Submitted:
02 March 2025
Posted:
03 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Characterization of Gold Nanoparticles and PVA/PEO/TEOS/AuNPs Hybrid Hydrogel Nanocomposite Membrane
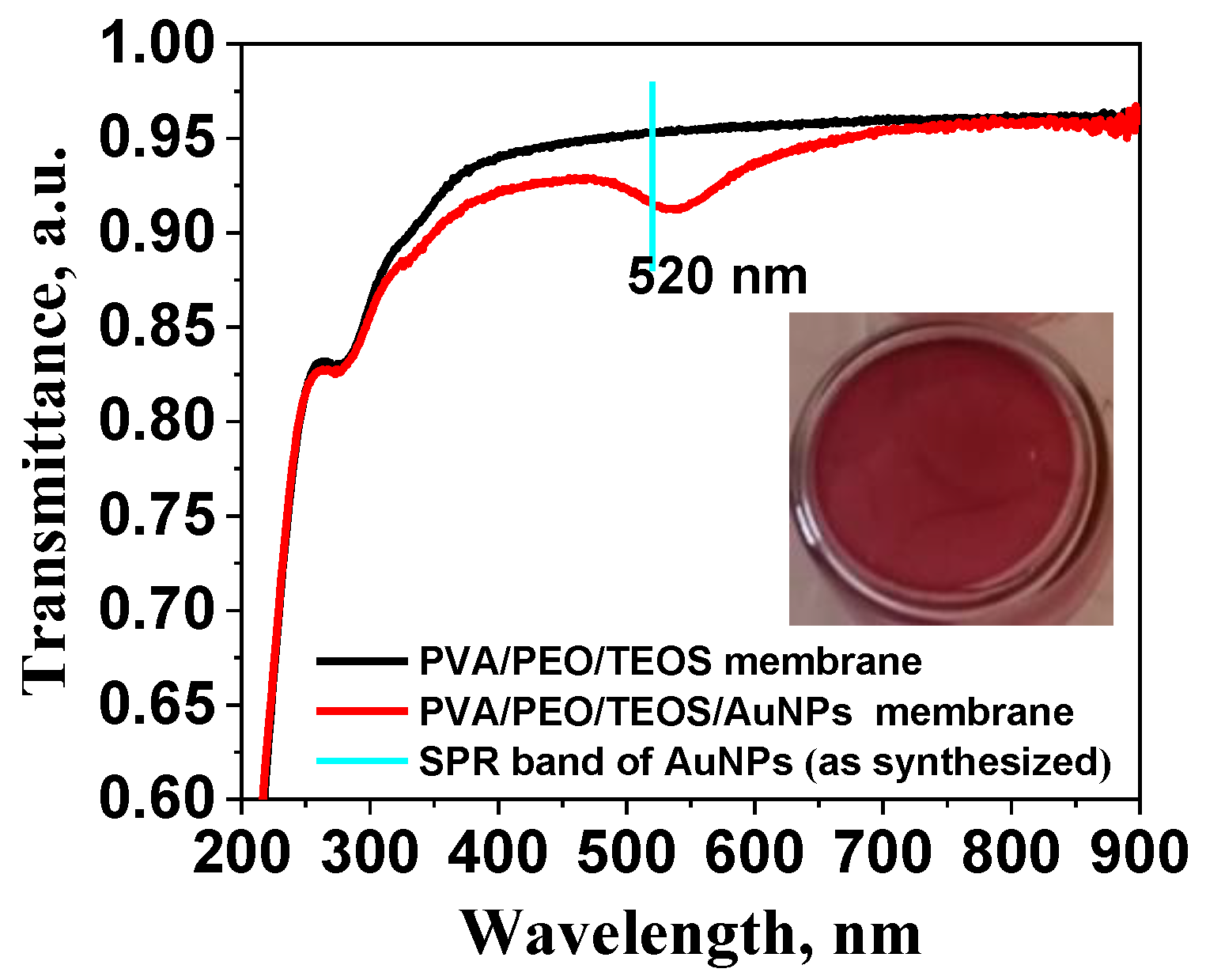
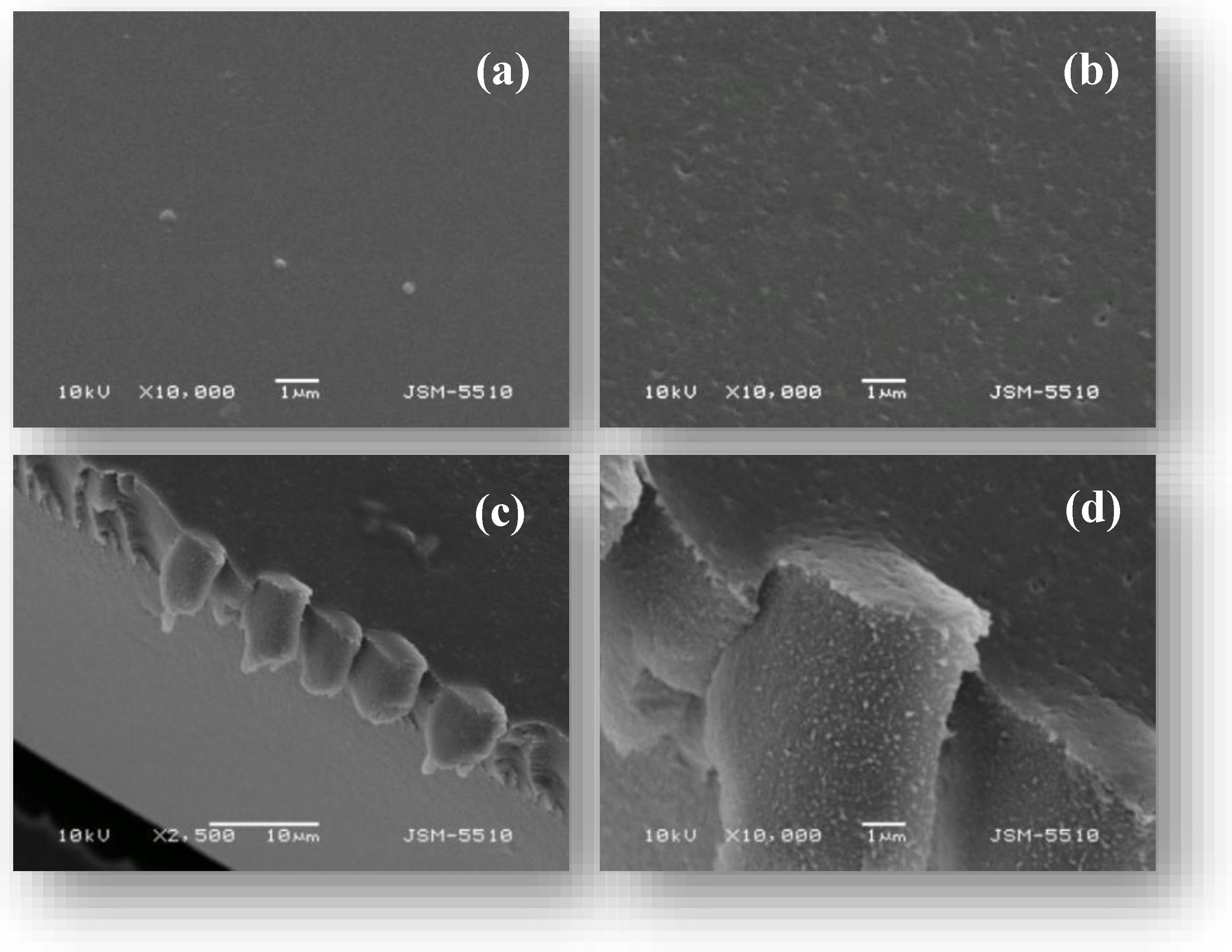
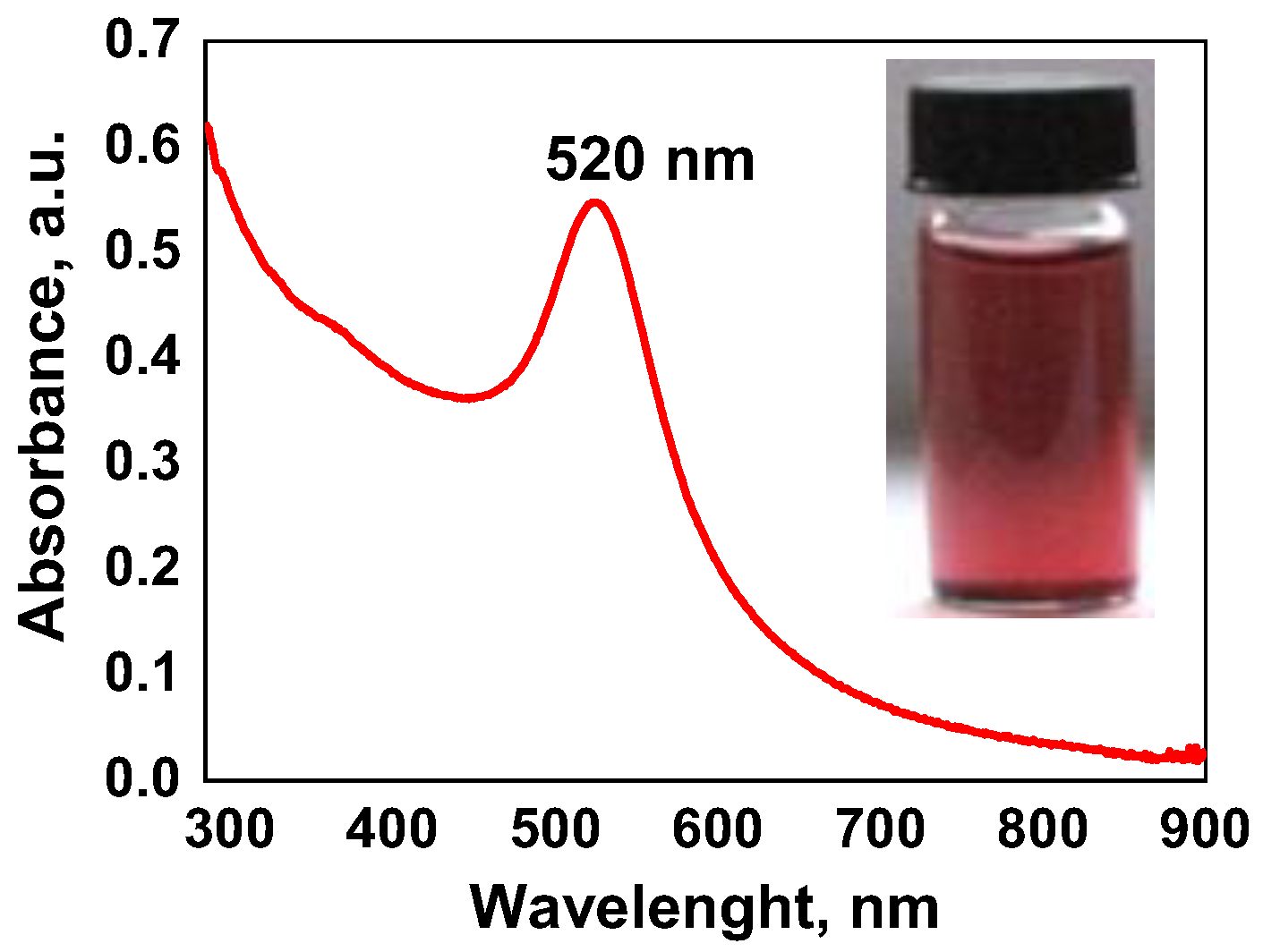
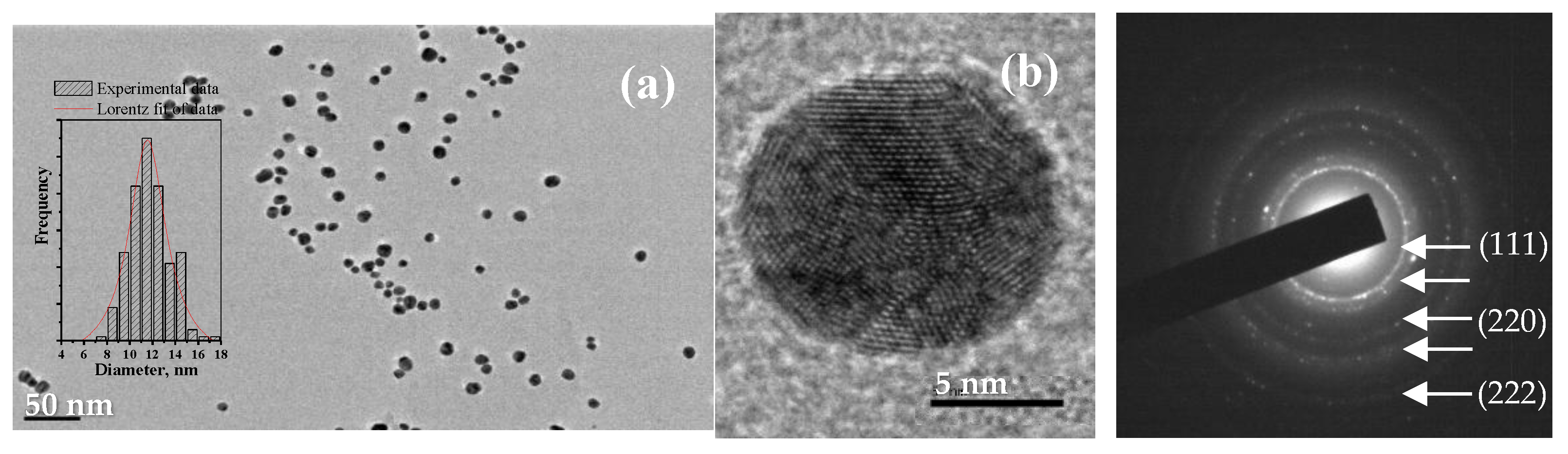
2.2. Adsorption Behavior of PVA/PEO/TEOS/AuNPs Hybrid Hydrogel Membrane toward Cr(III)/Cr(VI) and Mn(II)/Mn(VII)—Optimization Studies
2.3. Desorption Studies
2.4. Investigations on the Mechanism of Cr(III) and Mn(II) Adsorption onto PVA/PEO/TEOS/AuNPs Hydrogel Nanocomposite Membrane
2.4.1. Adsorption Isotherm Models
| Isotherm model | Parameters | Cr(III) | Mn(II) |
|---|---|---|---|
| Langmuir | Qm, mg/g | 0.2149 | 0.2256 |
| KL, L/mg | 71.66 | 154.8 | |
| RL (at C0 = 1 mg/L) | 0 < 0.0138 < 1 | 0 < 0.0064 < 1 | |
| R2 | 0.9998 | 0.9999 | |
| Freundlich | KF, mg/g | 0.1964 | 0.2126 |
| 1/n | 0.0908 << 1 | 0.0632 << 1 | |
| R2 | 0.7748 | 0.8127 | |
| DKR | Xm, mg/g | 0.2159 | 0.2161 |
| β, mol2/J2 | 1.156 × 10-8 | 2.192 × 10-11 | |
| E, J/mol | 6.578 | 151.0 | |
| R2 | 0.9900 | 0.8858 |
2.4.2. Modeling of Cr(III) Sorption Kinetics
| Kinetic sigmoidal model | Parameters | Cr(III) | Mn(II) |
|---|---|---|---|
| Slogistic1 | qe,calc, mg/g | 0.04446 | 0.04102 |
| τ, h | 8.895 | 8.301 | |
| k, dimensionless | 0.5115 | 0.6566 | |
| R2 | 0.9905 | 0.9811 | |
| Dose response | qe,calc, mg/g | 0.04313 | 0.04056 |
| qmin,calc, mg/g | 0.0025 | 0.00144 | |
| logEC50, h | 9.087 | 8.443 | |
| p, dimensionless | 0.2840 | 0.3310 | |
| R2 | 0.9945 | 0.9803 |
2.5. Analytical Applications
2.6. Analytical Figures of Merit
3. Conclusions
4. Materials and Methods
4.1. Materials, Reagents, and Instruments
4.2. Synthesis of Starch-Coated AuNPs
4.3. Preparation of PVA/PEO/TEOS/AuNPs Hydrogel Hybrid Nanocomposite Membrane
4.4. Static Adsorption/Desorption Experiments
4.5. Isotherm and Kinetic Studies
4.6. Analytical Procedure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metze, D.; Jakubowski, N.; Klockow, D. Species in the environment, food, medicine & occupational health. In Handbook of elemental speciation II: Species in the Environment, Food, Medicine and Occupational Health; Cornelis, R., Crews, H., Caruso, J., Heumann, K.G., Eds.; John Wiley&Sons, Ltd.: Chichester, United Kingdom, 2005; Chapter 2.6.1; pp. 120–135. [Google Scholar]
- Kot, A.; Namiesńik, J. The role of speciation in analytical chemistry. TrAC, Trends Anal. Chem. 2000, 19, 69–79. [Google Scholar] [CrossRef]
- Llaver, M.; Fiorentini, E.F.; Oviedo, M.N.; Quintas, P.Y.; Wuilloud, R.G. Elemental Speciation Analysis in Environmental Studies: Latest Trends and Ecological Impact. Int. J. Environ. Res. Public Health 2021, 19, 12135. [Google Scholar] [CrossRef]
- Rai, D.; Eary, L.E.; Zachara, J.M. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar] [CrossRef]
- Richard, F.C.; Bourg, A.C. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Chebeir, M.; Chen, G.; Liu, H. Emerging investigators series: frontier review: occurrence and speciation of chromium in drinking water distribution systems. Environ. Sci.:Water Res. Technol. 2016, 2, 906–914. [Google Scholar] [CrossRef]
- Katz, S.A.; Salem, H. The toxicology of chromium with respect to its chemical speciation—A review. J. Appl. Toxicol. 1993, 13, 217–224. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Jablońska-Czapla, M. Manganese and its speciation in environmental samples using hyphenated techniques: A review. J. Elem. 2015, 20, 1061–1075. [Google Scholar] [CrossRef]
- Witholt, R.; Gwiazda, R.H.; Smith, D.R. The neurobehavioral effects of sub-chronic manganese exposure in the presence and absence of pre-parkinsonism. Neurotoxicol. Teratol. 2000, 22, 851–861. [Google Scholar] [CrossRef]
- Yokel, R.A.; Crossgrove, J.S.; Bukaveckas, B.L. Manganese distribution across the blood-brain barrier. II. Manganese efflux from the brain does not appear to be carrier mediated. NeuroToxicology 2003, 24, 15–22. [Google Scholar] [CrossRef]
- Gómez, V.; Callao, M.P. Chromium determination and speciation since 2000. TrAC, Trends Anal. Chem. 2006, 25, 1006–1015. [Google Scholar] [CrossRef]
- Namieśnik, J.; Rabajczyk, A. Speciation Analysis of Chromium in Environmental Samples. Crit. Rev. Environ. Sci. Technol. 2011, 42, 327–377. [Google Scholar] [CrossRef]
- Rakhunde, R.; Deshpande, L.; Juneja, H.D. Chemical Speciation of Chromium in Water: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 776–810. [Google Scholar] [CrossRef]
- Dawra, N.; Dabas, N. Advances in spectrophotometric determination of Chromium(III) and Chromium(VI) in water: a review. J. Environ. Anal. Chem. 2022, 104, 2994–3015. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, B.; Komorowicz, I.; Sajnóg, A.; Belter, M.; Barałkiewicz, D. Chromium and its speciation in water samples by HPLC/ICP-MS – technique establishing metrological traceability: A review since 2000. Talanta 2015, 132, 814–828. [Google Scholar] [CrossRef]
- Rumsby, P.; Rockett, L.; Clegg, H.; Jonsson, J.; Benson, V.; Harman, M.; Doyle, T.; Rushton, L.; Wilkinson, D.; Warwick, P. Speciation of manganese in drinking water. Toxicol. Lett. 2014, 229 (Supplement), S120. [Google Scholar]
- Pearson, G.F.; Greenway, G.M. Recent developments in manganese speciation. Trends Analyt Chem. 2005, 24, 803–809. [Google Scholar] [CrossRef]
- Grygo-Szymanko, E.; Tobiasz, A.; Walas, S. Speciation analysis and fractionation of manganese—A review. Trends. Analyt. Chem. 2016, 80, 112–124. [Google Scholar] [CrossRef]
- Zhang, M.; Zhan, G.; Chen, Z. Iodometric Amplification Method for the Determinations of Microgram Amounts of Manganese(II), Manganese(VII), Chromium(III) and Chromium(VI) in Aqueous Solution. Anal. Sci. 2005, 14, 1077–1083. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Sadeghi, G.H. A nano-structured material for reliable speciation of chromium and manganese in drinking waters, surface waters and industrial wastewater effluents. Talanta 2012, 94, 201–208. [Google Scholar] [CrossRef]
- Kolekar, A.G.; Nille, O.S.; Gunjal, D.B.; Naik, V.M.; Ngoc, Q.N.; Sohn, D.; Kolekar, G.B.; Gokavi, G.S.; More, V.R. Prompt in situ synthesis of sulphur doped carbon dots from jaggery for parallel determination of iron, chromium and manganese in environmental samples. J. Photochem. Photobiol. A: Chem. 2024, 454, 115672. [Google Scholar] [CrossRef]
- Wakshe, S.B.; Dongare, P.R.; Gore, A.H.; Mote, G.V.; Salunkhe, S.Y.; Mahanwar, S.T.; Anbhule, P.V.; Kolekar, G.B. A highly sensitive and selective phthalazine derivative based fluorescent organic nanosheets for simultaneous detection of Cr6+ and Mn7+ in aqueous media. Inorganica Chim. Acta 2021, 526, 120534. [Google Scholar] [CrossRef]
- Hagarová, I.; Nemček, L. Application of Metallic Nanoparticles and Their Hybrids as Innovative Sorbents for Separation and Pre-concentration of Trace Elements by Dispersive Micro-Solid Phase Extraction: A Mini review. Front. Chem. 2021, 9, 672755. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Wang, X.Y.; Liu, X.; Yang, T.; Chen, M.L.; Wang, J.H. Ensuring high selectivity for preconcentration and detection of ultra-trace cadmium using a phage-functionalized metal–organic framework. Analyst 2020, 145, 5280–5288. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; et al. Nanomaterials-enabled water and wastewater treatment. NanoImpact 2016, 3, 22–39. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Pena-Crecente, R.M. Graphene and carbon nanotubes as solid phase extraction sorbents for the speciation of chromium: A review. Anal. Chim. Acta 2018, 1002, 1–7. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C. Layered double hydroxides: Emerging sorbent materials for analytical extractions. TrAC, Trends Anal. Chem. 2016, 75, 174–182. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, T.; Chen, M.L.; Wang, J.H. Recent advances in nanomaterials for analysis of trace heavy metals. Crit. Rev. Anal. Chem. 2021, 51, 353–372. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, L.; Zhao, B.; Chen, B.; Hu, B. Advanced functional materials in solid phase extraction for ICP-MS determination of trace elements and their species-A review. Anal. Chim. Acta 2017, 973, 1–24. [Google Scholar] [CrossRef]
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic nanoparticle based solid-phase extraction of heavy metal ions: A review on recent advances. Microchim. Acta 2018, 185, 160. [Google Scholar] [CrossRef] [PubMed]
- Samiey, B.; Cheng, C.H.; Wu, J. Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef]
- Rivas, B.L.; Urbano, B.F.; Sánchez, J. Water-soluble and insoluble polymers, nanoparticles, nanocomposites and hybrids with ability to remove hazardous inorganic pollutants in water. Front. Chem. 2018, 6, 320. [Google Scholar] [CrossRef]
- Shamsipur, M.; Fasihi, J.; Ashtari, K. Grafting of ion-imprinted polymers on the surface of silica gel particles through covalently surface-bound initiators: a selective sorbent for uranyl ion. Anal. Chem. 2007, 79, 7116–7123. [Google Scholar] [CrossRef]
- Buhani, B.; Narsito, N.; Nuryono, N.; Kunarti, E.S. Production of metal ion imprinted polymer from mercapto-silica through sol–gel process as selective adsorbent of cadmium. Desalination 2010, 251, 83–89. [Google Scholar] [CrossRef]
- Li, F.; Jiang, H.; Zhang, S. An ion-imprinted silica-supported organic–inorganic hybrid sorbent prepared by a surface imprinting technique combined with a polysaccharide incorporated sol–gel process for selective separation of cadmium(II) from aqueous solution. Talanta 2007, 71, 1487–1493. [Google Scholar] [CrossRef]
- Djerahov, L.; Vasileva, P.; Karadjova, I. Self-standing chitosan film loaded with silver nanoparticles as a tool for selective determination of Cr (VI) by ICP-MS. Microchem. J. 2016, 129, 23–28. [Google Scholar] [CrossRef]
- Vimala, K.; Murali Mohana, Y.; Samba Sivudu, K.; Varaprasad, K.; Ravindra, S.; Narayana Reddy, N.; Padma, Y.; Sreedhar, B.; Mohana Raju, K. Fabrication of porous chitosan films impregnated with silver nanoparticles: A facile approach for superior antibacterial application. Colloids Surf. B: Biointerfaces 2010, 76, 248–258. [Google Scholar] [CrossRef]
- Ščančar, J.; Milačič, R. A critical overview of Cr speciation analysis based on high performance liquid chromatography and spectrometric techniques. J. Anal. At. Spectrom. 2014, 29, 427–443. [Google Scholar] [CrossRef]
- Mladenova, E.K.; Dakova, I.G.; Karadjova, I.B. Chitosan membranes as sorbents for trace elements determination in surface waters. Environ Sci. Pollut. Res. 2011, 18, 1633–1643. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water-a review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Priastomo, Y.; Setiawan, H.R.; Kurniawan, Y.S.; Ohto, K. Simultaneous removal of lead (II), chromium (III), and copper (II) heavy metal ions through an adsorption process using C-phenylcalix [4] pyrogallolarene material. J. Environ. Chem. Eng. 2020, 8, 103971. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Fouad, Y.O.; Amin, N.K.; Mandor, H. Kinetic and thermodynamic aspects of cadmium adsorption onto raw and activated guava (Psidium guajava) leaves. Environ. Prog. Sustain. 2015, 34, 351–358. [Google Scholar] [CrossRef]
- Samadi, N.; Hasanzadeh, R.; Rasad, M. Adsorption isotherms, kinetic, and desorption studies on removal of toxic metal ions from aqueous solutions by polymeric adsorbent. J. Appl. Polym. Sci. 2015, 132, 41642. [Google Scholar] [CrossRef]
- Embaby, M.A.; Moniem, S.M.; Fathy, N.A.; El-Kady, A.A. Nanocarbon hybrid for simultaneous removal of arsenic, iron and manganese ions from aqueous solutions. Heliyon 2021, 7, e08218. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent—A review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Kaptso, K.G.; Njintang, Y.N.; Komnek, A.E.; Hounhouigan, J.; Scher, J.; Mbofung, C.M. Physical properties and rehydration kinetics of two varieties of cowpea (Vigna unguiculata) and bambara groundnuts (Voandzeia subterranea) seeds. J. Food Eng. 2008, 86, 91–99. [Google Scholar] [CrossRef]
- Cai, Q.; Turner, B.D.; Sheng, D.; Sloan, S. The kinetics of fluoride sorption by zeolite: Effects of cadmium, barium and manganese. J. Contam. Hydrol. 2015, 177, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Madaeni, S.S.; Vatanpour, V. Thermodynamic investigation and mathematical modeling of ion-imprinted membrane adsorption. J. Membr. Sci. 2012, 389, 334–342. [Google Scholar] [CrossRef]
- Martell, J.D.; Milner, P.J.; Siegelman, R.L.; Long, J.R. Kinetics of cooperative CO2 adsorption in diamine-appended variants of the metal–organic framework Mg2(dobpdc). Chem. Sci. 2020, 11, 6457–6471. [Google Scholar] [CrossRef]
- Quaranta, M.; Gehring, T.; Odell, B.; Brown, J.M.; Blackmond, D.G. Unusual inverse temperature dependence on reaction rate in the asymmetric autocatalytic alkylation of pyrimidyl aldehydes. J. Am. Chem. Soc. 2010, 132, 15104–15107. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.P.; Klussmann, M.; Iwamura, H.; Wells, D.H., Jr.; Armstrong, A.; Blackmond, D.G. A mechanistic rationalization of unusual kinetic behavior in proline-mediated C–O and C–N bond-forming reactions. Chem. Commun. 2006, 41, 4291–4293. [Google Scholar] [CrossRef]
- Calabrese, E. Hormesis and pharmacology. In Pharmacology: Principles and Practice, 1st ed.; Hacker, M., Messer, W.S., Bachmann, K.A., Eds.; Academic Press/Elsevier Inc.: Amsterdam, Netherlands, 2009; pp. 75–102. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-response 2009, 7, 358–383. [Google Scholar]
- Newberry, N.R.; Gilbert, M.J. Biphasic dose-response curve to muscarine on the rat superior cervical ganglion. Eur. J. Pharmacol. 1989, 163, 237–244. [Google Scholar] [CrossRef]
- Vasileva, P.; Donkova, B.; Karadjova, I.; Dushkin, C. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 382, 203–210.
- Yordanova, T.; Vasileva, P.; Karadjova, I. Noble metal nanocomposites as tools for fast and reliable speciation analysis of mercury in water samples. J. Environ. Anal. Chem. 2020, 105, 1152–1170. [Google Scholar] [CrossRef]
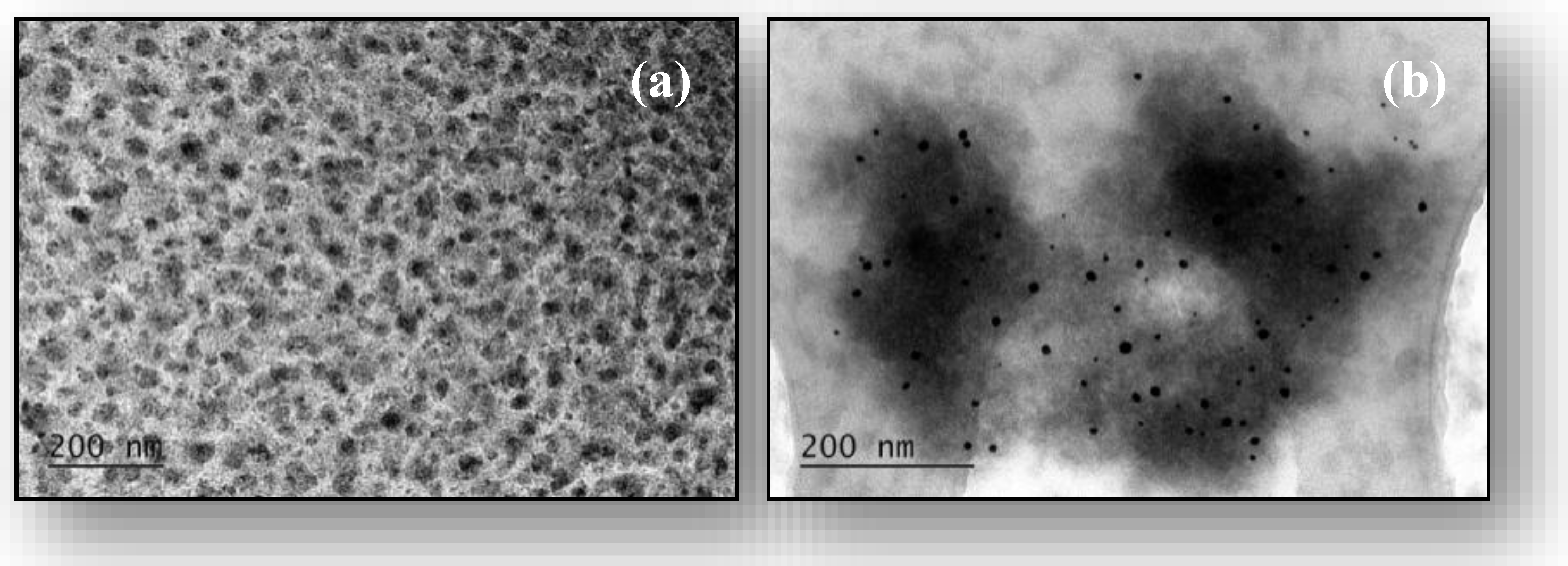
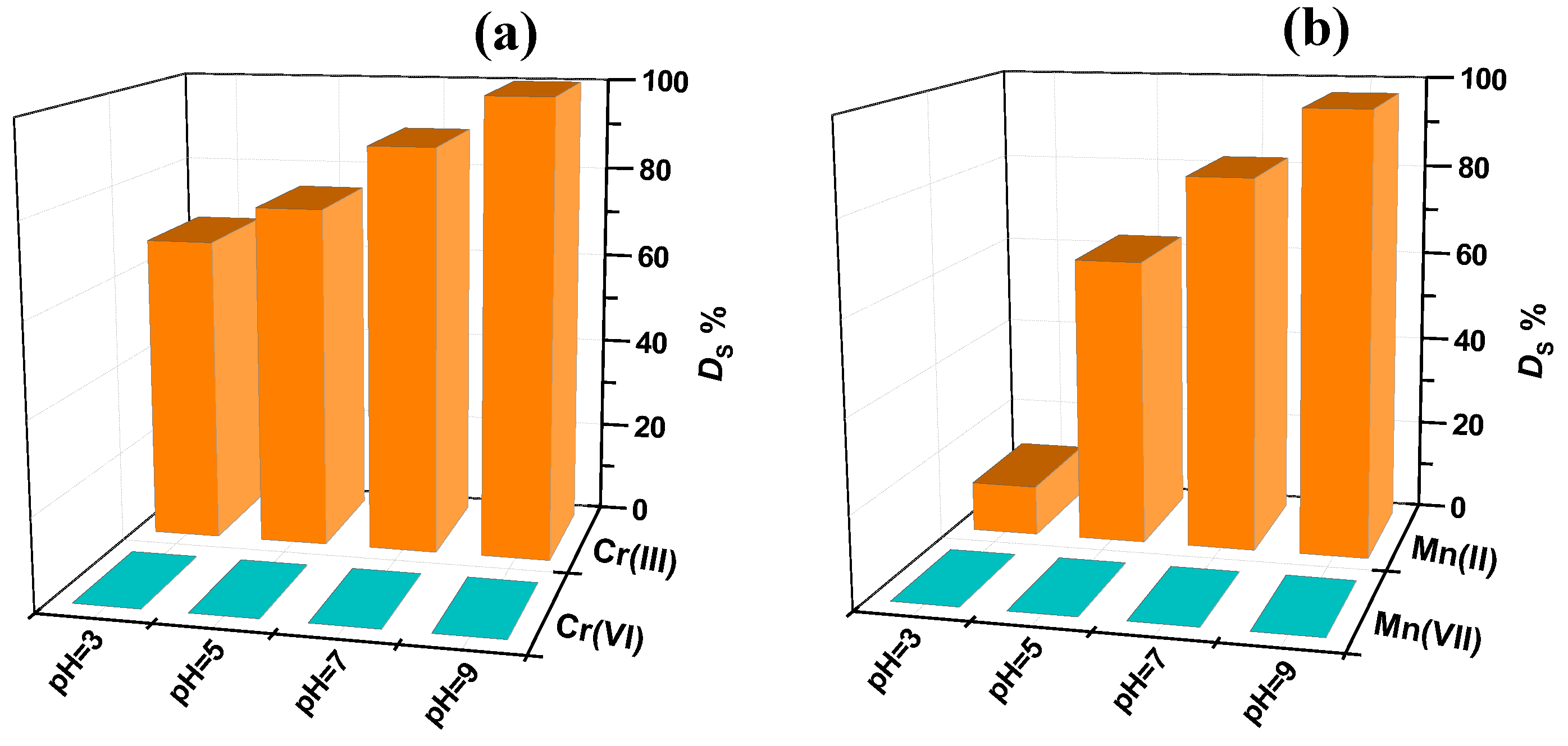
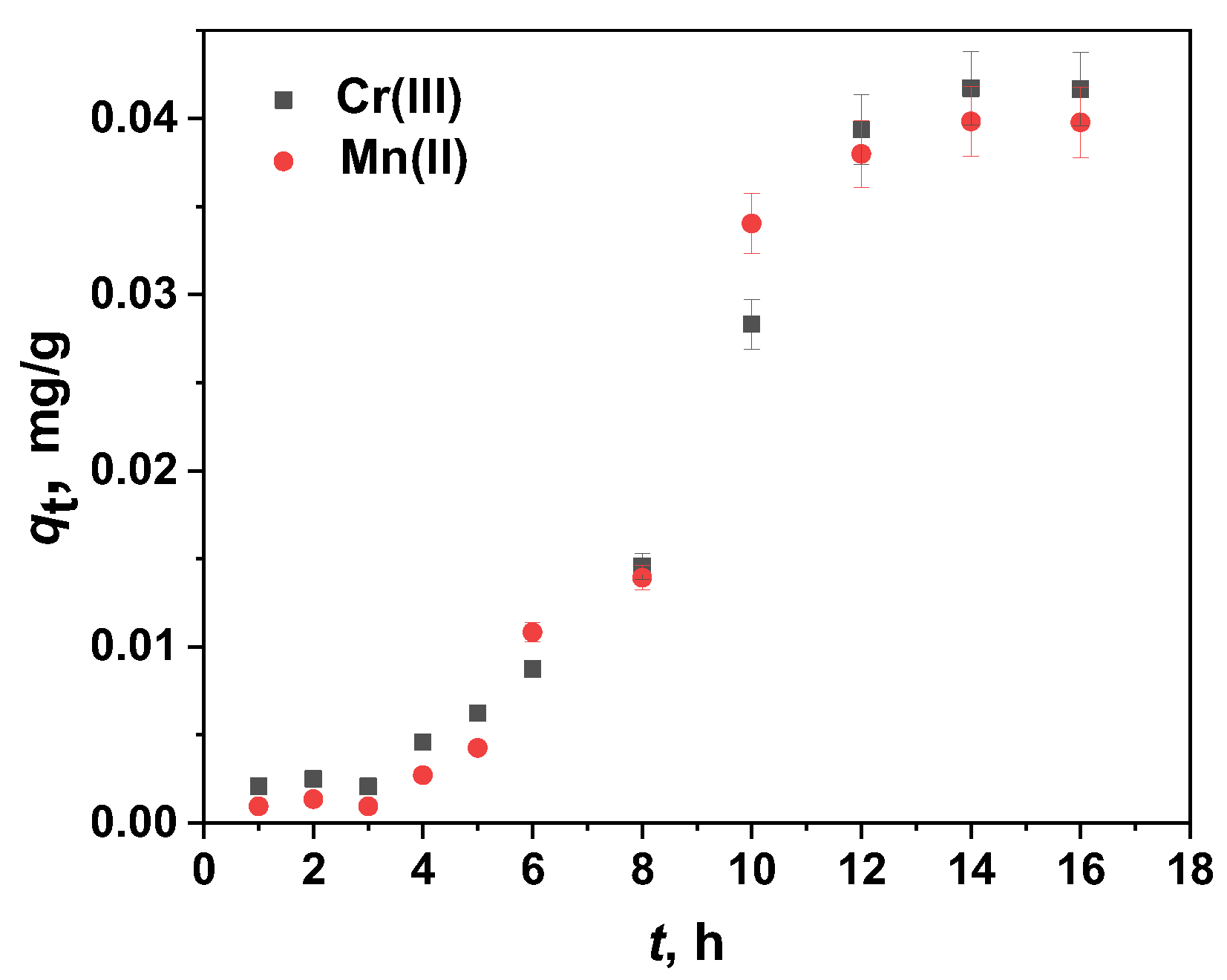
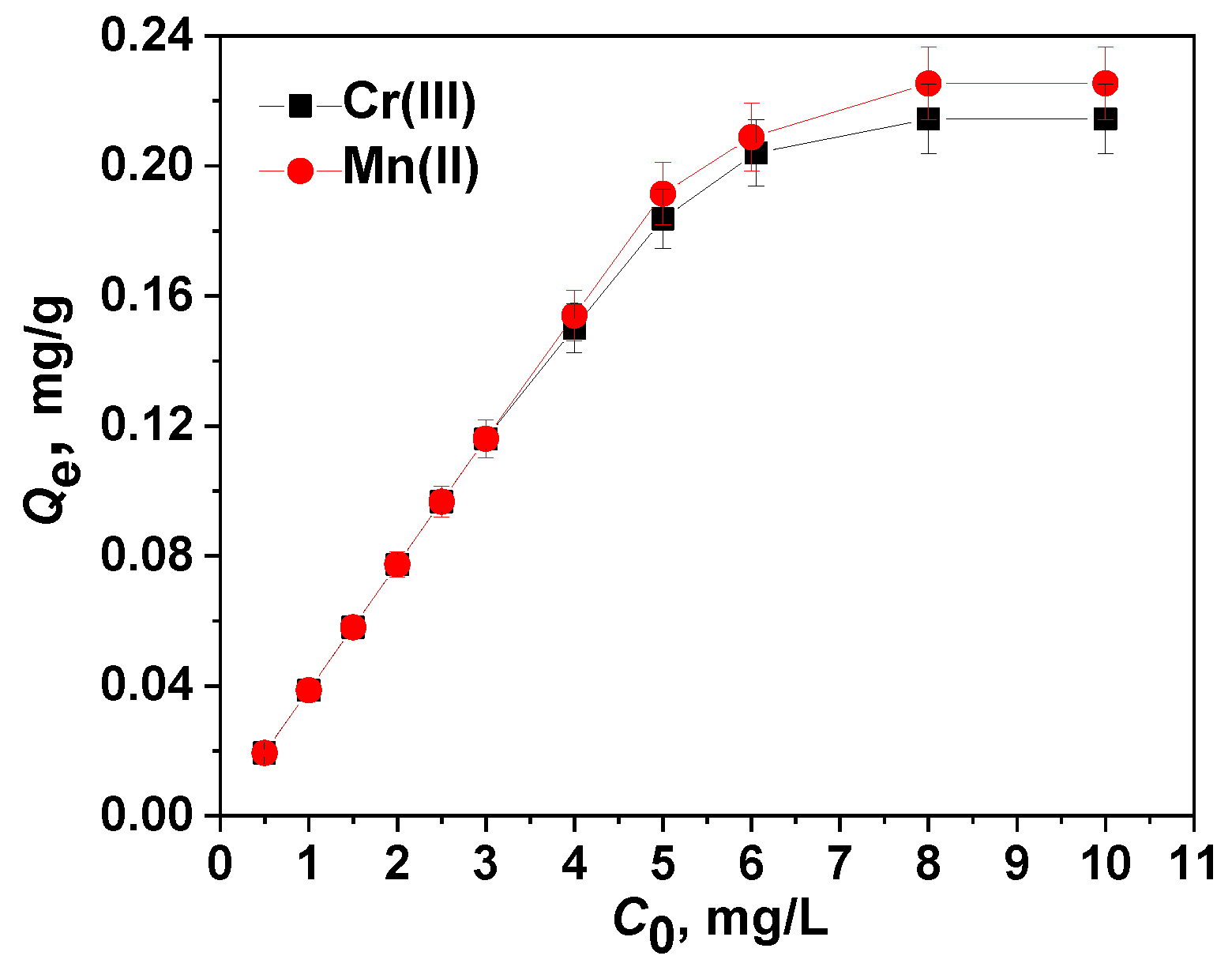
| Sample | Cr(III) | Cr(VI) | Mn(II) | Mn(VII) |
|---|---|---|---|---|
| River Iskar, µg/L | 0.15 ± 0.06 | <DL | 4.54 ± 0.5 | n.a. |
| Added, µg/L | 0.010 | |||
| Found, µg/L | 0.14 ± 0.08 | 0.009 ± 0.008 | 4.32 ± 0.06 | |
| Tap water, Bistritsa, µg/L | 0.092 ± 0.008 | <DL | 15.4 ± 0.5 | <DL |
| Added, µg/L | 0.05 | 2.00 | ||
| Found, µg/L | 0.093 ± 0.009 | 0.052 ± 0.007 | 14.9 ± 0.5 | 1.95 ± 0.45 |
| Wastewater | 1.23 ± 0.09 | 0.03 4± 0.004 | 256 ± 25 | 21 ± 2 |
| Added, µg/L | 0.10 | 10 | ||
| Found, µg/L | 1.34 ± 0.07 | 0.14 ± 0.006 | 249 ± 23 | 32 ± 3 |
| Cr (Cr(III)+Cr(VI)) | Mn (Mn(II)+Mn(VII)) | |
|---|---|---|
| Certified value, µg/L | 0.252 ± 0.012 | 2.12 ± 0.10 |
| Proposed procedure, µg/L | 0.245 ± 0.016 | 2.04 ± 0.09 |
| Recovery, % | 97.2 ± 0.3 | 96.2 ± 0.2 |
| Parameters | Cr(III) | Cr(VI) | Mn(II) | Mn(VII) |
|---|---|---|---|---|
| Detection limit, µg/L | 0.09 | 0.1 | 0.04 | 0.05 |
| Determination limit, µg/L | 0.26 | 0.3 | 0.12 | 0.15 |
| RSD, % in tap water for the species content (LOD-100 µg/L) | 3-7 | 4-7 | 3-7 | 4-7 |
| RSD, % in wastewaterfor the species content (LOD-500 µg/L) | 4-10 | 3-9 | 3-10 | 3-8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





