1. Introduction
The seed microbiome has emerged as a crucial frontier in agricultural research, offering promising avenues for enhancing crop performance and sustainability. Recent advances in seed science, particularly in the realm of beneficial microbes, have revealed the significant impact of seed-associated microorganisms on plant health, growth, and productivity. These microbes—including bacteria, fungi, and cyanobacteria—interact with seeds through biopriming, coating, and inoculation strategies, offering sustainable alternatives to chemical inputs.
Seeds shelter diverse microbial communities, collectively termed the seed microbiome, which play vital duty in seed germination, seedling establishment, and plant development [
1]. These microbial assemblages, comprising bacteria, fungi, and archaea, are unique among plant-associated microbiomes and have been shown to contain primarily beneficial microorganisms [
2]. The seed microbiome serves as a critical link between plant generations, facilitating the vertical transmission of microbial resources from parent plants to offspring [
3].
The importance of seed microbiomes extends beyond their role in plant development. They also contribute significantly to crop resilience against biotic and abiotic inflections, disease resistance, and overall plant fitness [
2]. Furthermore, seed microbiomes have implications for grain quality and food security, making them a subject of intense research interest in recent years [
1]. Studies have revealed that seed microbiomes are influenced by plant genotype, environmental factors, and developmental stages [
1]. The vertical transmission of microbes from parent plants to seeds has been observed, suggesting a mechanism for passing beneficial microbes to offspring [
4].
Seed-associated beneficial microbes have been shown to support seed germination, enhance seedling vigor, increase nutrient uptake, and improve plant resilience to various stresses [
5]. Plant growth-promoting bacteria (PGPB) and fungi have also demonstrated their abilities to increase crop production and improve plant performance under adverse conditions [
6,
7]. Innovative approaches to manipulate seed microbiomes, such as the endophytic microbial introduction(EMI) [
8] and synthetic microbial communities (SynComs) [
9], are being developed to enhance crop performance [
4]. Seed biopriming with beneficial microorganisms has also shown significant potential in improving germination rates, seedling vigor, and crop productivity [
6].
The use of beneficial seed microbes offers an environmentally friendly alternative to chemical fungicides and fertilizers, aligning with the goals of sustainable agriculture [
7]. These microorganisms can reduce the need for synthetic inputs while promoting natural disease suppression and nutrient cycling [
10]. Research has identified various beneficial microbial strains, including species of
Bacillus,
Pseudomonas, and
Trichoderma, which have shown auspicious results in enhancing plant growth and stress tolerance [
11,
12].
As our understanding of seed microbiomes continues to grow, so does the potential for harnessing these microbial communities to improve crop production, enhance food security, and promote sustainable agricultural practices.
2. Seed Microbiome Composition and Transmission
Recent research has noticeably advanced our knowledge of seed microbiome composition and transmission, revealing the complex and dynamic nature of microbial communities associated with seeds.
Seed microbiomes, composed of bacteria, fungi, and archaea, are crucial for plant health and development. They form a unique microbial community distinct from other plant-associated microbiomes [
2]. Seed microbiota composition varies widely, but most seeds share a core microbiome [
13]. Individual seeds are often dominated by a single bacterial taxon (>75% of reads), with high variability between and within plants [
14].
Vertical transmission of microbes from parent plants to seeds is a critical process in seed microbiome assembly. Parent seed and stem endosphere fungal and bacterial communities are key sources of progeny seed microbiomes [
15]. Seed-transmitted fungi and bacteria dominate juvenile crop plant microbiomes by abundance [
16]. Vertical transmission allows beneficial microbes to establish early founder populations, shaping the plant microbiome from the start through priority effects [
16].
Several factors contribute to the composition and transmission of seed microbiomes. Plant genotype, environmental factors, and developmental stages influence seed microbiome composition [
15]. Selection is the key ecological process driving dominant taxa succession during seed filling and maturation [
14]. Storage conditions can affect seed microbiota conservation, with initial seed drying before storage reducing microbial composition [
4].
Seed microbiome assembly and transmission involve complex processes. Abundance-based models classify microbes, with many late colonizers dominating at ripening. Temporal patterns are shaped by niche changes and neutrality [
15]. The transition from seed to seedling involves significant changes in microbial population sizes and community structure [
14].
Understanding seed microbiomes is crucial as seed-associated microbes support germination, protect against pathogens, and enhance seedling nutrition and vigor [
16]. The seed microbiome links the maternal and offspring environments, influencing plant ecology and evolution [
4]. Manipulating seed microbiomes offers potential for improving crop establishment and developing microbial-based solutions for agriculture [
4,
14,
17].
In other words, recent research has revealed the complexity of seed microbiome composition and transmission, highlighting its importance in plant health and agricultural practices. Further studies are needed to fully elucidate the mechanisms of microbial inheritance and their implications for sustainable agriculture.
3. Beneficial Effects of Seed-Associated Microbes
Seed microbes are vital for plant health, growth, and productivity. Recent research has revealed several key benefits of these microorganisms. Seed-associated microbes significantly improve germination rates and seedling vigor. Beneficial microbes boost germination, seedling vigor, biomass, and help overcome seed-related stresses during and after emergence [
5,
18,
19,
20].
Beneficial microbes initiate complex biochemical interactions during seed germination, priming metabolic pathways to enhance vigor and uniformity. Cyanobacteria such as Spirulina platensis enhance photosynthetic capacity in emerging seedlings by upregulating chlorophyll synthesis genes, particularly under cadmium stress [
5]. In a study on mung bean seeds, the addition of cultured microbes from domestic soil significantly enhanced germination frequency compared to controls [
21].
Seed microbes enhance plant growth, with PGPB boosting crop yields by 12-20% (
Figure 1) [
6]. Seed endophytic bacteria enhance crop growth and yields in plants like rice, maize, wheat, and tomatoes [
22]. Microbes produce phytohormones like IAA, gibberellins, and cytokinin, boosting root growth, biomass, and plant development [
22]. For example,
Bacillus subtilis and
Pseudomonas fluorescens secrete hydrolytic enzymes such as α-amylase and invertase, which mobilize stored carbohydrates in seeds, providing energy for radicle emergence [
5,
23]. These bacteria produce phytohormones like IAA, promoting cell elongation and lateral root growth in wheat and maize seedlings [
6].
Seed-associated microbes can also improve nutrient uptake and availability. Some microbes, like mycorrhizal fungi, increase the surface area of plant roots and improve nutrient uptake by creating an extensive mycelial network [
25]. Certain bacteria can even solubilize phosphorus, potassium, and zinc, making these nutrients more available to plants [
6].
Seed microbes enhance plant resilience to environmental stresses like drought and salinity [
7,
22,
26,
27]. Seed biopriming with beneficial microorganisms increases plant resilience and effectiveness under adverse conditions [
6].
Seed-associated microbes also play a crucial role in protecting plants from different pathogens. Some microbes produce compounds that inhibit the growth of harmful pathogens in the soil, reducing the risk of disease [
18,
19,
26,
27]. Seed biopriming offers eco-friendly biotic stress management, serving as an alternative to chemical fungicides [
6,
7,
18]. The seed microbiome influences plant ecology and evolution by enhancing nutrient uptake, pathogen resilience, and abiotic stress tolerance. It also aids plant establishment, colonization, and spread, offering insights into conservation and invasion ecology [
28].
In short, seed-associated microbes offer numerous benefits to plants, from improved germination and growth to enhanced stress tolerance and disease resistance. Understanding and harnessing these beneficial microbes can contribute significantly to sustainable agriculture and plant ecology.
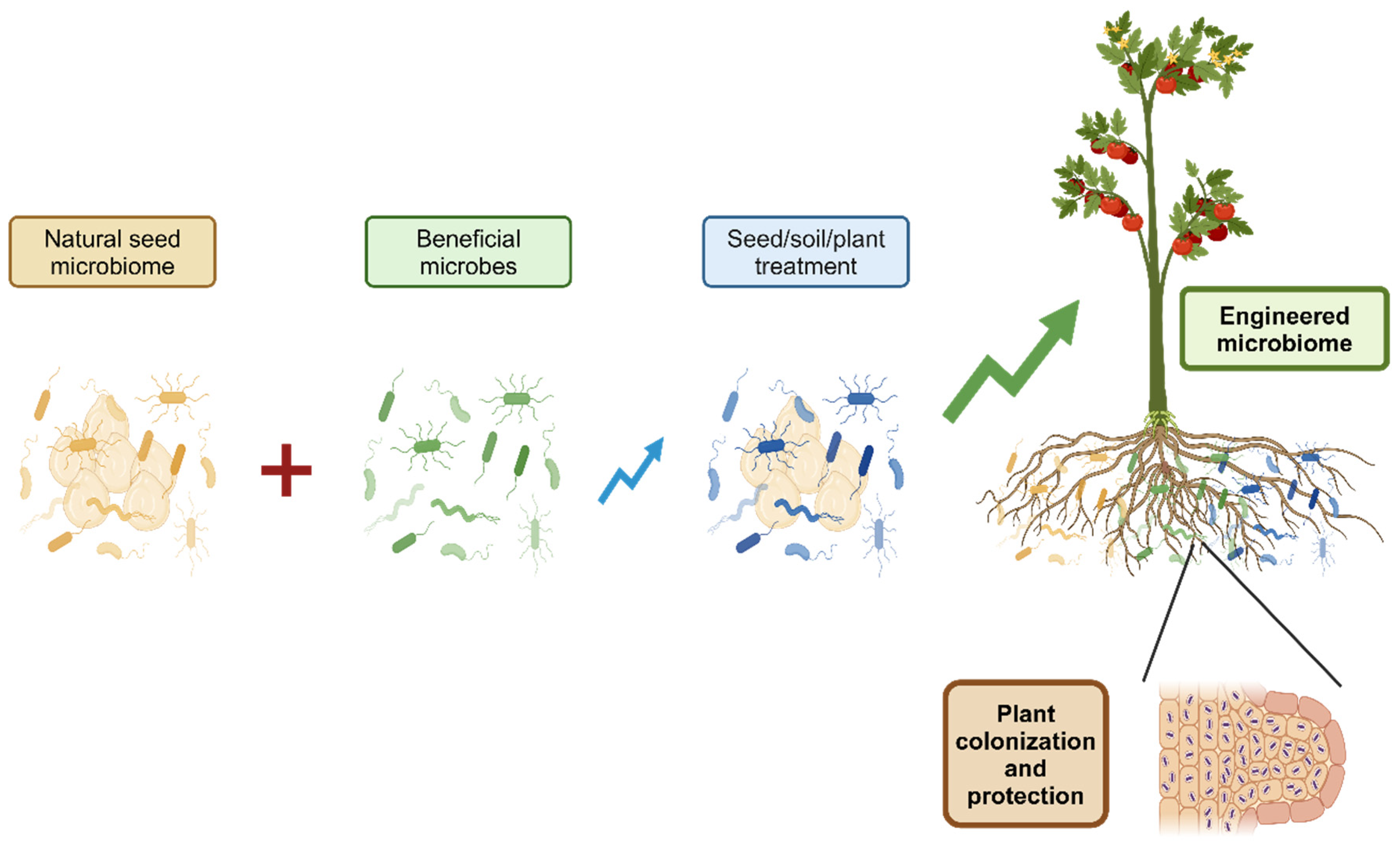
4. Innovative Approaches to Seed Microbiome Engineering
Recent advances in seed microbiome engineering have opened new possibilities for enhancing crop performance and sustainability (
Figure 2).
SynComs offer a promising method to engineer plant microbiota. A study in
FEMS Microbiology Ecology showed that seed inoculation with SynComs effectively shaped seedling microbiota, with SynComs comprising 80% of the community. Strain abundance on seeds was key to successful colonization [
9].
A new framework integrates top-down and bottom-up strategies to engineer natural microbiomes. Using herbicides and degrader inoculation, researchers guided microbiomes toward enhanced bioremediation. They also developed SuperCC, a metabolic modeling tool, to analyze interactions and predict microbiome performance [
30].
Recent research has focused on harnessing seed-associated endophytes for improved crop performance. A 2024 study used genome-wide association studies (GWAS) to identify genetic loci linked to bacterial seed endophyte diversity in fonio [
31]. The results suggested that Seed endophytes promote plant growth by enhancing nutrient availability and uptake [
31].
Understanding and manipulating microbial inheritance has emerged as a key approach in seed microbiome engineering. Researchers divide inheritance into three stages: plant-to-seed, seed dormancy, and seed-to-seedling [
2]. Vertical transmission of seed microbiomes is crucial for forming microbial communities and defending against phytopathogens [
1].
Recent studies have explored engineering seed microbiomes for targeted functions. Researchers have investigated the potential of seed microbe engineering for producing targeted metabolites or antimicrobial compounds to improve plant biomass and yield under stress conditions [
31]. Studies have focused on introducing beneficial bacteria at flowering to engineer the microbiomes of progeny seeds [
1].
These innovative approaches to seed microbiome engineering offer promising avenues for enhancing crop resilience, productivity, and sustainability in agriculture. As research in this field continues to advance, we can expect to see more sophisticated and targeted methods for manipulating seed microbiomes to improve crop performance.
5. Seed Biopriming with Beneficial Microorganisms
Seed biopriming with beneficial microorganisms has emerged as a promising approach for enhancing crop performance and sustainability. This technique merges seed priming with beneficial microbes, boosting plant growth, stress tolerance, and agricultural productivity.
Seed biopriming applies beneficial microbes to seeds with controlled hydration, enabling microbial colonization and metabolic activation without triggering germination [
32]. Many microorganisms or beneficial microbes have shown effectiveness in seed biopriming (
Table 1). For example,
Bacillus,
Pseudomonas, and
Azospirillum, and
Microbacterium species in Bacteria [
32,
33];
Trichoderma species in Fungi [
34]; and
Cyanobacteria, which is particularly useful for dryland restoration [
33].
Biopriming significantly improves seed germination rates, uniformity, and seedling vigor [
6]. Studies have reported increases in germination potential by up to 60% compared to control groups [
54]. Biopriming with PGPB can boost crop yields by 12-20% [
6]. This improvement is attributed to enhanced nutrient uptake, hormone production, and overall plant fitness [
32,
33]. Bioprimed seeds show increased resilience to both biotic and abiotic stresses, including improved tolerance to drought, salinity, and heavy metals [
33,
54] and enhanced resistance against soil-borne pathogens [
32,
33]. Seed biopriming offers an environmentally friendly alternative to chemical fungicides and fertilizers, aligning with sustainable agriculture goals [
6].
Recent research has demonstrated the effectiveness of biopriming across a diverse range of crops, highlighting its potential to enhance agricultural productivity and resilience. In wheat, biopriming has been shown to improve drought tolerance and boost germination potential [
54]. Carrot seeds treated with biopriming techniques have exhibited enhanced germination rates and overall plant growth promotion [
6]. Furthermore, the benefits of biopriming extend to other major crops such as maize, barley, pea, tomato, and sunflower, where significant improvements have been observed in germination rates, seed viability, and ultimately, crop yield [
33]. These findings collectively underscore the versatility and efficacy of biopriming as a promising approach in modern agriculture, offering potential solutions to various challenges faced by farmers across different crop types.
In conclusion, seed biopriming with beneficial microorganisms represents a significant advancement in sustainable agriculture, offering a multifaceted approach to improving crop performance and resilience. As research in this field continues to evolve, it holds exciting potential for addressing global food security challenges while promoting environmentally friendly farming practices.
6. Specific Microbial Strains and Their Effects
Recent studies show specific microbial strains significantly impact seed germination, plant growth, and stress tolerance (
Table 1).
T. harzianum demonstrated the greatest inhibition of seed mycoflora like
Alternaria sp. and
Fusarium spp. in cucumber seeds. It significantly improved seed germination (88.75%), shoot length (14.58 cm), root length (13.58 cm), and seedling vigor (2501.31) [
35].
T. viride and
T. virens also showed strong inhibition of seed mycoflora and improvements in seed germination and seedling growth, though slightly less than
T. harzianum [
35]. Applying T. viride with Pseudomonas fluorescens to cabbage seeds improved seedling vigor, root length, biomass, and chlorophyll content [
55].
Bacillus strains have demonstrated plant growth-promoting effects.
B. subtilis showed ability to solubilize potassium and phosphorus, and produce indole acetic acid (IAA), contributing to improved seed germination [
6]. However, in a comparative study with other bioagents,
B. subtilis was found to be less effective than
Trichoderma species in improving cucumber seed germination and seedling vigor [
35].
Pseudomonas fluorescens effectively controls soft rot caused by
Pectobacterium carotovorum in kale [
56]. When applied in combination with
T. viride to cabbage seeds,
P. fluorescens significantly improved seedling quality characteristics and yield-related traits [
55].
Mycorrhizal fungi (
Rhizophagus irregularis) and
Trichoderma spp. modulate root exudation patterns, releasing metabolites that attract symbiotic microbes while suppressing pathogens. In wheat,
Trichoderma harzianum increases phenolic compounds and peroxidase activity, reinforcing cell walls against oxidative stress. Similarly,
Azospirillum lipoferum enhances nitrogen fixation in maize, improving radicle growth and early biomass accumulation [
5] (
Table 1).
Recent research in FEMS Microbiology Ecology (2024) developed a method for engineering seedling microbiota using synthetic microbial communities (SynComs). This approach successfully modulated seed microbiota composition, with SynComs contributing 80% of the seedling microbiota. Strain abundance on seeds was identified as a key driver of colonization, with
Enterobacteriaceae and
Erwiniaceae as strong colonizers and
Bacillaceae and
Microbacteriaceae as weak colonizers [
9].
These findings demonstrate the diverse and significant effects of specific microbial strains on plant health and productivity. The research emphasizes the potential of these microorganisms in sustainable agriculture practices, particularly in enhancing crop resilience to biotic and abiotic stresses.
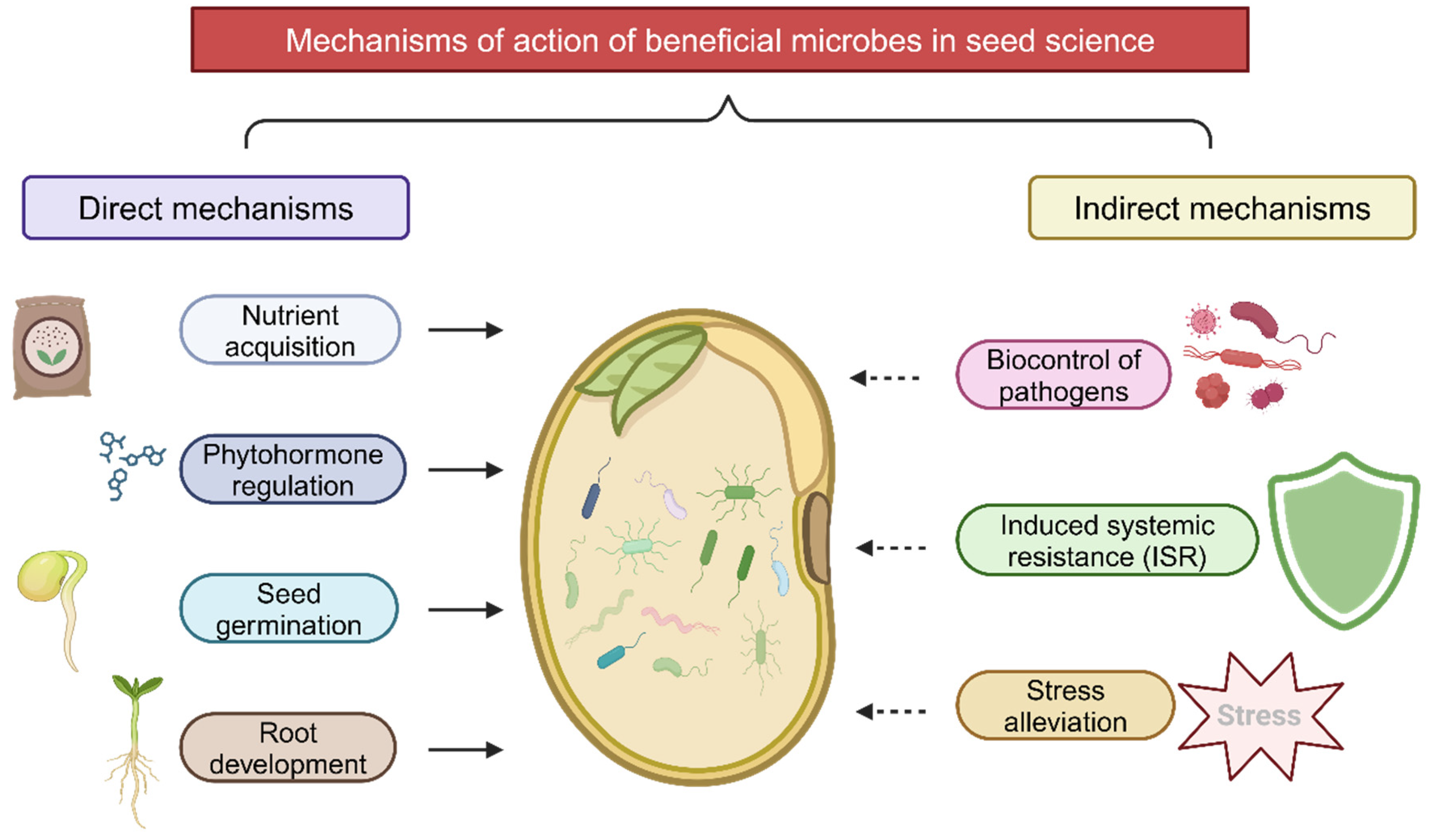
7. Mechanisms of Action
Recent research has revealed several key mechanisms through which beneficial seed-associated microbes promote plant growth and enhance stress tolerance. These mechanisms can be broadly categorized into direct growth promotion, stress tolerance enhancement, and disease suppression (
Figure 1).
Beneficial microbes promote plant growth by producing hormones like IAA, gibberellins, and cytokinins, which enhance root elongation, biomass, and development [
5]. Many beneficial bacteria can solubilize essential nutrients like phosphorus, potassium, and zinc, making them more readily available for plant uptake [
57]. Certain bacteria, especially rhizobia in legumes, can fix atmospheric nitrogen, reducing the need for synthetic fertilizers [
6]. For instance,
Bacillus gaemokensis upregulates salicylic acid (SA), ethylene (ET), and jasmonic acid (JA)pathways in cucumbers, conferring systemic resistance against bacterial pathogens and insect herbivores [
58]. Cyclodipeptides from
Bacillus strains activate defense-related genes, such as
PR-1 and
PDF1.2, even before pathogen exposure [
58]. In rice, biopriming with
Paenibacillus yonginensis alters DNA methylation patterns, enhancing drought tolerance through sustained expression of osmoprotectant genes [
6,
33].
Beneficial microbes boost drought tolerance by releasing osmolytes, improving seed germination and growth under water stress [
57]. Microbes like
Trichoderma harzianum improve the activities of antioxidant enzymes (SOD, APX, CAT, POD) in plants, enhancing their ability to cope with oxidative stress [
5]. For example, in sunflower,
Paraburkholderia phytofirmans upregulates superoxide dismutase (SOD) and catalase (CAT), lowering lipid peroxidation by 45% under 150 mM NaCl stress [
23,
33]. Some bacteria produce 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, which helps regulate ethylene levels in plants, improving stress tolerance [
57]. Microbial consortia mitigate drought through osmolyte synthesis.
Bacillus subtilis QST 713 increases proline levels in wheat seedlings, maintaining cell turgor and reducing electrolyte leakage by 30% [
6].
Beneficial microbes protect plants by producing antibiotics that suppress pathogens [
10,
19,
59]. Certain microbes activate plant defenses, enhancing resistance to various pathogens [
19,
20,
27,
60]. Beneficial microbes can outcompete pathogens for nutrients and space in the rhizosphere [
6,
7]. Seed treatments with
Bacillus velezensis CMRP 4490 produce lipopeptides (e.g., surfactin) that disrupt Fusarium graminearum hyphae, cutting infection rates by 70% in soybean [
5].
Trichoderma harzianum induces systemic resistance in tomatoes via β-1,3-glucanase and chitinase expression, reducing
Botrytis cinerea incidence by 60% [
5,
23].
Beneficial microbes directly influence seed germination and seedling development. Microbes can increase the activity of germination-related enzymes like α-amylase and invertase, improving seed germination and seedling vigor [
5,
26]. Microbes also help mobilize seed reserves, providing energy for seedling growth [
5].
Spirulina platensis coatings restrict cadmium translocation in maize, reducing shoot Cd accumulation by 57% through extracellular sequestration and glutathione-S-transferase activation [
5]. Similarly,
Enterobacter spp. in sunflower enhance phytochelatin synthesis, chelating lead and arsenic in root vacuoles [
33,
61].
These mechanisms demonstrate the multifaceted ways in which beneficial seed-associated microbes contribute to plant health and productivity. The complexity and diversity of these interactions highlight the potential for developing targeted microbial treatments to address specific agricultural challenges, from improving crop yields to enhancing resilience against climate change-induced stresses [
12,
57].
8. Conclusions and Future Directions
Recent advances in beneficial microbes for seed science have revealed promising avenues for enhancing crop performance and sustainability. This review synthesized the latest findings on microbial applications in seed science, emphasizing their role in agricultural productivity, abiotic/biotic stress mitigation, and ecosystem restoration. Several future directions are emerging.
The seed microbiome has been identified as a crucial factor in plant health, development, and resilience to environmental stresses. Research has shown that seed-associated microbes play vital roles in germination, seedling establishment, and long-term plant fitness. Microbial efficacy depends on strain-plant compatibility. For example,
Trichoderma virens isolates exhibit 40% variability in soybean germination outcomes, necessitating tailored formulations [
5]. Advances in nanoencapsulation (e.g., chitosan-coated
Pseudomonas spp.) prolong viability during storage, maintaining 90% cell viability after 12 months [
61]. Research is needed on thermotolerant strains (e.g.,
Geobacillus spp.) for seed treatments in warming climates. Preliminary trials show
Geobacillus stearothermophilus enhances rice germination at 42°C, though field validation is pending [
6].
Studies have demonstrated the vertical transmission of beneficial microbes from parent plants to seeds, suggesting a mechanism for passing advantageous traits to offspring. This finding opens new possibilities for harnessing seed microbiomes to improve crop performance across generations.
Seed biopriming with beneficial microorganisms has shown significant potential in improving germination rates, seedling vigor, and crop productivity. Research indicates that biopriming can increase crop production by ~20% and enhance plant resilience under adverse conditions.
Researchers are exploring innovative approaches to manipulate seed microbiomes for enhanced crop performance. The development of SynComs shows promise for seedling microbiota engineering. A 2024 study in
FEMS Microbiology Ecology demonstrated that SynCom inoculation on seeds could successfully modulate seedling microbiota composition, with SynComs contributing up to 80% of the seedling microbiota [
33]. There is growing interest in understanding and manipulating microbial inheritance and vertical transmission. Researchers are investigating ways to introduce beneficial bacteria at the flowering stage to engineer the microbiomes of progeny seeds [
62].
Future research aims to engineer seed microbiomes to produce targeted metabolites or antimicrobials, boosting plant biomass and yield under stress [
31]. The application of advanced -omics technologies is providing deeper insights into seed-microbe interactions. The 2024 study published in
Microbiome used GWAS to identify genetic loci associated with seed endophyte diversity in fonio millet. This approach opens up new possibilities for understanding the genetic basis of plant-microbe interactions in seeds [
31].
Research focuses on developing microbial inoculants to boost crop resilience to climate change by improving seed germination and seedling growth under stresses like drought, salinity, and extreme temperatures [
61]. The potential of seed-associated microbes in promoting sustainable agriculture and ecosystem restoration is gaining attention. A 2024 review in
Frontiers in Plant Science highlighted the potential of seed biopriming with beneficial microorganisms to enhance crop resilience and effectiveness under adverse conditions, potentially increasing crop production by 12-20% [
62]. Improving the formulation and delivery of beneficial microbes for seed applications is crucial. Research is needed to develop more effective seed coating technologies that ensure the viability and efficacy of beneficial microbes during storage and after planting [
61].
Beneficial microbes revolutionize seed science by bridging agronomic productivity and ecological sustainability. From molecular priming to large-scale ecosystem restoration, microbial technologies offer scalable solutions to global challenges like food security and climate change. Future success hinges on interdisciplinary collaboration—integrating microbiology, genomics, and precision agriculture—to optimize microbial consortia for diverse cropping systems. As seed coatings and biopriming gain traction, they promise to redefine agricultural paradigms, ensuring resilient food systems for generations to come [
23,
61].
References
- Sun, Z.; Adeleke, B.S.; Shi, Y.; Li, C. The Seed Microbiomes of Staple Food Crops. Microb. Biotechnol. 2023, 16, 2236–2249. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Tack, A.J.M.; Lobato, C.; Wassermann, B.; Berg, G. From Seed to Seed: The Role of Microbial Inheritance in the Assembly of the Plant Microbiome. Trends Microbiol. 2023, 31, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Hone, H.; Mann, R.; Yang, G.; Kaur, J.; Tannenbaum, I.; Li, T.; Spangenberg, G.; Sawbridge, T. Profiling, Isolation and Characterisation of Beneficial Microbes from the Seed Microbiomes of Drought Tolerant Wheat. Sci. Rep. 2021, 11, 11916. [Google Scholar] [CrossRef]
- Jonkers, W.; Gundel, P.E.; Verma, S.K.; White, J.F. Editorial: Seed Microbiome Research. Front. Microbiol. 2022, 13, 943329. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed Treatments with Microorganisms Can Have a Biostimulant Effect by Influencing Germination and Seedling Growth of Crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of Seed with Plant Growth-Promoting Bacteria for Improved Germination and Seedling Growth. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Yang, P.; Condrich, A.; Scranton, S.; Hebner, C.; Lu, L.; Ali, M.A. Utilizing Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture. Bacteria 2024, 3, 434–451. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. A New Approach to Modify Plant Microbiomes and Traits by Introducing Beneficial Bacteria at Flowering into Progeny Seeds. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Arnault, G.; Marais, C.; Préveaux, A.; Briand, M.; Poisson, A.-S.; Sarniguet, A.; Barret, M.; Simonin, M. Seedling Microbiota Engineering Using Bacterial Synthetic Community Inoculation on Seeds. FEMS Microbiol. Ecol. 2024, 100, fiae027. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Meza, C.; Valenzuela, F.; Echeverría-Vega, A.; Gomez, A.; Sarkar, S.; Cabeza, R.A.; Arencibia, A.D.; Quiroz, K.; Carrasco, B.; Banerjee, A. Plant-Growth-Promoting Bacteria from Rhizosphere of Chilean Common Bean Ecotype (Phaseolus Vulgaris L.) Supporting Seed Germination and Growth against Salinity Stress. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, A.; Katsenios, N.; Chanioti, S.; Giannoglou, M.; Djordjevic, N.; Katsaros, G. Effect of Foliar and Soil Application of Plant Growth Promoting Bacteria on Growth, Physiology, Yield and Seed Quality of Maize under Mediterranean Conditions. Sci. Rep. 2020, 10, 21060. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Briand, M.; Chesneau, G.; Rochefort, A.; Marais, C.; Sarniguet, A.; Barret, M. Seed Microbiota Revealed by a Large-Scale Meta-Analysis Including 50 Plant Species. New Phytol. 2022, 234, 1448–1463. [Google Scholar] [CrossRef] [PubMed]
- Chesneau, G.; Laroche, B.; Préveaux, A.; Marais, C.; Briand, M.; Marolleau, B.; Simonin, M.; Barret, M. Single Seed Microbiota: Assembly and Transmission from Parent Plant to Seedling. mBio 13, -22. [CrossRef]
- Kim, H.; Jeon, J.; Lee, K.K.; Lee, Y.-H. Longitudinal Transmission of Bacterial and Fungal Communities from Seed to Seed in Rice. Commun. Biol. 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Gutiérrez, J.P.; Lopez-Lavalle, L.A.B. Seed-Transmitted Bacteria and Fungi Dominate Juvenile Plant Microbiomes. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Yang, P.; Condrich, A.; Lu, L.; Scranton, S.; Hebner, C.; Sheykhhasan, M.; Ali, M.A. Genetic Engineering in Bacteria, Fungi, and Oomycetes, Taking Advantage of CRISPR. DNA 2024, 4, 427–454. [Google Scholar] [CrossRef]
- Yang, P. Exploring Plant-Microbe Interactions through the Lens of Beneficial Bacteria, The Ohio State University, 2023.
- Yang, P.; Yuan, P.; Liu, W.; Zhao, Z.; Bernier, M.C.; Zhang, C.; Adhikari, A.; Opiyo, S.O.; Zhao, L.; Banks, F.; et al. Plant Growth Promotion and Plant Disease Suppression Induced by Bacillus Amyloliquefaciens Strain GD4a. Plants 2024, 13, 672. [Google Scholar] [CrossRef]
- Yang, P.; Bokros, N.; Debolt, S.; Zhao, Z.; Xia, Y. Genome Sequence Source of Bacillus Amyloliquefaciens Strain GD4a, a Bacterial Endophyte Associated with Switchgrass Plants. Phytobiomes J. 2022, 6, 354–357. [Google Scholar] [CrossRef]
- Singam, R.; Azhari, A.; Patmanathan, S.N. Microbes Cultured from Garden Soil Positively Impact Seed Germination and Plant Growth. J. Emerg. Investig. 2021. [Google Scholar] [CrossRef]
- L’Hoir, M.; Duponnois, R. Combining the Seed Endophytic Bacteria and the Back to the Future Approaches for Plant Holonbiont Breeding. Front. Agron. 2021, 3. [Google Scholar] [CrossRef]
- Srivastava, S.; Tyagi, R.; Sharma, S. Seed Biopriming as a Promising Approach for Stress Tolerance and Enhancement of Crop Productivity: A Review. J. Sci. Food Agric. 2024, 104, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Kumar, A.; Babalola, O.O. The Role of Microbial Seed Endophytes in Agriculture: Mechanisms and Applications. Cereal Res. Commun. 2024, 1–13. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Yuan, P.; Zhao, Z.; Zhang, C.; Opiyo, S.O.; Adhikari, A.; Zhao, L.; Harsh, G.; Xia, Y. Plant Growth Promotion and Stress Tolerance Enhancement through Inoculation with Bacillus Proteolyticus OSUB18. Biology 2023, 12, 1495. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.O.; Xia, Y. Bacillus Proteolyticus OSUB18 Triggers Induced Systemic Resistance against Bacterial and Fungal Pathogens in Arabidopsis. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef]
- War, A.F.; Bashir, I.; Reshi, Z.A.; Kardol, P.; Rashid, I. Insights into the Seed Microbiome and Its Ecological Significance in Plant Life. Microbiol. Res. 2023, 269, 127318. [Google Scholar] [CrossRef]
- Compant, S.; Cassan, F.; Kostić, T.; Johnson, L.; Brader, G.; Trognitz, F.; Sessitsch, A. Harnessing the Plant Microbiome for Sustainable Crop Production. Nat. Rev. Microbiol. 2025, 23, 9–23. [Google Scholar] [CrossRef]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering Natural Microbiomes toward Enhanced Bioremediation by Microbiome Modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef]
- Tabassum, N.; Ahmed, H.I.; Parween, S.; Sheikh, A.H.; Saad, M.M.; Krattinger, S.G.; Hirt, H. Host Genotype, Soil Composition, and Geo-Climatic Factors Shape the Fonio Seed Microbiome. Microbiome 2024, 12, 11. [Google Scholar] [CrossRef]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed Biopriming with Plant Growth Promoting Rhizobacteria: A Review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef]
- Singh, P.; Vaishnav, A.; Liu, H.; Xiong, C.; Singh, H.B.; Singh, B.K. Seed Biopriming for Sustainable Agriculture and Ecosystem Restoration. Microb. Biotechnol. 2023, 16, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Sarangi, S.; Samal, P.; Khandual, A.; Jena, R.; Bhattacharyya, P.; Naik, S.K.; Mehetre, S.T.; et al. Seed Biopriming With Trichoderma Strains Isolated From Tree Bark Improves Plant Growth, Antioxidative Defense System in Rice and Enhance Straw Degradation Capacity. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Gupta, M. Effect of Bioagents on Cucumber Seed Mycoflora, Seed Germination, and Seedling Vigour. Sci. Rep. 2023, 13, 6052. [Google Scholar] [CrossRef] [PubMed]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed Treatment with Trichoderma Harzianum Alleviates Biotic, Abiotic, and Physiological Stresses in Germinating Seeds and Seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef]
- Mahmoodian, S.; Kowsari*, M.; Motallebi, M.; Zamani, M.; Jahromi, Z.M. Effect of Improved Trichoderma Harzianum on Growth and Resistance Promotion in Bean Plant. Braz. Arch. Biol. Technol. 2022, 65, e22210671. [Google Scholar] [CrossRef]
- Kthiri, Z.; Jabeur, M.B.; Machraoui, M.; Gargouri, S.; Hiba, K.; Hamada, W. Coating Seeds with Trichoderma Strains Promotes Plant Growth and Enhance the Systemic Resistance against Fusarium Crown Rot in Durum Wheat. Egypt. J. Biol. Pest Control 2020, 30, 139. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus Subtilis: A Plant-Growth Promoting Rhizobacterium That Also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Song, P.; Zhao, B.; Sun, X.; Li, L.; Wang, Z.; Ma, C.; Zhang, J. Effects of Bacillus Subtilis HS5B5 on Maize Seed Germination and Seedling Growth under NaCl Stress Conditions. Agronomy 2023, 13, 1874. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, R.; Liu, J. Effects of Bacillus Subtilis QM3 on Germination and Antioxidant Enzymes Activities of Wheat Seeds under Salt Stress. Open Access Libr. J. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Patel, M.; Islam, S.; Husain, F.M.; Yadav, V.K.; Park, H.-K.; Yadav, K.K.; Bagatharia, S.; Joshi, M.; Jeon, B.-H.; Patel, A. Bacillus Subtilis ER-08, a Multifunctional Plant Growth-Promoting Rhizobacterium, Promotes the Growth of Fenugreek (Trigonella Foenum-Graecum L.) Plants under Salt and Drought Stress. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S.; Jamil, N.; Iqbal, R.; Ali, B.; Ercisli, S.; et al. Multifaceted Impacts of Plant-Beneficial Pseudomonas Spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296–22315. [Google Scholar] [CrossRef] [PubMed]
- Nilmat, A.; Thepbandit, W.; Chuaboon, W.; Athinuwat, D. Pseudomonas Fluorescens SP007S Formulations in Controlling Soft Rot Disease and Promoting Growth in Kale. Agronomy 2023, 13, 1856. [Google Scholar] [CrossRef]
- Rivera-Conde, M.I.; Aranda-Ocampo, S.; Carrillo-Castañeda, G.; Gijón-Hernández, A.R.; Bueno-Aguilar, G.M.; Rivera-Conde, M.I.; Aranda-Ocampo, S.; Carrillo-Castañeda, G.; Gijón-Hernández, A.R.; Bueno-Aguilar, G.M. Effect of Fluorescent Pseudomonas on Tomato Seed Germination and Seedling Vigor. Rev. Chapingo Ser. Hortic. 2018, 24, 121–131. [Google Scholar] [CrossRef]
- Lally, R.D.; Galbally, P.; Moreira, A.S.; Spink, J.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Application of Endophytic Pseudomonas Fluorescens and a Bacterial Consortium to Brassica Napus Can Increase Plant Height and Biomass under Greenhouse and Field Conditions. Front. Plant Sci. 2017, 8, 2193. [Google Scholar] [CrossRef]
- Langendries, S.; Goormachtig, S. Paenibacillus Polymyxa, a Jack of All Trades. Environ. Microbiol. 2021, 23, 5659–5669. [Google Scholar] [CrossRef]
- Soni, R.; Rawal, K.; Keharia, H. Genomics Assisted Functional Characterization of Paenibacillus Polymyxa HK4 as a Biocontrol and Plant Growth Promoting Bacterium. Microbiol. Res. 2021, 248, 126734. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, S.-K.; Ryu, C.-M.; Park, S.-H. Chronicle of a Soil Bacterium: Paenibacillus Polymyxa E681 as a Tiny Guardian of Plant and Human Health. Front. Microbiol. 2019, 10, 467. [Google Scholar] [CrossRef]
- Acuña, J.J.; Rilling, J.I.; Inostroza, N.G.; Zhang, Q.; Wick, L.Y.; Sessitsch, A.; Jorquera, M.A. Variovorax Sp. Strain P1R9 Applied Individually or as Part of Bacterial Consortia Enhances Wheat Germination under Salt Stress Conditions. Sci. Rep. 2024, 14, 2070. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Humm, E.; Rubbi, L.; Del Vecchio, G.; Ha, S.M.; Pellegrini, M.; Gunsalus, R.P. Complete Genome of Variovorax Sp. EBFNA2, Isolated from a Surface-Sterilized Fava Bean Nodule. Microbiol. Resour. Announc. 2024, 13, e00762-24. [Google Scholar] [CrossRef]
- José, J.F.B. de S.; Hernandes, M.A.S.; Lisboa, B.B.; Volpiano, C.G.; Schlindwein, G.; Trindade, J.K. da; Lattuada, D.S.; Beneduzi, A.; Vargas, L.K. Seed Size and Azospirillum Brasilense Ab-V5 and Ab-V6 Inoculation Influences Germination and Early Seedling Vigor of Acacia Mearnsii. Ciênc. Florest. 2024, 34, e85546–e85546. [Google Scholar] [CrossRef]
- Méndez-Gómez, M.; Castro-Mercado, E.; López-Bucio, J.; García-Pineda, E. Azospirillum Brasilense Sp245 Triggers Cytokinin Signaling in Root Tips and Improves Biomass Accumulation in Arabidopsis through Canonical Cytokinin Receptors. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2021, 27, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Imran, M.; Kang, S.-M.; Khan, M.A.; Asaf, S.; Kim, W.-C.; Lee, I.-J. Seed Bio-Priming of Wheat with a Novel Bacterial Strain to Modulate Drought Stress in Daegu, South Korea. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Vij, S.; Sharma, N.; Sharma, M.; Mohanta, T.K.; Kaushik, P. Application of Trichoderma Viride and Pseudomonas Fluorescens to Cabbage (Brassica Oleracea L.) Improves Both Its Seedling Quality and Field Performance. Sustainability 2022, 14, 7583. [Google Scholar] [CrossRef]
- Dumigan, C.R.; Deyholos, M.K. Soil and Seed Both Influence Bacterial Diversity in the Microbiome of the Cannabis Sativa Seedling Endosphere. Front. Plant Sci. 2024, 15. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi; Lalmuanpuii, R.; Singh, P.K. Zothanpuia Plant Growth Promoting Bacteria (PGPB)-Induced Plant Adaptations to Stresses: An Updated Review. PeerJ 2024, 12, e17882. [CrossRef]
- Song, G.C.; Choi, H.K.; Kim, Y.S.; Choi, J.S.; Ryu, C.-M. Seed Defense Biopriming with Bacterial Cyclodipeptides Triggers Immunity in Cucumber and Pepper. Sci. Rep. 2017, 7, 14209. [Google Scholar] [CrossRef]
- Yang, P.; Condrich, A.; Scranton, S.; Hebner, C.; Lu, L.; Ali, M.A. Utilising Plant Growth-Promoting Rhizobacteria (PGPR) to Advance Sustainable Agriculture 2024.
- Yang, P.; Zhao, Z.; Virag, A.; Becker, T.; Zhao, L.; Liu, W.; Xia, Y. Botrytis Cinerea In-Vivo Inoculation Assays for Early-, Middle- and Late-Stage Strawberries. BIO-Protoc. 2023, 13. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An Attractive Tool for Sustainable Agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Hanif, M.S.; Tayyab, M.; Baillo, E.H.; Islam, M.M.; Islam, W.; Li, X. Plant Microbiome Technology for Sustainable Agriculture. Front. Microbiol. 2024, 15. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).