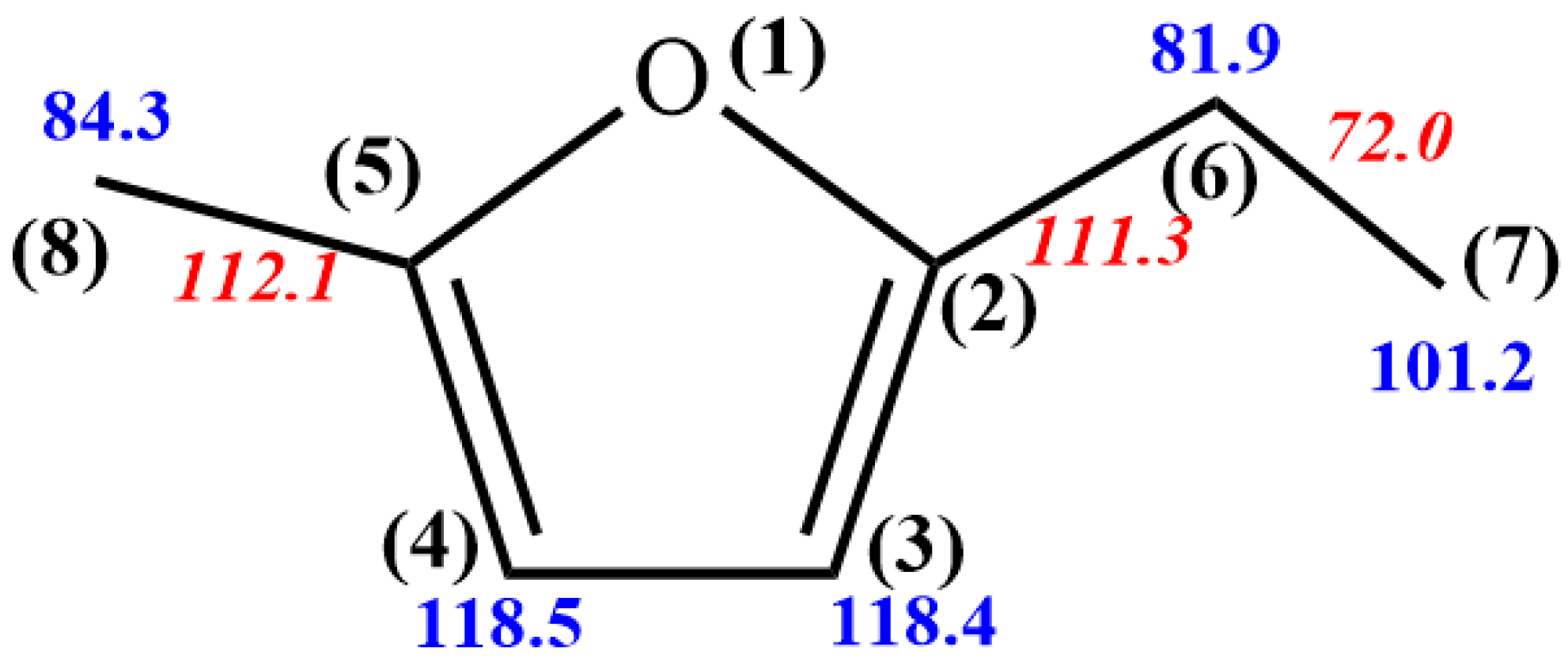
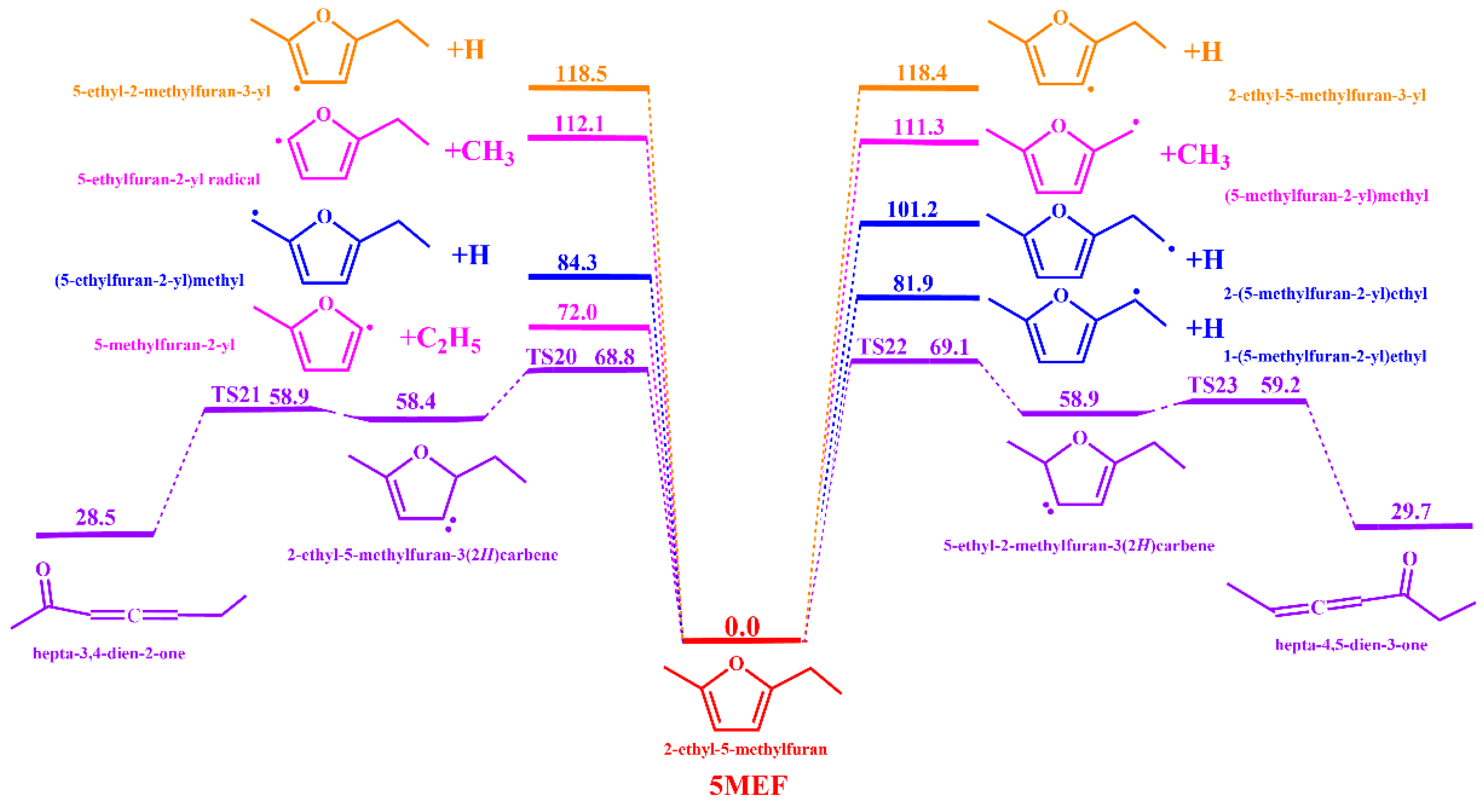
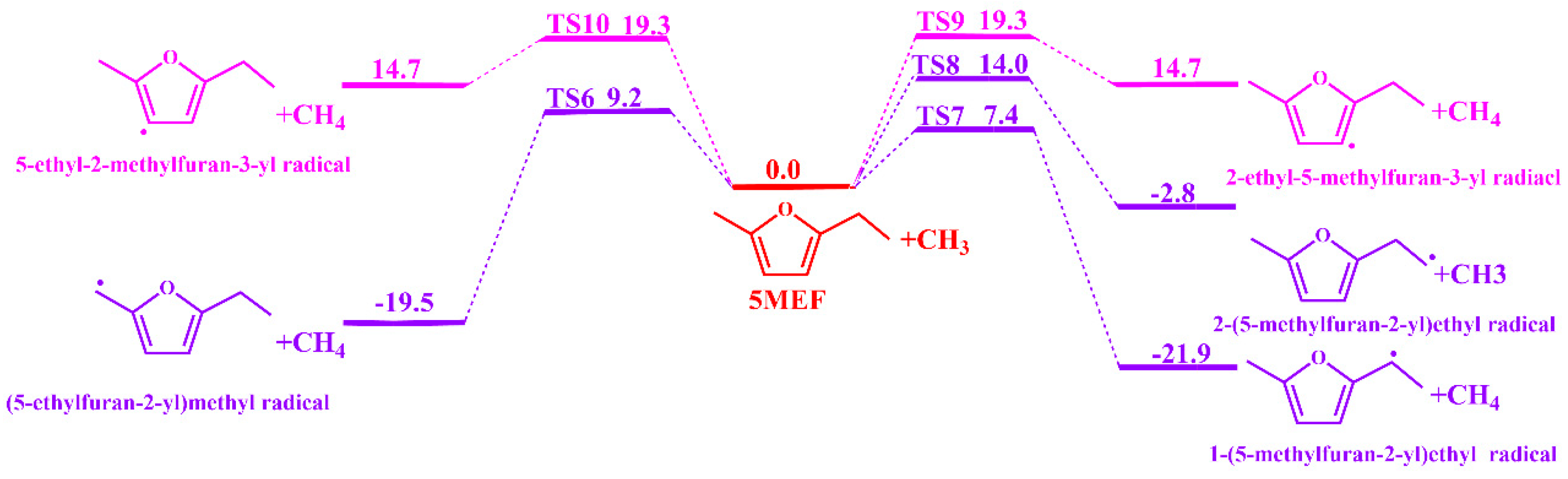
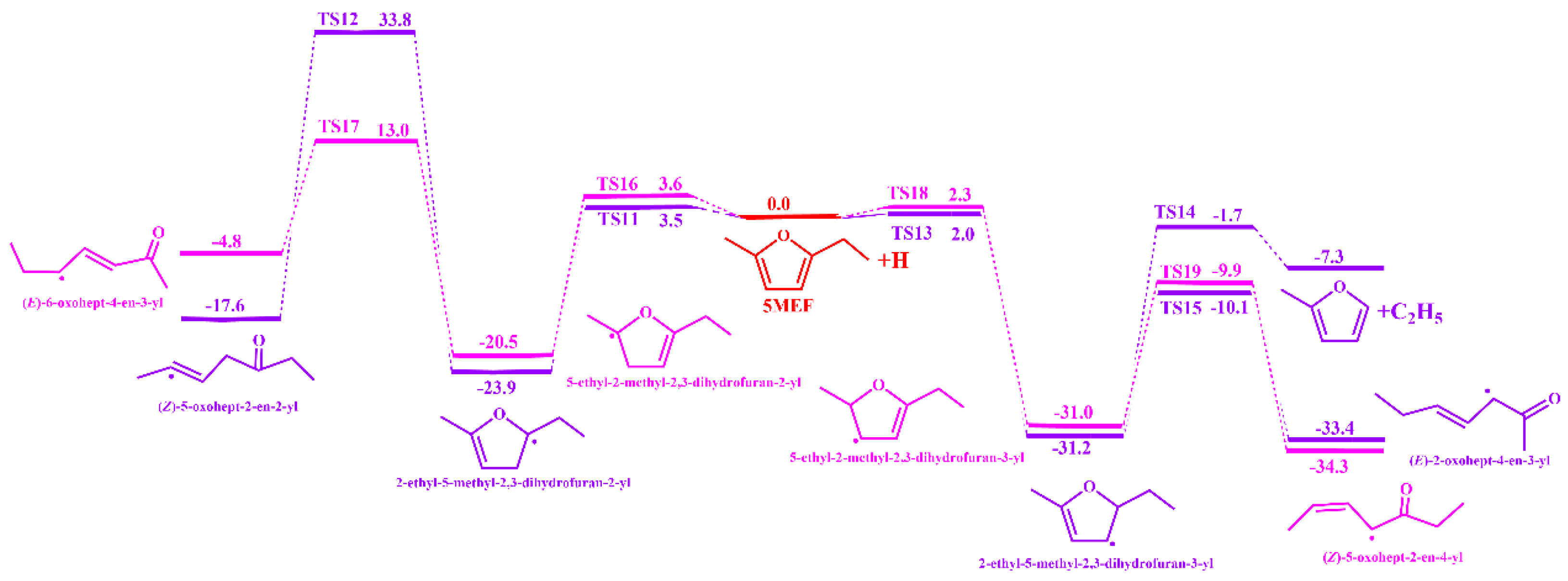
3.1.1. Unimolecular Dissociation Reaction Potential Energy Surface of 5-MEF
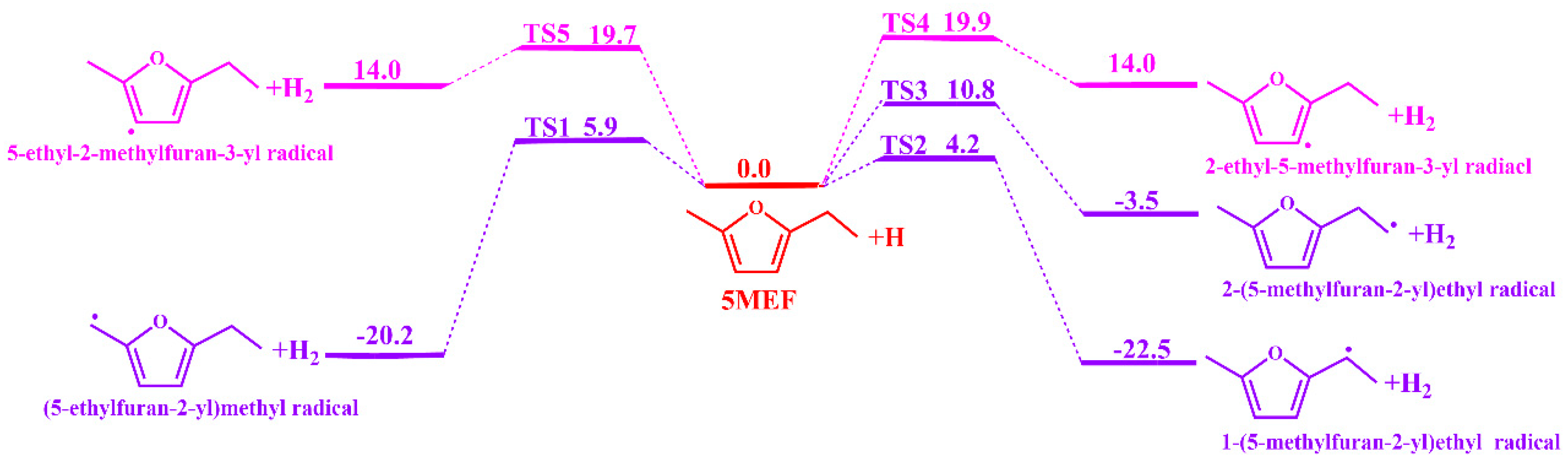
3.1.2. H-Transfer Decompositions Potential Energy Surface of 5-MEF
3.1.3. H-Abstraction Reaction Potential Energy Surface of 5-MEF
3.1.4. H-Addition Reaction Potential Energy Surface of 5-MEF
3.2. Temperature- and Pressure-Dependent Rate Coefficients
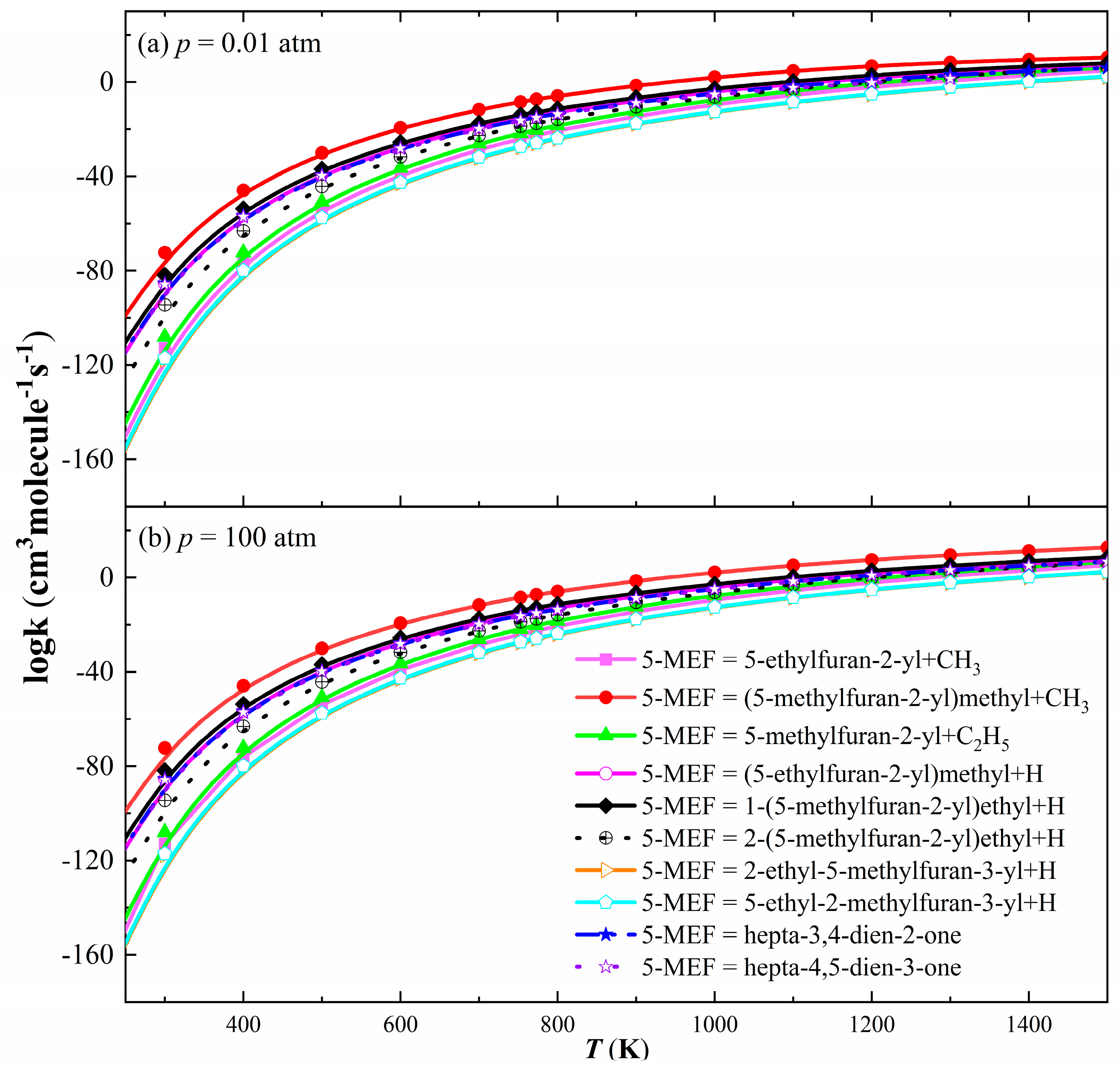
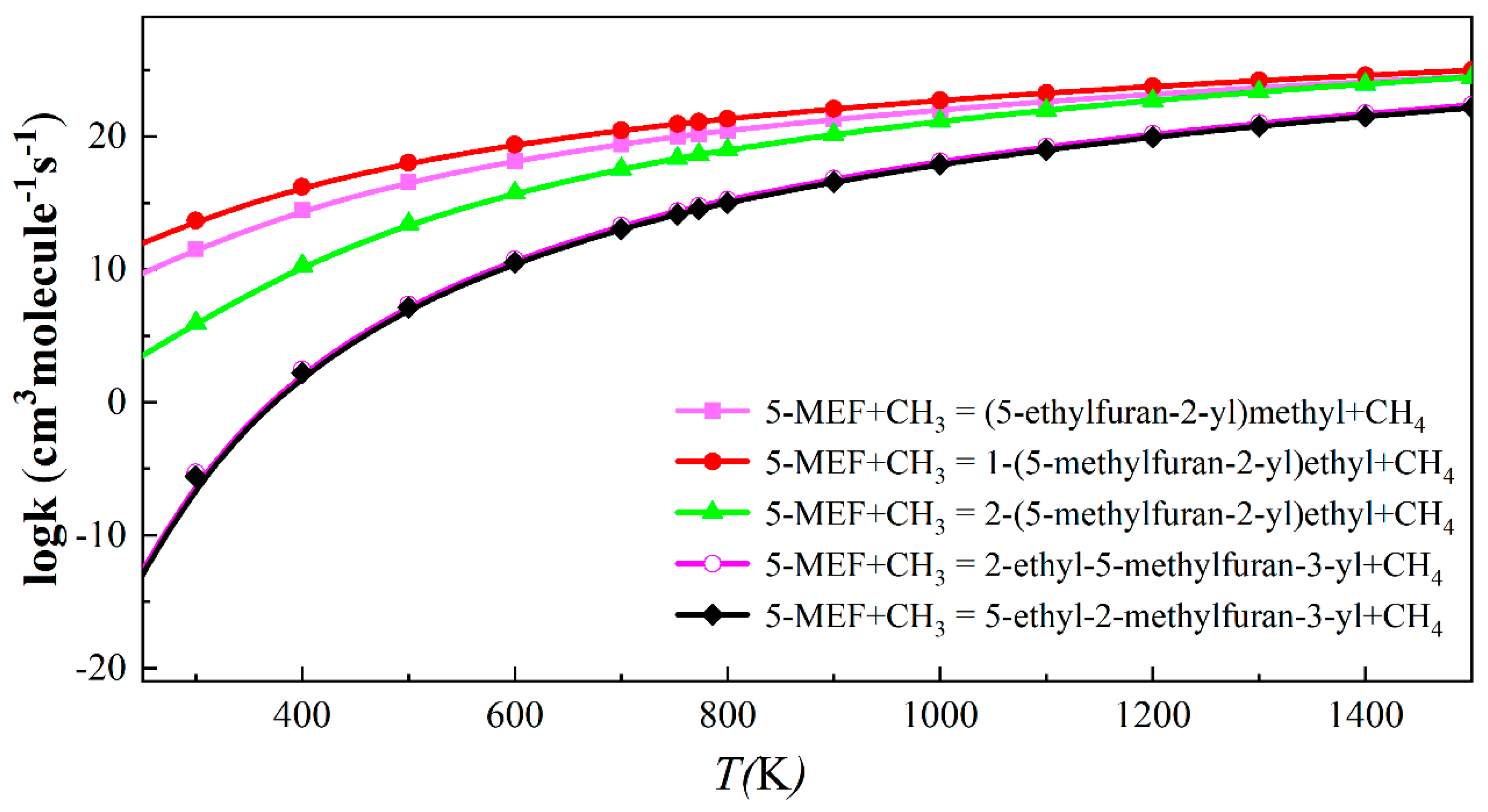
3.2.1. Unimolecular Dissociation and H-Transfer Decomposition Reaction Rate Constants for 5-MEF
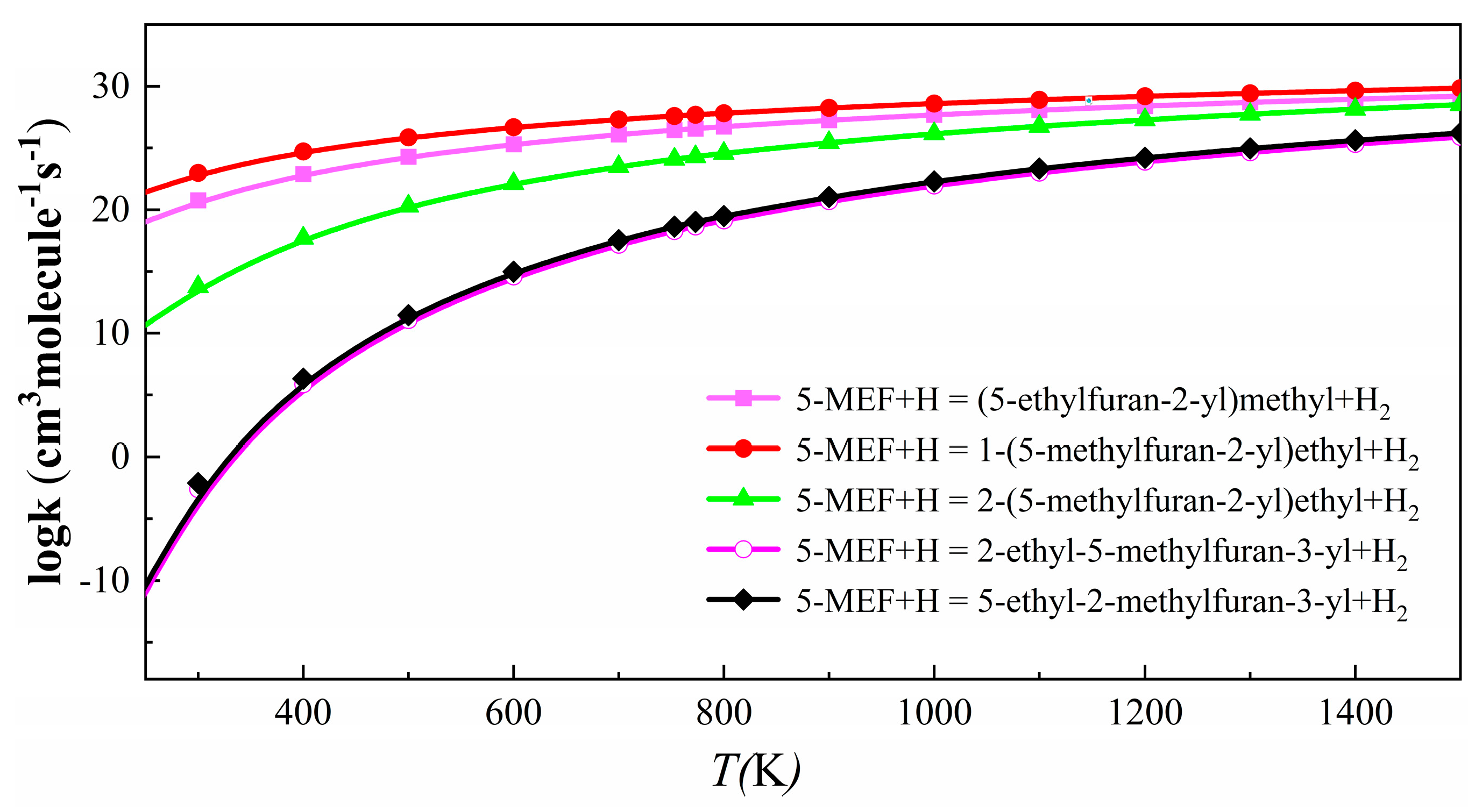
3.2.2. H-Abstraction Reaction Rate Constants for 5-MEF
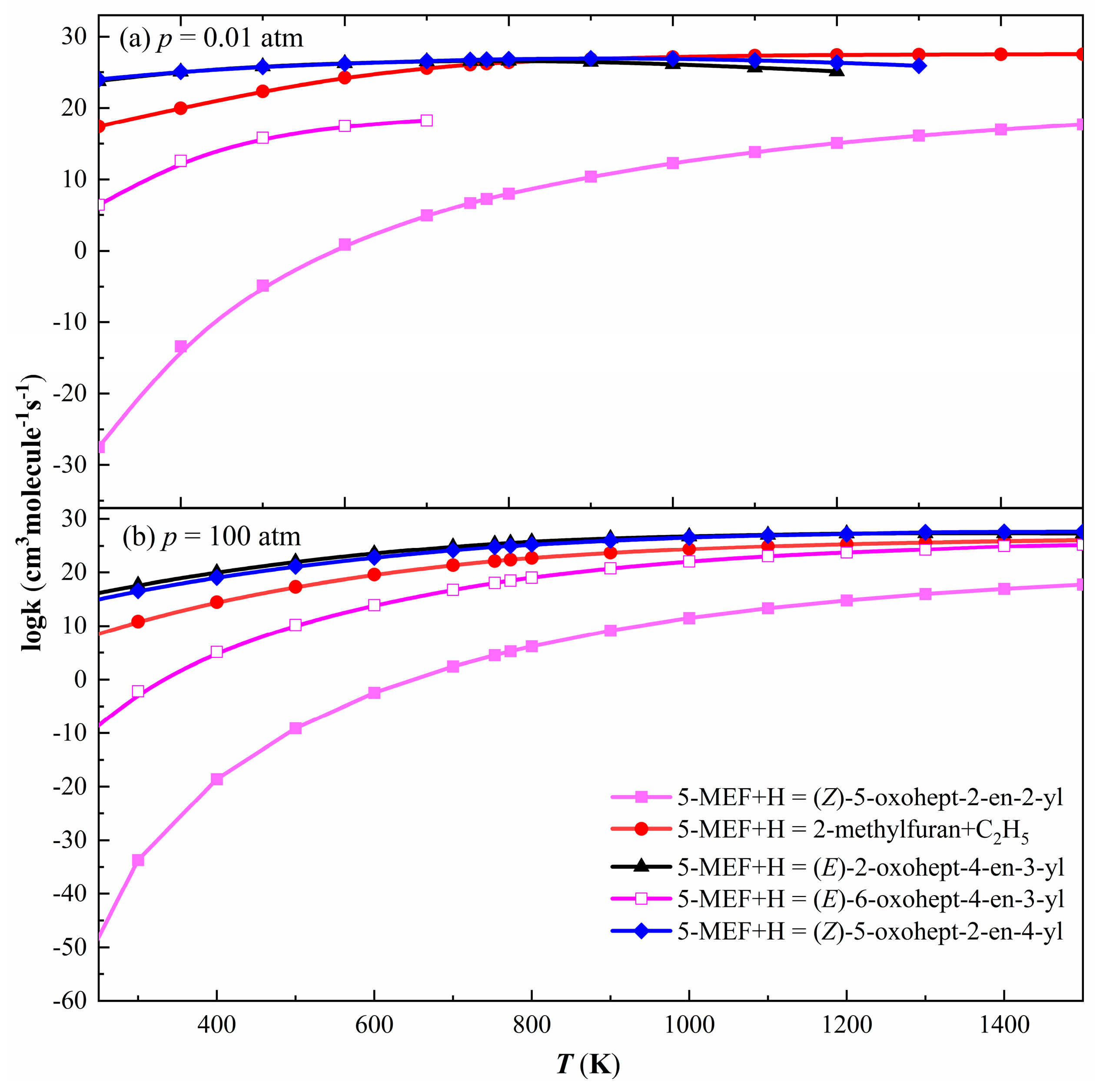
3.2.4. H-Addition Reaction Rate Constants for 5-MEF
4. Conclusions
Acknowledgments
References
- Jiang, Z.W.; Zeng, Y.J.; Hu, D.; Guo, R.C.; Yan, K.; Luque, R. , Chemical transformations of 5-hydroxymethylfurfural into highly added value products: present and future. Green Chemistry 2023, 25, 871–892. [Google Scholar]
- Yang, X.Y.; Zhang, J.P.; Zheng, J.; Liu, Z.C.; Liu, J.S.; Li, S.R.; Ye, Y.Y.; Xie, W.; Fan, J.Q.; Lan, H.Q.; Wang, D.C.; Zheng, Z.F. , In-situ and ex-situ catalytic pyrolysis of cellulose to produce furans over red mud-supported transition metal catalysts. Journal Of Analytical And Applied Pyrolysis 2023, 169. [Google Scholar]
- Zhu, Y.F.; Li, W.B.; Huang, Y.B.; Zheng, Y.W.; Wang, D.; Ye, Y.Y.; Li, S.R.; Zheng, Z.F. , Catalytic pyrolysis of cellulose over solid acidic catalysts: an environment-friendly method for furan production. Biomass Conversion And Biorefinery 2021, 11, 2695–2702. [Google Scholar]
- Ghosh, M.; Mishra, S.; Monir, K.; Hajra, A. , Copper-catalyzed regioselective synthesis of furan via tandem cycloaddition of ketone with an unsaturated carboxylic acid under air. Organic & Biomolecular Chemistry 2015, 13, 309–314. [Google Scholar]
- Gupta, K.; Rai, R.K.; Dwivedi, A.D.; Singh, S.K. , Catalytic Aerial Oxidation of Biomass-Derived Furans to Furan Carboxylic Acids in Water over Bimetallic Nickel-Palladium Alloy Nanoparticles. Chemcatchem 2017, 9, 2760–2767. [Google Scholar]
- Eldeeb, M.A.; Akih-Kumgeh, B. Recent Trends in the Production, Combustion and Modeling of Furan-Based Fuels. Energies 2018, 11. [Google Scholar]
- Eldeeb, M.A.; Akih-Kumgeh, B. , Investigation of 2,5-dimethyl furan and iso-octane ignition. Combustion And Flame 2015, 162, 2454–2465. [Google Scholar]
- Tanoue, K.; Takayama, T.; Ueno, S.; Mieno, T.; Irikura, K.; Kiritani, T.; Obata, K.; Naiki, T.; Watanabe, M. , Study on the combustion characteristics of furan- and nitromethane-added hydrocarbon fuels. Fuel 2021, 287. [Google Scholar]
- Whelan, C.A.; Eble, J.; Mir, Z.S.; Blitz, M.A.; Seakins, P.W.; Olzmann, M.; Stone, D. , Kinetics of the Reactions of Hydroxyl Radicals with Furan and Its Alkylated Derivatives 2-Methyl Furan and 2,5-Dimethyl Furan. Journal Of Physical Chemistry A 2020, 124, 7416–7426. [Google Scholar]
- Pintor, D.L.; Cho, S. , Effects of the stability of 2-methyl furan and 2, 5 dimethyl furan on the autoignition and combustion characteristics of a gasoline-like fuel. Fuel 2022, 312. [Google Scholar]
- Wu, S.H.; Tay, K.L.; Li, J.; Yang, W.M.; Yang, S.L. , Development of a compact and robust kinetic mechanism for furan group biofuels combustion in internal combustion engines. Fuel 2021, 298. [Google Scholar] [CrossRef]
- Tranter, R.S.; Lynch, P.T.; Randazzo, J.B.; Lockhart, J.P.A.; Chen, X.; Goldsmith, C.F. , High temperature pyrolysis of 2-methyl furan. Physical Chemistry Chemical Physics 2018, 20, 10826–10837. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Tang, C.L.; Meng, X.; Fan, X.S.; Tian, Z.M.; Huang, Z.H. , Experimental and Kinetic Study on the Ignition Delay Times of 2,5-Dimethylfuran and the Comparison to 2-Methylfuran and Furan. Energy & Fuels 2015, 29, 5372–5381. [Google Scholar]
- Wang, J.L.; Ding, W.M.; Gao, X.Z.; Wang, H.; Li, W.; Xu, Q.; Zhong, X.; Cheng, Z.J.; Wang, H.; Wang, Z.D.; Yang, J.Z.; Zhao, L.; Yan, B.B.; Chen, G.Y. , Experimental and kinetic model studies on the pyrolysis of 2-furfuryl alcohol at two reactors: Flow reactor and jet-stirred reactor. Combustion And Flame 2022, 244. [Google Scholar] [CrossRef]
- Li, P.D.; He, W.; Wang, J.L.; Song, S.B.; Wang, J.; Lv, T.L.; Yang, J.Z.; Cheng, Z.J.; Wei, L.X. , Experimental and kinetic modeling investigations on low-temperature oxidation of 2-ethylfuran in a jet-stirred reactor. Combustion And Flame 2022, 241. [Google Scholar] [CrossRef]
- Su, H.J.; Wang, J.L.; Zou, J.B.; Xu, Q.; Yang, J.Z.; Cheng, Z.J.; Wei, L.X. , Experimental and Kinetic Modeling Studies of 3-Methylfuran Pyrolysis at Low and Atmospheric Pressures. Energy & Fuels 2020, 34, 981–988. [Google Scholar]
- He, W.; Xu, Q.; Xie, C.; Yin, J.Z.; Li, P.D.; Wang, Z.D.; Zhang, L.D.; Wei, L.X. , Experimental and kinetic modeling studies of 2-acetylfuran pyrolysis at atmospheric pressure. Combustion And Flame 2022, 236. [Google Scholar] [CrossRef]
- Yan, B.B.; Wang, J.L.; Meng, Q.H.; Cheng, Z.J.; Wei, L.X.; Zhang, Y.; Cao, C.C.; Yang, J.Z.; Chen, G.Y. , Experimental and Kinetic Modeling Studies of Methyl 2-Furoate Pyrolysis at Atmospheric Pressure. Energy & Fuels 2019, 33, 4611–4620. [Google Scholar]
- He, W.; Zhang, Q.C.; Chen, K.X.; Nie, Y.S.; Li, Y.; Zhu, L.C.; Shen, K. , Theoretical Study of the Decomposition Reactions of 2-Vinylfuran. Acs Omega 2024, 9, 19063–19070. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. , A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. Journal of Chemical Physics 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Fukui, K. , The path of chemical reactions - the IRC approach. Accounts of Chemical Research 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Sirjean, B.; Fournet, R.; Glaude, P.-A.; Ruiz-López, M.F. , Extension of the composite CBS-QB3 method to singlet diradical calculations. Chemical Physics Letters 2007, 435, 152–156. [Google Scholar] [CrossRef]
- M.J. Frisch, G.W.T., H.B.Schlegel,G.E.Scuseria,M.A.Robb,J.R.Cheeseman,G.Scalmani, V.Barone,B.Mennucci,G.A.Petersson,H.Nakatsuji,M.Caricato,X.Li,H.P.Hratchian,A.F.Izmaylov,J.Bloino,G.Zheng,J.L.Sonnenberg,M.Hada,M.Ehara,K.Toyota,R.Fukuda,J.Hasegawa,M.Ishida,T.Nakajima,Y.Honda,O.Kitao,H.Nakai,T.Vreven,J.J.A.Montgomery,J.E.Peralta,F.Ogliaro,M.Bearpark,J.J.Heyd,E.Brothers,K.N.Kudin,V.N.Staroverov,T.Keith,R.Kobayashi,J.Normand,K.Raghavachari,A.Rendell,J.C.Burant,S.S.Iyengar,J.Tomasi,M.Cossi,N.Rega,J.M.Millam,M.Klene,J.E.Knox,J.B.Cross,V.Bakken,C.Adamo,J.Jaramillo,R.Gomperts,R.E.Stratmann,;O.Yazyev,A.J.A.,R.Cammi,C.Pomelli,J.W.Ochterski,R.L.Martin,K.Morokuma,V.G.Zakrzewski,G.A.Voth,P.Salvador,J.J.Dannenberg,S.Dapprich,A.D.Daniels,O.Farkas,J.B.Foresman,J.V.Ortiz,J.Cioslowski,D.J.Fox,Gaussian09,Gaussian,Inc.,WallingfordCT,2013.
- Eckart, C. , The Penetration of a Potential Barrier by Electrons. Physical Review 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Georgievskii, Y.; Miller, J.A.; Burke, M.P.; Klippenstein, S.J. , Reformulation and Solution of the Master Equation for Multiple-Well Chemical Reactions. The Journal of Physical Chemistry A 2013, 117, 12146–12154. [Google Scholar] [CrossRef]
- Y. Georgievskii, S.J.K., MESS, Argonne National Laboratory (2016).
- Klippenstein, S.J.; Miller, J.A. , From the time-dependent, multiple-well master equation to phenomenological rate coefficients. Journal of Physical Chemistry A 2002, 106, 9267–9277. [Google Scholar] [CrossRef]
- Date, N.; Hase, W.L.; Gilbert, R.G. , Collisional deactivation of highly vibrationally excited molecules. Dynamics of the collision event. The Journal of Physical Chemistry 1984, 88, 5135–5138. [Google Scholar] [CrossRef]
- Klippenstein, S.J. In From theoretical reaction dynamics to chemical modeling of combustion, 2017.
- Joback, K.G.; Reid, R.C. Estimation of pure-component properties from group-contributions. Chemical Engineering Communications 1987, 57, 233–243. [Google Scholar] [CrossRef]
- St John, P.C.; Guan, Y.F.; Kim, Y.; Kim, S.; Paton, R.S. , Prediction of organic homolytic bond dissociation enthalpies at near chemical accuracy with sub-second computational cost. Nature Communications 2020, 11. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




