1. Introduction
Cancer remains one of the most difficult diseases to be cured, and it is a major cause of morbidity and mortality. Cancer is so complex because it is heterogeneous, as it comes in numerous forms, grows at variable rates, and responds differently to treatment. Cancer kills about 10 million people every year, as reported by the World Health Organization (WHO), and is the number one cause of death worldwide.[
1] Despite advancements in treatments, survival chances continue to highly depend on the stage of detection. Prompt detection and proper diagnosis strongly increase treatment efficiency, cut down healthcare costs, and maximize survival rates. The earlier one responds to checks, which are strongly reliant on non-objective judgment and therefore introduces an absence of proper identification, the more unfortunate it becomes. To avoid all these issues, imaging modalities have evolved significantly by integrating advanced computational approaches such as artificial intelligence (AI) and machine learning (ML) that improve accuracy, speed, and reproducibility in cancer detection, diagnosis, and staging. [
2]
This review paper addresses the critical role of cancer as a global health challenge, emphasizing the importance of early detection, accurate diagnosis, and precise prognosis for improving patient outcomes. Recent advancements in image processing and pattern recognition have significantly enhanced cancer detection and prediction, bridging the gap between traditional radiology and modern artificial intelligence (AI)-based techniques. The paper explores key areas such as image preprocessing algorithms, tumor segmentation methods, feature extraction, and AI techniques like machine learning and deep learning (including CNNs, RNNs, and transformers). It delves into cancer prediction through radiomics and radiogenomics, AI-assisted histopathological diagnosis, and the integration of multimodal data for personalized cancer prognosis.
In addition, the review highlights emerging technologies, including explainable AI, 3D/4D imaging, nanotechnology-enhanced imaging, and cloud-based AI solutions, which have the potential to transform precision oncology and enable remote diagnostics. Despite these advancements, challenges such as poor data annotation, limited interpretability, and ethical and regulatory concerns remain, which the paper seeks to address. The paper also discusses promising trends in federated learning, quantum computing, and real-time AI applications, suggesting that these innovations could revolutionize cancer imaging and management. Ultimately, it underscores the potential of interdisciplinary research to foster more precise, equitable, and accessible cancer care in the future.
Oncology imaging devices have evolved a lot from the past years when these devices were used in traditional radiologic tests to the present with artificial intelligence (AI) aided diagnostic devices. Traditional tests utilized for cancer diagnosis and follow-up are X-rays, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and ultrasound. The imaging tests yield valuable anatomical and structural details, which inform clinicians regarding tumour localization, staging, and therapy planning. They do demand, however, radiologists to manually interpret them, and this is liable to human fallibility and interobserver variability. [
3] Medical image interpretation has been made more accurate, automatic, and data-driven with the development of radiomics, artificial intelligence, and deep learning. Artificial intelligence-driven image analysis has transformed cancer diagnosis with the automation of tumour segmentation, classification, and prediction of cancer risk, as well as higher diagnosis accuracy and enhanced patient outcomes. Deep learning approaches, in particular convolutional neural networks (CNNs), have proven to be excellent at detecting cancerous lesions, differentiating between benign and malignant tumours, and even predicting patient prognosis from imaging features. [
4]
The synthesis of image processing techniques and pattern recognition techniques has achieved a monumental leap in cancer therapy. Image processing is the term used for a set of computer techniques utilized for the improvement of the quality of medical images, extracting meaningful information, and allowing automatic analysis. Noise reduction, image segmentation, contrast enhancement, and feature extraction are all methods that work together to improve diagnostic accuracy. [
5] Pattern recognition but entails identification of distinctive features and patterns from images to refine classification and decision-making. The technologies have in cancer also found application in automatic identification of cancer, disease progression prediction, as well as patient-specific treatment planning. As a case in point, radiomics or quantitative imaging analysis separates massive amounts of imaging biomarkers referencing back to the tumor biology such that accurate prediction and diagnosis become possible. Besides, AI-powered histopathologic image analysis considerably improved cancer classification and grading with an end to diagnostic inconsistency between pathologists. [
6]
Detection, prediction, diagnosis, and prognosis in cancer are not mutually distinct processes but consecutive phases of managing cancer that all improve with technological improvements in imaging and computing. Cancer detection refers to the identification of abnormal growth or early lesions, which is typically accomplished by means of screening activities such as mammography in the case of breast cancer, low-dose computed tomography in the case of lung cancer, and colonoscopy in the case of colorectal cancer. Early diagnosis improves the chances of successful treatment and long-term survival. [
7] Cancer prediction is a computation of a perso
n’s likelihood of developing cancer based on imaging biomarkers, genetics, and environmental factors. Predictive algorithms based on AI integrate radiological information with patient-specific factors to determine high-risk groups and encourage early treatment. Cancer diagnosis involves ascertaining the presence or absence of cancer and its type, stage, and molecular features. Advanced imaging modalities and artificial intelligence-based histopathologic analysis can detect benign vs. malignant tumors, categorize cancer subtypes, and select targeted therapy. Cancer prognosis tries to predict disease progression, treatment, and survival based on imaging features, clinical parameters, and genomic information. Radiomics models predict recurrence and metastasis by measuring tumor heterogeneity, vascularity, and metabolic activity. [
8]
2. Fundamentals of Image Processing in Oncology
Image processing is of paramount importance in modern oncology because it is capable of improving the quality of medical images, yielding valuable information, and facilitating computer-aided tumor diagnosis, classification, and prediction. Advanced computational algorithms used together with imaging modalities such as MRI, CT, PET, and ultrasound have greatly improved cancer diagnosis and treatment planning. Building blocks of image processing in cancer including preprocessing, segmentation, feature extraction, and registration are discussed here. [
9]
2.1. Image Preprocessing Methods: Noise Reduction, Artifact Elimination, and Normalization
Medical images are prone to noise, artifacts, and variability due to differences in imaging modalities, patient motion, or hardware constraints. Preprocessing is necessary to improve image quality and yield accurate analysis. Noise reduction methods such as Gaussian filtering, median filtering, and wavelet-based denoising remove random variations without degrading significant structures, thus improving image clarity. [
10] Artifact correction procedures remedy distortions due to motion, metal implants, or imaging defects of imaging instruments. Methods such as bias field correction and inpainting procedures assist in maintaining fidelity of the image. Normalization assists in normalizing the intensity of images from scan to scan so that they can be processed more easily. Two of the top methods of keeping levels of brightness and contrast in step between sets are histogram equalization and intensity standardization. [
11]
2.2. Segmentation of Anatomical Structures and Tumors
Segmentation is an essential stage in medical image processing. It aids in the correct identification of anatomical regions and tumour sites. Segmentation consists of dividing an image into significant portions distinguishing between normal tissue and the area of interest. The conventional segmentation techniques include thresholding, region growing, and clustering, thresholding is a simple yet effective method for segmenting structures on the basis of intensity variations. [
12] Tumor regions are isolated from background tissues with global and adaptive thresholding methods. Region growing techniques start from a seed point and grow towards neighboring pixels depending on similarity conditions, so that similar tumor areas are identified. Clustering methods such as k-means clustering and fuzzy c-means (FCM) group similar pixels together and hence are appropriate for tumor segmentation with varying intensity distributions. [
13]
More advanced segmentation methods have been devised to improve accuracy and flexibility to complex anatomy. Active contour models (snakes) continuously update boundaries by solving for the minimum of an energy function that depends on object boundaries. Graph cuts consider segmentation as an optimization problem, producing globally optimal tumor definition. [
14] Deep learning-inspired methods, like U-Net architectures, revolutionized medical image segmentation through encoder-decoder architecture-based capture of spatial correlations and small features to render them most effective for segmenting tumors from radiological and histological images. [
15]
2.3. Feature Extraction: Texture, Shape, Intensity, and Higher-Order Features
Feature extraction plays a crucial role in oncologic image processing because it transforms image data into numerical descriptors that may be used for categorization, diagnosis, and prediction of prognosis. Texture features from gray-level co-occurrence matrix (GLCM) and local binary patterns (LBP) capture spatial variations of pixel intensity to differentiate between cancer and benign tumors. [
16] Tumor size, roundness, tightness, and border irregularity are all significant parameters for assessing tumor aggressiveness and staging. Intensity-based features, including mean, median, and standard deviation of pixel intensities, reflect tissue density and metabolic activity, particularly in PET and MRI imaging. Apart from this, higher-order characteristics, like radiomics, incorporate form, texture and intensity descriptors into prediction models for cancer prognosis as well as the evaluation of therapy response. [
17]
2.4. Image Registration: Aligning Multimodal Images for Comprehensive Analysis
Image registration is an important technique in cancer that registers images of different modalities (e.g., CT, MRI, PET) or time points and facilitates extensive analysis and longitudinal studies. Registration techniques improve tumor localization, treatment planning, and disease follow-up by mapping corresponding anatomical structures. Rigid registration registers images based on simple transformation such as translation and rotation and maintains relative distances. Affine registration includes scaling and shearing into rigid transformations to support patient placement variations. Non-rigid registration accounts for advanced deformations such as tissue movement caused by breathing or tumor growth, aligning anatomical structures appropriately. [
18]
3. Pattern Recognition Techniques in Cancer Analysis
Pattern recognition techniques are vital in cancer analysis because they can allow for automatic tumor diagnosis, classification, and prognosis prediction from images. The techniques apply machine learning and deep learning algorithms to recognize patterns in radiological, histological, and molecular imaging information, enhancing diagnostic accuracy and treatment planning. The blending of classical machine learning methods, deep learning frameworks, and combined approaches has enormously improved cancer imaging and analysis. Classical machine learning methods have been widely applied to cancer classification and diagnosis due to their ability to handle structured data and engineered features. [
19] Support Vector Machines (SVM) are useful in discriminating between benign and malignant tumors by determining a best hyperplane that possesses the largest margin between classifications. Kernel-based SVMs improve the accuracy of classification by projecting nonlinear data onto higher-dimensional feature spaces. Another well-known method is k-Nearest Neighbors (kNN), which groups tumors according to their similarity to the nearby samples and is applicable for the discrimination of tissue types with radiomics data. Random Forest, an ensemble learning algorithm, increases robustness and interpretability by combining multiple decision tree
s’ predictions, thereby reducing the risk of overfitting. Such classical methods, although effective, are mainly dependent on manual feature extraction, making them less useful for complex and high-dimensional medical image data.[
20]
Deep learning revolutionized cancer imaging through the possibility of end-to-end feature learning and auto-pattern detection. Convolutional Neural Networks (CNNs) are highly suitable for cancer image classification as they learn hierarchical features from unprocessed images without explicit feature engineering. CNN architectures like AlexNet, VGG, ResNet, and EfficientNet have demonstrated excellent accuracy in tumor detection and grading based on MRI, CT, PET, and histopathology imaging. These networks use convolutional layers to identify edge patterns and spatial correlations, pooling layers to subsample, and fully connected layers for classification. Sophisticated CNN-based architectures, including Mask R-CNN and U-Net, are widely applied in precise tumor segmentation and lesion location. [
21]
In situations where temporal relationships in medical imaging data need to be modeled, Recurrent Neural Networks (RNNs) and Transformers have shown potential. RNNs, specifically Long Short-Term Memory (LSTM) and Gated Recurrent Unit (GRU) networks, are used in longitudinal cancer research to monitor tumor development over time. Such models keep track of past information and therefore are suitable for time-series imaging data such as dynamic contrast-enhanced MRI. Transformers, being good at modeling long-range dependencies, have recently been applied in cancer studies to evaluate sequential imaging data and fuse multimodal information, including genetic and radiological information, to facilitate complete disease modeling.[
22]
One of the biggest challenges to cancer imaging is the lack of labeled data sets since labeling of medical images requires considerable time and trained radiologists. Transfer learning overcomes this by employing pre-trained deep neural network models based on large datasets such as ImageNet and adjusting them for specific tasks related to cancer. Transfer learning, which applies learned representations from generic image recognition tasks to cancer imaging, significantly enhances model performance on small datasets and decreases training time and computational resources. Pre-trained networks like ResNet, Inception, and DenseNet have been successfully used in tumor classification, segmentation, and outcome prediction. [
23,
24]
Ensemble methods and hybrid models enhance cancer analysis through the integration of multiple algorithm
s’ strengths. Ensemble methods such as bagging, boosting, and stacking blend predictions from many models to enhance resilience and generalization. Blending CNNs with traditional machine learning classifiers, such as Random Forest or SVM, may enhance interpretability and performance of minuscule medical datasets. Hybrid frameworks that incorporate deep learning, radiomics, genomes, and clinical information provide a more holistic method of diagnosing and prognosticating cancer and enabling precision treatment methods.[
25]
4. Applications in Cancer Detection
The use of artificial intelligence (AI) and image processing methods in oncology has led to significant breakthroughs in cancer detection. Computerized cancer screening systems, radiomic biomarker-based early tumour detection, and AI-based second opinions have all enhanced diagnostic precision, minimized human error, and enabled early intervention. These instruments are especially useful in breast, lung, and colorectal cancer screening programs in which early detection clearly affects survival. [
26]
Machine learning-based cancer screening devices have transformed early cancer detection by analyzing vast amounts of medical image data with accuracy. Deep learning algorithms are applied in mammography to detect microcalcifications and masses that are related to breast cancer, thus improving sensitivity and specificity. AI-based mammographic screening techniques help radiologists detect subtle abnormalities that may go unnoticed under human inspection. Besides, artificial intelligence-based evaluation of low-dose computed tomography (LDCT) scans aids diagnosis and assessment of pulmonary nodules for potential cancer. These machines offer rapid and accurate assessment of lung CT scans, and treatment may be started in the initial phases in high-risk patients such as smokers.[
27] Last but not least, computer colonoscopy screening adds AI-activated polyp detection software to it, which offers greater detection of precancerous polyps and also eliminates the possibility of development of colorectal cancer. Such AI-based screening tools not only improve detection rates, but they also improve workflow efficiency in the healthcare setting. [
28]
Radiomic biomarkers enhanced early detection of tumors via radiating quantitative features of medical images for identifying patterns associated with malignancy. Radiomics identifies malignant and benign lesions by studying the texture, outline, intensity, and heterogeneity of the tumor. Artificial intelligence-powered radiomic models have been employed in tumor aggressiveness prediction and treatment response in a chain of malignancies such as brain cancer, prostate cancer, and liver cancer. By integrating radiomic markers with clinical and genomic information, AI models are able to present comprehensive risk estimation to provide precision oncology strategies based on specific patients.[
29]
One of the major issues with cancer diagnosis is that there may be false positives and false negatives, which lead to over-treatment or under-diagnosis. Second-opinion systems using AI overcome this problem because they act as decision aids for radiologists and hence improve diagnostic accuracy. The systems review imaging data and attach confidence scores to detected abnormalities, enabling physicians to pick out cases requiring closer investigation. AI-based false-positive reduction measures minimize unnecessary biopsies and patient anxiety, while false-negative reduction measures optimize the detection of occult cancers. AI models get better with experience through ongoing learning from large amounts of data, allowing more accurate cancer detection systems to be developed.[
30]
5. Applications in Cancer Prediction
Clinical and imaging information predictive models are part of the identification of individuals with a high probability of developing cancer. Predictive analytics driven by AI can identify susceptibility to specific malignancies by analyzing a collection of demographic, lifestyle, and genetic parameters, in addition to medical imaging. Machine learning techniques like SVMs and deep networks analyze history data to search for weak patterns, which can indicate pre-cancerous disease.[
31] Artificial intelligence (AI) risk prediction models for lung cancer, for instance, utilize low-dose CT (LDCT) scans to identify nodular abnormalities at early stages and rank patients based on their likelihood of getting cancer. In the same manner, in breast cancer, AI models evaluate mammographic density and tissue patterns to estimate the risk level of a person in order to ensure preventive interventions early in life through changes in lifestyle and targeted screening initiatives.[
32]
Radio genomics, which is a new and emerging science, integrates the imaging characteristics with the genetic alterations for improved prediction of cancer. AI programs obtain radiomic characteristics from the image of the patient, such as tumor texture, shape, and heterogeneity, and relate these to the intrinsic genetic alterations. This enables characterization of the tumor without invasion, providing information about the aggressiveness of the tumor and the probability of progression. For example, in glioblastoma, radio genomic signatures link MRI-based tumor characteristics to informative genetic signatures of IDH mutation and MGMT methylation status regarding prognosis and treatment response. Radio genomics allows physicians to make more prudent decisions on tailored treatment strategies without the need for injurious biopsies.[
33]
Artificial intelligence (AI)-aided imaging analysis for predicting therapeutic response has revolutionized precision oncology by allowing physicians to predict how patients will react to different drugs. AI algorithms apply pre-treatment imaging, molecular, and clinical characteristics to predict chemotherapy, radiation, and immunotherapy responses. As an example, treatment planning can be adjusted in the case of immunotherapy response of patients with non-small cell lung cancer (NSCLC), where patient responsiveness to immunotherapy can be pre-empted with CT- and PET-generated radiomic biomarkers. [
34] Similarly, in cancer of the breast, tumor responses to neoadjuvant chemotherapy can be foretold via input from AI models using DCE-MRI data, in turn allowing changing treatment plans even before extensive growth of the disease. Therapists can potentially enhance the effectiveness of treatment and avoid undue toxicity by adapting regimens based on AI predictions.[
35]
6. Applications in Cancer Diagnosis
Histopathological imaging differential diagnosis is important in distinguishing between malignant and benign lesions and other cancers. Histological examination requires traditional manual inspection by pathologists, which is subjective and time-consuming. Computational models based on AI, like CNNs, are capable of processing full-slide images (WSIs) of tissue biopsies for the detection of cellular abnormalities, tumor type classification, and detection of salient morphological patterns. These algorithms improve diagnosis accuracy through training on large collections of labeled histopathology slides, decreasing inter-observer variation and enhancing overall efficiency.[
36]
Artificial intelligence-driven cell classification and grading systems have transformed the diagnosis of cancer by automating the evaluation of tumor malignancy. Machine learning algorithms grade the severity of cancer based on cell shape, size, density, and nuclear atypia, such as Gleason score in prostate cancer or Nottingham grading in breast cancer. Deep learning can accurately differentiate between normal, precancerous, and malignant cells, allowing pathologists to take better-informed decisions. Artificial intelligence computer programs examine blood smear images to distinguish distinctive forms of leukemia, thereby enhancing early detection and treatment planning.[
37]
Advanced computer algorithms for the identification of rare subtypes of cancer are very useful in the identification of rare malignancies that are difficult for human specialists to identify on the basis of low exposure and limited cases. AI systems that have been trained with enormous databases can detect minor histological changes, allowing for early and uniform detection of rare diseases such as angiosarcoma, medullary carcinoma, and neuroendocrine tumors. These artificial intelligence technologies drive precision medicine through providing patients with unusual cancers timely and appropriate care.[
38]
Real-time diagnostic software in clinical settings through augmented reality (AR) is becoming a revolutionizing oncology technology. Diagnostic software based on AR overlays AI-generated information onto histopathological or radiological images in real time to aid doctors in the identification of tumor margins, cancer subtype diagnosis, and guiding biopsy procedures. Such approaches improve visualization, surgical planning, and teleconsultation among pathologists and oncologists. Augmented reality (AR) powered by artificial intelligence (AI) is being researched for intraoperative tumor margin monitoring to ensure complete resection while preserving healthy tissue.[
39]
Quantitative imaging biomarkers for cancer classification provide objective approaches for the diagnosis and typing of cancers. AI models utilize imaging data to generate biomarkers that forecast malignancy, tumor grade, and genetic subtype. Biomarkers are utilized in radiology (e.g., distinguishing between malignant and benign lung nodules on CT scans) and histopathology (forecasting HER2 status of breast cancer from biopsy slides). Utilization of quantitative imaging biomarkers minimizes subjective interpretation but maximizes the repeatability of cancer diagnosis.[
40]
7. Integration with Multimodal Data
The complexity of cancer requires an integrated approach that brings together many sources of scientific information to enhance diagnosis, prediction, and outcome. Integration of imaging, genetic, proteomic, and clinical information provides a better insight into cancer, leading to individualized treatment options and improved patient outcomes.
Integration of imaging, genomic, proteomic, and clinical information for global cancer analysis is becoming more and more important in precision oncology. Conventional diagnostic methods are primarily based on imaging modalities such as MRI, CT, PET, and histopathology slides. However, by combining imaging data with genetic and proteomic profiles, clinicians are able to better understand tumor biology, patterns of progression, and therapeutic responses. For example, radio genomics relates imaging characteristics to genetic changes that can forecast tumor aggressiveness and therapeutic targets. Similarly, proteomic data elucidate
s’ protein expression and signaling pathways, which aid in characterizing cancer. Merging clinical data, such as patient demographics, comorbidities, and treatment history, with predictive modeling strengthens prediction and allows for more specific therapeutic approaches.[
41]
High-end oncology research is dependent on multi-omics technologies to enhance cancer prediction and prognosis. Multi-omics is the combination of numerous layers of biological information including genomics (DNA copy number alterations and mutations), transcriptomics (mRNA expression), proteomics (protein abundance and post-translational modifications), and metabolomics (metabolic pathways). These combined datasets give a comprehensive understanding of tumor heterogeneity and evolutionary processes. Multi-omics analysis through the power of artificial intelligence makes it possible to discover new biomarkers for detecting early cancer, prediction of the patient survival rate, and best choice of therapeutic regimens. For instance, a blend of genetic mutation and radiomic signatures can make possible recurrence and tumor cell resistance predictions. Furthermore, single-cell multi-omics profiling makes it possible to identify heterogeneous cellular subpopulations within the tumor, enabling the development of targeted therapy.[
42]
AI platforms that merge and comprehend a range of data types are revolutionizing cancer treatment and research. Machine learning approaches are being utilized to analyze and interpret vast, multi-dimensional sets of data in order to uncover patterns hard to identify for human beings. Complicated AI designs like deep learning-based fusion models enable smooth fusion of imaging, molecular, and clinical data. These platforms facilitate real-time decision-making, enhanced accuracy of diagnosis and treatment, and better outcomes. Digital pathology platforms driven by artificial intelligence, for instance, interpret histopathological images and DNA sequencing data in an attempt to more precisely classify tumors and predict outcomes in patients. Likewise, AI-facilitated cloud computing consolidates patient data from numerous disparate sources so that oncologists can make data-driven decisions more effectively.[
43]
8. Role of Explainable AI in Cancer Imaging
Artificial intelligence (AI) has made great strides in cancer imaging, enhancing diagnosis, treatment planning, and prognosis. Although effective, AI-based models are
“black boxes
” that provide only predictions without an explanation of how they reached their conclusions. Such transparency undermines the credibility and reliability of AI in clinical decision-making. Explainable AI (XAI) aims to address these concerns by making AI-based cancer diagnoses more interpretable and understandable to clinicians and patients. [
44]
Transparency of AI-based cancer diagnosis is required for extended clinical application. In contrast to conventional imaging methods, where radiologists would be able to offer explanation in terms of rationale about their interpretation based on perceivable cues and clinical experience, deep learning models have access to large amounts of information with complex mathematical computation, making the process decision-making opaque. Transparency, or lack thereof, triggers regulatory approval, juridical accountability, and clinician trust issues. Transparency in AI systems can identify possible biases, determine clinical validity, and relate AI-based decisions to professional clinical experience. Apart from this, explainability is extremely critical in situations of high risk such as cancer diagnosis where inaccurate predictions can be deadly in their outcomes like false diagnosis or inaccurate recommendations for treatment.[
45]
Techniques to describe AI choices are required for developing transparency of AI-cancer imaging. Different approaches have been designed to enable viewing and understanding the decision-making process of the AI model. An example of Grad-CAM (Gradient-weighted Class Activation Mapping) is one that shows areas in an image which were most responsible for the AI mode
l’s classification so physicians could see what areas of a tumor or lesion were responsible for the classification. The last two are SHAP (Shapley Additive Explanations) game-theoretic to assign each input feature a relevance score to the degree to which each contributed to the overall prediction.[
46] LIME (Local Interpretable Model-agnostic Explanations) is another that generates approximating models that mimic decisions of intricate AI models so that local predictions are rendered interpretable. Attention maps, which are extensively utilized in deep learning models like transformers, also identify the most significant image or text regions used in a prediction, allowing radiologists to validate AI-generated conclusions. Transparency tools like these promote interpretability by showing the reason why an AI model flagged a tumor as cancerous or predicted disease spread, rather than explaining the resultant conclusion.[
47]
Building patient-clinician trust through explainability is a crucial component of the use of AI in oncology. Clinicians will be inclined to embrace and trust AI-assisted diagnoses when they can see and confirm the rationale behind AI predictions. Explainability also promotes improved doctor-patient communication since doctors are in a position to use visual explanations when communicating diagnostic results and treatment options. This not only creates patient trust in AI-recommended choices, but also facilitates collaborative decision-making for cancer treatment. Furthermore, regulatory bodies such as the FDA and EMA also emphasize the need for explainability at the time of approval for AI-based medical devices, which encourages transparent AI models.[
48]
9. The Impact of 3D and 4D Imaging in Oncology
The application of 3D and 4D imaging for cancer has maximally enhanced the visualization, follow-up, and planning for therapy of tumors. 3D imaging modalities such as CT and MRI have maximized tumor visualization at high resolution, enabling precise measurement of tumor size, shape, and location. This maximally affects cancer staging and diagnosis. In addition, 3D imaging plays a critical role in preoperative planning since it enables the virtual modeling of the tumor and the adjacent tissues by the surgeons, enhancing the precision of surgery and minimizing damage to normal anatomy. In addition, 3D imaging also offers individualized treatment planning, especially in radiation therapy, by the precise delivery of doses of radiation to the tumor with sparing of adjacent healthy tissue.[
49]
4D imaging, which incorporates the time factor, enhances treatment planning by enabling time-dependent assessment. The technology is particularly beneficial in monitoring tumors that migrate with the body, e.g., tumors in the lungs or abdomen while breathing. 4D imaging makes it possible to deliver radiation therapy more accurately by enabling real-time monitoring of tumor motion, reducing damage to normal tissue and increasing the accuracy of the treatment. It also allows adaptive radiotherapy, i.e., real-time monitoring of tumor geometry and location and dynamic adjustment of radiation doses to optimize therapeutic effect during treatment.[
50]
The intersection of 3D and 4D imaging modalities with artificial intelligence is revolutionizing tumor identification and therapy planning. AI algorithms enable precise identification and segmentation of tumors with decreased human error and expedited manual reading. Furthermore, AI-based predictive models can learn the behavior of tumors and forecast response to various treatment modalities to assist personalized therapeutic intervention. Artificial Intelligence-assisted real-time imaging has made it conceivable to follow up with tumor development round-the-clock and empower doctors to accordingly alter the course of treatment, giving their patients an improved destiny. AI added to 3D and 4D imaging heightens cancer therapy effectiveness, accuracy, and patient individuality to ultimately make them experience improvement.[
51]
10. Role of Mobile and Cloud-Based AI Solutions
Mobile and cloud-based artificial intelligence (AI) technology is revolutionizing the future of cancer diagnosis and treatment by becoming more efficient and accessible. Possibly the most exciting development is possibly being able to diagnose cancer remotely using smartphone-based imaging techniques. As smartphones become more powerful, imaging technologies combined with AI software can now analyze diagnostic images like X-rays, CT scans, and MRIs directly from smartphones. This makes it possible for patients from rural or underprivileged regions to be assessed timely and accurately without needing to travel long distances. Smartphone imaging with AI can potentially identify the first signs of cancer so that treatment can be started early and results are better.[
52]
Cloud-based deep learning methods enhance the scalability and accessibility of cancer treatment. The technologies enable one to store and process large volumes of medical imaging data on distant computers, with the information being made accessible to oncologists and healthcare providers globally at any location and time. This offers a platform of collaboration where medical experts from around the world can exchange information, cross-check cases, and give second opinions. The capability of the cloud to manage vast quantities of data by the use of AI-based analysis streamlines the diagnosis process, making the healthcare system more efficient as a whole. This also ensures that patients even in areas where the healthcare system is not up to par are able to access sophisticated diagnostic equipment and experts.[
53]
Telemedicine integrated with artificial intelligence -driven virtual consultations is transforming cancer treatment by providing patients with specialist
s’ access without needing to appear in person. AI can be employed to assist in triaging cases, performing early diagnosis, and analyzing patient information like medical history and symptoms for use during consultations. Telemedicine platforms are accessed by patients to interact with oncologists in real time, receive personalized treatment recommendations, and track their treatment. This combination not only enhances patient accessibility but also corrects the problem of geographical inequalities, making healthcare more equally balanced. With continuous development in AI technology, cancer treatment on a global scale can transform and optimize accessibility as well as outcomes.[
54]
11. Application of Nanotechnology in Cancer Imaging
Nanotechnology has proven to be of great worth in the imaging of cancer, with enhanced early diagnosis, precise diagnosis, and enhanced monitoring of treatment. For instance, the development of contrast agents formed from nanoparticles has greatly advanced imaging techniques such as MRI, CT, and PET scans. As a result of their nanometer scale and surface chemistry, these nanoparticles, which are often engineered to possess new optical or magnetic characteristics, can enter tumor tissue more quickly than conventional contrast agents. This results in improved resolution imaging, allowing better visualization of the tumors and more precise determination of their size, location, and growth rate. Nanoparticle contrast agents increase the sensitivity to imaging, and cancer can be identified earlier, in an earlier curable phase.[
55]
The second typical use of nanotechnology-based cancer imaging is targeted imaging for the purpose of delivering accurate diagnosis. The particles may be used to tag specific ligands, antibodies, or other targeted drugs specifically bound to cancer cell tumor markers or receptors. The tailored approach makes provision for accurate placement of the tumor, thus avoiding misdiagnosis or unnecessary surgery. Targeted nanoparticles improve the sensitivity of imaging to identify extremely small or occult tumors and trace cancer at the cellular or molecular level. This is a very useful method for tumor heterogeneity detection, tracking therapeutic efficacy, and metastasis dissemination detection, and therefore to achieve more efficient and personalized treatment protocols.[
56]
The union of nanotechnology and AI is widening the frontiers of cancer imaging. Synergistic methods using the high sensitivity of nanotechnology and the miraculous analytical capabilities of AI are initiating more accurate and autonomous diagnosis. AI algorithms have the ability to untangle advanced imaging information from nanoparticle-augmented scans, which reveal patterns and relations imperceptible to the naked eye. This coupling enables faster, more precise tumor detection, computerized segmentation of the tumor area, and estimation of tumor growth. Furthermore, artificial intelligence-driven data analysis can be used to enhance nanoparticle design to make contrast agents for specific cancers. Artificial intelligence and nanotechnology are transforming cancer imaging with more precise, faster, and more personalized diagnosis that can lead to better treatments and more efficient treatments.[
57]
12. Ethical Considerations and Societal Implications
Application of AI technology in cancer imaging is of serious societal concern and ethics that must be dealt with to guarantee equitable and ethical use. Bias and fairness in artificial intelligence systems is arguably the most important concern. AI systems are trained on enormous amounts of data, and if the training data sets are unrepresentative, then the AI algorithms will produce biased results. This can lead to inconsistencies in cancer diagnosis and treatment regimens, particularly for disadvantaged or minority groups, who would be underrepresented in clinical trials. Bias within AI imaging equipment will also enhance disparities in health, so there has to be a representative and heterogeneous training set for this to be avoided. Also, regular checking and tuning of AI systems with respect to the detection and dampening of such biases have to be undertaken.[
58]
The second major ethical issue is patient confidentiality and protection of data. Cancer imaging with AI typically entails gathering and processing personal patient data, including medical images, personal health data, and genomic data. This poses a challenge from the viewpoint of patient confidentiality and misuse of information. Cyber-attacks and unauthorized access to health data can potentially result in infringement of patient privacy, resulting in loss of trust in healthcare organizations. To maintain protection of data and abuse, deploying strong encryption practices, adhering strictly to standards such as GDPR (General Data Protection Regulation) and HIPAA (Health Insurance Portability and Accountability Act), and frequent auditing of AI systems are critical.[
59]
To this end, policy regulations regarding the safe use of AI in oncology are required. Most importantly, there should be specialized standards for ethical use of AI in healthcare to ensure transparency, accountability, and fairness. It ought to be made mandatory for regulatory settings to make the AI technologies go through rigorous testing for fairness and accuracy in order to qualify for a license to use clinically and ongoing checks to track their performance in the field. Furthermore, governments should make it necessary that there is collaboration between health practitioners, data scientists, and ethicists to create AI technologies that are patient-centered and equity in society. Patient confidentiality should be guaranteed through the imposition of laws mandating safe sharing of information, where patient authorization is a necessary requirement. Lastly, ongoing training and education of healthcare professionals on the ethical use of AI and correct application should be conducted so as to further enhance the publi
c’s trust and so as to provide equitable use of AI technologies to the entire population of individuals.[
60]
13. Role of Open-Source Tools and Frameworks in Cancer Imaging Research
13.1. Popular Open-Source Tools for Cancer Image Analysis
13.1.1. TensorFlow
TensorFlow is a Google-developed one of the leading open-source machine and deep learning frameworks. It possesses a complete ecosystem for model training, model building, and artificial intelligence model deployment suitable for cancer image research. TensorFlow supports end-to-end pipelines such as model development, deployment, and data preprocessing. TensorFlow is dynamic in nature, enabling the researcher to design unique neural networks for tasks such as tumor segmentation, classification, and detection. Furthermore, TensorFlo
w’s TensorBoard visualization tool makes it possible for researchers to see and debug their models, and this eventually results in better performance and explainability. The scalability of the framework has improved it in handling giant-sized medical image data sets.[
62]
13.1.2. PyTorch
PyTorch, a framework built by the Facebook AI Research team, is another open-source platform that is very popular for cancer imaging research. PyTorch is particularly renowned for its dynamic computation graph that is more flexible and easier to work with, especially for prototyping and testing. The fact that the users find it easy with the convenient interface for them to build sophisticated deep learning models is what the users like. PyTorch has widespread usage in applications ranging from 3D medical image processing to tumor segmentation and survival prediction. Its increasing ecosystem, with libraries like TorchIO for medical imaging, makes it more useful in oncology research. Its community support and compatibility with other software make it the best among researchers.[
63]
13.1.3. MONAI (Medical Open Network for AI)
MONAI is a PyTorch-based open-source software framework specialized in medical imaging and healthcare artificial intelligence research. It is PyTorch- enabled and delivers domain-specific modules and pre-processing of medical imaging applications like tumor segmentation, classification, and registration. It delivers features like 3D imaging ability, training in multi-GPUs, and federated learning, which are all crucial for cancer imaging studies. MONAI enables reproducibility and standardization in order to solve shared problems in medical AI research. Its open community and partnership with academic and industrial collaborators provide it as a worthwhile contribution for the development of cancer imaging science.[
65]
13.1.4. ITK (Insight Toolkit)
Insight Toolkit (ITK) is an open-source software library for medical image analysis and processing. ITK provides a wide variety of image registration, feature extraction, and segmentation algorithms important in cancer imaging research. IT
K’s stable and thoroughly tested code makes it the best software to process intricate medical images like MRI, CT, and PET scan. ITK is typically linked with other packages, including the 3D Slicer framework for visualization, to build solid pipelines for analysis of cancer images. Its availability as open source and with abundant documentation renders it extremely accessible for all researchers in the world.[
65]
13.1.5. Public Datasets and Repositories
Public datasets and databases facilitate cancer imaging research by providing access to standardized, quality data for training and testing models. The Cancer Imaging Archive (TCIA) is the most commonly used database, with a large collection of de-identified medical images, including CT, MRI, and PET scans, and clinical data. TCIA enables research in tumor detection, classification, and prediction of treatment response. Some of the most valuable datasets such as the BraTS (Brain Tumor Segmentation) dataset for segmenting brain tumors and the NCI Genomic Data Commons that integrates imaging and genomic information are enabled. These enable scientists to compare, assess, and collaborate on large-scale studies, all of which drive cancer imaging innovation.[
66]
13.1.6. Collaborative Platforms
Open-source communities and collaborative platforms enable information sharing and collaboration, which encourages cancer imaging innovation. Platforms such as GitHub and GitLab enable researchers to share code, tools, and workflows so others can reproduce and extend their work. Competition in the form of funding by cultures such as Grand Challenge motivates researchers to build and test AI models on shared datasets, pushing the limits of what can be achieved in cancer imaging. Collective efforts such as the NVIDIA Clara platform and the Open Health Imaging Foundation (OHIF) offer platforms for constructing and deploying medical imaging systems. These sites not only speed up research but also make innovations reach the fingertips of individuals across the world.[
67]
14. Human-AI Collaboration in Cancer Care
Interoperability between clinicians and AI has the potential to revolutionize cancer care by building synergy-based models, which use the strengths of one another. Possibly one of the most significant distinctions is between artificial intelligence and augmented intelligence. Augmented intelligence highlights AI as an asset for decision-making support for clinicians rather than replacing human intelligence. AI boosts diagnostic accuracy and accelerates complex data analysis so that doctors can focus on tailored care and important decision-making. It is a collaborator in the health process, supplementing rather than replacing the human factor required for patient care.[
68]
CDSS illustrate this collaboration. AI-powered CDSS is a critical part of clinical workflows that improves diagnostic accuracy, patient outcomes, and productivity. The systems analyze vast volumes of patient information, medical history, and imaging reports to allow clinicians to make accurate decisions and thereby avoid diagnostic errors and timely interventions. Based on real-time data, AI assists doctors in developing personalized and evidence-based treatment plans.[
68]
Human-AI collaboration will become in the future more a question of hybrid models where human intelligence and AI competence are complementary roles. As AI advances by leaps and bounds, activities that demand a great level of sophistication such as identifying patterns beyond human perception and creating new treatment modalities will be within its purview while physicians will be busy balancing these results against the unique patien
t’s particular requirements. Human counselors and computers will join hands to achieve the best of technical advancement and human understanding. [
69]
15. Economic and Accessibility Implications of AI in Cancer Imaging
AI integration in cancer imaging holds vast potential not only to improve diagnostic performance but also to reduce the economic cost of cancer care. Cost-effectiveness of AI products is a key driver of wider implementation. With computerized time-consuming processes such as image analysis and interpretation of data, AI technology will be in a position to lower the cost of labor significantly, reduce the likelihood of diagnostic errors, and make possible faster decision-making on treatment, with resultant healthcare cost savings on long-term expenditures. However, the cost of acquiring AI technology and the infrastructure is still an impediment to overcome too.[
70]
AI for cancer imaging is hindered from being implemented in low-resource communities by infrastructure, expense, and expertise. Advanced AI software demands ongoing access to internet services, cutting-edge computer hardware, and highly trained healthcare workers, all of which might not be available in underserved communities. These barriers will be addressed by deliberate investment in technological and human capital development to make AI technologies accessible and useful to all populations regardless of geography or socioeconomic status. [
71]
Telemedicine and artificial intelligence offer paradigm-shifting technology for remote diagnosis and monitoring of cancer. AI-based solutions can enable time reduction to facilitate augmentation of remote consultation, which places oncologists in a position to make accurate diagnoses and treatment plans on electronically obtained images. It is particularly beneficial in underdeveloped or remote regions where access is limited to specialists. The integration of telemedicine with AI technologies allows patients to receive quality care from distant locations while ensuring timely intervention and increased access to necessary healthcare services. [
72]
In the area of AI technology in imaging cancer, an appeal for global health equity exists as well. To make AI technology available to marginalized groups means transcending the institutionalized inequities that exist within healthcare systems. This entails providing low-cost, available, and fitting training to the health providers among these populations. Policy initiatives that facilitate easier distribution of AI-based cancer imaging technologies in an equitable manner, i.e., either foreign technology transfer partnership or subsidies for poor health systems, will need to bridge the gap between developing and developed nations. [
73]
Policy suggestions should be centered on initiatives for increasing equitable access to AI technologies. Governments and international organizations might play a critical role by investing in the infrastructure of AI, training healthcare providers, and facilitating equal access to AI-powered cancer care technology. This may include establishing structures that render AI technologies affordable and accessible in various healthcare environments. Public-private collaboration has the potential to accelerate AI in oncolog
y’s adoption and ensure that high-risk populations experience its benefits. [
74]
16. Emerging Imaging Modalities and Their Integration with AI
Several novel imaging techniques hold excellent promise for the diagnosis and treatment of cancer, and artificial intelligence is making them more useful. Photoacoustic imaging, hyperspectral imaging, and optical coherence tomography all have specific benefits for the imaging of malignant tissue with high resolution and sensitivity. Photoacoustic imaging provides the high spatial resolution of ultrasound and the molecular sensitivity of optical imaging and therefore is well positioned for early detection of tumors. Hyperspectral imaging records more of the spectrum of light than conventional imaging, generating richer information about tissue composition. Optical coherence tomography creates high-resolution cross-sectional images of tissue, ideally suited to find superficial cancer such as in skin or eyes.[
75]
AI is advantageous in multimodal imaging fusion when data from novel imaging modalities is integrated with conventional imaging procedures. AI is able to provide a more global perspective of a tumo
r’s attributes, such as size, form, chemical traits, and milieu, by fusing information from different sources. Data fusion is likely to contribute to better diagnosis and better treatment planning options.[
77]
AI is also helping with real-time imaging, specifically intraoperative imaging and AI-guided biopsies. Surgeons are able to use real-time imaging to visualize tumors in 3D while operating so that they can make more precise cuts and avoid leaving behind cancerous tissue. AI-guided devices also can assist in guiding biopsy needles to the target locations of tumors, improving tissue sampling precision and diagnostic precision.[
78]
But technical, financial, and regulatory constraints prohibit the mass use of these novel modalities. Technical issues are the integration of new imaging technology into existing medical infrastructure and training physicians in the use of the same. Financially, access may be restricted by the expense of development and implementation of the technologies, particularly in low-resource settings. Novel imaging modalities also raise regulatory challenges, such that such technology has to be tested for safety and efficacy before mass application.[
79]
17. Challenges and Limitations
The application of AI in cancer imaging is not without limitation and challenge. The biggest data challenge is the availability of enough labeled datasets. AI model training needs massive amounts of properly labeled data, which are frequently limited, particularly in rare cancers or underrepresented groups. Moreover, variations in imaging data as a result of patient population changes, hardware, and imaging modalities can lead to inconsistencies in model performance. Imaging noise or distortions complicate the analysis and decrease the precision of AI systems, making models incapable of delivering consistent and reliable results across various clinical scenarios.[
80]
Model problems are another major obstacle. AI models, particularly deep learning models, overfit, i.e., they work well on training data but fail to generalize to new, unseen data. This is especially undesirable in clinical settings, where there is high variability. Another problem is that most AI models are not interpretable, and therefore clinicians cannot see how the model arrived at a specific diagnosis or recommendation. Such transparency can undermine physician trust in AI systems and complicate therapeutic decision-making. In addition, transferability across applications is a problem, as models that have been trained on certain data sets will do less than optimally when they are applied to data from different hospitals, countries, or scanners, which reduces their broader acceptability and utility.[
81]
Regulatory and ethical issues also contribute significantly to the implementation of AI and cancer image development. Data protection and confidentiality are critical because medical data is highly sensitive, and its infringement can be of significant concern. Designing fairness in AI systems to prevent bias affecting certain demographic groups with the potential to lead to differences in cancer treatment is also critical. Lastly, clinical validation must be done so that AI models are demonstrated to be safe and effective in actual clinical practice but is time-consuming, expensive, and complex.[
82]
18. Future Directions and Innovations
Over the next few years, several new trends will propel the next generation of AI-based cancer imaging developments. Self-supervised learning is especially promising since it enables models to learn from unlabeled data, eliminating the issue of limited amounts of annotated data. This approach is likely to dramatically enhance the ability of AI systems to generalize and predict well even from smaller or less prepared data sets. Federated learning is another burgeoning trend that supports AI model
s’ training on multiple institutions without any data leaving the local setting, reducing privacy threats while allowing strengths of varied sources of data to be shared. In addition, greater focus on explainable AI is enhancing interpretability of models, giving doctors information to be able to interpret and trust AI decision-making.[
83]
The potential of quantum computing to speed cancer image analysis is also being studied. Quantum computing has the potential to significantly enhance processing power to analyze large sets of images as well as accelerate analysis and also enhance accuracy. This can bring about easier early tumor detection as well as support more personalized planning for treatment based on more complex data with enhanced accuracy.[
84]
Another significant advance on the horizon is the shift toward precision and personalized imaging. AI-driven imaging platforms capable of tailoring their analysis to the patient in question can result in improved diagnosis, prognosis, and treatment planning, ultimately to individualized cancer therapy for the patient in question. By combining genetic, environmental, and lifestyle data with imaging data, AI can help construct a total picture of each patien
t’s cancer so that treatments can be custom-made for each patient based on their own individual needs.[
85]
19. Case Studies and Practical Implementations
Artificial intelligence applications in cancer imaging in the real world prove its usefulness and feasibility. Various AI-powered imaging machines have been effectively applied in cancer institutes, enhancing diagnosis procedures and clinical decision-making. For instance, AI has been utilized to assist radiologists in interpreting mammograms to identify breast cancer at an early stage, and the results are as good or even better than those achieved by humans. These technologies improve the quality of diagnoses while simultaneously reducing the radiologist workload, allowing them to focus on more complicated cases.[
86]
More cases of improved patient outcome with complex imaging analysis are being recorded. AI algorithms were used to detect subtle patterns on medical images such as metastasis or recurrence risk for cancer patients. Through early warnings, AI enabled timely intervention that significantly improved survival and health for most such patients. Sometimes, AI also aids in improving radiotherapy delivery to minimize damage to normal tissues and side effects. [
87]
Findings of clinical trials in real life and research collaborations highlight the remarkable potential of AI in cancer treatment. Collaborations between physicians, universities, and AI companies have resulted in the launch of new products that incorporate AI into clinical workflow. Collaborations have enabled the establishment of AI models that can be tailored to specific real-world applications, demonstrating the efficacy and dependability of AI-based solutions in a variety of specialties in cancer.[
88]
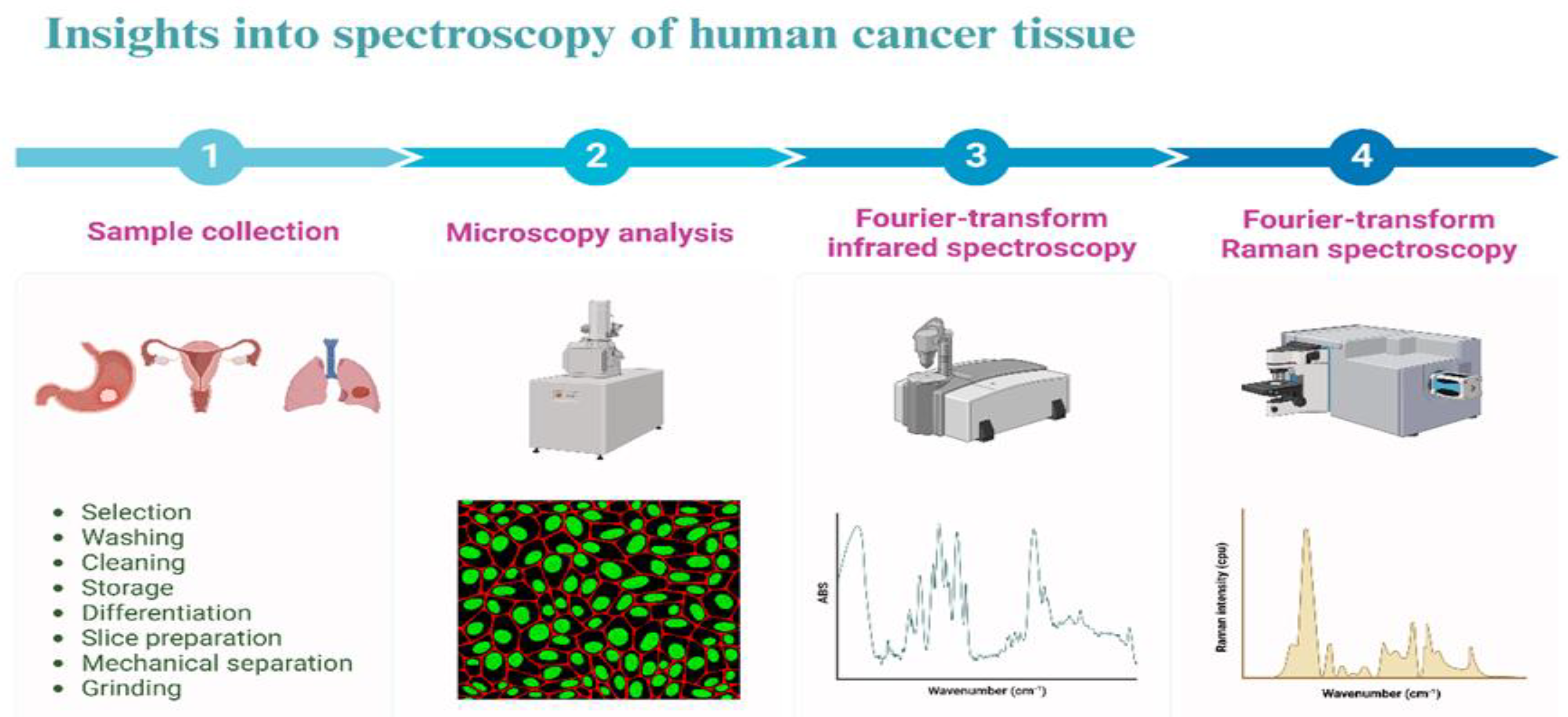
Figure 1.
Imaging process of cancer tissue.
Figure 1.
Imaging process of cancer tissue.
Table 1.
Methods and techniques in Image Processing for Cancer Detection.
Table 1.
Methods and techniques in Image Processing for Cancer Detection.
| Technique |
Description |
Applications in Cancer Detection |
Advantages |
| MRI (Magnetic Resonance Imaging) |
Uses strong magnetic fields and radio waves to generate detailed images of organs and tissues. |
Detection of brain, breast, and prostate cancers. |
Non-invasive, high-resolution images. |
| CT (Computed Tomography) |
X-ray technique that produces cross-sectional images of the body. |
Detection of lung, liver, and colorectal cancers. |
Quick, widely available, high sensitivity. |
| PET (Positron Emission Tomography) |
Combines imaging with radioactive tracers to detect cancerous tissues. |
Detection of metabolic activity in various cancers. |
High specificity for metabolic changes. |
| Ultrasound Imaging |
Uses sound waves to create images of internal body structures. |
Detection of breast, liver, and ovarian cancers. |
Safe, real-time, and cost-effective. |
| Optical Coherence Tomography (OCT) |
Non-invasive imaging method using light waves to capture tissue microstructures. |
Used in detecting early-stage skin, oral, and breast cancers. |
High-resolution, real-time imaging. |
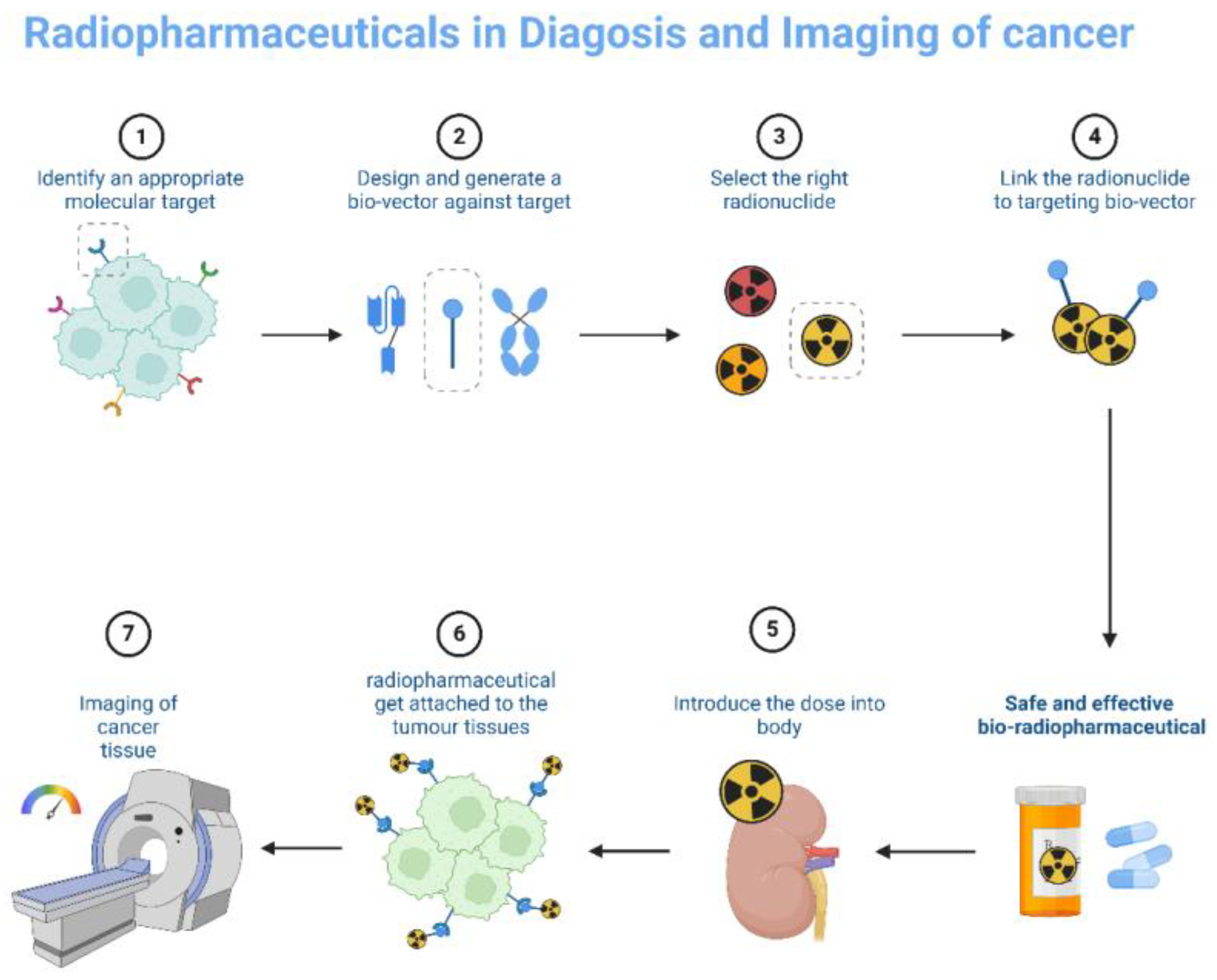
Figure 2.
Radiopharmaceuticals technique in cancer Imaging.
Figure 2.
Radiopharmaceuticals technique in cancer Imaging.
Table 2.
Pattern Recognition Algorithms in Cancer Diagnosis.
Table 2.
Pattern Recognition Algorithms in Cancer Diagnosis.
| Algorithm |
Description |
Applications in Cancer Diagnosis |
Advantages |
| Support Vector Machines (SVM) |
A supervised learning algorithm for classification and regression. |
Identifying cancer types from histopathological images. |
High accuracy, effective in high-dimensional spaces. |
| Artificial Neural Networks (ANNs) |
Computational models inspired by biological neural networks, capable of pattern recognition |
Classification of mammogram images for breast cancer. |
Can learn complex patterns, adaptive. |
| Convolutional Neural Networks (CNNs) |
A deep learning algorithm primarily used for image processing, especially in medical imaging. |
Tumor detection in medical images (e.g., CT, MRI). |
Highly effective in image classification and detection. |
| Random Forests |
Ensemble learning method for classification, using multiple decision trees. |
Classifying cancerous vs non-cancerous tissues. |
Robust, handles large datasets well. |
| K-Nearest Neighbors (KNN) |
A non-parametric classification algorithm that classifies based on the closest feature matches. |
Cancerous tissue identification in biopsies. |
Simple, interpretable, and effective in smaller datasets. |
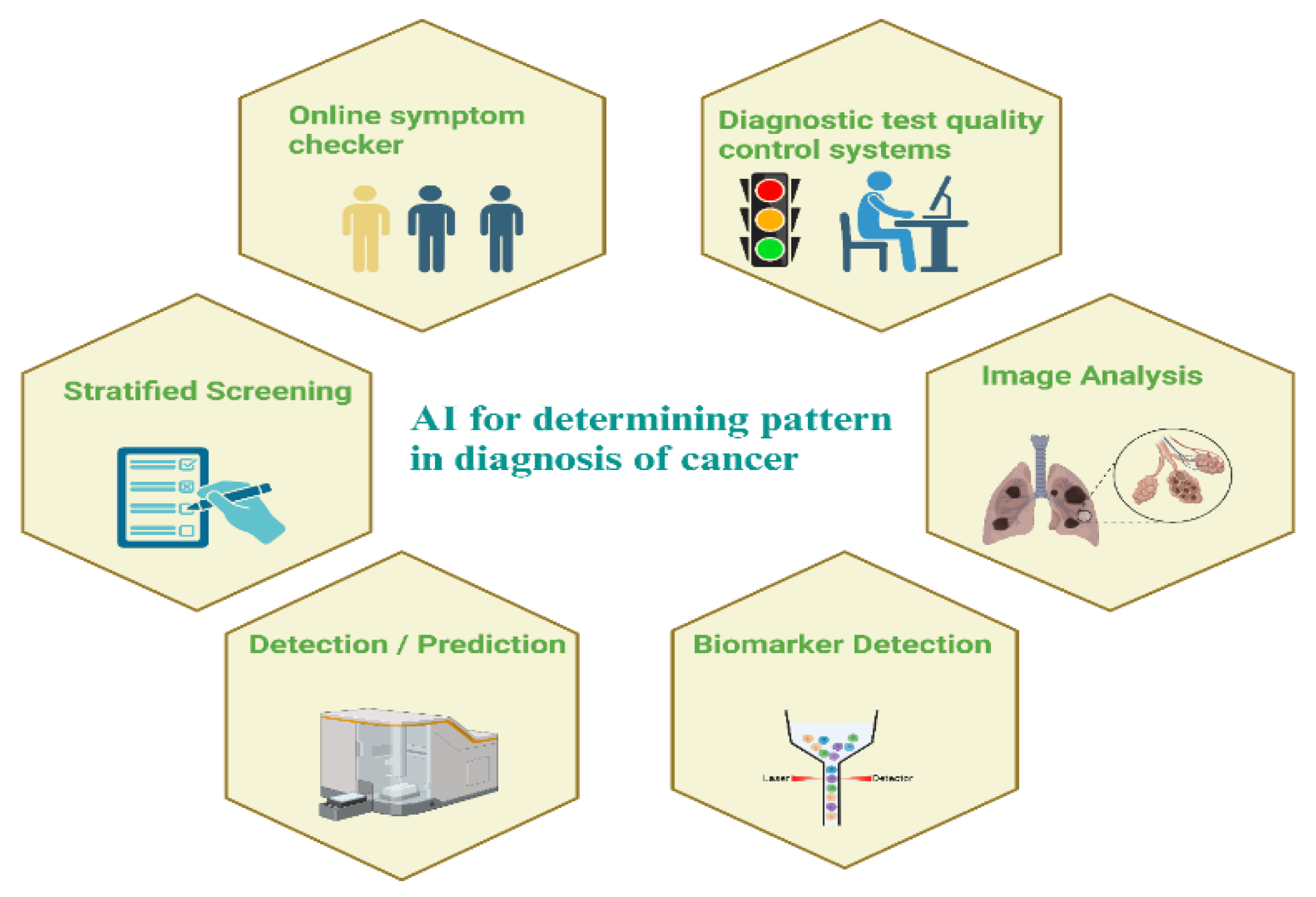
Figure 3.
Early Diagnosis of cancer using pattern recognition and AI.
Figure 3.
Early Diagnosis of cancer using pattern recognition and AI.
Table 3.
Advances in Image Processing and Pattern Recognition for Cancer Prognosis.
Table 3.
Advances in Image Processing and Pattern Recognition for Cancer Prognosis.
| Technique |
Application |
Advantages |
Challenges |
| Survival Analysis Models |
Predicting patient outcomes from imaging |
Integrates imaging data with clinical variables; improves prognosis accuracy |
Requires longitudinal data; complex to implement |
| Tumor Growth Modeling |
Simulating tumor progression over time |
Helps in understanding disease dynamics; aids in treatment planning |
Relies on assumptions; may not account for all biological variables |
| Biomarker Identification |
Linking imaging features to biomarkers |
Non-invasive; aids in monitoring treatment response |
Limited by imaging resolution; requires validation |
| AI-Driven Prognostic Tools |
Predicting recurrence and survival rates |
Provides personalized prognosis; improves patient management |
Ethical concerns; requires integration with clinical workflows |
| Multimodal Fusion |
Combining imaging with genomic data |
Enhances prognosis accuracy; provides holistic view of disease |
Data integration challenges; requires advanced computational methods |
20. Conclusions
Technological improvements in image analysis and pattern recognition have been a driving force behind the detection, prediction, diagnosis, and prognosis of cancer. Introduction of AI in oncology can potentially revolutionize cancer therapy by speeding it up, making it targeted, and personalized to the patient. Certain issues remain to be addressed, such as quality of data, model interpretability, and etiological explanations. The future of AI in cancer is bright, and new trends such as self-supervised learning, quantum computing, and real-time clinical use can potentially advance the application of AI in cancer treatment.
The future implications in oncology and care for patients are staggering, with the potential for more precise treatments, earlier detection, and improved survival rates. But the maximum potential of these technologies will be achieved only through continued inter-disciplinary collaboration by physicians, AI experts, ethicists, politicians, and healthcare practitioners alike-to ensure that AI is equitably and responsibly applied and serves all patients ultimately. By overcoming such challenges and facilitating cooperation, AI can revolutionize the world of cancer treatment, with more and improved treatments for patients all over the world.
Author Contributions
Conceptualization, A. K. S., P. K. P. and R. K. C.; methodology, M. A. and S. A.; software, A. M. A.; validation, A. M. A., M G. S., R. H. E., and A. K. S..; formal analysis, M. A.; investigation, M. A. and R. K. C; resources, A. M. A. .; data curation, M G. S.; writing—original draft preparation, M. A. and A. K. S.; writing—review and editing, P. K. P., A. K.S., and R. K. C.; visualization, M. G. S.; supervision, P. K. P and R. K. C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the participants for their patience and kindness.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. Lyon: International agency for research on cancer. 2020;20182020.
- Zhang, B.; Shi, H.; Wang, H. Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach. Vol. 16, Journal of Multidisciplinary Healthcare. Dove Medical Press Ltd.; 2023. p. 1779–91.
- Higgins, L.J.; Pomper, M.G. The evolution of imaging in cancer: Current state and future challenges. Semin Oncol. 2011, 38, 3–15. [Google Scholar] [PubMed]
- Bera, K.; Braman, N.; Gupta, A.; Velcheti, V.; Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Vol. 19, Nature Reviews Clinical Oncology. Nature Research; 2022. p. 132–46.
- Abut, S.; Okut, H.; Kallail, K.J. Paradigm shift from Artificial Neural Networks (ANNs) to deep Convolutional Neural Networks (DCNNs) in the field of medical image processing. Expert Syst Appl. 2024, 244, 122983. [Google Scholar] [CrossRef]
- Cheng, T.; Zhan, X. Pattern recognition for predictive, preventive, and personalized medicine in cancer. Vol. 8, EPMA Journal. BioMed Central Ltd.; 2017. p. 51–60.
- Frangioni, J.V. New technologies for human cancer imaging. Vol. 26, Journal of Clinical Oncology. 2008. p. 4012–21.
- Passaro A, Al Bakir M, Hamilton EG, Diehn M, André F, Roy-Chowdhuri S, et al. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Vol. 187, Cell. Elsevier B.V.; 2024. p. 1617–35.
- Mohammadzadeh, Z.; Safdari, R.; Ghazisaeidi, M.; Davoodi, S.; Azadmanjir, Z. Advances in optimal detection of cancer by image processing; experience with lung and breast cancers. Asian Pacific Journal of Cancer Prevention 2015, 16, 5613–8. [Google Scholar]
- Masoudi S, Harmon SA, Mehralivand S, Walker SM, Raviprakash H, Bagci U, et al. Quick guide on radiology image pre-processing for deep learning applications in prostate cancer research. Journal of Medical Imaging 2021, 8. [Google Scholar]
- Duffy BA, Zhao L, Sepehrband F, Min J, Wang DJ, Shi Y, et al. Retrospective motion artifact correction of structural MRI images using deep learning improves the quality of cortical surface reconstructions. Neuroimage 2021, 230. [Google Scholar]
- Ma, J.; He, Y.; Li, F.; Han, L.; You, C.; Wang, B. Segment anything in medical images. Nat Commun 2024, 15, 654. [Google Scholar]
- Alam MS, Rahman MM, Hossain MA, Islam MK, Ahmed KM, Ahmed KT, et al. Automatic human brain tumor detection in MRI image using template-based K means and improved fuzzy C means clustering algorithm. Big Data and Cognitive Computing 2019, 3, 27. [Google Scholar]
- Leymarie, F.; Levine, M.D. Tracking deformable objects in the plane using an active contour model. IEEE Trans Pattern Anal Mach Intell 1993, 15, 617–34. [Google Scholar]
- Yin XX, Sun L, Fu Y, Lu R, Zhang Y. [Retracted] U-Net-Based Medical Image Segmentation. J Healthc Eng 2022, 2022, 4189781. [Google Scholar]
- Kode, H.; Barkana, B.D. Deep Learning- and Expert Knowledge-Based Feature Extraction and Performance Evaluation in Breast Histopathology Images. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Arthur LE, McMann AH, Slattery LN, Fuhrman GM, Mackey AM, Rivere AE, et al. Factors That Predict Biological Aggressiveness in Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative/Lymph Node–Negative Breast Cancer. Ochsner Journal 2020, 20, 381–7. [Google Scholar]
- Rong Y, Rosu-Bubulac M, Benedict SH, Cui Y, Ruo R, Connell T, et al. Rigid and Deformable Image Registration for Radiation Therapy: A Self-Study Evaluation Guide for NRG Oncology Clinical Trial Participation. Pract Radiat Oncol 2021, 11, 282–98. [Google Scholar]
- Shekarian T, Valsesia-Wittmann S, Brody J, Michallet MC, Depil S, Caux C, et al. Pattern recognition receptors: immune targets to enhance cancer immunotherapy. Annals of Oncology 2017, 28, 1756–66. [Google Scholar]
- Huang, S.; Nianguang, C.A.I.; Penzuti Pacheco, P.; Narandes, S.; Wang, Y.; Wayne, X.U. Applications of support vector machine (SVM) learning in cancer genomics. Vol. 15, Cancer Genomics and Proteomics. International Institute of Anticancer Research; 2018. p. 41–51.
- Jiang, X.; Hu, Z.; Wang, S.; Zhang, Y. Deep Learning for Medical Image-Based Cancer Diagnosis. Vol. 15, Cancers. Multidisciplinary Digital Publishing Institute (MDPI); 2023.
- Zanjani FG, Panteli A, Zinger S, van der Sommen F, Tan T, Balluff B, et al. Cancer detection in mass spectrometry imaging data by recurrent neural networks. In: 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019). IEEE; 2019. p. 674–8.
- Ayana, G.; Dese, K.; Choe, S.W. Transfer learning in breast cancer diagnoses via ultrasound imaging. Vol. 13, Cancers. MDPI AG; 2021. p. 1–16.
- Chen, W.; Tan, X.; Zhang, J.; Du, G.; Fu, Q.; Jiang, H. A robust approach for multi-type classification of brain tumor using deep feature fusion. Front Neurosci. 2024, 18. [Google Scholar]
- Mohammed, M.; Mwambi, H.; Mboya, I.B.; Elbashir, M.K.; Omolo, B. A stacking ensemble deep learning approach to cancer type classification based on TCGA data. Sci Rep 2021, 11. [Google Scholar]
- Kolla, L.; Parikh, R.B. Uses and limitations of artificial intelligence for oncology. Vol. 130, Cancer. John Wiley and Sons Inc; 2024. p. 2101–7.
- da Silva HEC, Santos GNM, Leite AF, Mesquita CRM, de Souza Figueiredo PT, Stefani CM, et al. The use of artificial intelligence tools in cancer detection compared to the traditional diagnostic imaging methods: An overview of the systematic reviews. PLoS One 2023, 18. [Google Scholar]
- Yu, C.; Helwig, E.J. The role of AI technology in prediction, diagnosis and treatment of colorectal cancer. Artif Intell Rev 2022, 55, 323–43. [Google Scholar]
- Gillies, R.J.; Schabath, M.B. Radiomics improves cancer screening and early detection. Vol. 29, Cancer Epidemiology Biomarkers and Prevention. American Association for Cancer Research Inc.; 2020. p. 2556–67.
- Zeng, A.; Houssami, N.; Noguchi, N.; Nickel, B.; Marinovich, M.L. Frequency and characteristics of errors by artificial intelligence (AI) in reading screening mammography: a systematic review. Vol. 207, Breast Cancer Research and Treatment. Springer; 2024. p. 1–13.
- Vickers, A.J. Prediction models in cancer care. CA Cancer J Clin. 2011, 61, 315–26. [Google Scholar]
- Cellina M, Cacioppa LM, Cè M, Chiarpenello V, Costa M, Vincenzo Z, et al. Artificial Intelligence in Lung Cancer Screening: The Future Is Now. Vol. 15, Cancers. Multidisciplinary Digital Publishing Institute (MDPI); 2023.
- Chen H, Lan X, Yu T, Li L, Tang S, Liu S, et al. Development and validation of a radiogenomics model to predict axillary lymph node metastasis in breast cancer integrating MRI with transcriptome data: A multicohort study. Front Oncol 2022, 12. [Google Scholar]
- Jia LL, Zheng QY, Tian JH, He DL, Zhao JX, Zhao LP, et al. Artificial intelligence with magnetic resonance imaging for prediction of pathological complete response to neoadjuvant chemoradiotherapy in rectal cancer: A systematic review and meta-analysis. Vol. 12, Frontiers in Oncology. Frontiers Media S.A.; 2022.
- Lo Gullo R, Marcus E, Huayanay J, Eskreis-Winkler S, Thakur S, Teuwen J, et al. Artificial Intelligence-Enhanced Breast MRI: Applications in Breast Cancer Primary Treatment Response Assessment and Prediction. Vol. 59, Investigative Radiology. Lippincott Williams and Wilkins; 2024. p. 230–42.
- Komura, D.; Ochi, M.; Ishikawa, S. Machine learning methods for histopathological image analysis: Updates in 2024. Comput Struct Biotechnol J [Internet]. 2025, 27, 383–400. [Google Scholar]
- Hunter, B.; Hindocha, S.; Lee, R.W. The Role of Artificial Intelligence in Early Cancer Diagnosis. Cancers (Basel) 2022, 14. [Google Scholar]
- Kumar Y, Shrivastav S, Garg K, Modi N, Wiltos K, Woźniak M, et al. Automating cancer diagnosis using advanced deep learning techniques for multi-cancer image classification. Sci Rep [Internet] 2024, 14, 25006. [Google Scholar] [CrossRef]
- Douglas, D.B.; Wilke, C.A.; Gibson, J.D.; Boone, J.M.; Wintermark, M. Augmented reality: Advances in diagnostic imaging. Vol. 1, Multimodal Technologies and Interaction. MDPI AG; 2017.
- Tharmaseelan H, Hertel A, Rennebaum S, Nörenberg D, Haselmann V, Schoenberg SO, et al. The Potential and Emerging Role of Quantitative Imaging Biomarkers for Cancer Characterization. Cancers. 2022, 14. [Google Scholar]
- Li Y, Dou Y, Da Veiga Leprevost F, Geffen Y, Calinawan AP, Aguet F, et al. Proteogenomic data and resources for pan-cancer analysis. Cancer Cell [Internet] 2023, 41, 1397–406. [Google Scholar] [CrossRef]
- Heo, Y.J.; Hwa, C.; Lee, G.H.; Park, J.M.; An, J.Y. Integrative multi-omics approaches in cancer research: From biological networks to clinical subtypes. Mol Cells 2021, 44, 433–43. [Google Scholar]
- Boehm, K.M.; Khosravi, P.; Vanguri, R.; Gao, J.; Shah, S.P. Harnessing multimodal data integration to advance precision oncology. Vol. 22, Nature Reviews Cancer. Nature Research; 2022, 114–26.
- Alkhalaf S, Alturise F, Bahaddad AA, Elnaim BME, Shabana S, Abdel-Khalek S, et al. Adaptive Aquila Optimizer with Explainable Artificial Intelligence-Enabled Cancer Diagnosis on Medical Imaging. Cancers (Basel) 2023, 15. [Google Scholar]
- Smiley, A.; Reategui-Rivera, C.M.; Villarreal-Zegarra, D.; Escobar-Agreda, S.; Finkelstein, J. Exploring Artificial Intelligence Biases in Predictive Models for Cancer Diagnosis. Vol. 17, Cancers. Multidisciplinary Digital Publishing Institute (MDPI); 2025.
- Talaat, F.M.; Gamel, S.A.; El-Balka, R.M.; Shehata, M.; ZainEldin, H. Grad-CAM Enabled Breast Cancer Classification with a 3D Inception-ResNet V2: Empowering Radiologists with Explainable Insights. Cancers (Basel) 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Husby, U.E. Exploring Breast Cancer Diagnosis: A Study of SHAP and LIME in XAI-Driven Medical Imaging.
- Amann, J.; Blasimme, A.; Vayena, E.; Frey, D.; Madai, V.I. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak 2020, 20. [Google Scholar] [CrossRef]
- van Ineveld RL, van Vliet EJ, Wehrens EJ, Alieva M, Rios AC. 3D imaging for driving cancer discovery. EMBO J 2022, 41. [Google Scholar]
- Keall, P. 4-dimensional computed tomography imaging and treatment planning. Semin Radiat Oncol [Internet] 2004, 14, 81–90. [Google Scholar]
- Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019, 69, 127–57. [Google Scholar] [CrossRef] [PubMed]
- Cabral, B.P.; Braga, L.A.M.; Syed-Abdul, S.; Mota, F.B. Future of Artificial Intelligence Applications in Cancer Care: A Global Cross-Sectional Survey of Researchers. Current Oncology 2023, 30, 3432–46. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, E. High-dimensional role of AI and machine learning in cancer research. Vol. 126, British Journal of Cancer. Springer Nature; 2022. p. 523–32.
- Ghaneiyan M, Farjami P, Mehran H, Mohammadzadeh S, Ghaderi S, Niakan Y, et al. AI-Enhanced Virtual Clinics and Telemedicine for Cancer Treatment. 2024.
- Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H, et al. Nanoparticles for cancer imaging: The good, the bad, and the promise. Nano Today 2013, 8, 454–60. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Bhaumik, J.; Karver, M.R.; Sibel Erdem, S.; Weissleder, R. Targeted nanoagents for the detection of cancers. Vol. 4, Molecular Oncology. John Wiley and Sons Ltd.; 2010. p. 511–28.
- Soltani M, Kashkooli FM, Souri M, Harofte SZ, Harati T, Khadem A, et al. Enhancing clinical translation of cancer using nanoinformatics. Cancers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Far, B.F. Artificial intelligence ethics in precision oncology: balancing advancements in technology with patient privacy and autonomy. Explor Target Antitumor Ther 2023, 4, 685–9. [Google Scholar]
- Ziller A, Mueller TT, Stieger S, Feiner LF, Brandt J, Braren R, et al. Reconciling privacy and accuracy in AI for medical imaging. Nat Mach Intell 2024, 6, 764–74. [Google Scholar] [CrossRef]
- Hantel A, Clancy DD, Kehl KL, Marron JM, Eliezer ;, Van Allen M, et al. A Process Framework for Ethically Deploying Artificial Intelligence in Oncology [Internet]. 2022.
- Urban T, Ziegler E, Lewis R, Hafey C, Sadow C, Van Den Abbeele AD, et al. LesionTracker: Extensible open-source zero-footprint web viewer for cancer imaging research and clinical trials. Cancer Res 2017, 77, e119–22. [Google Scholar] [CrossRef]
- Gouda, W.; Sama, N.U.; Al-Waakid, G.; Humayun, M.; Jhanjhi, N.Z. Detection of Skin Cancer Based on Skin Lesion Images Using Deep Learning. Healthcare (Switzerland) 2022, 10. [Google Scholar] [CrossRef]
- Koh DM, Papanikolaou N, Bick U, Illing R, Kahn CE, Kalpathi-Cramer J, et al. Artificial intelligence and machine learning in cancer imaging. Vol. 2, Communications Medicine. Springer Nature; 2022.
- Cardoso MJ, Li W, Brown R, Ma N, Kerfoot E, Wang Y, et al. MONAI: An open-source framework for deep learning in healthcare 2022, Available from: http://arxiv.org/abs/2211. 0270.
- Caban, J.J.; Joshi, A.; Nagy, P. Rapid development of medical imaging tools with open-source libraries. J Digit Imaging. 2007, 20 (Suppl. 1), 83–93. [Google Scholar] [CrossRef]
- Alabduljabbar, A.; Khan, S.U.; Alsuhaibani, A.; Almarshad, F.; Altherwy, Y.N. Medical imaging datasets, preparation, and availability for artificial intelligence in medical imaging. J Alzheimers Dis Rep [Internet] 2024, 8, 1471–83. [Google Scholar] [CrossRef]
- Ziegler E, Urban T, Brown D, Petts J, Pieper SD, Lewis R, et al. Open Health Imaging Foundation Viewer: An Extensible Open-Source Framework for Building Web-Based Imaging Applications to Support Cancer Research [Internet]. JCO Clin Cancer Inform. 2020, 4. [Google Scholar]
- Heudel, P.E.; Crochet, H.; Blay, J.Y. Impact of artificial intelligence in transforming the doctor–cancer patient relationship. ESMO Real World Data and Digital Oncology [Internet]. 2024, 3, 100026. [Google Scholar]
- Elhaddad M, Hamam S. AI-Driven Clinical Decision Support Systems: An Ongoing Pursuit of Potential. Cureus 2024.
- Hemmer, P.; Schemmer, M.; Kühl, N. Human-AI Complementarity in Hybrid Intelligence Systems: A Structured Literature Review [Internet]. 2021. Available from: https://www.researchgate.net/publication/352882174.
- Ziegelmayer, S.; Graf, M.; Makowski, M.; Gawlitza, J.; Gassert, F. Cost-Effectiveness of Artificial Intelligence Support in Computed Tomography-Based Lung Cancer Screening. Cancers (Basel) 2022, 14. [Google Scholar]
- Mumuni, A.N.; Hasford, F.; Udeme, N.I.; Dada, M.O.; Awojoyogbe, B.O. A SWOT analysis of artificial intelligence in diagnostic imaging in the developing world: Making a case for a paradigm shift. In: Basic Sciences for Sustainable Development: Energy, Artificial intelligence, Chemistry, and Materials Science. De Gruyter; 2023. p. 51–84.
- Rai, H.M.; Pal, A.; Shukla, K.K.; Moqurrab, S.A. ; Amanzholova STRANSFORMATIVEIMPACTSOFAIANDTHEIOTONHEALTHCAREDELIVERY:, M. E.T.H.O.D.O.L.O.G.I.E.S.; ETHICALCONSIDERATIONS; ANDENHANCEMENTAVENUES Engineering Review 2024, 44, 116–37. [Google Scholar]
- Ethics and Governance of Artificial Intelligence for Health WHO Guidance. World Health Organization; 2021.
- Muniyandi, D.; Nandan, H. Artificial Intelligence in Education [Internet]. Available from: https://www.researchgate.net/publication/388594816.
- Mehrmohammadi, M.; Joon Yoon, S.; Yeager, D.; Y Emelianov, S. Photoacoustic Imaging for Cancer Detection and Staging. Curr Mol Imaging 2013, 2, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Suresh, T.; Garikipati, A.; Dangi, A.; Kothapalli, S.R. Modeling combined ultrasound and photoacoustic imaging: Simulations aiding device development and artificial intelligence. Photoacoustics [Internet]. 2021, 24, 100304, https://www.sciencedirect.com/science/article/pii/S2213597921000641. [Google Scholar]
- Carson, T.; Ghoshal, G.; Cornwall, G.B.; Tobias, R.; Schwartz, D.G.; Foley, K.T. Artificial Intelligence-enabled, Real-time Intraoperative Ultrasound Imaging of Neural Structures within the Psoas. Spine (Phila Pa 1976) 2021, 46, E146–52. [Google Scholar]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Vol. 10, Bioengineering. Multidisciplinary Digital Publishing Institute (MDPI); 2023.
- Cheung, H.M.C.; Rubin, D. Challenges and opportunities for artificial intelligence in oncological imaging. Vol. 76, Clinical Radiology. W.B. Saunders Ltd.; 2021. p. 728–36.
- Xu, C.; Coen-Pirani, P.; Jiang, X. Empirical Study of Overfitting in Deep Learning for Predicting Breast Cancer Metastasis. Cancers (Basel) 2023, 15. [Google Scholar]
- Hill DLG. AI in imaging: the regulatory landscape. Vol. 97, British Journal of Radiology. British Institute of Radiology; 2024. p. 483–91.
- Zheng, S.; Cui, X.; Ye, Z. Integrating artificial intelligence into radiological cancer imaging: from diagnosis and treatment response to prognosis. Cancer Biol Med [Internet] 2025, 22, 6–13, https://www.cancerbiomed.org/content/22/1/6. [Google Scholar] [CrossRef]
- Xiang Q, Li D, Hu Z, Yuan Y, Sun Y, Zhu Y, et al. Quantum classical hybrid convolutional neural networks for breast cancer diagnosis. Sci Rep 2024, 14, 24699. [Google Scholar]
- Liao J, Li X, Gan Y, Han S, Rong P, Wang W, et al. Artificial intelligence assists precision medicine in cancer treatment. Vol. 12, Frontiers in Oncology. Frontiers Media S.A.; 2023.
- Agrawal, S.; Vagha, S. A Comprehensive Review of Artificial Intelligence in Prostate Cancer Care: State-of-the-Art Diagnostic Tools and Future Outlook. Cureus 2024. [Google Scholar]
- Dong X, Chen G, Zhu Y, Ma B, Ban X, Wu N, et al. Artificial intelligence in skeletal metastasis imaging. Comput Struct Biotechnol J [Internet]. 2024, 23, 157–64, https://www.sciencedirect.com/science/article/pii/S200103702300421X. [Google Scholar] [CrossRef] [PubMed]
- Elemento, O.; Leslie, C.; Lundin, J.; Tourassi, G. Viewpoint Artificial Intelligence in cancer research, diagnosis and therapy.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).