1. Introduction
Lentinula edodes, commonly known as shiitake mushroom, is classified within the Basidiomycetes class, Agaricales order, and Tricholomataceae family, under the genus
Lentinula. This species is renowned for its high nutritional value, as it contains a wide range of essential amino acids, abundant mineral elements, and various trace elements [
1,
2]. Additionally,
L. edodes is rich in various medicinal compounds, including lentinan, which exhibits a range of therapeutic properties. Lentinan has been shown to possess anti-tumor, anti-viral, and antioxidant effects, as well as the ability to lower blood pressure and blood lipid levels. Due to these beneficial properties, it has been widely utilized in clinical applications [
3,
4]. Lentinan, the main secondary metabolite of
L. edodes, is a polysaccharide characterized by a β-(1→3)-D-glucan main chain. Traditionally, it is extracted from the fruiting bodies of the mushroom. However, the cultivation of fruiting bodies is a time-intensive and often inconsistent process, resulting in low lentinan yields and inefficient extraction of active compounds. These limitations hinder its broader development and application. Consequently, extracting and purifying active polysaccharides, such as lentinan, from mycelium through fermentation represents a highly promising and significant approach to overcome these challenges.
The selection of a strain capable of efficiently producing both biomass and bioactive compounds is crucial for optimizing the fermentation process. Microbial secondary metabolism is influenced by numerous factors, including strain characteristics [
5,
6] and fermentation conditions [
7,
8]. For instance, studies have shown significant variations in polysaccharide and ganoderic acid content among different Ganoderma lucidum strains, with strain-specific differences observed in the production of both intracellular and extracellular polysaccharides [
5,
6]. Fermentation techniques are primarily divided into two categories: static culture and shaking culture. These methods differ in cultivation approaches, oxygen supply, growth effects, and application scenarios. In liquid static culture, G. lucidum undergoes differentiation and morphological changes, leading to the formation of aerial mycelia and asexual spores, which are associated with high concentrations of ganoderic acids [
9,
10]. A two-stage fermentation approach, combining shaking culture followed by static culture, has been shown to enhance both the total triterpene content and yield [
11]. Given these findings, it is reasonable to infer that the growth and metabolic processes of
L. edodes may also exhibit distinct differences under shaking and static fermentation conditions. Understanding these variations is essential for optimizing the production of bioactive compounds in
L. edodes.
Mushroom polysaccharides exhibit a wide range of biological activities, including notable antioxidant effects. These activities are influenced by a combination of factors, such as extraction and purification methods, polysaccharide structure, culture conditions, and the regulation of exogenous substances. For example, polysaccharides extracted from
L. edodes at four different developmental stages demonstrated varying physicochemical properties and bioactivities. Among these stages, the immature phase was identified as the optimal harvest time for obtaining crude polysaccharides with higher biological activity [
10]. Heteropolysaccharides derived from Poria cocos mycelia exhibit varying antitumor activities depending on the strain and culture medium used [
12]. Similarly, polysaccharides extracted from
L. edodes demonstrate significant antioxidant activity in a concentration-dependent manner [
13,
14]. For instance, at a concentration of 3.0 mg mL-1, the scavenging rate for DPPH radicals reached 98.47%, comparable to that of vitamin C (Vc) [
15]. The bioactivities of polysaccharides are influenced by structural characteristics such as monosaccharide composition, molecular weight distribution, degree of branching, and degree of sulfation [
16,
17]. Among these factors, monosaccharide composition and molecular weight are the most straightforward and commonly measured indicators of polysaccharide properties.
Therefore, in this study, the mycelial biomass, polysaccharide content, and in vitro antioxidant activity of 19 L. edodes strains were evaluated under both static and shaking submerged culture conditions. Among these, strains XG21 and XG19 exhibited higher polysaccharide yields and superior antioxidant activity. Alongside the widely cultivated strain Xin808, further analyses were conducted to characterize the polysaccharide components, molecular weight distribution, and enzyme activities.
2. Materials and Methods
2.1. Experimental Materials
The strain Xin808, a commercially cultivated L. edoaes, was obtained from the Chengdu Academy of Agricultural and Forestry Sciences, while the remaining strains were generated through monosporal hybridization to ensure heterozygosity. For strain preservation and activation, Potato Sucrose Agar (PDA) medium was utilized, prepared with 200 g of potato, 20 g of glucose, 20 g of agar, and distilled water to make up 1 L. Three activated plug (diameter 5 mm) were inoculated into 50 mL fermentation medium,which consisted of 35 g sucrose, 5 g peptone, 2.5 g yeast powder, 1 g KH2PO4·H2O, 0.5 g MgSO4·7H2O, 0.5 g vitamin B1, adjusted to pH 7.0, and diluted to 1 L with distilled water. The cultures were incubated under two conditions: shaking (initial static period of 1 day followed by shaking at 150 r min-1 for 39 days at 25 ℃) and static (maintained at 25 ℃ for 40 days). Each treatment was performed in triplicate to ensure biological reproducibility.
2.2. Determination of Mycelial Biomass
The mycelium was separated from the liquid culture medium by filtration. The collected mycelium was rinsed three times with distilled water to remove any residual medium, followed by drying in an oven at 60 °C until a constant weight was achieved. The dry weight of the mycelium was then measured to determine the mycelial biomass.
2.3. Polysaccharide Extractions and Determinations
Polysaccharides were extracted using the hot water alcohol precipitation method as described previously [
8]. The polysaccharide content was quantified using the phenol-sulfuric acid method, with D-glucose serving as the standard [
18].
Intracellular polysaccharides (IPS) were extracted from the mycelium, and their yield was calculated using the formula: Y=mb/ma ×100 %, where Y represents the IPS yield (mg g-1), mb is the mass of IPS (mg), and ma is the mass of mycelial (g). Extracellular polysaccharides (EPS) were obtained from the filtered fermentation broth, and their yield was determined using the formula: w=mc/v, where w is the EPS yield (mg mL-1), mc is the mass of EPS (mg), and v is the volume of the fermentation broth volume (mL). The total polysaccharide (TPS) content was calculated by the formula G=Yg+wv, where G is the TPS content (mg), Y is the IPS yield (mg g-1), W is the EPS content (mg mL-1), g is the mycelium biomass (mg), and v is the volume of the liquid culture medium (mL).
2.4. In vitro Antioxidant Activities of Polysaccharides
The in vitro antioxidant activities of intracellular polysaccharides (IPS) and extracellular polysaccharides (EPS) were assessed based on their ability to scavenge the superoxide anion (O
2-), hydroxyl (·OH), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, following the methodology described by Xiang et al. [
8]. Solutions of IPS, EPS and Vitamin C (used as a positive control) were prepared at varying concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg mL
-1) for the antioxidant activity assays. Deionized water, replacing the polysaccharide solution, served as the negative control. The IC
50 value, defined as the concentration required to reduce 50% of the initial free radical concentration, was determined as per Liu et al. [
19].
2.5. Enzyme Activities and Expression Profiles of Key Genes in Polysaccharide Synthesis
Fresh mycelium (0.1 g) was washed three times with 1 mL of 20 mM phosphate buffer (pH 6.5), ground into a fine powder using liquid nitrogen, and then homogenized in 1 mL of the same phosphate buffer. The homogenate was centrifuged at 10,000 rpm for 15 minutes at 4 °C, and the resulting supernatant was collected as the crude enzyme solution. The activities of key enzymes involved in polysaccharide synthesis, including phosphoglucose isomerase (PGI), phosphoglucomutase (PGM), and UDPG-pyrophosphorylase (UGP) were determined according to previously established methods [
7]. Enzyme activity was quantified based on the molar extinction coefficient of NAD(P)H (ε340=6.22*10
3 mol L
-1 cm
-1), with one unit of enzyme activity defined as the amount required to oxidize 1 nmol of NAD(P)H per minute.
Total RNA was extracted using the TRIzol method and subsequently reverse transcribed into cDNA with the AMV First-Strand cDNA Synthesis Kit (Sangon Biotech, Shanghai, China). The transcriptional expression levels of
PGI,
PGM, and
UGP were quantified following the previously described protocol [
7].
2.6. Determination of Polysaccharide Monosaccharide Components and Molecular Weight
The monosaccharide composition of the crude polysaccharides was analyzed following the method described by Salvador et al. and Zhu et al. [
20,
21]. Briefly, approximately 5 mg of the polysaccharide sample was hydrolyzed using 2 M trifluoroacetic acid (TFA) at 121 °C for 2 hours in a sealed tube. The hydrolyzed sample was dried under a nitrogen stream, washed with methanol, and then dried again. This methanol washing step was repeated 2–3 times to ensure complete removal of residual TFA. The resulting residue was re-dissolved in deionized water and filtered through a 0.22 μm microporous membrane for further analysis.
High-performance anion-exchange chromatography (HPAEC) was performed on a CarboPac PA-20 anion-exchange column (3 × 150 mm; Dionex) coupled with a pulsed amperometric detector (PAD; Dionex ICS 5000 system). The analysis conditions were as follows: flow rate, 0.5 mL min-1; injection volume, 5 μL; solvent system A (ddH₂O), solvent system B (0.1 M NaOH), and solvent system C (0.1 M NaOH, 0.2 M NaAc). The gradient program was set as follows: 95:5:0 (A:B:C) at 0 min, 85:5:10 at 26 min, 85:5:10 at 42 min, 60:0:40 at 42.1 min, 60:40:0 at 52 min, 95:5:0 at 52.1 min, and 95:5:0 at 60 min. Data acquisition was performed using the ICS5000 system (Thermo Scientific), and the results were processed with Chromeleon 7.2 CDS software (Thermo Scientific). Quantified data were exported into Excel format for further analysis and interpretation.
The molecular weight of polysaccharides was measured according to the method reported before [
22,
23]. The polysaccharide samples were dissolved in a 0.1M NaNO
3 aqueous solution containing 0.02% NaN
3 at the concentration of 1 mg mL
-1 and filtered through a filter of 0.45 μm pore size. The homogeneity and molecular weight of the polysaccharide fractions were analyzed using size-exclusion chromatography coupled with multi-angle laser light scattering and refractive index detection (SEC-MALLS-RI).
The weight-average molecular (Mw), number-average molecular weight (Mn), and polydispersity index (Mw/Mn) were measured using a DAWN HELEOS-II laser photometer (Wyatt Technology Co., USA) equipped with three tandem columns (300 × 8 mm, Shodex OH-pak SB-805, 804, and 803; Showa Denko K.K., Tokyo, Japan). The columns were maintained at 45 °C using a column heater. The elution flow rate was set at 0.4 mL min-1. A differential refractive index detector (Optilab T-rEX, Wyatt Technology Co., USA) was connected in parallel to determine the concentration of the fractions and the specific refractive index increment (dn/dc). The dn/dc value for the polysaccharide fractions in the 0.1 M NaNO₃ aqueous solution containing 0.02% NaN₃ was determined to be 0.141 mL g-1. Data acquisition and processing were performed using ASTRA 6.1 software (Wyatt Technology). The quantified results were exported into Excel format for further analysis and interpretation.
2.7. Data Analysis
The Parson correlation coefficient (r2, ranging from +1 to −1) was calculated to analyze the relationship between the key enzyme activities involved in polysaccharide synthesis and the polysaccharide content. The analysis was conducted using SPSS 24.0 software (SPSS Inc., Chicago, IL, USA). Correlations were interpreted as follows: no correlation (r = 0), positive correlation (r > 0), or negative correlation (r < 0). All data are presented as means ± standard deviation (SD) derived from triplicate experiments. Statistical comparisons of means were performed using the least significant difference (LSD) test, with significance set at P ≤ 0.05.
3. Results
3.1. Polysaccharide Content of Mycelium from Different Strains Under Shaking and Static Culture Conditions
To identify the strain with the highest polysaccharide production, nineteen strains of L.edodes were cultivated under both shaking and static fermentation conditions. The mycelial biomass, intracellular polysaccharide (IPS) content, extracellular polysaccharide (EPS) content, and total polysaccharide content were systematically measured and compared across all strains.
Among the nineteen strains cultivated, six exhibited a mycelial biomass exceeding 0.20 g after 39 days of shaking culture. Strain XG24 demonstrated the highest mycelial biomass (0.55 g), which was 1.29 times greater than that of the reference strain L. edodes Xin808 (
Table S1). Additionally, seven strains showed intracellular polysaccharide (IPS) concentrations above 15 mg g
-1, with strains XG20 and XG21 displaying the highest IPS levels at 21.75 mg g
-1 and 17.97 mg g
-1, respectively. Furthermore, three strains- XG21, XG24, and XG30-produced extracellular polysaccharide (EPS) concentrations exceeding 0.50 mg mL
-1. Notably, strain XG21 exhibited the strongest EPS production capacity (0.70 mg mL
-1), representing an 89.82% increase compared to Xin808. When evaluating total polysaccharide content as a key indicator, strain XG21demonstrated superior polysaccharide production, with a total content of 44.06 mg, which was 88.31% higher than that of Xin808 (
Table 1).
Under static culture conditions, the biomass of the strains was generally higher compared to shaking culture, with only four strains exhibiting a biomass below 0.20 g. Strains XG14 and XG24 achieved the highest biomass, measuring 0.56 g and 0.57 g, respectively, which were 1.65 and 1.68 times greater than that of the reference strain Xin808. Among the strains, 14 displayed intracellular polysaccharide (IPS) concentrations exceeding 15 mg g-1. Strains XG20 and XG21 demonstrated strong IPS and EPS production capabilities, with IPS and EPS contents reaching 74.94 mg g-1 and 0.51 mg mL-1, respectively. These values were 5.77 and 0.91 times higher than those of Xin808. When evaluating total polysaccharide content as a key indicator, strain XG20 exhibited superior polysaccharide production under static culture, with a total content of 46.06 mg, representing a 1.31-fold increase over Xin808. Across the two culture conditions, strains XG21 and XG20 emerged as the top performers in mycelial polysaccharide production, with total polysaccharide contents of 78.80 mg and 75.15 mg, respectively.
3.2. In vitro Antioxidant Activity of Mycelium Polysaccharides from Different Strains Under Shaking and Static Culture Conditions
The antioxidant activities of mycelium intracellular polysaccharides (IPS) and extracellular polysaccharides (EPS) from 18 L. edodes strains were assessed using three distinct in vitro assays. As summarized in
Table 2, IPS and EPS derived from different strains under both static and shaking culture conditions demonstrated varying levels of antioxidant activity.
The mycelium IPS and EPS from strain XG5, cultured under shaking conditions, exhibited strong DPPH radical scavenging activity, with IC₅₀ values of 0.01 and 0.20 mg mL
-1, respectively (
Table S2). Similarly, under static culture conditions, IPS and EPS from strain XG4 demonstrated notable DPPH radical clearance, with IC₅₀ values of 0.01 and 0.20 mg mL
-1, respectively (
Table S3). For hydroxyl radical (·OH) scavenging ability, EPS from strain XG5 under shaking culture and IPS from strain XG14 under static culture displayed lower IC₅₀ values, measuring 0.07 and 0.01 mg mL
-1, respectively. Additionally, IPS and EPS from strain XG19, cultured under shaking conditions, showed excellent superoxide anion (O₂⁻) radical scavenging activity, with IC₅₀ values of 0.01 and 0.19 mg mL
-1, respectively. When considering the total IC₅₀ values as the screening criterion, polysaccharides from strain XG19 exhibited strong in vitro antioxidant activities, with total IC₅₀ values of 3.11 and 3.38 mg mL
-1 under shaking and static culture conditions, respectively (
Table 2).
3.3. Transcriptional Expression Levels and Enzyme Activities of Key Enzymes in Polysaccharide Biosynthesis
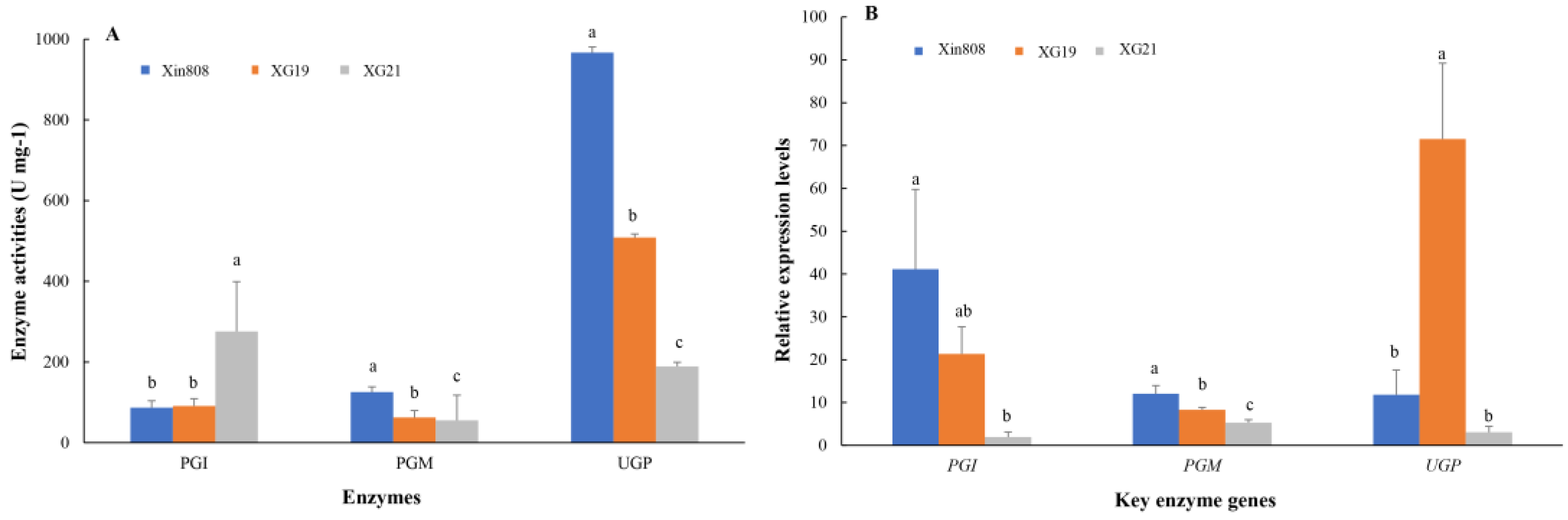
Polysaccharide biosynthesis is regulated by a complex network of enzymes, among which phosphoglucomutase (PGM), phosphoglucose isomerase (PGI), and UDPG-pyrophosphorylase (UGP) play pivotal roles [
24]. The activities of these three key enzymes in three L. edodes strains are illustrated in
Figure 1A. UGP exhibited the highest activity, while PGM showed the lowest. Strain Xin808 demonstrated the highest UGP activity (966.96 U mg
-1), which was 1.9 and 5.1 times greater than that of strains XG19 and XG21, respectively. In contrast, strain XG21 displayed the highest PGI activity (275.50 U mg-1), surpassing that of Xin808 and XG19 by 3.1 and 3.0 times, respectively.
The transcriptional expression levels of three key enzymes in the three strains are presented in
Figure 1B. The results revealed that the gene expression levels of
PGI and
PGM in strains XG19 and XG21 were lower than those in Xin808. Specifically, the expression levels of
PGI in Xin808 was 1.9 and 21.3 times higher than that in XG19 and XG21, respectively, while the expression level of
PGM in Xin808 was 1.4 and 2.3 times higher than that in XG19 and XG21. In contrast, the highest expression of
UGP was observed in strain XG19, which was 6.0 and 23.2 times greater than that in Xin808 and XG21, respectively.
Pearson correlation coefficient analysis, performed using SPSS 24.0, indicated no significant correlation between intracellular polysaccharide (IPS) content and the activities of the three key enzymes (
Table 3). However, extracellular polysaccharide (EPS) content showed a strong positive correlation with PGI activity (r = 0.981,
P < 0.01) and a significant negative correlation with PGM activity (r =-0.851,
P < 0.01). Additionally, the total polysaccharide content was positively correlated with PGI activity (r = 0.987,
P < 0.01) and negatively correlated with PGM activity (r = -0.782,
P < 0.05).
3.4. Monosaccharide Composition of Mycelial Polysaccharides
The structural features of polysaccharides play a critical role in determining and influencing their bioactivities. Monosaccharides, as the fundamental units and building blocks of polysaccharides, not only affect properties such as electrification, functional group composition, and bioactivity but also serve as one of the most straightforward and measurable indicators of polysaccharide structure [
16]. To further investigate these relationships, we analyzed the monosaccharide composition of two selected strains: XG21, which exhibited the highest polysaccharide production, and XG19, which demonstrated superior in vitro antioxidant activity. The widely cultivated strain Xin808 was also included as a reference for comparison.
The results revealed that the monosaccharide composition of polysaccharides varied among the strains, and differences were also observed between intracellular polysaccharides (IPS) and extracellular polysaccharides (EPS). Eight monosaccharides—fucose, galactose, glucose, xylose, mannose, ribose, galacturonic acid, and glucuronic acid—were identified in the IPS of the three L. edodes strains (
Table 4). In contrast, ribose was absent in all EPS samples, and xylose was not detected in the EPS of strain XG21, indicating that only six monosaccharides were present in the EPS of XG21. Mannose was the most abundant monosaccharide, with EPS containing a higher proportion of mannose (ranging from 51.41% to 51.91%) compared to IPS (ranging from 30.65% to 35.11%). These findings align with previous studies showing that mannose, glucose, and galactose are common monosaccharides in mushroom polysaccharides [
24].
Notably, IPS from strain XG19 contained a higher proportion of galacturonic acid (1.22%), which was 2.18 and 1.39 times greater than that of Xin808 and XG21, respectively. This observation is consistent with research indicating that an increase in uronic acid content enhances the antioxidant activity of polysaccharides, as demonstrated in Sagittaria sagittifolia L. [
25], and other studies showing a positive correlation between uronic acid content and antioxidant activity [
26].
These data clearly demonstrate that L. edodes polysaccharides are heterogeneous, with mannose and galactose dominating the IPS composition, while mannose and glucose are predominant in EPS. Arabinose (Ara) and galacturonic acid (GalA) appear to play critical roles in determining the IC₅₀ values for DPPH-scavenging activity [
27]. Interestingly, the IPS from the three strains with lower galacturonic acid content exhibited better DPPH scavenging activities, suggesting a complex relationship between monosaccharide composition and antioxidant properties.
3.5. Analysis of Molecular Weight of Mycelial Polysaccharides
Molecular weight is a critical structural feature that influences the bioactivity of polysaccharides. Studies have shown that low-molecular-weight (low-Mw) polysaccharides often exhibit superior immunomodulatory effects compared to their high-molecular-weight (high-Mw) counterparts [
28].
The molecular weight distributions of intracellular polysaccharides (IPS) and extracellular polysaccharides (EPS) from the three strains are summarized in
Table 5. The weight-average molecular weight (Mw) and number-average molecular weight (Mn) of IPS were consistently higher than those of EPS. Among the strains, XG19 exhibited the highest molecular weights for both IPS and EPS. Specifically, the Mw of IPS and EPS from strain XG19 was 702.924 kDa and 83.894 kDa, respectively, which were 2.60 and 1.05 times greater than those of Xin808 and 1.28 and 1.56 times higher than those of XG21.
The weight-average molecular weight (Mw) of polysaccharides from strain Xin808 was higher than that of the other two strains. The IPS and EPS of strain Xin808 measured 69.401 kDa and 83.894 kDa, respectively, which were 2.33 and 1.87 times greater than those of XG19 and 2.37 and 3.168 times higher than those of XG21. The ratio of Mw to Mn (Mw/Mn), known as the polydispersity index (PDI), reflects the uniformity of polysaccharide molecular weight distribution. Analysis of the PDI values revealed that IPS and EPS from Xin808 exhibited smaller PDIs (3.887 and 9.01, respectively), indicating a more uniform molecular weight distribution compared to the other strains.
Polysaccharides with molecular weights ranging from 4 to 100 kDa are generally associated with high DPPH radical scavenging activity [
27]. It is widely accepted that polysaccharides with lower molecular weights tend to exhibit stronger antioxidant activity [
29,
30]. In this study, the molecular weights of extracellular polysaccharides (EPS) were consistently lower than those of intracellular polysaccharides (IPS) across the three strains. This difference in molecular weight may partially explain the observed higher DPPH scavenging activity of EPS compared to IPS.
4. Conclusions
In this study, L. edodes strains XG21 and XG19 were identified as promising candidates due to their high polysaccharide productivity and strong in vitro antioxidant activities, respectively. Further analysis of polysaccharide components, molecular weight, and enzyme activities was conducted on XG19, XG21, and the widely cultivated strain Xin808. Strain XG19, which exhibited superior in vitro antioxidant activities, was characterized by higher uronic acid content and weight-average molecular weight (Mw). Specifically, the uronic acid content in its intracellular polysaccharides (IPS) (2.96%) was 2.22 and 1.14 times higher than that of Xin808 and XG21, respectively, while its Mw (702.924 kDa) was 2.60 and 1.28 times greater than that of Xin808 and XG21. Similarly, the uronic acid content in its extracellular polysaccharides (EPS) (8.26%) was 1.02 and 1.04 times higher than that of Xin808 and XG21, respectively, with an Mw (83.894 kDa) 1.05 and 1.56 times greater than that of Xin808 and XG21.
Correlation analysis revealed that the content of EPS and total polysaccharides (TPS) was positively correlated with phosphoglucose isomerase (PGI) activity and negatively correlated with phosphoglucomutase (PGM) activity. These findings suggest that strains XG19 and XG21 are potential candidates for further process optimization and scale-up studies aimed at enhancing polysaccharide production and bioactivities. However, additional research is needed to optimize fermentation conditions, extraction methods, and purification processes for these strains to maximize polysaccharide yield and bioactivity. Such efforts will help broaden the applications of L. edodes polysaccharides in various fields.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Mycelial biomass, intracellular and extracellular polysaccharide contents of 18 L.edodes strains under shaking and static culture conditions; Table S2: In vitro antioxidant activities of polysaccharide from 18 L.edodes strains under shake culture conditions; Table S3: In vitro antioxidant activities of polysaccharide from 18 L.edodes strains under static culture conditions.
Author Contributions
Conceptualization, Quanju Xiang; Data curation, Jihao Wen, Jie Zhang and Kanwal Rida; Funding acquisition, Yunfu Gu and Qiang Chen; Investigation, Quanju Xiang; Methodology, Quanju Xiang and Maoqiang He; Resources, Xiumei Yu and Quanju Xiang; Writing-original draft, Jie Zhang and Kanwal Rida; Writing-review & editing, Quanju Xiang.
Funding
This research was funded by Edible Fungus Innovation Team of Sichuan Province, grant number sccxtd-2025-07, Science and Technology Planning Project of Sichuan Province, grant number 2021YFYZ0026, and Central Guidance Local Science and Technology Development Special Project of Sichuan Province, grant number 2024ZYD0128.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IPS |
Intracellular polysaccharide |
| EPS |
Extracellular polysaccharide |
| TPS |
Total polysaccharide |
| PGI |
Phosphoglucose isomerase |
| PGM |
Phosphoglucomutase |
| UGP |
UDPG-pyrophosphorylase |
References
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A Review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food and Nutrition Sciences 2014; 5: 1095-1105. [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem Toxicol 2012; 50:191-197. [CrossRef]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol 2002; 60:258-274. [CrossRef]
- Bisen, P.S.; Baghel, R.K.;, Sanodiya, B.S.; Thakur, G. S.; Prasad, G.B.K.S. Lentinus edodes: A macrofungus with pharmacological activities. Curr Med Chem 2010; 17: 2419-2430. [CrossRef]
- Duan, Y.Q.; Xing, Z.C.; Xu, J.w. Screening of a high yield polysaccharide strain from ten edible and medicinal fungi and optimization of its culture conditions. Res J Biotechnol 2013; 8:11-15.
- Wei, Z.H.; Duan, Y.Y.; Qian, Y.Q.; Guo, X.F.; Li, Y.J.; Jin, S.H.; Zhou, Z.X.; Shan, S.Y.; Wang, C.R.; Chen, X.J. Screening of Ganoderma strains with high polysaccharides and ganoderic acid contents and optimization of the fermentation medium by statistical methods. Bioproc Biosyst Eng 2014; 37:1789-1797. [CrossRef]
- Adil, B.; Xiang, Q.J.; He, M.L.; Wu, Y.T.; Muhammad Ahsan,A.; Muhammad, A.; Qin, P.; Gu, Y.F.; Yu,X.M.; Zhao,K.; et al. Effect of sodium and calcium on polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Lentinus edodes. Amb Express 2020; 10:1-11. [CrossRef]
- Xiang, Q.J.; Zhang, H.J.; Chen, X.Q.; Hou, S.Y.; Gu, Y.F.; Yu,X.M.; Zhao,K.; Zhang, X.P.; Ma, M.G.; Chen, Q. Enhanced effects of iron on mycelial growth, metabolism and in vitro antioxidant activity of polysaccharides from Lentinula edodes. Bioengineering 2022; 9:581.
- Hu, G.; Zhai, M.; Niu, R.; Xu, X.; Liu, Q.; Jia, J. Optimization of culture condition for ganoderic acid production in Ganoderma lucidum liquid static culture and design of a suitable bioreactor. Molecules 2018; 23: 2563. [CrossRef]
- Hou, Z.; Cao, J. Comparative study of the P2X gene family in animals and plants. Purinergic Signal 2016; 12:269-281. [CrossRef]
- Luo,J.X.; Zhang, J.S.; Jia,W.; Feng, N.; Yang,Y.; Tang, Q.J.; Liu, Y.F.; Zhang, H. Influences of culture methods on the yield and antineoplastic activity of intracellular triterpene of Ganoderma lucidum mycelia. Acta Agriculturae Shanghai(In Chinese) 2014; 2:33-37.
- Jin, Y.; Lina Zhang, L.N.; Zhang M.; Chen, L.; Peter, C. K. C.; Oi, V.E.C.; Lin, Y.Y. Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media. Carbohydr Res 2003; 338: 1517-1521. [CrossRef]
- Chen, H.L.; Ju, Y.; Li, J.J.; Yu, M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int J Biol Macromol 2012; 50:214-218. [CrossRef]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem 2021; 358:129883. [CrossRef]
- Yin, C.M.; Li, C.; Ma, K.;Fan, X.Z.; Yao, F.; Shi,D.F.; Wu, W.J.; Qiu,J.H.;Hu,G.Y.; Gao,H. The physicochemical, antioxidant, hypoglycemic and prebiotic properties of γ-irradiated polysaccharides extracted from Lentinula edodes. Food Sci Biotechnol 2023:987-996.
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu,X.L.; Wang, L.; Wang, X.Q.; Li, Z.T.; Gao, M.J.; Yang, Y.H.; Wang,Q.; Li, N. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int J Biol Macromol 2024; 254: 127955. [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int J Biol Macromol 2009; 45:42-47. [CrossRef]
- B, Y.J.T.A.; A, W.Z.; C, J.J.Z. Performance analyses of a pH-shift and DOT-shift integrated fed-batch fermentation process for the production of ganoderic acid and ganoderma polysaccharides by medicinal mushroom Ganoderma lucidum. Bioresource Technol 2009; 100:1852-1859.
- Liu, X.Y.; Chen, Y.X.; Wu,L.X.; Wu, X.Q.; Huang,Y.F.; Liu,B. Optimization of polysaccharides extraction from Dictyophora indusiata and determination of its antioxidant activity. Int J Biol Macromol 2017;103: 175-181. [CrossRef]
- Salvador, L.D.; Suganuma, T.; Kitahara, K.; Tanoue, H.Y.; Ichiki, M. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. J. Agric. Food Chem. 2000; 48:3448-3454. [CrossRef]
- Zhu, M.; Huang, R.M.; Wen, P.; Song, Y.; He, B.L.; Tan, J.L.; Hao, H.L.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohyd Polym 2021; 254:117371. [CrossRef]
- Hu, T.; Huang, Q.L.; Wong, K.H.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int J Biol Macromol 2017;194:423-430. [CrossRef]
- Chen, P.; You, Q.; Li, X.; Chang, Q.; Zhang, Y.; Zheng, B.D.; Hu, X.K. Zeng, H.L. Polysaccharide fractions from Fortunella margarita affect proliferation of Bifidobacterium adolescentis ATCC 15703 and undergo structural changes following fermentation. Int J Biol Macromol 2019; 123:1070. [CrossRef]
- Li, S, Shah, N.P. Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments. Food Chem 2016; 197:240-249. [CrossRef]
- Feng, Y.; Juliet, I.C.; Wen, C.; Duan, Y.; Zhou, J.; He, Y.; Zhang, H.; Ma, H. Effects of multi-mode divergent ultrasound pretreatment on the physicochemical and functional properties of polysaccharides from Sagittaria sagittifolia L. Food Biosci 2021:42. [CrossRef]
- Wang, Y.; Li, X.; Zhao, P.; Qu, Z.; Bai, D.; Gao, X.; Zhao, C.; Chen, J.; Gao, W. Physicochemical characterizations of polysaccharides from Angelica Sinensis Radix under different drying methods for various applications - ScienceDirect. Int J Biol Macromol 2019; 121:381-389. [CrossRef]
- Li, Z.; Nie, K.; Wang, Z.; Luo, D. Quantitative structure activity relationship models for the antioxidant activity of polysaccharides. Plos One 2016; 11.1. [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohyd Polym 2012; 87:1206-1210. [CrossRef]
- Sun, T,; Zhou, D,; Xie, J,; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur Food Res Technol 2007; 225:451-456. [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki,J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohyd Res 2009; 344: 1690-1696. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).